Cisplatin, the Timeless Molecule
Abstract
1. Introduction
2. History of Cisplatin: An “Accidental” Discovery
3. Mechanism of Action of Cisplatin in Cancer
4. Side Effects of Cisplatin
5. Uses of Cisplatin in Cancer
5.1. Breast Cancer
5.2. Gastrointestinal Tumor
5.3. Lung Cancer
5.4. Cholangiocarcinoma
5.5. Testis Cancer
5.6. Ovarian Cancer
5.7. Head and Neck Cancer
5.8. Bladder Cancer
5.9. Brain Cancer
5.10. Oral Squamous Cell Carcinoma
5.11. Brain Metastases and Leptomeningeal Metastases
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEEP | Bevacizumab-etoposide-cisplatin |
| BC | Breast cancer |
| BCa | Bladder cancer |
| BEP | Bleomycin–etoposide–cisplatin |
| BM | Brain metastases |
| BMBC | Brain metastases from breast cancer |
| circRNAs | Circular RNAs |
| CVT | Cisplatin–vinblastine–temozolomide |
| EMT | Epithelial–mesenchymal transition |
| ES-SCLC | Extensive small cell lung cancer |
| FDA | Food and Drug Administration |
| GC | Gemcitabine and cisplatin |
| IVC | Impending visceral crisis |
| LM | Leptomeningeal |
| MBC | Metastatic breast cancer |
| mTNBC | Metastatic triple-negative breast cancer |
| NAX | Neoadjuvant chemotherapeutics |
| NCI | National Cancer Institute |
| NSCLC | Non-small cell lung cancer |
| PF | Cisplatin–fluorouracil |
| RRM | Relapsed refractory melanoma |
| SCLC | Small cell lung cancer |
| TACE | Transcatheter arterial chemoembolization |
| TNBC | Triple negative breast cancer |
| TP | Docetaxel–cisplatin |
| VC | Visceral crisis |
| VIP | Vindesine–ifosfamide–platinum |
| WRBT | Palliative whole-brain radiotherapy |
References
- Romani, A.M. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Koumaki, K.; Skarmalioraki, S.; Kosmidou, V.; Krikoni, L.; Goulielmaki, M.; Zoumpourlis, V.; Pintzas, A.; Souliotis, V.L. Antitumorigenic Effect of Combination Treatment with BRAF Inhibitor and Cisplatin in Colorectal Cancer In Vitro and In Vivo. Adv. Ther. 2025, 8, 2400250. [Google Scholar] [CrossRef]
- Lajous, H.; Lelièvre, B.; Vauléon, E.; Lecomte, P.; Garcion, E. Rethinking Alkylating (-like) Agents for Solid Tumour Management. Trends Pharmacol. Sci. 2019, 40, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Li, C.; Mu, X.; Mu, Q.; Zhang, X.; Su, X. ZNF703 Promotes Triple-Negative Breast Cancer Cell Progression and in Combination with STK11 Predicts Disease Recurrence (ZS− TNBC Model). Gene 2025, 942, 149258. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, K.; Krug, D. BRCAness Identifies Synthetic Cytotoxicity Between Cisplatin and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin Nephrotoxicity: New Insights and Therapeutic Implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Wang, D.; Wang, Y.; Zhou, Z.; Ma, X.; Liu, X.; Dong, Y. Cisplatin-Induced Ototoxicity: From Signaling Network to Therapeutic Targets. Biomed. Pharmacother. 2023, 157, 114045. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Atuati, S.F.; Oliveira, S.M. G Protein-Coupled Receptors and Ion Channels Involvement in Cisplatin-Induced Peripheral Neuropathy: A Review of Preclinical Studies. Cancers 2024, 16, 580. [Google Scholar] [CrossRef] [PubMed]
- León, I.E. Transition Metal Complexes: A New Generation of Anticancer Drugs. Fut. Med. Chem. 2024, 16, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, H.K.; Ortiz, C.J.C.; Arshad, T.; Padrón, J.M.; Khan, H. Advancements in Steroidal Pt (II) & Pt (IV) Derivatives for Targeted Chemotherapy (2000–2023). Eur. J. Med. Chem. 2024, 271, 116438. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Deb, P.; Basu, T.; Bardhan, S.; Patra, S.; Sukul, P.K. Advancements in Platinum-Based Anticancer Drug Development: A Comprehensive Review of Strategies, Discoveries, and Future Perspectives. Bioorg. Med. Chem. 2024, 112, 117894. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, L.; Wu, X.; Wang, Y.; Tian, H.; Zhang, G.; Wan, A.; Xiong, S.; Wang, C.; Zhou, Y.; et al. Taxane Combined with Lobaplatin or Anthracycline for Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: A Randomized, Controlled, Phase II Study. BMC Med. 2024, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Nie, P.; Herdewijn, P.; Sun, J.G. Unlocking the Synthetic Approaches and Clinical Application of Approved Small-Molecule Drugs for Gastrointestinal Cancer Treatment: A Comprehensive Exploration. Eur. J. Med. Chem. 2023, 262, 115928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, H.; Jiao, R.; Liu, H.; Qin, C.; Xu, L.; Chen, Y. Supramolecular Chemotherapy: Host–Guest Complexes of Heptaplatin-Cucurbit [7]Uril toward Colorectal Normal and Tumor Cells. Langmuir 2021, 37, 5475–5482. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J. Lobaplatin: A New Antitumour Platinum Drug. Exp. Opin. Investig. Drugs 2001, 10, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Malhotra, A.; Bansal, R. Synthetic cytotoxic drugs as cancer chemotherapeutic agents. In Medicinal Chemistry of Chemotherapeutic Agents; Elsevier: Amsterdam, The Netherlands, 2023; pp. 499–537. [Google Scholar]
- Acharya, P.C.; Kurosu, M. Medicinal Chemistry of Chemotherapeutic Agents: A Comprehensive Resource of Anti-Infective and Anti-Cancer Drugs; Academic Press: Cambridge, MA, USA, 2023; pp. 363–396. ISBN 9780323905756. [Google Scholar]
- Tanaka, T. Transarterial Chemoembolization for Hepatocellular Carcinoma: Current Role and Techniques. Intervent. Radiol. 2025, 10, e2024-0016. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Cai, M.; Shao, R.; Xia, G.; Zhao, W. Miriplatin-Loaded Liposome, as a Novel Mitophagy Inducer, Suppresses Pancreatic Cancer Proliferation through Blocking POLG and TFAM-Mediated mtDNA rRplication. Acta Pharm. Sin. B 2023, 13, 4477–4501. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kudo, M.; Aikata, H.; Nagamatsu, H.; Ishii, H.; Yokosuka, O.; Torimura, T.; Morimoto, M.; Ikeda, K.; Kumada, H.; et al. Transarterial Chemoembolization with Miriplatin vs. Epirubicin for Unresectable Hepatocellular Carcinoma: A Phase III Randomized Trial. J. Gastroenterol. 2018, 53, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Efficacy and Features of Balloon-Occluded Transarterial Chemoembolization for Hepatocellular Carcinoma: A Narrative Review. Transl. Gastroenterol. Hepatol. 2024, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Datta, A.; Das, A.; Adhikari, S. Metal-Based Drugs in Cancer Therapy. Int. J. Exp. Res. Rev. 2024, 37, 159–173. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Bełdzińska, P.; Galikowska-Bogut, B.; Zakrzewski, M.; Bury, K.; Jamrógiewicz, M.; Wyrzykowski, D.; Gołuński, G.; Sądej, R.; Piosik, J. Platinum as Both a Drug and Its Modulator—Do Platinum Nanoparticles Influence Cisplatin Activity? Chem. Biol. Interact. 2025, 407, 111365. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Nijiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Li, Z.; Men, L.; Zuo, H.; Gong, X. Cisplatin-Based Combination Therapies: Their Efficacy with a Focus on Ginsenosides Co-administration. Pharmacol. Res. 2024, 203, 107175. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, X.; Tang, Y.; Tang, H.; Xia, C. Natural Products Reverse Cisplatin Resistance in the Hypoxic Tumor Microenvironment. Cancer Lett. 2024, 598, 217116. [Google Scholar] [CrossRef] [PubMed]
- Zoń, A.; Bednarek, I. Cisplatin in Ovarian Cancer Treatment—Known Limitations in Therapy Force New Solutions. Int. J. Mol. Sci. 2023, 24, 7585. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, G.B.; Pentimalli, R.; Doldi, S.; Hall, M.D. Michele Peyrone (1813–1883), Discoverer of Cisplatin. Platin. Met. Rev. 2010, 54, 250–256. [Google Scholar] [CrossRef]
- The “Accidental” Cure—Platinum-Based Treatment for Cancer: The Discovery of Cisplatin. Available online: https://www.cancer.gov/research/progress/discovery/cisplatin (accessed on 28 April 2025).
