Zinc Complexes of Guanidine– and Amidine–Phenolate Ligands for the Ring-Opening Polymerization of Lactide
Abstract
1. Introduction
2. Results and Discussion
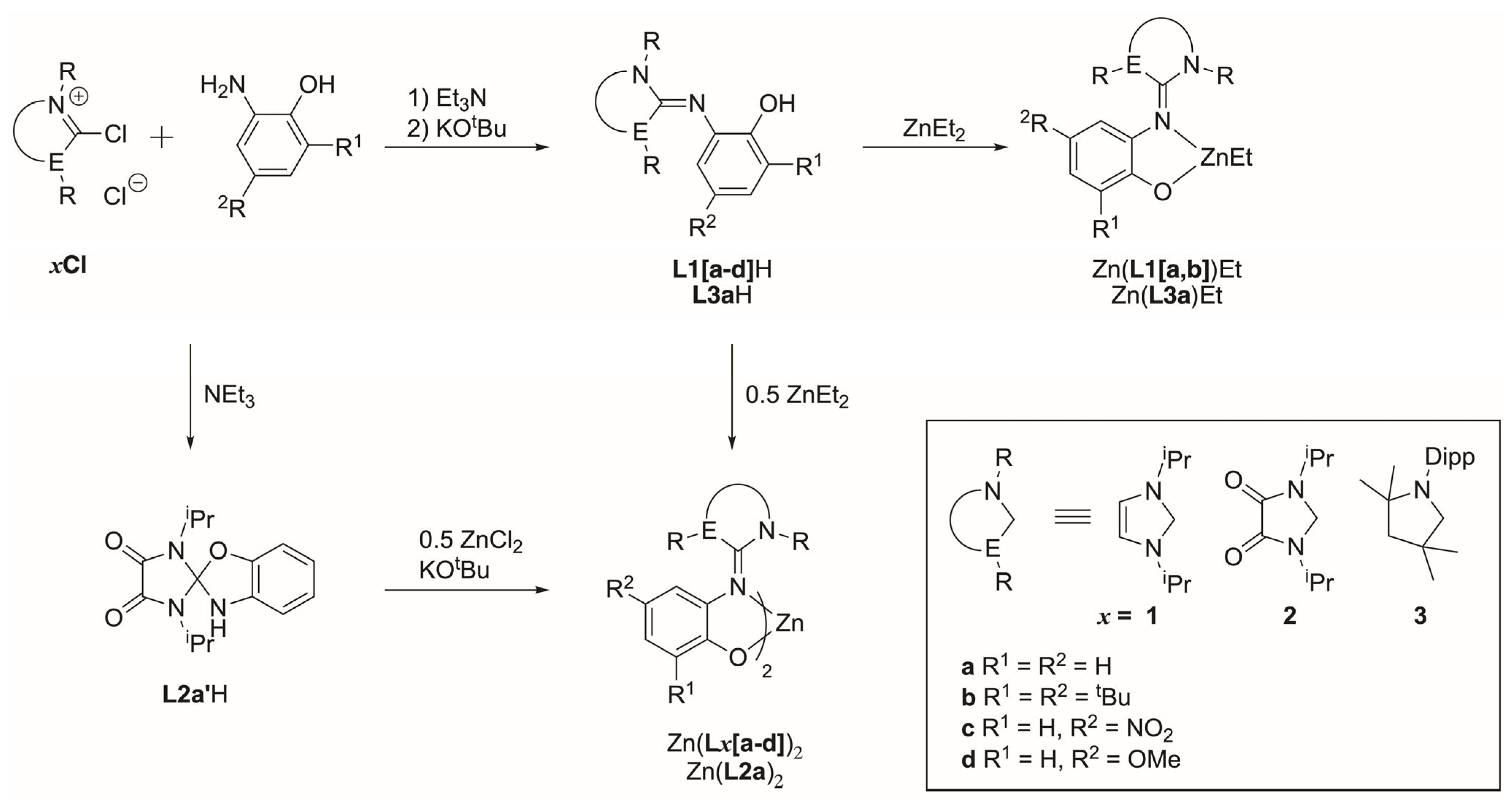
2.1. Synthesis of Ligands
2.2. Synthesis of Zn Complexes
2.3. Ring-Opening Polymerization of Rac-Lactide: Kinetics
2.4. Ring-Opening Polymerization of Rac-Lactide: Molecular Weight and Tacticity
3. Experimental Section
3.1. General Considerations
3.2. Solid-State Structure Determination
3.3. Polymers’ Molecular Weight Determination by Gel Permeation Chromatography
3.4. Synthesis of Ligands and Metal Complexes
3.5. General Procedure for the Polymerization of rac-Lactide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
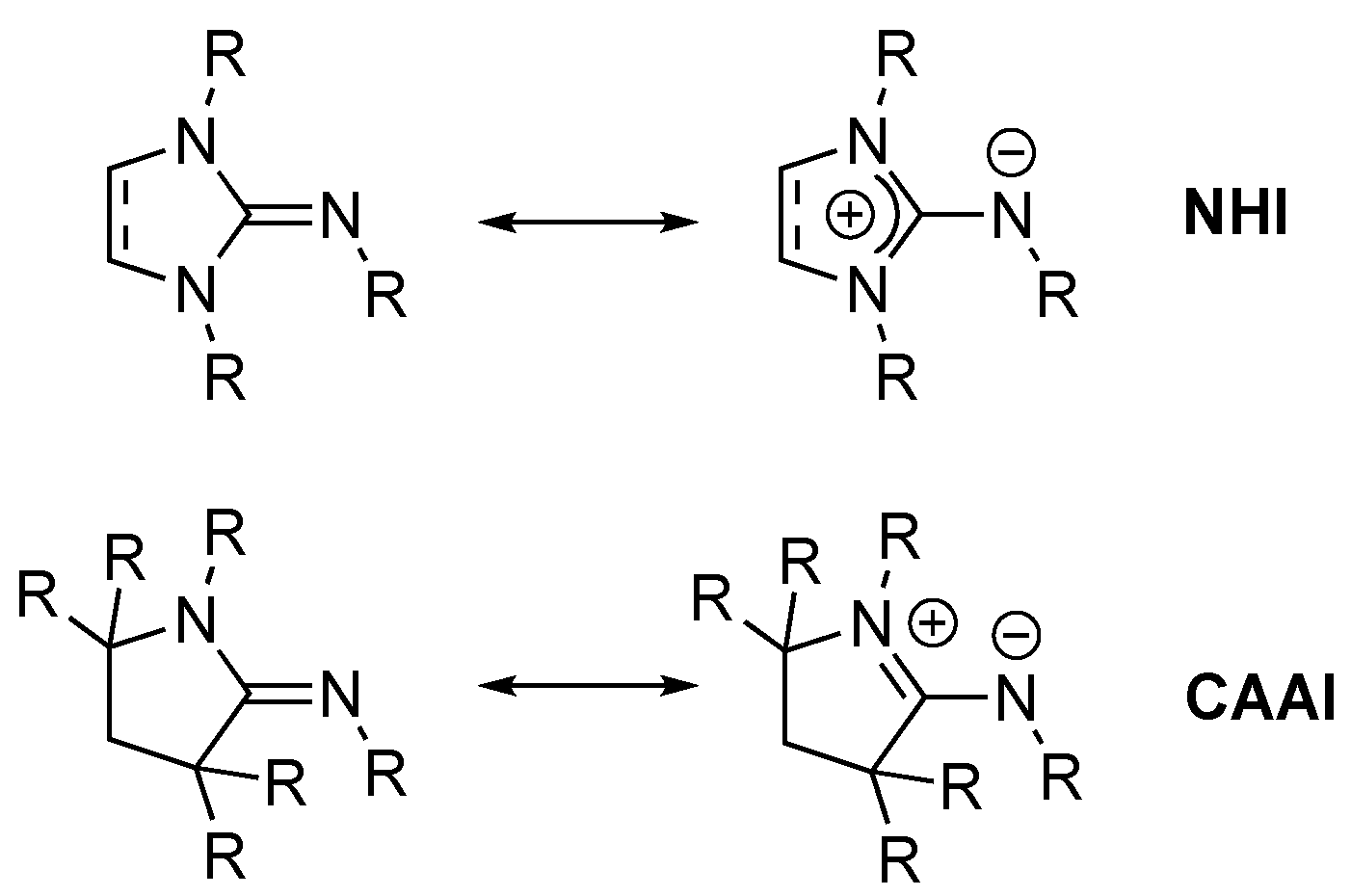
Abbreviations
| AMM | Activated monomer mechanism |
| BnOH | Benzyl alcohol |
| CAAC | Cyclic (alkyl)amino carbene |
| CAAI | Cyclic (alkyl)amino) imine |
| CCD | Charge-coupled device |
| CIM | Coordination-insertion mechanism |
| DCM | Dichloromethane |
| Dipp | 2,6-Diisopropylphenyl |
| Et | Ethyl |
| HMBC | Heteronuclear multiple bond correlation |
| kapp | Apparent (pseudo-first-order) rate constant |
| LA | Lactide |
| Mn | Number-average molecular weight |
| Mw | Weight-average molecular weight |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight |
| MeCN | Acetonitrile |
| MHz | Megahertz |
| NHC | N-Heterocyclic carbene |
| NHI | N-Heterocyclic imine |
| NMR | Nuclear magnetic resonance |
| ORTEP | Oak Ridge thermal-ellipsoid plot |
| PLA | Polylactic acid |
| ROP | Ring-opening polymerization |
| THF | Tetrahydrofuran |
References
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Mankaev, B.N.; Karlov, S.S. Metal Complexes in the Synthesis of Biodegradable Polymers: Achievements and Prospects. Materials 2023, 16, 6682. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A Circular Economy Approach to Plastic Waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.; Cheuk, K.; Lin, C. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, G.; So, Y.-M.; Pan, Y. Recent Advances in Zinc Complexes for Stereoselective Ring-Opening Polymerization and Copolymerization. Inorganics 2025, 13, 185. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, W.; Sun, W.-H. Progress of Ring-Opening Polymerization of Cyclic Esters Catalyzed by Iron Compounds. Organometallics 2023, 42, 1680–1692. [Google Scholar] [CrossRef]
