Synthesis and Application of Fe3O4–ZrO2 Magnetic Nanoparticles for Fluoride Adsorption from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Magnetic Fe3O4–ZrO2 Adsorbents
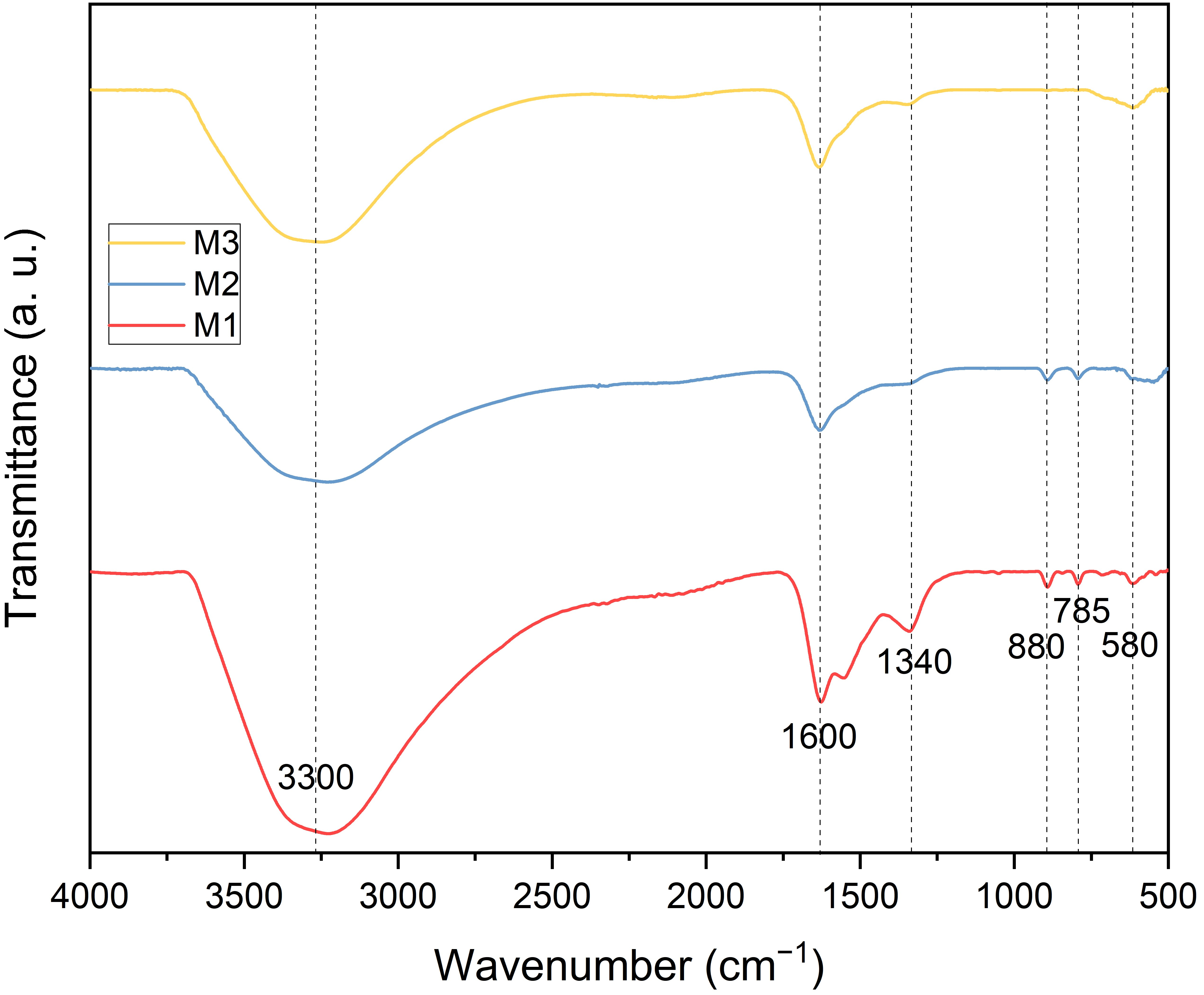
