Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs
Abstract
1. Introduction
2. Results and Discussion
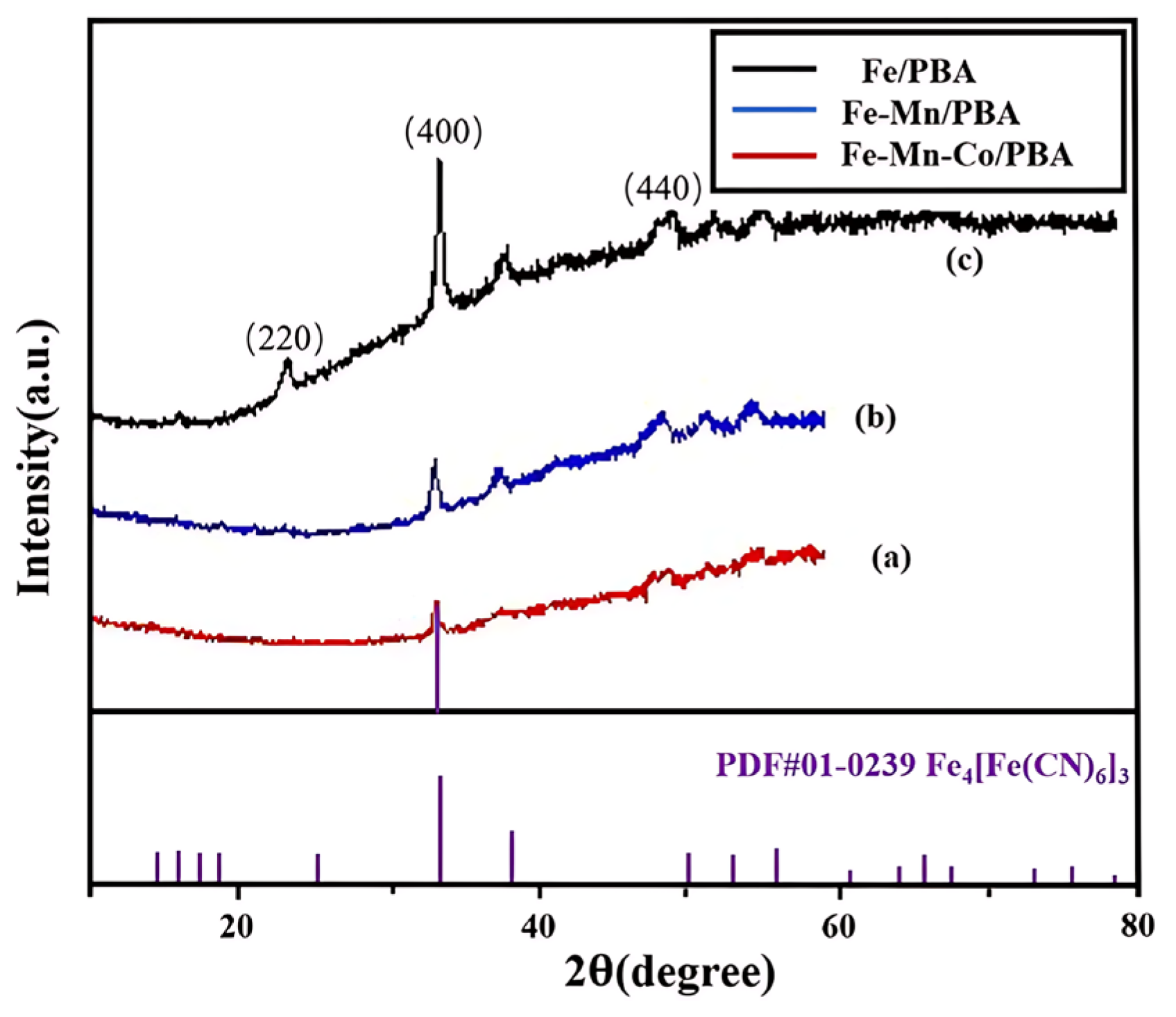
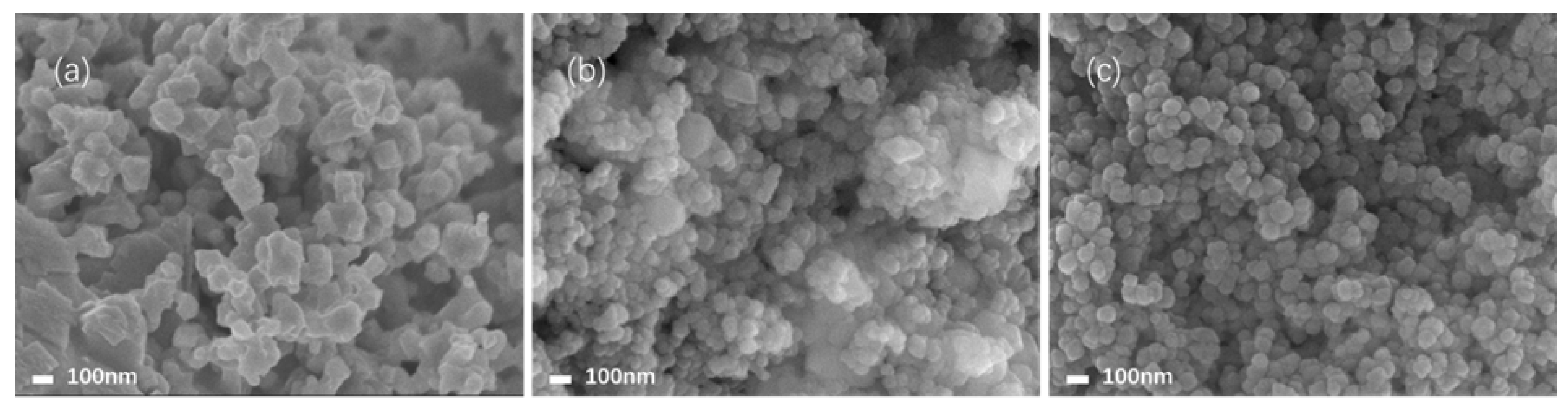
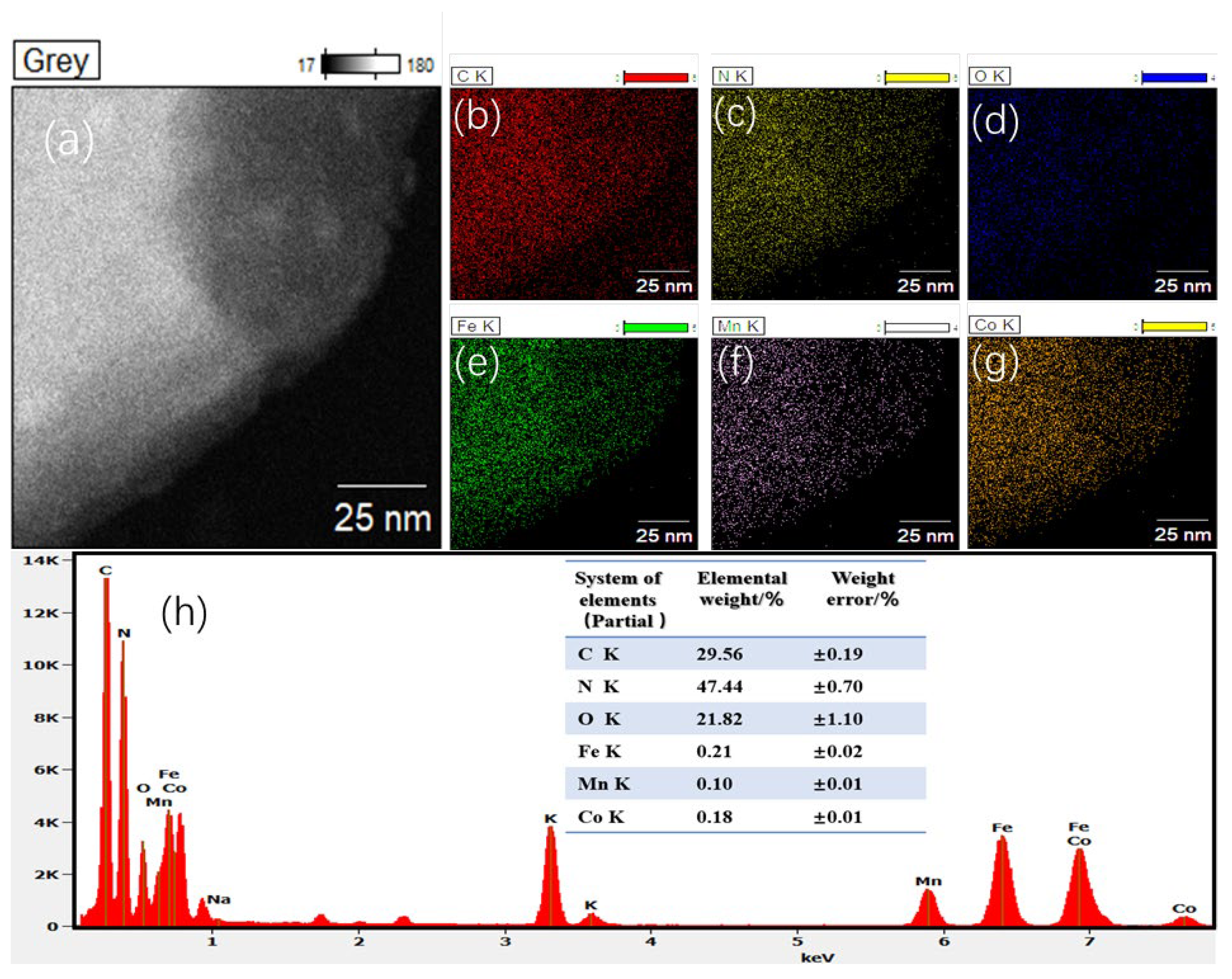
2.1. Characterization of the Electrocatalysts
| Sample Name | Reaction Equation | Outgrowth |
|---|---|---|
| Fe/PBA | 3Fe2+ + 2K4[Fe(CN)6] → Fe3[Fe(CN)6]2↓ + 6K+ + (4 − x) H2O | Fe3[Fe(CN)6]2 |
| Fe-Mn/PBA | Fe3[Fe(CN)6]2 + xMn2+ → (Fe3−xMnx)[Fe(CN)6]2 + x Fe2+ | (Fe, Mn)3[Fe(CN)6]2·nH2O |
| Fe-Mn-Co/PBA | xMn2+ + yCo2+ + zFe2+ + K4[Fe(CN)6] → (MnxCoyFez)[Fe(CN)6]↓ + 4K+ | (Mn, Co, Fe)3II[FeIII(CN)6]2⋅nH2O |
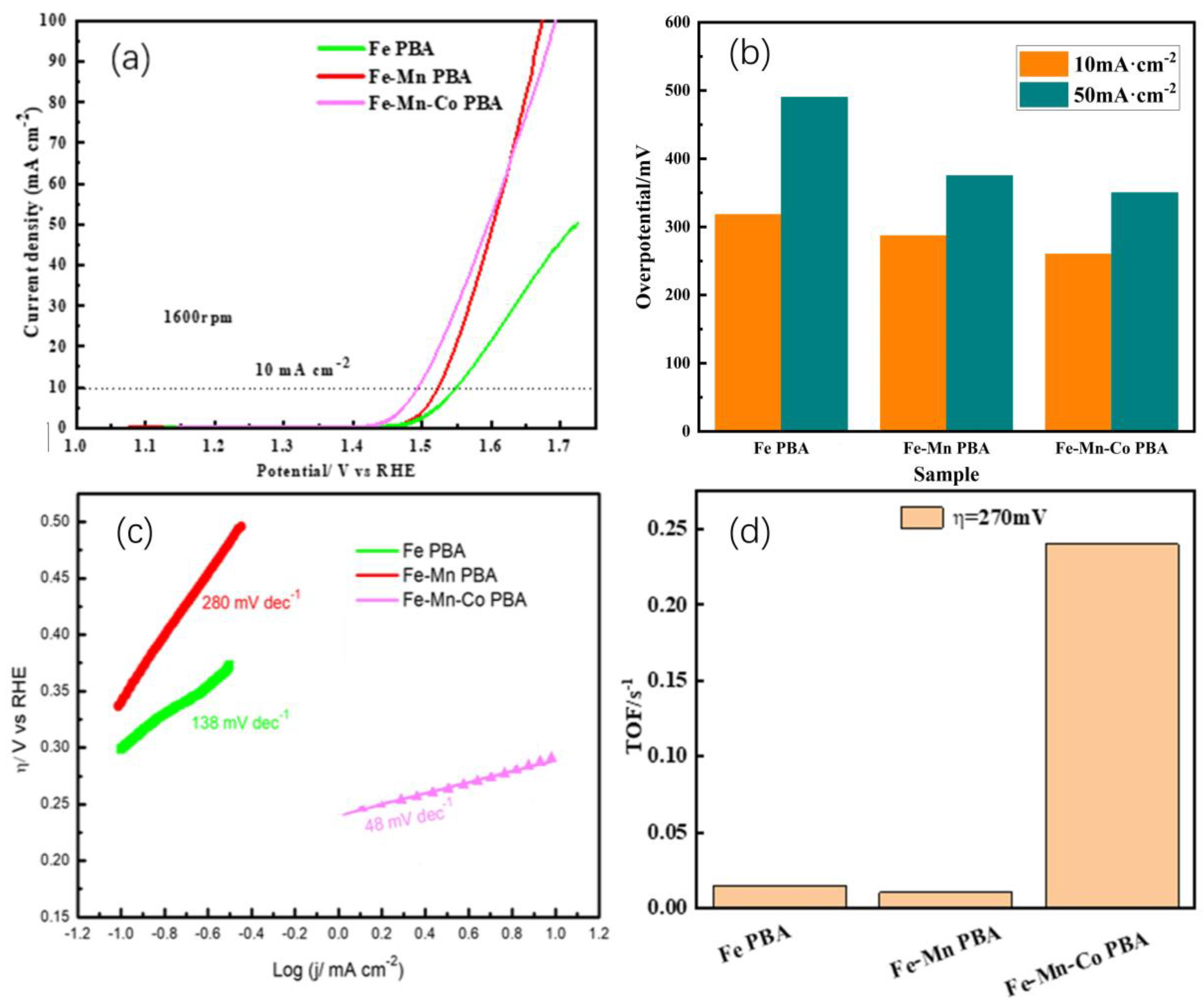
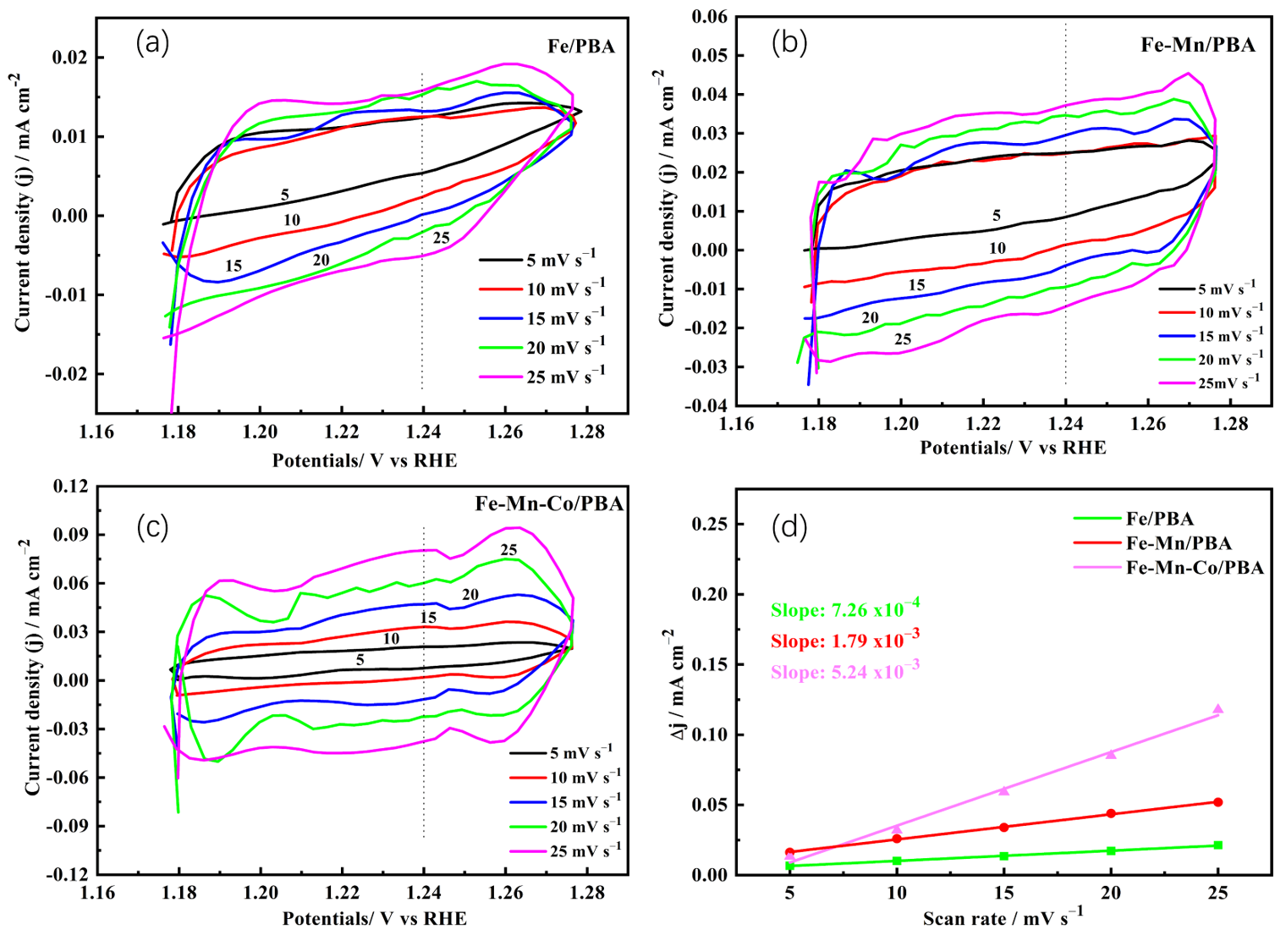
2.2. OER Activity
3. Materials and Methods
3.1. Preparation of Materials
3.2. Preparation of the Working Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, Z.-X.; Liang, G.-H.; Shen, L.-F.; Zhang, G.; Zheng, S.; Tian, J.-H.; Li, J.-F.; Zhang, H. Phase Engineering Facilitates O–O Coupling via Lattice Oxygen Mechanism for Enhanced Oxygen Evolution on Nickel–Iron Phosphide. J. Am. Chem. Soc. 2025, 147, 1334–1343. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Li, X.; Qin, S.; Li, C.; Zhang, Z.; Li, Z.; Meng, X. Engineering active and robust alloy-based electrocatalyst by rapid Joule-heating toward ampere-level hydrogen evolution. Nat. Commun. 2024, 15, 7475. [Google Scholar] [CrossRef]
