Abstract
Growing interest in the future applications of nanotechnology in medicine has led to groundbreaking developments in nanosensors. Nanosensors are excellent platforms that provide reliable solutions for continuous monitoring and real-time detection of clinical targets. Nanosensors have attracted great attention due to their remarkable sensitivity, portability, selectivity, and automated data acquisition. The exceptional nanoscale properties of nanomaterials used in the nanosensors boost their sensing potential even at minimal concentrations of analytes present in a clinical sample. Along with applications in diverse sectors, the beneficial aspects of nanosensors have been exploited in healthcare systems to utilize their applications in diagnosing, treating, and preventing diseases. Hence, in this review, we have presented an overview of the disease-prognostic applications of nanosensors in chronic diseases through a detailed literature analysis. We focused on the advances in various nanosensors in the field of major diseases such as cancer, cardiovascular diseases, diabetes mellitus, and neurodegenerative diseases along with other prevalent diseases. This review demonstrates various categories of nanosensors with different nanoparticle compositions and detection methods suitable for specific diagnostic applications in clinical settings. The chemical properties of different nanoparticles provide unique characteristics to each nanosensors for their specific applications. This will aid the detection of potential biomarkers or pathological conditions that correlate with the early detection of various diseases. The potential challenges and possible recommendations of the applications of nanosensors for disease diagnosis are also discussed. The consolidated information present in the review will help to better understand the disease-prognostic potentials of nanosensors, which can be utilized to explore new avenues in improved therapeutic interventions and treatment modalities.
1. Introduction
Research in nanotechnology and nanoscale materials is novel and fascinating. Because of their intrinsically small size and unique optical, magnetic, catalytic, and mechanical characteristics that are not present in bulk materials, nanoparticles can be used to create new technologies and applications. The revolutionary advancements in nanotechnology have led to the emergence of new-generation nanostructures with potentially diverse applications. The inclusion of nanotechnology and its allied systems in healthcare has significantly contributed to the provision of high-quality, innovative technologies [1]. In recent years, the unique properties of these nanomaterials have made a rapid evolution in the emergence of highly sensitive and selective nanosensors and led to various groundbreaking developments across various domains. Nanosensors are nanoscale devices that function to detect and transform various signals to convey information to the macroscopic world [2]. Nanostructured materials, including nanoparticles, carbon nanotubes (CNT) (extremely high surface area), thin films, polymer nanomaterials, and nanoscale wires (high detection sensitivity) are utilized to make nanosensors [3]. Over the past few decades, nanosensors have gained significant attention and experienced tremendous growth in the global market due to their greater sensitivity and specificity, facilitating precise detection methods. The large surface area to volume ratio of these nanodevices is the unique characteristic suitable for sensing applications. Nanosensors can be classified according to their energy source, structure, and applications. Based on their applications, four different kinds of sensors have been identified: chemical sensors, deployable nanosensors, electrometers, and biosensors. Nanosensors have been employed for application in diverse sectors such as healthcare, environment, industrial, security, and defense [3]. In the healthcare systems, by exploiting the beneficial aspects of nanosensors, efforts have been made to harness their applications in the diagnosis, treatment, and prevention of diseases.
Disease prognosis plays a key role in managing the clinical care of patients and decision-making of therapeutic interventions [4]. The assessment of prognostic factors, which correlate with the stages of disease progression, delineates the health characteristics and clinical manifestations among different populations. Of note, the timely detection of early-stage pathological changes offers immense potential in enhancing prognostic outcomes. Although conventional prognostic techniques offer valuable insights, their limitations necessitate the emergence of more robust approaches to the precision and applicability of prognostic predictions. The major limitations of conventional prognostic methods include lack of specificity, validation challenges, time-consuming nature, limited predictive power for specific therapies, and inconsistency in data quality. They often rely on population-level data generalization and may not be able to distinguish different subtypes and stages of disease progression, leading to limited personalized strategies. In addition, they face challenges in automation and integration with other diagnostic tools. In the field of medical science, various robust tools for deciphering prognostic markers are emerging which encourage prognosis prediction, recurrence detection, screening, early diagnosis, and therapy efficacy monitoring of various diseases [5]. Integrating prognostic biomarkers with emerging high-throughput techniques can have enormous impacts in the medical and clinical fields. Nanotechnology has also opened several avenues that utilize numerous materials at the nanoscale level to develop prognostic tools with superior sensitivity, selectivity, and lower cost [6]. The rapid and high sensitivity responses of nanosensors even with very small quantity of sample offers enhanced detection capacity. Of note, using the multiplexing, nanosensors provide the simultaneous detection of multiple biomarkers in a compact format. Personalized healthcare based on unique biomarker profiles of individual patients can also be achieved by the application of nanosensors in disease prognosis. Hence, the present review aims to consolidate the applications of nanosensors in the prognosis of various diseases. The review highlights the potential use of nanosensors as early warning systems in disease prognosis and will inspire future research to explore new avenues in improved therapeutic interventions and treatment modalities.
2. Types of Nanosensors
Nanosensors can be classified into various types based on the type and mechanism of signal detection. Chemical nanosensors can be classified into gas, ionic, and pH. While gas nanosensors detect gases and volatile organic compounds, ionic nanosensors detect specific ions in a solution. Metal oxide nanostructures and CNT are often used in chemical nanosensors. Ionic nanosensors mainly use ion-sensitive electrodes with nanoscale modifications for detection. The basicity or acidity of the analyte at the nanoscale level has been used in pH nanosensors. Biological nanosensors can be sub-categorized into DNA/RNA nanosensors, protein/enzyme nanosensors, and cellular nanosensors. As their name indicates, they detect specific biomolecules and cellular conditions. Physical nanosensors can have sub-divisions such as temperature nanosensors that detect temperature fluctuations using quantum dots and nanowires, piezoelectric nanosensors that convert mechanical force into electrical signals as a detection strategy, optical nanosensors that rely on light-based signals like fluorescence, absorbance, surface plasmon resonance, Raman scattering mainly using quantum dots or plasmonic nanoparticles, and magnetic nanosensors that detects the changes in magnetic field using magnetic nanoparticles. Electrical nanosensors use the fluctuations in the current intensity, voltage, or impedance in the presence of a specific analyte as a detection strategy. Nanotubes, graphene, CNT, and metal nanoparticles have been commonly used in these sensors to enhance the sensitivity. These diverse categories of nanosensors have been implicated in various fields like medicine, environmental, and industrial sectors. Of note this diversified class of detection strategies along with remarkable sensitivity and specificity makes them suitable for various medical applications.
3. Nanosensors in Medicine
Advances in the era of nanotechnology have assimilated different sensing approaches for the fabrication of versatile and sensitive nanosensors that can specifically detect various disease-related entities. Typically, a nanosensor consists of an analyte, sensor, transducer, and detector [7]. These nanosensors contain a biosensitive layer containing a biological recognition element linked to a transducer [8]. These biological recognition elements can be functional proteins, antibodies, enzymes, cells, or organisms. In particular, the sensor is the biological recognition element that can target ligands bound to nanoparticles. Depending on the functionality of the ligand, it can bind to a particular marker of interest (analyte) to produce physicochemical changes that can be transformed into a measurable effect such as electrical signals [7]. The basic structure of a nanosensor is depicted in Figure 1. Nanosensors can be categorized based on the constituent nanomaterial, detection targets, and the detection method [7]. Nanosensors based on nanoparticles and nanoclusters mainly include metal nanoparticles and semiconductor quantum dots, which work based on their optical and fluorescence properties. Another category of nanosensors is based on CNTs, nanofibers, and nanowires that work on their electrical properties [9,10]. Functionalized graphene is another important nanosensor with potential applications in chemical and biological sensors [11]. Furthermore, nanosensors have been created based on bulk nanostructured materials to enhance efficiency. The assembly of modified metal nanoparticles with pre-designed receptor units forming hybrid nanostructures was shown to exhibit improved performance of the nanosensors [12,13]. Numerous developments in the field of nanomaterial fabrication, manipulation, and characterization have significantly advanced the field of nanosensors owing to the emergence of highly advanced nanosensors with unique properties of high sensitivity, selectivity, and portability. Nanosensors can be used to detect chemical or mechanical information such as the presence of chemical species or monitor physical parameters such as temperature, and pH on the nanoscale.
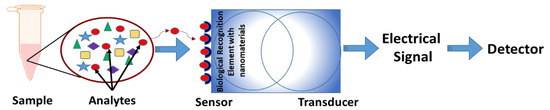
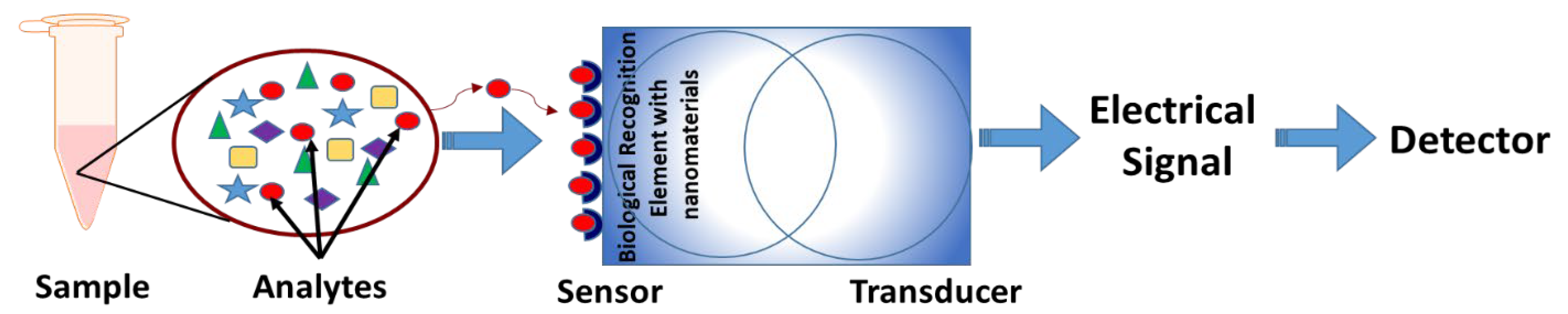
Figure 1.
Basic structure of a nanosensor. The analytes from the sample are identified through specific sensors that contain specialized nanoparticles as biological recognition elements. The transducer converts these physicochemical changes to an electrical signal that can be analyzed through detectors.
Advances in nanosensors have revolutionized the medical field with their remarkable applications in early diagnosis, monitoring the progression, and thereby personalized treatment of human diseases. The ability to target and detect disease-related molecules such as metabolites, proteins, nucleic acids, pathogens, and cells make them versatile tools for understanding and reporting various biological processes at the cellular level. The early-stage diagnosis of diseases through biomarker detection is one of the most significant applications of nanosensors in medicine [14,15]. Nanosensors have been developed for the early detection of various diseases including cancers [16], infectious disease progression [17], diabetes [18], cardiovascular diseases (CVD) [19], neurodegenerative disorders [20], and autoimmune diseases [21]. Furthermore, monitoring various cellular activities by nanosensors has shown remarkable applications in grading disease stages and associated therapeutic interventions [22]. In vivo and in vitro nanosensors working on biocatalytic dependent function of protease activity is one of such types as protease activity can be an indicator of various disease stages [22,23,24]. Gold nanoparticle (AuNP) and iron oxide nanoparticle (IONP)-based nanosensors are popular among them [24,25,26,27]. The sensor activation of another type of nanosensors is by the changes in the physiological environment such as pH, reactive oxygen species (ROS), etc., because abnormal pH and ROS are associated with various disease pathology. pH sensing using Mn(II)-containing layered double hydroxide nanoparticles and oxazine-conjugated nanoparticles that detect ROS are examples of them [28,29]. The nanosensors that detect the changes in casein kinase and alkaline phosphatase associated with different diseases include functionalized glutathione-modified CdSe/ZnS quantum dots with peptides [30,31]. In addition, CdTeS quantum dots linked to an organic dye have also been used for tumor detection based on fluorescence resonance energy transfer (FRET) [32]. The nanosensors with synthetic biomarkers conjugated to metal nanoparticles have been employed to identify the features of human disease and physiology for noninvasive urinary analysis [33]. Similarly, there are more nanosensors with diverse applications in medicine. As the success of clinical treatment is highly dependent on early detection, the current review focuses on the application of nanosensors in the prognosis of some chronic diseases. Figure 2 illustrates the various applications of nanosensors in medicine.