2.1.1. FTIR Spectra Analysis
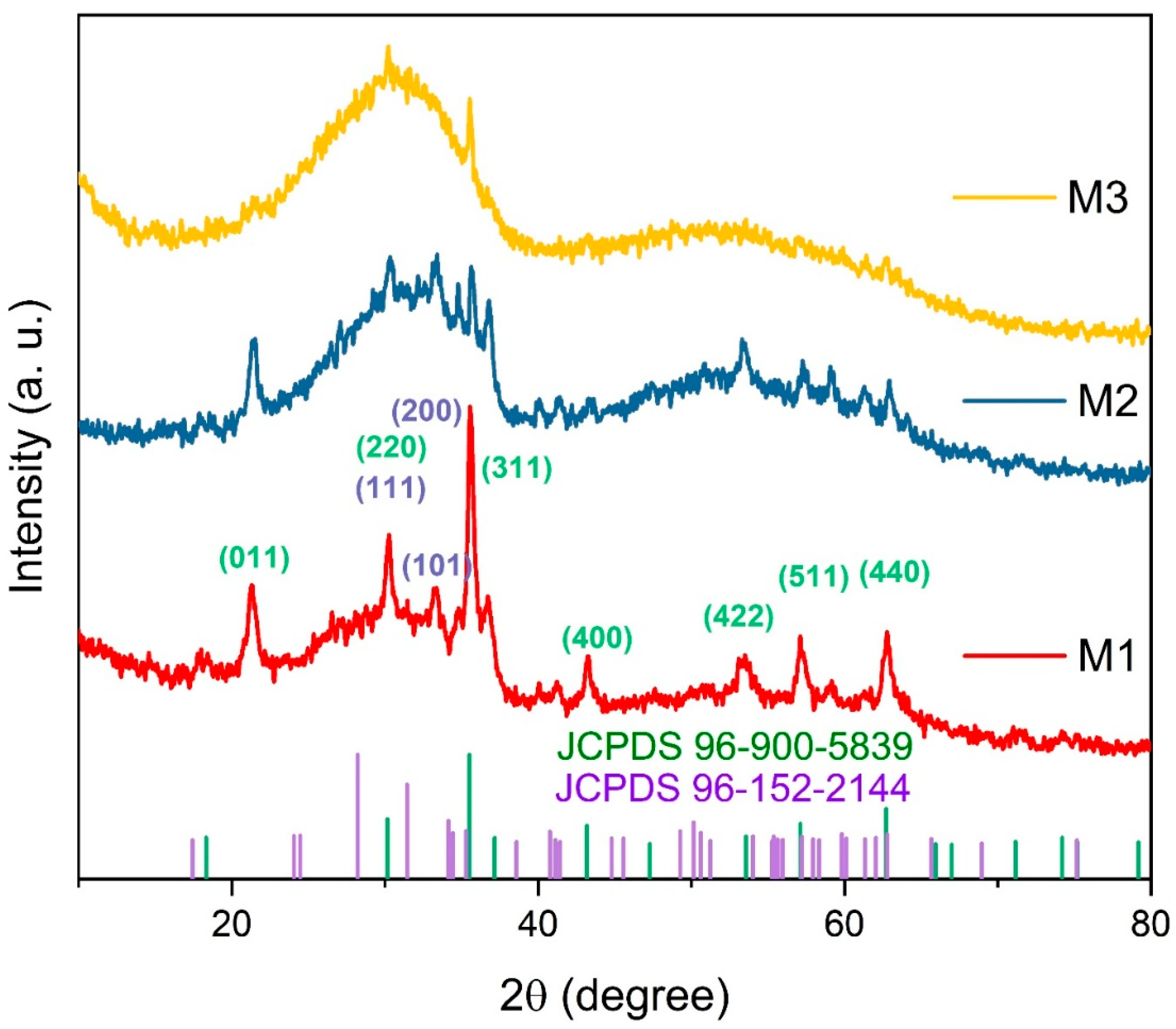
2.1.2. X-Ray Diffraction (XRD) Analysis
2.1.3. Morphology and Elemental Composition (SEM and EDS)
2.2. Fluoride Adsorption Studies
