A Rapid and Low-Cost Synthesis of ZSM-5 Single Crystals: The Inhibitory Effect of NH4F on Twinning
Abstract
1. Introduction
2. Results and Discussion
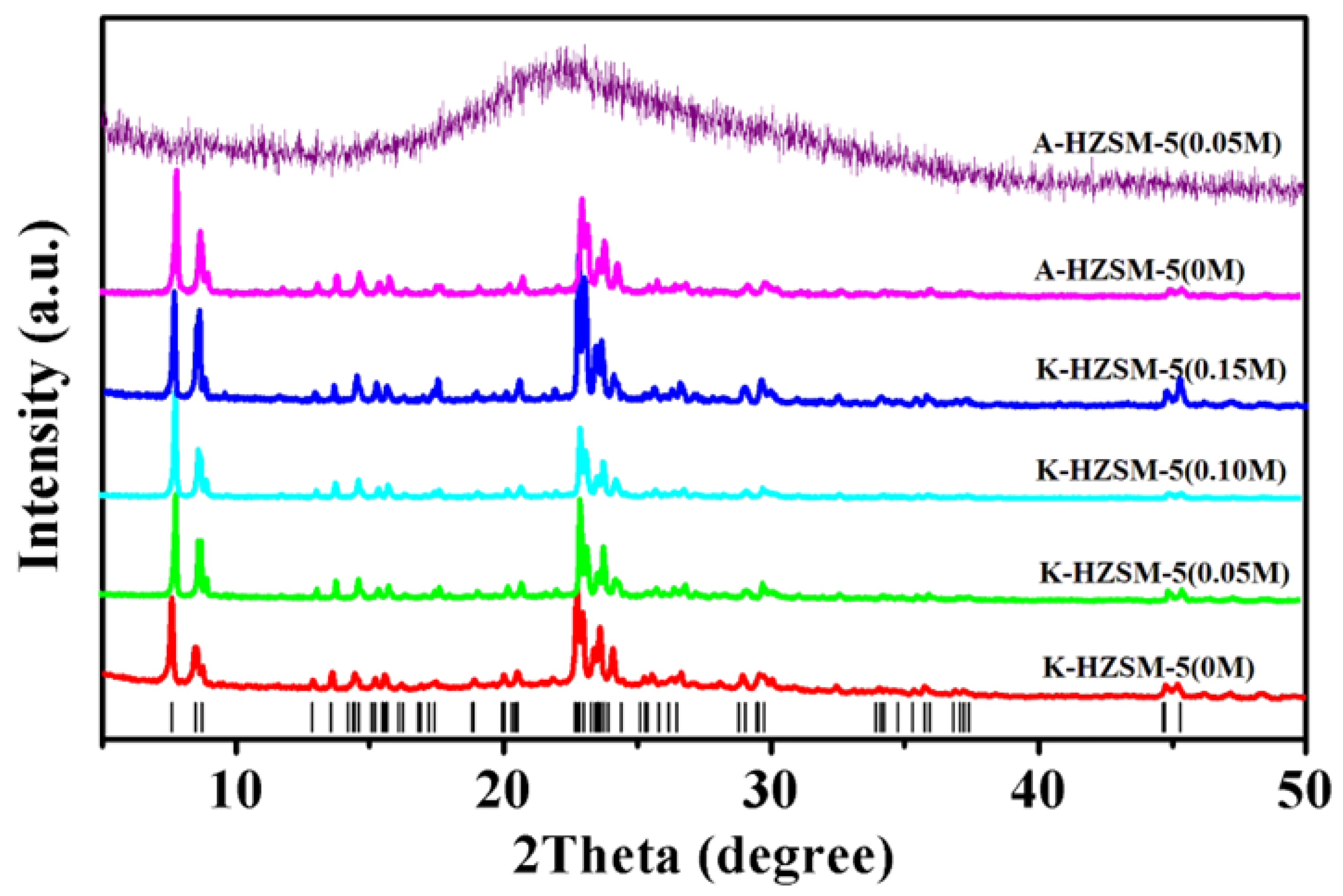
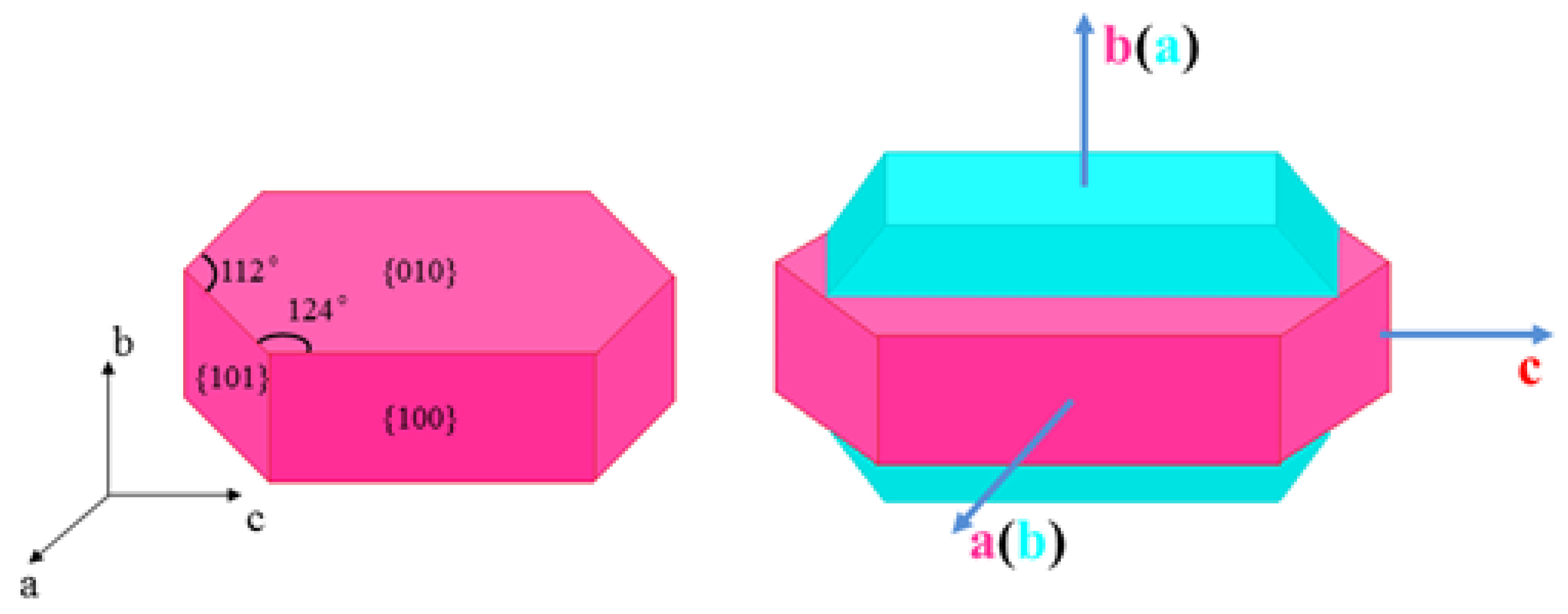
2.1. X-Ray Diffraction Analysis
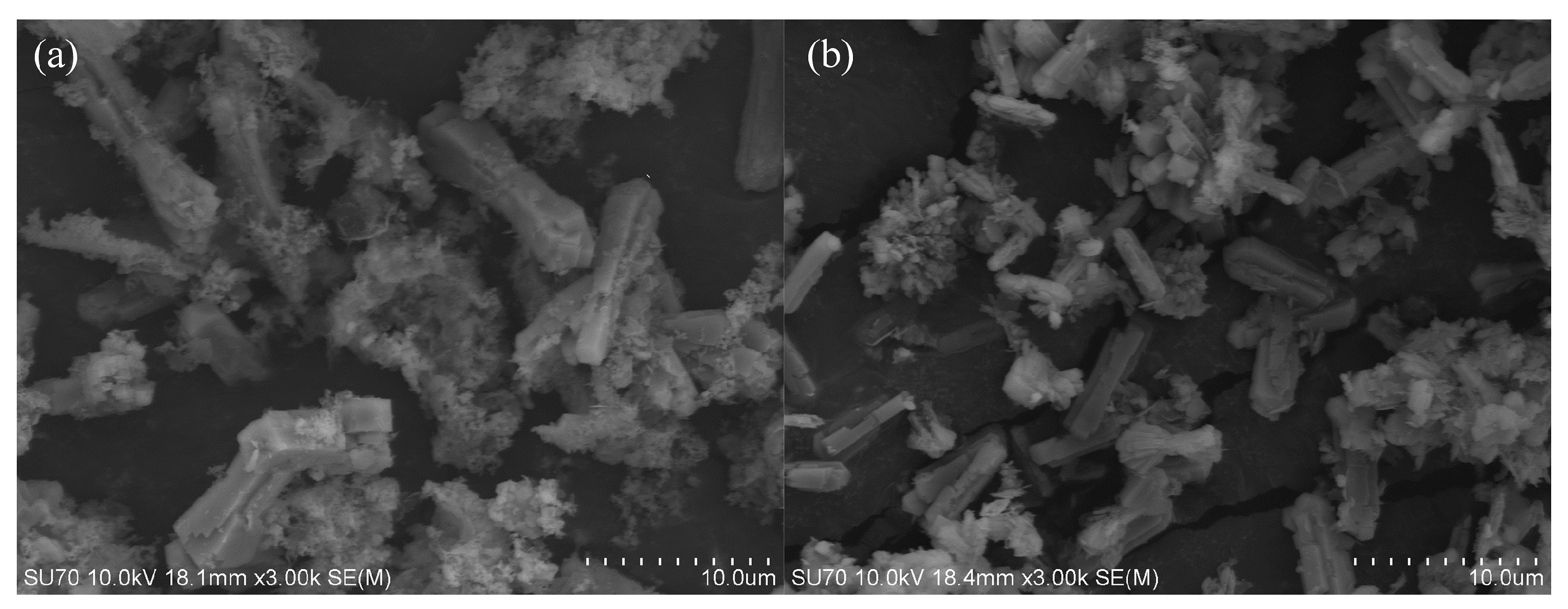
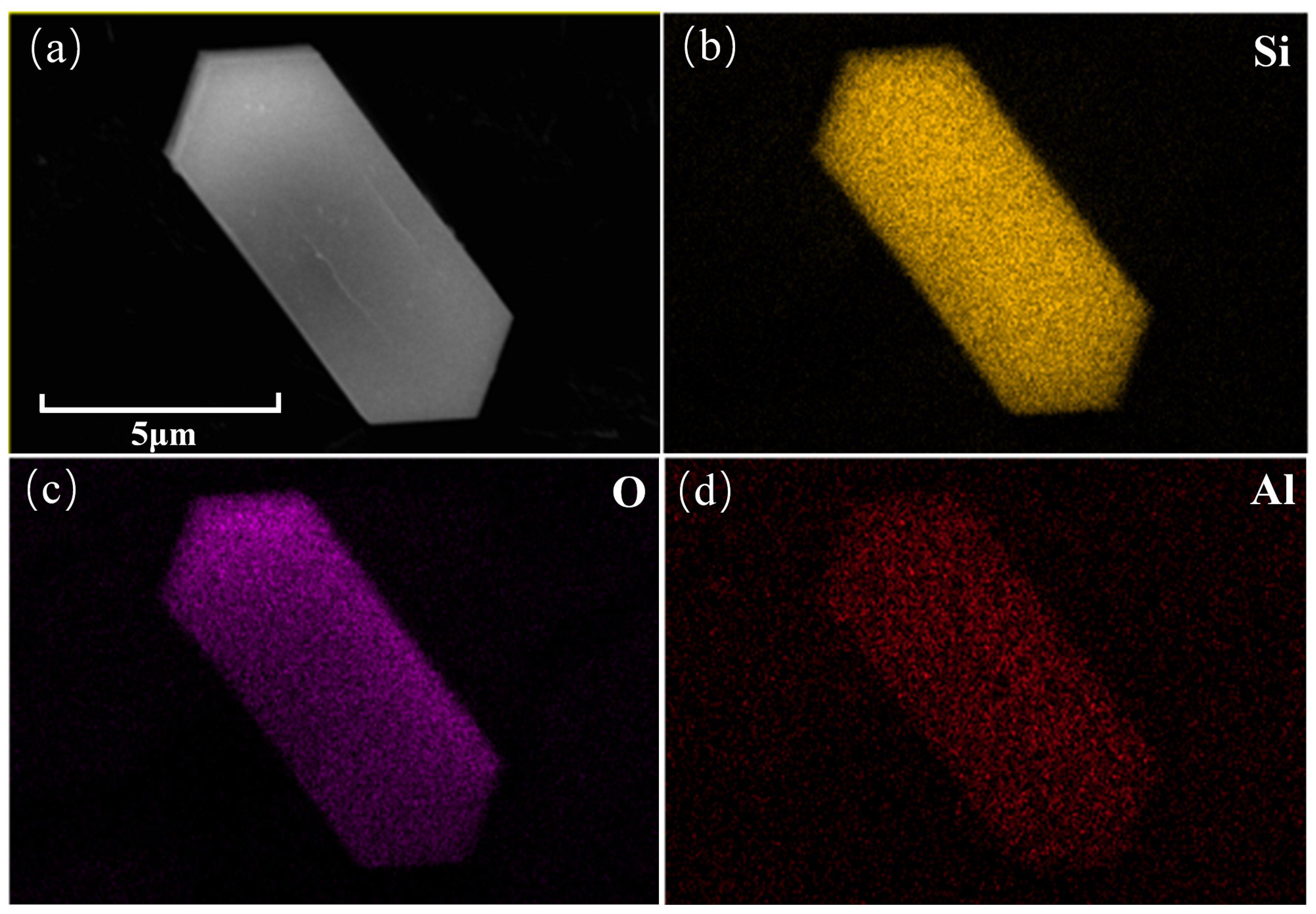
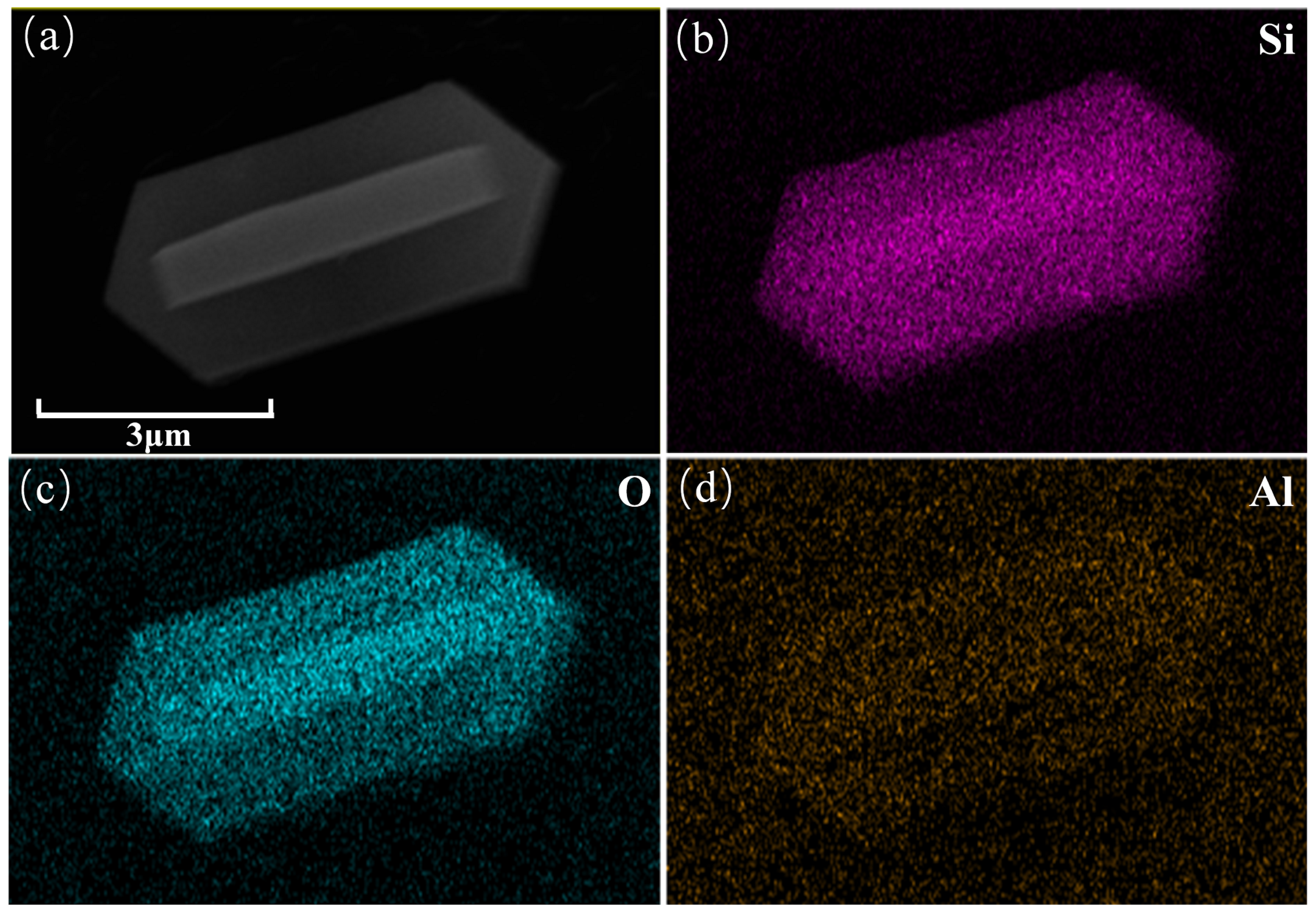
2.2. Surface Morphology and Elemental Analysis
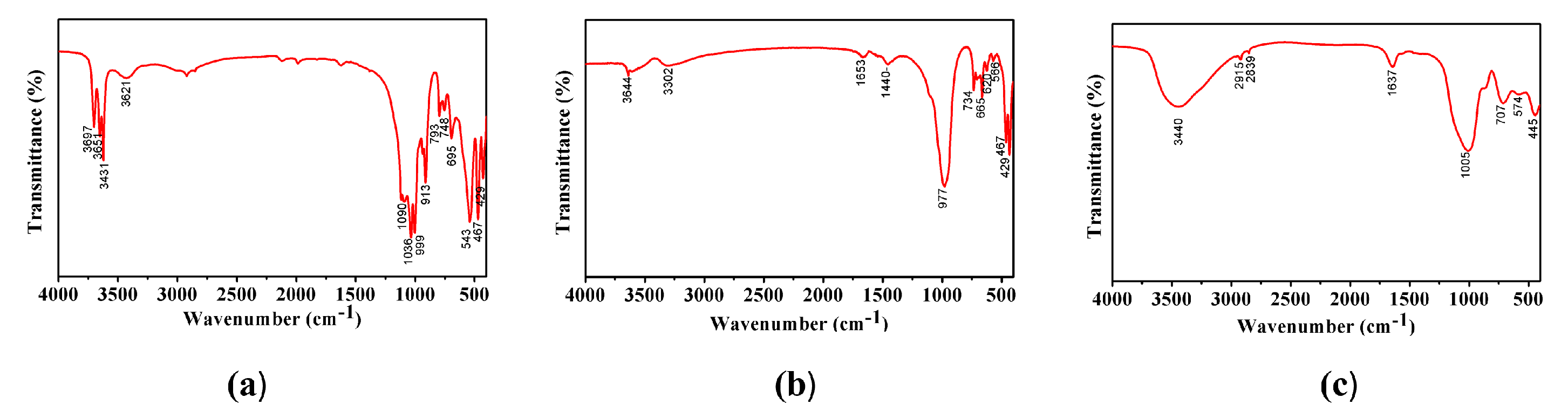
2.3. FT-IR Spectrum Analysis
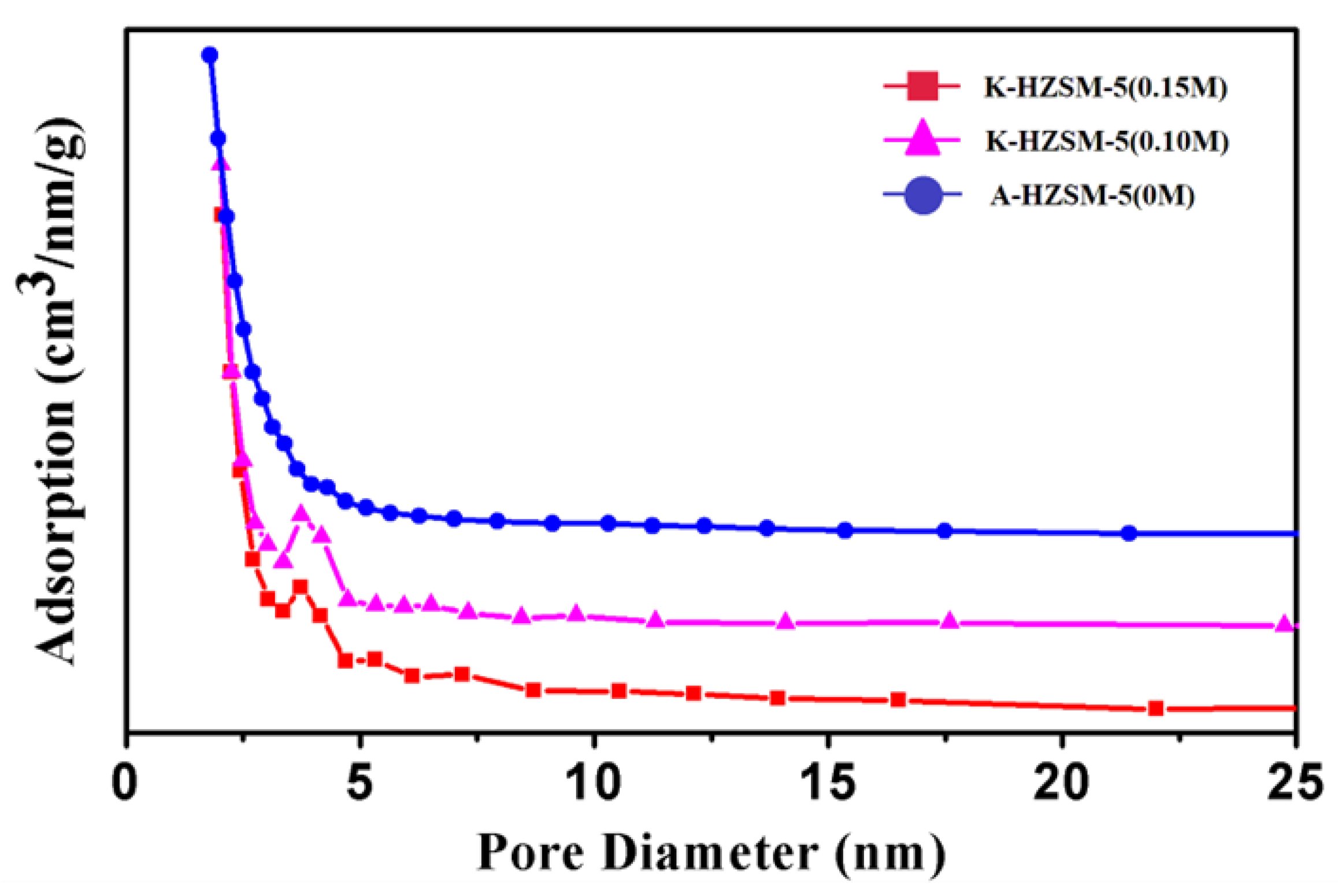
2.4. N Adsorption-Desorption Analysis
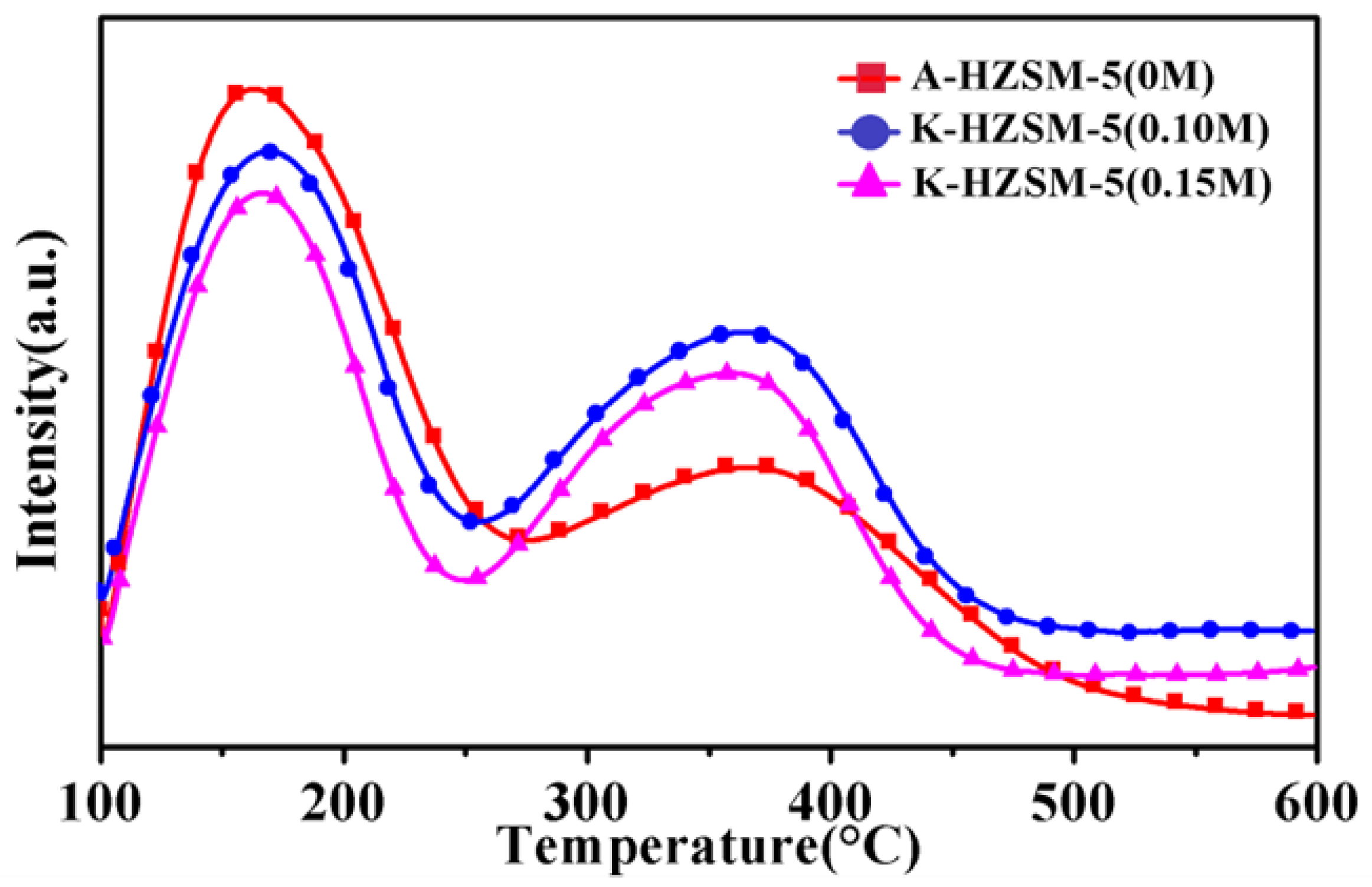
2.5. Evaluation of Surface Acidity
2.6. Discussion on the Formation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Pre-Treatment of Minerals
3.3. Synthesis of ZSM-5
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive X-ray Spectroscopy |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| TPABr | Tetrapropylammonium bromide |
| NH-TPD | Ammonia Temperature-Programmed Desorption |
| LT | Low-Temperature |
| HT | High-Temperature |
Appendix A

References
- Chi, C.Y.; Chang, C.C.; Hu, S.; Yeh, T.W.; Cronin, S.B.; Dapkus, P.D. Strain Effects on Wurtzite/Zinc-Blende Biphase InAs Nanowires. Nano Lett. 2013, 13, 2506–2515. [Google Scholar] [CrossRef]