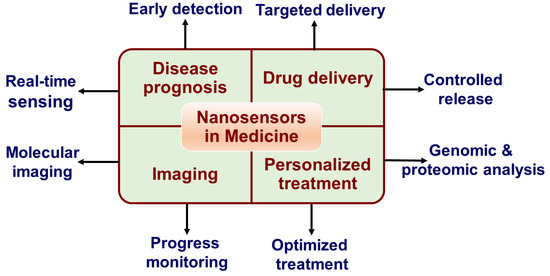
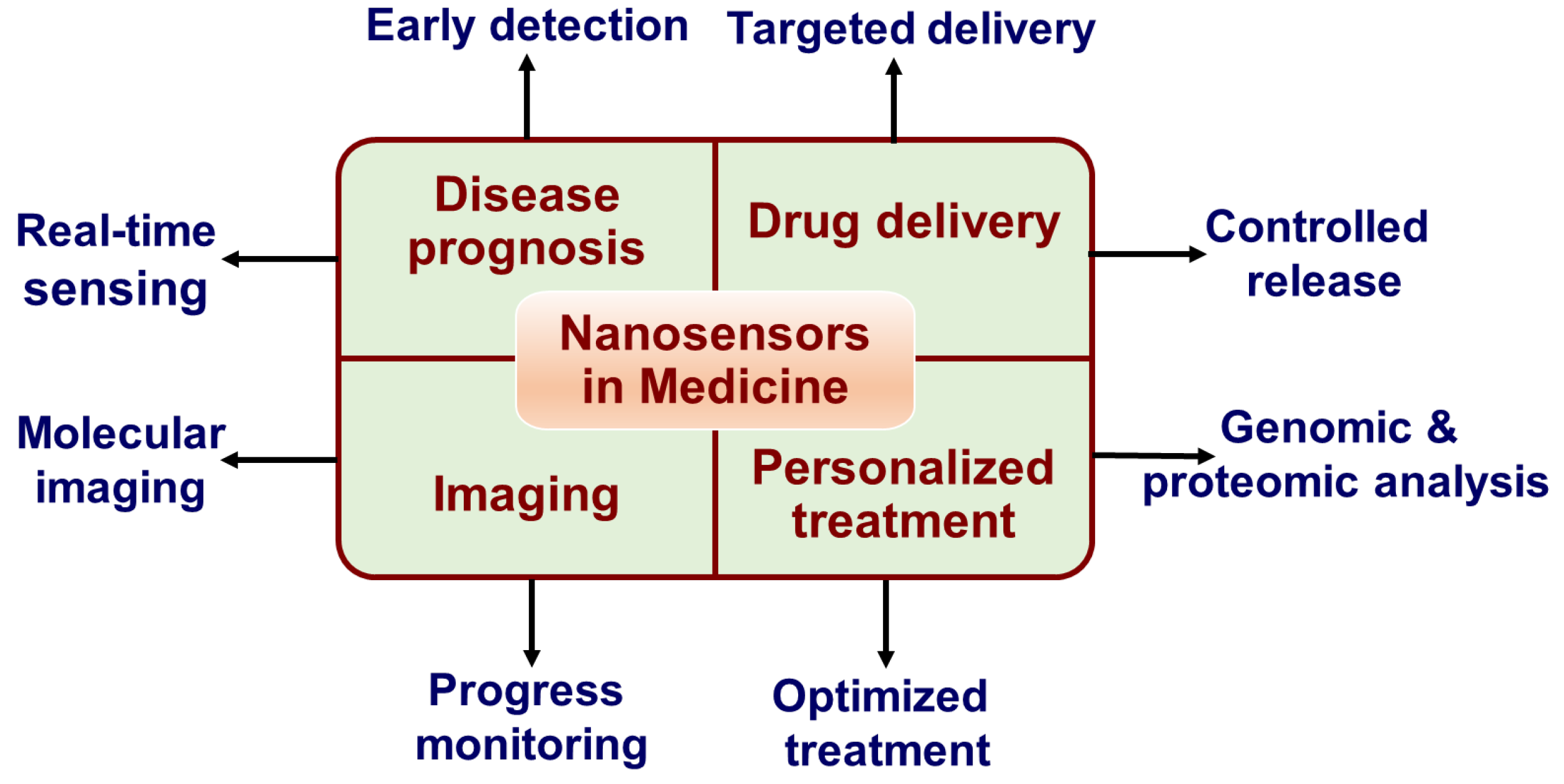
Figure 2.
Applications of nanosensors in medicine.
4. Disease Prognostic Applications of Nanosensors
Knowledge about the prognosis of chronic diseases is important for the systematic monitoring of disease progression and effective planning of specialized treatment. Early detection of disease pathology will support better quality of care and help to predict the outcomes of disease, which include recovery time, recurrence, etc. Nanosensors have been widely used in the early detection of various diseases that provide information necessary to select appropriate treatment modalities.
4.1. Disease Prognostic Applications of Nanosensors in Cancer
As cancer is a leading cause of death worldwide, the early detection of cancer that avoids delays in treatment can significantly improve the survival rate. Evidence-based strategies for early diagnosis are implemented for the prevention and management of cancer patients. In the last decade, various nanosensors have been developed for the early detection of various cancer types. Several biomarkers and detection targets have been utilized to develop more advanced nanosensors for cancer diagnosis and treatment.
A protease-based nanosensor has been shown to exhibit accurate detection of localized lung cancer with 100% specificity and 81% sensitivity [34]. These nanosensors are constructed based on exogenously administered mass-encoded peptides conjugated to nanoparticles that shed peptide fragments through urine in response to the protease activity of the tumor microenvironment. These activity-based nanosensors detects the dysregulated protease activity, which is the hallmark of various stages of cancer pathology. It helps to overcome the insensitivity of conventional biomarker assays and provide a highly specific urine-based readout. Another electrolyte-gated graphene field effect transistor nanosensor detected the lung cancer biomarker interleukin-6 with enhanced stability to diagnose cancer at its early stage [35]. The conformational change in the nanosensor aptamer after bonding to interleukin-6 alters the carrier concentration of graphene that can be detected with high consistency. Also, a nanosensor with an array of magnetic nanoparticles showed high-efficiency detection of tumor cells in lung cancer [36]. The miniature microfluidic chip with a dense array of magnetic pores can capture tumor cells from whole blood of lung cancer patients, which can be further characterized for mutational analysis. Another near-infrared-excited nanosensor with a metal–organic framework has been used to detect oxygen concentration inside tumors and it could successfully track the lung cancer lesions illustrating its exciting potential for cancer prognosis [37]. The donor and acceptor pairs present in the biological metal–organic framework precisely detects the oxygen concentration within the tumor cells using FRET with high sensitivity reversible hypoxic response. Furthermore, AuNP-based nanosensors have been used to diagnose and classify lung cancer [38,39]. These nanosensors in combination with techniques such as solid-phase microextraction, gas chromatography/mass spectrometry can identify a wide range of volatile organic compounds, which acts as lung cancer biomarkers, offering an inexpensive and non-invasive diagnostic tool for the early detection of lung cancer. A silicon-based lung metastasis initiating stem cells-functioned nanosensor has shown 100% diagnostic sensitivity in predicting metastatic lung cancers [40]. This study demonstrated that the nanosensor could detect the signatures of metastatic lung cancer cells accurately using not more than 5 μL of blood. In breast cancer diagnosis, metabolic profiling of cancer cell types has been characterized using mitochondrial pH nanosensors [41]. This study used fluorescence lifetime imaging microscopy to measure the intramitochondrial pH, which explains the relationship between mitochondrial pH, tumoral metabolism, and cancer. An AuNP-based fluorescent nanoprobe sensor has been used to visualize early breast cancer cell proliferation and invasion [42]. This nanosensor could detect markers of proliferation and invasion of breast cancer cells such as Ki-67 and urokinase plasminogen activator. Another study utilized a fluorescent gold–selenium nanoprobe-based sensor demonstrated a biomarker-mediated evaluation of the invasive potential of breast cancer cells [43]. This fluorescent nanoprobe could visualize the changes in urokinase-type plasminogen activator and matrix metalloproteinase-2, which are overexpressed in malignant tumors. Hence, it offers an in situ imaging of these potential biomarkers and real-time monitoring of their changes in living cells for prognostic applications in clinical settings. A fluorescence nanoprobe linked-AuNP-based nanosensor has been shown to monitor the changes in the oxygen and nutrient conditions on the migration and invasion of breast cancer cells [44]. Fluorescence imaging assays were used to assess the cell migration and invasion, along with the detection of changes in levels of cancer markers such as RAB-22a and MMP-2. Optical nanosensors have been used in the rapid differentiation of estrogen receptor (ER) status in breast cancer patients to improve the speed of subtyping and distribution of rapid detection methods [45]. This near-infrared optical nanosensor was designed using a single-walled CNT which acts as the transducer and an anti-ERα antibody which acts as the recognition element. Through a shift in the center wavelength, the nanosensor could differentiate the patient biopsies of ER− and ER+ upon sample addition offering the potential of further molecular marker profiling in breast cancer patients. The research on the integration of demethylation-activated DNAzyme with a single quantum dot nanosensor has shown great potential in the clinical diagnostics of malignant tumors in breast tissues [46]. The device could sensitively detect the dysregulations in O6-Methylguanine-DNA methyltransferase, which can be implicated in malignant tumors in comparison with healthy persons.
By using the NaIO4 oxidation method, an anti-epithelial cell adhesion (EpCAM) molecule monoclonal antibody conjugated silica-coated fluorescent nanoparticles-based nanosensors demonstrated a sensitive and selective detection of colon cancer cells [47]. The nanosensor could distinguish different types of colon cancer cell types, such as colo205, sw480, and NCM460 through fluorescence microscopy imaging and flow cytometer with the distribution and abundance of EpCAM in these cells. Also, the surface-enhanced Raman scattering (SERS) technique has been used to detect colon cancer biomarkers in nanosensors with magnetized Fe3O4/Au/Ag nanoparticles [48]. Using this technique, cytidine, an early prognostic marker of colon cancer, can be quantified even at a small concentration of 1 nM. A study shows that liver cancer cells can be detected using a nanosensor with anti-CD155 and anti-CD112 monoclonal antibodies conjugated to fluorescent mesoporous silica nanoparticles [49]. The FRET between Rhodamine 6G and fluorescein are exploited as a detection strategy in this nanosensor, which showed high sensitivity and specificity for detecting liver cancer SMMC-7721 and HHCC cells. Another study has demonstrated the efficacy of a CNT-based optical nanosensor implant for the noninvasive detection of ovarian cancer [50]. The nanosensor was able to measure the key biomarker of ovarian cancer, human epididymis protein 4, through the modulation of the nanotube optical bandgap even in the early stages of cancer progression. Also, prostate cancer can be detected using reduced graphene oxide-gold nanostructure-based electrochemical nanosensors [51]. The nanosensor construct contains an electrode with reduced graphene oxide/AuNPs (GO/AuNPs), anti-total prostate-specific antigen monoclonal antibody, and anti-free PSA antibody in which cyclic voltammetry and electrochemical impedance spectroscopy have been used to detect cancer antigen with high sensitivity. Researchers have developed a silver nanoparticle (AgNP) functionalized carbon nanofibers-based nanosensors to detect salivary nitrite for the prodiagnosis of oral cancer [52]. The fabrication of the nanosensor was performed using co-axial electrospinning in which thermal treatment and chemical reduction methods were used for the functionalization. SERS has been used to detect the salivary nitrite within the clinically relevant range. Various nanosensors have also been employed for the identification of thyroid tumor biomarkers [53]. Sucrose-powered liposome nanosensors have been utilized for the urinary glucometer-based detection of cancer cells [54]. The extraintestinal metabolic inertness and efficiency renal clearance of sucrose has been exploited to design the nanosensor. Here, sucrose has been used to convert tumor-specific esterase activity into glucose meter readout for timely detection of cancer using urine samples in early stages. Also, mobile nanosensors have been developed to detect the changes in the biomarker levels in the blood [55]. These injectable mobile nanosensors are activated in the presence of cancer biomarkers at the tumor site, based on the concentration of biomarkers followed by the validation of the obtained results by particle-based simulations for cancer prognosis. Another study shows that chitosan-derived colloidal nitrogen-doped graphene quantum dots can be used in nanosensors to detect cancer biomarkers [56]. This study has utilized an atmospheric pressure microplasma system to convert a natural bioresource chitosan into nitrogen-doped graphene quantum dots, which will help in the photoluminescence-based detection of multiple cancer specific markers simultaneously with high selectivity and sensitivity. A biocompatible polystyrene nanoparticles-loaded nanosensor functionalized with a pH-responsive fluorescein dye can be used to correlate the altered extracellular pH in cancer conditions [57]. This will advance the understanding of tumor cellular microenvironments to differentiate cancer and normal cells early. An ultrasensitive super lattice nanosensor, self-functionalized for SERS has been shown to have 100% sensitivity and 97% specificity in the detection of glioblastoma, the most aggressive and lethal brain cancer [58]. Here, they have utilized a biomarker-free approach in which the signals from extracellular vesicles of glioblastoma cancer stem cells have been used for the prognosis of cancer from patient serum. Cancer research advances aiming for rapid and accurate cancer diagnosis have developed the combination of 3D-printed nanosensors with several detection modalities, such as SERS, electrochemical detection, and fluorescence [59]. The findings demonstrate enhanced cancer biomarker sensitivity and specificity, allowing for the early identification of circulating tumor cells. Hence, applying nanosensors in cancer for identifying tumor-specific biomarkers, circulating tumor cells, or changes in the tumor microenvironment may aid in the early detection and advanced treatment modalities to improve the long-term survival of cancer patients. The disease prognostic applications of nanosensors in various cancers have been summarized in Table 1.

