Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges
Abstract
1. Introduction
2. Fabrication Strategies for Constructing Solid–Solid Interfaces in Hierarchically Structured Anodes
2.1. In Situ Transformation Methods
2.2. Deposition Methods
2.3. Thermal Synthesis Methods
3. The Critical Roles of Solid–Solid Interfaces in DSE Electrodes
3.1. Enhancing Electron Transfer
3.2. Enhancing Mass Transport
3.3. Strengthening Interface Bonding Force
3.4. Enhancing Intrinsic Catalytic Performance
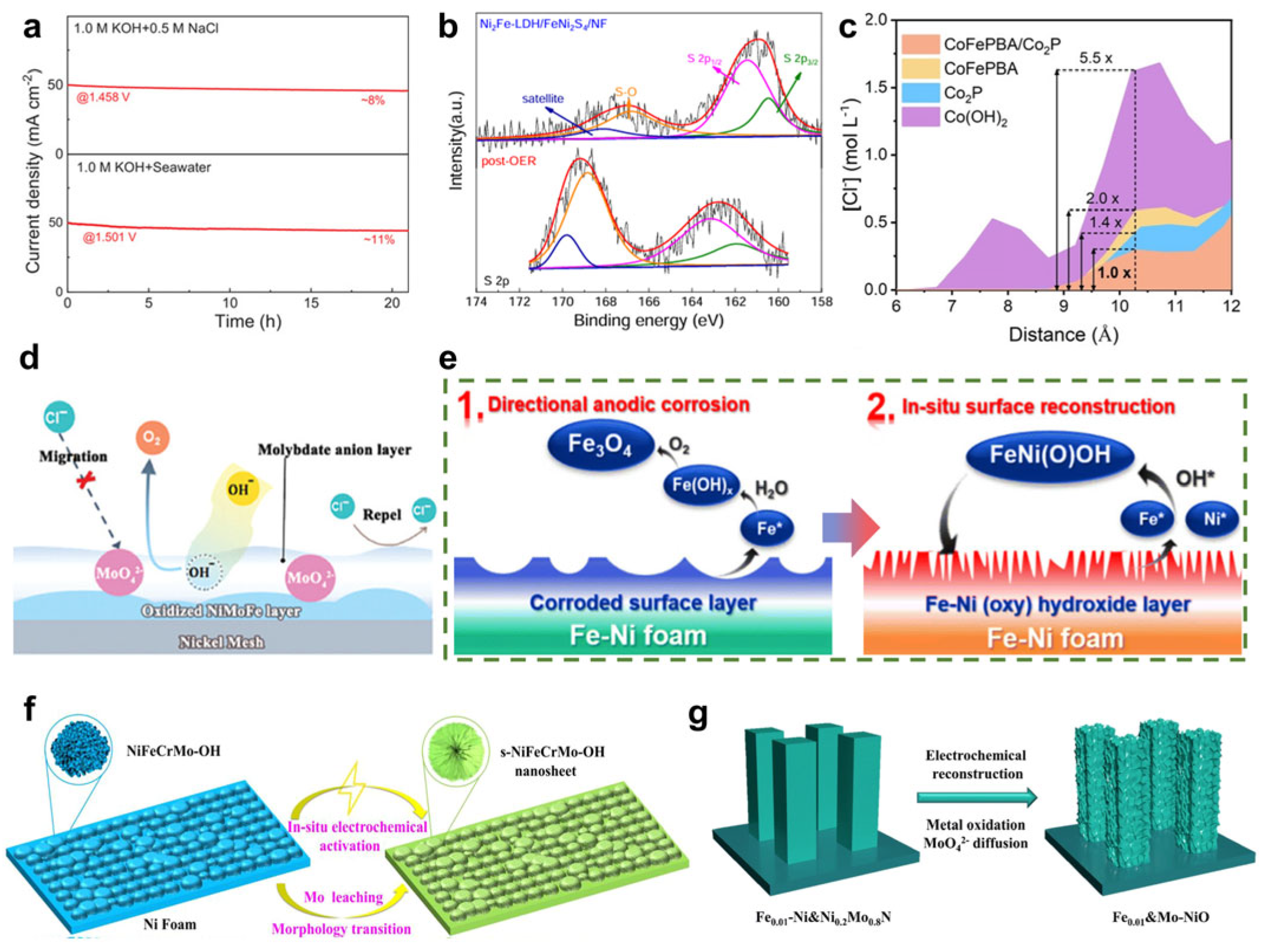
3.5. Chlorine Blocking Effects
3.6. Anti-Scaling Effects
4. Summary and Outlook
- (1)
- Mechanistic insights and strategies for multi-ion corrosion resistance. While most current studies on DSE anodes focus on mitigating electrode corrosion by Cl−, recent reports indicate that bromide ions (Br−) may induce even more severe and mechanistically distinct degradation [127]. Cl− often corrodes to form narrow and deep pits, due to its own rapid diffusion kinetics, while Br− corrodes to form wide and shallow pits, due to its lower reaction energy. The experimental results prove that the corrosion induced by Br− will cause the catalyst layer to peel off from the substrate over a large area, resulting in the rapid deactivation of the anode. Recently, some researchers have proposed solutions to this problem. For example, Fan et al. utilized the electrostatic complexation of biomass-derived polysaccharides to form gradient negative-charge interfaces [128]. This interface achieves a stable operation of 1300 h in natural seawater electrolytes by repelling Br−. Therefore, conventional interfacial designs optimized for Cl− resistance may fall short under real seawater conditions, where multi-ion co-corrosion occurs. Future efforts should focus on developing interface structures that can resist complex ion interactions and on constructing a mechanistic framework for multi-ion corrosion processes [129,130].
- (2)
- Understanding interfacial stress responses in dynamic environments. The integration of seawater electrolysis with intermittent renewable energy sources such as wind or solar power induces dynamic operating conditions [131,132,133]. The fluctuating operating conditions of intermittent renewable energy sources can lead to frequent fluctuations in electrode potential [134], triggering redox cyclic stress and damaging the electrode structure. Recently, Liu et al. placed the seawater electrolysis system on a floating seawater hydrogen production platform and added an energy storage device that achieved a stable energy supply [135]. Although this offers temporary solutions, it also introduces added system complexity and costs. Alternatively, the dynamic repair interface of NiCoP-Cr2O3 designed by Sha et al. can be transformed in situ to form a phosphate passivation layer when the DSE system is turned on, preventing insoluble precipitation in seawater from blocking the cathode. When the DSE system is shut down, the phosphate ions that are converted and adsorbed on the electrode surface electrostatically repel chloride ions in seawater, preventing the electrode from being corroded. Therefore, the synthesized electrode can work stably for 10,000 h under fluctuating working conditions [134]. Advanced interfacial designs that are capable of accommodating stress fluctuations without performance degradation are urgently needed.
- (3)
- Integration of advanced in situ/operando characterization techniques. A variety of in situ and operando tools have emerged as being indispensable for elucidating the dynamic behavior of solid–solid interfaces under DSE conditions. Techniques such as in situ X-ray absorption spectroscopy can track local structural and chemical changes, shedding light on interface formation and evolution. In situ X-ray photoelectron spectroscopy can reveal electronic structure shifts and compositional changes during operation, while in situ transmission electron microscopy and X-ray diffraction enable the visualization of morphological and phase changes in real time. Moreover, in situ Raman spectroscopy captures the dynamics of the surface intermediates involved in interface reconstruction, and in situ EIS quantifies changes in interfacial charge and mass transfer resistance. These complementary techniques provide a multi-dimensional understanding of how solid–solid interfaces function and degrade in realistic DSE environments. Such insights are critical for predicting long-term electrode durability and for guiding the design of more robust, efficient interface architectures in future DSE systems.