- Babu, R.R.; Sethuraman, K.; Gopalakrishnan, R.; Ramasamy, P. Growth and characterization of L-arginine acetate single crystals. J. Cryst. Growth 2006, 297, 356–360. [Google Scholar]
- Golzarroshan, B.; Siddegowda, M.S.; Li, H.Q.; Yathirajan, H.S.; Narayana, B.; Rathore, R.S. Crystal structure, Hirshfeld surface analysis and DFT studies of (E)-1-(4-chlorophenyl)-3-(4-nitrophenyl)prop-2-en-1-one. J. Mol. Struct. 2012, 1018, 107–112. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Centi, G.; Lanzafame, P.; Perathoner, S. Use of zeolites in the catalytic conversion of bio-ethanol to light olefins. Catal. Sci. Technol. 2016, 6, 2485–2501. [Google Scholar] [CrossRef]
- de Pietre, M.K.; dos Santos, F.S.; de Almeida Ribeiro, J.A.; Neri, J.A.M.; de Oliveira, D.F. Removal of reactive dyes from aqueous solution by adsorption on natural zeolite. Water Sci. Technol. 2017, 76, 3441–3451. [Google Scholar]
- Hou, X.; Qiu, Y.; Yuan, E.; Li, F.; Li, Z.; Ji, S.; Yang, Z.; Liu, G.; Zhang, X. Catalytic cracking of Jatropha oil for hydrocarbon liquid fuel over hierarchical ZSM-5 zeolite. Appl. Catal. A Gen. 2017, 543, 51–60. [Google Scholar] [CrossRef]
- Rasouli, M.; Atashi, H.; Mohebbi-Kalhori, D.; Yaghobi, N. A kinetic study of n-hexane isomerization over Pt/HZSM-5 catalyst. J. Taiwan Inst. Chem. Eng. 2017, 78, 438–446. [Google Scholar] [CrossRef]