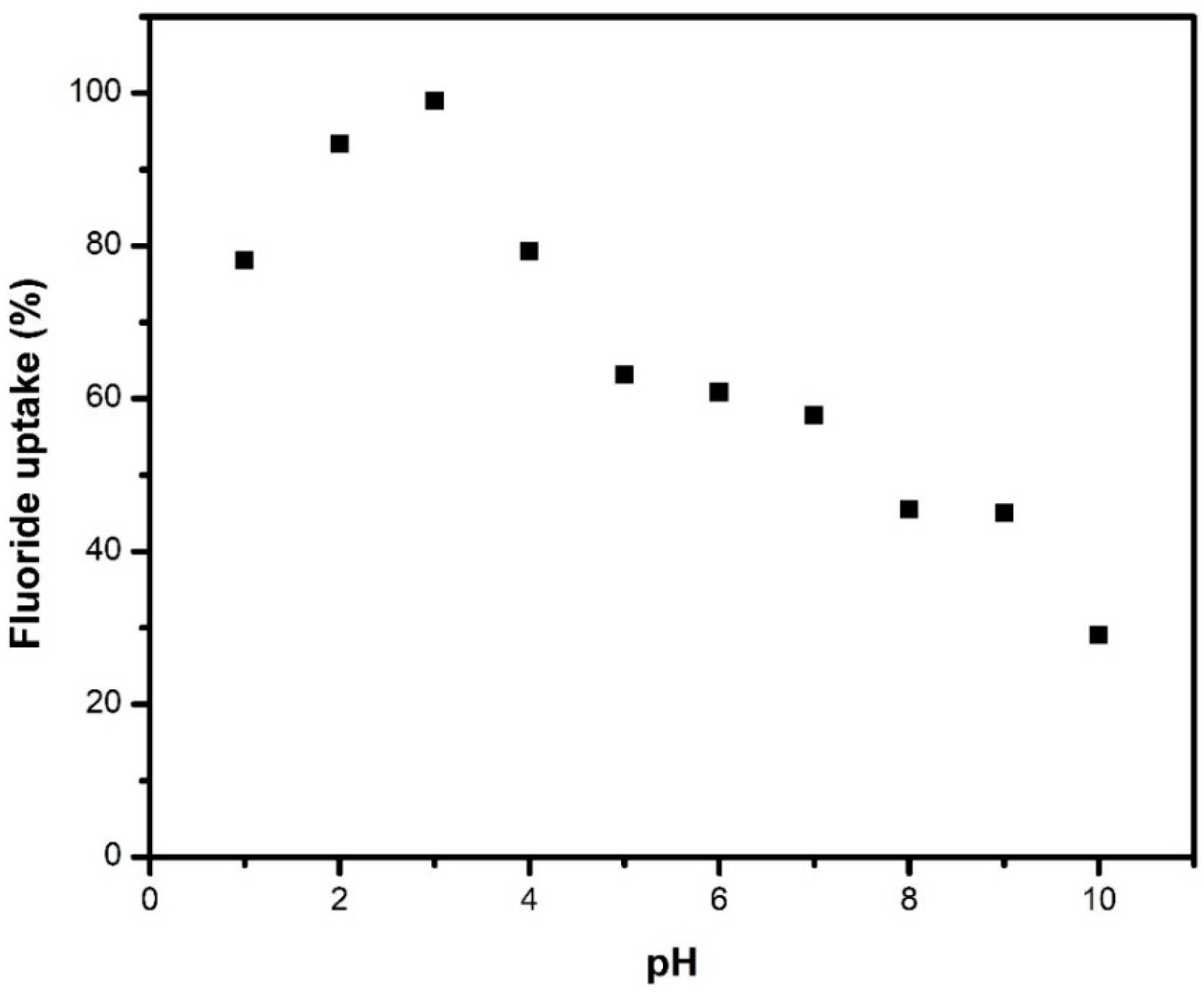
2.2.1. Effect of pH on Fluoride Removal
2.2.2. Kinetics Analysis
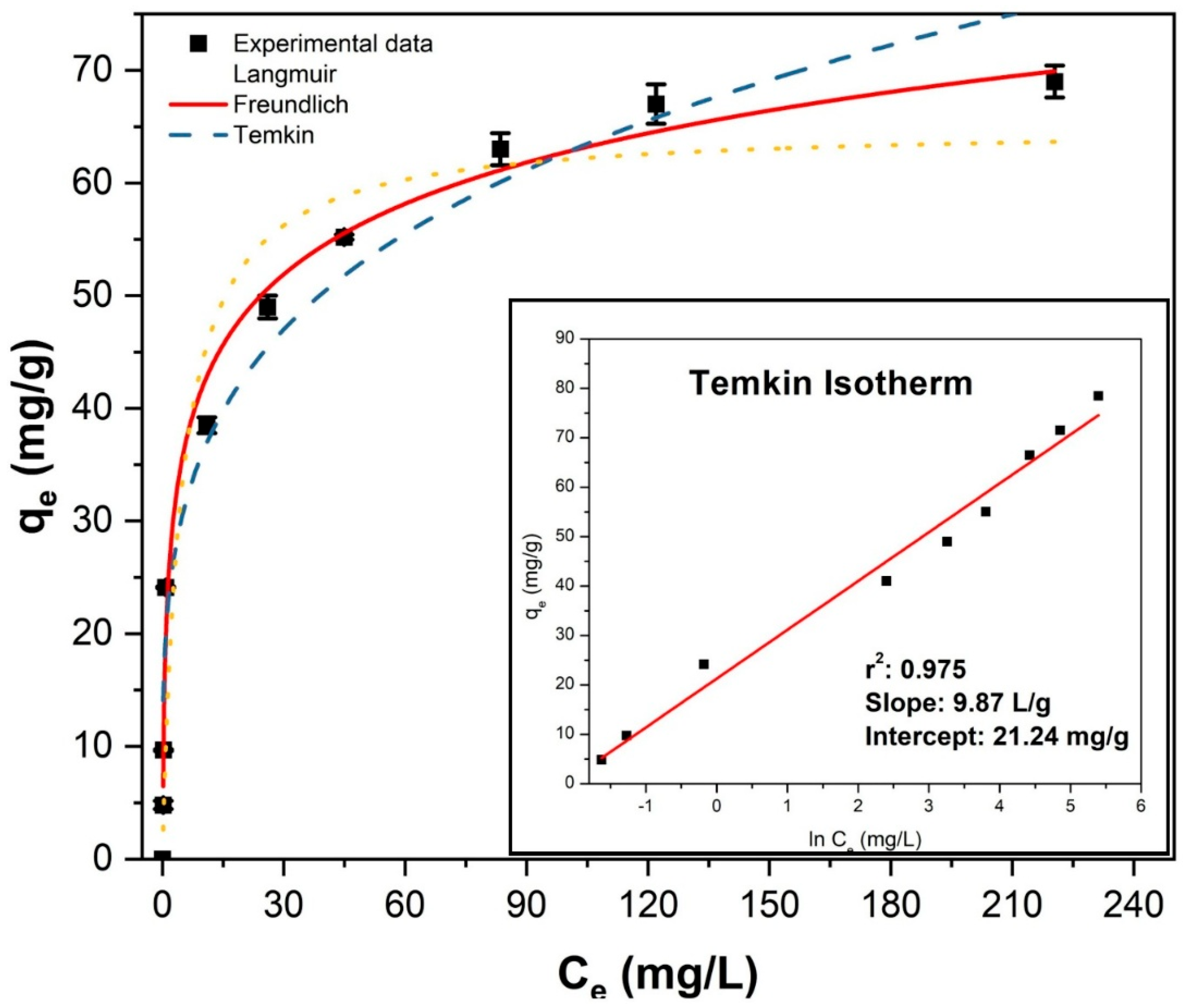
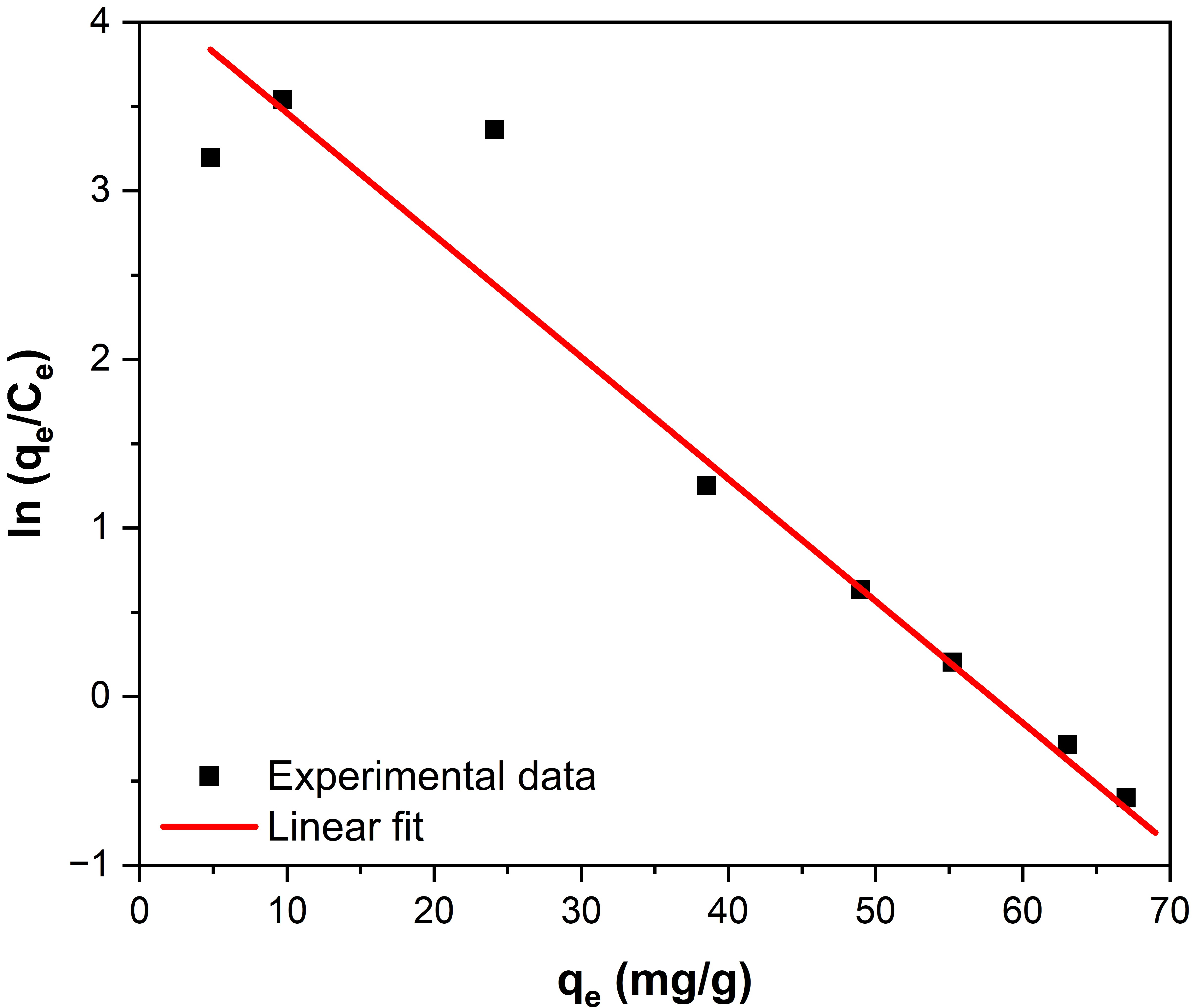
