Abstract
The toxicity of lead in conventional perovskites and their inherent chemical instability impede the commercialization of perovskite-based optoelectronics. Therefore, it is vital to develop chemically stable and environmentally friendly Pb-free alternatives. Recently, zero-dimensional (0D) all-inorganic Cs2ZnCl4 doped with Sn(II) has emerged as a promising candidate, exhibiting superior chemical robustness, minimal biotoxicity, and exceptional optoelectronic properties. In this work, pressure effects on structure and optical properties in Sn(II)-doped all-inorganic zero-dimensional halide perovskite are investigated both experimentally and theoretically. The structure–property relationship of Sn(II)-doped Cs2ZnCl4 is studied using high-pressure techniques. Piezochromism, accompanied by a remarkable change in emission color from orange/red and green to orange/yellow, was obtained from 1 atm to 22.5 GPa. Angle dispersive synchrotron X-ray diffraction (ADXRD) patterns and Raman spectra manifest that the material underwent an isostructural phase transition followed by amorphization with increasing pressure. The piezochromism and band gap engineering originate from the pressure-induced lattice compression and isostructural phase transition. This work advances STE emission studies and provides a robust strategy to boost emission efficiency and to construct multifunctional materials with piezochromism in environmentally friendly perovskites, thus facilitating diverse future applications.
1. Introduction
Metal halide perovskites have attracted tremendous attention in the application of light-emitting diodes (LEDs) due to their high photoluminescence (PL) efficiency and tunable light emission [1,2,3]. Lead-based perovskites represent benchmark metal halide semiconductors due to their exceptional optoelectronic properties. However, severe environmental and toxicity concerns associated with lead critically hinder their commercial viability. Consequently, developing environmentally benign lead-free alternatives has become imperative. Inorganic perovskites offer distinct advantages over hybrid counterparts, exhibiting enhanced stability, scalable processability, broader application compatibility, and intrinsic eco-friendliness [4]. Recently, much research effort has been devoted to replacing the toxic Pb in the perovskite framework [5,6,7,8,9,10,11]. Sn2+, Bi3+, and Sb3+ represent three of the most promising Pb-substitution candidates in perovskite architectures, owing to their isoelectronic configurations with Pb2+ [12,13]. Among Pb-substitution candidates, Bi3+-based variants, refs. [14,15,16,17,18] constrained by indirect band gaps and low-dimensional electronic confinement, demonstrate inferior photovoltaic efficiency relative to lead-based analogs but have found utility in photodetectors, capacitors, and memory devices. Similarly, Sb3+-based perovskites underperform in optoelectronic applications compared to conventional lead halide systems [5,10,19,20]. Sn2+-based perovskites exhibit direct band gaps and competitive optoelectronic performance in photovoltaics and photodetectors, yet remain hampered by rapid oxidation. Despite these limitations, the vast compositional space of lead-free perovskites remains largely unexplored, warranting intensified investigation to unlock their untapped potential for next-generation optoelectronics. Recently, Zn2+-based perovskites offer key advantages, including non-toxicity (lead-free), exceptional chemical and thermal stability, and unique intrinsic broadband luminescence (particularly via self-trapped exciton emission). These properties make them promising candidates for stable optoelectronic devices, particularly deep-blue LEDs and scintillators. However, due to the large band gap of Cs2ZnCl4, Wu et al. introduced Sn(II) doping, creating impurity states that simultaneously reduce the band gap and localize band-edge orbitals [11]. Moreover, photoexcitation induces Jahn–Teller distortion in the [SnCl4]2− units, driving STE emission through symmetry-breaking lattice reorganization. Despite its significance for advancing solid-state emission studies and establishing a framework for developing lead-free perovskite variants in illumination applications, this work does not provide mechanistic depth in correlating structural evolution with optical responses critical gap that warrants targeted investigation.
Pressure, as a fundamental thermodynamic variable independent of temperature and chemical composition, enables precise modulation of interatomic distances without altering stoichiometry. This unique capability directly perturbs intermolecular interactions and modifies intrinsic atomic-scale properties through controlled lattice compression. As we all know, the crystal structure has a close correlation with the optical properties of all-inorganic lead-free halide perovskites [21,22]. The adjustable structure of perovskite can easily respond to most external pressure stimuli [4,23]. Thus, high-pressure techniques serve as a powerful strategy for probing the intrinsic structure–property correlations in functional materials, refs. [24,25,26], enabling non-destructive modulation of lattice parameters while preserving chemical composition [27,28,29,30,31]. Consequently, high-pressure methodology serves as a critical experimental paradigm for probing structure–optoelectronic property correlations in perovskite materials through controlled lattice compression without altering stoichiometry.
In this work, Sn(II)-doped Cs2ZnCl4 was synthesized with a STE emission centered at ca. 678.0 nm and a full width at half-maximum (FWHM) of ca.120.0 nm. Piezochromism in Sn(II)-doped Cs2ZnCl4 was obtained by in situ pressure PL, with an emission color from orange/red to green and orange/yellow. Furthermore, the band gap exhibits a non-monotonic trend under pressure, undergoing three sequential stages: initial reduction followed by subsequent enlargement. This pressure-dependent band gap evolution originates from progressive lattice compression and an isostructural phase transition under hydrostatic loading. Angle dispersive synchrotron X-ray diffraction (ADXRD) patterns and Raman spectra manifest that the material underwent an isostructural phase transition followed by amorphization with increasing pressure. This study investigated pressure effects on structure and optical properties in Sn(II)-doped all-inorganic zero-dimensional halide perovskite, both experimentally and theoretically.