- (4)
- Machine learning-assisted interface design. Theoretical modeling and machine learning offer powerful tools for accelerating electrode development [18,136]. By encoding the interfacial properties and material combinations discussed in this review as descriptors, it is possible to construct comprehensive databases for data-driven screening. DFT-based pre-evaluation, such as the successful prediction of the Fe-Ni2Pv/NF system [137], provides a promising pathway for rational electrode design. Integrating these computational approaches with experimental validation will significantly enhance the discovery and optimization of high-performance multilayer electrodes for DSE.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Accelerated durability test |
| CC | Carbon cloth |
| CF | Carbon fiber |
| ClER | Chloride evolution reaction |
| DFT | Density functional theory |
| DOS | Density of states |
| DSE | Direct seawater electrolysis |
| ECSA | Electrochemically active surface area |
| EIS | Electrochemical impedance spectroscopy |
| FT-EXAFS | Frontier-extended X-ray absorption fine structure |
| GDY | Graphdiyne |
| GO | Graphene oxide |
| GQD | Graphene quantum dot |
| LDH | Layered double hydroxide |
| LSV | Linear sweep voltammetry |
| MS | Melamine sponge |
| NF | Nickel foam |
| OER | Oxygen evolution reaction |
| SEM | Scanning electron microscope |
| STEM | Scanning transmission electron microscopy |
| TOF-SIMS | Time-of-flight secondary ion mass spectrometry |
| XPS | X-ray photoelectron spectroscopy |
References
- Kaufman, D.S.; Broadman, E. Revisiting the Holocene global temperature conundrum. Nature 2023, 614, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Emam, A.S.; Hamdan, M.O.; Abu-Nabah, B.A.; Elnajjar, E. A review on recent trends, challenges, and innovations in alkaline water electrolysis. Int. J. Hydrogen Energy 2024, 64, 599–625. [Google Scholar] [CrossRef]
- Tüysüz, H. Alkaline Water Electrolysis for Green Hydrogen Production. Acc. Chem. Res. 2024, 57, 558–567. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nat. Commun. 2022, 612, 673–678. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Fuel Production from Seawater and Fuel Cells Using Seawater. ChemSusChem 2017, 10, 4264–4276. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Z.; Yu, E.; Ye, H.; Li, Z.; Guo, X.; Zhou, D.; Wang, C.; Sha, Q.; et al. A Review of Hydrogen Production via Seawater Electrolysis: Current Status and Challenges. Catalysts 2024, 14, 691. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater electrolysis for hydrogen production: A solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Harvey, C.; Delacroix, S.; Tard, C. Unraveling the competition between the oxygen and chlorine evolution reactions in seawater electrolysis: Enhancing selectivity for green hydrogen production. Electrochim. Acta 2024, 497, 144534. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Attfield, J.P.; Yang, M. Metal nitrides for seawater electrolysis. Chem. Soc. Rev. 2024, 53, 163–203. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.T.; Chen, L.; Yuan, Z.Y. Electrocatalytic seawater splitting from direct electrolysis to hybrid electrolysis: Challenges and opportunities. Mater. Today 2025, 86, 282–316. [Google Scholar] [CrossRef]
- Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent Advances in Seawater Electrolysis. Catalysts 2022, 12, 123. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Li, J.; Fu, G.; Sheng, X.; Li, G.; Chen, H.; Shu, K.; Dong, Y.; Wang, T.; Deng, Y. A comprehensive review on catalysts for seawater electrolysis. Adv. Powder Mater. 2024, 3, 100227. [Google Scholar] [CrossRef]
- Din, M.A.U.; Krishnan, M.R.; Alsharaeh, E.H. Design strategies for cost-effective high-performance electrocatalysts in seawater electrolysis to produce hydrogen. J. Energy Chem. 2025, 102, 497–515. [Google Scholar] [CrossRef]
- Wang, X.H.; Ling, Y.; Wu, B.; Li, B.L.; Li, X.L.; Lei, J.L.; Li, N.B.; Luo, H.Q. Doping modification, defects construction, and surface engineering: Design of cost-effective high-performance electrocatalysts and their application in alkaline seawater splitting. Nano Energy 2021, 87, 106160. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, H.; Wang, D.; Song, J.; Yang, Y. Multifunctional Design of Catalysts for Seawater Electrolysis for Hydrogen Production. Materials 2024, 17, 4057. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xue, Y.; Wu, H.; Chen, S.; Zheng, X.; Xing, C.; Li, Y. Self-Organized Gradually Single-Atom-Layer of Metal Osmium for an Unprecedented Hydrogen Production from Seawater. J. Am. Chem. Soc. 2024, 146, 10573–10580. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Zhang, D.; Zhao, X.; Jiao, X.; Xia, Y.; Chen, D. Polarization Manipulation of NiO Nanosheets Engineered with Fe/Pt Single Atoms for High-Performance Electrocatalytic Overall Alkaline Seawater Splitting. ACS Catal. 2023, 13, 7868–7878. [Google Scholar] [CrossRef]