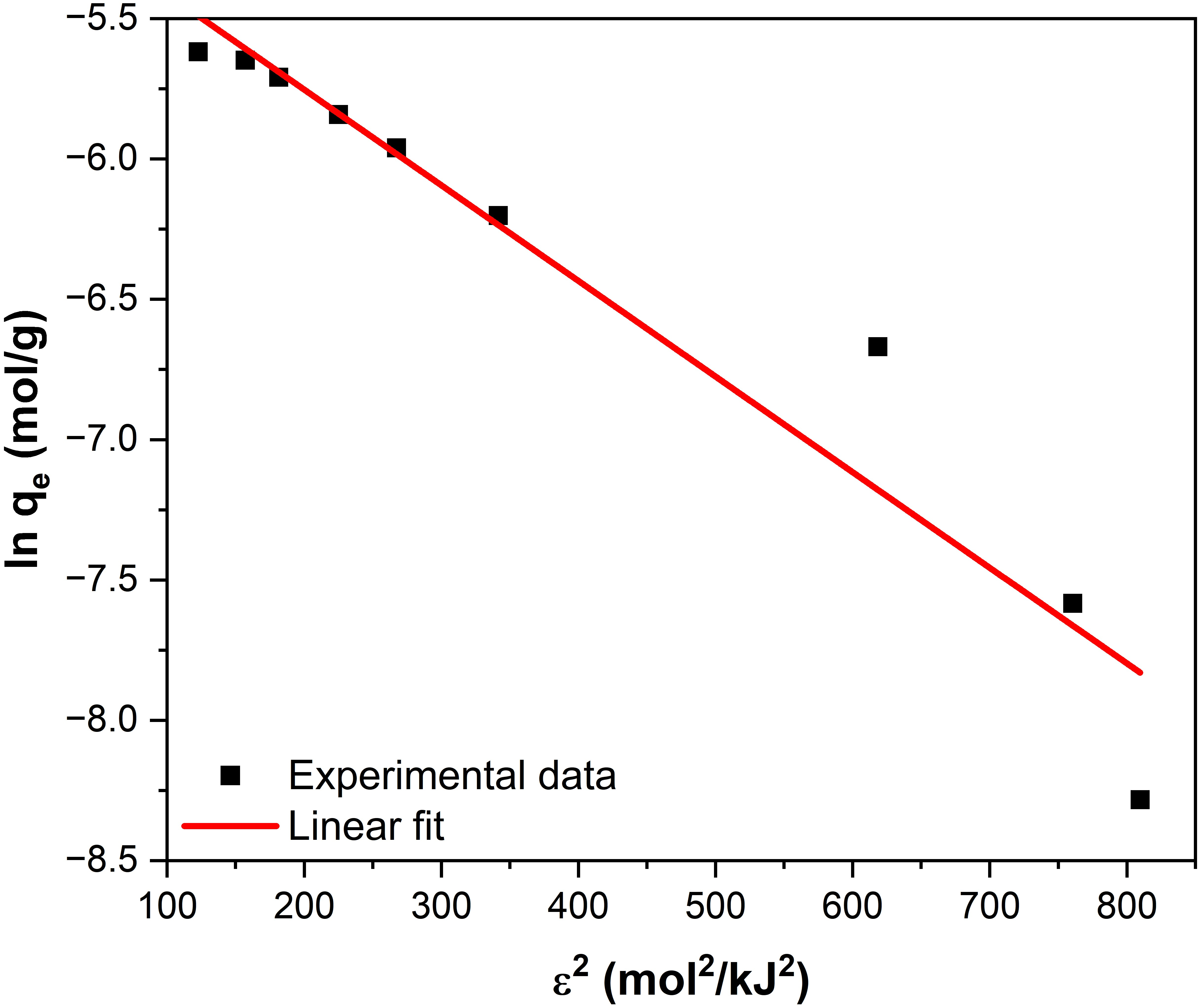
2.2.3. Adsorption Isotherm Analysis
- (i)
- Physisorption if EDR < 8 kJ/mol;
- (ii)
- Ion exchange if EDR = 8.0–16.0 kJ/mol;
- (iii)
- Chemisorption if EDR > 16.0 kJ/mol [60].
