Abstract
The recovery and control of volatile organic compounds (VOCs) have gained significant attention. Supported sulfonic acid materials show potential in converting aromatic VOCs into non-volatile sulfonic acid derivatives. However, their effectiveness is closely tied to the anchoring state of the sulfonic acid groups. In this study, two supported sulfonic acids, SSA@CdO and SSA@CaO, were synthesized via the respective reactions of CdO and CaO with chlorosulfonic acid to investigate how the properties of the supports influence sulfonic acid anchoring and reactivity toward o-xylene. Comprehensive characterization and performance tests revealed that sulfonic acid groups on CdO were covalently bonded, forming positively charged sites ([O0.5Cd–O]ɗ−–SO3Hɗ+) with high loading (9.7 mmol/g), enabling excellent o-xylene removal (≥95.6%) and adsorption capacity (51.67–91.59 mg/g) at 130–150 °C. In contrast, ion-paired bonding on CaO formed negatively charged sites ([O0.5Ca]+:OSO3H−), which were inactive in electrophilic sulfonation. This work provides new insights for enhancing supported sulfonic acid materials in VOC treatment.
1. Introduction
Volatile organic compounds (VOCs), which encompass a wide range of substances including alkanes, alkenes, aromatic hydrocarbons, halogenated hydrocarbons, oxygenated organic compounds, and numerous other species, are significant pollutants present in both industrial processes and residential settings [1,2,3]. Owing to their high volatility and high diffusivity, VOCs present significant health concerns and are a major contributor to environmental problems such as photochemical fog and haze [4,5]. Among these hazards are metabolic abnormalities, pulmonary conditions, sensory discomfort, nervous system impairment, and, in severe cases, carcinogenic effects that pose a substantial threat to human health [6,7]. Consequently, governments worldwide have established stringent emission standards and regulatory frameworks to control VOC emissions from relevant industries [8,9]. As a result, the mitigation and recovery of VOCs have become focal points of research and development, representing one of the most significant domains in the field of separation and purification technologies [10,11].
The mitigation and recovery of VOCs can be achieved through various treatment technologies, including extraction, condensation, membrane separation, photocatalytic degradation, thermal catalytic oxidation, and microbial degradation [12,13,14,15,16,17,18,19]. Because of its high efficiency, relatively low operating costs, ease of operation, process safety, and potential for adsorbent reuse, the adsorption method is widely acknowledged as the best strategy for reducing VOCs [20]. Temperature swing adsorption (TSA) and pressure swing adsorption (PSA) have both been widely used in industrial settings [21,22,23]. Both techniques enable adsorbent re-utilization and adsorbate recovery. However, in both processes, adsorption occurs through physical mechanisms under either high-pressure or relatively low-temperature conditions, while desorption requires either a vacuum or a heating environment. It is well established that the textural properties of the adsorbent, including pore volume and specific surface area, and pore size, significantly influence its adsorption capacity [24,25]. However, these characteristics tend to deteriorate over time due to pore collapse or clogging and surface contamination [26,27], which may limit the long-term effectiveness and broad applicability of the adsorbent. Therefore, in order to improve the technological capabilities for processing VOCs, it is essential to develop more advanced adsorption methods and materials.
In our prior study, a new approach utilizing reactive adsorption based on supported sulfonic acid was introduced for the effective removal and recovery of aromatic VOCs, including benzene, toluene, xylene, and ethylbenzene [28]. In this approach, silica-supported sulfonic acids (SSSA), synthesized by treating silica gel with sulfuric acid, effectively remove o-xylene from gaseous streams through a sulfonation reaction mechanism. Unlike conventional PSA and TSA processes, this approach provides the distinct advantage of simultaneously removing aromatic pollutants, recovering the adsorbed aryl sulfonic acid, and recycling the solid support, thereby demonstrating considerable potential for mitigating and recovering aromatic VOCs from waste gas streams. According to our recent research [28,29,30], when sulfuric acid was used as a precursor for the preparation of SSSA, sulfonic acid groups were anchored on the silica support surface in two distinct forms: a chemically bound state (C-state) and a physically adsorbed state (P-state). Interestingly, compared to its P-state analog, the C-state sulfonic acid exhibited significantly higher reactivity toward o-xylene, a typical aromatic molecule. Furthermore, previous studies have indicated that the percentage of C-state sulfonic acid groups in SSSA materials can be effectively controlled by modifying the composition or structure of the support [28], the preparation method [29], and the reaction medium [30]. These factors mainly regulate the concentration of hydroxyl groups present on the surface of the silica support, consequently affecting the percentage of sulfonic acid groups in the C-state that are attached through Si–O–S bonding. If the silicon element in the Si–O–S bond were replaced with other elements, it would be worthwhile to investigate whether the number of sulfonic acid groups bonded through the oxygen bridge and their reactivity would change as a result. This is because the electronic structure of the sulfur element may be affected by the substitution of alternative elements.
Therefore, this study initially investigated the effects of substituting SiO2 with CdO and CaO as supports, regarding the loading capacity of C-state sulfonic acid groups and their reactive adsorption efficiency in eliminating o-xylene. Considering that calcium (Ca) and cadmium (Cd) belong to Group IIA and Group IIB elements, respectively, and exhibit a notable difference in electronegativity [31], commercial CaO and CdO powders with comparable particle sizes, specific surface areas, and pore volumes were selected as supports. This approach aimed to emphasize the influence of the electronegativity of the cationic central element in oxide supports on the characteristics of the generated sulfonic acid groups. Additionally, both CaO and CdO supports were allowed to react with ClSO3H acid in a CH2Cl2 solvent, resulting in the selective formation of the corresponding supported sulfonic acids in their C-state form (SSA@CaO and SSA@CdO) [32], as depicted in Scheme 1. It was found that the sulfonic acid group loading capacity in SSA@CdO was significantly greater compared to that in SSA@CaO. More importantly, SSA@CdO demonstrated superior reactive adsorption performance for o-xylene. Under the specified experimental conditions, the breakthrough reactive adsorption capacity achieved 91.60 mg/g, whereas SSA@CaO showed negligible reactivity adsorption ability. This marked difference in reactive adsorption activity for o-xylene can be attributed to the differing electrical characteristics of the sulfonic acid groups present in the two functional materials. Therefore, this study can provide both theoretical and experimental foundations for the development of advanced supported sulfonic acid materials and their application in VOC resource-oriented processing.