- Michell, R.M.; Ladelta, V.; Da Silva, E.; Müller, A.J.; Hadjichristidis, N. Poly(lactic acid) stereocomplexes based molecular architectures: Synthesis and crystallization. Prog. Polym. Sci. 2023, 146, 101742. [Google Scholar] [CrossRef]
- Pan, Y.; Hao, M.; Li, X.; Meng, Y.; Kang, X.; Zhang, G.; Sun, X.; Song, X.-Z.; Zhang, L.; So, Y.-M. Anilido-Oxazoline-Ligated Iron Alkoxide Complexes for Living Ring-Opening Polymerization of Cyclic Esters with Controllability. Inorg. Chem. 2025, 64, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Liu, S.; Liu, J.; Zhang, Z.; Qu, R.; Gu, Y.; Qin, Y. Novel epoxide-promoted polymerization of lactides mediated by a zinc guanidine complex: A potential strategy for the tin-free PLA industry. Polym. Chem. 2023, 14, 4652–4658. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, X.; Kang, X.; Hou, X.; Wan, C.; Song, X.; Leung, W.-H.; So, Y.-M. Flexible Coordination of the Bis(amino-oxazoline) Ligand in Rare-Earth Metal Complexes: Synthesis, Structure, and Their Reactivity and Polymerization Performance. Inorg. Chem. 2022, 61, 18828–18841. [Google Scholar] [CrossRef]
- Kowalski, A.; Libiszowski, J.; Duda, A.; Penczek, S. Polymerization of L,L-Dilactide Initiated by Tin(II) Butoxide. Macromolecules 2000, 33, 1964–1971. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Stricker, A. Polylactones 48. SnOct2 -Initiated Polymerizations of Lactide: A Mechanistic Study. Macromolecules 2000, 33, 702–709. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and Mechanism of Cyclic Esters Polymerization Initiated with Tin(II) Octoate. 3. Polymerization of L,L-Dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Lunt, J. Large-Scale Production, Properties and Commercial Applications of Polylactic Acid Polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Hayes, P.G.; Ireland, B.J. Complexes of Mg, Ca and Zn as Homogeneous Catalysts for Lactide Polymerization. Dalton Trans. 2009, 25, 4832–4846. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, Z.; Xu, X.; Ren, C.; Wei, X.; Yang, Y.; Li, J.; Jiang, D.; Zhang, K.; Wang, B.; et al. Efficient synthesis of polylactide and copolymers under industrial conditions by multinuclear β-ketoimide zinc complexes. Polym. Chem. 2025, 16, 3030–3040. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Benchaphanthawee, W.; Teng, H.-H.; Huang, N.; Yang, J.-H.; Sun, J.-R.; Lee, G.-H.; Kungwan, N.; Peng, C.-H. Ring-Opening Polymerization of Cyclic Esters Mediated By Zinc Complexes Coordinated With Benzotriazo-Based Imino-Phenoxy Ligands. Polymer 2023, 267, 125687. [Google Scholar] [CrossRef]