- Monneret, C. Platinum anticancer drugs. From Serendipity to Rational Design. Ann. Pharm. Fr. 2011, 69, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, V.; Fuertes, M.A.; Castilla, J.; Alonso, C.; Quevedo, C.; Pérez, J.M. Biochemical Mechanisms of Cisplatin Cytotoxicity. Anticancer Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Alderden, R.A.; Hall, M.D.; Hambley, T. The Discovery and Development of Cisplatin. J. Chem. Educ. 2006, 83, 728–734. [Google Scholar] [CrossRef]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by Cisplatin Requires p53 Mediated p38alpha MAPK Activation through ROS Generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Xu, J.; Chong, J.; Wang, D. Molecular basis of transcriptional pausing, stalling, and transcription-coupled repair initiation. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194659. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Jebrouni, F.; Bailal, H.; Omari, M.; Khater, K.; Bali, A.; Al Jarroudi, O.; Brahmi, S.A.; Afqir, S. Management of Cisplatin-Induced Encephalopathy: A Case Report and Literature Review. Cureus 2024, 16, e62176. [Google Scholar] [CrossRef] [PubMed]
- Abass, S.A.; Elgazar, A.A.; El-Kholy, S.S.; El-Refaiy, A.I.; Nawaya, R.A.; Bhat, M.A.; Farrag, F.A.; Hamdi, A.; Balaha, M.; El-Magd, M.A. Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations. Molecules 2024, 29, 1927. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, X.; Li, X.; Li, Q.; Fang, X. Effects of ginsenoside Rh2 on cisplatin-induced nephrotoxicity in renal tubular epithelial cells by inhibiting endoplasmic reticulum stress. J. Biochem. Mol. Toxicol. 2024, 38, e23768. [Google Scholar] [CrossRef]
- Kazmi, I.; Altayb, H.N.; Al-Abbasi, F.A.; Alharbi, K.S.; Almalki, N.A.R.; Moglad, E.; Al-Qahtani, S.D.; Bawadood, A.S.; Sayyed, N. Rosiridin Prevents Cisplatin-Induced Renal Toxicity by Inhibiting Caspase-3/NF-κB/Bcl-2 Signaling Pathways in Rats and In Silico Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 5895–5913. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Chang, D.; Du, X.; Sun, C. Berberrubine Protects Against Cisplatin-Induced Ototoxicity by Promoting Folate Biosynthesis. Front. Pharmacol. 2025, 15, 1496917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qian, J.; Wei, B.; Zhang, B. Flavonoids as modulators of Nrf2 signaling pathway in alleviating cisplatin-induced organ toxicity. Yangtze Med. 2025, 9, 52–77. [Google Scholar] [CrossRef]
- Aktas, I.; Gur, F.M.; Bilgiç, S. Protection of Lutein against the Toxic Effect of Cisplatin on Liver in Male Rat. Prostaglandins Other Lipid Mediat. 2025, 178, 106995. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Liu, S.J.; Lee, R.; Boyd, J.; Geary, K.; Zhang, D. Using Cimetidine to Mitigate Cisplatin-Induced Ototoxicity. Toxicol. In Vitro 2025, 104, 106025. [Google Scholar] [CrossRef] [PubMed]
- Hazem, S.H.; Saad, K.M.; Samaha, M.M. Protective Effects of BTK Inhibition by Acalabrutinib on Cisplatin-Induced Renal and Testicular Injury in Mice: Modulation of mTOR/AMPK, NLRP3/GSDMD-N, and Apoptotic Pathways. Int. Immunopharmacol. 2025, 149, 114256. [Google Scholar] [CrossRef] [PubMed]
- Anbar, H.S.; Radi, A.W.; Shaker, H.A.; Janoudi, L.F.; Zain, H.N.A.; Hassan, H.M.; Hersi, F.; El-Gamal, M.; Dohle, W.; Potter, B.V.L.; et al. Protective Potential of Irosustat, STX140 and the Sulfonate Derivative 1G in Counteracting Cisplatin-Induced Renal and Hepatic Toxicities: An In Vivo Comparative Study. Life Sci. 2025, 378, 123793. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Fujihara, H.; Maita, K.; Miyakawa, M.; Sakai, Y.; Nakayama, R.; Ito, Y.; Hasebe, M.; Kawaguchi, K.; Hamada, Y. Combinatorial Effects of Cisplatin and PARP Inhibitor Olaparib on Survival, Intestinal Integrity, and Microbiome Modulation in Murine Model. Int. J. Mol. Sci. 2025, 26, 1191. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Valencia, A.C.; Velasco-Hidalgo, L.; Martínez-Avalos, A.; Castillejos-López, M.; Torres-Espíndola, L.M. Effect of N-Acetylcysteine on Cisplatin Toxicity: A Review of the Literature. Biol. Targets Ther. 2024, 18, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Seddiek, H.; Hanna, M.; Hamoud, A.E.M.; Elbaset, M.A.; Akabawy, A.M.; Kotb, M.Z.; Khalifa, M.M. Deferiprone Ameliorates Cisplatin Induced Peripheral Neurotoxicity via Ferritinophagy Adjustment. Sci. Rep. 2025, 15, 4485. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in Understanding Cisplatin-Induced Toxicity: Molecular Mechanisms and Protective Strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Tian, Y.; Gu, M.; Wang, Y.; Ashrafizadeh, M.; Aref, A.R.; Cañadas, I.; Klionsky, D.J.; Goel, A.; et al. Autophagy-Driven Regulation of Cisplatin Response in Human Cancers: Exploring Molecular and Cell Death Dynamics. Cancer Lett. 2024, 587, 216659. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, S.; Oura, K.; Morishita, A.; Masaki, T. Cisplatin in liver cancer therapy. Int. J. Mol. Sci. 2023, 24, 10858. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present, and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Meshesha, S.M.; Hong, C.E. Synergistic Effects of Mistletoe Lectin and Cisplatin on Triple-Negative Breast Cancer Cells: Insights from 2D and 3D In Vitro Models. Int. J. Mol. Sci. 2025, 26, 366. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, R.; Hassannia, H.; Arabnozari, H.; Enderami, S.E.; Habibi, E. Natural Polysaccharides in Breast Cancer: Fucoidan’s Role in Enhancing Cisplatin Cytotoxicity and Reducing Chemotherapy Resistance. In Latest Research on Breast Cancer; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Chen, H.; Wang, Y.; Zhang, J.; Song, D.; Tan, Y.; Guo, Z.; Wang, X. Platinum(IV) Complexes Trigger Death Receptors and Natural Killer Cells to Suppress Breast Cancer. J. Med. Chem. 2025, 68, 9162–9175. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Hanif, M.; Yang, P. Beyond Cisplatin: New Frontiers in Metallodrugs for Hard-to-Treat Triple Negative Breast Cancer. Coord. Chem. Rev. 2024, 499, 215507. [Google Scholar] [CrossRef]
- Mason, S.R.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum-based Chemotherapy for Early Triple-Negative Breast Cancer. Cochrane Database Syst. Rev. 2023, 9, CD014805. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, A.; Rea, C.; Parati, M.C.; Borgonovo, K.; Ghidini, M.; Ruatta, F.; Zaniboni, A.; Luciani, A.; Garrone, O.; et al. Platinum Dose in Neoadjuvant Therapy for Triple-Negative Breast Cancer: A Systematic Review and Network Meta-Analysis. Curr. Probl. Cancer 2024, 50, 101096. [Google Scholar] [CrossRef] [PubMed]
- Püsküllüoğlu, M.; Pieniążek, M.; Rudzińska, A.; Pietruszka, A.; Pacholczak-Madej, R.; Grela-Wojewoda, A.; Ziobro, M. Cisplatin Monotherapy as a Treatment Option for Patients with HER-2 Negative Breast Cancer Experiencing Hepatic Visceral Crisis or Impending Visceral Crisis. Oncol. Ther. 2024, 12, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, Z.; Shen, G.; Wang, T.; Li, J.; Wang, M.; Liu, Z.; Zhao, F.; Ren, D.; Zhao, J. Efficacy and safety of first-line treatment for metastatic triple-negative breast cancer: A network meta-analysis. Cancer Pathog. Ther. 2024, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, T.J.; Batista de Carvalho, A.L.; Vojtek, M.; Laginha, R.C.; Marques, M.P.M.; Diniz, C.; Gil, A.M. Pd2Spermine as an Alternative Therapeutics for Cisplatin-Resistant Triple-Negative Breast Cancer. J. Med. Chem. 2024, 67, 6839–6853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Yin, W.; Wang, Y.; Lu, J. Metronomic Paclitaxel and Cisplatin Chemotherapy for Metastatic Breast Cancer: Insights from a Target Trial Emulation. Lancet Reg. Health–West. Pacif. 2025, 55, 101457. [Google Scholar] [CrossRef]
- Smothers, A.R.; Beasley, M.E.; Warren, H.S.; Kegel, O.G.; Edenfield, W.J.; O’Connell, J.J.; Booth, B.W. Tumor-Treating Fields and Concurrent Cisplatin: An In Vitro Demonstration of Efficacy in Triple-Negative Breast Cancer. Am. J. Cancer Res. 2025, 15, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, J.; Liu, J.; Wang, H.; Wang, T.; Li, S.; Niu, L.; Wei, Y. FOXD1 Activates KIFC1 To Modulate Aerobic Glycolysis and Reinforce Cisplatin Resistance of Breast Cancer. Reproduct. Biol. 2025, 25, 100969. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.; Podder, S.; Dang, H.; Lin, D. Advances in Immunotherapy in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 1936. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current Developments in Gastric Cancer: From Molecular Profiling to Treatment Strategy. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Depotte, L.; Palle, J.; Rasola, C.; Broudin, C.; Afrăsânie, V.A.; Mariani, A.; Zaanan, A. New Developments and Standard of Care in the Management of Advanced Gastric Cancer. Clin. Res. Hepatol. Gastroenterol. 2023, 48, 102245. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xu, S.; Zhang, P.; Wang, Y. Transcription Factor E2F8 Promotes Cisplatin Resistance in Hepatocellular Carcinoma by Regulating DNA Damage via NUSAP1. Int. J. Toxicol. 2023, 42, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.; Wahab, H.A.; Attiq, A.; Amjad, M.W.; Bukhari, S.N.A.; Ahmad, W. Platinum-Based Targeted Chemotherapies and Reversal of Cisplatin Resistance in Non-Small Cell Lung Cancer (NSCLC). Mut. Res. Fundament. Mol. Mech. Mutagen. 2024, 828, 111856. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tong, W.; Jiang, M.; Liu, H.; Meng, C.; Wang, K.; Mu, X. Mitochondria-targeted multifunctional nanoprodrugs by inhibiting metabolic reprogramming for combating cisplatin-resistant lung cancer. ACS Nano 2024, 18, 21156–21170. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, P.; Zhang, Q.; Wang, B.; Zhao, Q.; Liu, F.; Ma, X.; Zhao, C.; Zhou, X.; Chen, R.; et al. Transcriptome Analysis Reveals PRKCA as a Potential Therapeutic Target for Overcoming Cisplatin Resistance in Lung Cancer Through Ferroptosis. Heliyon 2024, 10, e30780. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.; Pu, X.; Ren, T.; Zhang, F.; Shen, M.; Zhu, Y.; Kros, A.; Yang, J. Combating Cisplatin-Resistant Lung Cancer Using a Coiled-Coil Lipopeptides Modified Membrane Fused Drug Delivery System. J. Control. Release 2025, 379, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Li, P.; Li, Y.; Wu, J.; Wang, J.; Zhao, S.; Li, G.; Xu, S.; Chen, H.; et al. Cost-effectiveness Analysis of Pembrolizumab Combined with Gemcitabine and Cisplatin for the First-line Treatment for Advanced Cholangiocarcinoma. Heliyon 2025, 11, e42553. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Kerns, S.L.; Dinh, P.C., Jr.; Monahan, P.O.; Stump, T.; Fung, C.; Sesso, H.D.; Feldman, D.R.; Hamilton, R.J.; Vaughn, D.J.; Huddart, R.; et al. Factors Associated with Longitudinal Progression of the Cumulative Burden of Morbidity and Overall Mortality after Cisplatin-Based Chemotherapy for Testicular Cancer. J. Natl. Cancer Inst. 2025, djaf014. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian Cancer Today and Tomorrow: A Global Assessment by World Region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular Matrix in High-Grade Serous Ovarian Cancer: Advances in Understanding of Carcinogenesis and Cancer Biology. Matrix Biol. 2023, 118, 16–46. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Kim, H.Y.; Lee, G.H.; Lim, Y.J.; Chae, H.J.; Kim, J.Y.; Yoon, H. Mechanistic Insights into Epithelial-Mesenchymal Transition Mediated Cisplatin Resistance in Ovarian Cancer. Sci. Rep. 2025, 15, 3053. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Marques, I.S.; Melo, I.G.D.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Xu, S.; Zhu, A.; Zheng, Z.; Chen, W.; Jiang, S. AHR Suppresses Cisplatin-Induced Apoptosis in Ovarian Cancer Cells by Regulating XIAP. Biochem. Pharmacol. 2025, 231, 116640. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Adelstein, D.; Van Gestel, D.; Vermorken, J.B. Low-dose vs. High-Dose Cisplatin: Lessons Learned from 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front. Oncol. 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Schaeffers, A.W.M.A.; van Neerven, C.B.; van Balen, D.; Crul, M.; Slingerland, M.; Luelmo, S.A.; de Boer, J.P.; Voortman, J.; van Zuilen, A.D.; de Bree, R.; et al. Effect of Hydration Schedules on Dose-Limiting Toxicity in Patients with Head and Neck Squamous Cell Carcinoma Treated with Cisplatin. Br. J. Clin. Pharmacol. 2025, 91, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Schaeffers, A.W.; Devriese, L.A.; van Gils, C.H.; Dankbaar, J.W.; Voortman, J.; de Boer, J.P.; Slingerland, M.; Hendrix, M.P.; Smid, E.J.; Frederix, G.W.J.; et al. Low Dose Cisplatin Weekly versus High Dose Cisplatin Every Three Weeks in Primary Chemoradiotherapy in Head and Neck Cancer Patients with Low Skeletal Muscle Mass: The CISLOW-Study Protocol. PLoS ONE 2023, 18, e0294147. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.V.; Koot, M.A.; van Balen, D.E.; Schaeffers, A.W.; Zuur, C.L.; Devriese, L.A.; de Ridder, M.; Hoetink, A.E.; Schutte, T.; Crul, M. Comparison of Cisplatin-Induced Hearing Loss in Different Durations of Infusion and Volume of Hydration Schedules in Head and Neck Squamous Cell Carcinoma Patients Treated with Cisplatin-Based Chemoradiation. Oral Oncol. 2025, 164, 107246. [Google Scholar] [CrossRef] [PubMed]
- Jantarat, A.; Thamlikitkul, L.; Thephamongkhol, K.; Setakornnukul, J.; Phisalprapa, P.; Kositamongkol, C.; Srithongkul, T.; Ithimakin, S. Efficacy and Safety of Short Intravenous Hydration for Preventing Nephrotoxicity from High-Dose Cisplatin: A Randomized, Open-Label, Phase II Trial. JCO Glob. Oncol. 2025, 11, e2400515. [Google Scholar] [CrossRef] [PubMed]
- Geynisman, D.M.; Broughton, E.; Hao, Y.; Zhang, Y.; Le, T.; Huo, S. Real-World Treatment Patterns and Clinical Outcomes among Patients with Advanced Urothelial Carcinoma in the United States. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 195.e1–195.e11. [Google Scholar] [CrossRef] [PubMed]
- Bladder Cancer Statistics|World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/bladder-cancer-statistics (accessed on 28 April 2025).
