Metallic Lanthanum (III) Hybrid Magnetic Nanocellulose Composites for Enhanced DNA Capture via Rare-Earth Coordination Chemistry
Abstract
1. Introduction
2. Results and Discussion
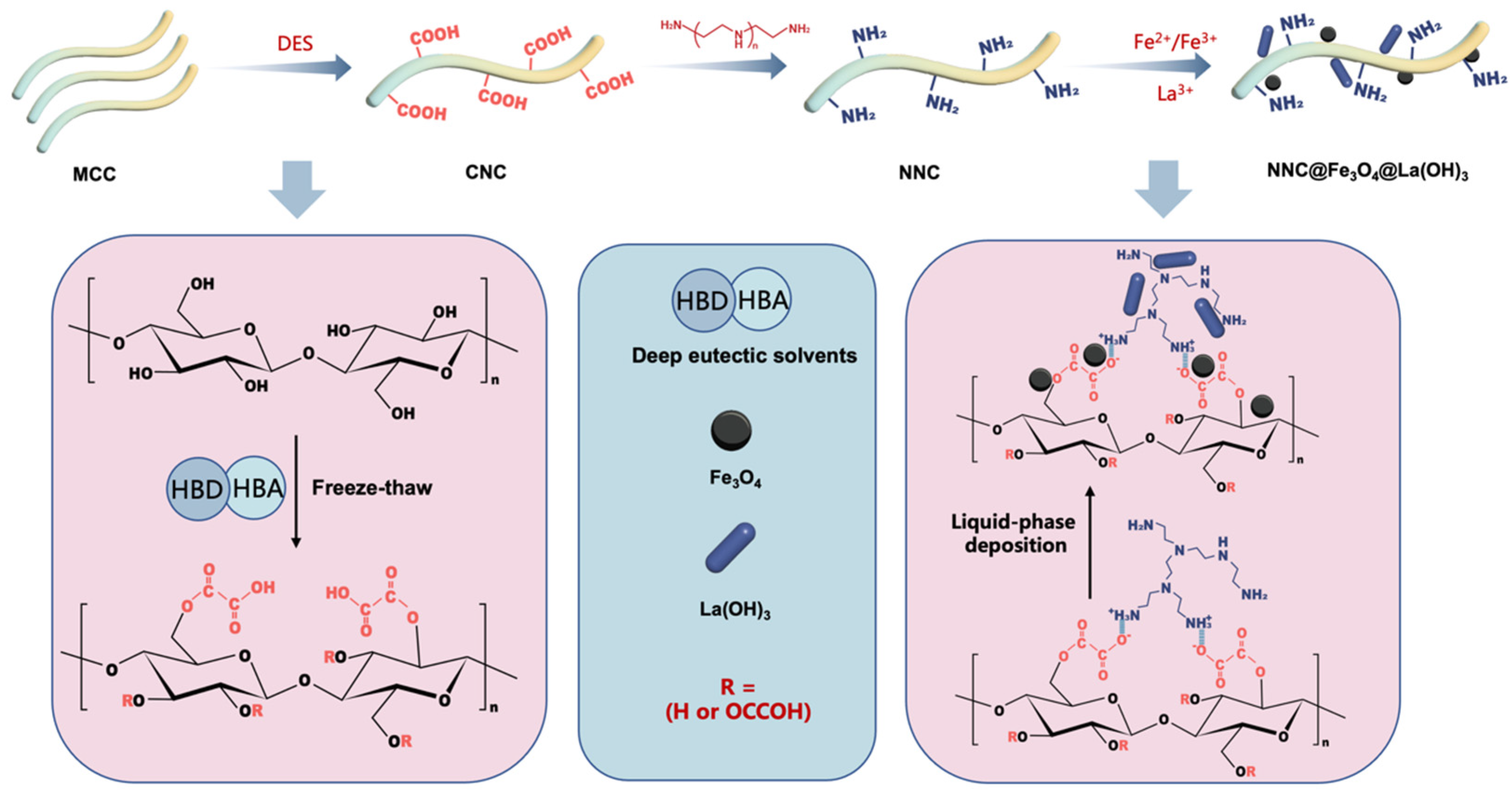
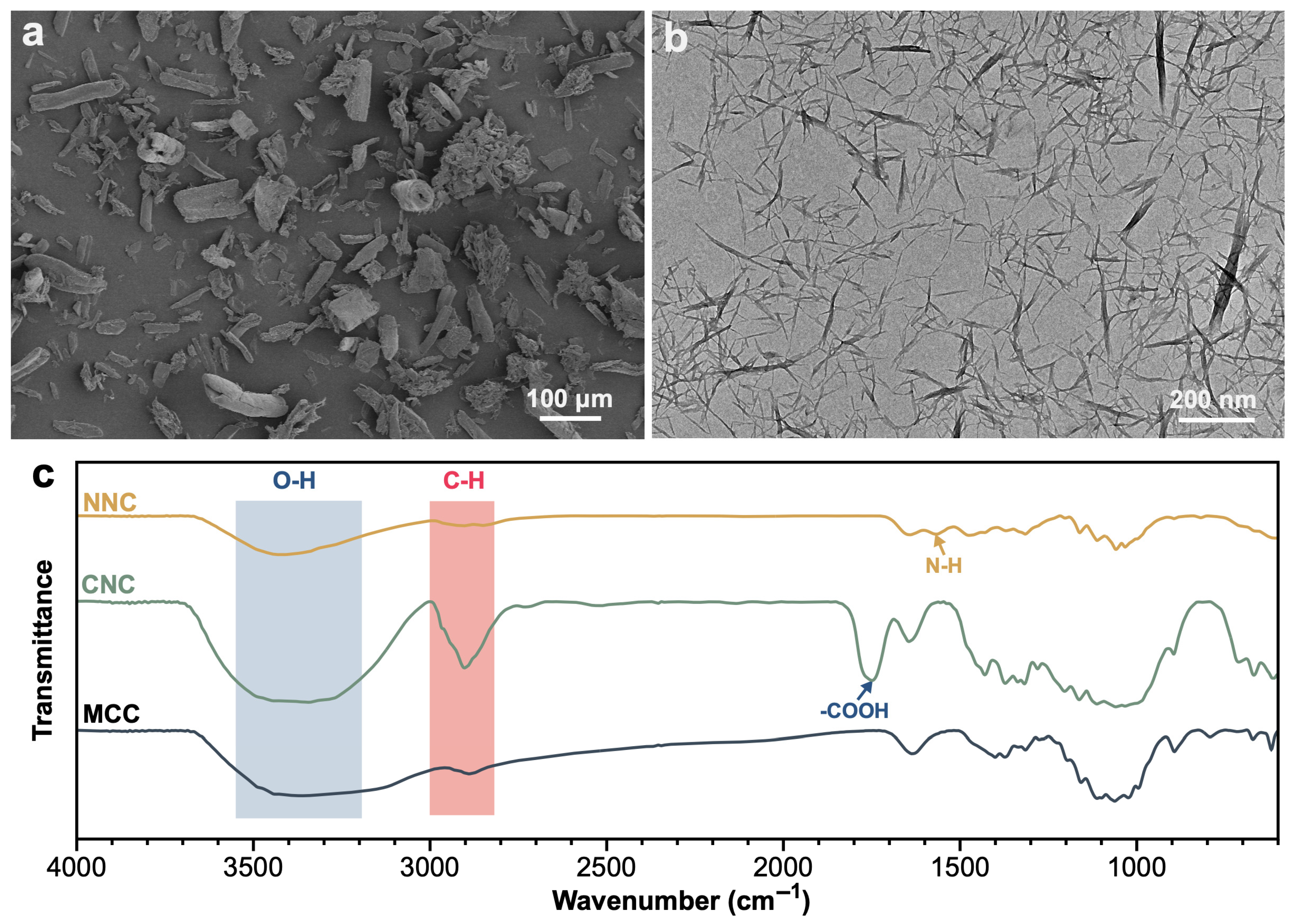
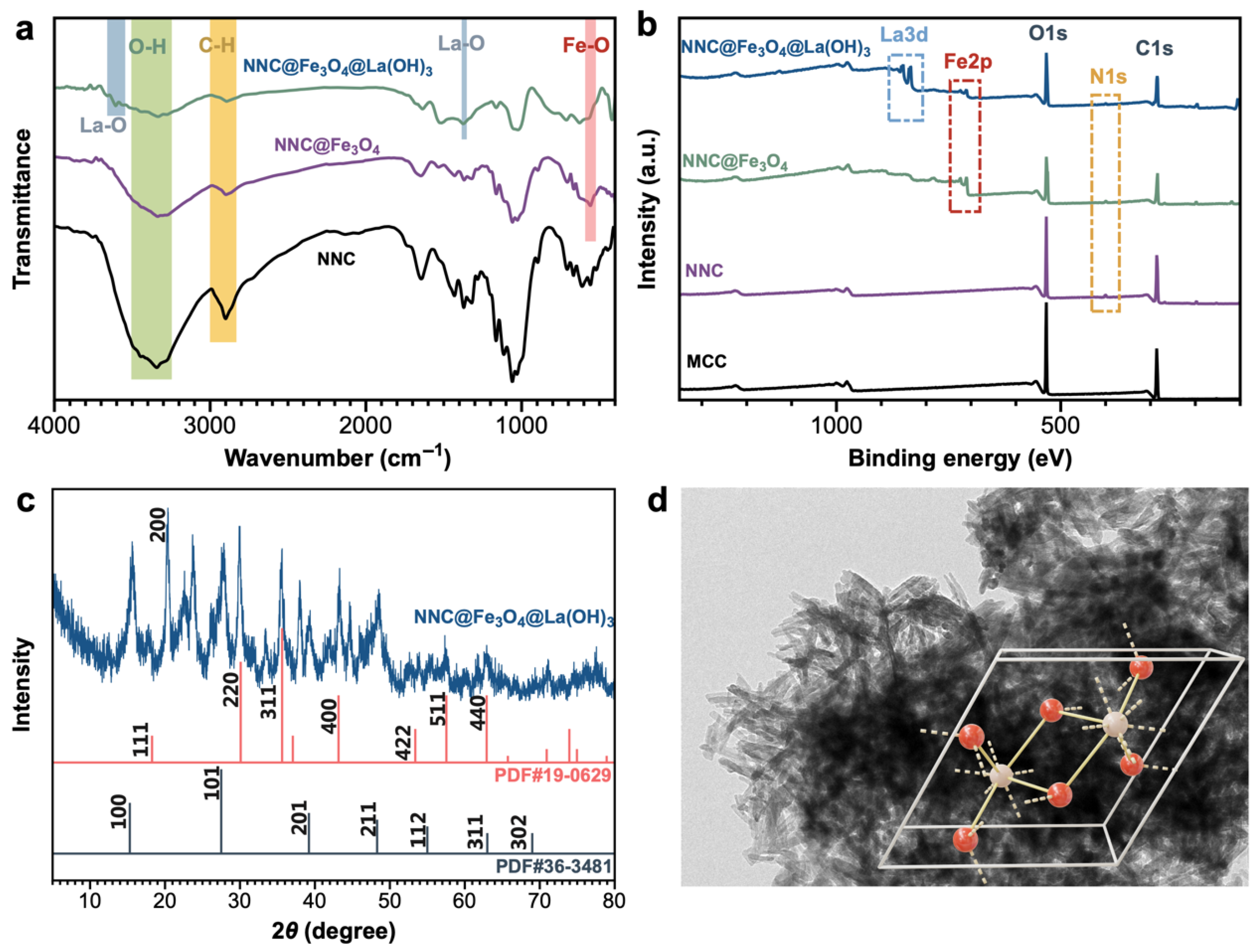
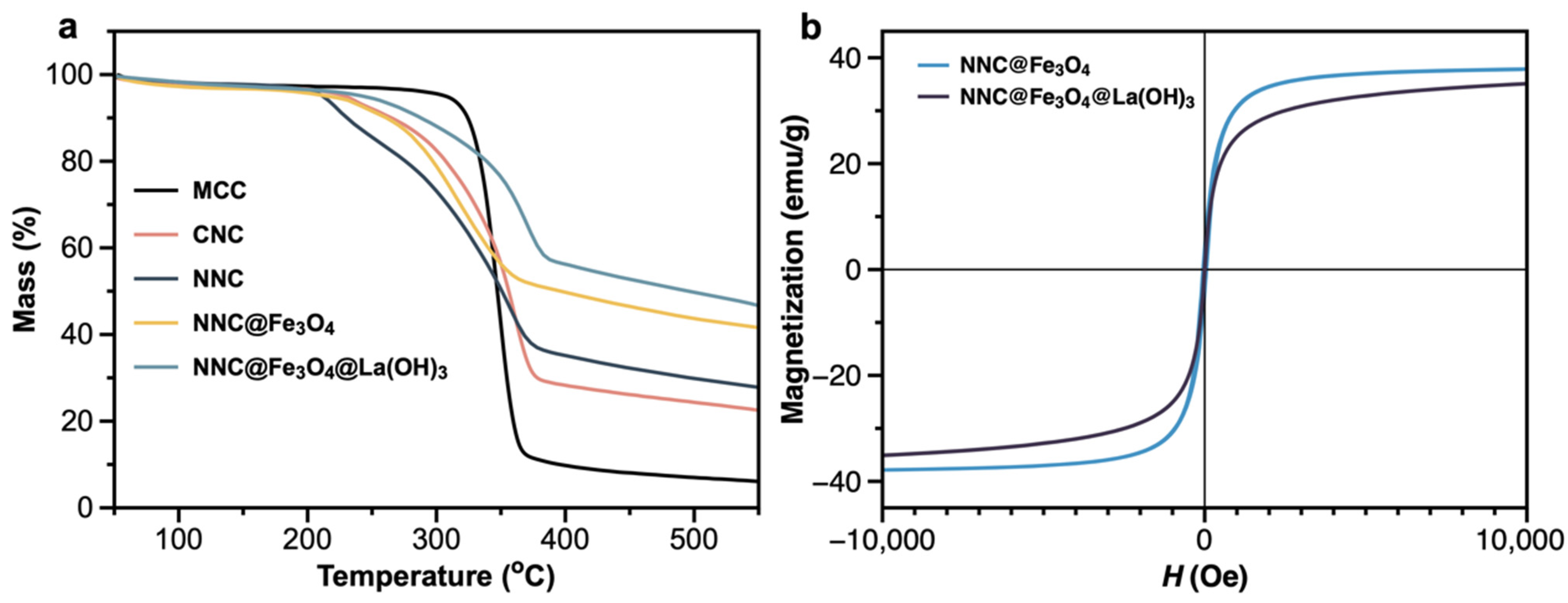
2.1. Structure and Characteristics of Lanthanum Hybrid Magnetic Nanocellulose
2.2. Adsorption Behavior and Kinetics
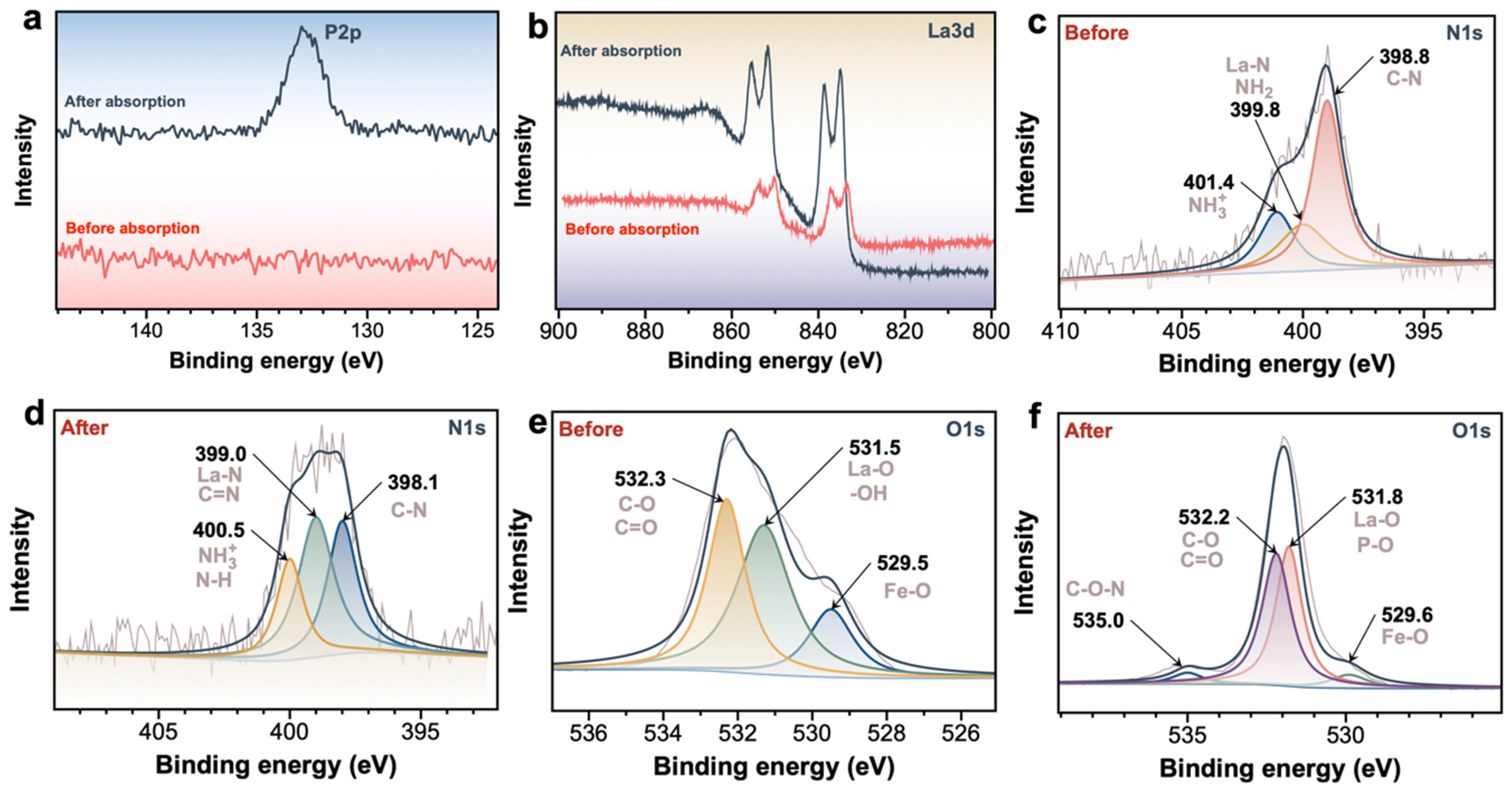
2.3. Mechanism Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Carboxylated Nano-Cellulose (CNC)
3.3. Preparation of Amino-Modified Nanocellulose (NNC)
3.4. Synthesis of NNC@Fe3O4
3.5. Synthesis of NNC@Fe3O4@La(OH)3
3.6. Characterization
3.7. Preparation of DNA Solution
3.8. Adsorption Behavior of NNC@Fe3O4@La(OH)3
3.9. Kinetics of Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youssef, H.; Sedykh, A.E.; Becker, J.; Taydakov, I.V.; Mueller-Buschbaum, K. 3-(2-Pyridyl)pyrazole Based Luminescent 1D-Coordination Polymers and Polymorphic Complexes of Various Lanthanide Chlorides Including Orange-Emitting Cerium(III). Inorganics 2022, 10, 10120254. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Y.; Ren, J.; Qu, X. Recent progress in lanthanide complexes for DNA sensing and targeting specific DNA structures. Inorganica Chim. Acta 2016, 452, 50–61. [Google Scholar] [CrossRef]