2. Results and Discussion
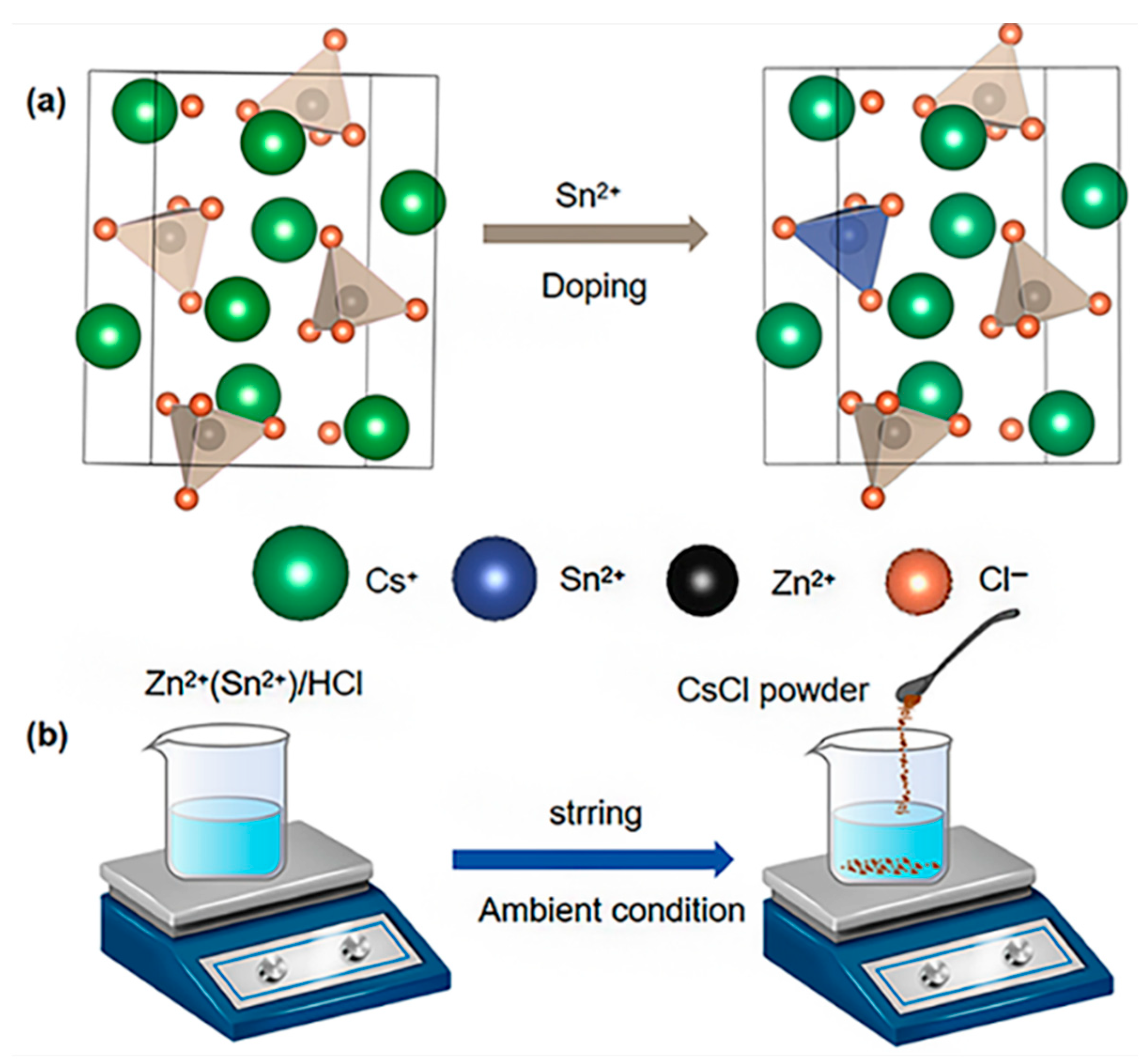
The Sn(II)-doped Cs2ZnCl4 was synthesized following the methodology reported in a previous study. Detailed information was provided in the Experimental section of the Supporting Information. Due to the identical valency of Zn(II) and Sn(II), a simple Sn/Zn substitution is introduced into a pristine Cs2ZnCl4 cell model, constructing an Sn(II)-doped model. After full optimization, the Cs2ZnCl4 and Sn(II)-doped Cs2ZnCl4 models are visualized as in Figure 1a. Figure 1b demonstrates the schematics of the preparation process of both the Sn(II)-doped Cs2ZnCl4 crystals, synthesized by the precipitation method. The required crystals are immediately precipitated by dropping CsCl powder into the clear hydrochloride precursor solution. Sn(II)-doped Cs2ZnCl4 is further investigated via X-ray diffraction (XRD). The XRD pattern of doped crystals is shown in Figure S1a. There is no new XRD peak or variation in the pattern observed, elucidating that the original lattice structure of Cs2ZnCl4 is unaltered with Sn(II) doping. Additionally, the XRD patterns of Cs2ZnCl4 are simulated, which are in accordance with the experimental ones, confirming the availability of our facile synthesis method. No phase transition of the lattice structure is observed, which is consistent with the experimental results (Figure S1). The synthesized Sn(II)-doped Cs2ZnCl4 crystallizes in space group Pnam, with lattice parameters a = 9.75251 Å, b = 12.9811 Å, c = 7.40238 Å, and α = β = γ = 90°. The unit cell of Sn(II)-doped Cs2ZnCl4 is schematically described in Figure 1a. Within the unit cell, each tetrahedron is separated from the other, forming a zero-dimensional perovskite structure.
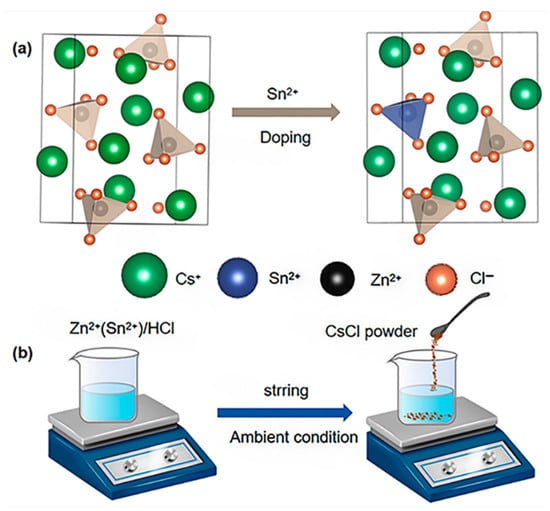
Figure 1.
(a) The crystal structure diagrams of Cs2ZnCl4 and Sn(II)-doped Cs2ZnCl4; (b) A schematic diagram of the synthesis process of the pristine and Sn(II)-doped Cs2ZnCl4 crystals.