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zheng, Z.; Chen, W.; Li, D.; Zhang, H.; Zhu, Y.; Mo, Q.; Zhao, X.; Fan, Q.; Deng, F.; et al. Regulation of Cisplatin Resistance in Bladder Cancer by Epigenetic Mechanisms. Drug Resist. Updat. 2023, 68, 100938. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, H.; Huang, Y.; Li, D.; Zheng, Z.; Xie, K.; Cao, C.; Wang, Q.; Zhao, X.; Huang, Z.; et al. Single-cell Transcriptome Analysis Reveals the Association Between Histone Lactylation and Cisplatin Resistance in Bladder Cancer. Drug Resist. Updates 2024, 73, 101059. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, Y.; Wang, X.; Chen, S.; Liu, Z.; Li, Z.; Tang, X.; Zhang, Q. PTBP1-Mediated Biogenesis of circATIC Promotes Progression and Cisplatin Resistance of Bladder Cancer. Int. J. Biol. Sci. 2024, 20, 3570–3589. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Huang, S.; Yan, Z.; He, Q.; Liu, C.; Cheng, L.; Cong, Y.; Chen, K.; Song, Y.; Xing, Y. CircUGGT2 Facilitates Progression and Cisplatin Resistance of Bladder Cancer Through Nonhomologous End-Joining Pathway. Cell Signal. 2024, 119, 111164. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, K.; Huang, X.; Tian, S.; Wang, H.; Zhang, C.; Ye, J.; Dong, Y.; An, Z.; Ma, X.; et al. EIF4A3-Mediated Biogenesis of circSTX6 Promotes Bladder Cancer Metastasis and Cisplatin Resistance. J. Exp. Clin. Cancer Res. 2024, 43, 2. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gao, D.; Shi, Y.; Fan, G.; Yu, X.; Yang, E.; Cheng, H.; Tian, J.; Ding, H.; Liu, S.; et al. SRC Enhanced Cisplatin Resistance in Bladder Cancer by Reprogramming Glycolysis and Pentose Phosphate Pathway. Comm. Biol. 2025, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Entezari, M.; Clark, C.; Zabolian, A.; Ranjbar, E.; Farahani, M.V.; Saleki, H.; Sharifzadeh, S.O.; Far, F.B.; Ashrafizadeh, M.; et al. Targeted Regulation of Autophagy Using Nanoparticles: New Insight into Cancer Therapy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166326. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Tang, Y.; Xu, W.; Wang, M.; Liu, J.; Li, Y.; He, J.; Peng, Y.; Sun, P.; Liao, D.; et al. Engineered Biomimetic Cisplatin-Polyphenol Nanocomplex for Chemo-Immunotherapy of Glioblastoma by Inducing Pyroptosis. J. Nanobiotechnol. 2025, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, J.; Li, Y.; Li, W. Pluronic F127-Complexed PEGylated Poly(glutamic acid)-Cisplatin Nanomedicine for Enhanced Glioblastoma Therapy. Macromol. Rapid Commun. 2024, 45, e2400662. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Rajkumar, S.; Shepard, M.J.; Wegner, R.E. National Trends in Radiation Treatment for Small Cell Lung Cancer Brain Metastases in the Modern Era. Adv. Rad. Oncol. 2025, 10, 101720. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Wang, T.; Xu, T.; Zhou, Q. Comparing the Effectiveness and Safety of First-Line Interventions in Patients with Advanced Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer, with Particular Focus on Brain Metastatic Status: A Systematic Review and Network Meta-analysis. Clin. Oncol. 2025, 40, 103776. [Google Scholar] [CrossRef] [PubMed]