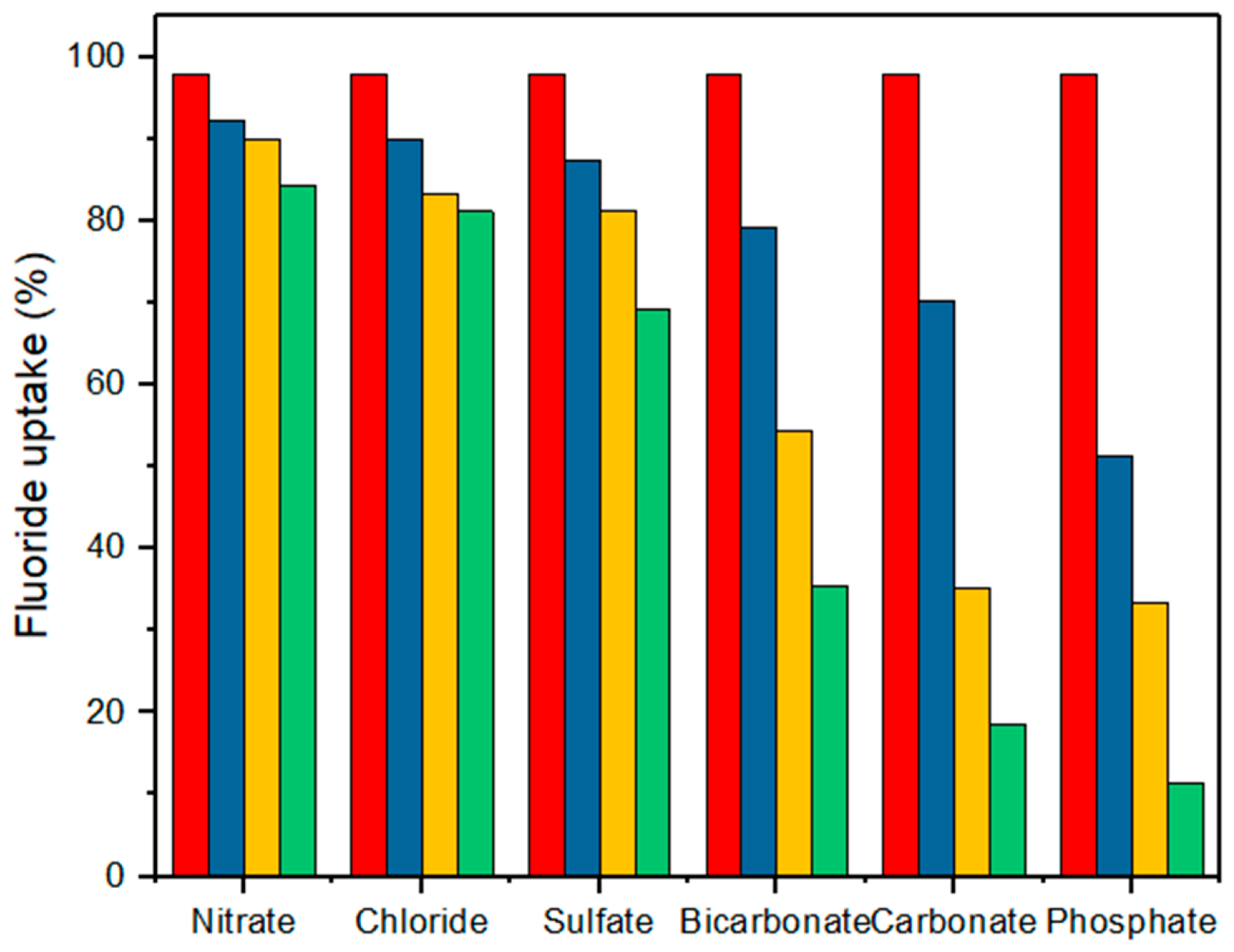
2.2.4. Effects of Interfering Anions on Fluoride Adsorption
2.2.5. Comparison of Fe3O4–ZrO2 with Other Fluoride Adsorbents
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Fe3O4–ZrO2 Nanoparticles
3.2.1. Characterization
3.2.2. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Liu, X.; Zhu, Y.; Zhang, J.; Zhu, L. A Zr-based coordination polymer for detection and adsorption of fluoride in water. Polym. Bull. 2024, 81, 335–350. [Google Scholar] [CrossRef]
- Poursaberi, T.; Hassanisadi, M.; Torkestani, K.; Zare, M. Development of zirconium (IV)-metalloporphyrin grafted Fe3O4 nanoparticles for efficient fluoride removal. Chem. Eng. J. 2012, 189–190, 117–125. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Fluoride Removal from Water Sources by Adsorption on MOFs. Separations 2023, 10, 467. [Google Scholar] [CrossRef]
- Ali, F.A.A.; Alam, J.; Qaid, S.M.H.; Shukla, A.K.; Al-Fatesh, A.S.; Alghamdi, A.M.; Fadhillah, F.; Osman, A.I.; Alhoshan, M. Fluoride removal using nanofiltration-ranged polyamide thin-film nanocomposite membrane incorporated titanium oxide nanosheets. Nanomaterials 2024, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in drinking water and defluoridation of water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, S.; Lataye, D.H.; Arfin, T.; Rayalu, S. Defluoridation by nano-materials, building materials and other miscellaneous materials: A systematic review. IJIRSET 2015, 4, 11998–12010. [Google Scholar]
- WHO. Fluoride in Drinking-Water; WHO Press: Geneva, Switzerland, 2006; Available online: https://www.who.int/ (accessed on 24 June 2024).