- Fuchs, M.; Schmitz, S.; Schäfer, P.M.; Secker, T.; Metz, A.; Ksiazkiewicz, A.N.; Pich, A.; Kögerler, P.; Monakhov, K.Y.; Herres-Pawlis, S. Mononuclear Zinc(II) Schiff Base Complexes as Catalysts for the Ring-Opening Polymerization of Lactide. Eur. Polym. J. 2020, 122, 109302. [Google Scholar] [CrossRef]
- Li, M.; Behzadi, S.; Chen, M.; Pang, W.; Wang, F.; Tan, C. Phenoxyimine Ligands Bearing Nitrogen-Containing Second Coordination Spheres for Zinc Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2019, 38, 461–468. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Nyamori, V.O.; Omondi, B. N,O-Amino-phenolate Mg(II) and Zn(II) Schiff Base Complexes: Synthesis and Application in Ring-Opening Polymerization of ε-Caprolactone and Lactides. Inorg. Chim. Acta 2019, 487, 264–274. [Google Scholar] [CrossRef]
- McKeown, P.; McCormick, S.N.; Mahon, M.F.; Jones, M.D. Highly Active Mg(II) and Zn(II) Complexes for the Ring Opening Polymerisation of Lactide. Polym. Chem. 2018, 9, 5339–5347. [Google Scholar] [CrossRef]
- Nuñez-Dallos, N.; Posada, A.F.; Hurtado, J. Coumarin Salen-Based Zinc Complex for Solvent-Free Ring Opening Polymerization of ε-Caprolactone. Tetrahedron Lett. 2017, 58, 977–980. [Google Scholar] [CrossRef]
- Jones, M.D.; Davidson, M.G.; Keir, C.G.; Hughes, L.M.; Mahon, M.F.; Apperley, D.C. Zinc(II) Homogeneous and Heterogeneous Species and Their Application for the Ring-Opening Polymerisation of rac-Lactide. Eur. J. Inorg. Chem. 2009, 2009, 635–642. [Google Scholar] [CrossRef]
- Pongpanit, T.; Saeteaw, T.; Chumsaeng, P.; Chasing, P.; Phomphrai, K. Highly Active Homoleptic Zinc and Magnesium Complexes Supported by Constrained Reduced Schiff Base Ligands for the Ring-Opening Polymerization of Lactide. Inorg. Chem. 2021, 60, 17114–17122. [Google Scholar] [CrossRef]
- Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Highly Isoselective and Active Zinc Catalysts for rac-Lactide Polymerization: Effect of Pendant Groups of Aminophenolate Ligands. Macromolecules 2017, 50, 7911–7919. [Google Scholar] [CrossRef]
- Williams, C.K.; Breyfogle, L.E.; Choi, S.K.; Nam, W.; Young, V.G.; Hillmyer, M.A.; Tolman, W.B. A Highly Active Zinc Catalyst for the Controlled Polymerization of Lactide. J. Am. Chem. Soc. 2003, 125, 11350–11359. [Google Scholar] [CrossRef]
- Upitak, K.; Thomas, C.M. Combining Two Mechanistically Distinct Reactions from a Single Iron Complex: A Tandem Approach to Thermally Stable and Recyclable Polymers. Angew. Chem. Int. Ed. 2025, 64, e202418908. [Google Scholar] [CrossRef]