Scheme 1.
Preparation process for SSA@CdO and SSA@CaO materials.
2. Results and Discussion
2.1. Basic Characterization of CdO and CaO Support Materials
In accordance with the fundamental research nature of this study, commercially available reagent-grade CdO and CaO powders were directly selected as support materials for sulfonic acid immobilization. Figure 1 presents the XRD patterns and SEM images of CdO and CaO. It was evident that CdO exhibited sharp diffraction peaks at 2θ angles of 33.0°, 38.3°, 55.3°, 65.9° and 69.3° (Figure 1A), which were in agreement with the standard diffraction card PDF#05-0640 [33]. This indicates that CdO possesses a cubic crystal structure (lattice parameters a = b = c = 4.695 Å) and belongs to the Fm-3m space group. The morphology of CdO particles revealed micrometer-sized rectangular blocks with regular and relatively smooth surfaces (Figure 1B). Furthermore, CaO displayed characteristic peaks at 2θ angles of 32.2°, 37.3°, 53.8°, 64.7° and 67.37° (Figure 1C), which aligned closely with the standard diffraction card PDF#37-1497 [34]. This confirms that CaO also has a cubic structure (lattice parameters a = b = c = 4.811 Å) and belongs to the Fm-3m space group. However, its particles exhibited an irregular nodular morphology at the micrometer level, with relatively smooth surfaces (Figure 1D).

Figure 1.
(A) XRD pattern of CdO; (B) SEM image of CdO; (C) XRD pattern of CaO; and (D) SEM image of CaO.
The thermogravimetric analysis (TGA) curves presented in Figure 2A demonstrated that the weight loss of CaO and CdO was merely 0.2% and 0.3%, respectively, when heated from 50 °C to 350 °C at rate of 5 °C/min. However, FTIR (Figure 2B) analysis revealed a prominent absorption peak corresponding to hydroxyl groups at approximately 3450 cm−1 for both materials [35]. These findings indicate that following pre-drying treatment, the surfaces of CaO and CdO exhibit abundant and stable bonded hydroxyl groups, while their moisture adsorption capacity remains negligible. Consequently, these materials are deemed suitable as supports for sulfonic acid group immobilization [36,37]. Furthermore, Figure 2C and Figure 2D respectively depict the nitrogen adsorption–desorption isotherms of CdO and CaO. It was observed that both materials exhibited characteristics inconsistent with Type III isotherms as classified by IUPAC [38,39], with negligible hysteresis loop phenomena between the adsorption and desorption branches. This finding confirms that CdO and CaO are non-porous materials with low specific surface areas, which aligns with the results obtained from SEM and XRD characterizations. Additionally, through fitting analysis of the desorption branch isotherms using the BET model, the specific surface areas of CdO and CaO were determined to be 10.5 m2/g and 8.9 m2/g, respectively, demonstrating relatively close values. This similarity facilitates further investigation into the influence of intrinsic property differences between the two materials on their sulfonic acid functionalization.

Figure 2.
(A) TG curves and DTG curves of CdO and CaO; (B) FT-IR spectra of CdO and CaO; (C) N2 adsorption–desorption isotherm of CdO; (D) N2 adsorption–desorption isotherm of CaO.
2.2. Comparison of Sulfonic Acid Loading in SSA@CaO and SSA@CdO Materials with Basic Characterization
According to the methodology outlined in Section 3.2, the pre-dried CdO and CaO materials were subjected to chlorosulfonic acid treatment. Figures S1A and S1B show the XRD patterns of SSA@CdO and SSA@CaO, respectively. The main diffraction peaks of both the SSA@CdO and SSA@CaO samples were found to be largely consistent with those of the respective pristine CdO and CaO supports. Furthermore, several weak additional diffraction peaks were observed in both samples, which can be attributed to the formation of CdSO4 (PDF#53-0841) and CaSO4 (PDF#37-1496) [40,41]. Therefore, the XRD results suggest that in a CH2Cl2 medium, ClSO3H primarily reacts with surface hydroxyl groups on CaO or CdO (as shown in Scheme 1), leading to the formation of chemically bonded supported sulfonic acid groups. Titration analysis, performed based on the principle of acid–base neutralization in aqueous solution, indicated that the loadings of sulfonic acid groups for both SSA@CdO and SSA@CaO were 9.7 mmol/g and 2.2 mmol/g, respectively. This demonstrates that the sulfonic acid group loading on SSA@CdO is significantly higher than that on SSA@CaO, approximately 4.4 times greater. Furthermore, the TGA and DTG curves (Figure S2) revealed a substantial weight loss stage within the temperature range of approximately 120–270 °C for both SSA@CdO and SSA@CaO, with corresponding weight loss rates of 79.5% and 18.3%, respectively. The ratio of these weight loss rates is approximately 4.3, which aligns well with the titration analysis data for sulfonic acid groups presented earlier. This suggests that the observed weight loss is primarily resulting from the breakdown of sulfonic acid groups in the C-state, which are located on the surface of CdO and CaO [28,29]. Additionally, calculations based on the peak area of the NH3-TPD curve at the gas–solid interface (see Figure S3) showed that the ratio of sulfonic acid site densities in SSA@CdO to those in SSA@CaO was 4.6, a value that was consistent with the titration analysis results. These two sets of experimental data collectively confirm that the sulfonic acid group loading of SSA@CdO is substantially higher than that of SSA@CaO. As illustrated in Scheme 1, this difference can be ascribed to the varying densities of surface hydroxyl groups [42], indicating that CdO possesses a higher concentration of hydroxyl groups.