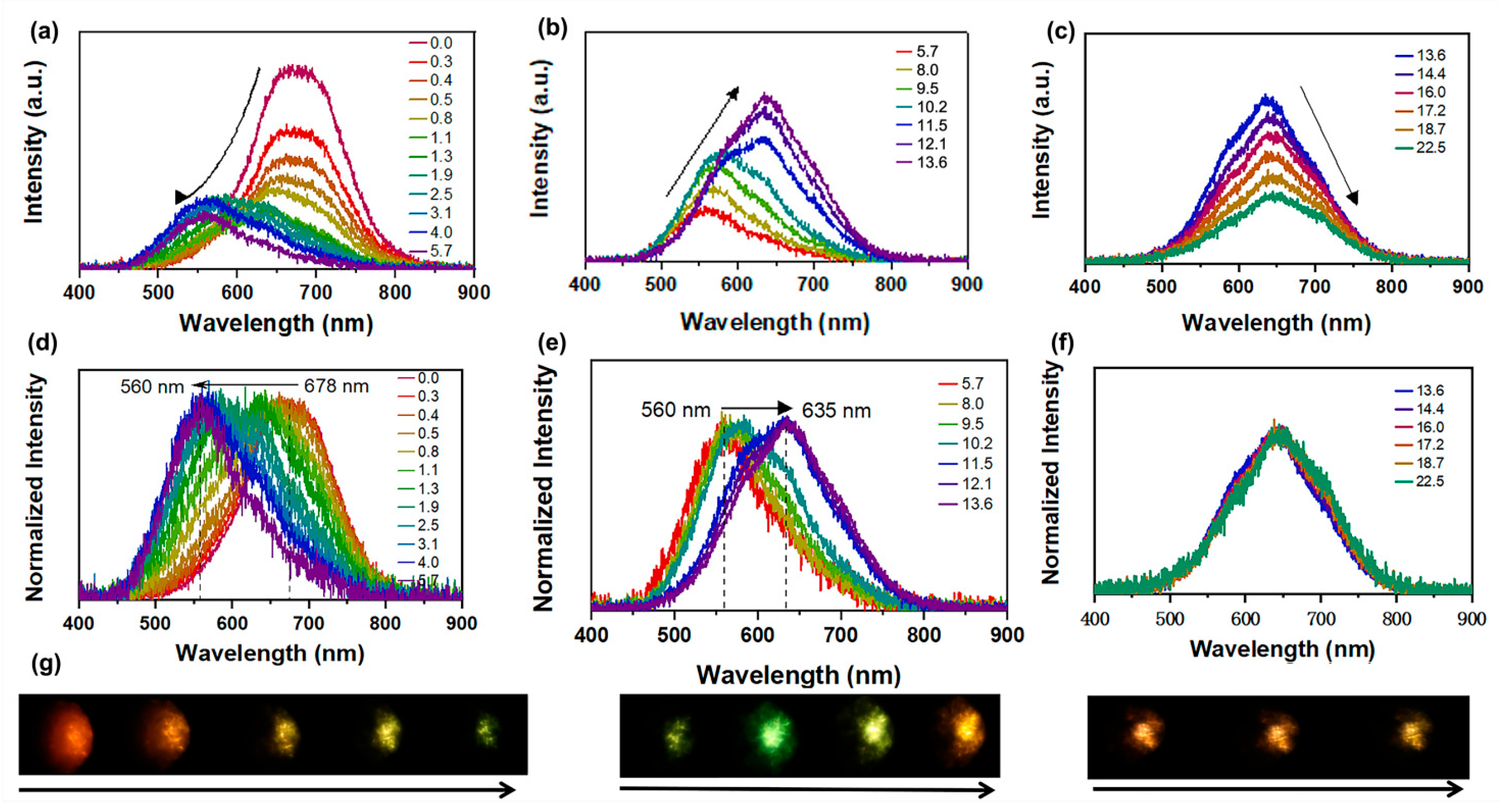
The pressure-dependent PL spectra of Sn(II)-doped Cs2ZnCl4 crystals were measured using in situ PL spectroscopy during both compression and decompression processes. The Sn(II)-doped Cs2ZnCl4 crystals exhibit broad emission, associated with the inorganic lattice’s local distortion, which was detected at ambient conditions in Figure 2a. The single PL peak centered at ca. 678.0 nm, with a full width at half-maximum (FWHM) of ca.120.0 nm, was observed at ambient conditions. This PL peak could be attributed to the STE emission of the disphenoidal [SnCl4]2− units in the host matrix. For certain ions with ns2 outer electronic configuration (Sn2+ with 5s2), the singlet electronic ground state was denoted as 1S0 and the nsnp excited state was split into singlet 1P and triplet 3P states [12]. The triplet 3P state further splits into three non-degenerate states denoted as 3Pn (n = 0, 1, 2) [32]. The transition rule indicates that the 1P1 to 1S0 transition is allowed and the 3P1 to 1S0 transition is partially allowed due to the spin–orbit coupling of heavy atoms. However, 3P2 to 1S0 and 3P0 to 1S0 are forbidden transitions. Under UV radiation, the emission of Sn(II)-doped Cs2ZnCl4 is centered at approximately 678 nm, which is consistent with the previously reported results [11]. With the increase in pressure, the STE emission experienced an unambiguous pressure-sensitive evolution. The PL intensity decreases significantly, and the PL peak blue-shifts from 678 nm to 560 nm at 1.9 GPa (Figure 2a–c). The increase in PL intensity observed at 5.7 GPa is attributed to an increase in the concentration of self-trapped excitons (STEs), resulting from lattice compression. When the pressure was increased to 8.0 GPa, the PL intensity continued to rise, accompanied by a red shift from 560 nm to 635 nm, originating from a pressure-induced isostructural phase transition occurring at approximately 8.0 GPa. When the pressure reaches 11.5 GPa, the original emission at λ = 678 nm gradually disappears. After that, the PL intensity decreases again, while the peak position remains almost unchanged. As shown in Figure 2d,e, the normalized spectra revealed the shift in peak positions during the pressure variation process. When the pressure was increased to 22.6 GPa (Figure 2f), the PL peak position remains virtually unchanged, accompanied by a gradual decrease in fluorescence intensity. In addition, the fluorescent micrographs of Sn(II)-doped Cs2ZnCl4 clearly demonstrated the PL brightness changes with increasing pressure. Upon completely releasing pressure to the ambient conditions, a pressure-driven PL peak reversibility of Sn(II)-doped Cs2ZnCl4 was identified (Figure S2). While the PL intensity shows a slightly smaller value than the initial state, which should be related to the incomplete recrystallization process.
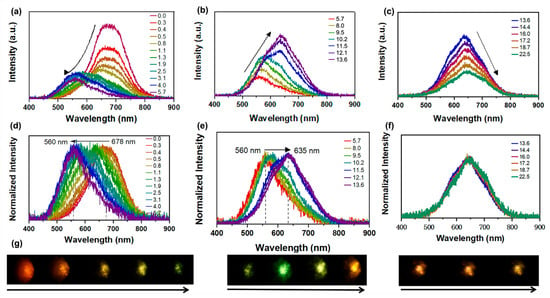
Figure 2.
(a–c) High-pressure PL evolution of Sn(II)-doped Cs2ZnCl4 upon compression to 22.5 GPa; (d–f) Normalized PL spectra of Sn(II)-doped Cs2ZnCl4; (g) PL micrographs of Sn(II)-doped Cs2ZnCl4 at ambient conditions and under compression up to 22.5 GPa.
