Synthesis, Characterization, and Biological Evaluation of Some 3d Metal Complexes with 2-Benzoylpyridine 4-Allylthiosemicarbazone
Abstract
1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Synthesis
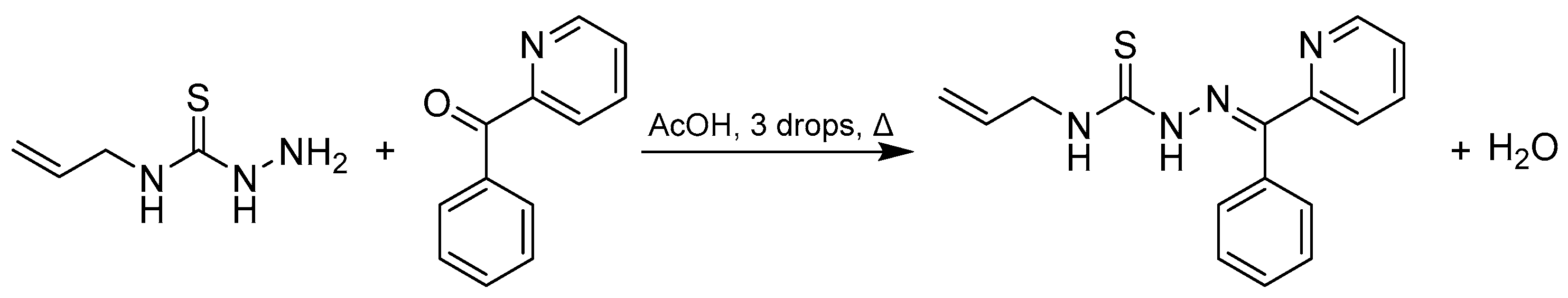
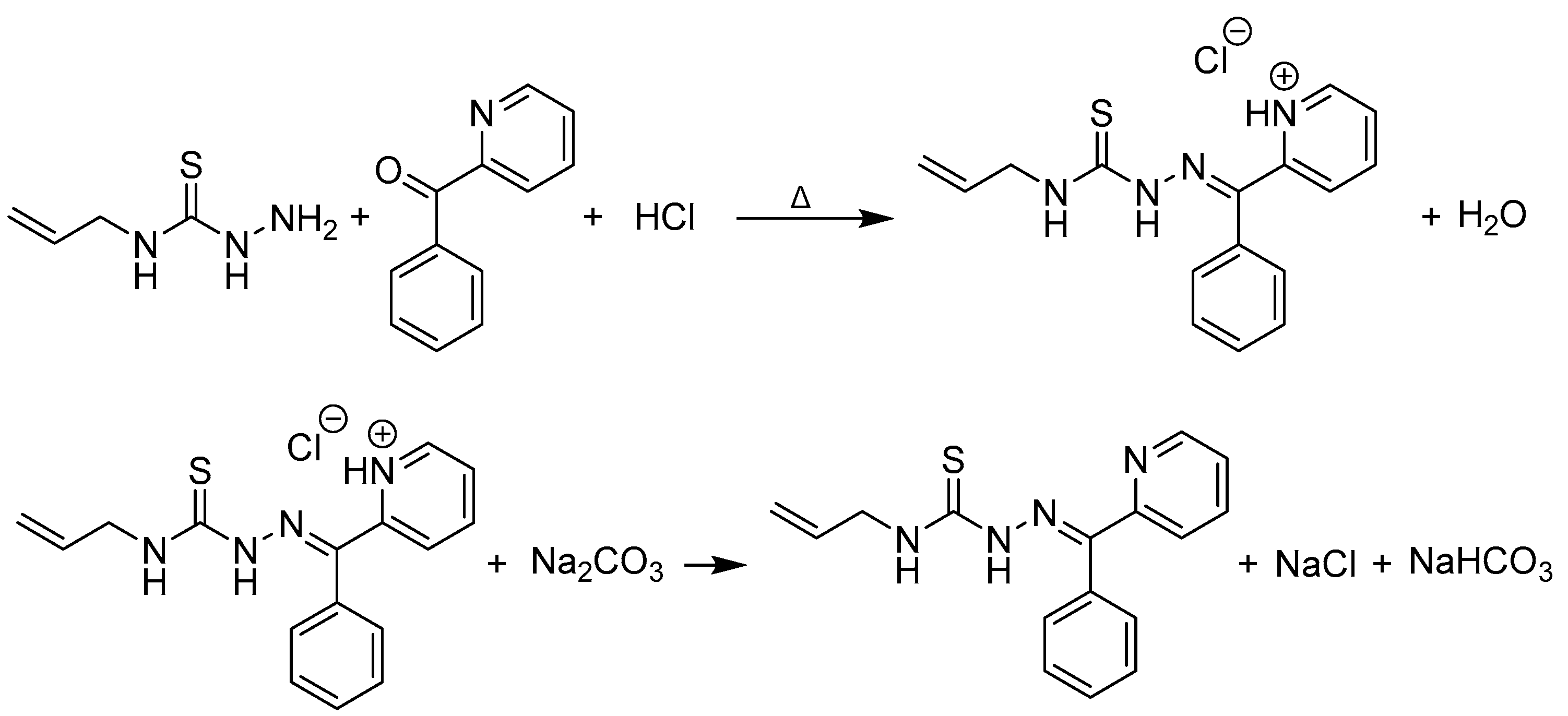
3.2.1. Synthesis of 2-benzoylpyridine 4-allylthiosemicarbazone
Method 1
Method 2
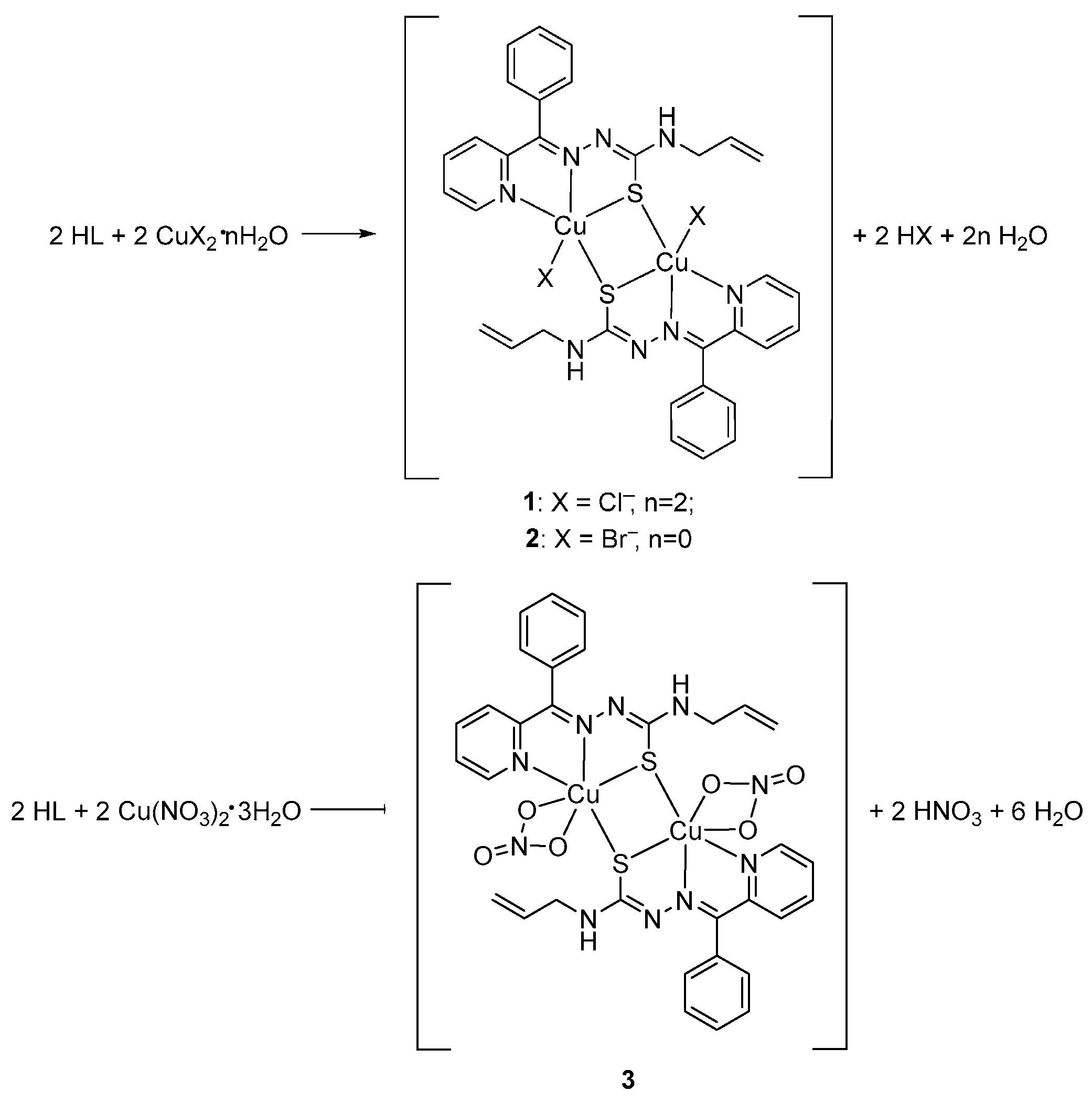
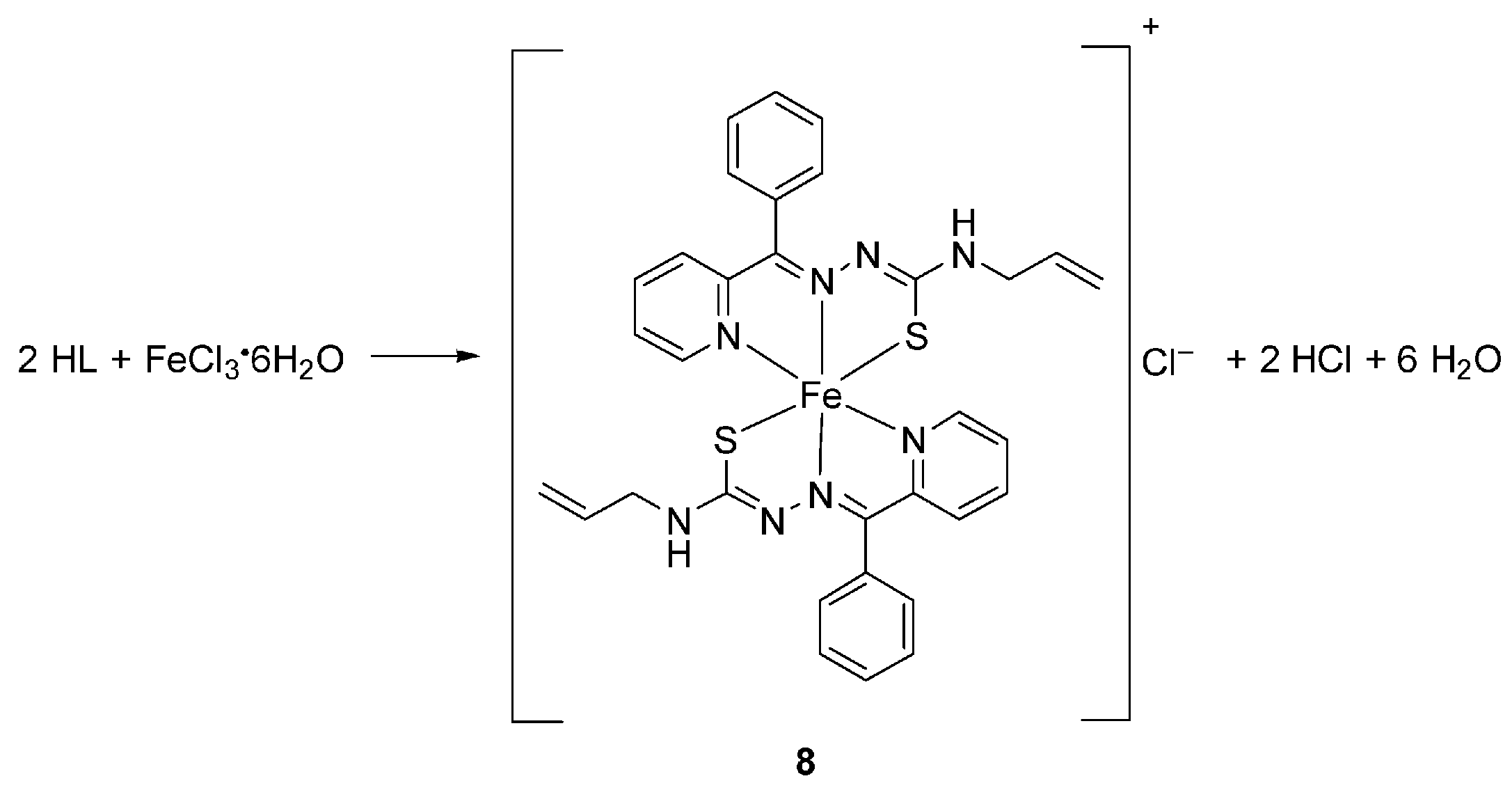
3.2.2. Synthesis of Coordination Compounds
[Cu(L)Cl]2 (1)
[Cu(L)Br]2 (2)
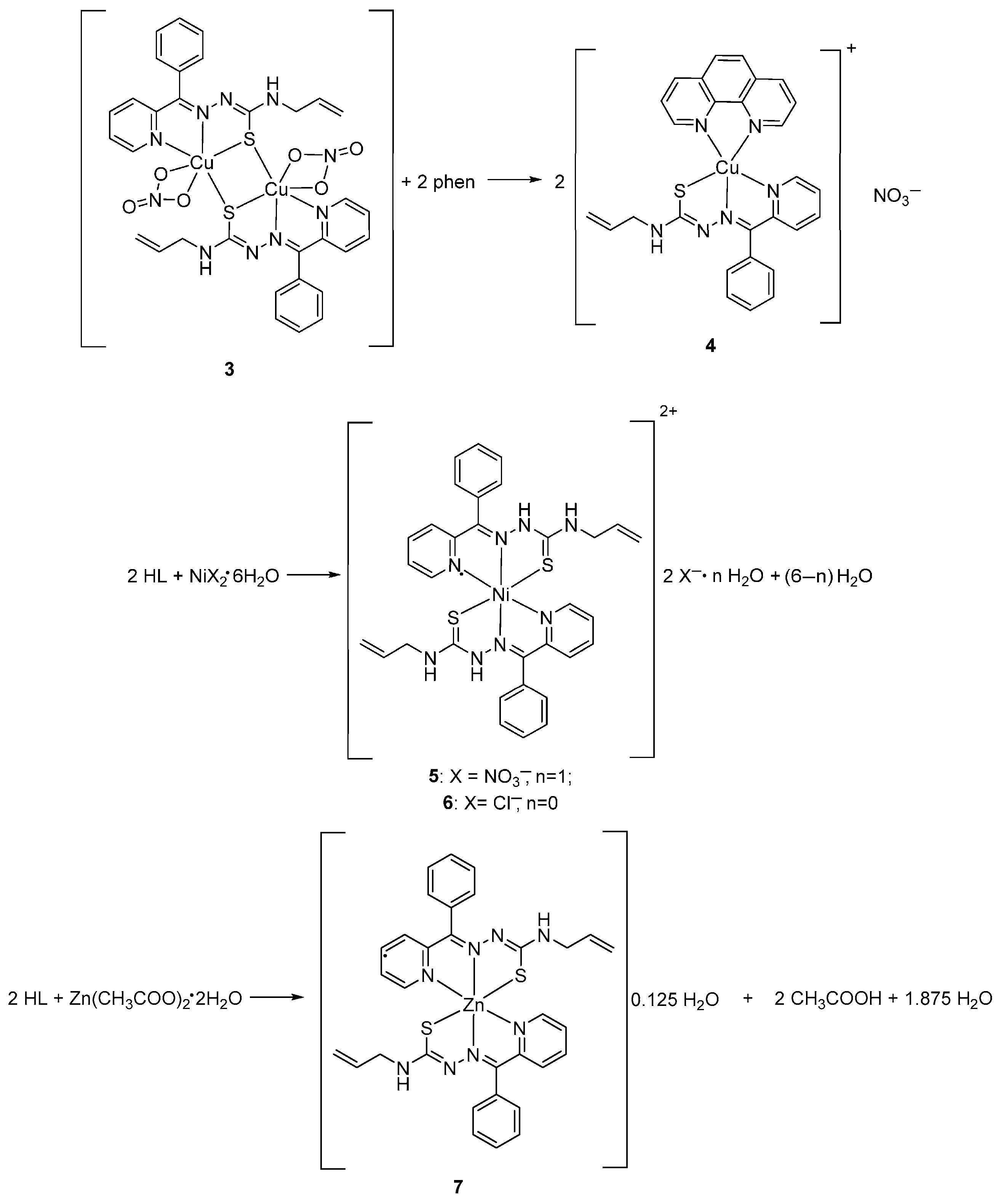
[Cu(L)(NO3)]2 (3)
[Cu(phen)(L)]NO3 (4)
[Ni(HL)2](NO3)2·H2O (5)
[Ni(HL)2]Cl2 (6)
[Zn(L)2]·0.125H2O (7)
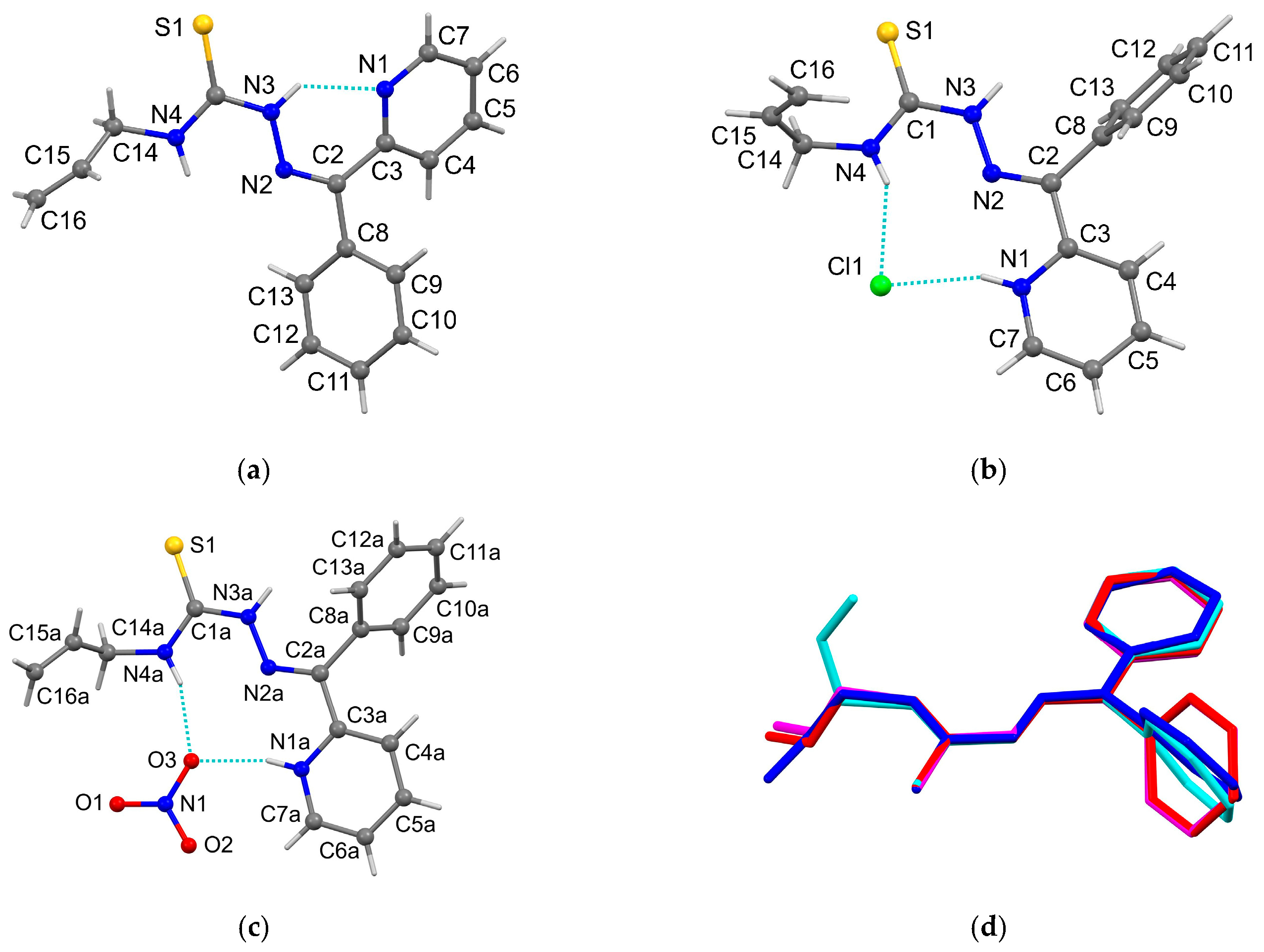
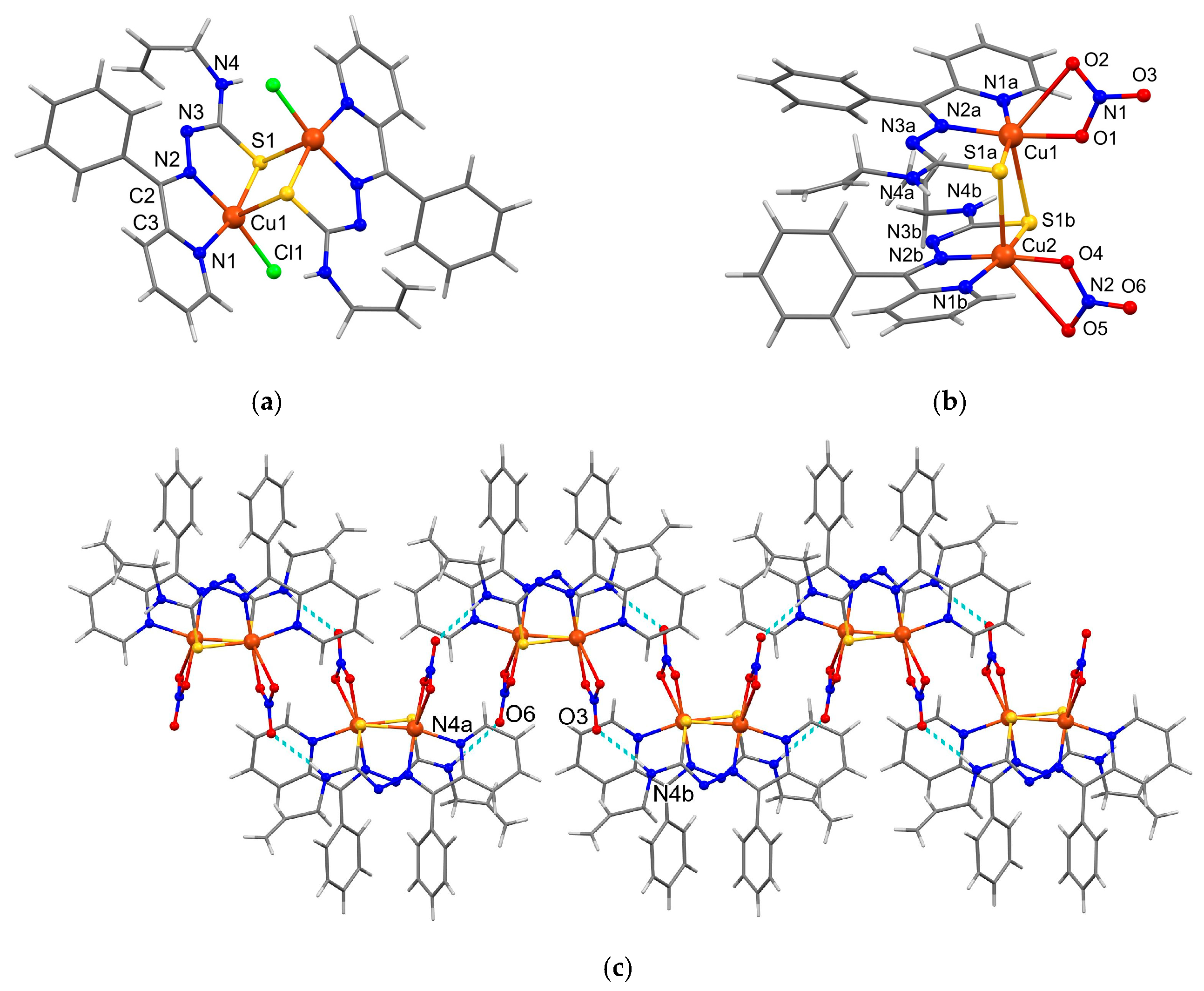
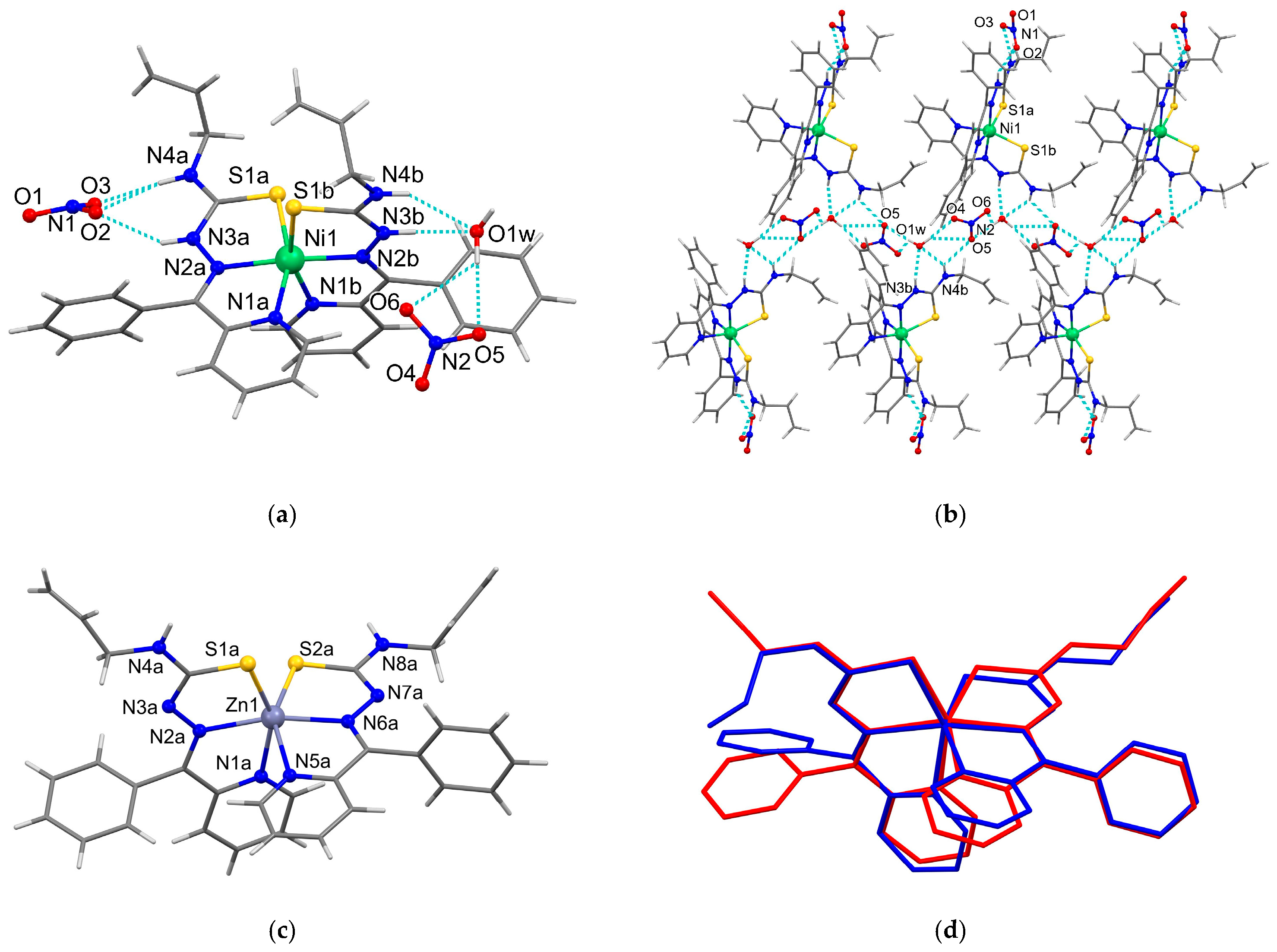
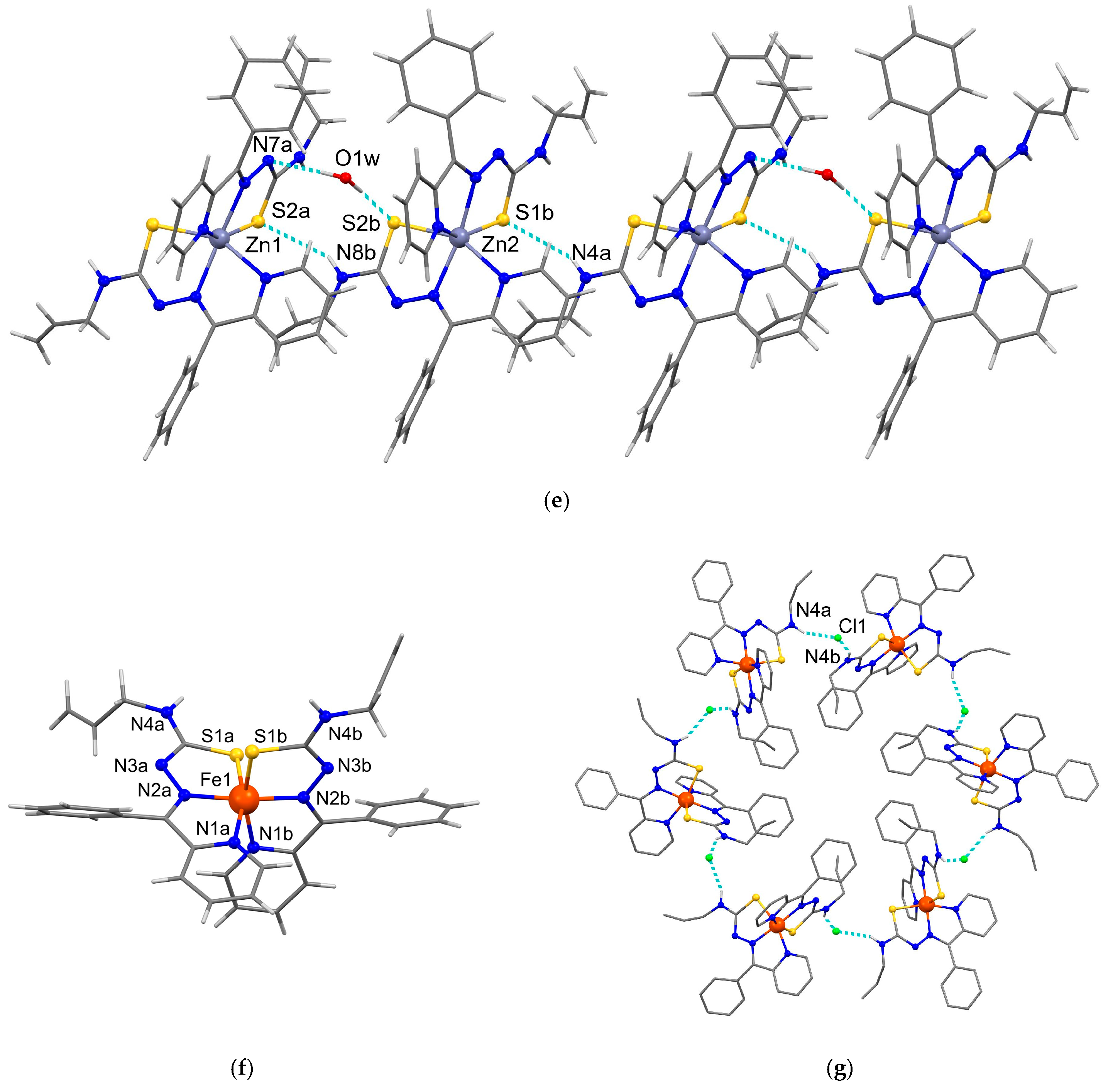
3.3. X-Ray Crystallography
3.4. Antibacterial and Antifungal Activity
3.5. Antiradical Activity
3.6. Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beraldo, H.; Gambino, D. The Wide Pharmacological Versatility of Semicarbazones, Thiosemicarbazones and Their Metal Complexes. Mini-Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [PubMed]
- Bavin, E.M.; Rees, R.J.W.; Robson, J.M.; Seiler, M.; Seymour, D.E.; Suddaby, D. The Tuberculostatic Activity of Some Thiosemicarbazones. J. Pharm. Pharmacol. 1950, 2, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Kune, G.A. To-Day’s Drugs: Methisazone. Br. Med. J. 1964, 2, 621. [Google Scholar] [PubMed]
- Tojal, J.G.; Orad, A.G.; Serra, J.L.; Pizarro, J.L.; Lezama, L.; Arriortua, M.I.; Rojo, T.J. Synthesis and spectroscopic properties of copper(II) complexes derived from thiophene-2-carbaldehyde thiosemicarbazone. Structure and biological activity of [Cu(C6H6N3S2)2]. Inorg. Biochem. 1999, 75, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Garg, B.S.; Bhoon, Y.K. Characterization of copper(II) complexes of N4,N4-disubstituted thiosemicarbazones of 2-acetylpyridine by combined evaluation of electronic and ESR parameters. Spectrochim. Acta Part A Mol. Spectrosc. 1986, 42, 959–968. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Ferandes, L.; Carrilho, D.; Pinto, M.C.X.; Leite, M.F.; Fagundes, E.M.S.; Speziali, N.L.; Mendes, I.C.; Beraldo, H. 2-Benzoylpyridine-N(4)-tolyl thiosemicarbazones and their palladium(II) complexes: Cytotoxicity against leukemia cells. Bioorg. Med. Chem. 2009, 17, 7138–7144. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, A.P.; Vieites, M.; Gambino, D.; Piro, O.E.; Castellano, E.E.; Zani, C.L.; Fagundes, E.M.S.; Teixeira, L.R.; Batista, A.A.; Beraldo, H.J. Palladium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activity. Inorg. Biochem. 2005, 99, 698–706. [Google Scholar]
- Graminha, A.E.; Rodrigues, C.; Batista, A.A.; Teixeira, L.R.; Fagundes, E.S.; Beraldo, H. Ruthenium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones with cytotoxic activity against human tumor cell lines. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2008, 69, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, A.P.; Ayala, J.D.; Lima, G.M.; Marchini, N.; Bombieri, G.; Zani, C.L.; Fagundes, E.M.S.; Beraldo, H. Structural studies and cytotoxic activity of N(4)-phenyl-2-benzoylpyridine thiosemicarbazone Sn(IV) complexes. Eur. J. Med. Chem. 2005, 40, 467–472. [Google Scholar] [CrossRef]
- Costa, R.F.; Rebolledo, A.P.; Matencio, T.; Calado, H.D.; Ardisson, J.D.; Cortés, M.E.; Bernardo, L.R.; Beraldo, H. Metal complexes of 2-benzoylpyridine-derived thiosemicarbazones: Structural, electrochemical and biological studies. J. Coord. Chem. 2005, 58, 1307–1319. [Google Scholar] [CrossRef]
- Joseph, M.; Kuriakose, M.; Kurup, M.P.; Suresh, E.; Kishore, A.; Bhat, S.G. Structural, antimicrobial and spectral studies of copper (II) complexes of 2-benzoylpyridine N (4)-phenyl thiosemicarbazone. Polyhedron 2006, 25, 61–70. [Google Scholar] [CrossRef]