Figure 3A and Figure 3B respectively present the SEM images of SSA@CdO and SSA@CaO. It is evident that, in comparison to the pristine CdO (Figure 1B) and CaO (Figure 1D), the morphologies of the modified SSA@CdO and SSA@CaO underwent significant reconstruction. Specifically, the originally well-defined edges and faces were no longer visible for SSA@CdO, and prominent gap structures emerged on the surface. Meanwhile, SSA@CaO underwent decomposition into finer particles. These morphological changes can be ascribed to the etching outcome induced by ClSO3H during the modification process [43,44]. The etching effect and the resultant anchoring of sulfonic acid groups may lead to the reduction in the specific surface area of SSA@CdO and SSA@CaO. This hypothesis is supported by the N2 adsorption–desorption isotherms presented in Figure 3C,D. The specific surface area of SSA@CdO was determined to be 1.5 m2/g, which was 9.0 m2/g lower than that of the support material, while the specific surface area of SSA@CaO was measured to be 7.0 m2/g, representing a reduction of 1.9 m2/g compared to the support, based on the BET model. It is speculated that the substantial decrease in the specific surface area of SSA@CdO may be strongly associated with its higher loading of sulfonic acid groups [45]. The FTIR spectra of both SSA@CdO and SSA@CaO are shown in Figure S4. Compared to the FTIR spectra of the aforementioned supports, four characteristic absorption peaks corresponding to sulfonic acid groups emerged at similar positions. Specifically, the peaks observed at 589 cm−1 and 678 cm−1 were associated with S-O bending vibrations, while the peak at 888 cm−1 was linked to S-O stretching vibration. Additionally, the peak detected at 1278 cm−1 was assigned to the symmetric stretching vibration of the S=O bond [43,44]. Furthermore, qualitative analysis via EDS (Figure 4) revealed that both SSA@CdO and SSA@CaO incorporated sulfur elements. Notably, the spectral peak intensity of sulfur in SSA@CdO was higher than that in SSA@CaO. These findings further support the conclusions drawn from the aforementioned quantitative analysis of sulfonic groups.

Figure 3.
(A) SEM images of SSA@CdO; (B) SEM images of SSA@CaO; (C) N2 adsorption–desorption isotherms of SSA@CdO; (D) N2 adsorption–desorption isotherms of SSA@CaO.

Figure 4.
(A) EDS spectra of CdO; (B) EDS spectra of SSA@CdO; (C) EDS spectra of CaO; (D) EDS spectra of SSA@CaO.
2.3. Comparison of SSA@CdO and SSA@CaO Reactivity for o-Xylene Removal
The reactivity between supported sulfonic acid materials and aromatic VOCs can be evaluated by examining the trend curve of the removal efficiency with temperature change [28,29,30]. As shown in Figure 5A, the relationship curves between Xo−x (the removal rate of o-xylene) and T for SSA@CdO and SSA@CaO materials, along with those for their respective supports (CdO and CaO), are presented. Among these results, one set aligned with expectations; that is, unmodified CdO and CaO exhibited a removal rate of less than 24.2% for o-xylene due to physical adsorption. Unexpectedly, a significant difference was observed in the removal behavior of o-xylene between SSA@CdO and SSA@CaO. Consistent with the previously reported removal behavior of SiO2-supported sulfonic acid for o-xylene [29], SSA@CdO demonstrated typical temperature-dependent removal characteristics, where Xo−x followed an inverted “U” profile as a function of temperature. A removal efficiency of over 95.6% was attained within the temperature interval of 130 °C to 150 °C, which is designated as the reactive adsorption temperature zone. This suggests that SSA@CdO exhibits excellent reactivity in the elimination of o-xylene. Conversely, across the entire testing temperature range, the removal rate of o-xylene by SSA@CaO consistently remained below 26.3%. This performance aligns with the physical adsorption behavior observed for pristine CaO, indicating a lack of chemical reactivity of SSA@CaO.
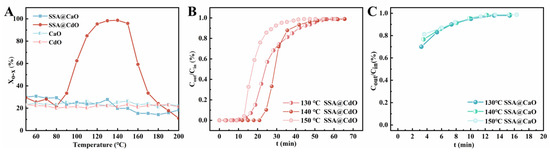
Figure 5.
(A) Relationship curves between Xo−x and T; (B) reactive adsorption breakthrough curves in SSA@CdO at different temperatures; (C) reactive adsorption breakthrough curves in SSA@CaO at different temperatures.
Additionally, the effectiveness of reactive adsorption of both SSA@CdO and SSA@CaO for o-xylene was quantitatively assessed and compared through isothermal reactive adsorption breakthrough curves. Specifically, Figure 5B and Figure 5C present the breakthrough curves for SSA@CdO and SSA@CaO, respectively, at the designated temperatures within the reactive adsorption temperature range. The exact values of tB and QB for each material are summarized in Table 1, which were obtained from the corresponding breakthrough curves. When SSA@CdO was used as the adsorbent bed material for o-xylene removal, its reactive adsorption behavior exhibited significant temperature dependence. At 130 °C, 140 °C, and 150 °C, the tB value varied from 12.92 min to 22.90 min, while the QB value ranged from 51.67 mg/g to 91.59 mg/g, indicating relatively high adsorption efficiency. Notably, the optimal performance was achieved at 140 °C. This optimized temperature aligns closely with that of SiO2-based analogues, albeit with relatively lower tB and QB values [29]. In contrast, the corresponding curve of SSA@CaO indicated that the o-xylene pollutant broke through immediately upon input, leading to both the tB and QB values being zero. These findings collectively suggest that there are fundamental differences in the reactivity of SSA@CdO and SSA@CaO during the removal of o-xylene. This discrepancy cannot be adequately explained solely by the numerical difference in the loading amount of sulfonic acid groups between the two materials. Hence, it is imperative to further investigate the underlying mechanisms.

Table 1.
Adsorption parameters of SSA@CdO and SSA@CaO for o-xylene at different temperatures.