- Alsaç, E.P.; Ülker, E.; Nune, S.V.K.; Dede, Y.; Karadas, F. Tuning the electronic properties of prussian blue analogues for efficient water oxidation electrocatalysis: Experimental and computational studies. Chem. Eur. J. 2018, 24, 4856. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Popa, I.M.; Negahdar, L.; Palkovits, S.; Kaufmann, B.; Pilaski, M.; Hoster, H.; Palkovits, R. Elucidating the Influence of Intercalated Anions in NiFe LDH on the Electrocatalytic Behavior of OER: A Kinetic Study. ChemElectroChem 2023, 10, e202300235. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zhang, L.-L.; Fu, X.-Y.; Yan, B.; Yang, X.-L. Synergistic Modification of Fe-Based Prussian Blue Cathode Material Based on Structural Regulation and Surface Engineering. ACS Appl. Mater. Interfaces 2022, 14, 43308–43318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, H.; Chen, Y.; Zhao, D.; Gao, X. Efficient charge redistribution enabled by lithiophilic Ag/NiCo-PBA heterojunction for dendrite-free lithium metal anode. Electrochim. Acta 2025, 535, 146688. [Google Scholar] [CrossRef]
- Li, Y.; Yu, W.; Chen, X.; Miao, X.; Chen, S.; Lu, Q.; Si, C.; Han, X. Experimental and DFT investigations of CoFe PBA derived hollow porous electrocatalysts for superior OER and ORR. Int. J. Hydrogen Energy 2025, 101, 627–635. [Google Scholar] [CrossRef]