- Chan, M.; Doan, H.; Dang-Vu, T. An Investigation of Lanthanum Recovery from an Aqueous Solution by Adsorption (Ion Exchange). Inorganics 2024, 12, 12090255. [Google Scholar] [CrossRef]
- Lin, G.; Xiong, Y.; Wang, G.; Li, S.; Jiang, R.; Lu, B.; Chen, Y.; Huang, B. Selective and efficient removal of lanthanum ion in aqueous solution by P-doped porous carbon. J. Water Process Eng. 2023, 55, 104116. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Alexander, C.; Guo, Z.; Glover, P.B.; Faulkner, S.; Pikramenou, Z. Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics. Chem. Rev. 2025, 125, 2269–2370. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.; Chen, J.K. Lanthanide-Based Optical Probes of Biological Systems. Cell Chem. Biol. 2020, 27, 921–936. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H.J. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef]
- Fremy, G.; Raibaut, L.; Cepeda, C.; Sanson, M.; Boujut, M.; Seneque, O. A novel DOTA-like building block with a picolinate arm for the synthesis of lanthanide complex-peptide conjugates with improved luminescence properties. J. Inorg. Biochem. 2020, 213. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, H.; Zhang, H.; Wang, X.; Fu, Y.; Wang, Q.; Liu, H.; Yong, Y.-c.; Guo, J.; Liu, J. Biosafety consideration of nanocellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2024, 265, 130900. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, T.; Cheng, Y.; Zhao, K.; Meng, Z.; Huang, J.; Cai, W.; Lai, Y. Recent advances in functional cellulose-based materials: Classification, properties, and applications. Adv. Fiber Mater. 2024, 6, 1343–1368. [Google Scholar] [CrossRef]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef]
- Qin, Z.x.; Wang, C.y.; Zhang, J.s.; Wang, Z.y.; Wei, Y.; Li, Y.t.; Dai, S.q.; Tay, F.R.; Niu, L.n. DNA-Based Materials Inspired by Natural Extracellular DNA. Adv. Funct. Mater. 2023, 33, 2211669. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Choi, M.-H.; Ok, K.M.; You, T.-S. Complex Structure, Chemical Bonding, and Electrical Transport Properties of a La-Doped Zintl Phase. Inorganics 2024, 12, 12120333. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Nie, Z.; Yao, S.Z. A label-free and time-resolved luminescence strategy for the detection of proteins based on DNA-Tb3+ luminescence quenched by graphene oxide. Analyst 2015, 140, 6386–6391. [Google Scholar] [CrossRef]