- Graur, V.; Chumakov, Y.; Garbuz, O.; Hureau, C.; Tsapkov, V.; Gulea, A. Synthesis, structure, and biologic activity of some copper, nickel, cobalt, and zinc complexes with 2-formylpyridine N4-Allylthiosemicarbazone. Bioinorg. Chem. Appl. 2022, 2022, 2705332. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Yu, Y.; Sharpe, P.C.; Islam, M.; Liao, Y.T.; Lovejoy, D.B.; Kumar, N.; Bernhardt, P.; Richardson, D.R. Design, synthesis, and characterization of novel iron chelators: Structure− activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J. Med. Chem. 2007, 50, 3716–3729. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Yale, H.L.; Losee, K.; Holsing, M.; Martins, J.; Lott, W.A. The chemotherapy of experimental tuberculosis. III. The synthesis of thiosemicarbazones and related compounds. J. Am. Chem. Soc. 1951, 73, 906–912. [Google Scholar] [CrossRef]
- Graur, V.; Graur, I.; Gulea, A.; Teleuca, I.; Lozan-Tirsu, C.; Balan, G. Copper(II) coordination compounds with 2-acetylpyridine N4-(bicyclo[2.2.1]heptan-2-yl)thiosemicarbazone as potential antibacterial agents. Stud. Univ. Mold. 2003, 1, 206–213. [Google Scholar] [CrossRef]
- Chumakov, Y.; Graur, V.; Graur, I.; Tsapkov, V.; Garbuz, O.; Gulea, A. Insights into Antiradical Behavior: Crystal Structures and DFT Analysis of 2-Formylpyridine N 4-Allylthiosemicarbazone Salts. Acta Chim. Slov. 2024, 71, 609–613. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Divilov, V.; Lewis, J.S. Role of metalation in the topoisomerase IIα inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes. J. Med. Chem. 2011, 54, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, M. Synthesis and characterization of some multi-substituted thiosemicarbazones as the multi-dental ligands of metal ions. Chin. J. Org. Chem. 2001, 21, 681–684. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.; Perrin, D.R. Purification of Laboratory Chemicals, 4th ed.; Butter-worth-Heinemann, Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Leovac, V.M.; Vojinović, L.S.; Mesaroš-Sečenji, K.F.; Češljević, V.I. Transition metal complexes with thiosemicarbazide-based ligands, part 46: Synthesis and physico-chemical characterization of mixed ligand cobalt (III)-complexes with salicylaldehyde semi-, thiosemi-an. J. Serb. Chem. Soc. 2003, 68, 919–927. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, O.; Gudumac, V.; Toderas, I.; Gulea, A. Antioxidant Properties of Synthetic Compounds and Natural Products. Action Mechanisms; CEP USM: Chișinău, Moldova, 2023; ISBN 978-9975-62-516-6. [Google Scholar]
