An Introduction to the Role of Molybdenum and Tungsten in Biology
Abstract
1. Introduction

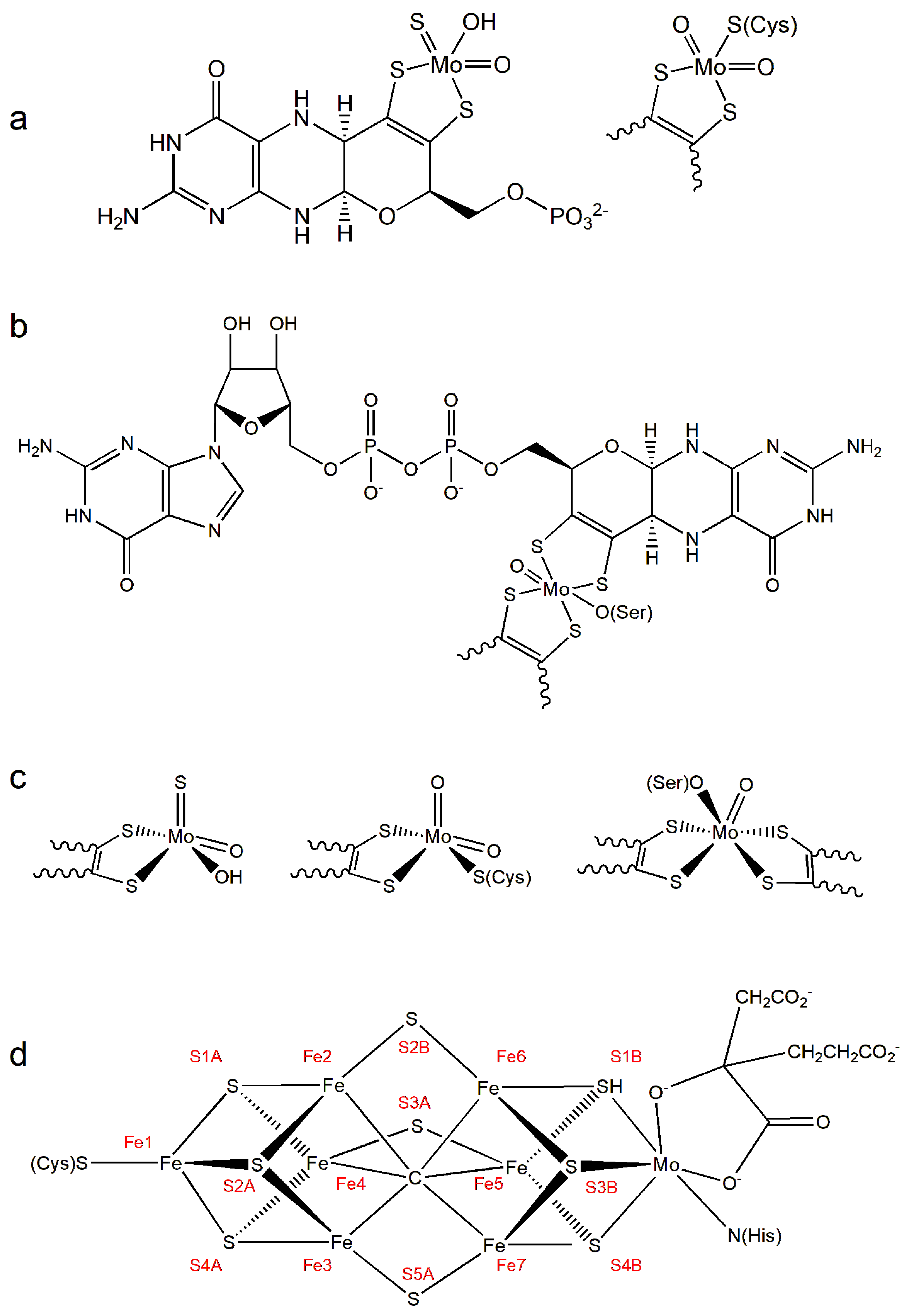
2. Molybdenum in Biology: An Overview
3. Tungsten in Biology: An Overview