- Sha, Q.; Shen, J.; Yang, G.; Li, T.; Liu, W.; Kuang, Y.; Sun, X. A single-atom Au catalyst boosts high-efficiency electrochemical seawater oxidation. Catalysts 2024, 14, 348. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Zhang, G.; Wu, S.; Chen, Z.; Ranganathan, H.; Sun, S.; Shi, Z. Mn-doped nickel–iron phosphide heterointerface nanoflowers for efficient alkaline freshwater/seawater splitting at high current densities. Chem. Eng. J. 2023, 454, 140061. [Google Scholar] [CrossRef]
- He, Y.; Hu, Y.; Zhu, Z.; Li, J.; Huang, Y.; Zhang, S.; Balogun, M.S.; Tong, Y. High-performance multidimensional-structured N-doped nickel modulated Mo2N/FeOxNy bifunctional electrocatalysts for efficient alkaline seawater splitting. Chem. Eng. J. 2024, 489, 151348. [Google Scholar] [CrossRef]
- Feng, C.; Zhou, Y.; Chen, M.; Zou, L.; Li, X.; An, X.; Zhao, Q.; Xiaokaiti, P.; Abudula, A.; Yan, K.; et al. High-entropy spinel (FeCoNiMnAl)3O4 with three-dimensional microflower structure for stable seawater oxidation. Appl. Catal. B Environ. 2024, 349, 123875. [Google Scholar] [CrossRef]
- Sun, H.; Sun, J.; Song, Y.; Zhang, Y.; Qiu, Y.; Sun, M.; Tian, X.; Li, C.; Lv, Z.; Zhang, L. Nickel–Cobalt Hydrogen Phosphate on Nickel Nitride Supported on Nickel Foam for Alkaline Seawater Electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 22061–22070. [Google Scholar] [CrossRef]
- Lin, F.; Dong, Z.; Yao, Y.; Yang, L.; Fang, F.; Jiao, L. Electrocatalytic Hydrogen Evolution of Ultrathin Co-Mo5N6 Heterojunction with Interfacial Electron Redistribution. Adv. Energy Mater. 2020, 10, 2002176. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Z.; Jiao, L. Development Strategies in Transition Metal Borides for Electrochemical Water Splitting. Energy Environ. Sci. 2022, 5, 470–485. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Han, X.; Wang, H.; Hua, Y.; Wu, D.; Deng, P.; Xiao, J.; Tian, X.; Li, J. Introducing sulfur to nickel-iron selenide for high-efficiency alkaline seawater electrolysis. Sci. China Chem. 2024, 67, 2747–2754. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Zhang, S.; Cao, Y.; Wang, H.; Han, C.; Sun, J. High Corrosion Resistance of NiFe-Layered Double Hydroxide Catalyst for Stable Seawater Electrolysis Promoted by Phosphate Intercalation. Small 2022, 18, 2203852. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, W.; Jin, H.; Xu, J.; Xi, P.; Dong, J.; Zheng, Y.; Qiao, S.-Z. High-Performance Alkaline Seawater Electrolysis with Anomalous Chloride Promoted Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202311674. [Google Scholar]
- Bamba, J.N.; Dumlao, A.T.; Lazaro, R.M.; Matienzo, D.J.D.; Ocon, J. Green hydrogen from seawater electrolysis: Recent developments and future perspectives. Curr. Opin. Electrochem. 2024, 48, 101592. [Google Scholar] [CrossRef]
- Bahuguna, G.; Patolsky, F. Routes to Avoiding Chlorine Evolution in Seawater Electrolysis: Recent Perspective and Future Directions. ACS Mater. Lett. 2024, 6, 3202–3217. [Google Scholar] [CrossRef]
- Zhang, R.; Zhai, T.; Wang, H.; Lu, S. Recent Advances in High-Performance Direct Seawater Electrolysis for “Green” Hydrogen. Adv. Energy Sust. Res. 2024, 5, 2400085. [Google Scholar] [CrossRef]
- Li, P.; Zhao, S.; Huang, Y.; Huang, Q.; Xi, B.; An, X.; Xiong, S. Corrosion Resistant Multilayered Electrode Comprising Ni3N Nanoarray Overcoated with NiFe-Phytate Complex for Boosted Oxygen Evolution in Seawater Electrolysis. Adv. Energy Mater. 2024, 14, 2303360. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.; Li, J.; Fan, L.; Liu, Z.; Shi, J.; Cai, W. Surface-growing organophosphorus layer on layered double hydroxides enables boosted and durable electrochemical freshwater/seawater oxidation. Appl. Catal. B Environ. 2023, 332, 122749. [Google Scholar] [CrossRef]
- Wang, J.; Tran, D.T.; Chang, K.; Prabhakaran, S.; Zhao, J.; Kim, D.H.; Kim, N.H.; Lee, J.H. Hierarchical Ni@CNTs-bridged MoxC/Ni2P heterostructure micro-pillars for enhanced seawater splitting and Mg/seawater battery. Nano Energy 2023, 111, 108440. [Google Scholar] [CrossRef]
- Chen, L.; Yu, C.; Dong, J.; Han, Y.; Huang, H.; Li, W.; Zhang, Y.; Tan, X.; Qiu, J. Seawater electrolysis for fuels and chemicals production: Fundamentals, achievements, and perspectives. Chem. Soc. Rev. 2024, 53, 7455–7488. [Google Scholar] [CrossRef]
- Xia, C.; Wang, H.; Kim, J.K.; Wang, J. Rational Design of Metal Oxide-Based Heterostructure for Efficient Photocatalytic and Photoelectrochemical Systems. Adv. Funct. Mater. 2021, 31, 2008247. [Google Scholar] [CrossRef]
- Wang, B.; Cao, J.-T.; Liu, Y.-M. Recent progress of heterostructure-based photoelectrodes in photoelectrochemical biosensing: A mini review. Analyst 2020, 145, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, D.; Qiao, F.; Liu, C.; Wang, J.; Zhang, Y.; Wang, J.; Zhang, Q.; Notten, P.H.L. Heterostructured transition metal chalcogenides with strategic heterointerfaces for electrochemical energy conversion/Storage. Coord. Chem. Rev. 2023, 496, 215405. [Google Scholar] [CrossRef]