2.4. Analysis of Anchoring States of Sulfonic Acid Groups and o-Xylene Removal Mechanism
As previously discussed, the loading of sulfonic acid groups in SSA@CdO (9.7 mmol/g) surpassed that in SSA@CaO (2.2 mmol/g). This can be ascribed to the higher density of surface hydroxyl groups in SSA@CdO compared to SSA@CaO. It is assumed that the sulfonic acid groups immobilized in SSA@CdO and SSA@CaO exhibit a homogeneous distribution and demonstrate equivalent reactivity towards o-xylene sulfonation; it could be inferred that SSA@CaO would demonstrate certain reactivity and adsorption performance rather than zero. However, this assumption does not align with the experimental results obtained in this study. Therefore, we further deduce that the sulfonic acid groups in SSA@CdO and SSA@CaO may exist in different anchoring states, which are determined by their generation via Cd–OH or Ca–OH. Considering the relatively small electronegativity difference (Δ = 1.75) between Cd (1.69) and O (3.44) [31], as depicted in Scheme 1, it is reasonable to deduce that when Cd–OH reacts with chlorosulfonic acid, the resulting sulfonic acid groups are covalently bonded (CB) to the surface of the CdO support ([O0.5Cd–O]ɗ−–SO3Hɗ+). As shown in Figure 6A, the CB state sulfonic acid sites in SSA@CdO material possess a positive charge, which enables them to participate in electrophilic substitution reactions, particularly sulfonation processes involving o-xylene compounds [46,47]. Conversely, due to the significant difference in electronegativity (Δ = 2.44) between Ca (1.00) and O (3.44), a strong ionic character is expected in their bonding interaction [31], the sulfonic acid groups originating from Ca-OH are anchored to the surface of the CaO support through ion-pair (IP) interactions. The sulfonic acid groups are changed into negatively charged hydrogen sulfate groups ([O0.5Ca]+:OSO3H−) by this interaction, preventing their involvement in the electrophilic reaction of sulfonation with o-xylene molecules (as illustrated in Figure 6A).

Figure 6.
(A) Reactive adsorption equations of CB-state sulfonic acid in SSA@CdO and IP-state sulfonic acid in SSA@CaO with o-xylene; (B) NH3-TPD curves of SSA@CdO and SSA@CaO; (C) XPS spectrum of S2p of SSA@CdO; (D) XPS spectrum of S2p of SSA@CaO.
The distinction between the sulfonic acid groups in the CB-state and IP-state of SSA@CdO and SSA@CaO was further confirmed through NH3-TPD and XPS characterization. As shown in Figure 6B, within the temperature range of 50 °C to 450 °C, the NH3-TPD analysis of both SSA@CdO and SSA@CaO exhibited a single desorption peak for each material. Nevertheless, notable differences were observed in terms of the central position, intensity, and area of these peaks. The maximum desorption temperature of ammonia from the SSA@CaO surface (330 °C) was considerably higher than that from the SSA@CdO surface (185 °C). This suggests that the binding strength of ammonia to the sulfonic acid sites in SSA@CaO is stronger than that in SSA@CdO, which aligns with the characteristics of the anchored sulfonic acid groups in these two materials. Prior to the TPD analysis, hydrogen bonds are formed between NH3 molecules and the sulfonic acid groups present in the SSA@CdO and SSA@CaO materials, subsequently adsorbing onto their surfaces from the gaseous phase. Given that the acidity of [O0.5Ca]+:OSO3H− is stronger than that of [O0.5Cd–O]ɗ−–SO3Hɗ+, it is reasonable to infer that the hydrogen bond affinity at the IP-state sites exceeds that observed at the CB-state sites. Clearly, the desorption peak temperature of ammonia corresponding to SSA@CdO is lower than that observed for SSA@CaO. Moreover, the ratio of the ammonia desorption peak area for SSA@CdO relative to SSA@CaO was calculated to be 4.6. This value indicates the proportional relationship between the densities of anchored sulfonic acid groups in the two materials and aligns with the conclusions drawn in the preceding analysis.
Furthermore, Figure 6C and Figure 6D present the S2p core-level spectra of SSA@CdO and SSA@CaO, respectively. Subsequently, each single peak in both materials was resolved into doublet peaks, corresponding to S 2p3/2 and S 2p1/2, which were attributed to sulfonic acid groups [48]. The results indicate that the two signals of SSA@CdO are located at 169.90 eV and 168.80 eV, respectively, which are higher than the corresponding values for SSA@CaO (169.65 eV and 168.41 eV). These findings suggest that the sulfur element in SSA@CdO exhibits a higher positive charge compared to that in SSA@CaO. This result further corroborates the hypothesis that there are differences in the anchoring states of sulfonic acid groups between the two materials.
In summary, the sulfonic acid groups in the CB state of SSA@CdO exhibit significant sulfonation reactivity, which contributes to its outstanding reactive adsorption performance toward o-xylene. In conjunction with previous research on the elimination of o-xylene using SiO2-supported sulfonic acids [29], it can be inferred that the reactive adsorption product of SSA@CdO and o-xylene is 3,4-dimethylbenzenesulfonic acid (Figure 6A). To validate this anticipation, the product was extracted using methanol and subsequently characterized in detail through HPLC-MS, FT-IR, and 1H-NMR. As shown in Figure 7A, a distinct molecular ion peak at m/z = 185, corresponding to a molecular weight of 186, was visible in the mass spectra acquired in negative ion mode [35]. This value is in excellent agreement with the theoretical molecular weight of the expected product. Further structural characterization was performed using FT-IR analysis via the liquid film method (Figure 7B). A broad O-H stretching vibration at 3435 cm−1, along with asymmetric and symmetric C–H stretching vibrations of methyl groups observed at 2919 and 2842 cm−1, were identified as characteristic absorption bands visible in the FT-IR spectrum [49]. Additionally, the spectrum exhibited aromatic C=C skeletal vibrations at 1640, 1500, and 1450 cm−1, a methyl C-H bending vibration at 1380 cm−1 [50], and distinct O=S=O stretching vibrations at 1189 and 1046 cm−1 [35]. Notably, diagnostic peaks at 885 and 820 cm−1 confirmed the 1,3,4-trisubstitution pattern on the benzene ring [51]. Moreover, 1H-NMR analysis (Figure 7C) provided further structural insights. Three distinct aromatic proton signals were identified at δ7.04, δ7.28, and δ7.35, corresponding to three chemically inequivalent hydrogen atoms on the benzene ring. A characteristic singlet at δ2.20 with an integration ratio of 6H was attributed to the methyl group protons. The observed 1:1:1:6 integration ratio of the four proton groups corroborated the hydrogen distribution pattern [52]. Collectively, these findings confirm that the adsorbed product is 3,4-dimethylbenzenesulfonic acid.