Table 1.
Disease prognostic applications of nanosensors in various cancers.
4.2. Disease Prognostic Applications of Nanosensors in Cardiovascular Diseases
As a major cause of death universally and a major contributor to healthcare costs, significant advancements have been made in the timely detection of CVD. Nanosensors that integrate the benefits of sensing platforms with nanomaterials have shown promising potential in the rapid diagnosis of CVD. Various electrochemical, optical, pressure, and paper-based nanosensors have been utilized for the early detection of CVD. In the following section, we aim to discuss the possible applications of nanosensors in CVD.
A magnetic resonance nanosensor functionalized with IONP and a fibrin-binding peptide that specifically targets thrombus has been developed to detect the early-stage progression of CVD [60]. This MRI nanosensor is labeled with fluorescent dye to enable optical imaging, which can generate specific signals based on the presence or absence or age of thrombin at the thrombus site. A nanosensor comprising Simian virus 40 (SV40)-based nanoparticles containing quantum dots has been demonstrated to have potential applications in the imaging of the various stages of atherosclerosis along with its effective drug delivery potential [61]. This study has used near-infrared quantum dots encapsulated in the SV40 virus-like particles to produce optical properties for imaging, which could detect vascular cell adhesion molecule-1, macrophages, and fibrin to differentiate early, developmental, and late stages of atherosclerosis. This targeted nanosensor can also be used to deliver anticoagulant drug to rapture atherosclerosis plaques. A porous hybrid hydrogel composed of polyethylene glycol diacrylate and gelatin-encapsulated nanosensors with photonic barcodes has been utilized for the simultaneous multiplex detection of CVD biomarkers [62]. This nanosensor was designer for the detection of cardiac troponin I, B-type natriuretic peptide, and myoglobin, which are key biomarkers for the early diagnosis of acute myocardial infarction and heart failure. Thus, the nanosensor offers multiplex detection of cardiovascular biomarkers in a single sample even with small quantity. An implantable nanosensor fabricated with core/shell polyvinylidene difluoride/hydroxylamine hydrochloride organic piezoelectric nanofibers and spatial self-oriented β-phase nanocrystals has demonstrated remarkable sensitivity and accuracy in capturing the micropressure variations in the cardiovascular walls that reflect changes in the cardiovascular elasticity and incidence of CVD [63]. This will help to distinguish the fluctuations in the elasticity of cardiac wall in response to cardiac complications, which can be used as an early diagnostic tool for thrombosis and atherosclerosis. A pulse nanosensor with an AgNP-reinforced polydimethylsiloxane membrane, working based on piezo-thermic transduction showed high sensitivity and good linearity in monitoring radial arterial pulse waves [64]. The device could sense subtle pulse waveforms by adjusting the air gap volume fraction and silver particle volume fraction of the structured material, which provide pivotal information about the auxiliary diagnosis of CVD. An electrochemical paper-based nanosensor constructed using a phosphocholine-based recognition system for determining high-sensitivity C-reactive protein levels showed potential application in evaluating the risk of future cardiovascular events [65]. The phosphocholine-modified screen-printed carbon electrodes, which has high sensitivity to the clinical range of C-reactive protein, have been used in this nanosensor device to reproduce the results with minimum bias. Another nanosensor containing nanoporous gold networks directly grown on a titanium substrate and co-immobilized cholesterol metabolizing enzymes has displayed high selectivity and high sensitivity for the clinical determination of cholesterol [66]. The main enzymes used in this device for the clinical determination of cholesterol include cholesterol oxidase, cholesterol esterase, and horseradish peroxidase. A nitrogen-doped carbon dots-based nanosensor also showed a sensitive, selective, and cost-effective method for the detection of cholesterol in whole blood samples [67]. The principle used in this nanosensor is the production of hydrogen peroxide as the product of cholesterol oxidation in the presence of cholesterol oxidase, followed by the strong adsorption of hydrogen peroxide on the graphitic N positions of the nitrogen-doped carbon dots that offers the sensitive detection of blood cholesterol. The atherosclerosis-associated disease diagnosis was performed using a paper-based, SERS nanosensing platform working on the specific identification and quantification of cytokine targets in the blood [68]. The cytokines, interleukin-10, and monocyte chemoattractant protein 1, which are important for the early diagnosis of atherosclerosis, have been used in a sandwich design in this nanosensor. The device exhibits high sensitivity with low-nonspecific binding and acceptable cross-reactivity for the multi-target prognosis of atherosclerosis. Also, SERS nanosensors have been utilized for the detection of hypertension-induced changes in erythrocytes in animal models as a diagnostic strategy for CVD [69]. This study used plasmonic SERS nanosensors to analyze the properties of erythrocytes under normotensive and hypertensive conditions, in which hypertension caused decrease in the plasma membrane fluidity of erythrocyte and mobility of the heme of the membrane-bound hemoglobin. These findings can be used in the diagnosis of CVD. A single-walled CNT-based nanosensors exhibited highly sensitive and specific detection of interleukin-6, which plays a critical role in the progression of inflammatory diseases including CVD [70]. Fluorescent-antibody-conjugated single-walled CNT and a polymer, poly-l-lysine was used to detect interleukin-6 in human serum samples. A nanosensor based on MoS2 nanosheet-powered CRISPR/Cas12a sensing strategy was shown to have high sensitivity in the determination of miR-499, a microRNA marker having superior potential than other protein markers in the prognosis of acute myocardial infarction [71]. Based on the presence of miR-499, even at trace level, the nanosensor could induce a significantly enhanced fluorescence signal, which can be detected in human serum samples with high sensitivity. Similarly, another electrochemiluminescence nanosensor has shown fast and ultrasensitive microRNA detection for the rapid diagnosis of acute myocardial infarction [72]. In this construct, a covalently hemin-modified spherical nucleic acid enzyme (Enzyme) and a truncated triangular pyramid DNA nanoplatform were attached to two probe strands on the electrode, hybridized with miRNAs that are having promising role in the prognosis of acute myocardial infarction. This specialized construction improves the electrochemiluminescence, reduces the background, and enables the rapid detection of the target microRNA. Of note, various aptamer-based nanosensors have been developed for the sensitive detection of myoglobin as a biomarker for the early diagnosis of acute myocardial infarction [73]. As an early marker of acute myocardial infarction, myoglobin offers promising prognostic potential to detect cardiac damage. The changes in pH and protein phosphorylation due to the inflammatory microenvironment associated with atherosclerosis have been monitored in blood using a dual-detection fluorescence nanosensor to assess the early progression of atherosclerotic disease in mouse models [74]. The pH-sensitive group piperazine and the Ziv node of the nanosensor construct has been utilized for the simultaneous detection and imaging of pH and phosphorylation in the vascular endothelium. Likewise, a ratiometric nanosensor with metal–organic frameworks and fluorescent nanocluster wool-balls showed changes in the protein phosphorylation related to the signaling pathways associated with atherosclerosis progression in mice models [75]. While the specific interaction between active center Zr(IV) and phosphate aids in the detection of phosphorylation sites, the two-photon property of porphyrin reduces the background for sensitive detection. Using the fluorescence imaging of protein phosphorylation level, this study demonstrated significant changes between normal and atherosclerotic mice that provides a valuable tool for tracing the disease progression. Another nanoflare-based DNA nanosensor assembled with an AuNP has shown excellent sensitivity and specificity in detecting ClO-, a typical marker of atherosclerosis, in cells and mice models [76]. The ClO--specific nanoflare probe was constructed using a ClO--responsive phosphorothioate inserted into DNA assembled with an AuNP core. The hydrolysis between phosphorothioate and ClO- produces fluorescence signal on the probe and the nanoflare probes helps in tracing the variations in endogenous and exogenous ClO-, which demonstrate potential implications in the clinical prognosis of atherosclerosis. The aforementioned nanosensors clearly demonstrate that the accurate, sensitive, selective, and rapid determination of potential biomarkers or pathological conditions that correlate with the early detection of cardiovascular function by the recent advances in nanosensors have revolutionized the clinical diagnosis and therapeutic outcome of CVD. The disease prognostic applications of nanosensors in cardiovascular diseases have been summarized in Table 2.

Table 2.
Disease prognostic applications of nanosensors in cardiovascular diseases.
4.3. Disease Prognostic Applications of Nanosensors in Diabetes Mellitus
Diabetes mellitus, a chronic and multifactorial metabolic syndrome, is a rapidly growing public health problem that affects the quality of life and leads to increased healthcare costs worldwide. It is mainly characterized by high blood glucose levels that in turn cause serious pathophysiological complications resulting in several comorbidities [77]. Therefore, to minimize the adverse complications of diabetes, tight monitoring and controlling blood glucose is required. The limitations of the conventional diagnostic methods have necessitated research focusing on developing improved methods to measure glucose and to diagnose and manage diabetic complications effectively. Over the past few years, nanosensors have played pivotal roles in this scenario with remarkable sensitivity, selectivity, and rapid detection systems.