- Liao, W.; Yang, Q.; Jiang, H.; Liu, P. Construction of P/Ag@AgCo PBA hollow cuboid and its bifunctional activity for water splitting. Renew. Energy 2025, 242, 122383. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Xu, Y.; Wang, G.; Xu, T.; Zhang, W.; Si, C. Heteroatom-doped and graphitization-enhanced lignin-derived hierarchically porous carbon via facile assembly of lignin-Fe coordination for high-voltage symmetric supercapacitors. J. Colloid Interface Sci. 2024, 659, 374–384. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, K.; Li, J.; Wang, L.; Yang, Y.; Li, Z.; Shao, M.; Duan, X. Boosting electrochemical oxygen reduction to hydrogen peroxide coupled with organic oxidation. Nat. Commun. 2024, 15, 6098. [Google Scholar] [CrossRef]
- Ji, Y.-R.; Guo, Y.-F.; Liu, X.; Wang, P.-F.; Yi, T.-F. Insights on rational design and regulation strategies of Prussian blue analogues and their derivatives towards high-performance electrocatalysts. Chem. Eng. J. 2023, 471, 144743. [Google Scholar] [CrossRef]
- Yu, T.; Gao, P.; Wang, Y.; Muhetaer, M.; Du, H. Improving the Electrocatalytic Activity of a Core/Shell NiCo–ZIF@PBA Catalyst by Co–O–Fe Bridge Bonds for Water Oxidation. Inorg. Chem. 2024, 63, 20964–20974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, P.; Li, Y.; Zhai, X.; Lu, K.; Chen, X.; Yang, J.; Wang, Z.; Zhang, H.; Ge, G. Prussian blue analogues derived Fe-NiCoP reveals the cooperation of Fe doping and phosphating for enhancing OER activity. Appl. Surf. Sci. 2023, 615, 156378. [Google Scholar] [CrossRef]