- Li, W.; Song, Q.; Li, M.; Yuan, Y.; Zhang, J.; Wang, N.; Yang, Z.; Huang, J.; Lu, J.; Li, X. Chemical Heterointerface Engineering on Hybrid Electrode Materials for Electrochemical Energy Storage. Small Methods 2021, 5, 2100444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nie, K.; Yi, L.; Li, B.; Yuan, Y.; Liu, Z.; Huang, W. Recent Advances in Engineering of 2D Materials-Based Heterostructures for Electrochemical Energy Conversion. Adv. Sci. 2023, 10, 2302301. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Yu, P.-C.; Sun, S.-P.; Shi, L.; Yang, P.-P.; Wu, Z.-Z.; Chi, L.-P.; Zheng, Y.-R.; Gao, M.-R. In situ ammonium formation mediates efficient hydrogen production from natural seawater splitting. Nat. Commun. 2024, 15, 9462. [Google Scholar] [CrossRef]
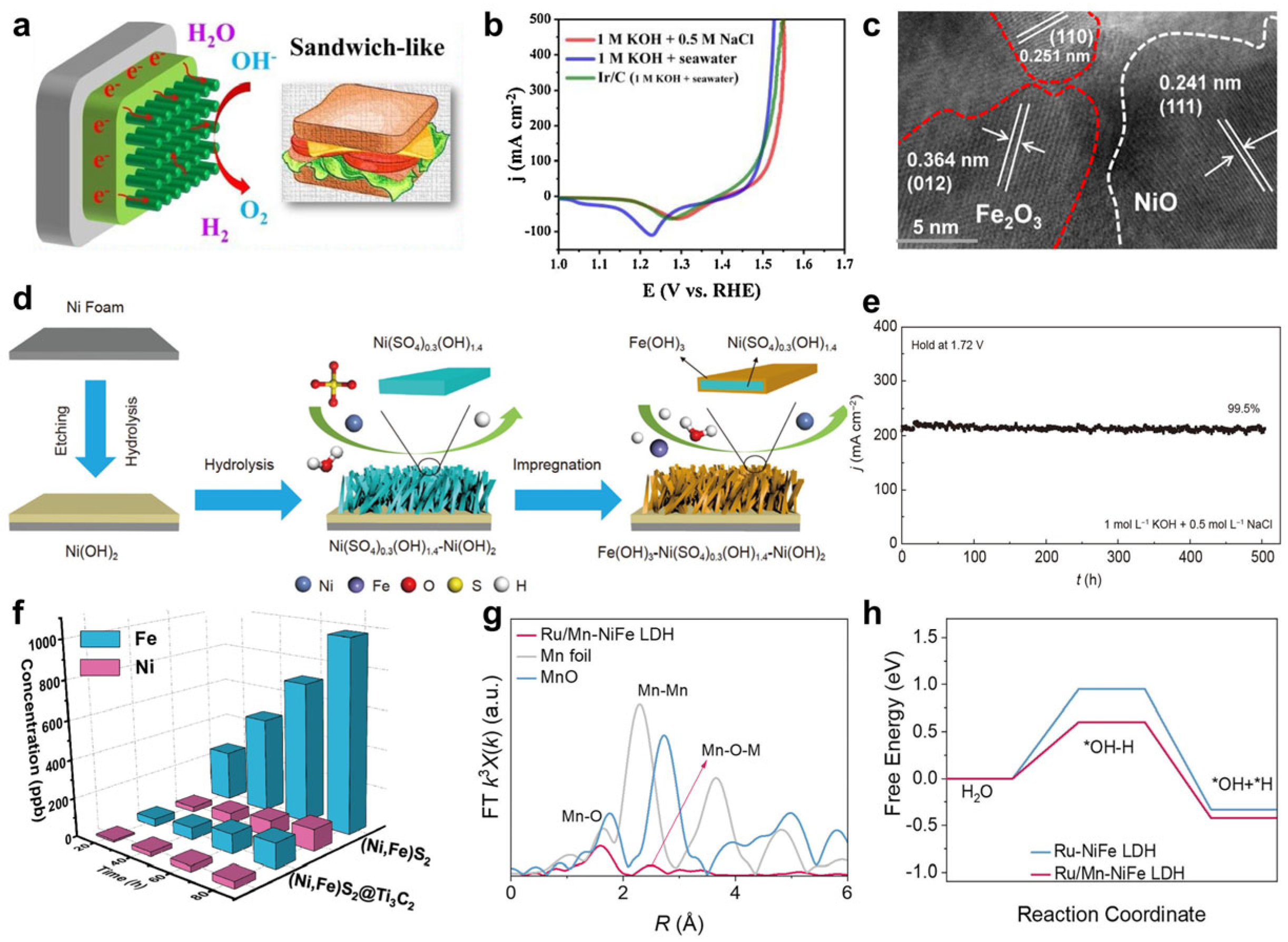
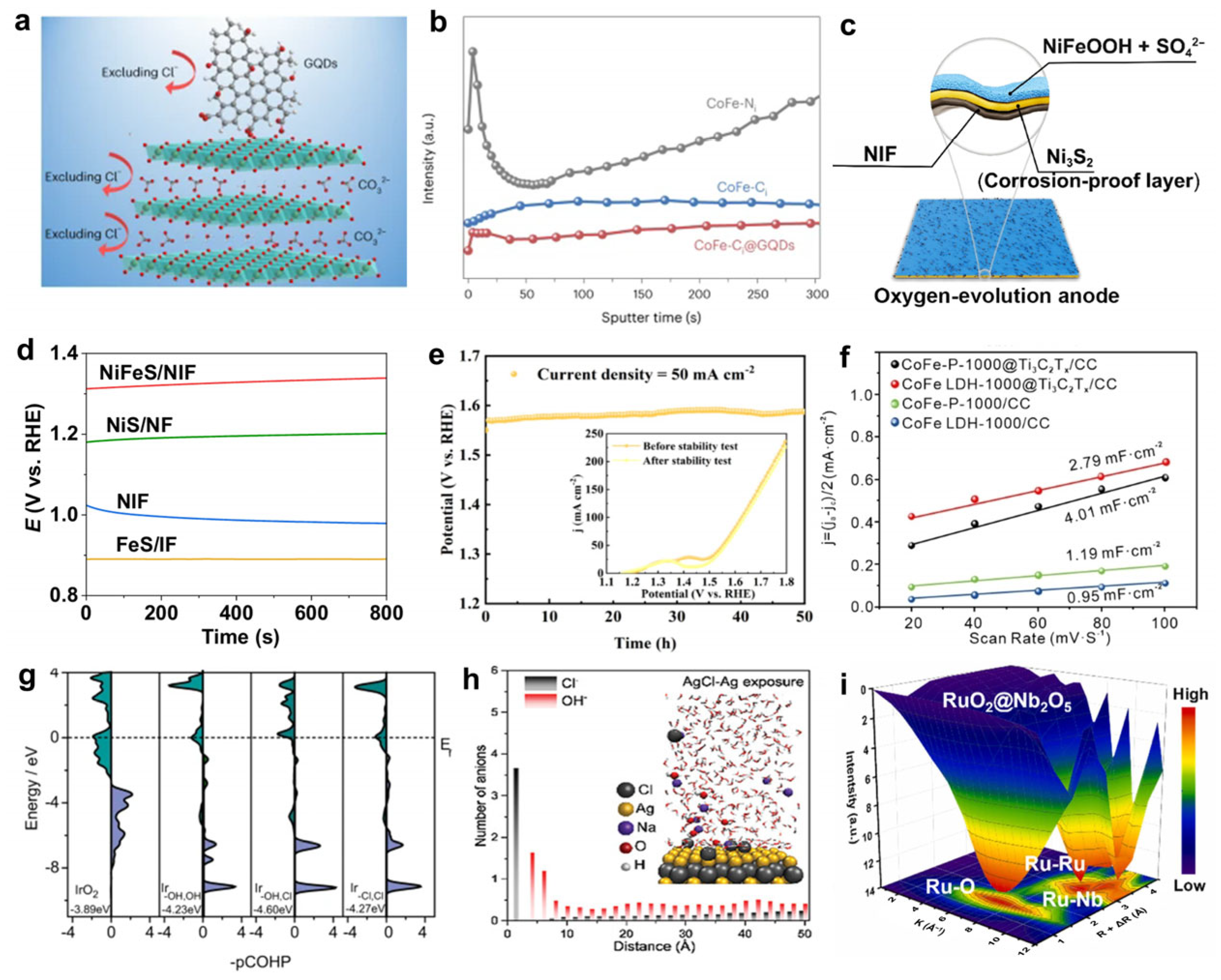
- Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z. The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes. Angew. Chem. 2021, 133, 22922–22926. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Liu, F.; Liu, J.; Xu, W.; Hu, H.; Chen, X.; Wang, W.; Cheng, F. Anionic formulation of electrolyte additive towards stable electrocatalytic oxygen evolution in seawater splitting. J. Energy Chem. 2022, 72, 361–369. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Menezes, P.W. Effect of surface-adsorbed and intercalated (oxy) anions on the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202207279. [Google Scholar] [CrossRef]
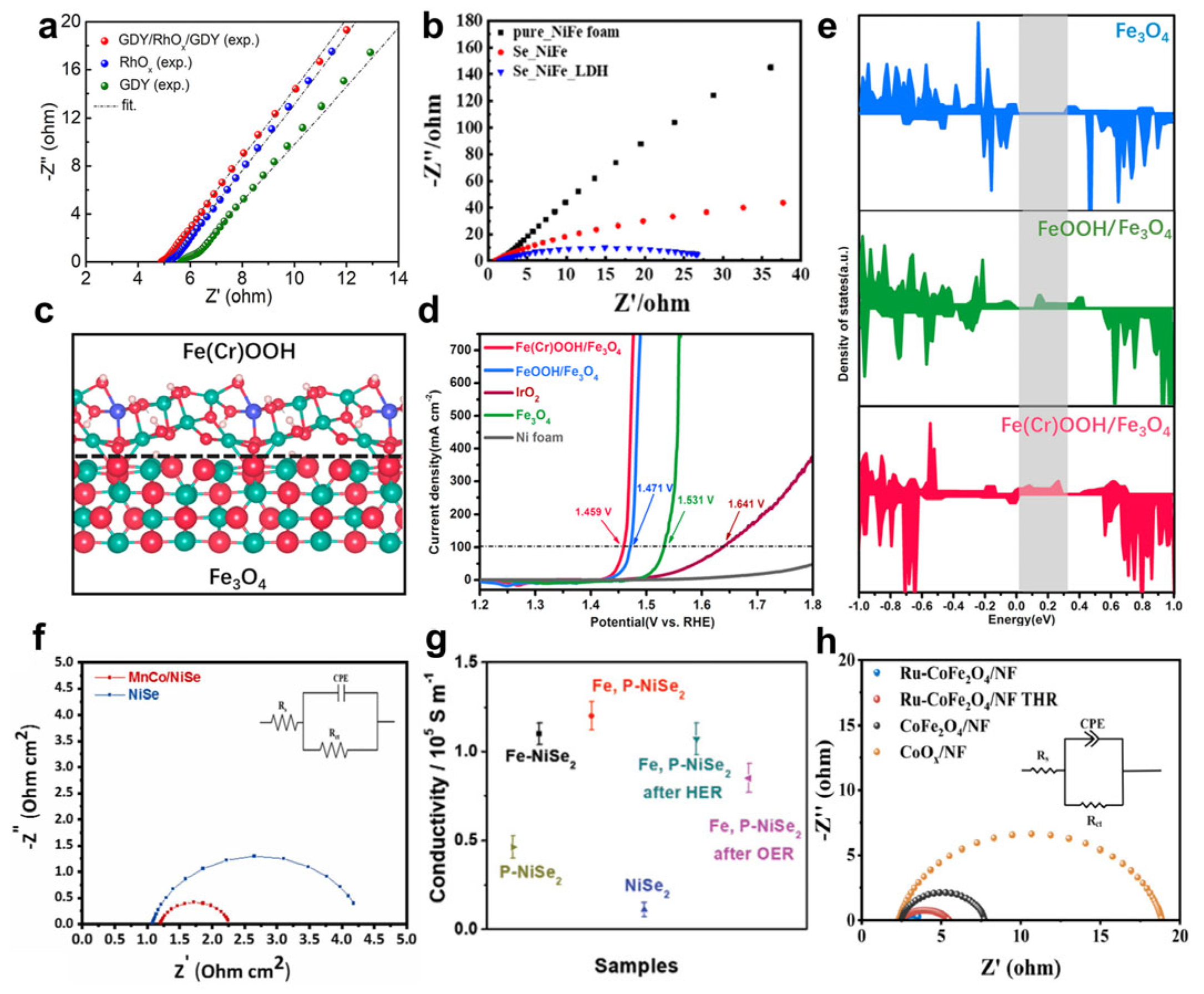
- Shao, L.; Han, X.; Shi, L.; Wang, T.; Zhang, Y.; Jiang, Z.; Yin, Z.; Zheng, X.; Li, J.; Han, X.; et al. In Situ Generation of Molybdate-Modulated Nickel-Iron Oxide Electrodes with High Corrosion Resistance for Efficient Seawater Electrolysis. Adv. Energy Mater. 2024, 14, 2303261. [Google Scholar] [CrossRef]
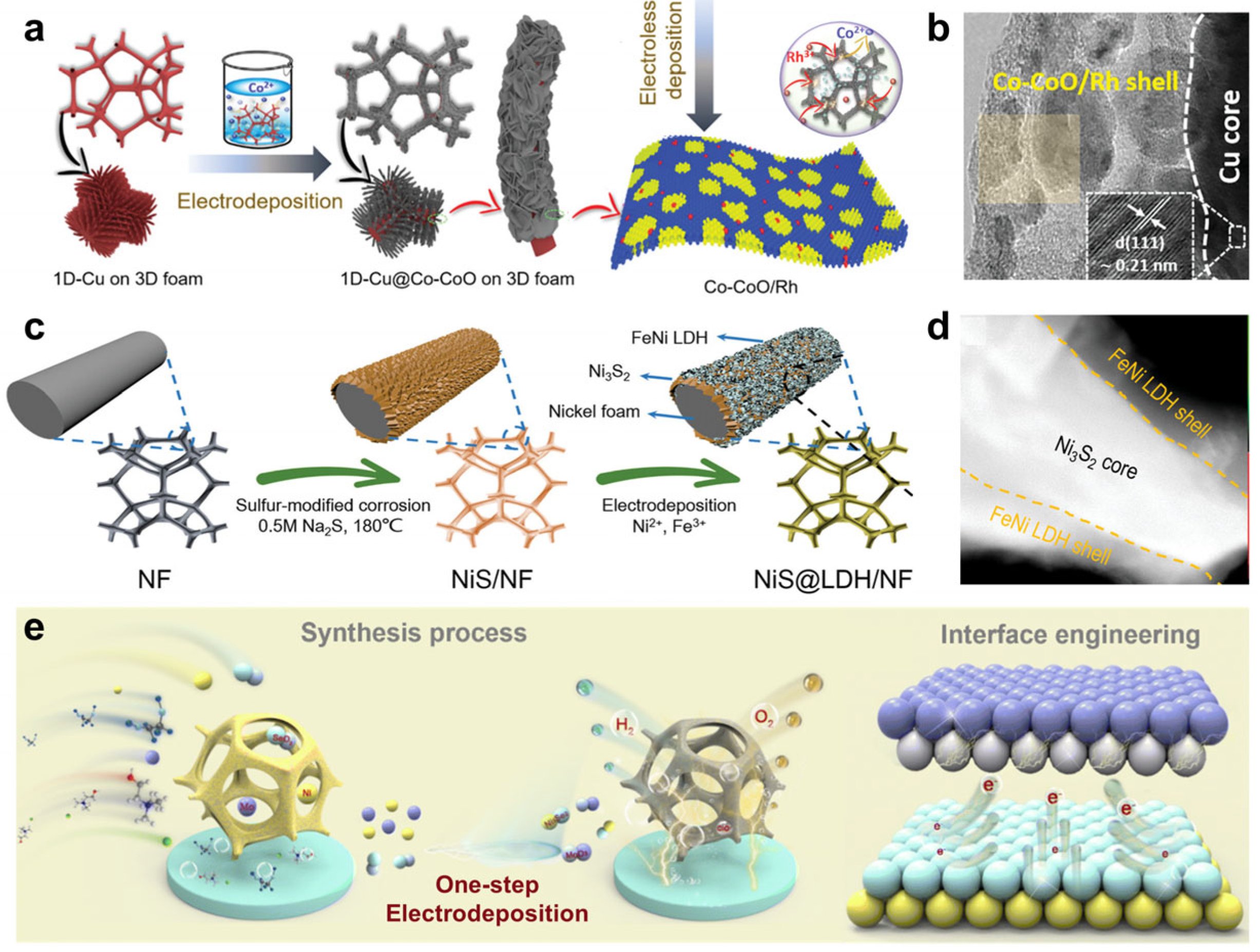
- Tan, L.; Yu, J.; Wang, C.; Wang, H.; Liu, X.; Gao, H.; Xin, L.; Liu, D.; Hou, W.; Zhan, T. Partial Sulfidation Strategy to NiFe-LDH@FeNi2S4 Heterostructure Enable High-Performance Water/Seawater Oxidation. Adv. Funct. Mater. 2022, 32, 2200951. [Google Scholar] [CrossRef]
- Liu, W.; Yu, J.; Sendeku, M.G.; Li, T.; Gao, W.; Yang, G.; Kuang, Y.; Sun, X. Ferricyanide armed anodes enable stable water oxidation in saturated saline water at 2 A/cm2. Angew. Chem. Int. Ed. 2023, 135, e202309882. [Google Scholar]
- Fan, R.-Y.; Zhang, X.-Y.; Yu, N.; Wang, F.-G.; Zhao, H.-Y.; Liu, X.; Lv, Q.-X.; Liu, D.-P.; Chai, Y.-M.; Dong, B. Rapid “self-healing” behavior induced by chloride anions to renew the Fe–Ni(oxy)hydroxide surface for long-term alkaline seawater electrolysis. Inorg. Chem. Front. 2022, 9, 4216–4224. [Google Scholar] [CrossRef]
- Pan, S.; Li, R.; Wang, J.; Zhang, Q.; Wang, M.; Shi, B.; Wang, P.; Zhao, Y.; Zhang, X. Floating Seawater Splitting Device Based on NiFeCrMo Metal Hydroxide Electrocatalyst and Perovskite/Silicon Tandem Solar Cells. ACS Nano 2023, 17, 4539–4550. [Google Scholar] [CrossRef]
- Ning, M.; Zhang, F.; Wu, L.; Xing, X.; Wang, D.; Song, S.; Zhou, Q.; Yu, L.; Bao, J.; Chen, S.; et al. Boosting efficient alkaline fresh water and seawater electrolysis via electrochemical reconstruction. Energy Environ. Sci. 2022, 15, 3945–3957. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Zhang, C.; Liu, F.; Wang, L.; Xu, G.-R. Self-supported electrocatalysts for high-current-density water/seawater electrolysis. J. Alloys Compd. 2023, 968, 172286. [Google Scholar] [CrossRef]
- Tran, P.K.L.; Tran, D.T.; Malhotra, D.; Prabhakaran, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Highly Effective Freshwater and Seawater Electrolysis Enabled by Atomic Rh-Modulated Co-CoO Lateral Heterostructures. Small 2021, 17, 2103826. [Google Scholar] [CrossRef]
- Ren, J.-T.; Chen, L.; Tian, W.-W.; Song, X.-L.; Kong, Q.-H.; Wang, H.-Y.; Yuan, Z.-Y. Rational Synthesis of Core-Shell-Structured Nickel Sulfide-Based Nanostructures for Efficient Seawater Electrolysis. Small 2023, 19, 2300194. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tan, L.; Liu, X.; Wen, Y.; Hou, W.; Zhan, T. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J. Colloid Interface Sci. 2022, 613, 349–358. [Google Scholar] [CrossRef]
- Feng, S.; Gu, C.; Yu, Y.; Rao, P.; Deng, P.; Li, J.; Kang, Z.; Tian, X.; Wu, Z. A two-dimensional heterogeneous structured Ni3Se2@MoO3 catalyst for seawater electrolysis. J. Mater. Chem. A 2023, 11, 11740–11747. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Getaye Sendeku, M.; Zhang, X.; Xu, L.; Wang, Z.; Wang, S.; Duan, X.; Liu, H.; Liu, W.; et al. Phosphate-decorated Ni3Fe-LDHs@CoPx nanoarray for near-neutral seawater splitting. Chem. Eng. J. 2023, 460, 141413. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.-Z. Stable and Highly Efficient Hydrogen Evolution from Seawater Enabled by an Unsaturated Nickel Surface Nitride. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, L.; Zhou, H.; Li, D.; Tang, D.; Yu, F. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Mater. Today Phys. 2022, 26, 100727. [Google Scholar] [CrossRef]