Nanosensors have great potential in continuous and noninvasive monitoring of blood sugar levels. An amperometric nanosensor based on ferrocene-modified multiwalled CNT nanocomposites has shown to be effective in glucose measurement [78]. This was constructed by the adsorption of ferrocenecarboxaldehyde on multiwalled CNT which will mediate the electrical communication between glucose oxidase and electrode. Chitosan was used to immobilize the glucose oxidase on the nanofilm that in turn form a reagentless amperometric sensor for the determination of glucose. Another amperometric nanosensor with silicon dioxide coated magnetic nanoparticle and multiwalled CNTs exhibited excellent glucose detection limit [79]. The nanosensor was constructed by the catalytic chemical vapor decomposition of acetylene over rare earth-based AB alloy hydride catalyst. Further chemical reduction was performed to coat biocompatible SiO2 on multiwalled CNT impregnated with magnetic Fe3O4 nanoparticles followed by the deposition of glucose oxidase on the electrode. This will enable the retention of the catalytic activity of the enzyme for the rapid and sensitive detection of glucose. An electrochemical nanosensor with a polymer electrode modified with a gold/MoS2/gold nanofilm showed more sensitivity with the help of electron transfer by MoS2 [80]. The sputter deposition of gold, spin coating of MoS2, and sputter deposition of gold on the flexible polymer electrode have been utilized for the fabrication of the nanosensor. The immobilized glucose oxidase enzyme on the flexible polymer electrode modified with nanofilm overcomes the low sensitivity of the conventional flexible biosensors, making it an excellent wearable biosensing system for clinical applications. A CNT nano-yarn fiber-based nanosensor has showed superior efficiency in sensing glucose than traditional sensors [81]. Here, a chemical vapor deposition gas flow reaction, in which ethanol and acetone as the carbon source and an iron nano-catalyst, has been used to construct CNT nano-yarn fiber, which in turn compacted into multiple layers to form a nanoporous network structure. Glutaraldehyde crosslinking in the presence of bovine serum albumin has been used to immobilize glucose oxidase enzyme for effective detection of glucose. A chemiresistive nanosensors with poly(4-vinylpyridine) and single-walled CNT displayed high selectivity and instant response within 3 sec in glucose detection [82]. The polymer-single-walled carbon nanotube composite and the glass substrate were covalently connected by the treatment of glass substrate containing gold electrodes with 3-bromopropyltrichlorosilane. The pyridyl moieties in poly(4-vinylpyridine) on the surface and those quaternized using 2-bromoethanol provide the highly charged hydrophilic surface. This will enable the improved biocompatibility of glucose oxidase for the successful functionalization on the nanocomposite offering highly sensitive detection of glucose. An excellent selectivity, good reproducibility, and stability has been shown by a molecularly imprinted electrochemical nanosensor with nano-carbon-dots and chitosan [83]. The construct demonstrates an environmentally friendly approach in the electrode construction with active area and electron-transport ability. Using glucose as a template and 3-aminobenzeneboronic acid as a functional monomer, a molecularly imprinted polymer screen was created in order to detect glucose using differential pulse voltammetry. Of note, the nanosensor showed successful application in determining the glucose concentration in human samples.
Another nanosensor based on Fe3O4 nanoparticles, cross-linked on an electrode with chitosan covered with a thin nafion film could successfully eliminate the interference during the glucose detection in human blood samples for practical clinical application [84]. The intrinsic peroxidase-like activity of Fe3O4 nanoparticles and the anti-interference ability of the nafion film has been utilized to eliminate interference during glucose detection in human samples, making the nanosensor a potential tool in clinical settings. An enzymatic glucose nanosensor fabricated with palladium nanoparticles dispersed onto poly(3,4-ethylenedioxythiophene) nanofibers displayed outstanding performance in glucose detection with high sensitivity [85]. The electrodeposition of poly(3,4-ethylenedioxythiophene) nanofibers was performed by using a surfactant and co-surfactant followed by the immobilization of glucose oxidase and electrochemical deposition of palladium nanoparticles to fabricate the nanosensor construct. A renewable Ni nanoparticle-loaded carbon nanofiber paste electrode, prepared by the combination of electrospinning technique with thermal treatment has been used to fabricate a nonenzymatic nanosensor for glucose determination [86]. A combination of electrospinning technique with thermal treatment was used for the nanocomposite preparation. The renewable Ni nanoparticle-loaded carbon nanofiber paste electrode demonstrated strong and fast amperometric response to glucose without any interference with other ions like chloride. The electrocatalytic performance of the firmly embedded Ni nanoparticles and the chemical inertness of the carbon nanofiber-based electrode provided high sensitivity and stability to the nanosensor. Another nanosensor which is working on FRET process between terbium (III)-1, 10-phenanthroline (Tb-phen) complex and AgNPs has shown ultra-sensitive performance in glucose detection enabling the rapid detection of glucose in blood samples of diabetes patients in clinical settings [87]. Because of the special emission characteristics of the Tb-phen complex probe, common interfering species such ascorbic acid, fructose, and galactose did not induce internal interference. The interaction of the Tb-phen complex with AgNPs that produce fluorescence intensity corresponding to glucose residues will make the nanosensor an alternative to existing optical and electrochemical methods.
Studies have also been performed in diabetic animal models to show the prognostic applications of various nanosensors in diabetes. A conjugated copolymer-based fluorescence nanosensor (DPA-PFNP-Cu(II)) with 2,7-dibromofluorene and 4,7-bis (2-bromothiophen-5-yl)-2-1-3-benzothiadiazole has been used to detect the level of homocysteine (Hcy), one of the key markers of diabetes pathology, in diabetic mice model [88]. In this nanosensor, the fluorescence emission by the competitive coordination between Hcy and Cu(II) allows the correlation analysis of diabetic severity for screening. Another study has used a tandem of nanosensors to measure nitric oxide and peroxynitrite in hypertensive rats with diabetes [89]. The study aimed to explore the mechanism of beneficial effects of atorvastatin in diabetes-induced hypertension using nanosensors. Thus, the emergence of these nanosensors that help to detect or monitor glucose levels and associated biomarkers in diabetes may improve the clinical outcome in the management of diabetes treatment. The disease prognostic applications of nanosensors in Diabetes mellitus have been summarized in Table 3.

Table 3.
Disease prognostic applications of nanosensors in diabetes mellitus.
4.4. Disease Prognostic Applications of Nanosensors in Neurodegenerative Diseases
The progressive loss of structure and function of neurons in the central nervous system is a hallmark of neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, mental illness, and amyotrophic lateral sclerosis (ALS), which pose a significant burden on individuals, families, and societies around the world [90]. Even though scientific advances have developed various medical or surgical solutions for these neurodegenerative diseases, there are concerns regarding the long-term benefits of these remedies due to brain barrier impairment, which hinders their clinical acceptance. Hence, better illness outcomes would be possible with early diagnosis with advanced techniques.
Nanosensors have proven to be very useful in the diagnosis of several neurodegenerative disorders, especially in detecting the fluctuating level of various neurotransmitters associated with neurodegenerative diseases. SERS nanosensing has been widely employed in the monitoring and imaging of various aspects of neurological disorders [91]. These nanosensors demonstrate faster and cheaper alternatives for diagnosing neuropathological alterations as reflected by the changes in the level of key neurotransmitters like dopamine, serotonin, etc., in the brain and biological fluids. A nanocomposite composed of magnetite and AgNPs, with surface modification of iron nitriloacetic acid, has been utilized as a platform for SERS-mediated nanosensing of dopamine in biological fluids at even low femtomolar concentrations [92]. The separation of dopamine was achieved by the magnetic properties of this nanocomposite along with SERS mediated by silver. The biocompatibility of nanosensor was further reinforced by the joining of silver and magnetite nanoparticles by carboxymethyl chitosan, which in turn enhances the sensitivity of the detection due to its carboxyl groups. Another SERS nanosensing probe made of magnetite Fe3O4 and Au nanoparticles (Fe3O4/AuNPs) with coexisting electric-field effect and charge transfer enhancement was demonstrated to have enhanced dopamine sensitivity in biological fluids and tissues [93]. Here, Fe3O4 aids in the specific chemical interaction with dopamine molecules and enhances the Raman signal of dopamine by acting as a SERS substrate. This enhances the efficient separation and sensitive detection of dopamine from complex specimens and various tissues. A graphene-Au nanopyramid heterostructure-based nanosensor has shown ultrahigh sensitivity with SERS in detecting dopamine and serotonin in body fluids [94]. The sensitivity of SERS was obtained by the high-density and high-homogeneity hotspots boosting by the quasi-periodic Au structure, enabling the detection and differentiation of dopamine and serotonin from each other. The AgNP synthesized on the surface of Fe, forming a metal–organic framework of the nanosensor has been demonstrated as an excellent SERS substrate for the detection of dopamine by utilizing the enzyme-linked immunosorbent assay [95]. Herein, in situ synthesis of AgNPs on the surface of MIL-101 (Fe) acts as a SERS substrate that combines the numerous Raman hot spots between the high-density AgNPs and the excellent adsorption performance of metal–organic framework. This helps in the highly sensitive ERS detection by concentrating analytes in close proximity to the Raman hot spots domains between the nearby AgNPs. The colorimetric substrate, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS) cats as a SERS marker for enzyme-linked immunosorbent assay-based detection. SERS of 4-mercaptophenylboronic acid coupled with Ag nanostructure was also used for the detection of dopamine in human serum [96]. One-step gas-flow sputtering was performed to construct the Ag nanostructure, followed by the vapor-based deposition of 4-mercaptophenylboronic acid, which as a reporter molecule for dopamine binding. The nanosensor showed remarkable selectivity against other analytes like glucose, creatinine, and uric acid. Ultrasensitive SERS nanosensing of dopamine in human serum and urine samples has also been demonstrated using silver nanocubes (AgNCs) and nanoporous silver film (AgNF) modified with 4-mercaptobenzene boronic acid [97]. While AgNF with mercaptopropionic acid aids in the specific capture of dopamine, AgNCs with 4-mercaptobenzene boronic acid acts as a Raman reporter for the quantitative detection of dopamine. A nanosensor with Ag-plated Au bimetallic nanocluster as SERS substrate showed reliable and quantitative detection of dopamine levels in human blood plasma with Parkinsonism [98]. The combination of electrodeposition and electroless plating methods have been utilized for the construction of the bimetallic nanosensor cluster. The device could successfully differentiate the dysregulations in dopamine levels in healthy subjects and patients with Parkinsonism. Fe(III)-sensitized nanogaps in AuNP monolayer films were reported for the SERS-nanosensing of dopamine [99]. Integration of Fe(III) sensitizers into the precisely defined <1 nm nanogaps has been devised to target dopamine and oxygen plasma cleaning protocols has been utilized for the reusability after different analyte sensing. Another nanosensor with aggregated gold nanospheres encapsulated with polyvinylpyrrolidone has been used in the SERS detection of five neurotransmitters such as dopamine, epinephrine, norepinephrine, serotonin, and histamine [100]. Here in, physicochemical trapping method offers high signal-to-noise ratio and spectral consistency even at nm levels. A nanosensor composed of AuNP coupled with chitosan has been used to measure epinephrine levels [101]. Box–Behnken design has been used to optimize the increased signal sensibility of epinephrine enabling stable signal intensity for the quantification. The SERS nanosensor with AuNP suspension has demonstrated to have application in the ultrasensitive quantitative detection of norepinephrine and epinephrine [102]. The measurement of Raman signal intensity over time coupled with chemometric tools offers the ultrasensitive detection of norepinephrine and epinephrine with effective removal of outliers.
Metalloporphyrin nanosensors have been used to detect the levels of peroxynitrite (ONOO¯), produced in the neurological system in response to ROS produced during neurodegenerative diseases [103]. This electrochemical metalloporphyrin nanosensor demonstrated promising applications in the early medical diagnosis of neurological illnesses through the real-time monitoring of nitroxidative stress. A nanoplasmonic fiber tip nanosensor demonstrated the simultaneous monitoring of Aβ42 generation and tau phosphorylation, which are the pathological hallmarks of AD [104]. The nanoplasmonic fiber tip probe contains a nanoscale optical fiber with a single gold nanorod biosensor on its tip, which specifically detects Aβ42 generation and tau phosphorylation. Another label-free optical nanosensor could also successfully detect Aβ42 and T-tau in cerebrospinal fluid to diagnose AD [105]. An adenosine-imprinted and non-imprinted poly(2-hydroxyethyl methacrylate-methacrylic acid) surface plasmon resonance (SPR) nanosensor showed effective detection of adenosine nucleoside, which can be correlated with the progression of neurodegenerative diseases [106]. A graphene electrolyte-gated transistor nanosensor demonstrated the detection of neuron-derived exosomal Aβ42 in serum for the reliable diagnosis of AD [107]. Another near-infrared optical nanosensor also showed effectiveness in detecting Aβ levels [108]. The device consists of single-walled CNT functionalized with Aβ. Through solvatochromic regulation of the near-infrared emission of the nanotube, Aβ nanosensors respond to Aβ selectively in live cells enabling the identification of Aβ neurotoxicity. A near-infrared (near-IR) fluorescent single walled-CNT optical nanosensor has been developed for the detection of cholinesterase enzymes such as acetylcholinesterase and butyrylcholinesterase, which are known to be dysregulated in various neurodegenerative diseases [109]. A nanosensor embedded in mesoporous silica nanoparticles showed high sensitivity and specificity in detecting potassium levels that affect the membrane potential and neuronal activity [110]. To detect the ATP levels in the cerebrospinal fluid, a silver nanocluster-based ratiometric fluorescent nanosensor has been used and demonstrated high level of ATP in the brain with AD [111]. Thus, the present review explores wide applications of nanosensors in neuroscience and highlights the importance of integrating the effectiveness of these nanosensors into conventional neurochemistry techniques. The disease prognostic applications of nanosensors in neurodegenerative diseases have been summarized in Table 4.