Figure 7.
(A) ESI-MS spectrum of adsorption product; (B) FTIR spectrum of adsorption product; (C) 1H-NMR spectrum of adsorption product.
3. Materials and Methods
3.1. Reagents and Materials
Cadmium oxide (CdO, ≥99.0%) and calcium oxide (CaO, ≥98.0%) were provided by Aladdin Reagent Co., Ltd. (Shanghai, China). Potassium hydrogen phthalate (KHC8H4O4, ≥99.5%), sodium hydroxide (NaOH, ≥96.0%), and hydrochloric acid (HCl, mass fraction 36–38%) were purchased from Yongda Chemical Reagent Co., Ltd. (Tianjin, China). Chlorosulfonic acid (ClSO3H, ≥98%) was obtained from AiKeDa Chemical Reagent Company (Chengdu, China). Absolute ethanol (C2H5OH, ≥99.7%), methanol (CH3OH, ≥98%), o-xylene (C8H10, ≥99%) and dichloromethane (CH2Cl2, ≥98%) were supplied by Damao Chemical Reagent Co., Ltd. (Tianjin, China). A laboratory water purification system (model no. SCSJ-IV, manufactured by Shanghai Hetai Instrument Co., Ltd., Shanghai, China) was utilized to prepare the deionized water used in the experiment.
3.2. Preparation of SSA@CdO and SSA@CaO
SSA@CdO and SSA@CaO were prepared by the chlorosulfonic acid modification method [35]. The specific procedures were as follows: Firstly, CdO and CaO powders were placed into a drying oven and heated at 120 °C for 8 h to remove surface-adsorbed moisture. After cooling the powders to room temperature, 3.0 g of the support material was accurately weighed and dispersed in 15 mL of CH2Cl2. The mixture was continuously stirred using a magnetic stirrer for 30 min to obtain a uniformly dispersed suspension, which was labeled as solution A. Subsequently, solution B was prepared by diluting 2.5 mL of ClSO3H with 10 mL of CH2Cl2. Solution B was then transferred into a dropping funnel under controlled pressure and slowly added to solution A under continuous stirring. When the reaction had progressed for 3 h, the solid product was isolated via suction filtration. It was subsequently washed with 10 mL of CH2Cl2 and re-isolated through suction filtration. This procedure was repeated twice to ensure adequate purification. The obtained pure product was subjected to vacuum drying at a temperature of 60 °C for a duration of 12 h, cooled to room temperature, and then ground and sieved using a 425 mm (40 mesh) sieve. Thus, SSA@CdO and SSA@CaO were successfully synthesized and stored in a desiccator prior to use.
3.3. Quantification of the Loading Amount of Sulfonic Acid Groups
To determine the quantity of sulfonic acid groups loaded (QS, mmol/g) in SSA@CdO and SSA@CaO, a precise sample weight (approximately 0.05 g, denoted as W) was measured using an analytical balance. At room temperature, the weighed sample was transferred into a 50 mL conical flask containing 10.00 mL (V1) of 0.1 mol/L (C1) NaOH solution. The mixture was continuously stirred for 30 min using a magnetic stirrer to ensure complete neutralization of the sulfonic acid loading in the sample. Subsequently, Phenolphthalein was used as an indicator, and the remaining sodium hydroxide was titrated using a 0.1 mol/L (C2) HCl solution. During the titration process, the volume of HCl consumed was measured (V2). Based on the experimental data obtained, the QS value can be calculated through the corresponding Formula (1):
The NaOH solution was standardized using potassium hydrogen phthalate (KHC8H4O4) as a reference material to determine its precise concentration (C1). This standardized NaOH solution was then employed to titrate and determine the concentration of the HCl solution (C2).
3.4. Dynamic Adsorption Experiment of o-Xylene
Dynamic adsorption tests were carried out to evaluate the efficiency of SSA@CdO materials in removing o-xylene from gaseous streams. A custom-designed fixed-bed column arrangement was used in these trials. Ultra-pure N2 was used as the carrier gas in all trials, with its flow rate (Vg) precisely controlled and maintained at 0.050 L/min by means of a mass flow controller. The gaseous o-xylene stream was generated by passing the nitrogen through a gas washing bottle containing liquid o-xylene, which was maintained at 0 °C via a low-temperature circulating pump serving as a cooling medium. This setup ensured a consistent inlet o-xylene concentration (Cin) of 7.5 mg/L. The simulated gas then passed through a quartz tube (6 mm inner diameter × 40 cm length), packed with a mixture of the adsorbent (50 mg) and inert quartz sand (100 mg, particle size range 0.180–0.425 mm). The outlet o-xylene concentration (Cou) was monitored using a GC7900 gas chromatograph (Tianmei, Shanghai, China). A programmed temperature ramping technique was used to determine the temperature range for the interaction between the adsorbent and o-xylene (from 50 °C to 250 °C at a heating rate of 2 °C/min). To investigate the effect of adsorption temperature on the samples’ adsorption capability, breakthrough curves were recorded. Finally, using Equations (2) and (3), the breakthrough adsorption capacity (QB, mg/g) and elimination effectiveness of o-xylene (Xo−x, %) were calculated.