- Xing, S.; Liu, N.; Li, Q.; Liang, M.; Liu, X.; Xie, H.; Yu, F.; Ma, J. Reactive P and S co-doped porous hollow nanotube arrays for high performance chloride ion storage. Nat. Commun. 2024, 15, 4951. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ding, H.; Huang, T.; Liu, X.; Xiao, J.; Xie, M.; Xu, G.; Zhang, L. Fe-Doped Ni/Co-Based Selenide Hierarchical Nanosheet Arrays as Self-Supporting Bifunctional Electrocatalysts for Overall Water Splitting. ACS Appl. Nano Mater. 2024, 7, 11530–11540. [Google Scholar] [CrossRef]
- Zhang, H.; Geng, S.; Ouyang, M.; Mao, M.; Xie, F.; Riley, D.J. Using Metal Cation to Control the Microstructure of Cobalt Oxide in Energy Conversion and Storage Applications. Small 2022, 18, 2106391. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Ye, L.; Fu, H.; Cao, R.; Yang, J. Optimizing Mn in Prussian blue analogs with double redox active sites to induce boosted Zn2+ storage. J. Colloid Interface Sci. 2024, 664, 423–432. [Google Scholar] [CrossRef]
- Rahamathulla, N.; Vadivel, N.; Theerthagiri, J.; Raj, R.S.; Moon, C.J.; Murthy, A.P.; Kheawhom, S.; Choi, M.Y. Underlying aspects of surface amendment strategies adopted in electrocatalysts for overall water splitting under alkaline conditions. Curr. Opin. Electrochem. 2024, 43, 101428. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Dong, L.; Yang, W.; Liu, X.; Long, L.; Liu, C.; Dong, S.; Jia, J. Synergistic effect between atomically dispersed Fe and Co metal sites for enhanced oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 4369–4375. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Wang, F.; Zhang, L.; Li, L.; Chen, H.; Wei, Z. Rational design of porous Ni-Co-Fe ternary metal phosphides nanobricks as bifunctional electrocatalysts for efficient overall water splitting. Appl. Catal. B Environ. 2022, 310, 121353. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Hou, Y.; Liang, H.; Zhou, Z.; Yang, H. Construction of Defect-Rich Ni-Fe-Doped K0.23MnO2 Cubic Nanoflowers via Etching Prussian Blue Analogue for Efficient Overall Water Splitting. Small 2020, 16, 1905223. [Google Scholar] [CrossRef]
- Tran, T.A.; Tran, H.C.; Nghiem, N.T.; Truong-Son, L.V.; Imanova, G.T.; Jabarov, S.H. Effect of doping Mn ion on the crystal structure and cation distribution in Co1-xMnxFe2O4 compounds. J. Mater. Sci. Mater. Eng. 2025, 20, 5. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, X.; Bai, J.; Wang, H.; Wang, G. Prussian blue analogs (PBA) derived porous bimetal (Mn, Fe) selenide with carbon nanotubes as anode materials for sodium and potassium ion batteries. Chem. Eng. J. 2020, 382, 123050. [Google Scholar] [CrossRef]
- Purusottam Reddy, B.; Reddy Prasad, P.; Mallikarjuna, K.; Chandra Sekhar, M.; Lee, Y.-W.; Suh, Y.; Park, S.-H. Mn–Co Prussian blue analogue cubic frames for efficient aqueous Zn ion batteries. Microporous Mesoporous Mater. 2023, 362, 112793. [Google Scholar] [CrossRef]