Table 4.
Disease prognostic applications of nanosensors in neurodegenerative diseases.
5. Disease Prognostic Applications of Nanosensors in Other Diseases
Nanosensors have been demonstrated to have wide applications in diagnosing various diseases. An intravital electrochemical nanosensor has been shown to detect ROS/nitrogen species (ROS/RNS) in the liver, which can be correlated with the live pathologies such as acute liver injury, partial hepatectomy and hepatocellular carcinoma (HCC) [112]. A ratiometric fluorescence nanosensor with carbon dots-doped mesoporous silica and fluorescein-based fluorescent probes showed effectiveness in detecting cysteine in biological samples to monitor the pathological progression of liver damage, AD, weakness, and CVD [113]. A fluorescent metal–organic frameworks (MOFs) nanoprobe containing UiO-66(OH)2 and Cu-MOFs has been used to detect the abnormal level of glutathione and phosphate levels in various organs to evaluate the degree of hyperthyroidism-induced liver injury in the early clinical stage [114]. A dual-aptamer modified reduced graphene oxide (RGO) field-effect transistor (AAP-GFET) nanosensor with AuNP demonstrated sensitive quantification of cancerous microvesicles for the diagnosis of HCC [115]. Another nanosensor with multiwalled CNTs and zein nanoparticles has been utilized for the electrochemical sensing of hydrogen peroxide using a glassy carbon electrode in liver cancer cells even at a very low detection limit [116]. Carbon dot-graphene oxide-based luminescent nanosensors exhibited outstanding consistency and specificity in detecting creatinine in human urine samples for the diagnosis of kidney dysfunction [117]. The multifunctional iridium complex and polymeric micellar nanoparticle form of a multifunctional molecule (rubrene) has been used to construct an afterglow nanosensor for the detection of pathologically overproduced superoxide in cisplatin-induced kidney injury [118]. Another spectroelectrochemical nanosensor could effectively detect cystatin C in blood for the monitoring of AD and kidney failure diseases [119].
An orally administered ultrasmall platinum nanoclusters (PtNCs)-based nanosensors has been effective in monitoring the progression of inflammatory bowel disease [120]. Quantum dot-based nanosensors have been widely used for the detection of Mycobacterium tuberculosis as a promising approach for the diagnosis of tuberculosis [121]. A nanosensor, integrated with gold nanocrown and 1,3,3,1′,3′,3′-hexamethyl-2,2′-indotricarbocyanine iodide showed excellent sensitivity, reproducibility, and rapid detection of the SARS-CoV-2 S1 protein and demonstrates effectiveness in the early detection of COVID-19 [122]. For the detection of SARS-CoV-2 Spike protein in saliva, a nanosensor with Zn-Cu-In-Se-P quantum dots has shown a wide linear detection range and a low detection limit, along with excellent selectivity against nonspecific protein targets [123]. A nanosensor constructed by coupling dithiobis (succinimidylpropionate) on the surface of AuNPs showed good sensitivity and selectivity in the detection of histamine, the key biomarker of allergic diseases [124]. In the bacterial lipopolysaccharides-treated cell culture model, an optical aptamer-based nanosensor with single-walled CNTs exhibited rapid and sensitive detection of inflammatory cytokine which can be implicated in the diagnosis of acute and chronic inflammatory diseases [125]. The development of a chronic arthritic disease, osteoarthritis, has been predicted using a nitric oxide nanosensor constructed by encapsulating 4-amino-5-methylamino-2′,7′-difluorofluorescein Diaminofluorescein-FM, a nitric oxide sensing molecules within the biodegradable poly(lactic-co-glycolic acid) nanoparticles [126]. An amine-functionalized graphene-based nanosensor conjugated with antibodies that detect key biomarkers associated with human immunodeficiency virus (HIV) infection and related CVD and rheumatoid arthritis demonstrated excellent performance with high sensitivity in real samples [127].
6. Potential Challenges and Possible Recommendations for the Disease Prognostic Applications of Nanosensors
Even though nanosensors hold a great promise in the early prognosis of chronic diseases, several challenges hinder their widespread implementation [128,129,130,131,132]. Noise and interference can affect nanosensors, especially those that use individual nanoparticles. Careful signal processing and calibration are necessary because background signals, fluctuations, or environmental contaminants might compromise the precision and dependability of measurements. In some instances where the biomarker concentrations are relatively low, false positives or cross-reactivity with other factors may reduce the sensitivity of the detection. Inconsistencies in the detection can also be caused by environmental factors such as pH temperature and humidity. Nanoparticles used in the sensors may exhibit variations in their physicochemical properties that may cause challenges in reproducibility and continuing stability of sensor performance. As biocompatibility and potential toxicity of nanoparticles is very important for biomedical applications, to guarantee their safe and efficient application, the interaction between the nanosensors and living systems should be thoroughly evaluated. The variations in the electrical properties of some nanoconstructs used may cause variability in sensor performance. The manufacturing complexity of some nanosensors also poses significant challenges. The complex and expensive production and functionalization of nanosensors may limit their applications in clinical settings. Timely standardization and quality control are essential to maintain consistent performance, reliability, and reproducibility. Current research and technological advancements aim to overcome these challenges and increase the potential of nanosensors in various biomedical applications.
Environmental factors pose constant challenges to sensor operation across a broad spectrum of applications such as industrial monitoring, environmental monitoring, medical applications, and consumer products. Temperature fluctuations could lead to sensor output drift via electrical and physical property variations, while elevated humidity could lead to condensation, corrosion, or swelling of materials—most troublesome for biosensors. Dust and particulates may obstruct optical paths or get into sensitive surfaces, whereas chemical impurities can destroy gas and biosensors by corrosion or poisoning. These issues have been addressed in the recent past using temperature compensation algorithms, hydrophobic protective coatings for wearable devices, periodic calibration methods for mobile air quality sensors and artificial intelligence (AI)-facilitated signal processing to remove environmental noise [133,134,135]. In the coming future, efficient methods such as sensor fusion, nanomaterials, AI-deployed real-time correction, intelligent protective cases, autonomous calibration schedules, and open standardization efforts will be expected to enhance the resilience and precision of sensors. With these technologies, next-generation sensor systems can become more reliable and resilient despite adverse environmental conditions.
Given the growing demand for more accurate and cost-effective strategies in the medical field, the applications of advanced nanosensor-based approaches to improve the performance of health systems have been considered [131]. Hence, possible recommendations that can improve the efficiency and effectiveness of nanosensors should be examined. To increase the sensitivity and selectivity of nanosensors, the use of advanced nanomaterials such as carbon nanotubes, quantum dots, and engineered metal nanoparticles that can amplify the weak signals of target molecules is recommended [3]. Biocompatible coating materials can be used to minimize the adverse reactions in the biological system, thereby improving the biocompatibility [136]. Proper optimization of nanosensors can improve the timely response and rapid identification of risk factors that detect disease progression. To mitigate the effects of environmental factors that cause inconsistencies in the detection, the use of nanomaterials with high resistance to environmental changes is viable strategy. This may help sensors to be self-adjusting to provide accurate results without the need for frequent calibration. The high cost of manufacturing nanosensors is the main obstacle for their widespread clinical utility Advanced research focusing on the improved cost-effective fabrication techniques that substitute for complex nanomaterials are warranted to resolve this challenge. Most importantly, to conserve the security and privacy of the medical data generated by nanosensors, unauthorized access and misuse should be prevented. This can be achieved by using sophisticated encryption technologies, data storage on secure servers, and authentication techniques.
7. Artificial Intelligence in the Disease Prognostic Applications of Nanosensors
As we discussed in the previous sections, nanotechnology has revolutionized the field of sensors with high-efficiency detection capabilities. The emergence of AI has further enhanced the diagnostic accuracy of nanosensors with their deep learning and detection properties that facilitate robust analysis of highly complex systems [137]. The machine learning algorithms in AI help filter noise in nanosensors due to various environmental factors, thereby improving signal clarity and enhancing sensor reliability [138]. For immediate analysis and faster responses to changing conditions, real-time data processing is very important for nanosensors. The machine learning of capacity AI helps in the real-time data processing that offers several advantages to the functioning of nanosensors [139]. For the early detection of diagnostic markers, this AI-mediated real-time processing helps in the timely decision-making through its rapid response. Also, a vast amount of data can be processed by AI algorithms from possible patterns and anomalies that might go unnoticed by human analysts. The adaptive and predictive capabilities of AI-enhanced nanosensors will improve the accuracy and efficiency of detection and analysis of various disease prognosis markers. AI mediates the continuous monitoring of physiological parameters and disease-specific biomarkers that aid in early disease diagnosis [140]. By analyzing massive datasets, predicting treatment outcomes in real-time, and customizing treatments for each patient based on their genetic and molecular profiles, AI-assisted nanosensors provide personalized medicine in the healthcare industry. The cost of integrating sophisticated technology for AI sensors may pose significant barriers to its widespread adoption. So future research is warranted to drive down the cost to develop more robust AI-enhanced nanosensors. The effective collaboration of researchers from interdisciplinary fields that can integrate AI with nanotechnology is crucial for overcoming challenges and translating these technologies into real-world solutions for the disease prognosis applications in clinical settings.
8. Conclusions
The advances in nanosensors have revolutionized the field of medicine with the timely detection of early-stage pathological changes that enable advanced treatment modalities to improve clinical outcomes. In the present review, we have consolidated the wide range of prognostic applications of nanosensors in various disease pathologies. Special focus was given to the scientific advances of nanosensors in the field of major diseases such as cancer, CVD, diabetes mellitus, and neurodegenerative diseases along with their applications in other prevalent diseases. The nanoscale properties of nanosensors integrated with high-quality detection techniques help in the effective targeting and detection of disease-related molecules, making them versatile tools for understanding and reporting various biological processes at the cellular level. This may help to improve or modulate the therapeutic strategies according to the disease states that may help in the long-term survival of patients. This review highlights the importance of integrating the effectiveness of these nanosensors into conventional therapeutic modalities. In addition, the advanced knowledge and the applications of these nanosensors with high sensitivity and specificity will aid in the early detection of disease progression and will have enormous impacts in the medical and clinical fields. This review serves as a starting point for future research focusing on more advanced nanosensors coupled with emerging technologies, which have higher sensitivity and accuracy in the simultaneous measurements of multiple targets. Of these, multimodal nanosensors can respond to multiple types of stimuli to provide information about the different types of disease targets. Of note, the current era of AI integrated with disease prognostic applications of nanosensors offers real-time detection of various disease specific markers by utilizing multi omics profiling. These technological advances will help to overcome data integration challenges with increased sensitivity for improved applications in clinical settings.