3.5. Materials Analysis and Characterization
A D8 advanced Kα X-ray diffractometer was employed to characterize the adsorbent by X-ray diffraction (XRD). The X-ray diffractometer is equipped with a copper target X-ray source. With a scanning rate of 20°/min and a step size of 0.02°, the measurement range was adjusted to 2θ = 10° − 80° (λ = 0.154 nm; Bruker, Bremen, Germany). A SU-8600 field emission scanning electron microscope (FESEM, equipped with a Bruker XFlash7|6 energy dispersive X-ray spectrometer, Bruker, Bremen, Germany) was used to investigate energy-dispersive morphology observation and X-ray spectroscopy (EDS) analysis at an acceleration voltage of 10 kV in order to examine the materials’ microstructure and chemical makeup. A Kubo X1000 automated specific surface area and pore size analyzer was used to assess the N2 adsorption–desorption isotherms at 77 K following degassing under vacuum at 120 °C for 2 h (Beijing Builder Electronic Technology Co., Ltd., Beijing, China). The Brunauer–Emmett–Teller (BET) linear fitting equation was calculated using the specific surface area. Fourier transform infrared (FT-IR) analysis was performed using a Bruker AXS Tensor 27 spectrometer (Bruker, Bremen, Germany). The spectral acquisition range was set from 500 to 4000 cm−1. Thermogravimetric analysis (TGA) was carried out using a TA-Q600 SDT analyzer(TA Instruments, New Castle, DE, USA). A sample mass of 10–15 mg was heated from room temperature to 400 °C at a heating rate of 20 °C/min under a nitrogen flow of 50 mL/min.
The PCA-1200 chemical adsorption analyzer (Beijing Builder Electronic Technology Co., Ltd., Beijing, China) was employed to perform ammonia temperature-programmed desorption (NH3-TPD) tests on SSA@CdO and SSA@CaO samples in a helium atmosphere at a heating rate of 10 °C/min. Prior to analysis, 10.0 mg of the sample was accurately weighed and placed in the sample tube. Subsequently, under the condition of continuous purging with argon at a flow rate of 30 mL/min, the sample was pretreated at 100 °C for 1 h to ensure complete dehydration. Then, the sample was cooled to room temperature, and pure ammonia was introduced at a flow rate of 30 mL/min for 1 h to allow the sulfonic acid groups in the sample to fully combine with ammonia molecules. Finally, continuous purging was carried out under the protection of helium at the same flow rate until the instrument baseline stabilized.
Adsorption products were dissolved in chromatographic-grade methanol and subsequently analyzed by a Q-TOF liquid chromatography-mass spectrometry (LC-MS) system (Agilent Technologies, Santa Clara, CA, USA) in negative ion mode for molecular weight determination. Nuclear magnetic resonance (NMR) spectra were acquired using an AVANCE NEO-500 NMR spectrometer (Bruker, Bremen, Germany) operating at 500 MHz, with deuterated dimethyl sulfoxide (DMSO-d6) as the solvent, and 1H-NMR spectra were recorded. X-ray photoelectron spectroscopy (XPS) analysis was performed using an ESCALAB QXi XPS spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). A pass energy of 50 eV was used to produce high-resolution spectra of particular elements (S 2p), which were then calibrated relative to the C 1s peak at 284.8 eV.
4. Conclusions
Using commercial CdO and CaO powders with comparable particle sizes and textural properties as supports, two supported sulfonic acid materials (SSA@CdO and SSA@CaO) were synthesized under consistent conditions via the chlorosulfonic acid modification method. It was found that SSA@CdO had a significantly larger loading amount of sulfonic acid groups (9.7 mmol/g) than SSA@CaO (2.2 mmol/g), which was credited to the higher density of surface hydroxyl groups in SSA@CdO compared to SSA@CaO. More importantly, the substantial difference in electronegativity between the Cd and Ca elements led to the sulfonic acid groups being anchored onto the surfaces of CdO and CaO in the CB−state and IP−state, respectively. Therefore, the SSA@CdO demonstrated excellent removal efficiency (≥95.6%) and superior breakthrough reactive adsorption capacity (ranging from 51.67 mg/g to 91.59 mg/g) for o-xylene within the temperature range of 130 °C to 150 °C, whereas SSA@CaO exhibited negligible reactive adsorption ability. Furthermore, the reactive adsorption product of SSA@CdO with o-xylene was identified as 3,4-dimethylbenzenesulfonic acid through HPLC-MS, FT-IR, and 1H-NMR techniques. These findings can provide crucial strategic insights for the development of efficient supported sulfonic acid materials for resource-oriented treatment of aromatic VOCs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13080275/s1, Figure S1: XRD pattern of the samples: (A) SSA@CdO; (B) SSA@CaO; Figure S2: TG curves and DTG curves of SSA@CdO and SSA@CaO; Figure S3: NH3-TPD curves of SSA@CdO and SSA@CaO; Figure S4. FT-IR spectra of SSA@CdO and SSA@CaO; Table S1: XRF analysis result of the SSA@CaO sample.