- Théron, B.; Vaillant-Coindard, V.; Balan, C.; Rousselin, Y.; Bayardon, J.; Malacea-Kabbara, R.; Le Gendre, P. Al and Zn Phenoxy-Amidine Complexes for Lactide ROP Catalysis. Dalton Trans. 2023, 52, 7854–7868. [Google Scholar] [CrossRef]
- Vaillant-Coindard, V.; Théron, B.; Printz, G.; Chotard, F.; Balan, C.; Rousselin, Y.; Richard, P.; Tolbatov, I.; Fleurat-Lessard, P.; Bodio, E.; et al. Phenoxy-Amidine Ligands: Toward Lactic Acid-Tolerant Catalysts for Lactide Ring-Opening Polymerization. Organometallics 2022, 41, 2920–2932. [Google Scholar] [CrossRef]
- Rao, W.; Cai, C.; Tang, J.; Wei, Y.; Gao, C.; Yu, L.; Ding, J. Coordination Insertion Mechanism of Ring-Opening Polymerization of Lactide Catalyzed by Stannous Octoate. Chin. J. Chem. 2021, 39, 1965–1974. [Google Scholar] [CrossRef]
- Storey, R.F.; Sherman, J.W. Kinetics and Mechanism of the Stannous Octoate-Catalyzed Bulk Polymerization of ε-Caprolactone. Macromolecules 2002, 35, 1504–1512. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Mechanism of Cyclic Ester Polymerization Initiated with Tin(II) Octoate. 2. Macromolecules Fitted with Tin(II) Alkoxide Species Observed Directly in MALDI-TOF Spectra. Macromolecules 2000, 33, 689–695. [Google Scholar] [CrossRef]
- Dubois, P.; Jacobs, C.; Jérôme, R.; Teyssié, P. Macromolecular Engineering of Polylactones and Polylactides. 4. Mechanism and Kinetics of Lactide Homopolymerization By Aluminum Isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. Activated Monomer Mechanism (AMM) in Cationic Ring-Opening Polymerization. The Origin of the AMM and Further Development in Polymerization of Cyclic Esters. ACS Macro Lett. 2021, 10, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, I.; Tedesco, C.; Mazzeo, M.; Pellecchia, C. New homoleptic bis(pyrrolylpyridiylimino) Mg(II) and Zn(II) complexes as catalysts for the ring opening polymerization of cyclic esters via an “activated monomer” mechanism. Dalton Trans. 2017, 46, 12217–12225. [Google Scholar] [CrossRef] [PubMed]
- Platel, R.H.; Hodgson, L.M.; Williams, C.K. Biocompatible Initiators for Lactide Polymerization. Polym. Rev. 2008, 48, 11–63. [Google Scholar] [CrossRef]
- Baśko, M.; Kubisa, P. Cationic Copolymerization of ε-Caprolactone and L,L-Lactide By an Activated Monomer Mechanism. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7071–7081. [Google Scholar] [CrossRef]
- D’Auria, I.; Ferrara, V.; Tedesco, C.; Kretschmer, W.; Kempe, R.; Pellecchia, C. Guanidinate Zn(II) Complexes as Efficient Catalysts for Lactide Homo- and Copolymerization under Industrially Relevant Conditions. ACS Appl. Polym. Mater. 2021, 3, 4035–4043. [Google Scholar] [CrossRef]