Author Contributions
Project administration: S.S.P. (Saumya S. Pillai), Conceptualization: S.S.P. (Saumya S. Pillai), S.S.P. (Sneha S. Pillai), P.K.R.; Writing—original draft preparation: N.K.M., N.S.M., S.M.S., and T.M.D.; Review and editing: S.S.P. (Saumya S. Pillai), S.S.P. (Sneha S. Pillai), and P.K.R.; Supervision: S.S.P. (Saumya S. Pillai). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. [Google Scholar] [CrossRef]
- Guruprasath, N.; Sankarganesh, P.; Adeyeye, S.A.O.; Babu, A.S.; Parthasarathy, V. Review on emerging applications of nanobiosensor in food safety. J. Food Sci. 2024, 89, 3950–3972. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abd-Elaziem, W.; Elsheikh, A.; Zayed, A.A. Advancements in nanomaterials for nanosensors: A comprehensive review. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef]
- Halabi, S.; Owzar, K. The importance of identifying and validating prognostic factors in oncology. Semin. Oncol. 2010, 37, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Tousignant-Laflamme, Y.; Houle, C.; Cook, C.; Naye, F.; LeBlanc, A.; Decary, S. Mastering Prognostic Tools: An Opportunity to Enhance Personalized Care and to Optimize Clinical Outcomes in Physical Therapy. Phys. Ther. 2022, 102, pzac023. [Google Scholar] [CrossRef] [PubMed]
- Savaliya, R.; Shah, D.; Singh, R.; Kumar, A.; Shanker, R.; Dhawan, A.; Singh, S. Nanotechnology in Disease Diagnostic Techniques. Curr. Drug Metab. 2015, 16, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Dhahi, T.S.; Yousif Dafhalla, A.K.; Tayfour, O.E.; Mubarakali, A.; Alqahtani, A.S.; Tayfour Ahmed, A.E.; Elobaid, M.E.; Adam, T.; Gopinath, S.C.B. Advances in nano sensors for monitoring and optimal performance enhancement in photovoltaic cells. iScience 2024, 27, 109347. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials 2024, 14, 1085. [Google Scholar] [CrossRef]
- Jeykumari, D.R.; Narayanan, S.S. Fabrication of bienzyme nanobiocomposite electrode using functionalized carbon nanotubes for biosensing applications. Biosens. Bioelectron. 2008, 23, 1686–1693. [Google Scholar] [CrossRef]
- Gosai, A.; Khondakar, K.R.; Ma, X.; Ali, M.A. Application of Functionalized Graphene Oxide Based Biosensors for Health Monitoring: Simple Graphene Derivatives to 3D Printed Platforms. Biosensors 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Harimech, P.K.; Brown, T.; Kanaras, A.G. Gold nanoparticles and fluorescently-labelled DNA as a platform for biological sensing. Nanoscale 2013, 5, 9503–9510. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.G.; Gonzalez-Gonzalez, R.B.; Martinez-Ruiz, M.; Coronado-Apodaca, K.G.; Reyes-Pardo, H.; Morreeuw, Z.P.; Oyervides-Munoz, M.A.; Sosa-Hernandez, J.E.; Barcelo, D.; Parra-Saldivar, R.; et al. Expanding the Scope of Nanobiocatalysis and Nanosensing: Applications of Nanomaterial Constructs. ACS Omega 2022, 7, 32863–32876. [Google Scholar] [CrossRef] [PubMed]
- Nezami, A.; Dehghani, S.; Nosrati, R.; Eskandari, N.; Taghdisi, S.M.; Karimi, G. Nanomaterial-based biosensors and immunosensors for quantitative determination of cardiac troponins. J. Pharm. Biomed. Anal. 2018, 159, 425–436. [Google Scholar] [CrossRef]
- Dehghani, S.; Nosrati, R.; Yousefi, M.; Nezami, A.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Alibolandi, M.; Ramezani, M. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosens. Bioelectron. 2018, 110, 23–37. [Google Scholar] [CrossRef]
- Salvati, E.; Stellacci, F.; Krol, S. Nanosensors for early cancer detection and for therapeutic drug monitoring. Nanomedicine 2015, 10, 3495–3512. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, S.; Liu, Y.; Liu, C.; Sun, J. Nanosensors for Diagnosis of Infectious Diseases. ACS Appl. Bio Mater. 2021, 4, 3863–3879. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Y.; Guan, W.; Zhou, W.; Wei, P. Advances in nanosensors for cardiovascular disease detection. Life Sci. 2022, 305, 120733. [Google Scholar] [CrossRef]
- Palaniyandi, T.; Kanagavalli, B.; Prabhakaran, P.; Viswanathan, S.; Wahab, M.R.A.; Natarajan, S.; Moorthy, S.K.K.; Kumarasamy, S. Nanosensors for the diagnosis and therapy of neurodegenerative disorders and inflammatory bowel disease. Acta Histochem. 2023, 125, 151997. [Google Scholar] [CrossRef]
- Teniou, A.; Rhouati, A.; Marty, J.L. Recent Advances in Biosensors for Diagnosis of Autoimmune Diseases. Sensors 2024, 24, 1510. [Google Scholar] [CrossRef]
- Arndt, N.; Tran, H.D.N.; Zhang, R.; Xu, Z.P.; Ta, H.T. Different Approaches to Develop Nanosensors for Diagnosis of Diseases. Adv. Sci. 2020, 7, 2001476. [Google Scholar] [CrossRef]
- Bremer, C.; Tung, C.H.; Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001, 7, 743–748. [Google Scholar] [CrossRef]
- Gandhi, S.; Arami, H.; Krishnan, K.M. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett. 2016, 16, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, F.; Aronova, M.; Zhu, L.; Lin, X.; Quan, Q.; Liu, G.; Zhang, G.; Choi, K.Y.; Kim, K.; et al. Manipulating the power of an additional phase: A flower-like Au-Fe3O4 optical nanosensor for imaging protease expressions in vivo. ACS Nano 2011, 5, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Waterman, P.; Thurber, G.; Groves, K.; Rajopadhye, M.; Panizzi, P.; Marinelli, B.; Aikawa, E.; Pittet, M.J.; Swirski, F.K.; et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arter. Thromb. Vasc. Biol. 2009, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cha, E.J.; Park, K.; Lee, S.Y.; Hong, J.K.; Sun, I.C.; Kim, S.Y.; Choi, K.; Kwon, I.C.; Kim, K.; et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. 2008, 47, 2804–2807. [Google Scholar] [CrossRef]
- Li, B.; Gu, Z.; Kurniawan, N.; Chen, W.; Xu, Z.P. Manganese-Based Layered Double Hydroxide Nanoparticles as a T(1) -MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity. Adv. Mater. 2017, 29, 1700373. [Google Scholar] [CrossRef]
- Panizzi, P.; Nahrendorf, M.; Wildgruber, M.; Waterman, P.; Figueiredo, J.L.; Aikawa, E.; McCarthy, J.; Weissleder, R.; Hilderbrand, S.A. Oxazine conjugated nanoparticle detects in vivo hypochlorous acid and peroxynitrite generation. J. Am. Chem. Soc. 2009, 131, 15739–15744. [Google Scholar] [CrossRef]
- Freeman, R.; Finder, T.; Gill, R.; Willner, I. Probing protein kinase (CK2) and alkaline phosphatase with CdSe/ZnS quantum dots. Nano Lett. 2010, 10, 2192–2196. [Google Scholar] [CrossRef]
- Lowe, S.B.; Dick, J.A.; Cohen, B.E.; Stevens, M.M. Multiplex sensing of protease and kinase enzyme activity via orthogonal coupling of quantum dot-peptide conjugates. ACS Nano 2012, 6, 851–857. [Google Scholar] [CrossRef]
- Li, X.; Deng, D.; Xue, J.; Qu, L.; Achilefu, S.; Gu, Y. Quantum dots based molecular beacons for in vitro and in vivo detection of MMP-2 on tumor. Biosens. Bioelectron. 2014, 61, 512–518. [Google Scholar] [CrossRef]
- Kwong, G.A.; von Maltzahn, G.; Murugappan, G.; Abudayyeh, O.; Mo, S.; Papayannopoulos, I.A.; Sverdlov, D.Y.; Liu, S.B.; Warren, A.D.; Popov, Y.; et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 2013, 31, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.D.; Warren, A.D.; Soleimany, A.P.; Westcott, P.M.K.; Voog, J.C.; Martin-Alonso, C.; Fleming, H.E.; Tammela, T.; Jacks, T.; Bhatia, S.N. Urinary detection of lung cancer in mice via noninvasive pulmonary protease profiling. Sci. Transl. Med. 2020, 12, eaaw0262. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Pan, Y.; Huang, C.; Wang, Z.; Zhao, X. Sensitive detection of lung cancer biomarkers using an aptameric graphene-based nanosensor with enhanced stability. Biomed. Microdevices 2019, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Earhart, C.M.; Hughes, C.E.; Gaster, R.S.; Ooi, C.C.; Wilson, R.J.; Zhou, L.Y.; Humke, E.W.; Xu, L.; Wong, D.J.; Willingham, S.B.; et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab. Chip 2014, 14, 78–88. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Z.; Jin, J.; Liu, Y.; Chen, C.; Tang, Z. Optimizing Energy Transfer in Nanostructures Enables In Vivo Cancer Lesion Tracking via Near-Infrared Excited Hypoxia Imaging. Adv. Mater. 2020, 32, e1907718. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef]
- Barash, O.; Peled, N.; Tisch, U.; Bunn, P.A., Jr.; Hirsch, F.R.; Haick, H. Classification of lung cancer histology by gold nanoparticle sensors. Nanomedicine 2012, 8, 580–589. [Google Scholar] [CrossRef]
- Premachandran, S.; Dhinakaran, A.K.; Das, S.; Venkatakrishnan, K.; Tan, B.; Sharma, M. Detection of lung cancer metastasis from blood using L-MISC nanosensor: Targeting circulating metastatic cues for improved diagnosis. Biosens. Bioelectron. 2024, 243, 115782. [Google Scholar] [CrossRef]
- Ripoll, C.; Roldan, M.; Contreras-Montoya, R.; Diaz-Mochon, J.J.; Martin, M.; Ruedas-Rama, M.J.; Orte, A. Mitochondrial pH Nanosensors for Metabolic Profiling of Breast Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 3731. [Google Scholar] [CrossRef]
- Luan, M.; Yu, L.; Li, Y.; Pan, W.; Gao, X.; Wan, X.; Li, N.; Tang, B. Visualizing Breast Cancer Cell Proliferation and Invasion for Assessing Drug Efficacy with a Fluorescent Nanoprobe. Anal. Chem. 2017, 89, 10601–10607. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Li, X.; Zang, L.; Xu, K. An Au-Se nanoprobe for the evaluation of the invasive potential of breast cancer cells via imaging the sequential activation of uPA and MMP-2. Analyst 2020, 145, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, B.; Luan, M.; Li, N.; Tang, B. A fluorescence nanoprobe for detecting the effect of different oxygen and nutrient conditions on breast cancer cells’ migration and invasion. Biomater. Sci. 2021, 9, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.V.; Rahman, N.; Ghosh, P.; Ng, D.; Williams, R.M. Rapid differentiation of estrogen receptor status in patient biopsy breast cancer aspirates with an optical nanosensor. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Han, Y.; Li, D.L.; Han, Q.; Ma, F.; Zhang, C.Y. Integration of Demethylation-Activated DNAzyme with a Single Quantum Dot Nanosensor for Sensitive Detection of O(6)-Methylguanine DNA Methyltransferase in Breast Tissues. Anal. Chem. 2024, 96, 4487–4494. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, K.; Sun, Y.; Jin, B.; Zhang, Z.; Yang, K. Anti-epithelial cell adhesion molecule monoclonal antibody conjugated fluorescent nanoparticle biosensor for sensitive detection of colon cancer cells. Biosens. Bioelectron. 2012, 35, 186–192. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, H.; Guo, X.; Wu, Y.; Ying, Y.; Wen, Y.; Yang, H. Surface enhanced Raman detection of the colon cancer biomarker cytidine by using magnetized nanoparticles of the type Fe(3)O(4)/Au/Ag. Microchim. Acta 2018, 185, 195. [Google Scholar] [CrossRef]