- Singh, J.; Singh, P.; Singh, A. Fluoride ions vs removal technologies: A study. Arab. J. Chem. 2016, 9, 815–824. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Pandian, S.; Panchal, H. Overview of fluoride removal from water using separation techniques. Environ. Technol. Innov. 2021, 21, 101246. [Google Scholar] [CrossRef]
- Gai, W.Z.; Deng, Z.Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, X.; Shang, Y.; Gao, B. Removal of fluoride by carbohydrate-based material embedded with hydrous zirconium oxide nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 27982–27991. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2007, 1, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.B.; Gupta, A.; Dubey, S.; Shafeeq, M.; Banerjee, P.; Sinha, A.S.K. Sol–gel synthesis of nanoparticles of gamma alumina and their application in defluoridation of water. J. Sol-Gel Sci. Technol. 2016, 77, 416–422. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.; Daneshvar, E.; Maljanen, M.; Lähde, A.; Jokiniemi, J.; Naushad, M.; Bhatnagar, A. Optimization of fluoride removal from aqueous solution by Al2O3 nanoparticles. J. Mol. Liq. 2017, 238, 254–262. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, C.; Yuan, K.; Gan, X.; Feng, C.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Characterization and adsorption mechanism of ZrO2 mesoporous fibers for health-hazardous fluoride removal. J. Hazard. Mater. 2018, 346, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Babaeivelni, K.; Khodadoust, A.P. Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions. J. Colloid. Interface Sci. 2013, 394, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Zhang, Y.; Wang, H.; Wang, T.; Wang, Y. Performance of granular zirconium-iron oxide in the removal of fluoride from drinking water. Water Res. 2011, 45, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Savari, A.; Hamidi, A.; Farjadfard, S.; Omidvar, M.; Ramavandi, B. Zirconium-based materials for fluoride removal from aqueous environments: A literature review and scientometric analysis. Colloids Interface Sci. Commun. 2023, 55, 100722. [Google Scholar] [CrossRef]
- Muhizi, P.; Dandautiya, R.; Sahu, O. The efficiency of nano metakaolin modified with zirconium oxide for fluoride adsorption from aqueous solution. Next Nanotechnol. 2023, 3–4, 100024. [Google Scholar] [CrossRef]
- Riahi, F.; Bagherzadeh, M.; Hadizadeh, Z. Modification of Fe3O4 superparamagnetic nanoparticles with zirconium oxide; Preparation, characterization and its application toward fluoride removal. RSC Adv. 2015, 5, 72058–72068. [Google Scholar] [CrossRef]
- Bombuwala Dewage, N.; Liyanage, A.S.; Pittman, C.U.; Mohan, D.; Mlsna, T. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour. Technol. 2018, 263, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lei, M.; Zeng, W.; Li, Y.; Li, B.; Liu, D.; Liu, C. Synthesis of magnetic Fe3O4@SiO2-(-NH2/-COOH) nanoparticles and their application for the removal of heavy metals from wastewater. Ceram. Int. 2023, 49, 20470–20479. [Google Scholar] [CrossRef]
- Kouchakipour, S.; Hosseinzadeh, M.; Qaretapeh, M.Z.; Dashtian, K. Sustainable large-scale Fe3O4/carbon for enhanced polystyrene nanoplastics removal through magnetic adsorption coagulation. J. Water Process Eng. 2024, 58, 104919. [Google Scholar] [CrossRef]
- Abbas, R.F.; Hassan, M.J.M.; Rheima, A.M. Adsorption of fast green dye onto Fe3O4 MNPs and GO/Fe3O4 MNPs synthesized by photo-irradiation method: Isotherms, thermodynamics, kinetics, and reuse studies. Sustain. Chem. Environ. 2024, 6, 100104. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Soltys, L.; Macyk, W. Magnetic adsorbents for removal of pharmaceuticals: A review of adsorption properties. J. Mol. Liq. 2023, 384, 122174. [Google Scholar] [CrossRef]
- Liu, M.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Enhanced fluoride adsorption from aqueous solution by zirconium (IV)-impregnated magnetic chitosan graphene oxide. Int. J. Biol. Macromol. 2021, 182, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Sagredo, V.; Torres, T.; Márquez, G. Characterization of magnetite nanoparticles synthesized by the coprecipitation method. Cienc. Ing. 2019, 40, 39–44. [Google Scholar]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, F.; Yu, Z.; Wei, J.; Yang, Z.; Ma, C.; Li, Z.; Xu, Z.Y.; Zeng, G. Performance of magnetic zirconium-iron oxide nanoparticle in the removal of phosphate from aqueous solution. Appl. Surf. Sci. 2017, 396, 1783–1792. [Google Scholar] [CrossRef]
- Alam, E.; Feng, Q.; Yang, H.; Fan, J.; Mumtaz, S.; Begum, F. Synthesis of Fe3O4@mZrO2-Re (Re = Y/La/Ce) by using uniform design, surface response methodology, and orthogonal design & its application for As3+ and As5+ removal. Nanomaterials 2021, 11, 2177. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Khodikar, R.; Ghafuri, H. Nanomagnetic zirconia-based sulfonic acid (Fe3O4@ZrO2-Pr-SO3H): A new, efficient and recyclable solid acid catalyst for the protection of alcohols: Via HMDS under solvent free conditions. RSC Adv. 2016, 6, 63480–63487. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafy, H.I.; Mansour, M.S.M. Removal of pyrene and benzo(a)pyrene micropollutant from water via adsorption by green synthesized iron oxide nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015006. [Google Scholar] [CrossRef]
- Bashir, M.; Riaz, S.; Naseem, S. Magnetic properties of Fe3O4 stabilized zirconia. IEEE Trans. Magn. 2014, 50, 1102204. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic nanoparticles in medical diagnostic applications: Synthesis, characterization and proteins conjugation. Curr. Nanosci. 2015, 12, 455–468. [Google Scholar] [CrossRef]
- Altass, H.M.; Khder, A.E.R.S. Surface and catalytic properties of triflic acid supported zirconia: Effect of zirconia tetragonal phase. J. Mol. Catal. A Chem. 2015, 411, 138–145. [Google Scholar] [CrossRef]