- Xiao, L.; Qi, L.; Sun, J.; Husile, A.; Zhang, S.; Wang, Z.; Guan, J. Structural regulation of covalent organic frameworks for advanced electrocatalysis. Nano Energy 2024, 120, 109155. [Google Scholar] [CrossRef]
- Fang, W.; Tang, T.; Li, M.; Xin, S.; Wu, Y.; Cao, Y.; Yu, Z.; Cui, W.; Li, Z.; Zhao, H. Advanced design of metal single-atom (Pt, Mn, Fe or Cu) loaded S-Ni(OH)2 nanosheets array catalysts to achieve high-performance overall water splitting. Chem. Eng. J. 2024, 499, 156013. [Google Scholar] [CrossRef]
- Naito, T.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Gas Crossover Regulation by Porosity-Controlled Glass Sheet Achieves Pure Hydrogen Production by Buffered Water Electrolysis at Neutral pH. ChemSusChem 2022, 15, e202102294. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Kang, B.; Lee, J.Y. Understanding the synergistic effects of dual-atom catalysts NiSn on carbon dioxide reduction. Appl. Surf. Sci. 2023, 638, 158109. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Li, J.; Gao, Y.; Sun, J.; Geng, S.; Meng, Y. High-valence Mo, Mn co-doped amorphous bimetallic sulfide for efficient overall alkaline water/seawater electrolysis. Fuel 2024, 371, 132111. [Google Scholar] [CrossRef]