- Tao, L.; Song, C.; Huo, C.; Sun, Y.; Zhang, C.; Li, X.; Yu, S.; Sun, M.; Jin, B.; Zhang, Z.; et al. Anti-CD155 and anti-CD112 monoclonal antibodies conjugated to a fluorescent mesoporous silica nanosensor encapsulating rhodamine 6G and fluorescein for sensitive detection of liver cancer cells. Analyst 2016, 141, 4933–4940. [Google Scholar] [CrossRef]
- Williams, R.M.; Lee, C.; Galassi, T.V.; Harvey, J.D.; Leicher, R.; Sirenko, M.; Dorso, M.A.; Shah, J.; Olvera, N.; Dao, F.; et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci. Adv. 2018, 4, eaaq1090. [Google Scholar] [CrossRef]
- Akbari Jonous, Z.; Shayeh, J.S.; Yazdian, F.; Yadegari, A.; Hashemi, M.; Omidi, M. An electrochemical biosensor for prostate cancer biomarker detection using graphene oxide-gold nanostructures. Eng. Life Sci. 2019, 19, 206–216. [Google Scholar] [CrossRef]
- Sunil, N.; Unnathpadi, R.; Pullithadathil, B. Ag nanoisland functionalized hollow carbon nanofibers as a non-invasive, label-free SERS salivary biosensor platform for salivary nitrite detection for pre-diagnosis of oral cancer. Analyst 2024, 149, 4443–4453. [Google Scholar] [CrossRef]
- Xu, K.; Wu, X. Recent development on nanomaterial-based biosensors for identifying thyroid tumor biomarkers. Biotechnol. Appl. Biochem. 2024, 71, 1329–1338. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Z.; Pan, L.; Wang, Y.; Yang, J.; Gao, Y.; Song, Y. Sucrose-Powered Liposome Nanosensors for Urinary Glucometer-Based Monitoring of Cancer. Angew. Chem. Int. Ed. 2024, 63, e202404493. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, R.; Ahmadzadeh, A.; Wicke, W.; Jamali, V.; Schober, R.; Nasiri-Kenari, M. Early Cancer Detection in Blood Vessels Using Mobile Nanosensors. IEEE Trans. Nanobiosci. 2019, 18, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Kurniawan, D.; Mousavi, S.M.; Fedotov, P.V.; Obraztsova, E.D.; Chiang, W.H. Bioresource-derived colloidal nitrogen-doped graphene quantum dots as ultrasensitive and stable nanosensors for detection of cancer and neurotransmitter biomarkers. J. Mater. Chem. B 2022, 10, 9654–9661. [Google Scholar] [CrossRef] [PubMed]
- Kromer, C.; Katz, A.; Feldmann, I.; Laux, P.; Luch, A.; Tschiche, H.R. A targeted fluorescent nanosensor for ratiometric pH sensing at the cell surface. Sci. Rep. 2024, 14, 12302. [Google Scholar] [CrossRef]
- Premachandran, S.; Haldavnekar, R.; Ganesh, S.; Das, S.; Venkatakrishnan, K.; Tan, B. Self-Functionalized Superlattice Nanosensor Enables Glioblastoma Diagnosis Using Liquid Biopsy. ACS Nano 2023, 17, 19832–19852. [Google Scholar] [CrossRef]
- Gupta, B.; Malviya, R.; Srivastava, S.; Ahmad, I.; Rab, S.O.; Singh, D.P. 3D Printed Nanosensors for Cancer Diagnosis: Advances and Future Perspective. Curr. Pharm. Des. 2024, 30, 2993–3008. [Google Scholar] [CrossRef]
- Ta, H.T.; Arndt, N.; Wu, Y.; Lim, H.J.; Landeen, S.; Zhang, R.; Kamato, D.; Little, P.J.; Whittaker, A.K.; Xu, Z.P. Activatable magnetic resonance nanosensor as a potential imaging agent for detecting and discriminating thrombosis. Nanoscale 2018, 10, 15103–15115. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Zhang, X.; Qi, M.; Zhang, Z.; Zhang, X.E.; Cui, Z. In Vivo Targeting and Imaging of Atherosclerosis Using Multifunctional Virus-Like Particles of Simian Virus 40. Nano Lett. 2016, 16, 6164–6171. [Google Scholar] [CrossRef]
- Ji, J.; Lu, W.; Zhu, Y.; Jin, H.; Yao, Y.; Zhang, H.; Zhao, Y. Porous Hydrogel-Encapsulated Photonic Barcodes for Multiplex Detection of Cardiovascular Biomarkers. ACS Sens. 2019, 4, 1384–1390. [Google Scholar] [CrossRef]
- Li, T.; Feng, Z.Q.; Qu, M.; Yan, K.; Yuan, T.; Gao, B.; Wang, T.; Dong, W.; Zheng, J. Core/Shell Piezoelectric Nanofibers with Spatial Self-Orientated beta-Phase Nanocrystals for Real-Time Micropressure Monitoring of Cardiovascular Walls. ACS Nano 2019, 13, 10062–10073. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, S.; Wang, L.; Zhu, R. A Wearable Sensor Using Structured Silver-Particle Reinforced PDMS for Radial Arterial Pulse Wave Monitoring. Adv. Healthc. Mater. 2019, 8, e1900633. [Google Scholar] [CrossRef]
- Boonyasit, Y.; Chailapakul, O.; Laiwattanapaisal, W. A folding affinity paper-based electrochemical impedance device for cardiovascular risk assessment. Biosens. Bioelectron. 2019, 130, 389–396. [Google Scholar] [CrossRef]
- Ahmadalinezhad, A.; Chen, A. High-performance electrochemical biosensor for the detection of total cholesterol. Biosens. Bioelectron. 2011, 26, 4508–4513. [Google Scholar] [CrossRef]
- Kitchawengkul, N.; Prakobkij, A.; Anutrasakda, W.; Yodsin, N.; Jungsuttiwong, S.; Chunta, S.; Amatatongchai, M.; Jarujamrus, P. Mimicking Peroxidase-Like Activity of Nitrogen-Doped Carbon Dots (N-CDs) Coupled with a Laminated Three-Dimensional Microfluidic Paper-Based Analytical Device (Laminated 3D-muPAD) for Smart Sensing of Total Cholesterol from Whole Blood. Anal. Chem. 2021, 93, 6989–6999. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Zhou, X.; Wang, Y. A paper-based SERS assay for sensitive duplex cytokine detection towards the atherosclerosis-associated disease diagnosis. J. Mater. Chem. B 2020, 8, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Nikelshparg, E.I.; Baizhumanov, A.A.; Bochkova, Z.V.; Novikov, S.M.; Yakubovsky, D.I.; Arsenin, A.V.; Volkov, V.S.; Goodilin, E.A.; Semenova, A.A.; Sosnovtseva, O.; et al. Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors. Biosensors 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.; Rahman, N.; Parikh, R.; Crespo, J.; Cohen, Z.; Williams, R.M. Optical Nanosensor Passivation Enables Highly Sensitive Detection of the Inflammatory Cytokine Interleukin-6. ACS Appl. Mater. Interfaces 2024, 16, 27102–27113. [Google Scholar] [CrossRef]
- Li, P.; Ye, Y.; Li, Y.; Xie, Z.; Ye, L.; Huang, J. A MoS(2) nanosheet-based CRISPR/Cas12a biosensor for efficient miRNA quantification for acute myocardial infarction. Biosens. Bioelectron. 2024, 251, 116129. [Google Scholar] [CrossRef]
- Shi, L.; Liu, C.; Wang, H.; Zheng, J.; Wang, Q.; Shi, L.; Li, T. Framework and Spherical Nucleic Acids Synergistically Enhanced Electrochemiluminescence Nanosensors for Rapidly Diagnosing Acute Myocardial Infarction Based on Circulating MicroRNA Levels. Anal. Chem. 2022, 94, 14394–14401. [Google Scholar] [CrossRef]
- Asl, S.K.; Rahimzadegan, M. The recent progress in the early diagnosis of acute myocardial infarction based on myoglobin biomarker: Nano-aptasensors approaches. J. Pharm. Biomed. Anal. 2022, 211, 114624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, N.; Zhang, W.; Li, P.; Yin, X.; Zhang, W.; Wang, H.; Tang, B. Assessing the Progression of Early Atherosclerosis Mice Using a Fluorescence Nanosensor for the Simultaneous Detection and Imaging of pH and Phosphorylation. Angew. Chem. Int. Ed. 2023, 62, e202215178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Zhao, N.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Ratiometric fluorescence biosensor for imaging of protein phosphorylation levels in atherosclerosis mice. Anal. Chim. Acta 2022, 1208, 339825. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yao, C.; Yang, D.; Liu, D. A functional DNA nanosensor for highly sensitive and selective imaging of ClO(-) in atherosclerotic plaques. Biosens. Bioelectron. 2022, 209, 114273. [Google Scholar] [CrossRef]
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2020, 16, 249–251. [Google Scholar] [CrossRef]
- Qiu, J.D.; Zhou, W.M.; Guo, J.; Wang, R.; Liang, R.P. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta 2010, 80, 2016–2022. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.W.; Choi, H.K.; Lee, T.; Choi, J.W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS(2)/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, W.; Burugapalli, K.; Moussy, F.; Li, Y.L.; Zhong, X.H. Nano-yarn carbon nanotube fiber based enzymatic glucose biosensor. Nanotechnology 2010, 21, 165501. [Google Scholar] [CrossRef]
- Soylemez, S.; Yoon, B.; Toppare, L.; Swager, T.M. Quaternized Polymer-Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor. ACS Sens. 2017, 2, 1123–1127. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, H.; Jiang, Y.; Xu, J.; Li, X.; Zhang, W.; Qiu, F. A molecularly-imprinted-electrochemical-sensor modified with nano-carbon-dots with high sensitivity and selectivity for rapid determination of glucose. Anal. Biochem. 2018, 555, 42–49. [Google Scholar] [CrossRef]
- Yang, L.; Ren, X.; Tang, F.; Zhang, L. A practical glucose biosensor based on Fe(3)O(4) nanoparticles and chitosan/nafion composite film. Biosens. Bioelectron. 2009, 25, 889–895. [Google Scholar] [CrossRef]
- Santhosh, P.; Manesh, K.M.; Uthayakumar, S.; Komathi, S.; Gopalan, A.I.; Lee, K.P. Fabrication of enzymatic glucose biosensor based on palladium nanoparticles dispersed onto poly(3,4-ethylenedioxythiophene) nanofibers. Bioelectrochemistry 2009, 75, 61–66. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, H.; Hou, H.; You, T. Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, G.; Shaghaghi, M.; Alizadeh, P. A novel ultrasensitive and non-enzymatic “turn-on-off” fluorescence nanosensor for direct determination of glucose in the serum: As an alternative approach to the other optical and electrochemical methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Wang, M.; Li, P.; Ding, C.; Zhang, W.; Wang, H.; Tang, B. Copolymer-Based Fluorescence Nanosensor for In Situ Imaging of Homocysteine in the Liver and Kidney of Diabetic Mice. Anal. Chem. 2020, 92, 16221–16228. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Corbalan, J.J.; Jacob, R.F.; Dawoud, H.; Malinski, T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J. Physiol. Pharmacol. 2015, 66, 65–72. [Google Scholar]
- Xu, Y.; Qiu, S.; Tu, W.; Xu, J. Editorial: Molecular biomarkers in the prediction, diagnosis, and prognosis of neurodegenerative diseases. Front. Neurosci. 2023, 17, 1226675. [Google Scholar] [CrossRef]
- Boudries, R.; Williams, H.; Paquereau-Gaboreau, S.; Bashir, S.; Hojjat Jodaylami, M.; Chisanga, M.; Trudeau, L.E.; Masson, J.F. Surface-Enhanced Raman Scattering Nanosensing and Imaging in Neuroscience. ACS Nano 2024, 18, 22620–22647. [Google Scholar] [CrossRef]
- Ranc, V.; Markova, Z.; Hajduch, M.; Prucek, R.; Kvitek, L.; Kaslik, J.; Safarova, K.; Zboril, R. Magnetically assisted surface-enhanced raman scattering selective determination of dopamine in an artificial cerebrospinal fluid and a mouse striatum using Fe(3)O(4)/Ag nanocomposite. Anal. Chem. 2014, 86, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ge, M.; Cao, C.; Lin, D.; Yang, L. High-affinity Fe(3)O(4)/Au probe with synergetic effect of surface plasmon resonance and charge transfer enabling improved SERS sensing of dopamine in biofluids. Analyst 2019, 144, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.H. Label-Free SERS Selective Detection of Dopamine and Serotonin Using Graphene-Au Nanopyramid Heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, P.; Yang, L.; Huang, C.; Li, Y. Facile in Situ Synthesis of Silver Nanoparticles on the Surface of Metal-Organic Framework for Ultrasensitive Surface-Enhanced Raman Scattering Detection of Dopamine. Anal. Chem. 2015, 87, 12177–12182. [Google Scholar] [CrossRef]