- Kalaiyarasi, J.; Pandian, K. Selective and sensitive electrochemical detection of ATP in human serum samples at ZrO2 hallow spheres modified GCE. J. Electrochem. Soc. 2021, 168, 057524. [Google Scholar] [CrossRef]
- Nakhaei, A.; Davoodnia, A.; Yadegarian, S. Nano-Fe3O4@ZrO2-SO3H as highly efficient recyclable catalyst for the green synthesis of fluoroquinolones in ordinary or magnetized water. Iran. J. Catal. 2018, 8, 47–52. [Google Scholar]
- Imran, M.; Riaz, S.; Sanaullah, I.; Khan, U.; Sabri, A.N.; Naseem, S. Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles. Ceram. Int. 2019, 45, 10106–10113. [Google Scholar] [CrossRef]
- Bashir, M.; Riaz, S.; Naseem, S. Fe3O4 stabilized zirconia: Structural, mechanical and optical properties. J. Sol-Gel Sci. Technol. 2015, 74, 281–289. [Google Scholar] [CrossRef]
- Dou, X.; Mohan, D.; Pittman, C.U.; Yang, S. Remediating fluoride from water using hydrous zirconium oxide. Chem. Eng. J. 2012, 198–199, 236–245. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Lu, G.; Cao, Z.; Cheng, J.; Wang, K.; Wen, X.; Ma, W.; Wu, D.; Liu, C. Facile fabrication of high-contrast and light-colored marking on dark thermoplastic polyurethane materials. ACS Omega 2019, 4, 20787–20796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, T.J.; Jiang, Y.; Fok, J. Synthesis and properties of a high-capacity iron oxide adsorbent for fluoride removal from drinking water. Appl. Surf. Sci. 2017, 425, 272–281. [Google Scholar] [CrossRef]
- Blackwell, J.A.; Carr, P.W. Study of the fluoride adsorption characteristics of porous microparticulate zirconium oxide. J. Chromatogr. A 1991, 549, 43–57. [Google Scholar] [CrossRef]
- Chang, C.; Chang, C.; Hsu, T. Preparation and adsorptive application of novel superparamagnetic zirconia material. Colloids Surf. A: Physicochem. Eng. Asp. 2008, 327, 64–70. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, B.; Yang, W.; Jia, Y.; Jiang, P.; Zhang, Q.; Kong, L.; Liu, J. Fluoride removal performance and mechanism of superparamagnetic Fe3O4 nanoparticles. Desalination Water Treat. 2021, 233, 281–291. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Sharma, A.K.; Philip, L. Manganese-oxide-coated alumina: A promising sorbent for defluoridation of water. Water Res. 2006, 40, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, Q.; Li, Q.; Yan, J.; Zhao, X.; Huang, L.; Zhao, S.; Zhang, H. Experimental and DFT calculation study on the efficient removal of high fluoride wastewater from metallurgical wastewater by kaolinite. Environ. Res. 2024, 260, 119604. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, S.I.; Wang, H.; He, Y.; Yan, L.; Jiang, Y.; Wu, B.; Wang, T.; Gang, H.; Huang, L.; Jin, L.; et al. Fluoride remediation from on-site wastewater using optimized bauxite nanocomposite (Bx-Ce-La@500): Synthesis maximization, and mechanism of F─ removal. J. Hazard. Mater. 2022, 430, 128401. [Google Scholar] [CrossRef] [PubMed]
- Aurich, A.; Hofmann, J.; Oltrogge, R.; Wecks, M.; Gläser, R.; Blömer, L.; Mauersberger, S.; Müller, R.A.; Sicker, D.; Giannis, A. Improved Isolation of Microbiologically Produced (2R,3S)-Isocitric Acid by Adsorption on Activated Carbon and Recovery with Methanol. Org. Process Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Xiao, X. Efficient Fluoride Removal Using a CeO2/Attapulgite (ATP) Composite. Nanomaterials 2025, 15, 357. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: An overview of linear and nonlinear approach and error analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef] [PubMed]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.; Radushkevich, L.V. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Ghosh, A.; Chakrabarti, S.; Biswas, K.; Ghosh, U.C. Agglomerated nanoparticles of hydrous Ce(IV) + Zr(IV) mixed oxide: Preparation, characterization and physicochemical aspects on fluoride adsorption. Appl. Surf. Sci. 2014, 307, 665–676. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Carvajal Flórez, E. Adsorption isotherms for copper and lead removal from landfill leachate. Ing. Compet. 2024, 26, e-20912457. [Google Scholar] [CrossRef]
- Qiu, H.; Liang, C.; Zhang, X.; Chen, M.; Zhao, Y.; Tao, T.; Xu, Z.; Liu, G. Fabrication of a biomass-based hydrous zirconium oxide nanocomposite for preferable phosphate removal and recovery. ACS Appl. Mater. Interfaces 2015, 7, 20835–20844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tan, X.; Yu, T. Effectively arsenic(V) and fluoride removal in geothermal water using magnetic Fe3O4@MgO nanoparticles. Chin. Chem. Lett. 2023, 34, 107748. [Google Scholar] [CrossRef]
- Sarwar, A.; Wang, J.; Khan, M.S.; Farooq, U.; Riaz, N.; Nazir, A.; Mahmood, Q.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Iron oxide (Fe3O4)-supported SiO2 magnetic nanocomposites for efficient adsorption of fluoride from drinking water: Synthesis, characterization, and adsorption isotherm analysis. Water 2021, 13, 1514. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; McKay, G.; Kadhim, A.; Joybari, M.M.; Balarak, D. Activated carbon prepared from hazelnut shell waste and magnetized by Fe3O4 nanoparticles for highly efficient adsorption of fluoride. Biomass Convers. Biorefinery 2024, 14, 4687–4702. [Google Scholar] [CrossRef]
- Sarwar, A.; Wang, J.; Riaz, N.; Khan, M.S.; Zeb, B.S.; Khan, I.A.; Akmal, M.; Khalid, A.; Khan, A.; Al-Harrasi, A.; et al. Optimizing the fluoride removal from drinking water through adsorption with mesoporous magnetic calcite nanocomposites. Results Eng. 2024, 22, 102100. [Google Scholar] [CrossRef]
- Bao, X.; Qiang, Z.; Chang, J.H.; Ben, W.; Qu, J. Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. J. Environ. Sci. 2014, 26, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Ma, C.; He, H.; Wei, L.; Xia, F. Preparation of Fe3O4/NiO Nanomaterials by Electrodeposition and Their Adsorption Performance for Fluoride Ions. Coatings 2024, 14, 739. [Google Scholar] [CrossRef]