| Compound | HL | [H2L]Cl | [H2L]NO3 | 1 |
|---|---|---|---|---|
| Deposition number | 2465802 | 2465803 | 2465804 | 2465805 |
| Empirical formula | C16H16N4S1 | C16H17Cl1N4S1 | C16H17N5O3S1 | C32H30Cl2Cu2N8S2 |
| Formula weight | 296.39 | 332.85 | 359.40 | 788.74 |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic |
| Space group | P21/c | P21/c | P21/c | |
| Unit cell dimensions | ||||
| a (Å) | 11.7586(11) | 12.2246(6) | 23.5603(10) | 9.4554(6) |
| b (Å) | 17.1938(15) | 13.3282(6) | 17.6702(6) | 9.6175(6) |
| c (Å) | 8.0748(9) | 9.9981(4) | 18.1932(7) | 10.4901(9) |
| α (°) | 90 | 90 | 90 | 76.988(6) |
| β (°) | 109.660(11) | 91.703(4) | 107.144(5) | 69.140(7) |
| γ (°) | 90 | 90 | 90 | 74.564(6) |
| V (Å3) | 1537.4(3) | 1628.29(12) | 7237.6(5) | 850.21(12) |
| Z | 4 | 4 | 16 | 1 |
| ρcalc (g cm−3) | 1.281 | 1.358 | 1.319 | 1.540 |
| μMo (mm−1) | 0.209 | 0.364 | 0.204 | 1.567 |
| F(000) | 524 | 696 | 3008 | 402 |
| Crystal size (mm) | 0.60 × 0.08 × 0.03 | 0.50 × 0.40 × 0.10 | 0.61 × 0.52 × 0.38 | 0.35 × 0.25 × 0.20 |
| θ Range (°) | 2.93–25.05 | 3.01–25.50 | 2.95–25.05 | 3.27–25.55 |
| Index range | −14 ≤ h ≤ 9, −20 ≤ k ≤ 19, −9 ≤ l ≤ 9 | −12 ≤ h ≤ 14, −10 ≤ k ≤ 16, −12 ≤ l ≤ 12 | −28 ≤ h ≤ 27, −21 ≤ k ≤ 13, −21 ≤ l ≤ 15 | −10 ≤ h ≤ 11, −8 ≤ k ≤ 11, −12 ≤ l ≤ 12 |
| Reflections collected/unique | 5046/2716 (Rint = 0.0314) | 5813/3009 (Rint = 0.0196) | 25994/12757 (Rint = 0.0424) | 5435/3155 (Rint = 0.0281) |
| Completeness (%) | 99.6 (θ = 25.05°) | 99.5 (θ = 25.24°) | 99.6 (θ = 25.05°) | 99.7 (θ = 25.24°) |
| Reflections with I > 2σ(I) | 1734 | 2277 | 6035 | 2555 |
| Number of refined parameters | 191 | 207 | 957 | 222 |
| Goodness-of-fit (GOF) | 1.005 | 1.004 | 1.000 | 1.001 |
| R (for I > 2σ(I)) | R1 = 0.0583, wR2 = 0.1410 | R1 = 0.0408, wR2 = 0.1021 | R1 = 0.0754, wR2 = 0.1827 | R1 = 0.0483, wR2 = 0.1235 |