- Li, W.; Kong, L.; Wang, Z.; Han, Z.; Zhang, B.; Shi, M.; Qian, K.; Yang, J.; He, X.; Ma, J. Electronic structure reconstruction of Fe-Mn diatomic pair for disentangling activity-stability tradeoff in Fenton-like reactions. Appl. Catal. B Environ. Energy 2025, 365, 124920. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Jia, S.; Wang, X.; Lyu, K.; Peng, Y.; Zheng, H.; Wei, X.; Ren, H.; Xiao, L.; et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells. Nat. Commun. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Hou, C.; Cui, Z.; Zhang, S.; Yang, W.; Gao, H.; Luo, X. Rapid large-scale synthesis of ultrathin NiFe-layered double hydroxide nanosheets with tunable structures as robust oxygen evolution electrocatalysts. RSC Adv. 2021, 11, 37624–37630. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Sun, S.; Bao, S. Study on the behavior and mechanism of NiFe-LDHs used for the degradation of tetracycline in the photo-Fenton process. RSC Adv. 2023, 13, 31528–31540. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Vernon, L.J.; Martinez, E.; Voter, A.F. The relationship between grain boundary structure, defect mobility and grain boundary sink efficiency. Sci. Rep. 2015, 5, 9095. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, X.; Ye, L.; Wu, Z.; Yang, J.; Shi, M.; Ang, E.H. Optimizing Fe in Mn-based Prussian blue analogs with dual redox-active sites to enhance operating voltage and durability in Zn-ion batteries. Chem. Eng. J. 2025, 506, 160308. [Google Scholar] [CrossRef]