- Khan, B.S.; Flores-Romero, V.; LeBlanc, J.; Lavoie, G.G. Lactide Polymerization Using Zinc Dichloride Complexes Containing a Neutral Bidentate Ligand with a Diacylated Cyclic Guanidine. Organometallics 2022, 41, 2668–2677. [Google Scholar] [CrossRef]
- Hermann, A.; Becker, T.; Schäfer, M.A.; Hoffmann, A.; Herres-Pawlis, S. Effective Ligand Design: Zinc Complexes with Guanidine Hydroquinoline Ligands for Fast Lactide Polymerization and Chemical Recycling. ChemSusChem 2022, 15, e202201075. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.M.; Herres-Pawlis, S. Robust Guanidine Metal Catalysts for the Ring-Opening Polymerization of Lactide under Industrially Relevant Conditions. ChemPlusChem 2020, 85, 1044–1052. [Google Scholar] [CrossRef]
- Flores-Romero, V.; LeBlanc, J.; Chen, Z.; Lavoie, G.G. Ti and Zr Complexes Bearing Guanidine-Phenolate Ligands: Coordination Chemistry and Polymerization Studies. RSC Adv. 2024, 14, 25889–25899. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shu, X.; Cai, Z.; Eisen, M.S. Synthesis, Structures, and Norbornene Polymerization Behavior of Neutral Nickel(II) and Palladium(II) Complexes Bearing Aryloxide Imidazolidin-2-imine Ligands. Organometallics 2018, 37, 1172–1180. [Google Scholar] [CrossRef]
- Li, M.; Cai, Z.; Eisen, M.S. Neutral Nickel(II) Complexes Bearing Aryloxide Imidazolin-2-imine Ligands for Efficient Copolymerization of Norbornene and Polar Monomers. Organometallics 2018, 37, 4753–4762. [Google Scholar] [CrossRef]
- Larocque, T.G.; Dastgir, S.; Lavoie, G.G. Coordination and Reactivity Study of Titanium and Zirconium Complexes of the First Imidazol-2-imine Ethenolate Ligand. Organometallics 2013, 32, 4314–4320. [Google Scholar] [CrossRef]
- Goettel, J.T.; Gao, H.; Dotzauer, S.; Braunschweig, H. MeCAAC = N–: A Cyclic (Alkyl) (Amino) Carbene Imino Ligand. Chem. Eur. J. 2020, 26, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Huynh, S.; Arrowsmith, M.; Meier, L.; Dietz, M.; Härterich, M.; Michel, M.; Gärtner, A.; Braunschweig, H. Cyclic Alkyl(amino)iminates (CAAIs) as Strong 2σ,4π-Electron Donor Ligands for the Stabilisation of Boranes and Diboranes(4): A Synthetic and Computational Study. Dalton Trans. 2023, 52, 3869–3876. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl) (Amino) Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef]
- Lavoie, G.G.; Campos-Rivera, L.; LeBlanc, J.; Flores-Romero, V. (York University, Toronto, Canada). Personal communication, 2025.