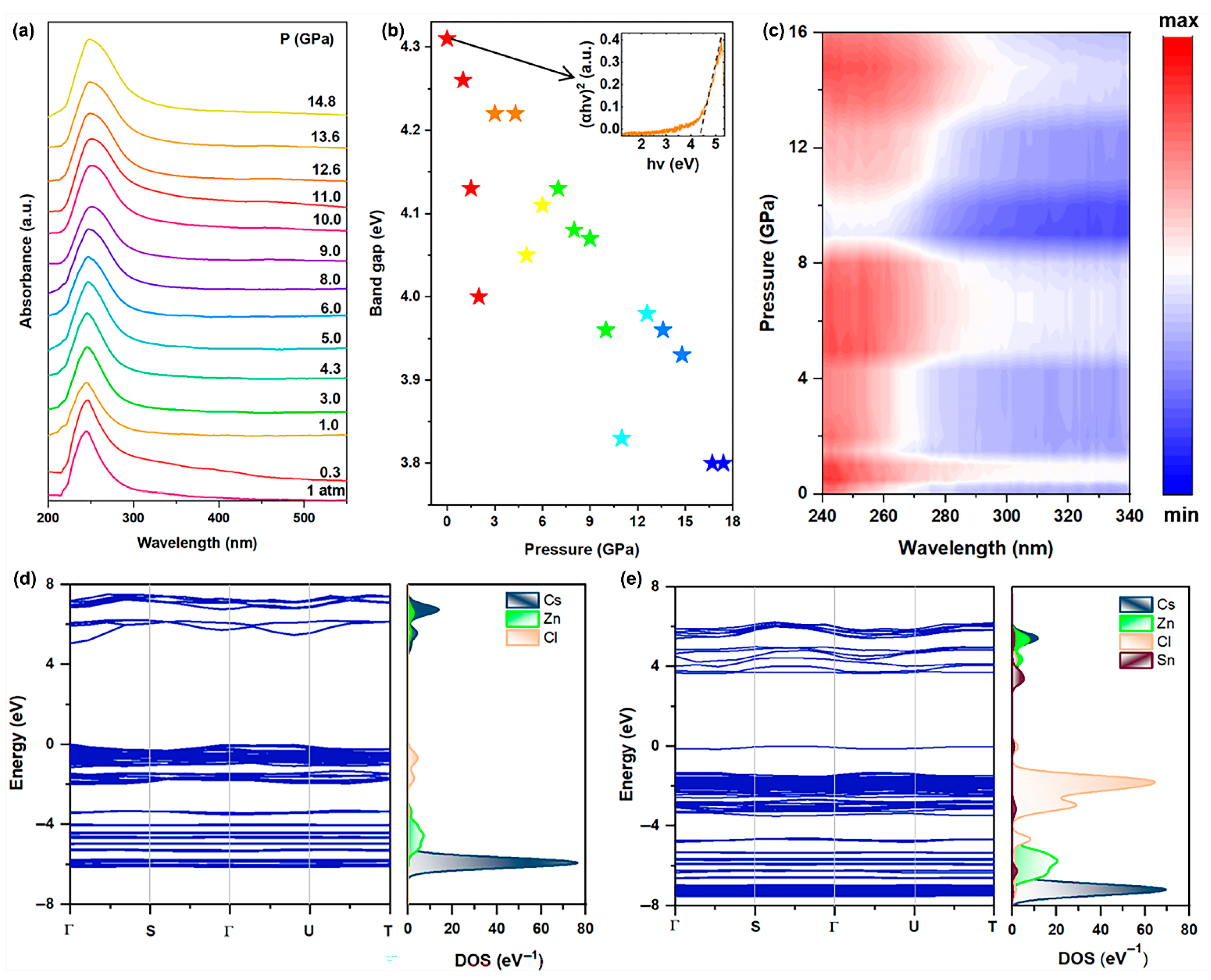
Likewise, in situ high-pressure ultraviolet−visible (UV-vis) absorption spectroscopy experiments were conducted to investigate the band gap evolution of Sn(II)-doped Cs2ZnCl4 under high pressure (Figure 3a). The absorption edge was observed to be approximately located at 287 nm under ambient conditions and experienced an obvious red shift with an increase in pressure. The Tauc plot was fitted to assess the band gap values of Sn(II)-doped Cs2ZnCl4 as a function of pressure by considering the direct band gap nature (Figure 3b). The pristine Sn(II)-doped Cs2ZnCl4 presents a large direct band gap of about 4.31 eV. The band gap of the Sn(II)-doped Cs2ZnCl4 underwent a reduction from 4.31 to 3.80 eV upon compression. At a relatively low pressure of 1.5 GPa, the band gap initially displayed a gradual decrease. The band gap began to increase from 1.5 to 4.3 GPa, which is in accordance with the blue shift in PL. Upon further pressure increase to about 5.0 GPa, the band gap decreased once more. Subsequently, the band gap increased again until around 8.0 GPa. As the pressure exceeded 11.0 GPa, the band gap continued to decrease, a trend that corresponds to the red shift in the PL peak. As demonstrated in Figure 3c, the band gap underwent significant modifications under applied pressure, with its values systematically shifting as pressure increases. Upon the complete release of pressure, the band gap shows a slightly smaller value than the initial state (Figure S3), which should be related to the incomplete recrystallization process.
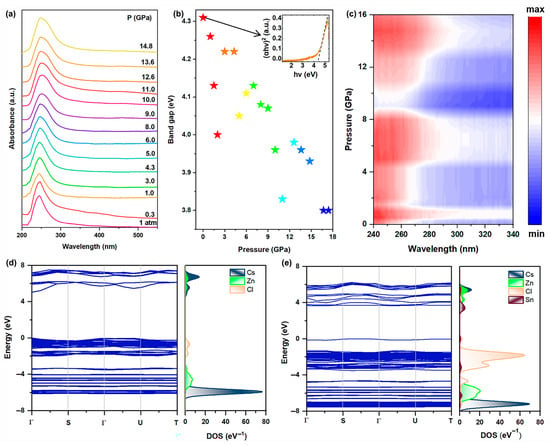
Figure 3.
(a) UV-vis absorption spectroscopy of Sn(II)-doped Cs2ZnCl4 under high pressure; (b) Band gap evolution of Sn(II)-doped Cs2ZnCl4 upon compression; (c) Absorption heatmap of Sn(II)-doped Cs2ZnCl4 upon compression; (d) DFT-calculated band structure and projected density of states of Cs2ZnCl4 and (e) Sn(II)-doped Cs2ZnCl4 under ambient conditions.
First-principles calculations of the band structure of Cs2ZnCl4 and Sn(II)-doped Cs2ZnCl4 were performed using density functional theory (DFT). The band structures and density of states (DOS) are exhibited as depicted in Figure 3d,e. The pristine Cs2ZnCl4 presents a large direct band gap of about 5.06 eV. The VBM is derived from a mixture of Cl p and Zn p orbitals, and the CBM is mainly contributed by the p-orbital of Cl. The band gap decreased with Sn2+ doping, introducing an impurity band above the VBM (Figure S4), which is mainly composed of Sn s orbitals, and the CBM is mainly composed of Sn p orbitals. Thus, the band gap is reduced to 3.54 eV. It is indicated that both the pristine and doped samples will mainly absorb photons in the ultraviolet (UV) region, which is per our experimental results. It is readily observed that both the VBM and CBM are localized near the disphenoidal [SnCl4]2−, indicating that the transitions mainly exist near [SnCl4]2− units, and distortion will influence the radiative energy.