Author Contributions
Conceptualization, H.W. and Z.M.; methodology, X.Z.; software, X.Z.; validation, H.W. and Y.N.; formal analysis, H.W.; investigation, H.W. and Y.N.; resources, Z.M.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, Z.M.; visualization, Z.M.; supervision, Z.M.; project administration, Z.M.; funding acquisition, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the Key Research and Development Program of Hebei (22327503D) and the National Natural Science Foundation of China (22176049).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sui, H.; Liu, H.; An, P.; He, L.; Li, X.; Cong, S. Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Q.; Wang, F.; Xie, J.; Li, Y.; Li, J.; Wu, S. Emission behavior; environmental impact and priority-controlled pollutants assessment of volatile organic compounds (VOCs) during asphalt pavement construction based on laboratory experiment. J. Hazard. Mater. 2020, 398, 122904. [Google Scholar] [CrossRef]
- Siu, B.; Chowdhury, A.R.; Yan, Z.; Humphrey, S.M.; Hutter, T. Selective adsorption of volatile organic compounds in metal-organic frameworks (MOFs). Coord. Chem. Rev. 2023, 485, 215119. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics 2022, 10, 29. [Google Scholar] [CrossRef]
- Ha Chi, N.N.; Kim Oanh, N.T. Photochemical smog modeling of PM2.5 for assessment of associated health impacts in crowded urban area of Southeast Asia. Environ. Technol. Innov. 2020, 21, 101241. [Google Scholar] [CrossRef]
- Hao, X.; Dai, L.; Deng, J.; Liu, Y.; Jing, L.; Wang, J.; Pei, W.; Zhang, X.; Hou, Z.; Dai, H. Nanotubular OMS-2 supported single-atom platinum catalysts highly active for benzene oxidation. J. Phys. Chem. C 2021, 125, 17696–17708. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.-t. Recent advances in surface and interface design of photocatalysts for the degradation of volatile organic compounds. Adv. Colloid Interface Sci. 2020, 284, 102275. [Google Scholar] [CrossRef] [PubMed]
- Kolade, M.A.; Kogelbauer, A.; Alpay, E. Adsorptive reactor technology for VOC abatement. Chem. Eng. Sci. 2009, 64, 1167–1177. [Google Scholar] [CrossRef]
- Velinova, R.; Kaneva, N.; Ivanov, G.; Kovacheva, D.; Spassova, I.; Todorova, S.; Atanasova, G.; Naydenov, A. Synthesis and Characterization of Pd/La2O3/ZnO Catalyst for Complete Oxidation of Methane, Propane and Butane. Inorganics 2025, 13, 17. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Liu, G.; Shi, C. Research status of volatile organic compound (VOC) removal technology and prospect of new strategies: A review. Environ. Sci. Process. Impacts 2023, 25, 727–740. [Google Scholar] [CrossRef]
- Baskaran, D.; Dhamodharan, D.; Behera, U.S.; Byun, H.-S. A comprehensive review and perspective research in technology integration for the treatment of gaseous volatile organic compounds. Environ. Res. 2024, 251, 118472. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Teng, Y.-J.; Li, Z.-G.; Liu, W.-H.; Lee, M.-R. Magnetic nanoparticles used in headspace extraction coupled with DSI-GC-IT/MS for analysis of VOCs in dry Traditional Chinese Medicine. Chin. Chem. Lett. 2016, 27, 178–184. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Favre, E. Energy efficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy 2016, 95, 291–302. [Google Scholar] [CrossRef]
- Lyu, J.; Zhou, L.; Shao, J.; Zhou, Z.; Gao, J.; Dong, Y.; Wang, Z.; Li, J. TiO2 hollow heterophase junction with enhanced pollutant adsorption; light harvesting; and charge separation for photocatalytic degradation of volatile organic compounds. Chem. Eng. J. 2022, 391, 123602. [Google Scholar] [CrossRef]
- Fatima, S.; Govardhan, B.; Kalyani, S.; Sridhar, S. Extraction of volatile organic compounds from water and wastewater by vacuum-driven membrane process: A comprehensive review. Chem. Eng. J. 2022, 434, 134664. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Zong, C.; Li, Y.; Jin, W. Study on membrane performance in vapor permeation of VOC/N2 mixtures via modified constant volume/variable pressure method. Sep. Purif. Technol. 2018, 200, 273–283. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Soltan, W.B.; Peng, J.; Cao, Z.; Fu, Z.; Liu, H. Bimetallic Fe-Mn loaded H-ZSM-5 zeolites for excellent VOCs catalytic oxidation at low-temperatures: Synergistic effects and catalytic mechanisms. Chem. Eng. J. 2023, 475, 146251. [Google Scholar] [CrossRef]
- Masi, M.; Nissim, W.G.; Pandolfi, C.; Azzarello, E.; Mancuso, S. Modelling botanical biofiltration of indoor air streams contaminated by volatile organic compounds. J. Hazard. Mater. 2022, 422, 126875. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Guo, Y.; Zhu, T.; Xu, W. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J. 2021, 411, 128558. [Google Scholar] [CrossRef]
- Dammak, N.; Fakhfakh, N.; Fourmentin, S.; Benzina, M. Treatment of gas containing hydrophobic VOCs by adsorption process on raw and intercalated clays. Res. Chem. Intermed. 2014, 41, 5475–5493. [Google Scholar] [CrossRef]
- Ambrożek, B.; Zwarycz-Makles, K. Theoretical and experimental studies of the recovery of volatile organic compounds from waste air streams in the thermal swing adsorption system with closed-loop regeneration of adsorbent. Energy Convers. Manag. 2014, 85, 646–654. [Google Scholar] [CrossRef]
- Ramalingam, S.G.; Pré, P.; Giraudet, S.; Le Coq, L.; Le Cloirec, P.; Baudouin, O.; Déchelotte, S. Different families of volatile organic compounds pollution control by microporous carbons in temperature swing adsorption processes. J. Hazard. Mater. 2012, 221–222, 242–247. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Alston, S.; Xiao, Y.; Ajayan, P.; Bu, X.; Feng, P. Multi-Stage optimization of pore size and shape in pore-space-partitioned Metal–Organic Frameworks for highly selective and sensitive benzene capture. Angew. Chem. Int. Ed. 2024, 64, e202415576. [Google Scholar] [CrossRef]