- Rahman, M.M.; Pyle, D.J.; Bisz, E.; Dziuk, B.; Ejsmont, K.; Lalancette, R.; Wang, Q.; Chen, H.; Szostak, R.; Szostak, M. Evaluation of Cyclic Amides as Activating Groups in N–C Bond Cross-Coupling: Discovery of N-Acyl-δ-valerolactams as Effective Twisted Amide Precursors for Cross-Coupling Reactions. J. Org. Chem. 2021, 86, 10455–10466. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Petrovic, D.; Randoll, S.; Beer, S.; Bannenberg, T.; Jones, P.G.; Grunenberg, J. Structural and Theoretical Investigation of 2-Iminoimidazolines—Carbene Analogues of Iminophosphoranes. Org. Biomol. Chem. 2007, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xiao, X.; Zhang, Y.; Chao, J.; Chen, X. Beta-Pyridylenolate Zinc Catalysts for the Ring-Opening Homo- and Copolymerization of ε-Caprolactone and Lactides. Dalton Trans. 2017, 46, 9846–9858. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes With Pyridylmethylamide Ligands: Structural Analysis With a New Four-Coordinate Geometry Index, τ. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Börner, J.; Flörke, U.; Huber, K.; Döring, A.; Kuckling, D.; Herres-Pawlis, S. Lactide Polymerisation with Air-Stable and Highly Active Zinc Complexes with Guanidine–Pyridine Hybrid Ligands. Chem. Eur. J. 2009, 15, 2362–2376. [Google Scholar] [CrossRef]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, J.E. The Reaction Mechanism for the Organocatalytic Ring-Opening Polymerization of L-Lactide Using a Guanidine-Based Catalyst: Hydrogen-Bonded or Covalently Bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef]
- Zell, M.T.; Padden, B.E.; Paterick, A.J.; Thakur, K.A.M.; Kean, R.T.; Hillmyer, M.A.; Munson, E.J. Unambiguous Determination of the 13C and 1H NMR Stereosequence Assignments of Polylactide Using High-Resolution Solution NMR Spectroscopy. Macromolecules 2002, 35, 7700–7707. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of Lactide Polymerization with Chiral Catalysts: New Opportunities for Stereocontrol Using Polymer Exchange Mechanisms. J. Am. Chem. Soc. 2002, 124, 1316–1326. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Iyer, S.S.; McCollum, D.G.; Pagel, M.; Werner-Zwanziger, U. Microstructure of Poly(lactide). Phase-Sensitive HETCOR Spectra of Poly(meso-lactide), Poly(rac-lactide), and Atactic Poly(lactide). Macromolecules 1999, 32, 963–973. [Google Scholar] [CrossRef]