- Samanta, A.; Jana, S. Ni-, Co-, and Mn-Doped Fe2O3 Nano-Parallelepipeds for Oxygen Evolution. ACS Appl. Nano Mater. 2021, 4, 5131–5140. [Google Scholar] [CrossRef]
- Wang, M.; Dong, C.L.; Huang, Y.C.; Shen, S. Operando Spectral and Electrochemical Investigation into the Heterophase Stimulated Active Species Transformation in Transition-Metal Sulfides for Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2020, 10, 1855. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Liu, B.; Xiang, H.; Ren, F.; Xu, T.; Liu, J.; Xu, H.; Ding, H.; Zhu, Y.; Liu, F. Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs. Inorganics 2025, 13, 258. https://doi.org/10.3390/inorganics13080258
Ding Y, Liu B, Xiang H, Ren F, Xu T, Liu J, Xu H, Ding H, Zhu Y, Liu F. Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs. Inorganics. 2025; 13(8):258. https://doi.org/10.3390/inorganics13080258
Chicago/Turabian StyleDing, Yanhong, Bin Liu, Haiyan Xiang, Fangqi Ren, Tianzi Xu, Jiayi Liu, Haifeng Xu, Hanzhou Ding, Yirong Zhu, and Fusheng Liu. 2025. "Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs" Inorganics 13, no. 8: 258. https://doi.org/10.3390/inorganics13080258
APA StyleDing, Y., Liu, B., Xiang, H., Ren, F., Xu, T., Liu, J., Xu, H., Ding, H., Zhu, Y., & Liu, F. (2025). Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs. Inorganics, 13(8), 258. https://doi.org/10.3390/inorganics13080258