4. Examples of Molybdenum-Dependent Enzymes
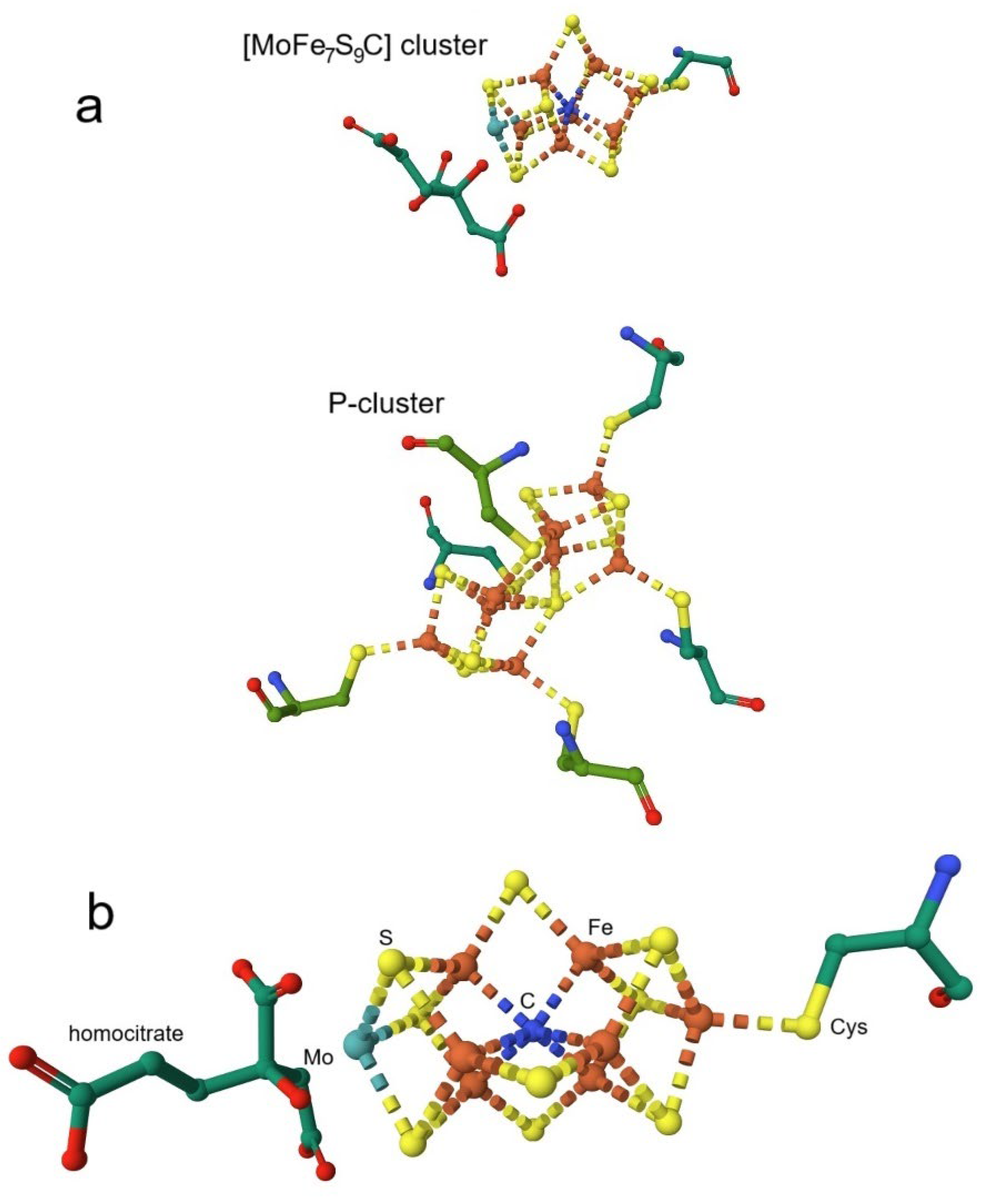
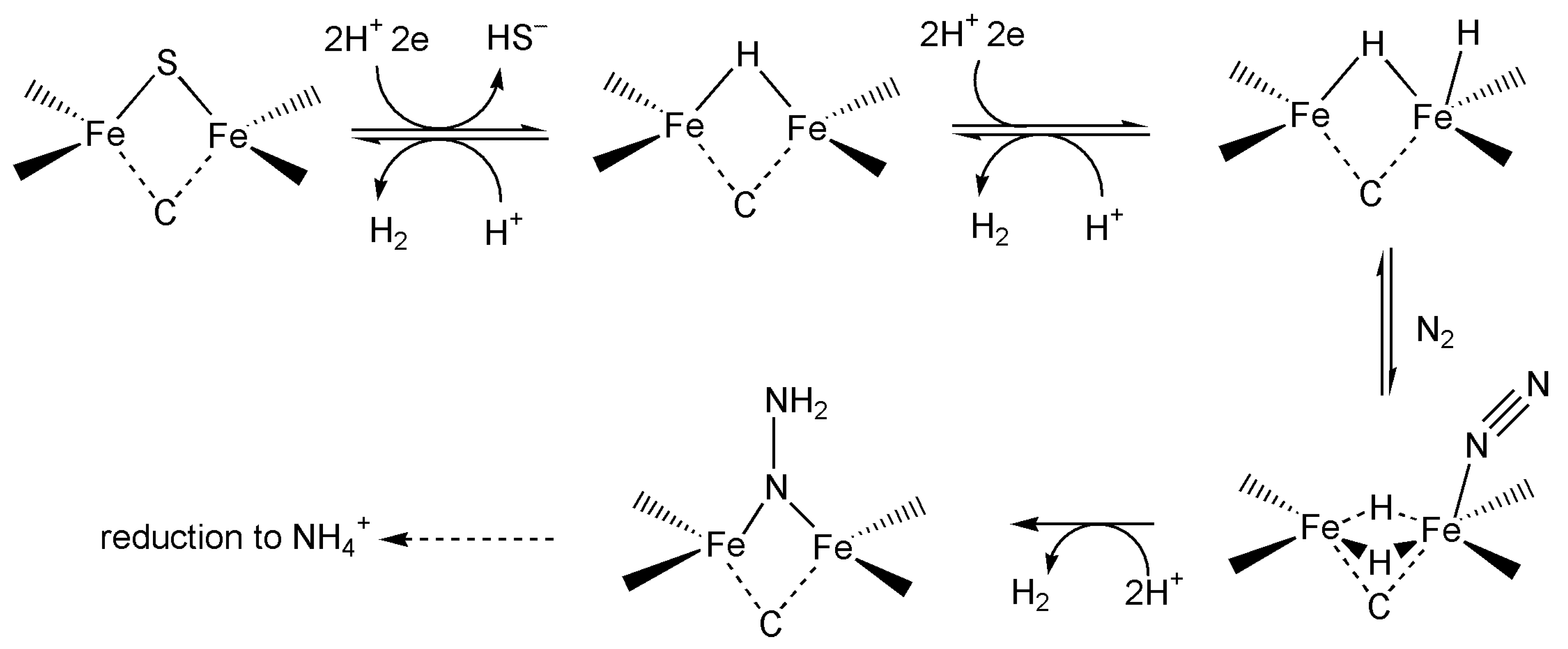