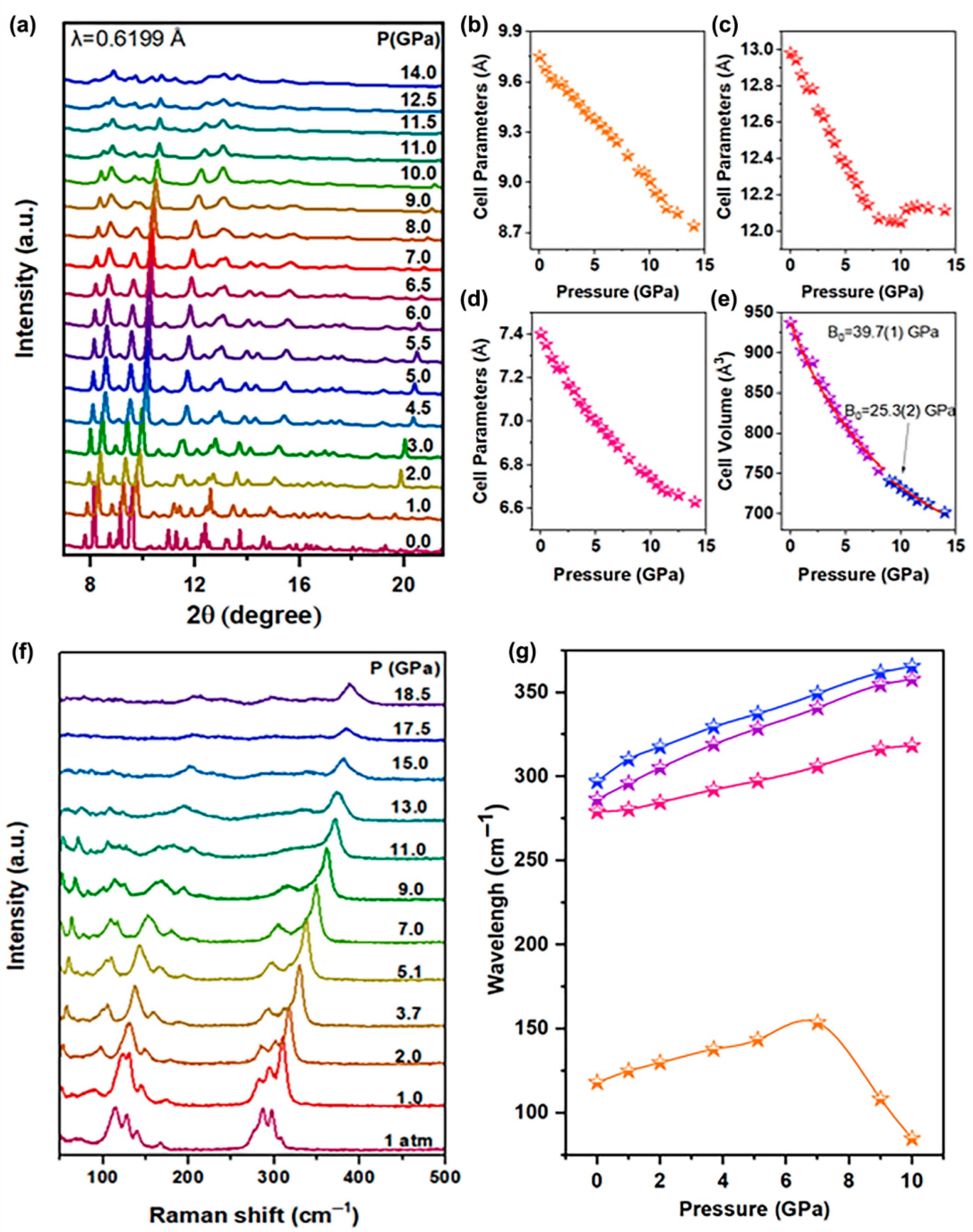
The optical properties of the Sn(II)-doped Cs2ZnCl4 strongly depend on the response of the structure. In order to figure out the relationship between the optical properties and structure of Sn(II)-doped Cs2ZnCl4, the in situ high-pressure angle dispersive synchrotron X-ray diffraction (ADXRD) experiments were performed at room temperature. The ADXRD data under pressure are shown in Figure 4a. Under ambient conditions, the refined ADXRD pattern (Figure S5) demonstrated that the initial Sn(II)-doped Cs2ZnCl4 crystallized in orthorhombic structure (Table S1), with the space group Pnam (lattice parameters a = 9.75251 Å, b = 12.9811 Å, c = 7.40238 Å and α = β = γ = 90°). Upon compression, all Bragg diffraction peaks of the Sn(II)-doped Cs2ZnCl4 shift toward higher diffraction angles, owing to the contraction in the volume under pressure. When the pressure reaches about 8.0 GPa, the splitting of the initial two diffraction peaks at about 9.5° occurs without concomitant emergence of new diffraction peaks, indicating that the pressure-induced isostructural phase transition begins. When the pressure exceeds 8.0 GPa, the diffraction peaks exhibit obvious broadening and weakening, and tend to disappear at 11.0 GPa, demonstrating that the original ordered crystal structure of the material is destroyed under high pressure, leading to the transition of the crystal to amorphization. Furthermore, Sn(II)-doped Cs2ZnCl4 is reverted into its original phase after fully releasing pressure (Figure S6). The diffraction peaks of the recovered sample are obviously broadened and weakened, as well as partially disappeared, illustrating the incomplete recrystallization and the presence of residual amorphous phase in the quenched sample. The variations in unit cell parameters were shown in panels Figure 4b–d. With the increase in pressure, the lattice parameters of the a-axis and c-axis decrease continuously. An abrupt change in the pressure response of the b-axis lattice parameter of Sn(II)-doped Cs2ZnCl4 crystals occurs at about 8.0 GPa, originating from a pressure-induced isostructural phase transition (Table S1). In addition, the experimental pressure−volume (P−V) curves are fitted using the third-order Birch−Murnaghan equation of state (Figure 4e):
where V0 is the volume at 1 atm, B0 is the bulk modulus at 1 atm, and B0′ is the derivative of the bulk modulus with respect to pressure. With increasing pressure, the bulk modulus of the unit cell for Sn(II)-doped Cs2ZnCl4 gradually decreases from B0 (39.7(1) GPa) to B0 (25.3(2) GPa) near 8.0 GPa, which can be attributed to the pressure-induced electronic structural phase transition. This phenomenon is consistent with the aforementioned fluorescence variation pattern. The decrease in bulk modulus indicates the weakening of the material’s compressibility.
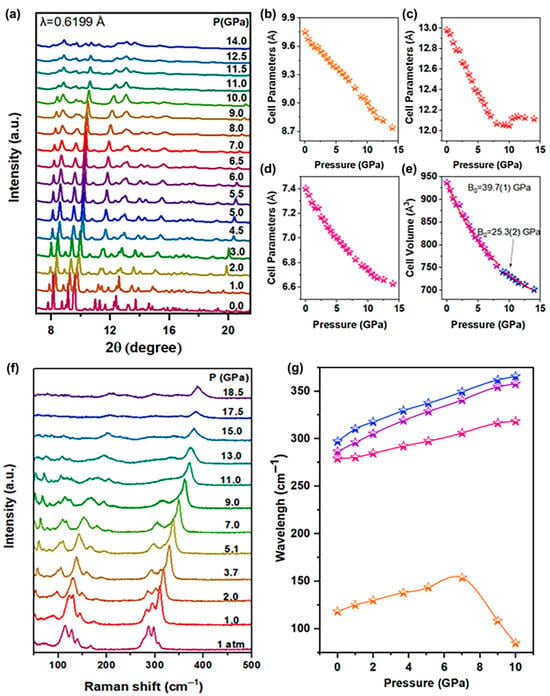
Figure 4.
(a) Typical XRD patterns of the Sn(II)-doped Cs2ZnCl4 at different pressures; (b–d) cell parameters and (e) cell volume under high pressure; (f) in situ Raman spectra under high pressure; (g) shift in Raman peaks for different vibrational modes with pressure.