- Fang, Y.; Tang, J.; Huang, X.; Shen, W.; Song, Y.; Sun, C. Aromatization of methanol over Zn-modified ZSM-5 zeolites. Chin. J. Catal. 2010, 31, 264–266. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Cen, J.; Li, X. Alkylation of Benzene with Methanol over HZSM-5 Zeolites with Different Si/Al Ratios. Catal. Lett. 2015, 145, 715–722. [Google Scholar] [CrossRef]
- Hartanto, D.; Kurniawati, R.; Pambudi, A.B.; Utomo, W.P.; Leaw, W.L.; Nur, H. The synthesis of ZSM-5 from kaolin without organic template. Solid State Sci. 2019, 87, 150–154. [Google Scholar] [CrossRef]
- Hartati, H.; Widati, A.A.; Dewi, T.K.; Prasetyoko, D. Synthesis of ZSM-5 from Kaolin Clay using a Hydrothermal Method without an Organic Template. Bull. Chem. React. Eng. Catal. 2017, 12, 251. [Google Scholar] [CrossRef]
- Holmes, S.M.; Khoo, S.H.; Kovo, A.S. The synthesis of zeolites from mineral sources. Green Chem. 2011, 13, 843–858. [Google Scholar]
- Youssef, H.F.; Hegazy, W.H.; Abo-Almaged, H.H.; El-Bassyouni, G.T. Synthesis and Characterization of Zeolite A from Egyptian Kaolin. Bioinorg. Chem. Appl. 2015, 2015, 428121. [Google Scholar]
- Liu, H.; Liu, G.; Zhang, X.; Zhao, D.; Wang, L. Synthesis of ZSM-5 zeolite with small crystal size from silica-alumina gel for catalytic cracking of n-heptane. Microporous Mesoporous Mater. 2017, 244, 164–170. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Isa, Y.M.; Mdleleni, M.M.; Sincadu, N.; Key, D.; Tshabalala, T. Synthesis of ZSM-5 from South African kaolin. Appl. Clay Sci. 2016, 119, 213–221. [Google Scholar] [CrossRef]