| R (for all reflections) | R1 = 0.1016, wR2 = 0.1666 | R1 = 0.0594, wR2 = 0.1125 | R1 = 0.1628, wR2 = 0.2233 | R1 = 0.0613, wR2 = 0.1337 |
| Δρ>max/Δρmin (e·Å−3) | 0.191/−0.208 | 0.762/−0.607 | 0.496/−0.367 | 0.593/−0.652 |
| Compound | 3 | 5 | 7 | 8 |
| Deposition number | 2465806 | 2465807 | 2465808 | 2465809 |
| Empirical formula | C32H30Cu2N10O6S2 | C32H34N10Ni1O7S2 | C32H30.25N8O0.12S2Zn1 | C32H30Cl1Fe1N8S2 |
| Formula weight | 841.86 | 793.52 | 658.38 | 682.06 |
| Crystal system | triclinic | orthorhombic | triclinic | trigonal |
| Space group | Pca21 | |||
| Unit cell dimensions | ||||
| a (Å) | 12.1561(7) | 39.059(3) | 10.5417(4) | 43.3336(19) |
| b (Å) | 12.7795(7) | 9.0777(7) | 12.0147(4) | 43.3336(19) |
| c (Å) | 14.3203(6) | 10.3722(8) | 12.0147(4) | 9.1757(6) |
| α (°) | 98.110(4) | 90 | 100.250(3) | 90 |
| β (°) | 103.014(4) | 90 | 90.593(3) | 90 |
| γ (°) | 114.503(5) | 90 | 92.543(3) | 120 |
| V (Å3) | 1902.06(19) | 3677.6(5) | 3255.6(2) | 14921.7(16) |
| Z | 2 | 4 | 4 | 18 |
| ρcalc (g cm−3) | 1.470 | 1.433 | 1.343 | 1.366 |
| μMo (mm−1) | 1.283 | 0.701 | 0.918 | 0.697 |
| F(000) | 860 | 1648 | 1365 | 6354 |
| Crystal size (mm) | 0.20 × 0.15 × 0.10 | 0.30 × 0.16 × 0.08 | 0.60 × 0.50 × 0.04 | 0.60 × 0.15 × 0.06 |
| θ Range (°) | 3.03–25.05 | 3.03–25.05 | 3.09–25.05 | 3.00–25.04 |
| Index range | −14 ≤ h ≤ 14, −15 ≤ k ≤ 15, −17 ≤ l ≤ 11 | −46 ≤ h ≤ 46, −8 ≤ k ≤ 10, −5 ≤ l ≤ 12 | −12 ≤ h ≤ 8, −14 ≤ k ≤ 14, −31 ≤ l ≤ 31 | −49 ≤ h ≤ 17, −45 ≤ k ≤ 43, −4 ≤ l ≤ 10 |
| Reflections collected/unique | 11930/6709 (Rint = 0.0637) | 7997/4663 (Rint = 0.0405) | 20382/11519 (Rint = 0.0340) | 8163/5821 (Rint = 0.0355) |
| Completeness (%) | 99.7 (θ =25.05°) | 99.5 (θ = 25.05°) | 99.7 (θ = 25.05°) | 99.5 (θ = 25.04°) |
| Reflections with I > 2σ(I) | 3283 | 2678 | 5711 | 3726 |
| Number of refined parameters | 457 | 515 | 826 | 826 |
| Goodness-of-fit (GOF) | 0.974 | 0.995 | 1.006 | 1.006 |
| R (for I > 2σ(I)) | R1 = 0.0697, wR2 = 0.0950 | R1 = 0.0506, wR2 = 0.0584 | R1 = 0.0539, wR2 = 0.0898 | R1 = 0.0572, wR2 = 0.1168 |