- Tagore, S.; Caprio, L.; Amin, A.D.; Bestak, K.; Luthria, K.; D’Souza, E.; Barrera, I.; Melms, J.C.; Wu, S.; Abuzaid, S.; et al. Single-cell and Spatial Genomic Landscape of Non-small Cell Lung Cancer Brain Metastases. Nat. Med. 2025, 31, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Yin, H.; Li, Q.; Fan, B.; Jiang, B.; Xie, A.; Guo, D.; Hao, H.; Zhang, B. Roles of SPOCK1 in the Formation Mechanisms and Treatment of Non-Small-Cell Lung Cancer and Brain Metastases from Lung Cancer. Onco Targets Ther. 2025, 18, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Miccio, J.A.; Tian, Z.; Mahase, S.S.; Lin, C.; Choi, S.; Zacharia, B.E.; Sheehan, J.P.; Brown, P.D.; Trifiletti, D.M.; Palmer, J.D.; et al. Estimating the Risk of Brain Metastasis for Patients Newly Diagnosed with Cancer. Comm. Med. 2024, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.-W.; Dai, M.-S.; Tseng, L.-M.; Chen, S.-C.; Chao, T.-Y.; Chao, T.-C.; Chang, Y.-C.; Chiu, C.-F.; Liu, C.-T.; Lin, C.-H.; et al. Whole-Brain Radiotherapy Alone vs Preceded by Bevacizumab, Etoposide, and Cisplatin for Untreated Brain Metastases From Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2024, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.K.L.; Trybula, M.; Sheng, I.Y.F.; Rothermel, L.D.; Sidiropoulos, J.; Goolamier, G.; Costello, T.M.; Mangla, A. Cisplatin-Vinblastine-Temozolomide Regimen for Patients with Relapsed-Refractory Melanoma who Are Ineligible for Clinical Trials: A Tertiary Care Center Experience. J. Clin. Oncol. 2024, 42, e21540. [Google Scholar] [CrossRef]
- Pandit, K.; Yuen, K.; Puri, D.; Yodkhunnatham, N.; Millard, F.; Bagrodia, A. Metastasis-directed Therapy in Testicular Cancer. Curr. Opin. Urol. 2024, 34, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; He, Q.; Yang, Y.; Wang, X.; Han, F. Clinical Characteristics and Prognostic Factors in Nasopharyngeal Carcinoma with Brain Metastasis: A Retrospective, Single-Center Study. Head Neck 2024, 46, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Sunaga, N.; Yatomi, M.; Wakamatsu, I.; Muto, S.; Ikota, H.; Yamaguchi, R.; Ohtaki, Y.; Nagashima, T.; Kubo, N.; et al. A Case of Primary Lung Adenocarcinoma with Recurrent Brain Metastasis due to Transformation to Small Cell Carcinoma During Adjuvant Atezolizumab Therapy. Thorac. Cancer 2025, 16, e15512. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, M.; Cao, J.; Wang, J.; Tao, Z.; Wang, B.; Hu, X.; Zhang, J. A Phase II Trial Combining SHR-1316, Bevacizumab, and Cisplatin/Carboplatin in Patients with TNBC with Active Brain Metastases. J. Clin. Oncol. 2024, 42, e13133. [Google Scholar] [CrossRef]
- Ozair, A.; Wilding, H.; Bhanja, D.; Mikolajewicz, N.; Glantz, M.; Grossman, S.A.; Sahgal, A.; Le Rhun, E.; Weller, M.; Weiss, T.; et al. Leptomeningeal Metastatic Disease: New Frontiers and Future Directions. Nat. Rev. Clin. Oncol. 2024, 22, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Remsik, J.; Boire, A. The path to leptomeningeal metastasis. Nat. Rev. Cancer 2024, 24, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Seddiq, W.; Chen, K.S.; Kajiwara, Y.; Moreno Lama, L.; Borges, P.; Kuroda, S.; Wakimoto, H.; Shah, K. Engineered Allogeneic Stem Cells Orchestrate T Lymphocyte Driven Immunotherapy in Immunosuppressive Leptomeningeal Brain Metastasis. J. Natl. Cancer Inst. 2025, 117, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Jan, I.S.; Chang, D.Y.; Lin, C.H.; Chen, I.C.; Chen, H.M.; Cheng, A.L.; Lu, Y.S. Systemic Treatment of Breast Cancer with Leptomeningeal Metastases Using Bevacizumab, Etoposide and Cisplatin (BEEP regimen) Significantly Improves Overall Survival. J. Neurooncol. 