- Baltrun, M.; Watt, F.A.; Schoch, R.; Wölper, C.; Neuba, A.G.; Hohloch, S. A New Bis-Phenolate Mesoionic Carbene Ligand for Early Transition Metal Chemistry. Dalton Trans. 2019, 48, 14611–14625. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, W.-X.; Xi, Z. Metal-free synthesis of cyclic di-oxoguanidines via one-pot sequential transformation of amines, carbodiimides and acyl dichlorides. Org. Biomol. Chem. 2012, 10, 6266–6270. [Google Scholar] [CrossRef] [PubMed]
- LaPierre, E.A.; Watanabe, L.K.; Patrick, B.O.; Rawson, J.M.; Tuononen, H.M.; Manners, I. Synthesis of a Carbene-Stabilized (Diphospha)aminyl Radical and Its One Electron Oxidation and Reduction to Nonclassical Nitrenium and Amide Species. J. Am. Chem. Soc. 2023, 145, 9223–9232. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement With SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
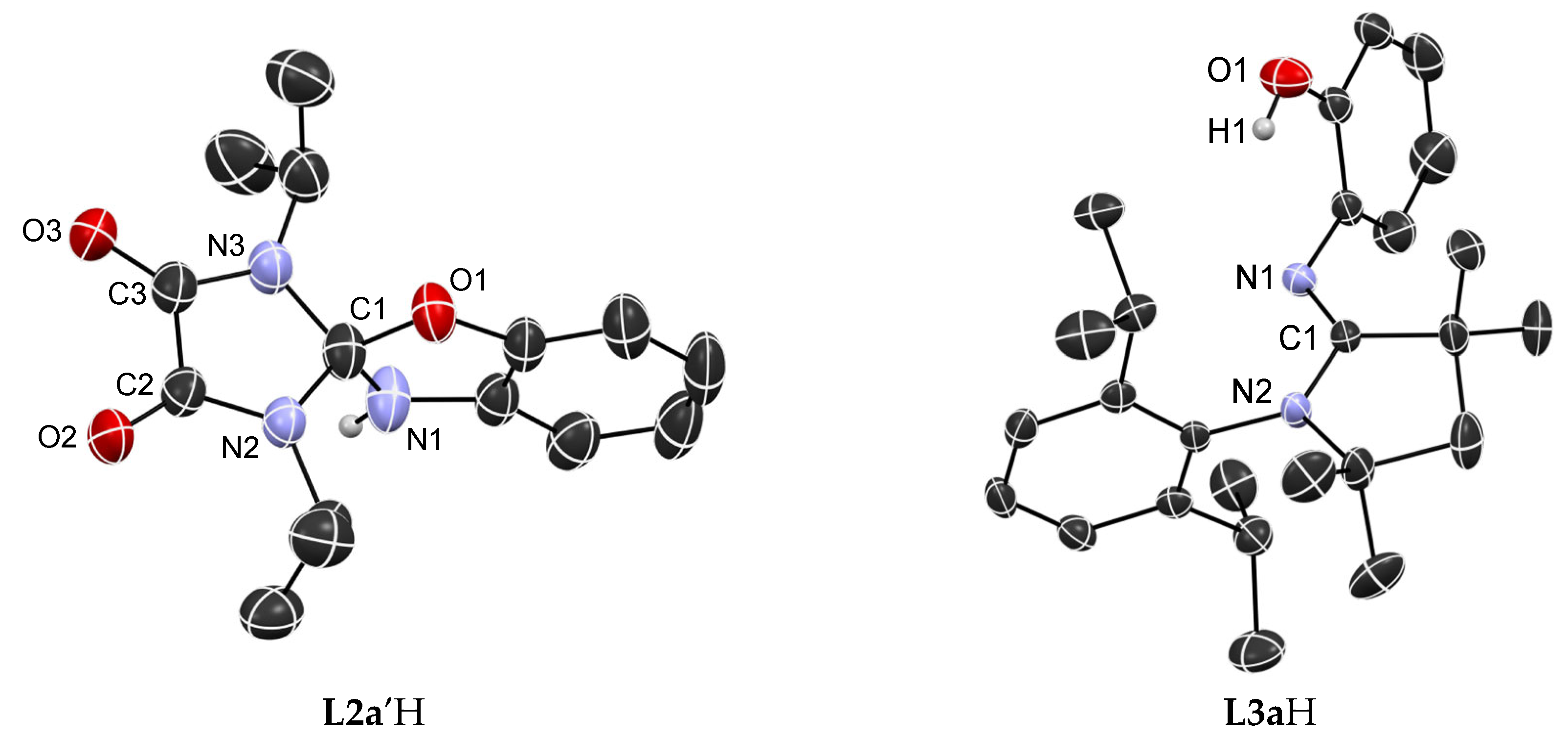

| L2′aH | L3aH | ||
|---|---|---|---|
| Bond lengths (Å) | |||
| N1–C1 | 1.428(5) | N1–C1 | 1.287(2) |
| N2–C1 | 1.453(5) | N2–C1 | 1.362(2) |
| N3–C1 | 1.444(5) | ||
| N2–C2 | 1.341(5) | ||
| N3–C3 | 1.349(5) | ||
| O2–C2 | 1.226(5) | ||
| O3–C3 | 1.223(5) | ||
| Bond angles (deg) | |||
| N1–C1–N2 | 114.7(3) | N1–C1–N2 | 120.3(2) |
| N1–C1–N3 | 115.1(3) | N1–C1–C4 | 131.2(2) |
| N2–C1–N3 | 103.2(3) | N2–C1–C4 | 108.6(1) |
| O1–C1–N3 | 110.0(3) | ||
| O1–C1–N2 | 109.3(3) | ||
| O1–C1–N1 | 104.6(3) | ||
| Selected Bond Lengths (Å) | |||
|---|---|---|---|
| Zn1–N1 | 2.002(11) | Zn1–N4 | 1.949(11) |
| Zn1–O1 | 1.938(10) | Zn1–O2 | 1.943(10) |
| N1–C1 | 1.336(17) | N4–C16 | 1.337(17) |
| N2–C1 | 1.360(18) | N5–C16 | 1.359(17) |
| N3–C1 | 1.394(18) | N6–C16 | 1.358(17) |
| Selected Bond Angles (deg) | |||
| N1–Zn–O1 | 85.73(4) | N4–Zn–O2 | 85.67(4) |
| N1–Zn–O2 | 125.59(5) | N4–Zn–O1 | 124.19(5) |
| O1–Zn–O2 | 117.40(5) | N1–Zn–N4 | 122.82(5) |
| N1–Zn–O1 | 85.73(4) | N4–Zn–O2 | 85.67(4) |