| R (for all reflections) | R1 = 0.1537, wR2 = 0.1152 | R1 = 0.1060, wR2 = 0.0639 | R1 = 0.1200, wR2 = 0.1052 | R1 = 0.1000, wR2 = 0.1358 |
| Δρmax/Δρmin (e·Å−3) | 0.591/−0.483 | 0.504/−0.219 | 0.321/−0.286 | 0.483/−0.385 |
| D–H···A | d(H···A) | d(D···A) | ∠(DHA) | Symmetry Transformation for Acceptor |
|---|---|---|---|---|
| HL | ||||
| N(3)–H(1N)···N(1) | 2.00 | 2.670(3) | 134 | x, y, z |
| [H2L]Cl | ||||
| N(3)–H(1N)···Cl(1) | 2.21 | 3.010(2) | 160 | x, y, z |
| N(4)–H(3N)···Cl(1) | 2.45 | 3.242(2) | 153 | x, y, z |
| N(3)–H(2N)···S(1) | 3.05 | 3.709(2) | 135 | −x + 1, −y, −z + 1 |
| [H2L]NO3 | ||||
| N(1A)–H(1N)···O(3) | 1.91 | 2.742(4) | 162 | x, y, z |
| N(4A)–H(3N)···O(3) | 2.02 | 2.851(5) | 162 | x, y, z |
| N(1B)–H(4N)···O(6) | 1.84 | 2.750(4) | 167 | x, y, z |
| N(4B)–H(6N)···O(6) | 2.09 | 2.918(5) | 163 | x, y, z |
| N(1C)–H(7N)···O(8) | 1.90 | 2.732(4) | 163 | x, y, z |
| N(4C)–H(9N)···O(8) | 2.05 | 2.884(5) | 162 | x, y, z |
| N(1D)–H(10N)···O(12) | 1.79 | 2.713(5) | 162 | x, y, z |
| N(4D)–H(12N)···O(12) | 2.00 | 2.833(5) | 164 | x, y, z |
| 3 | ||||
| N(4A)–H(1N)···O(6) | 2.25 | 2.990(7) | 144 | −x + 2, −y + 1, −z + 2 |
| N(4B)–H(2N)···O(3) | 2.11 | 2.949(6) | 164 | −x + 1, −y, −z + 1 |
| 5 | ||||
| N(3A)–H(3A)···O(2) | 2.03 | 2.787(19) | 146 | x, y, z |
| N(3A)–H(3A)···O(2#) | 1.90 | 2.72(3) | 159 | x, y, z |
| N(4A)–H(2N)···O(2) | 2.44 | 3.132(17) | 137 | x, y, z |
| N(4A)–H(2N)···O(3) | 1.99 | 2.838(18) | 167 | x, y, z |
| N(4A)–H(2N)···O(2#) | 2.31 | 3.06(2) | 146 | x, y, z |
| N(4A)–H(2N)···O(3#) | 2.13 | 2.96(2) | 156 | x, y, z |
| N(3B)–H(3B)···O(1W) | 2.04 | 2.828(7) | 152 | x, y, z |
| N(4B)–H(4N)···O(5) | 2.38 | 2.981(8) | 127 | −x, −y + 1, z − 1/2 |
| N(4B)–H(4N)···O(1W) | 2.31 | 3.057(8) | 145 | x, y, z |
| O(1W)–H(1W)···O(5) | 2.15 | 3.052(8) | 179 | x, y, z |
| O(1W)–H(1W)···O(6) | 2.46 | 3.086(9) | 126 | −x, −y + 1, z − 1/2 |
| O(1W)–H(2W)···O(4) | 1.94 | 2.751(8) | 177 | −x, −y + 1, z − 1/2 |
| 7 | ||||
| N(4A)–H(1N)···S(1B) | 2.71 | 3.422(3) | 142 | x, y + 1, z |
| N(8B)–H(4N)···S(2A) | 2.71 | 3.422(3) | 142 | x − 1, y, z |
| O(1W)–H(1W)···N(7A) | 2.07 | 2.970(13) | 178 | x, y, z |