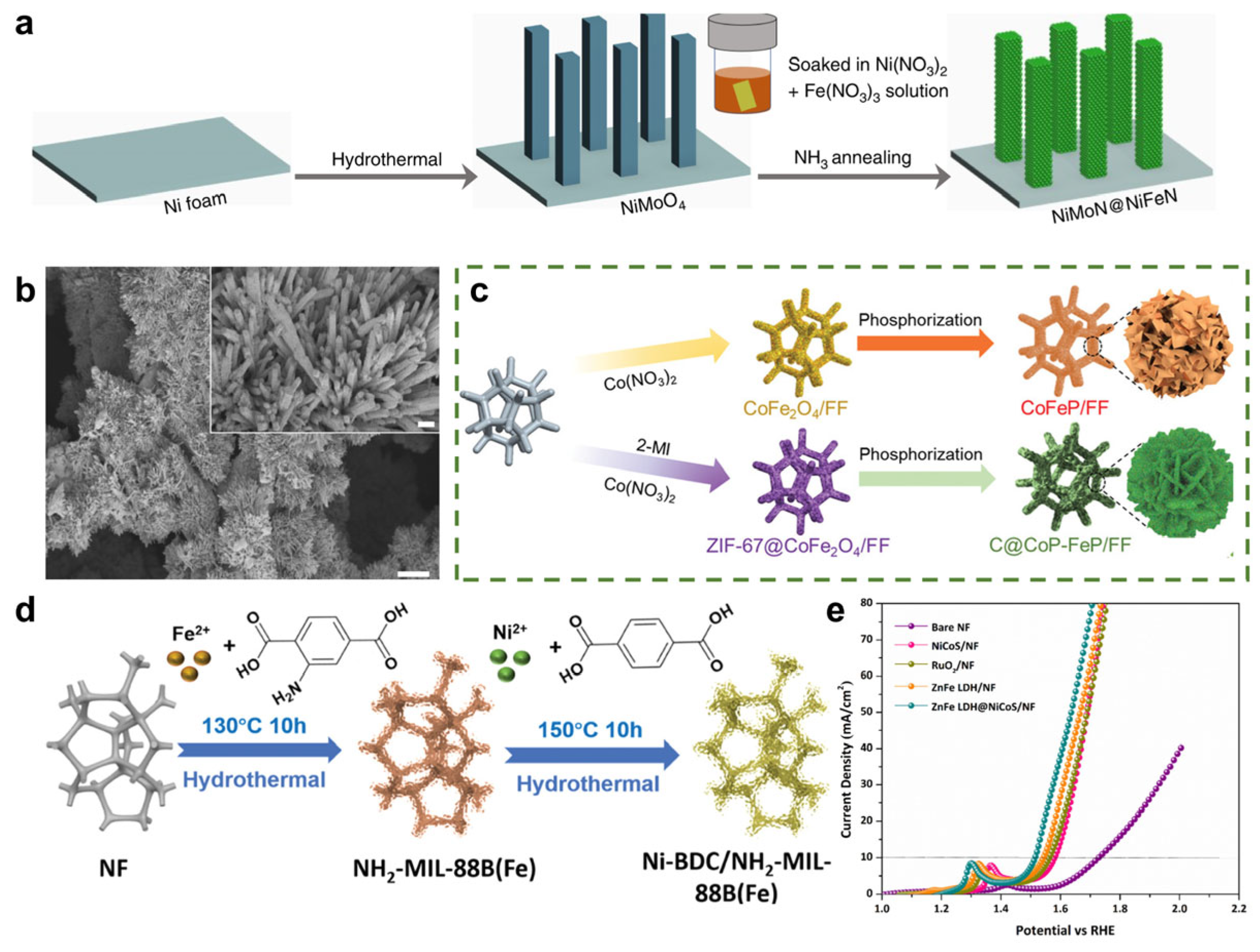
- Li, J.; Hu, Y.; Huang, X.; Zhu, Y.; Wang, D. Bimetallic Phosphide Heterostructure Coupled with Ultrathin Carbon Layer Boosting Overall Alkaline Water and Seawater Splitting. Small 2023, 19, 2206533. [Google Scholar] [CrossRef]
- Bao, Y.; Ru, H.; Wang, Y.; Zhang, K.; Yu, R.; Wu, Q.; Yu, A.; Li, D.-S.; Sun, C.; Li, W.; et al. Hetero MOF-On-MOF of Ni-BDC/NH2-MIL-88B(Fe) Enables Efficient Electrochemical Seawater Oxidation. Adv. Funct. Mater. 2024, 34, 2314611. [Google Scholar] [CrossRef]
- Vedanarayanan, M.; Chen, C.-M.; Sethuraman, M.G. Efficient Hydrogen and Oxygen Evolution: Dual-Functional Electrocatalyst of Zinc Iron Layered Double Hydroxides and Nickel Cobalt Sulfides on Nickel Foam for Seawater Splitting. ACS Appl. Energy Mater. 2024, 7, 7260–7271. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, X.; Liu, Y.; Li, S.; Guan, W.; Du, Z.; Ai, W. Stabilizing Lithium-Metal Host Anodes by Covalently Binding MgF2 Nanodots to Honeycomb Carbon Nanofibers. ACS Appl. Mater. Interfaces 2024, 16, 4530–4539. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, B.; Li, D. Partial-sacrificial-template Synthesis of Fe/Ni Phosphides on Ni Foam: A Strongly Stabilized and Efficient Catalyst for Electrochemical Water Splitting. Electrochim. Acta 2017, 242, 260–267. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Sun, H.; Zhou, T.; Chen, X.; Na, G.; Qiu, G.; Li, D.; Yang, N.; Zheng, H.; et al. Coupling Ir single atom with NiFe LDH/NiMo heterointerface toward efficient and durable water splitting at large current density. Appl. Catal. B Environ. 2025, 360, 124548. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jin, B.; Vasileff, A.; Jiao, Y.; Qiao, S.-Z. Interfacial nickel nitride/sulfide as a bifunctional electrode for highly efficient overall water/seawater electrolysis. J. Mater. Chem. A 2019, 7, 8117–8121. [Google Scholar] [CrossRef]
- Kasani, A.; Maric, R.; Bonville, L.; Bliznakov, S. Catalysts for Direct Seawater Electrolysis: Current Status and Future Prospectives. ChemElectroChem 2024, 11, e202300743. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Tranca, D.C.; Rodríguez-Hernández, F.; Seifert, G.; Zhuang, X. Theoretical models for hydrogen evolution reaction at combined Mo2C and N—doped graphene. J. Catal. 2020, 381, 234–247. [Google Scholar] [CrossRef]
- Song, H.J.; Yoon, H.; Ju, B.; Lee, D.-Y.; Kim, D.-W. Electrocatalytic Selective Oxygen Evolution of Carbon-Coated Na2Co1–xFexP2O7 Nanoparticles for Alkaline Seawater Electrolysis. ACS Catal. 2020, 10, 702–709. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Qiu, H.; Tao, Y.; Yin, J.; Li, J.; Wang, W.; Li, Z. Synthesis of Ni nanoparticles/N-doped carbon sheets for freshwater and seawater electrolysis. Fuel 2024, 371, 131995. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Zhang, Q.; Chen, S.; Wu, Y.; Sun, Z. Fe-Doped and Carbon Composite Multiphase Hetero-structured Catalysts Based on the Ion-Exchange Strategy for Seawater Electrolysis. ACS Sustain. Chem. Eng. 2023, 11, 15338–15349. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; He, F.; Li, Y. Controlled growth of a high selectivity interface for seawater electrolysis. Proc. Natl. Acad. Sci. USA 2022, 119, e2206946119. [Google Scholar] [CrossRef]
- Hung, W.H.; Xue, B.Y.; Lin, T.M.; Lu, S.Y.; Tsao, I.Y. A highly active selenized nickel–iron electrode with layered double hydroxide for electrocatalytic water splitting in saline electrolyte. Mater. Today Energy 2021, 19, 100575. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, S. Constructing the Fe/Cr double (oxy)hydroxides on Fe3O4 for boosting the electrochemical oxygen evolution in alkaline seawater and domestic sewage. Appl. Catal. B Environ. 2022, 302, 120847. [Google Scholar] [CrossRef]
- Andaveh, R.; Sabour Rouhaghdam, A.; Ai, J.; Maleki, M.; Wang, K.; Seif, A.; Barati Darband, G.; Li, J. Boosting the electrocatalytic activity of NiSe by introducing MnCo as an efficient heterostructured electrocatalyst for large-current-density alkaline seawater splitting. Appl. Catal. B Environ. 2023, 325, 122355. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G.; et al. Dual-Doping and Synergism toward High-Performance Seawater Electrolysis. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef]
- Zeng, Y.-T.; Xu, M.-Y.; Wang, T.; Wu, S.-Y.; Zhang, J.; Mu, S.-C.; Yu, J. Ru-decorated cobalt-iron oxide nanosheet arrays derived from MOF and LDH double-precursors for overall water splitting in alkali and seawater. Electrochim. Acta 2023, 444, 142004. [Google Scholar] [CrossRef]
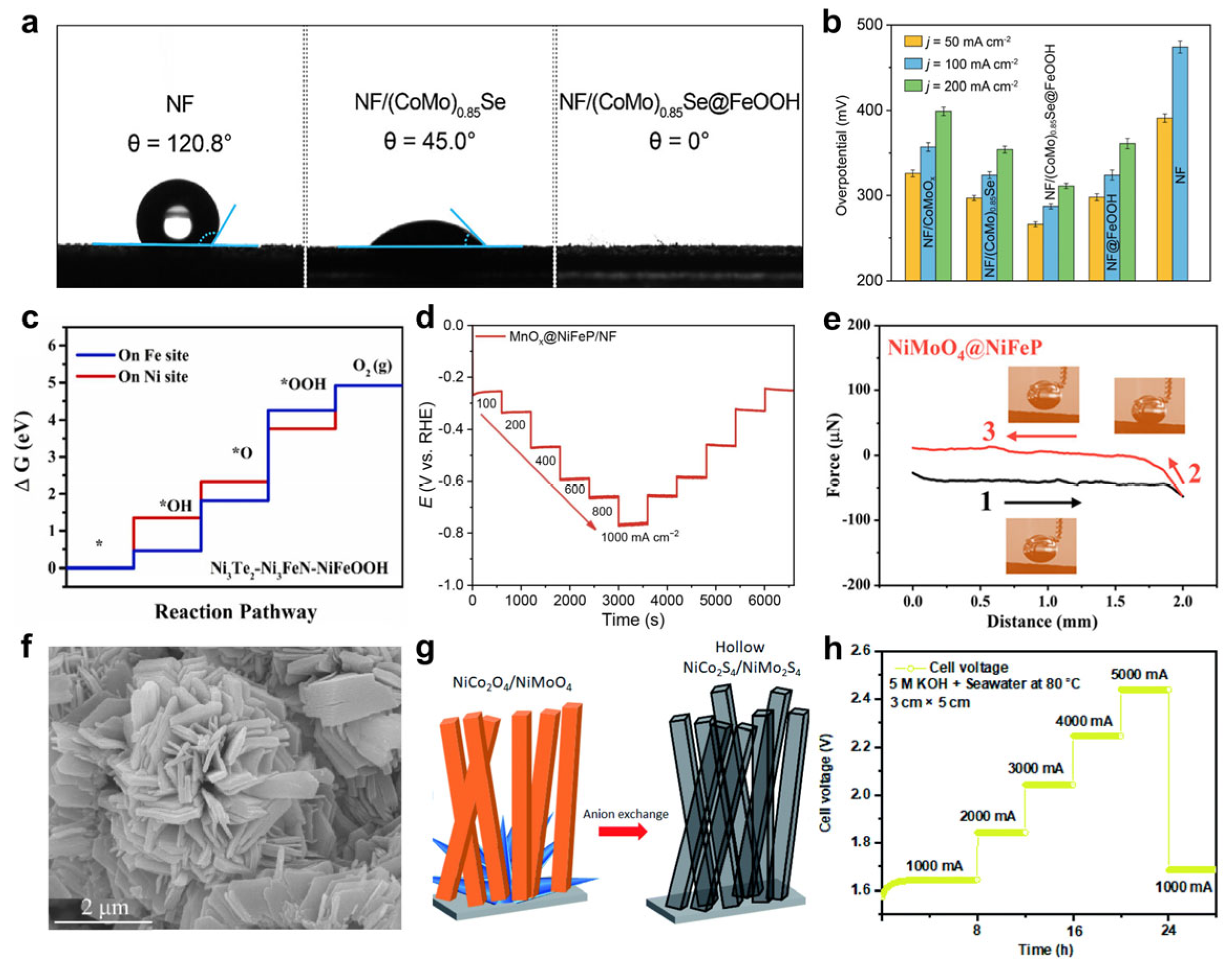