2020, 148, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.N.; Tsai, Y.J.; Chiu, H.H.; Chen, T.W.W.; Lin, C.H.; Lu, Y.S.; Kuo, C.H. Evaluation of the Association Between Bevacizumab Concentration and Clinical Outcomes in Patients with Breast Cancer Brain Metastasis. Heliyon 2025, 11, e41390. [Google Scholar] [CrossRef] [PubMed]
- Arjani, S.; Jeon, H.; Chadha, B.; Yousuf, H.; Castellucci, E. Leptomeningeal carcinomatosis in gastric cancer: A Review. Gastric Cancer 2025, 28, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.S.; Fox, E.; Dennie, C.; Morgan, L.B.; McCully, C.L.; Balis, F.M. Plasma and Cerebrospinal Fluid Pharmacokinetics of Intravenous Oxaliplatin, Cisplatin, and Carboplatin in Nonhuman Primates. Clin. Cancer Res. 2005, 11, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
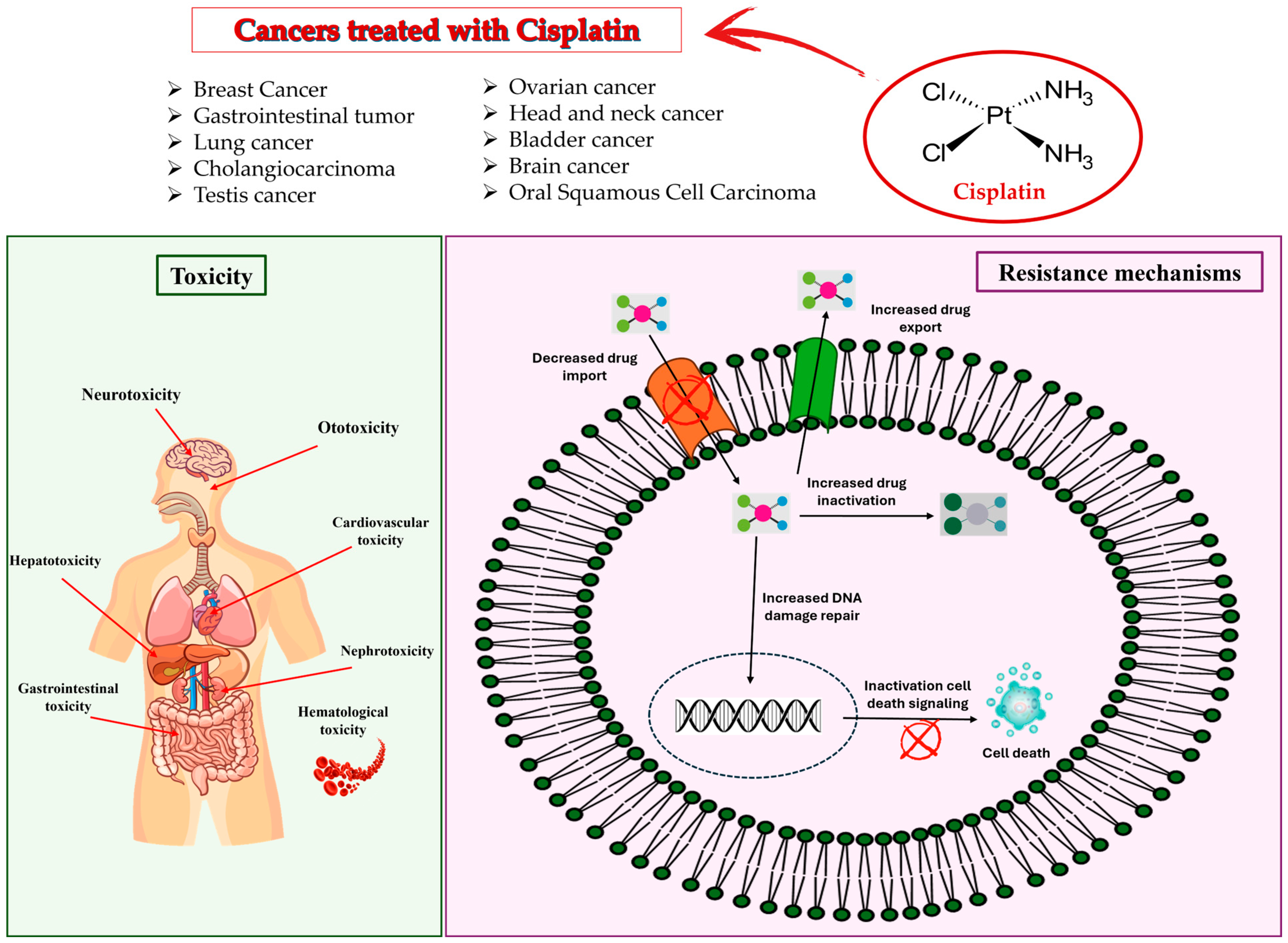
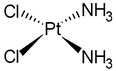
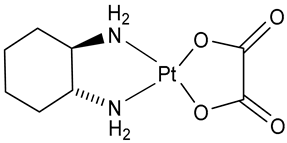
| Compound | Name | Pt Oxidation State | Approved | Company | Brand Name | First Approval |
|---|---|---|---|---|---|---|
 | Cisplatin (DDP) | Pt(II) | Worldwide | Nippon Kayaku Co., Ltd., Tokyo, Japan | Randa® | 1978 |
 | Carboplatin (CBDCA) | Pt(II) | Worldwide | Bristol-Myers Squibb Co., Tokyo, Japan | Paraplatin® | 1986 |
 | Oxalilplatin (OXA) | Pt(II) | Worldwide | Sanofi-Synthelabo Inc.; New York, NY, USA Yakult Co., Ltd., Tokyo, Japan | Eloxatin® Elplat® | 2002 |
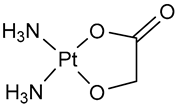 | Nedaplatin (NDP) | Pt(II) | Japan | Shionogi & Co., Ltd., Osaka, Japan | Aqupla® | 1995 |
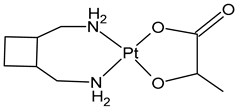 | Lobaplatin | Pt(II) | China | ASTA Pharma AG Company (Frankfurt, Germany) | Lobaplatin | 1998 |
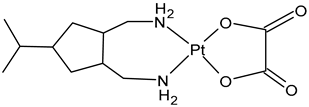 | Heptaplatin | Pt(II) | North and South Korea | Sk Chemicals | Eptaplatin or Sunpla | 1999 |
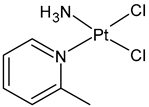 | Picoplatin | Pt(II) | Failed | - | - | NA |
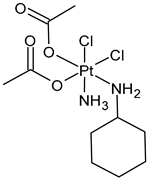 | Satraplatin (JM216) | Pt(IV) | Failed | - | - | NA |
 | Iproplatin (JM-9) | Pt(IV) | Failed | - | - | NA |
 | Ormaplatin or Tetraplatin | Pt(IV) | Failed | - | - | NA |
 | Miriplatin | Pt(II) | Japan | Sumitomo Dainippon Pharma, Japan | Miripla | 2010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariconda, A.; Ceramella, J.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Cisplatin, the Timeless Molecule. Inorganics 2025, 13, 246. https://doi.org/10.3390/inorganics13070246
Mariconda A, Ceramella J, Catalano A, Saturnino C, Sinicropi MS, Longo P. Cisplatin, the Timeless Molecule. Inorganics. 2025; 13(7):246. https://doi.org/10.3390/inorganics13070246
Chicago/Turabian StyleMariconda, Annaluisa, Jessica Ceramella, Alessia Catalano, Carmela Saturnino, Maria Stefania Sinicropi, and Pasquale Longo. 2025. "Cisplatin, the Timeless Molecule" Inorganics 13, no. 7: 246. https://doi.org/10.3390/inorganics13070246
APA StyleMariconda, A., Ceramella, J., Catalano, A., Saturnino, C., Sinicropi, M. S., & Longo, P. (2025). Cisplatin, the Timeless Molecule. Inorganics, 13(7), 246. https://doi.org/10.3390/inorganics13070246