- Choi, Y.; Jeon, C.S.; Kim, K.B.; Kim, H.J.; Pyun, S.H.; Park, Y.M. Quantitative detection of dopamine in human serum with surface-enhanced Raman scattering (SERS) of constrained vibrational mode. Talanta 2023, 260, 124590. [Google Scholar] [CrossRef]
- Lu, D.; Fan, M.; Cai, R.; Huang, Z.; You, R.; Huang, L.; Feng, S.; Lu, Y. Silver nanocube coupling with a nanoporous silver film for dual-molecule recognition based ultrasensitive SERS detection of dopamine. Analyst 2020, 145, 3009–3016. [Google Scholar] [CrossRef]
- Phung, V.D.; Jung, W.S.; Nguyen, T.A.; Kim, J.H.; Lee, S.W. Reliable and quantitative SERS detection of dopamine levels in human blood plasma using a plasmonic Au/Ag nanocluster substrate. Nanoscale 2018, 10, 22493–22503. [Google Scholar] [CrossRef]
- Niihori, M.; Foldes, T.; Readman, C.A.; Arul, R.; Grys, D.B.; Nijs, B.; Rosta, E.; Baumberg, J.J. SERS Sensing of Dopamine with Fe(III)-Sensitized Nanogaps in Recleanable AuNP Monolayer Films. Small 2023, 19, e2302531. [Google Scholar] [CrossRef]
- Vander Ende, E.; Bourgeois, M.R.; Henry, A.I.; Chavez, J.L.; Krabacher, R.; Schatz, G.C.; Van Duyne, R.P. Physicochemical Trapping of Neurotransmitters in Polymer-Mediated Gold Nanoparticle Aggregates for Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 9554–9562. [Google Scholar] [CrossRef]
- Dowek, A.; Voisin, F.; Le, L.; Tan, C.; Mallet, J.M.; Carn, F.; Caudron, E. Self-assembly of gold nanoparticles by chitosan for improved epinephrine detection using a portable surface enhanced Raman scattering device. Talanta 2023, 251, 123752. [Google Scholar] [CrossRef]
- Dowek, A.; Berge, M.; Prognon, P.; Legrand, F.X.; Larquet, E.; Tfayli, A.; Le, L.M.M.; Caudron, E. Discriminative and quantitative analysis of norepinephrine and epinephrine by surface-enhanced Raman spectroscopy with gold nanoparticle suspensions. Anal. Bioanal. Chem. 2022, 414, 1163–1176. [Google Scholar] [CrossRef]
- Alsiraey, N.; Dewald, H.D. Nitroxidative stress in human neural progenitor cells: In situ measurement of nitric oxide/peroxynitrite imbalance using metalloporphyrin nanosensors. J. Inorg. Biochem. 2025, 263, 112785. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, Y.; Hong, W.; Dong, Y.; Xie, Z.; Quan, Q. Direct Tracking of Amyloid and Tu Dynamics in Neuroblastoma Cells Using Nanoplasmonic Fiber Tip Probes. Nano Lett. 2016, 16, 3989–3994. [Google Scholar] [CrossRef]
- Song, C.; Deng, P.; Que, L. Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer’s disease in cerebrospinal fluid. Nanomedicine 2018, 14, 1845–1852. [Google Scholar] [CrossRef]
- Kurt, Z.T.; Cimen, D.; Denizli, A.; Bereli, N. Development of Optical-Based Molecularly Imprinted Nanosensors for Adenosine Detection. ACS Omega 2023, 8, 18839–18850. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, W.; Jin, D.; Yu, Y.; Xiao, M.M.; Zhang, Z.Y.; Zhang, G.J. Nanosensor-Driven Detection of Neuron-Derived Exosomal Abeta(42) with Graphene Electrolyte-Gated Transistor for Alzheimer’s Disease Diagnosis. Anal. Chem. 2023, 95, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Antman-Passig, M.; Wong, E.; Frost, G.R.; Cupo, C.; Shah, J.; Agustinus, A.; Chen, Z.; Mancinelli, C.; Kamel, M.; Li, T.; et al. Optical Nanosensor for Intracellular and Intracranial Detection of Amyloid-Beta. ACS Nano 2022, 16, 7269–7283. [Google Scholar] [CrossRef] [PubMed]
- Loewenthal, D.; Kamber, D.; Bisker, G. Monitoring the Activity and Inhibition of Cholinesterase Enzymes using Single-Walled Carbon Nanotube Fluorescent Sensors. Anal. Chem. 2022, 94, 14223–14231. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Wang, Y.; Pan, L.; Lin, P.; Zhang, B.; Zheng, Y.; Xu, Y.; Liao, H.; Ko, G.; et al. A sensitive and specific nanosensor for monitoring extracellular potassium levels in the brain. Nat. Nanotechnol. 2020, 15, 321–330. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, P.; Yin, Y.; Tian, Y. A ratiometric fluorescent DNA nanoprobe for cerebral adenosine triphosphate assay. Chem Commun. 2019, 55, 9955–9958. [Google Scholar] [CrossRef]
- Abakumova, T.; Vaneev, A.; Naumenko, V.; Shokhina, A.; Belousov, V.; Mikaelyan, A.; Balysheva, K.; Gorelkin, P.; Erofeev, A.; Zatsepin, T. Intravital electrochemical nanosensor as a tool for the measurement of reactive oxygen/nitrogen species in liver diseases. J. Nanobiotechnol. 2022, 20, 497. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Z.; Zhao, Z.; Zhu, L.; Tang, M.; Yu, R.; Wang, J. Dual-channel fluorescent signal readout strategy for cysteine sensing. Talanta 2021, 231, 122331. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Evaluating Hyperthyroidism-Induced Liver Injury Based on In Situ Fluorescence Imaging of Glutathione and Phosphate via Nano-MOFs Sensor. Anal. Chem. 2020, 92, 8952–8958. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, Y.; Jin, D.; Xiao, M.M.; Zhang, Z.Y.; Zhang, G.J. Dual-Aptamer Modified Graphene Field-Effect Transistor Nanosensor for Label-Free and Specific Detection of Hepatocellular Carcinoma-Derived Microvesicles. Anal. Chem. 2020, 92, 4006–4015. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, H.; Akhond, M.; Ghorbankhani, G.A.; Absalan, G. Electrochemical sensing of hydrogen peroxide using a glassy carbon electrode modified with multiwalled carbon nanotubes and zein nanoparticle composites: Application to HepG2 cancer cell detection. Mikrochim. Acta 2020, 187, 105. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kukkar, D.; Yadav, A.K. Carbon dot-graphene oxide-based luminescent nanosensor for creatinine detection in human urine. Mikrochim. Acta 2024, 191, 745. [Google Scholar] [CrossRef]
- Anjong, T.F.; Choi, H.; Yoo, J.; Bak, Y.; Cho, Y.; Kim, D.; Lee, S.; Lee, K.; Kim, B.G.; Kim, S. Multifunction-Harnessed Afterglow Nanosensor for Molecular Imaging of Acute Kidney Injury In Vivo. Small 2022, 18, e2200245. [Google Scholar] [CrossRef]
- Hassanain, W.A.; Izake, E.L.; Ayoko, G.A. Spectroelectrochemical Nanosensor for the Determination of Cystatin C in Human Blood. Anal. Chem. 2018, 90, 10843–10850. [Google Scholar] [CrossRef]
- Zhou, D.; Yin, Y.; Zhu, Z.; Gao, Y.; Yang, J.; Pan, Y.; Song, Y. Orally Administered Platinum Nanomarkers for Urinary Monitoring of Inflammatory Bowel Disease. ACS Nano 2022, 16, 18503–18514. [Google Scholar] [CrossRef]
- Nikolaev, V.V.; Lepekhina, T.B.; Alliluev, A.S.; Bidram, E.; Sokolov, P.M.; Nabiev, I.R.; Kistenev, Y.V. Quantum Dot-Based Nanosensors for In Vitro Detection of Mycobacterium tuberculosis. Nanomaterials 2024, 14, 1553. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Zhao, Y.; Li, J.Q.; Vo-Dinh, T. Dual-Modal Colorimetric and Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunoassay for Ultrasensitive Detection of SARS-CoV-2 Using a Plasmonic Gold Nanocrown. Anal. Chem. 2024, 96, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, K.O.; Oyinlola, K.; Achadu, O.J.; Menard, H.; Grillo, F.; Yang, Z.; Adegoke, O. Molecularly Imprinted Viral Protein Integrated Zn-Cu-In-Se-P Quantum Dots Superlattice for Quantitative Ratiometric Electrochemical Detection of SARS-CoV-2 Spike Protein in Saliva. ACS Appl. Nano Mater. 2024, 7, 17630–17647. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Liu, Y.; Zhu, D.; An, W.; Geng, J.; Li, L.; Yu, C.; Wei, J.F. Colorimetric visualization of histamine secreted by basophils based on DSP-functionalized gold nanoparticles. Anal. Methods 2022, 14, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.K.; Rahman, S.; Williams, R.M. Optical Aptamer-Based Cytokine Nanosensor Detects Macrophage Activation by Bacterial Toxins. ACS Sens. 2024, 9, 3697–3706. [Google Scholar] [CrossRef]
- Jin, P.; Wiraja, C.; Zhao, J.; Zhang, J.; Zheng, L.; Xu, C. Nitric Oxide Nanosensors for Predicting the Development of Osteoarthritis in Rat Model. ACS Appl. Mater. Interfaces 2017, 9, 25128–25137. [Google Scholar] [CrossRef]
- Islam, S.; Shukla, S.; Bajpai, V.K.; Han, Y.K.; Huh, Y.S.; Kumar, A.; Ghosh, A.; Gandhi, S. A smart nanosensor for the detection of human immunodeficiency virus and associated cardiovascular and arthritis diseases using functionalized graphene-based transistors. Biosens. Bioelectron. 2019, 126, 792–799. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, H.; Feder-Kubis, J.; Nguyen, D.D. Recent advances in nanobiosensors for sustainable healthcare applications: A systematic literature review. Environ. Res. 2023, 238, 117177. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and their applications in early diagnosis of cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Kim, C.; Kang, M.S.; Raja, I.S.; Oh, J.W.; Joung, Y.K.; Han, D.W. Current issues and perspectives in nanosensors-based artificial olfactory systems for breath diagnostics and environmental exposure monitoring. TrAC Trends Anal. Chem. 2024, 174, 117656. [Google Scholar] [CrossRef]
- Ghorbian, M.; Ghobaei-Arani, M.; Babaei, M.R.; Ghorbian, S. Nanotechnology and nanosensors in personalized healthcare: A comprehensive review. Sens. Bio-Sens. Res. 2025, 47, 100740. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Chapter 9—Nanosensors for medical diagnosis. In Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–213. [Google Scholar] [CrossRef]
- Su, M.; Chen, Y.; Li, Q.; Wei, Y.; Liu, J.; Chang, Z.; Liu, X.; Zhang, A. Temperature compensation model for non-dispersive infrared CO2 gas sensor based on WOA-BP algorithm. Front. Energy Res. 2024, 12, 1407630. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.R.; Grinstaff, M.W. Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chem. Rev. 2012, 112, 1615–1632. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, e1901989. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhou, H.; Xu, S.; Lee, C. Advances in Machine-Learning Enhanced Nanosensors: From Cloud Artificial Intelligence Toward Future Edge Computing at Chip Level. Small Struct. 2024, 5, 2300325. [Google Scholar] [CrossRef]
- Arellano Vidal, C.L.; Govan, J.E. Machine Learning Techniques for Improving Nanosensors in Agroenvironmental Applications. Agronomy 2024, 14, 341. [Google Scholar] [CrossRef]
- Taha, B.A.; Abdulrahm, Z.M.; Addie, A.J.; Haider, A.J.; Alkawaz, A.N.; Yaqoob, I.A.M.; Arsad, N. Advancing optical nanosensors with artificial intelligence: A powerful tool to identify disease-specific biomarkers in multi-omics profiling. Talanta 2025, 287, 127693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).