| Entry | Catalyst | [LA]:[Cat]:[BnOH] | kapp b (10–4 s–1) | Mn c (Da) | Mw c (Da) | Ð d | Pr |
|---|---|---|---|---|---|---|---|
| 1 | Zn[L1a]2 | 100:1:0 | 4.26 | 1800 | 2500 | 1.4 | 0.58 |
| 2 | Zn[L1a]2 | 100:1:1 | 4.37 | 1400 | 2100 | 1.5 | 0.58 |
| 3 | Zn[L1a]Et | 100:1:1 | 2.01 | 1700 | 2600 | 1.6 | 0.55 |
| 4 | Zn[L1b]2 | 100:1:0 | 4.01 | 3200 | 4900 | 1.5 | 0.54 |
| 5 | Zn[L1b]Et | 100:1:1 | 2.24 | 1100 | 1600 | 1.4 | 0.54 |
| 6 | Zn[L1c]2 | 100:1:0 | 1.11 | 400 | 700 | 1.8 | 0.47 |
| 7 | Zn[L1d]2 | 100:1:0 | 1.41 | 3000 | 3800 | 1.3 | 0.52 |
| 8 | Zn[L2a]2 | 100:1:0 | 0.71 | 800 | 1200 | 1.5 | 0.54 |
| 9 | Zn[L3a]Et | 100:1:1 | 4.08 | 2700 | 3700 | 1.4 | 0.62 |
| 10 | L1aH | 50:1:0 | 2.45 | 1700 | 2500 | 1.5 | 0.48 |
| 11 | L1bH | 50:1:0 | 2.26 | 1700 | 2000 | 1.2 | 0.50 |
| 12 | L1cH | 50:1:0 | 2.11 | 1900 | 2700 | 1.4 | 0.50 |
| 13 | L1dH | 50:1:0 | 2.41 | 1100 | 1600 | 1.4 | 0.51 |
| 14 | L2a’H | 50:1:0 | 0.30 | 1300 | 1900 | 1.5 | 0.50 |
| 15 | L3aH | 100:1:0 | 1.22 | 1000 | 1300 | 1.3 | 0.49 |
| 16 | ZnEt2 | 200:1:2 | 1.41 | 1200 | 1500 | 1.3 | 0.56 |
| 17 | Sn(Oct)2 | 100:1:1 | 15.1 | 2100 | 3100 | 1.5 | 0.68 |
| 18 | Sn(Oct)2 | 100:1:0 | 12.4 | 3300 | 4700 | 1.4 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Romero, V.; LeBlanc, J.; Lavoie, G.G. Zinc Complexes of Guanidine– and Amidine–Phenolate Ligands for the Ring-Opening Polymerization of Lactide. Inorganics 2025, 13, 265. https://doi.org/10.3390/inorganics13080265
Flores-Romero V, LeBlanc J, Lavoie GG. Zinc Complexes of Guanidine– and Amidine–Phenolate Ligands for the Ring-Opening Polymerization of Lactide. Inorganics. 2025; 13(8):265. https://doi.org/10.3390/inorganics13080265
Chicago/Turabian StyleFlores-Romero, Víctor, Jesse LeBlanc, and Gino G. Lavoie. 2025. "Zinc Complexes of Guanidine– and Amidine–Phenolate Ligands for the Ring-Opening Polymerization of Lactide" Inorganics 13, no. 8: 265. https://doi.org/10.3390/inorganics13080265
APA StyleFlores-Romero, V., LeBlanc, J., & Lavoie, G. G. (2025). Zinc Complexes of Guanidine– and Amidine–Phenolate Ligands for the Ring-Opening Polymerization of Lactide. Inorganics, 13(8), 265. https://doi.org/10.3390/inorganics13080265