- Li, P.; Zhao, S.; Huang, Y.; Huang, Q.; Yang, Y.; Yang, H. Multiscale Structural Engineering of a Multilayered Nanoarray Electrode Realizing Boosted and Sustained Oxygen Evolution Catalysis in Seawater Electrolysis. ACS Catal. 2023, 13, 15360–15374. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Xu, R.; Li, Y.; Xue, J.; Zhang, J.; et al. Synergistic interface engineering and structural optimization of non-noble metal telluride-nitride electrocatalysts for sustainably overall seawater electrolysis. Appl. Catal. B Environ. 2022, 318, 121834. [Google Scholar] [CrossRef]
- Tang, X.; Yang, N.; Li, Z.; Cai, Z.; Dai, Q.; Wang, H.; He, X.; Yao, Y.; Li, T.; Guo, J.; et al. NiFe-based arrays with manganese dioxide enhance chloride blocking for durable alkaline seawater oxidation. J. Colloid Interface Sci. 2025, 684, 64–72. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Z.; Zong, M.-Y.; Fan, C.; Zheng, W.; Wang, D. Engineered Superhydrophilic/Superaerophobic Array Electrode Composed of NiMoO4@NiFeP for High-Performance Overall Water/Seawater Splitting. ACS Sustain. Chem. Eng. 2023, 11, 8362–8373. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Chen, Y.; Zhang, X.; Xiao, H.; Zhao, M.; Zhao, C.; Ma, X.; Hu, T.; Luo, E.; et al. In-situ fabrication of bimetallic FeCo2O4-FeCo2S4 heterostructure for high-efficient alkaline freshwater/seawater electrolysis. J. Colloid Interface Sci. 2024, 653, 821–832. [Google Scholar] [CrossRef]
- Seenivasan, S.; Kim, D.-H. Engineering the surface anatomy of an industrially durable NiCo2S4/NiMo2S4/NiO bifunctional electrode for alkaline seawater electrolysis. J. Mater. Chem. A 2022, 10, 9547–9564. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Chen, W.; Lu, Z.; Xu, J.; Pei, A.; Peng, Y.; Zheng, X.; Zhang, Z.; Chu, S.; et al. Breathing-Mimicking Electrocatalysis for Oxygen Evolution and Reduction. Joule 2019, 3, 557–569. [Google Scholar] [CrossRef]
- Liu, G.; Wong, W.S.Y.; Kraft, M.; Ager, J.W.; Vollmer, D.; Xu, R. Wetting-regulated gas-involving (photo)electrocatalysis: Biomimetics in energy conversion. Chem. Soc. Rev. 2021, 50, 10674–10699. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhou, D.; Shi, B.; Chen, F.; Luo, L.; Kumar, A.; Wang, C.; Lin, X.; Sheng, S.; Xu, W.; et al. Understanding of Dynamic Contacting Behaviors of Underwater Gas Bubbles on Solid Surfaces. Langmuir 2020, 36, 11422–11428. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Luo, Y.; Cui, Z.; Yu, Q.; Gao, Z.; Zhang, Z.; Yang, F.; Kang, X.; Ge, S.; et al. Dual interfacial engineering of a Chevrel phase electrode material for stable hydrogen evolution at 2500 mA cm−2. Nat. Commun. 2022, 13, 6382. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Li, J.; Zhang, K.; Xia, Y.; Hui, B.; Yang, D. Wood aerogel-derived sandwich-like layered nanoelectrodes for alkaline overall seawater electrosplitting. Appl. Catal. B Environ. 2021, 293, 120215. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Zhu, D.; Liu, D.; Liu, Y.; Yang, S. Fe2O3/NiO Interface for the Electrochemical Oxygen Evolution in Seawater and Domestic Sewage. ACS Appl. Mater. Interfaces 2021, 13, 37152–37161. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Y.; Liang, H.-P. Divalent anion intercalation and etching-hydrolysis strategies to construct ultra-stable electrodes for seawater splitting. Sci. China Chem. 2024, 67, 687–695. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Yang, G.; Jiao, Y.; Dong, Y.; Tian, C.; Yan, H.; Fu, H. MXene-Assisted NiFe sulfides for high-performance anion exchange membrane seawater electrolysis. Nat. Commun. 2025, 16, 1319. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.-Q.; Ding, R.; Zeng, Z.-W.; Liu, B.-W.; Zeng, F.-R.; Wang, Y.-Z.; Zhao, H.-B. Satellite-like shielding for dual single-atom catalysis, boosting ampere-level alkaline seawater splitting. Matter 2024, 7, 3189–3204. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Duan, D.; Liu, X.; Li, R.; Wang, J.; Chen, H.; Chen, S.-W.; Wu, Y.; Wang, H.; et al. Empowering multicomponent alloys with unique nanostructure for exceptional oxygen evolution performance through self-replenishment. Joule 2024, 8, 2920–2937. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Zhang, H.; Hu, Y.; Li, C. Heterogeneous interface engineered atomic configuration on ultrathin Ni(OH)2/Ni3S2 nanoforests for efficient water splitting. Appl. Catal. B Environ. 2019, 242, 60–66. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Li, S.; Zhou, L.; Li, Z.; Li, J.; Shao, M. Interface engineering of (Ni, Fe)S2@MoS2 heterostructures for synergetic electrochemical water splitting. Appl. Catal. B Environ. 2019, 247, 107–114. [Google Scholar] [CrossRef]
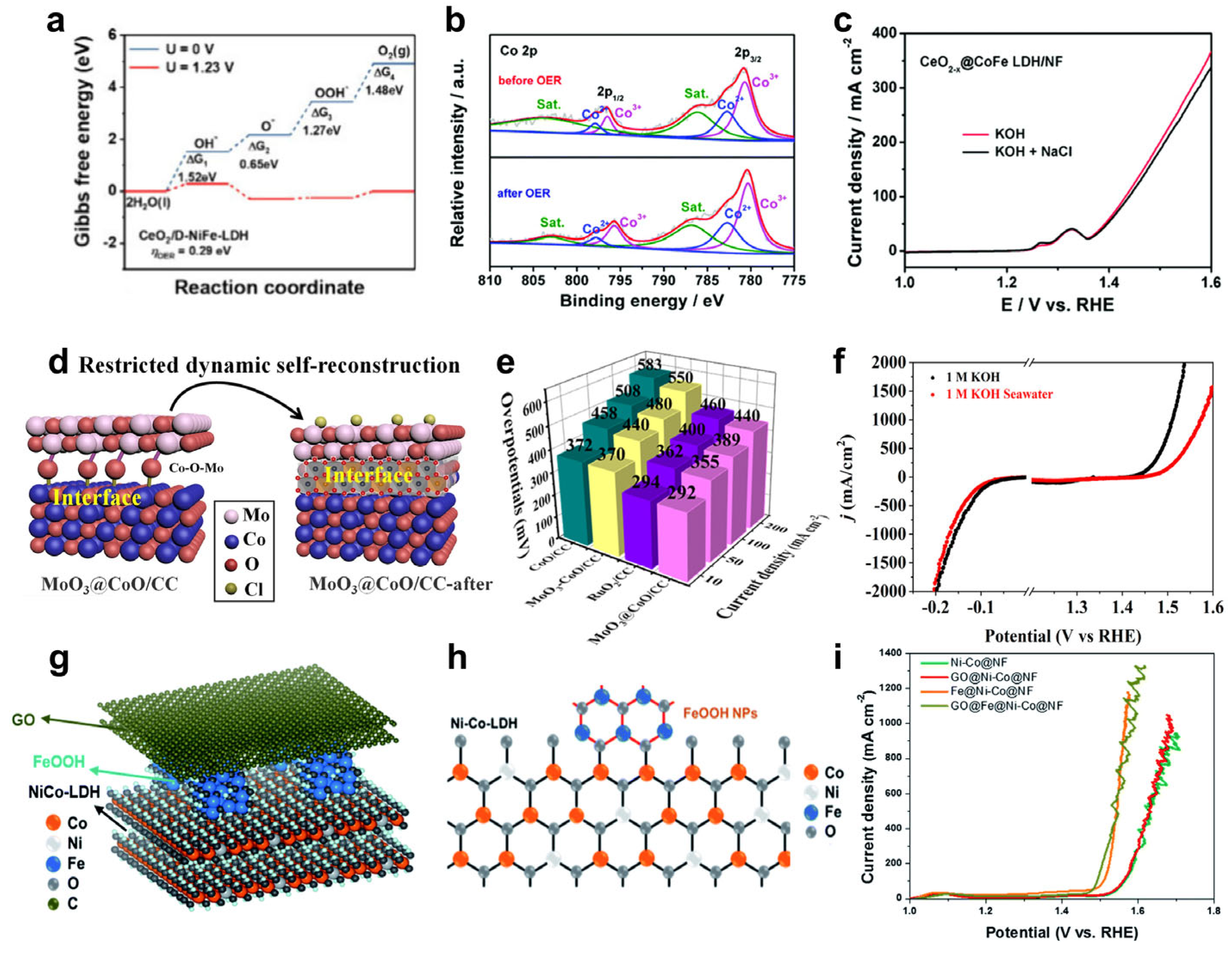