- Kimiagar, S.; Mirazimi, H. Hydrothermal synthesis of Fe3O4-ZnO nanocomposites for removing fluoride from water. Progr Phys. Appl. Mat. 2024, 4, 83–91. [Google Scholar] [CrossRef]
- Kamoun, N.; Raissi, S.; Younes, M.K.; Elfil, H. Multifunctional nanoparticles as effective adsorbents for fluoride removal from synthetic and drinking waters: Equilibrium, kinetics, and thermodynamics. React. Kinet. Mech. Catal. 2024, 137, 3393–3415. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Li, Y.; Qin, Z.; Li, X.; Li, Y.; Zhang, K. Double cross-linked chitosan sponge encapsulated with ZrO2/soy protein isolate amyloid fibrils nanoparticles for the fluoride ion removal from water. Int. J. Biol. Macromol. 2024, 279, 135520. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cai, X.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Meng, F.; Liu, N.; Wang, X.; Kong, L.; et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal. J. Colloid. Interface Sci. 2016, 484, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, W.; Cao, M.; Yang, H.; Li, Y. Constructing hydrangea-like hierarchical zinc-zirconium oxide microspheres for accelerating fluoride elimination. J. Mol. Liq. 2020, 317, 114133. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Chen, L.; Jia, Y.; Wang, L.; Huang, X.J.; Liu, J. Excellent fluoride removal performance by CeO2-ZrO2 nanocages in water environment. Chem. Eng. J. 2013, 231, 198–205. [Google Scholar] [CrossRef]
- Rahman, N.; Nasir, M. Development of Zr(IV)—Doped polypyrrole/zirconium (IV) iodate composite for efficient removal of fluoride from water environment. J. Water Process Eng. 2017, 19, 172–184. [Google Scholar] [CrossRef]
- Mohamed, A.; Valadez Sanchez, E.P.; Bogdanova, E.; Bergfeldt, B.; Mahmood, A.; Ostvald, R.V.; Hashem, T. Efficient fluoride removal from aqueous solution using zirconium-based composite nanofiber membranes. Membranes 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]


| Model | Equation | qe (mg/g) | Constant(s) | R2 | Reduced χ2 |
|---|---|---|---|---|---|
| Pseudo-first order | qt = qe · (1 − e^(−k1·t)) | 9.69 ± 0.08 | k1 = 0.2135 ± 0.0186 (1/min) | 0.8063 | 0.04012 |
| Pseudo-second order | qt = (qe2·k2·t)/(1 + qe·k2·t) | 9.86 ± 0.04 | k2 = 0.0731 ± 0.0057 (g/mg·min) | 0.9724 | 0.00572 |
| Elovich | qt = (1/β)·ln(1 + α·β·t) | – | α = 7.04 × 1019 ± 1.18 × 1021 (mg/g·min) | 0.6095 | 0.08089 |
| β = 5.46 ± 1.79 (g/mg) |
| Isotherm | Equation | Parameters | |||
|---|---|---|---|---|---|
| Model | R2 | χ2 | |||
| Langmuir | KL | ||||
| (mg/g) | (L/g) | ||||
| Nonlinear | 70.40 | 0.21 | 0.905 | 56.52 | |
| Linear | 70.42 | 0.43 | 0.942 | - | |
| Freundlich | KF | n | |||
| (mg/g) | |||||
| Nonlinear | 20.80 | 0.24 | 0.948 | 31.30 | |
| Linear | 14.62 | 0.33 | 0.88 | - | |
| Temkin | bT | AT | |||
| (J/mol) | (L/g) | ||||
| Nonlinear | 274.4 | 10.42 | 0.987 | 7.91 | |
| Linear | 251.1 | 8.60 | 0.975 | - | |
| Fe3O4–ZrO2 | |
|---|---|
| Intersection, (ln K0) | 4.185 |
| ΔG0(kJ/mol) | −10.37 |
| R2 | 0.9461 |
| qDR (mg/g) | KDR (mol2/kJ2) | EDR (kJ/mol) | R2 |
|---|---|---|---|
| 119.12 | 3.41 × 10−3 | 12.11 | 0.932 |
| Reference | Material | Max Adsorption Capacity (mg/g) | pH Range/Optimal | Contact Time | Synthesis Method | Magnetic/ Calcination/ Scalable |
|---|---|---|---|---|---|---|
| [62] | Fe3O4@MgO | 98.4 | 2–11/7 | 30 min | SE, C 700 °C | Yes/Yes/No |
| [63] | Fe3O4-SiO2 | 5.6 | ND/6.8 | 10 min | CP, C 500 °C | Yes/Yes/No |
| [64] | Fe3O4-Activated Carbon | 146.2 | 4–11/7 | 75 min | CP, CA: 650 °C | Yes/Yes/No |
| [65] | Fe3O4-CaCO3 | 17.95 | 2–12/6.8 | 60 min | WI, C 500 °C | Yes/Yes/No |
| [66] | Fe3O4@SiO2/Cdots | 21.4 | ND/5.5 | 60 min | HT, SG | Yes/No/No |
| [67] | Fe3O4@NiO | 63.86 | ND/4 | 120 min | ED | Yes/No/No |
| [68] | Fe3O4-ZnO | 7.8 | ND | 80 min | HT | Yes/No/No |
| [69] | SO42−-ZrO2–CeO2 | 10.37 | 2–10/7 | 35 s | SG | No/No/No |
| [70] | ZrO2-Chitosan Composite | 44.44 | 3–11/4–10 | 600 min | SG, CL | No/No/No |
| [71] | ZrO2-MOFs | 102.4 | ND/7 | 24 h | ST | No/No/No |
| [72] | ZrO2-ZnO | 107.41 | ND/7 | 24 h | HT | No/Yes/No |
| [73] | CeO2-ZrO2 | 175 | ND/4 | 24 h | ST | No/No/No |
| [74] | Zr(IV)-Polypyrrole-Zr iodate | 183.5 | ND/6.8 | Column | P | No/No/No |
| [75] | UiO-66-NH2 Zr metal-organic frameworks | 97 | ND/8 | 60 min | ST, ES | No/No/No |
| This work | Fe3O4–ZrO2 | 70.8 | 1–10/3 | 120 min | CP | Yes/No/Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Águila-Martínez, I.; Pérez-Tavares, J.A.; González-Aguiñaga, E.; Cardoso-Avila, P.E.; Pérez Ladrón de Guevara, H.; Patakfalvi, R. Synthesis and Application of Fe3O4–ZrO2 Magnetic Nanoparticles for Fluoride Adsorption from Water. Inorganics 2025, 13, 248. https://doi.org/10.3390/inorganics13070248
Águila-Martínez I, Pérez-Tavares JA, González-Aguiñaga E, Cardoso-Avila PE, Pérez Ladrón de Guevara H, Patakfalvi R. Synthesis and Application of Fe3O4–ZrO2 Magnetic Nanoparticles for Fluoride Adsorption from Water. Inorganics. 2025; 13(7):248. https://doi.org/10.3390/inorganics13070248
Chicago/Turabian StyleÁguila-Martínez, Israel, José Antonio Pérez-Tavares, Efrén González-Aguiñaga, Pablo Eduardo Cardoso-Avila, Héctor Pérez Ladrón de Guevara, and Rita Patakfalvi. 2025. "Synthesis and Application of Fe3O4–ZrO2 Magnetic Nanoparticles for Fluoride Adsorption from Water" Inorganics 13, no. 7: 248. https://doi.org/10.3390/inorganics13070248
APA StyleÁguila-Martínez, I., Pérez-Tavares, J. A., González-Aguiñaga, E., Cardoso-Avila, P. E., Pérez Ladrón de Guevara, H., & Patakfalvi, R. (2025). Synthesis and Application of Fe3O4–ZrO2 Magnetic Nanoparticles for Fluoride Adsorption from Water. Inorganics, 13(7), 248. https://doi.org/10.3390/inorganics13070248