- Liu, Y.; Han, S.; Guan, D.; Chen, S.; Wu, Y.; Yang, Y.; Jiang, N. Synthesis of ZSM-5 zeolite from illite mineral. Microporous Mesoporous Mater. 2019, 280, 324–330. [Google Scholar] [CrossRef]
- Wang, J.Q.; Huang, Y.X.; Pan, Y.; Mi, J.X. Synthesis of zeolite A from kaolin by a new method. Microporous Mesoporous Mater. 2014, 199, 50–56. [Google Scholar] [CrossRef]
- Bolshakov, A.; Kosinov, N.; Romero Hidalgo, D.E.; Mezari, B.; van Hoof, A.J.F.; Hensen, E.J.M. Controlled generation of mesopores in ZSM-5 zeolite by treatment with aqueous solutions of NH4F and NaOH. Catal. Sci. Technol. 2019, 9, 4239–4247. [Google Scholar] [CrossRef]
- Přech, J.; Bozhilov, K.N.; El Fallah, J.; Barrier, N.; Valtchev, V. Top-down synthesis of nanosized FAU-type zeolite. Microporous Mesoporous Mater. 2019, 280, 297–305. [Google Scholar] [CrossRef]
- Si, D.Y.; Zhu, M.H.; Sun, X.M.; Xue, M.; Li, Y.Q.; Wu, T.; Gui, T.; Kumakiri, I.; Chen, X.S.; Kita, H. Formation process and pervaporation of high aluminum ZSM-5 zeolite membrane with fluoride-containing and organic template-free gel. Sep. Purif. Technol. 2021, 257, 117891. [Google Scholar] [CrossRef]
- Meng, X.; Qin, Y.; Zhang, Y.; Li, M.; Huang, H.; Peng, J.; Zhou, L.; Feng, J. The Synergistic Impact of Crystal Seed and Fluoride Ion in the Synthesis of Silicalite-1 Zeolite in Low-Template Systems. Materials 2024, 17, 266. [Google Scholar] [CrossRef]