- Cao, M.; Wang, J.; Liu, X.; Pei, Y.; Gao, M.; Wang, W.; Yang, H. Bio-inspired adsorbent with ultra-uniform and abundance sites accelerate breaking the trade-off effect between adsorption capacity and removal efficiency. Chem. Eng. J. 2023, 465, 142790. [Google Scholar] [CrossRef]
- Pak, S.-H.; Jeon, M.-J.; Jeon, Y.-W. Study of sulfuric acid treatment of activated carbon used to enhance mixed VOC removal. Int. Biodeterior. Biodegrad. 2016, 113, 195–200. [Google Scholar] [CrossRef]
- Gao, K.; Ma, M.; Liu, Y.; Ma, Z. A comparative study of the removal of o-xylene from gas streams using mesoporous silicas and their silica supported sulfuric acids. J. Hazard. Mater. 2021, 409, 124965. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Gao, K.; Ma, Z.; Ding, J. Influence of preparation method on the adsorptive performance of silica sulfuric acid for the removal of gaseous o-xylene. Sep. Purif. Technol. 2021, 265, 118484. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, M.; Qian, J.; Wang, Y.; Ma, Z.; Ma, X. Influence of impregnation medium on the adsorptive performance of silica sulfuric acid for the removal of Gaseous o-xylene: Comparison on ethyl acetate and water. Catalysts 2022, 12, 737. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Zolfigol, M.A. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 2001, 57, 9509–9511. [Google Scholar] [CrossRef]
- Banoqitah, E.M.; Saleh, M.A.; Damoom, M.M.; Alhawsawi, A.M.; Kasmani, R.M.; Al-Hada, N.M. One-step synthesis of bunsenite cadmium oxide nanoparticles. Appl. Sci. 2022, 13, 438. [Google Scholar] [CrossRef]
- Hong, J.; Zhao, Y.; Wu, J.; Xie, X.; Zhao, P.; Li, S.; Yao, H.; Luo, G.; Liu, Z.; Yang, X. Fabrication of Al2O3/CaO with anti-sintering for efficient removal of As2O3 in simulated flue gas: Experimental and DFT study. Fuel 2022, 307, 121812. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Wen, X.; Su, X.; Chu, Y.; Liang, J. Heteroatom Sr doped MCM-41 molecular sieve for VOC adsorption: Study of the surface functionalization and adsorption performance. Chem. Eng. J. 2024, 488, 150924. [Google Scholar] [CrossRef]
- Zhou, G.; Cheng, Y.; Yu, Z.; Liu, X.; Chen, D.; Wang, J.; Hang, Y.; Xu, Y.; Li, C.; Lu, Z. Regulation of coordination and doping environment via target molecular transformation for boosting selective photocatalytic ability. Chem. Commun. 2022, 58, 10036–10039. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Guo, X.; Ma, S. Revealing the mechanism of hydration on the CO2 kinetic adsorption of CaO surface via ReaxFF MD simulations with experiments. Appl. Surf. Sci. 2024, 670, 160652. [Google Scholar] [CrossRef]
- Ghotekar, S.; Ravikumar, C.R.; Chauhan, A.; Hikku, G.S.; Lin, K.-Y.A.; Rahdar, A.; Hitler, L.; Jabir, M.S.; Marzban, A.; Oza, R. Eco-friendly fabrication of CdO nanoparticles using Polyalthia longifolia leaves extract for antibacterial and electrochemical sensing studies. J. Sol-Gel Sci. Technol. 2024, 110, 221–232. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, S.; Pal, B. Superior adsorptive removal of brilliant green and phenol red dyes mixture by CaO nanoparticles extracted from egg shells. J. Nanostruct. Chem. 2021, 12, 207–221. [Google Scholar] [CrossRef]
- Li, X.; Lei, Z.; Qu, J.; Zhou, X.; Li, Z.; Zhang, Q. Separation of Cu(ii) from Cd(ii) in sulfate solution using CaCO3 and FeSO4 based on mechanochemical activation. RSC Adv. 2017, 7, 2002–2008. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tuan, W.-H.; Lai, P.-L. Transformation from calcium sulfate to calcium phosphate in biological environment. J. Mater. Sci. Mater. Med. 2021, 32, 146. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Cross, H.E.; Brown, D.R.; Düren, T.; Williams, J.J.; Lee, A.F.; Wilson, K. Interdependent lateral interactions; hydrophobicity and acid strength and their influence on the catalytic activity of nanoporous sulfonic acid silicas. Green Chem. 2010, 12, 1383–1391. [Google Scholar] [CrossRef]
- Lv, S.; Ma, X.; Wang, Y.; Zheng, Y.; Ma, Z.; Liu, T. Cyclic siloxane removal by ring-opening polymerization on silica gel-supported sulfuric acid. Chem. Eng. J. 2025, 504, 158842. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, R.; He, Y.; Ma, Z.; Ma, X. Efficient reactive adsorption of hexamethyldisiloxane on MCM-41 supported sulfuric acid. Renew. Energy 2024, 224, 120174. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Wang, H.; Zhao, D.; Liu, Y.; Ma, Z. Enhancement of gaseous o-xylene elimination by chlorosulfonic acid-modified H-zeolite socony mobil-5. Molecules 2024, 29, 3507. [Google Scholar] [CrossRef]
- Parreño, R.P. The correlation of sulfonation reaction kinetics with the degree of sulfonation (DS) and its effects on microstructure and morphology of electrospun fibers for the membrane of fuel cells. RSC Adv. 2023, 13, 2523–2529. [Google Scholar] [CrossRef]
- Wang, P.C.; Chen, J.; Lu, M. Electrophilic aromatic nitration: Substituent effects of monosubstituted benzenes. J. Chin. Chem. Soc. 2013, 57, 967–971. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Tan, M.; Jiang, B.; Zheng, J.; Tsubaki, N.; Wu, M. Monodispersed hollow SO3H-functionalized carbon/silica as efficient solid acid catalyst for esterification of oleic acid. ACS Appl. Mater. Interfaces 2015, 7, 26767–26775. [Google Scholar] [CrossRef]
- Karthick, N.K.; Arivazhagan, G.; Kannan, P.P.; Kumbharkhane, A.C.; Joshi, Y.S. Homo/hetero interactions in the binary solutions of toluene with acetonitrile: FTIR spectroscopic; theoretical and dielectric studies. J. Mol. Struct. 2019, 1192, 208–216. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, B.; Fan, S.; Wang, P.; Wang, X.; Long, D.; Zhang, L.; Yang, X.; Li, X. In situ FTIR spectra investigation of the photocatalytic degradation of gaseous toluene over a novel hedgehog-like CaFe2O4 hollow-structured materials. Catal. Commun. 2019, 130, 105754. [Google Scholar] [CrossRef]
- Aripova, S.F.; Abdilalimov, O. Convolacine a new alkaloid from convolvulus subhirsutus. Chem. Nat. Compd. 1993, 29, 74–75. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).