The in situ high-pressure Raman scattering spectra of the Sn(II)-doped Cs2ZnCl4 are collected in Figure 4f. The main vibration modes of Sn(II)-doped Cs2ZnCl4 are related to the [ZnCl4]2− cluster and [SnCl4]2− cluster. Among them, the Raman band at about 118.18 cm−1 corresponds to the bending vibration of Cl-Zn-Cl, the Raman band at about 286.51 cm−1 corresponds to the stretching vibration of Zn-Cl, and the Raman band at about 297.01 cm−1 corresponds to the stretching vibration of Sn-Cl. The Raman band at about 279.10 cm−1 corresponds to the stretching vibration of Cs-Cl (Table S2). As can be discerned, with the increase in pressure, Raman peaks collectively shift toward higher wavenumbers, accompanied by the shortening of chemical bond lengths under compression, as well as the increase in vibration frequency and the decrease in interplanar spacing. When the pressure exceeds 15.0 GPa, partial Raman peaks vanish, originating from the amorphization of crystals. When the pressure is increased to about 8.0 GPa, the bending vibration mode of Cl-Zn-Cl exhibits an inflection point, indicating a new structural transition, which is associated with the change in the inorganic Sn(II)-doped Cs2ZnCl4. After the pressure was fully released, the Raman scattering spectra eventually returned to the original crystal structure (Figure S6).
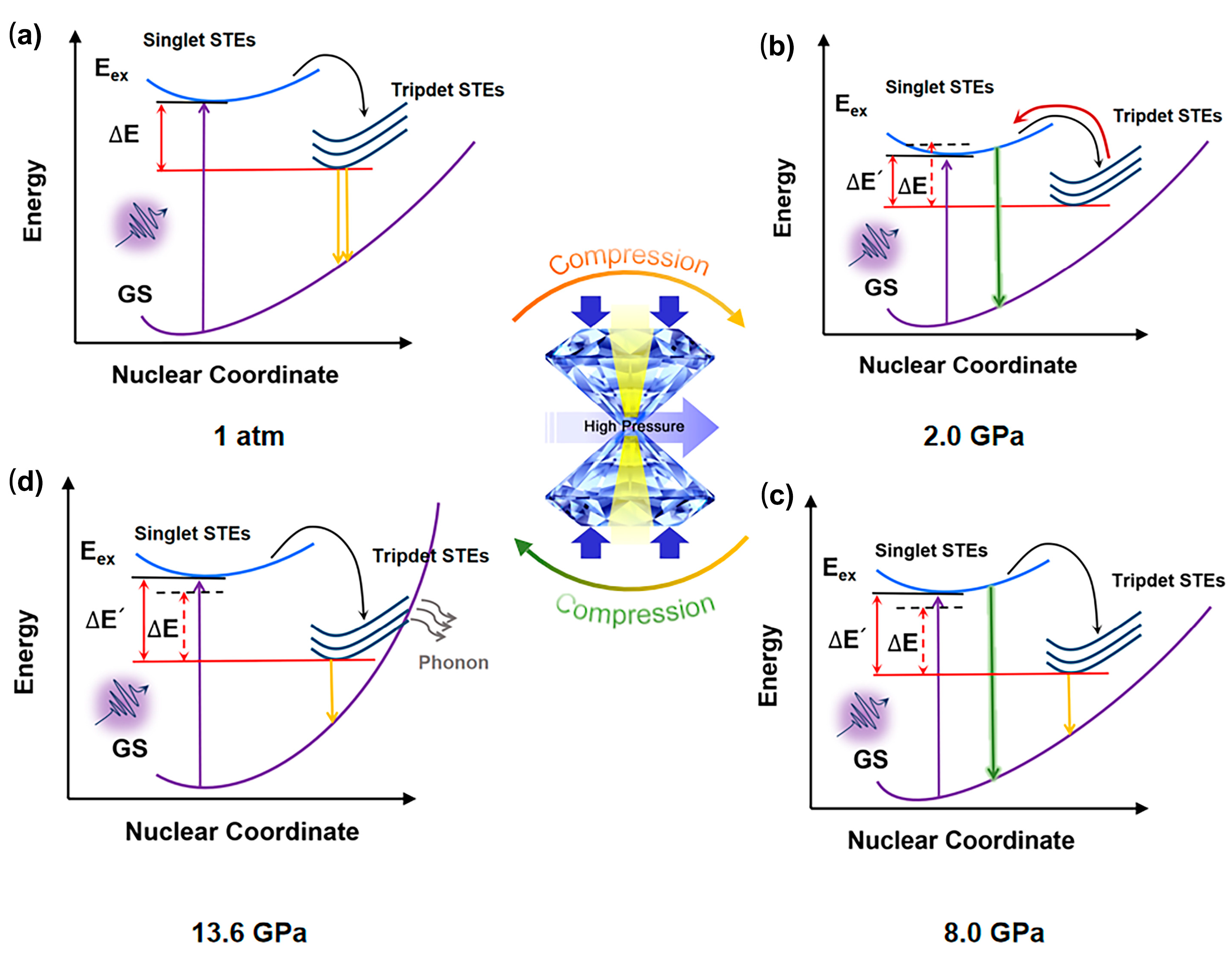
The mechanism of piezochromism for Sn(II)-doped Cs2ZnCl4 was illustrated in Figure 5. Under ambient conditions, a broad PL spectrum was observed in the measurement, which also exists widely in multiple different STE emission systems. The broad STE emission of Sn(II)-doped Cs2ZnCl4 originated from the electron and hole being bound to form an exciton trapped in the distorted disphenoidal [SnCl4]2− units. [SnCl4]2− will act as the radiative recombination center for STE emission, and STE emission can be regarded as the 3P1 → 1S0 transition of Sn2+ from excited to ground states. A pressure-driven reverse intersystem crossing (RISC) from triplet excited states back to singlet states was established principally by the difference (∆E) between the two states. With increasing pressure up to 2.0 GPa, the adjacent [SnCl4]2− moved closer, and Sn(II)-doped Cs2ZnCl4 was compressed under pressure, resulting in a gradual decrease in ∆E. Therefore, more excitons were trapped in high-energy excited singlet states, which is consistent with the change in PL peak blue-shift from 678 nm to 560 nm. As the pressure further increased and exceeded 5.7 GPa, the STEs exhibited an obvious increase, which is attributed to an increase in the concentration of self-trapped excitons (STEs), resulting from lattice compression. When the pressure reaches 8.0 GPa, an abrupt change in the pressure response of the b-axis lattice parameter of Sn(II)-doped Cs2ZnCl4 crystals occurs. The PL intensity continues to rise, accompanied by a red shift from 560 nm to 635 nm, originating from a pressure-induced isostructural phase transition occurring at approximately 8.0 GPa. Therefore, the band gap increased again, and the emission with two PL peaks centered at ca. 560 nm and 678 nm, respectively. As pressure further increased and exceeded 13.6 GPa, due to the further compressed structure, the highly structural distortion and the strong electron–phonon coupling led to a high crossover between STE states and the ground state, making the trapped excitons easily annihilated through the phonon-assisted pathway. Thus, facilitating the formation of bound excitons to dominate the decrease in PL intensity.