- Feriancová, A.; Pajtášová, M.; Paliesková, J.; Ondrušová, D.; Kopcová, M.; Jóna, E.; Mojumdar, S.C. Thermal analysis and infrared spectroscopy of kaolin. J. Therm. Anal. Calorim. 2013, 112, 1047–1052. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Alshameri, A.; Zhou, S.; Ma, Y.; Sun, T.; Liang, H.; Gong, Y.; Wang, H.; Yan, C. A study on the thermal behavior of kaolinite. Ceram. Int. 2014, 40, 11751–11758. [Google Scholar] [CrossRef]
- Buhl, J.C.; Stemme, F.; Poltz, I. Synthesis and characterization of nitrate containing sodalite. Microporous Mesoporous Mater. 2009, 126, 276–282. [Google Scholar] [CrossRef]
- Malonda Shabani, J.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis and Characterization of Sodalite Zeolite from South African Coal Fly Ash. Catalysts 2019, 9, 1033. [Google Scholar]
- Cannane, N.O.A.; Rajendran, M.; Selvaraju, R. Synthesis and characterization of silica-alumina mixed oxides. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Peng, H.; Wen, L.; Qiu, G.; Hu, M.; Bai, C. Preparation of amorphous silica-alumina from kaolin. ISIJ Int. 2014, 54, 734–742. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Zhang, Y.; Chevella, D.; Li, G.; Chen, Y.; Guo, X.; Liu, J.; Yu, J. A hierarchical ZSM-5 zeolite with intracrystalline mesopores for the catalytic cracking of n-dodecane. Chem. Eng. J. 2020, 388, 124322. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.X.; Pan, Y.; Mi, J.X. Synthesis of ZSM-5 zeolite from kaolin using a two-step crystallization method. Microporous Mesoporous Mater. 2020, 303, 110247. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Yang, J. Synthesis of hierarchical ZSM-5 zeolite by a dual-template method for the methanol to propylene reaction. Catal. Lett. 2020, 150, 1454–1461. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Krishnan, P.S.; Ramesh, A.; Shanthi, K. Synthesis and characterization of ZSM-5 catalysts for the conversion of ethanol to hydrocarbons. Polyhedron 2018, 154, 314–324. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Methanol to olefin conversion over nano-ZSM-5 catalysts: Effect of crystal size on catalyst performance. J. Nat. Gas Sci. Eng. 2015, 22, 260–269. [Google Scholar] [CrossRef]
- Yue, Y.; Kang, Y.; Bai, Y.; Gu, L.; Liu, H.; Bao, J.; Wang, T.; Yuan, P.; Zhu, H.; Bai, Z.; et al. Synthesis of hierarchical ZSM-5 zeolite from natural kaolin for catalytic cracking of n-hexane. Appl. Clay Sci. 2018, 158, 177–185. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Chen, J.; Wei, Y.; Li, J.; Fan, D.; Yu, Z.; Qi, Y.; He, Y.; Xu, S.; et al. Synthesis of hierarchical ZSM-5 zeolites with tunable mesoporosity and acidity for the methanol to propylene reaction. RSC Adv. 2014, 4, 21479–21491. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Yin, C.; Jiang, N. Synthesis of hierarchical ZSM-5 zeolite from diatomite for catalytic cracking of n-heptane. Microporous Mesoporous Mater. 2019, 275, 223–228. [Google Scholar] [CrossRef]
- Wang, J.J.; Tan, Z.C.; Zhu, C.C.; Miao, G.; Kong, L.Z.; Sun, Y.H. Green synthesis of hierarchical ZSM-5 zeolite with tunable acidity for the catalytic conversion of glucose to 5-hydroxymethylfurfural. Green Chem. 2016, 18, 452–460. [Google Scholar] [CrossRef]
- Schmidt, F.; Lohe, M.R.; Büchner, B.; Giordanino, F.; Bonino, F.; Kaskel, S. Hierarchical ZSM-5 zeolites: A comparative study of steaming and desilication. Microporous Mesoporous Mater. 2013, 165, 148–157. [Google Scholar] [CrossRef]
- van Koningsveld, H.; Jansen, J.C.; van Bekkum, H. The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 1990, 10, 235–242. [Google Scholar] [CrossRef]
- Kashchiev, D. On the Kossel-Stranski Theory of Crystal Growth. Cryst. Growth Des. 2012, 12, 3257–3262. [Google Scholar] [CrossRef]




| Sample | S a | S c | S b | V d | V f | V e |
|---|---|---|---|---|---|---|
| (mg) | (mg) | (mg) | (cmg) | (cmg) | (cmg) | |
| A-HZSM-5 (0 M) | 322 | 198 | 123 | 0.16 | 0.12 | 0.04 |
| K-HZSM-5 (0.10 M) | 351 | 280 | 71 | 0.18 | 0.15 | 0.03 |
| K-HZSM-5 (0.15 M) | 385 | 316 | 69 | 0.19 | 0.16 | 0.03 |