| O(1W)–H(2W)···S(2B) | 2.26 | 3.283(12) | 178 | x + 1, y, z |
| 8 | ||||
| N(3A)–H(1N)···Cl(1) | 2.32 | 3.116(4) | 154 | x, y, z |
| N(4B)–H(2N)···Cl(1) | 2.66 | 3.146(9) | 113 | x − y + 1/3, x − 1/3, −z + 5/3 |
| Bonds | 1, (Å) M=Cu(1) | 3, (Å) M=Cu(1)/Cu(2) A/B |
|---|---|---|
| M–N(1) | 2.011(3) | 1.983(5)/2.000(5) |
| M–N(2) | 1.972(3) | 1.947(5)/1.958(5) |
| M–S(1) | 2.2487(10) | 2.258(2)/2.268(2) |
| M–S(1) */S(1B/1A) * | 3.047(1) | 2.859(2)/2.794(2) |
| M–Cl(1)/O(1)/O(4) | 2.2260(10) | 1.975(4)/1.979(4) |
| M–O(2)/O(5) | 2.749(5)/2.746(7) | |
| Bonds | 5, (Å) M=Ni(1), A/B | 8, (Å) M=Fe(1) A/B |
| M–N(1) | 2.065(6)/2.067(6) | 1.971(3)/1.997(3) |
| M–N(2) | 2.017(5)/2.016(5) | 1.922(3)/1.918(3) |
| M–S(1) | 2.391(2)/2.410(2) | 2.2214(12)/2.2097(12) |
| Bonds | 7, (Å) M=Zn(1) A | 7, (Å) M=Zn(2) B |
| M–N(1) | 2.230(3) | 2.250(3) |
| M–N(2) | 2.137(3) | 2.169(3) |
| M–S(1) | 2.4397(12) | 2.4350(10) |
| M–N(5) | 2.270(3) | 2.209(3) |
| M–/N(6) | 2.150(3) | 2.150(3) |
| M–S(2) | 2.4327(11) | 2.4554(12) |
| Compound | Staphylococcus aureus ATCC 25923 | Bacillus cereus ATCC 11778 | Escherichia coli ATCC 25922 | Acinetobacter baumannii BAA-747 | Candida albicans ATCC 90028 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| HL | 1.95 | 3.91 | 1.95 | 1.95 | >1000 | >1000 | >1000 | >1000 | 125 | 500 |
| 1 | 0.122 | 0.244 | 0.122 | 0.122 | 125 | 125 | 125 | 125 | 0.977 | 1.95 |
| 2 | 0.244 | 0.244 | 0.244 | 0.244 | 125 | 125 | 125 | 125 | 1.95 | 1.95 |
| 3 | 0.244 | 0.488 | 0.244 | 0.244 | 125 | 250 | 62.5 | 62.5 | 1.95 | 1.95 |
| 4 | 0.977 | 1.95 | 0.977 | 1.95 | 31.3 | 62.5 | 31.3 | 62.5 | 15.6 | 31.3 |
| 6 | 250 | 500 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 7.81 | 7.81 |
| 7 | 0.244 | 0.488 | 0.244 | 0.244 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 8 | 3.91 | 7.81 | 62.5 | 62.5 | >1000 | >1000 | >1000 | >1000 | 31.3 | 62.5 |
| Furacillinum | 9.3 | 9.3 | 4.7 | 4.7 | 4.7 | 9.4 | 18.5 | 37.5 | - | - |
| Nystatine | - | - | - | - | - | - | - | - | 80 | 80 |
| Fluconazole | - | - | - | - | - | - | - | - | 15.6 | 31.3 |