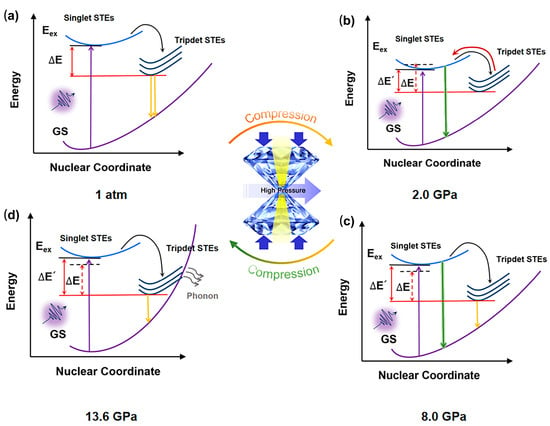
Figure 5.
Configuration coordinate models for Sn(II)-doped Cs2ZnCl4 at (a) 1 atm, (b) 2.0 GPa, (c) 8.0 GPa, and (d) 13.6 GPa. STEs: self-trapped states; GS: ground state.
3. Conclusions
In summary, pressure effects on structure and optical properties in Sn(II)-doped Cs2ZnCl4 all-inorganic zero-dimensional halide perovskite were methodically studied by high-pressure technology. The considerable piezochromism, accompanied by a remarkable change in emission color from orange/red to green, then to orange/yellow, and band gap engineering, originates from the pressure-induced changes in the lattice parameters resulting from the isostructural phase transition. First-principles calculations and comprehensive characterizations, including PL spectra, UV/Vis absorption, Raman spectroscopy, and synchrotron ADXRD, were performed on Sn(II)-doped Cs2ZnCl4. These results indicated that Sn(II)-doped Cs2ZnCl4 experiences an isostructural phase transition at about 8.0 GPa, which was responsible for the piezochromism phenomenon and the change in band gap. The results of the analysis agree very well with the experimental results. Our work provides a robust strategy to boost emission efficiency and to construct multifunctional materials with piezochromism in environmentally friendly perovskites, thus facilitating diverse applications in display technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13080264/s1, Figure S1: The XRD (wave length: 0.6199 Å) of Sn(II)-doped Cs2ZnCl4 ((a) experimental XRD; (b): simulated XRD). Figure S2: PL spectra of the Sn(II)-doped Cs2ZnCl4 at ambient conditions and decompression, respectively. Figure S3: UV/Vis absorption spectra of the Sn(II)-doped Cs2ZnCl4 at ambient conditions and decompression, respectively. Figure S4: Refinements of n(II)-doped Cs2ZnCl4 at 0.5 GPa, 7.0 GPa and 9.0 GPa. Figure S5: Selected ADXRD patterns of Sn(II)-doped Cs2ZnCl4 upon decompression. Figure S6: Selected Raman spectroscopy of Sn(II)-doped Cs2ZnCl4 upon decompression. Table S1: Raman peaks of Sn(II)-doped Cs2ZnCl4. Table S2: Refinement cell parameters and refinement statistics at ambient pressure and 9.0 GPa for Sn(II)-doped Cs2ZnCl. References [33,34,35] are cited in the Supplementary Materials.
Author Contributions
Software, M.W., Y.Q. and Z.C.; Validation, T.G. and M.W.; Data curation, C.Z. and Y.L.; Writing—review & editing, T.G., M.W., Y.Q., A.Z., C.Z., Y.L. and G.X.; Project administration, Y.L. and G.X.; Funding acquisition, Y.L. and G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation of China (12174144, 12474009), the Zhejiang Provincial Natural Science Foundation of China (LR22B010001), BIGC Project (Nos: Ea202412 and 27170124039), and Innovation and Entrepreneurship Training Program (202510015027, S202510015044).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This work was mainly performed at BL15U1 at the Shanghai Synchrotron Radiation Facility (SSRF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Dong, B.; Hagfeldt, A.; Luo, J.; Graetzel, M. Chemically tailored molecular surface modifiers for efficient and stable perovskite photovoltaics. SmartMat 2021, 2, 33–37. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, Y.; Ma, F.; Zhang, C.-X.; Deng, H.; Gao, F.; Ye, Q.; Meng, J.; Yin, Z.; Zhang, X. Large cation ethylammonium incorporated perovskite for efficient and spectra stable blue light-emitting diodes. Nat. Commun. 2020, 11, 4165. [Google Scholar] [CrossRef]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Current development toward commercialization of metalalide perovskite photovoltaics. Adv. Opt. Mater. 2021, 9, 2100390. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Geng, T.; Lv, P.; Zhao, D.; Li, Y.; Dong, Q.; Xiao, G.; Zou, B. Pressure-induced emission and remarkable piezochromism of two-dimensional cesium antimony bromide perovskites. Mater. Res. Lett. 2024, 12, 500–506. [Google Scholar] [CrossRef]
- Das Adhikari, S.; Echeverria-Arrondo, C.; Sanchez, R.S.; Chirvony, V.S.; Martinez-Pastor, J.P.; Agouram, S.; Munoz-Sanjose, V.; Mora-Sero, I. White light emission from lead-free mixed-cation doped Cs2SnCl6 nanocrystals. Nanoscale 2022, 14, 1468–1479. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, L.; Guo, D.; Pan, Y. Te4+-doped zero-dimensional Cs2ZnCl4 single crystals for broadband yellow light emission. J. Mater. Chem. C 2022, 10, 204–209. [Google Scholar] [CrossRef]
- Chang, T.; Wei, Q.; Zeng, R.; Cao, S.; Zhao, J.; Zou, B. Efficient Energy Transfer in Te(4+)-doped Cs2ZrCl6 vacancy-ordered perovskites and ultrahigh moisture stability via A-Site Rb-alloying strategy. J. Phys. Chem. Lett. 2021, 12, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Q.; Fu, Y.; Qi, J.; Chen, L.; Yan, S.; Liu, W.; Guo, S. Phase engineering for achieving full-color tunable emission from blue to red and multi-level information security in isomeric hybrid copper halides. Angew. Chem. Int. Ed. 2025, 64, e202506748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zaffalon, M.L.; Pinchetti, V.; Brescia, R.; Moro, F.; Fasoli, M.; Fanciulli, M.; Tang, A.; Infante, I.; De Trizio, L.; et al. Bright blue emitting Cu-doped Cs2ZnCl4 colloidal nanocrystals. chemistry of materials: A publication of the American. Chem. Soc. 2020, 32, 5897–5903. [Google Scholar]
- Wang, X.; Shen, Q.; Chen, Y.; Ali, N.; Ren, Z.; Bi, G.; Wu, H. Bright luminescence of Sb doping in all-inorganic zinc halide perovskite variant. J. Alloys Compd. 2022, 895, 162610. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Q.; Chen, Y.; Ali, N.; Ren, Z.; Bi, G.; Wu, H. Self-trapped exciton emission in an Sn(II)-doped all-inorganic zero-dimensional zinc halide perovskite variant. Nanoscale 2021, 13, 15285–15291. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, Y.; Zhao, J.; Xia, Z. Sb3+ doping-induced triplet self-trapped excitons emission in lead-free Cs2SnCl6 nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7439–7444. [Google Scholar] [CrossRef]