- Ru, X.-M.; Yang, Z.-Y.; Ran, S.-Y. Lanthanide ions induce DNA compaction with ionic specificity. Int. J. Biol. Macromol. 2022, 210, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.D.; Santana, L.R.; Oliveira, R.M.; Valério, A.; Carreno, N.L.V.; Wolke, S.I.; da Silva, R.; Fajardo, A.R.; Dias, A.R.G.; Zavareze, E.D. Cellulose, cellulose nanofibers, and cellulose acetate from Butia fruits (Butia odorata): Chemical, morphological, structural, and thermal properties. Int. J. Biol. Macromol. 2024, 281, 136151. [Google Scholar] [CrossRef]
- Velinova, R.; Kaneva, N.; Ivanov, G.; Kovacheva, D.; Spassova, I.; Todorova, S.; Atanasova, G.; Naydenov, A. Synthesis and Characterization of Pd/La2O3/ZnO Catalyst for Complete Oxidation of Methane, Propane and Butane. Inorganics 2025, 13, 13010017. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Ozturkkan, F.E.; Teymouri, E.; Yuksek, M.; Yildiz, E.A.; Sertcelik, M.; Alemi, A.; Necefoglu, H.; Hokelek, T. Synthesis, structural characterization and determination of the nitro-containing explosives detection potential of supramolecular lanthanum complex and Lanthana nanoparticles. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2023, 290, 116331. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, W.; Yu, Y.; Jiang, S.; Xu, Y.; Zong, E. Magnetic ZrO2/PEI/Fe3O4 functionalized MWCNTs composite with enhanced phosphate removal performance and easy separability. Compos. Part B-Eng. 2022, 237, 109861. [Google Scholar] [CrossRef]
- Zong, E.; Zhang, C.; Wu, S.; Gao, Y.; Yang, J.; Liu, X.; Song, P. Titanium dioxide nanoparticles functionalized chitosan toward bio-based antibacterial adsorbent for enhanced phosphate capture. Int. J. Biol. Macromol. 2023, 241, 124511. [Google Scholar] [CrossRef]
- Yang, R.D.; Xie, C.; Wan, X.; Li, H.R.; Ge, L.Y.; Li, X.F.; Zhao, G.L. In situ growth of ultrathin covalent triazine frameworks on unmodified cellulose II beads for enhanced dye pollutant removal. Chem. Eng. J. 2024, 498, 155017. [Google Scholar] [CrossRef]
- Zong, E.; Wang, X.; Zhang, L.; Yang, J.; Liu, X. A Recyclable Magnetic Aminated Lignin Supported Zr-La Dual-Metal Hydroxide for Rapid Separation and Highly Efficient Sequestration of Phosphate. Molecules 2023, 28, 28072923. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, E.; Arıkan, B.; Yücel, O.; Çakır, O.; Kara, N.T.; İyim, T.B.; Gürdağ, G.; Emik, S. Synthesis and characterization of amino functional poly(acrylamide) coated Fe3O4 nanoparticles and investigation of their potential usage in DNA isolation. Chem. Pap. 2022, 76, 5747–5759. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Zhang, H.; Yang, Q.; Wei, X.; Xu, P.; Zhou, Y. Solid-phase extraction of DNA by using a composite prepared from multiwalled carbon nanotubes, chitosan, Fe3O4 and a poly (ethylene glycol)-based deep eutectic solvent. Microchim. Acta 2017, 184, 4133–4140. [Google Scholar] [CrossRef]
- Pan, X.; Cheng, S.; Su, T.; Zuo, G.; Zhang, C.; Wu, L.; Jiao, Y.; Dong, W. Poly (2-hydroxypropylene imines) functionalized magnetic polydopamine nanoparticles for high-efficiency DNA isolation. Appl. Surf. Sci. 2019, 498, 143888. [Google Scholar] [CrossRef]
- Niu, B.; Zhou, Y.; Wen, T.; Quan, G.; Singh, V.; Pan, X.; Wu, C. Proper functional modification and optimized adsorption conditions improved the DNA loading capacity of mesoporous silica nanoparticles. Colloid. Surface. A 2018, 548, 98–107. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Li, B.; Tian, N.; Yang, M.; Zhang, H.; You, C.; Zhang, J. Adsorption of DNA by using polydopamine modified magnetic nanoparticles based on solid-phase extraction. Anal. Biochem. 2019, 579, 9–17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fei, J.; Wang, H.; Li, Y. Metallic Lanthanum (III) Hybrid Magnetic Nanocellulose Composites for Enhanced DNA Capture via Rare-Earth Coordination Chemistry. Inorganics 2025, 13, 257. https://doi.org/10.3390/inorganics13080257
Yang J, Fei J, Wang H, Li Y. Metallic Lanthanum (III) Hybrid Magnetic Nanocellulose Composites for Enhanced DNA Capture via Rare-Earth Coordination Chemistry. Inorganics. 2025; 13(8):257. https://doi.org/10.3390/inorganics13080257
Chicago/Turabian StyleYang, Jiayao, Jie Fei, Hongpeng Wang, and Ye Li. 2025. "Metallic Lanthanum (III) Hybrid Magnetic Nanocellulose Composites for Enhanced DNA Capture via Rare-Earth Coordination Chemistry" Inorganics 13, no. 8: 257. https://doi.org/10.3390/inorganics13080257
APA StyleYang, J., Fei, J., Wang, H., & Li, Y. (2025). Metallic Lanthanum (III) Hybrid Magnetic Nanocellulose Composites for Enhanced DNA Capture via Rare-Earth Coordination Chemistry. Inorganics, 13(8), 257. https://doi.org/10.3390/inorganics13080257