| Compound | Trolox | HL | 1 * | 2 * | 3 * | 4 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| IC50, μM | 33.3 ± 0.2 | 17.3 ± 0.6 | 52.9 ± 0.8 | >100 | >100 | >100 | 16.3 ± 0.3 | 4.69 ± 0.34 | >100 |
| Compound | Inhibition of Cell Proliferation, % | ||
|---|---|---|---|
| 10 μM | 1 μM | 0.1 μM | |
| HL | 99.5 ± 1.2 | 98.2 ± 1.5 | 96.7 ± 0.6 |
| 1 * | 100.0 ± 0.3 | 99.4 ± 1.0 | 93.5 ± 0.5 |
| HAp4alT | 98.7 ± 1.7 | 96.1 ± 0.8 | 92.4 ± 0.9 |
| [Cu(Ap4alT)Cl] | 100.0 ± 0.2 | 98.2 ± 0.7 | 82.5 ± 2.5 |
| HFp4alT [17] | 100.3 ± 2.2 | 99.6 ± 0.4 | 1.8 ± 0.1 |
| [Cu(Fp4alT)Cl] [17] | 99.4 ± 0.6 | 99.2 ± 0.2 | 40.2 ± 2.8 |
| Doxorubicin [17] | 95.0 ± 0.6 | 92.9 ± 0.7 | 16.5 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graur, V.; Graur, I.; Bourosh, P.; Kravtsov, V.; Lozan-Tirsu, C.; Balan, G.; Garbuz, O.; Tsapkov, V.; Gulea, A. Synthesis, Characterization, and Biological Evaluation of Some 3d Metal Complexes with 2-Benzoylpyridine 4-Allylthiosemicarbazone. Inorganics 2025, 13, 249. https://doi.org/10.3390/inorganics13070249
Graur V, Graur I, Bourosh P, Kravtsov V, Lozan-Tirsu C, Balan G, Garbuz O, Tsapkov V, Gulea A. Synthesis, Characterization, and Biological Evaluation of Some 3d Metal Complexes with 2-Benzoylpyridine 4-Allylthiosemicarbazone. Inorganics. 2025; 13(7):249. https://doi.org/10.3390/inorganics13070249
Chicago/Turabian StyleGraur, Vasilii, Ianina Graur, Pavlina Bourosh, Victor Kravtsov, Carolina Lozan-Tirsu, Greta Balan, Olga Garbuz, Victor Tsapkov, and Aurelian Gulea. 2025. "Synthesis, Characterization, and Biological Evaluation of Some 3d Metal Complexes with 2-Benzoylpyridine 4-Allylthiosemicarbazone" Inorganics 13, no. 7: 249. https://doi.org/10.3390/inorganics13070249
APA StyleGraur, V., Graur, I., Bourosh, P., Kravtsov, V., Lozan-Tirsu, C., Balan, G., Garbuz, O., Tsapkov, V., & Gulea, A. (2025). Synthesis, Characterization, and Biological Evaluation of Some 3d Metal Complexes with 2-Benzoylpyridine 4-Allylthiosemicarbazone. Inorganics, 13(7), 249. https://doi.org/10.3390/inorganics13070249