- Dave, K.; Huang, W.-T.; Leśniewski, T.; Lazarowska, A.; Jankowski, D.; Mahlik, S.; Liu, R.-S. Photoluminescence enhancement study in a Bi-doped Cs2AgInCl6 double perovskite by pressure and temperature-dependent self-trapped exciton emission. Dalton Trans. 2022, 51, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kavanagh, S.R.; Napari, M.; Palgrave, R.G.; Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Davies, D.W.; Laitinen, M.; Julin, J.; Isaacs, M.A.; et al. Bandgap lowering in mixed alloys of Cs2Ag(SbxBi1−x)Br6 double perovskite thin films. J. Mater. Chem. A 2020, 8, 21780–21788. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Ghini, M.; Melcherts, A.E.M.; Zito, J.; Goldoni, L.; Infante, I.; Guizzardi, M.; Scotognella, F.; Kriegel, I.; et al. Colloidal Bi-doped Cs2Ag1-x NaxInCl6 nanocrystals: Undercoordinated surface Cl ions limit their light emission efficiency. ACS Mater. Lett. 2020, 2, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Singh, V. Exploring double luminescence in Bi3+-doped Cs2ZrCl6 perovskite crystals: Insight into multiexcitonic emission processes for optoelectronic applications. Energy Fuels 2024, 38, 15681–15690. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhang, X.-S.; Gong, X.-K.; Yuan, X.-R.; Chen, M.-X.; Huang, S.-W.; Zhou, B.-Z.; Xu, J.-P.; Li, L. Enhancing broadband blue luminescence efficiency and stability in Bi 3+-doped Cs2ZnCl4 nanocrystals from STEs and advancing energy applications. Inorg. Chem. Front. 2024, 11, 71–84. [Google Scholar] [CrossRef]
- Ye, W.; Lin, H.Z.; Li, M.; Jiang, L.; Chen, D.; Lu, J.M. Dimensional reduction in Cs2AgBiBr6 enables long-term stable Perovskite-based gas sensing. Nat. Commun. 2025, 16, 4820. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.; Fang, S.; Zhong, H.; Tian, B.; Wang, Y.; Li, H.; Hu, H.; Shi, Y. Efficient White Photoluminescence from Self-Trapped Excitons in Sb3+/Bi3+-Codoped Cs2NaInCl6 Double perovskites with tunable dual-emission. ACS Energy Lett. 2021, 6, 3343–3351. [Google Scholar] [CrossRef]
- Zhu, D.; Zaffalon, M.L.; Zito, J.; Cova, F.; Meinardi, F.; De Trizio, L.; Infante, I.; Brovelli, S.; Manna, L. Sb-doped metal halide nanocrystals: A 0D versus 3D comparison. ACS Energy Lett. 2021, 6, 2283–2292. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, W.; Li, L.; Shang, X.; Huang, P.; Yi, X.; Chen, X. Multiphoton-excited upconversion luminescence and amplified spontaneous emission from Te4+-doped Cs2SnCl6 nanocrystals. ACS Nano 2025, 19, 18866–18873. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wang, J.; Wu, X.; Yuan, Y.; Shahzadi, U.; Guo, H. Ion-doping and compression effects on the emission properties of halide double perovskites: Transition from non-luminescence to significant emission enhancement. Chem. Eng. J. 2025, 531, 162923. [Google Scholar] [CrossRef]
- Geng, T.; Shi, Y.; Liu, Z.; Zhao, D.; Ma, Z.; Wang, K.; Dong, Q.; Xiao, G.; Zou, B. Pressure-induced emission from all-Inorganic two-dimensional vacancy-ordered lead-free metal halide perovskite nanocrystals. J. Phys. Chem. Lett. 2022, 13, 11837–11843. [Google Scholar] [CrossRef]
- Geng, T.; Wang, M.; Chen, Z.; Li, Y.; Zhang, A.; Li, F.; Wu, W.; Xiao, G. Pressure effect on all-inorganic lead-free halide perovskite materials: Structural and optical properties. ChemNanoMat 2025, 11, e202400677. [Google Scholar] [CrossRef]
- Matheu, R.; Ke, F.; Breidenbach, A.; Wolf, N.R.; Lee, Y.; Liu, Z.; Leppert, L.; Lin, Y.; Karunadasa, H.I. Charge reservoirs in an expanded halide perovskite analog: Enhancing high-pressure conductivity through redox-active molecules. Angew. Chem. Int. Ed. 2022, 61, e202202911. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, G.; Jiang, J.; Che, L.; Sui, L.; Wang, X.; Zeng, X.; Wu, G.; Yuan, K.; Yang, X. High-pressure modulation of NIR Luminescence in Cr3+-doped Cs2AgInCl6 double perovskites: The role of ultrafast energy transfer. Laser Photonics Rev. 2025, 19, 2401000. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, S.; Wang, S.; Yang, X.; Guo, F.; Zhang, Y.; Gu, Z.; Song, Y. Pressure engineering on perovskite structures, properties, and devices. Adv. Funct. Mater. 2024, 34, 2315918. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S.; Su, Y.; Qin, J.; Xiao, G.; Shang, Y.; Yin, X.; lv, P.; Wang, F.; Yang, J.; et al. Pressure encryption toward physically uncopiable anti-counterfeiting. Nat. Commun. 2025, 16, 6203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, S.; Luo, H.; Zhou, C.; Lin, H.; Ma, X.; Hu, Q.; Du, M.-h.; Ma, B.; Yang, W.; et al. Reaching 90% Photoluminescence quantum yield in one-dimensional metal halide C4N2H14PbBr4 by pressure-suppressed nonradiative loss. J. Am. Chem. Soc. 2020, 37, 16001–16006. [Google Scholar] [CrossRef]
- Xie, Z.; Deng, H.; Ge, X.; Chi, Z.; Liu, B. Mechanoluminescence from amorphous organic luminogens. J. Am. Chem. Soc. 2025, 15, 12722–12729. [Google Scholar] [CrossRef]
- Jin, N.; Nagaoka, Y.; Liu, Z.; Li, R.; Chen, O. Pressure-modulated energy transfer dynamics in Mn2+-Doped CdS/ZnS Core/Shell Quantum Dots. J. Am. Chem. Soc. 2025, 9, 7965–7973. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, Z.; Hu, M.; Chen, C.; Luo, J.; Li, S.; Gao, L.; Xiao, Z.; Niu, G.; Tang, J. Antimony doped Cs2SnCl6 with bright and stable emission. Front. Optoelectron. 2019, 12, 352–364. [Google Scholar] [CrossRef]
- Leng, M.; Yang, Y.; Zeng, K.; Chen, Z.; Tan, Z.; Li, S.; Li, J.; Xu, B.; Li, D.; Hautzinger, M.P.; et al. All-inorganic bismuth-based perovskite quantum dots with bright blue photoluminescence and excellent stability. Adv. Funct. Mater. 2018, 28, 1704446. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673. [Google Scholar] [CrossRef]
- Jansen, E.; Schafer, W.; Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Crystallogr. 1994, 27, 492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).