| Sample | Weak Acidity | Strong Acidity | Total Acidity |
|---|---|---|---|
| (mmol g) | (mmol g) | (mmol g) | |
| A-HZSM-5 (0 M) | 5.91 | 1.25 | 7.16 |
| K-HZSM-5 (0.10 M) | 3.50 | 2.44 | 5.94 |
| K-HZSM-5 (0.15 M) | 3.59 | 2.53 | 6.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wan, X.; Song, C.; Wu, K.; Yang, W.; Liu, B.; Yang, Q.; Fang, J.; Razzaq, A. A Rapid and Low-Cost Synthesis of ZSM-5 Single Crystals: The Inhibitory Effect of NH4F on Twinning. Inorganics 2025, 13, 272. https://doi.org/10.3390/inorganics13080272
Du J, Wan X, Song C, Wu K, Yang W, Liu B, Yang Q, Fang J, Razzaq A. A Rapid and Low-Cost Synthesis of ZSM-5 Single Crystals: The Inhibitory Effect of NH4F on Twinning. Inorganics. 2025; 13(8):272. https://doi.org/10.3390/inorganics13080272
Chicago/Turabian StyleDu, Juan, Xiang Wan, Caixiong Song, Kangsheng Wu, Wenbing Yang, Beiye Liu, Qi Yang, Jingjing Fang, and Ayesha Razzaq. 2025. "A Rapid and Low-Cost Synthesis of ZSM-5 Single Crystals: The Inhibitory Effect of NH4F on Twinning" Inorganics 13, no. 8: 272. https://doi.org/10.3390/inorganics13080272
APA StyleDu, J., Wan, X., Song, C., Wu, K., Yang, W., Liu, B., Yang, Q., Fang, J., & Razzaq, A. (2025). A Rapid and Low-Cost Synthesis of ZSM-5 Single Crystals: The Inhibitory Effect of NH4F on Twinning. Inorganics, 13(8), 272. https://doi.org/10.3390/inorganics13080272