- Wang, Z.; Wang, L.; Chu, L.; Yang, M.; Wang, G. CeO2 Nanoparticles Anchored in Cation-Vacancies NiFe-LDH toward Efficient Oxygen Evolution Reactions in Green Sustainable Seawater Electrolysis. ACS Sustain. Chem. Eng. 2024, 12, 11628–11637. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Jiang, K.; Xu, L.; Guan, M.; Bao, J.; Ji, H.; Li, H. Constructing a CeO2−x@CoFe-layered double hydroxide heterostructure as an improved electrocatalyst for highly efficient water oxidation. Inorg. Chem. Front. 2020, 7, 4461–4468. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, D.; Wu, L.; Guan, Z.; Zou, C.; Jin, H.; Fang, G.; Chen, X.a.; Wang, S. A restricted dynamic surface self-reconstruction toward high-performance of direct seawater oxidation. Nat. Commun. 2024, 15, 2481. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Yu, F.; Pang, H.; Zhou, X.; Li, D.; Ma, W.; Zhou, Q.; Mo, Y.; Zhou, H. Engineering Multilevel Collaborative Catalytic Interfaces with Multifunctional Iron Sites Enabling High-Performance Real Seawater Splitting. ACS Nano 2023, 17, 1681–1692. [Google Scholar] [CrossRef]
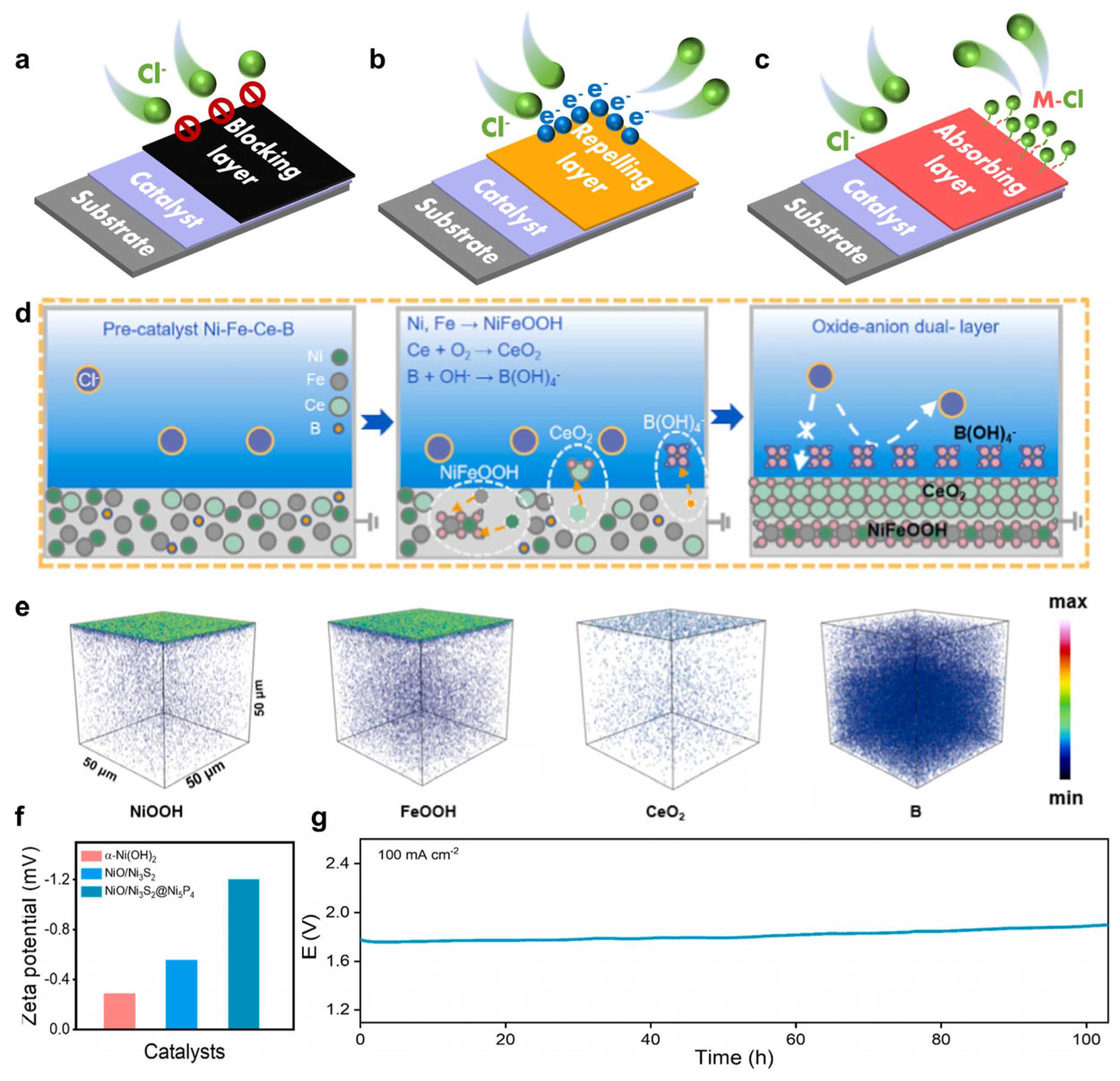
- Jadhav, A.R.; Kumar, A.; Lee, J.; Yang, T.; Na, S.; Lee, J.; Luo, Y.; Liu, X.; Hwang, Y.; Liu, Y.; et al. Stable complete seawater electrolysis by using interfacial chloride ion blocking layer on catalyst surface. J. Mater. Chem. A 2020, 8, 24501–24514. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Ye, C.; Ye, M.; Shen, J. In-situ constructing oxide-anion dual-layer on Ce-B-containing electrode electrolyte interface towards highly corrosive seawater splitting. Appl. Catal. B Environ. 2024, 343, 123560. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, X.; Li, J.; Xiao, Y.; Shi, X.; Rao, P.; Deng, P.; Wen, H.; Tian, X. Ni-based heterostructure with protective phosphide layer to enhance the oxygen evolution reaction for the seawater electrolysis. Int. J. Hydrogen Energy 2024, 51, 1373–1380. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrogen Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Liu, C.; Li, Z.; Huang, H.; Feng, J.; Li, Z.; Zou, Z. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nat. Sustain. 2024, 7, 158–167. [Google Scholar] [CrossRef]
- Han, Y.; Shao, L.; Liu, Y.; Li, G.; Wang, T.; Zheng, X.; Li, J.; Han, X.; Hu, W.; Deng, Y. Sulfate-assisted Ni/Fe-based electrodes for anion exchange membrane saline splitting. Nano Res. 2024, 17, 5985–5995. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Gao, Y.; Li, J.; Sun, J.; Wang, D.; Wang, L.; Meng, Y. Enhancing electronic communication in amorphous-crystalline NiCoS-CeOx heterostructure for efficient overall water/simulated seawater electrolysis. Fuel 2024, 375, 132652. [Google Scholar] [CrossRef]
- Song, S.; Xia, M.; Feng, Y.; Zhang, X. Synergistic Coupling Effect and Anionic Modulation of CoFe LDH@MXene for Triggered and Sustained Alkaline Water/Seawater Electrolysis. Chem.-Asian J. 2025, 20, e202401295. [Google Scholar] [CrossRef]
- Duan, X.; Sha, Q.; Li, P.; Li, T.; Yang, G.; Liu, W.; Yu, E.; Zhou, D.; Fang, J.; Chen, W.; et al. Dynamic chloride ion adsorption on single iridium atom boosts seawater oxidation catalysis. Nat. Commun. 2024, 15, 1973. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, P.; Tang, X.; Zhang, S.; Chen, H.; Yang, Q.; Chen, X.; Tian, Z.; Dai, S.; et al. Ag Nanoparticle-Induced Surface Chloride Immobilization Strategy Enables Stable Seawater Electrolysis. Adv. Mater. 2024, 36, 2306062. [Google Scholar] [CrossRef]
- Feng, S.; Li, G.; Wei, Q.; Wang, T.; Hua, Y.; Li, J.; Wang, W.; Ling, P.; Wu, D.; Yuan, Y.; et al. Hard Lewis acid induced chloride repulsion for durable neutral seawater electrolysis. Nano Energy 2025, 136, 110714. [Google Scholar] [CrossRef]
- Deng, P.-J.; Xue, R.; Lu, J.; Tsiakaras, P. Strategies for Designing Anti-Chlorine Corrosion Catalysts in Seawater Splitting. Adv. Energy Mater. 2025, 15, 2405749. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Z.; Zhang, K.; Du, Z.; Fang, H.; Chen, L.; Jiang, H.; Wang, M.; Wu, M. Comprehensive Chlorine Suppression: Advances in Materials and System Technologies for Direct Seawater Electrolysis. Nano-Micro Lett. 2025, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, X.; Lu, J.; Wang, X.; Liu, K.; Cai, Y.; Qi, Y.; Wang, L.; Ai, H.; Wang, Z. Efficient and Ultrastable Seawater Electrolysis at Industrial Current Density with Strong Metal-Support Interaction and Dual Cl−-Repelling Layers. Adv. Mater. 2024, 36, 2408982. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cai, Z.; He, X.; Luo, Y.; Zheng, D.; Sun, S.; Liu, Q.; Li, L.; Chu, W.; Alfaifi, S.; et al. Electroreduction of alkaline/natural seawater: Self-cleaning Pt/carbon cathode and on-site co-synthesis of H2 and Mg hydroxide nanoflakes. Chem 2024, 10, 3067–3087. [Google Scholar] [CrossRef]
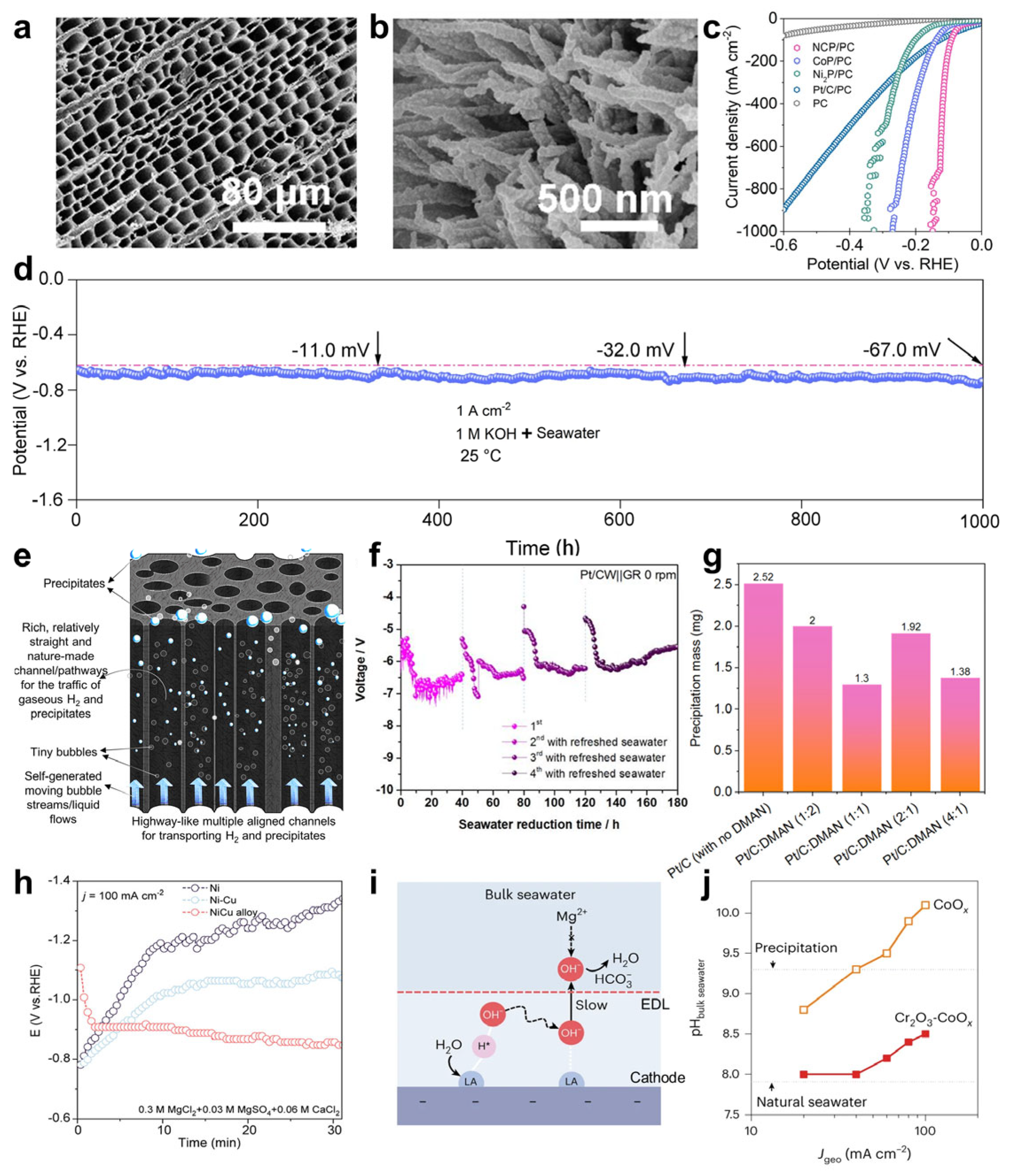
- Liang, J.; Cai, Z.; Li, Z.; Yao, Y.; Luo, Y.; Sun, S.; Zheng, D.; Liu, Q.; Sun, X.; Tang, B. Efficient bubble/precipitate traffic enables stable seawater reduction electrocatalysis at industrial-level current densities. Nat. Commun. 2024, 15, 2950. [Google Scholar] [CrossRef]
- Yi, L.; Chen, X.; Wen, Y.; Chen, H.; Zhang, S.; Yang, H.; Li, W.; Zhou, L.; Xu, B.; Xu, W.; et al. Solidophobic Surface for Electrochemical Extraction of High-Valued Mg(OH)2 Coupled with H2 Production from Seawater. Nano Lett. 2024, 24, 5920–5928. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Li, S.; Wang, Z.; Chen, H.; Yi, L.; Chen, X.; Yang, Q.; Xu, W.; Wang, A.; et al. Concerning the stability of seawater electrolysis: A corrosion mechanism study of halide on Ni-based anode. Nat. Commun. 2023, 14, 4822. [Google Scholar] [CrossRef]
- Fan, W.; Zhu, C.; Wang, X.; Wang, H.; Zhu, Y.; Chen, J.; Tian, W.; Wu, J.; Yu, G. All-natural charge gradient interface for sustainable seawater zinc batteries. Nat. Commun. 2025, 16, 1273. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Fornasiero, P.; Tian, G.; Strasser, P.; Yang, X.Y. Long-term Durability of Seawater Electrolysis for Hydrogen: From Catalysts to Systems. Angew. Chem. Int. Ed. 2024, 136, e202412087. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Chen, Z.; Chen, X.; Wang, J.; Wei, S.; Liu, S.; Wang, Z.; Dai, F.; Wang, M. Self-Adapting Oxyanion Armor Achieves Highly Stable and Efficient Seawater Electrolysis at Ampere-Level Current Densities. Adv. Funct. Mater. 2024, 35, 2418940. [Google Scholar] [CrossRef]
- Temiz, M.; Dincer, I. Development of solar and wind based hydrogen energy systems for sustainable communities. Energy Convers. Manag. 2022, 269, 116090. [Google Scholar] [CrossRef]
- Okunlola, A.; Davis, M.; Kumar, A. The development of an assessment framework to determine the technical hydrogen production potential from wind and solar energy. Renew. Sustain. Energy Rev. 2022, 166, 112610. [Google Scholar] [CrossRef]
- Shi, X.; Qian, Y.; Yang, S. Fluctuation Analysis of a Complementary Wind–Solar Energy System and Integration for Large Scale Hydrogen Production. ACS Sustain. Chem. Eng. 2020, 8, 7097–7110. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, S.; Yan, L.; Feng, Y.; Zhang, Z.; Li, S.; Guo, X.; Li, T.; Li, H.; Zhuang, Z.; et al. 10,000-h-stable intermittent alkaline seawater electrolysis. Nature 2025, 639, 360–367. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Tang, W.; Chen, Y.; Lan, C.; Zhu, L.; Jiang, W.; Wu, Y.; Wang, Y.; Yang, Z.; et al. In-situ direct seawater electrolysis using floating platform in ocean with uncontrollable wave motion. Nat. Commun. 2024, 15, 5305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, W.; Peng, Y.; Guo, Y.; Zhou, J.; Wang, S.; Yuan, J.; Liao, Y.; Liu, L.; Zhang, Y.; et al. Corrosion-resistant single-atom catalysts for direct seawater electrolysis. Natl. Sci. Rev. 2025, 12, nwaf060. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Q.; Qu, X.; Wang, X.; Chi, J.; Wang, L. Manipulating Electron Redistribution in Ni2P for Enhanced Alkaline Seawater Electrolysis. Adv. Mater. 2024, 36, 2307395. [Google Scholar] [CrossRef]
| Sample | Preparation Cost (USD) | |
|---|---|---|
| In situ transformation methods | Ni2Fe-LDH/FeNi2S4/NF | 1.2545 |
| CoFePBA/Co2P | 1.3402 | |
| NiMoFe/NM | 0.0401 | |
| Deposition methods | Cu@Co-CoO/Rh | 5.6913 |
| NiS@FeNi/NF | 0.1606 | |
| S-NiMoO4@NiFe-LDH/NF | 0.4861 | |
| Thermal synthesis methods | NiMoN@NiFeN/NF | 1.0064 |
| C@CoP-FeP/FF | 0.9154 | |
| ZnFe LDH@NiCoS | 1.2967 | |
| Sample | Ref. | Overpotential (mV @ 100 mA/cm2) | Tafel Slope (mV/dec) | Durability (h) | |
|---|---|---|---|---|---|
| In situ transformation methods | Ni2Fe-LDH/FeNi2S4/NF | [51] | 261 | 55.9 | >20 @ 50 mA/cm2 |
| CoFePBA/Co2P | [52] | 297 | 43.5 | >1000 @ 1000 mA/cm2 | |
| NiMoFe/NM | [50] | 296 | 52 | >1500 @ 100 mA/cm2 | |
| Deposition methods | Cu@Co-CoO/Rh | [57] | ~440 | 124.8 | >12 @ 10 mA/cm2 |
| NiS@FeNi/NF | [58] | 258 | Not mentioned | >100 @ 200 mA/cm2 | |
| S-NiMoO4@NiFe-LDH/NF | [59] | 273 | 90 | >20 @ 60 mA/cm2 | |
| Ni3Se2@MoO3/CF | [60] | 280 | 74.7 | >200 @ 100 mA/cm2 | |
| Thermal synthesis methods | NiMoN@NiFeN/NF | [64] | 286 | 58.6 | >100 @ 500 mA/cm2 |
| C@CoP-FeP/FF | [66] | 297 | 59.09 | >28 @ 100 mA/cm2 | |
| Ni-BDC/NH2-MIL-888(Fe) | [67] | 299 | 66.8 | >28 @ 330 mA/cm2 | |
| ZnFe LDH@NiCoS | [68] | >460 | 85.7 | >50 @ 10 mA/cm2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Wu, T.; Dong, Y.; Zhan, Y.; Wei, F.; Zhang, D.; Long, X. Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges. Inorganics 2025, 13, 183. https://doi.org/10.3390/inorganics13060183
Zhou B, Wu T, Dong Y, Zhan Y, Wei F, Zhang D, Long X. Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges. Inorganics. 2025; 13(6):183. https://doi.org/10.3390/inorganics13060183
Chicago/Turabian StyleZhou, Bowei, Tong Wu, Yilin Dong, Yinbo Zhan, Fei Wei, Dongliang Zhang, and Xia Long. 2025. "Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges" Inorganics 13, no. 6: 183. https://doi.org/10.3390/inorganics13060183
APA StyleZhou, B., Wu, T., Dong, Y., Zhan, Y., Wei, F., Zhang, D., & Long, X. (2025). Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges. Inorganics, 13(6), 183. https://doi.org/10.3390/inorganics13060183