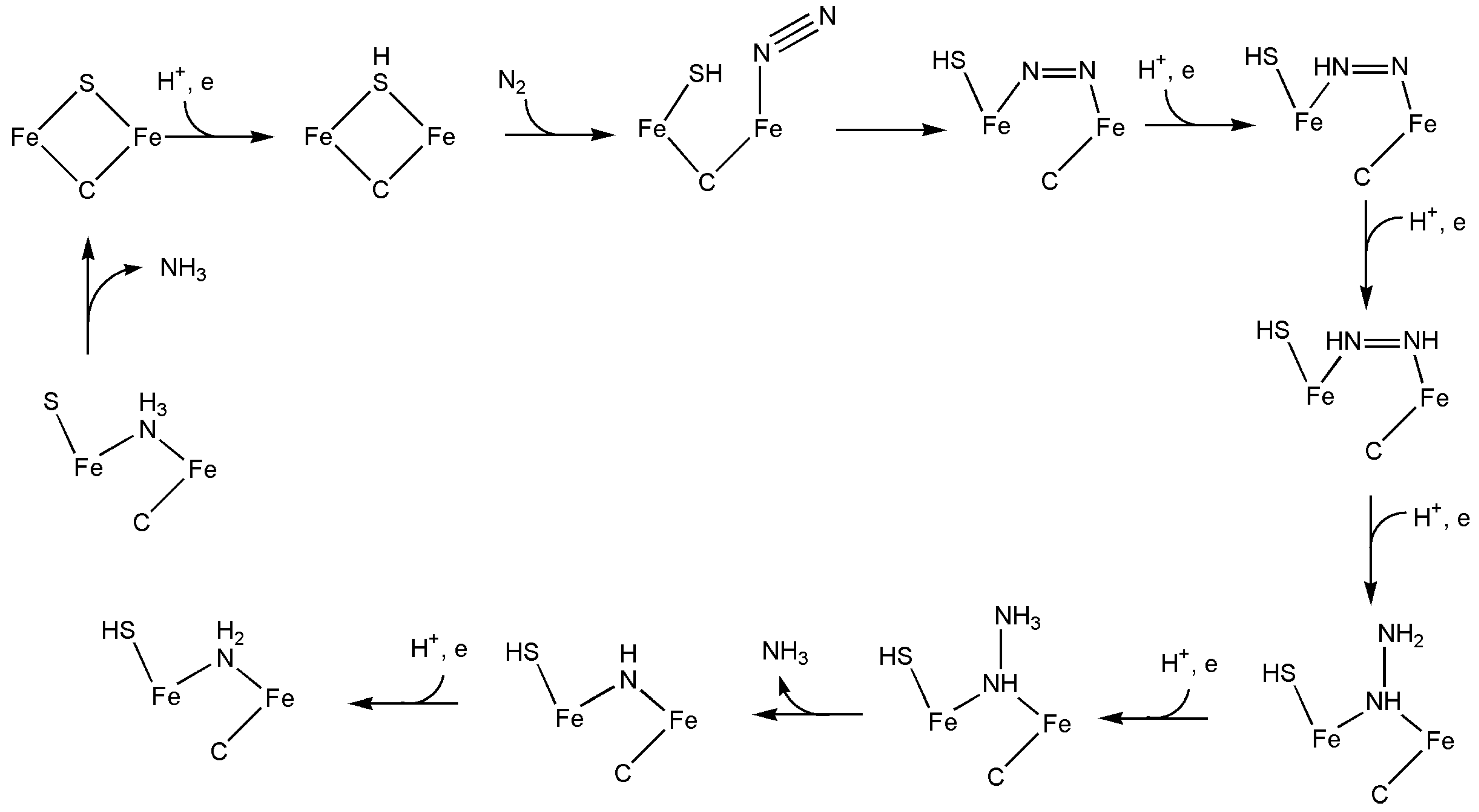
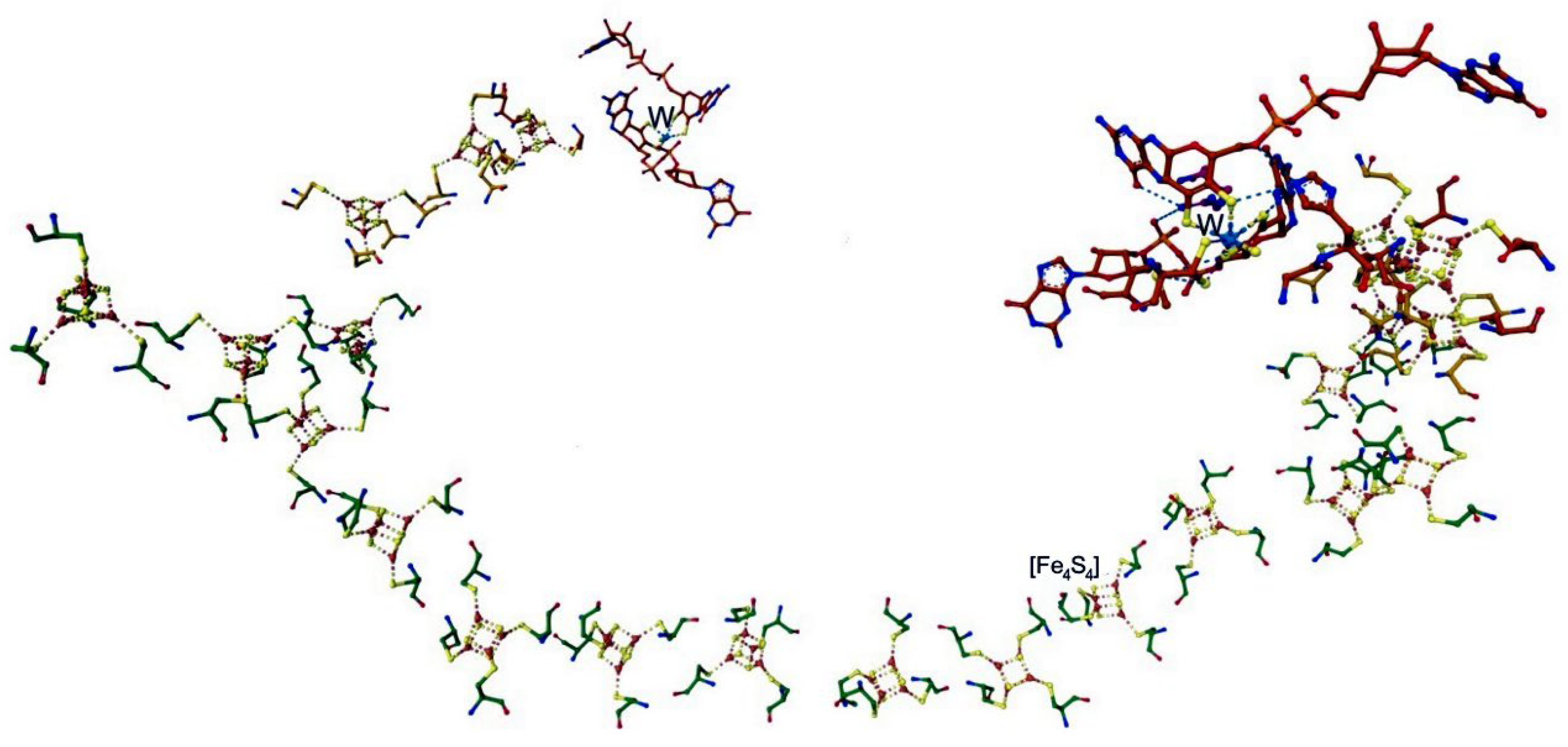
4.1. Nitrogenase
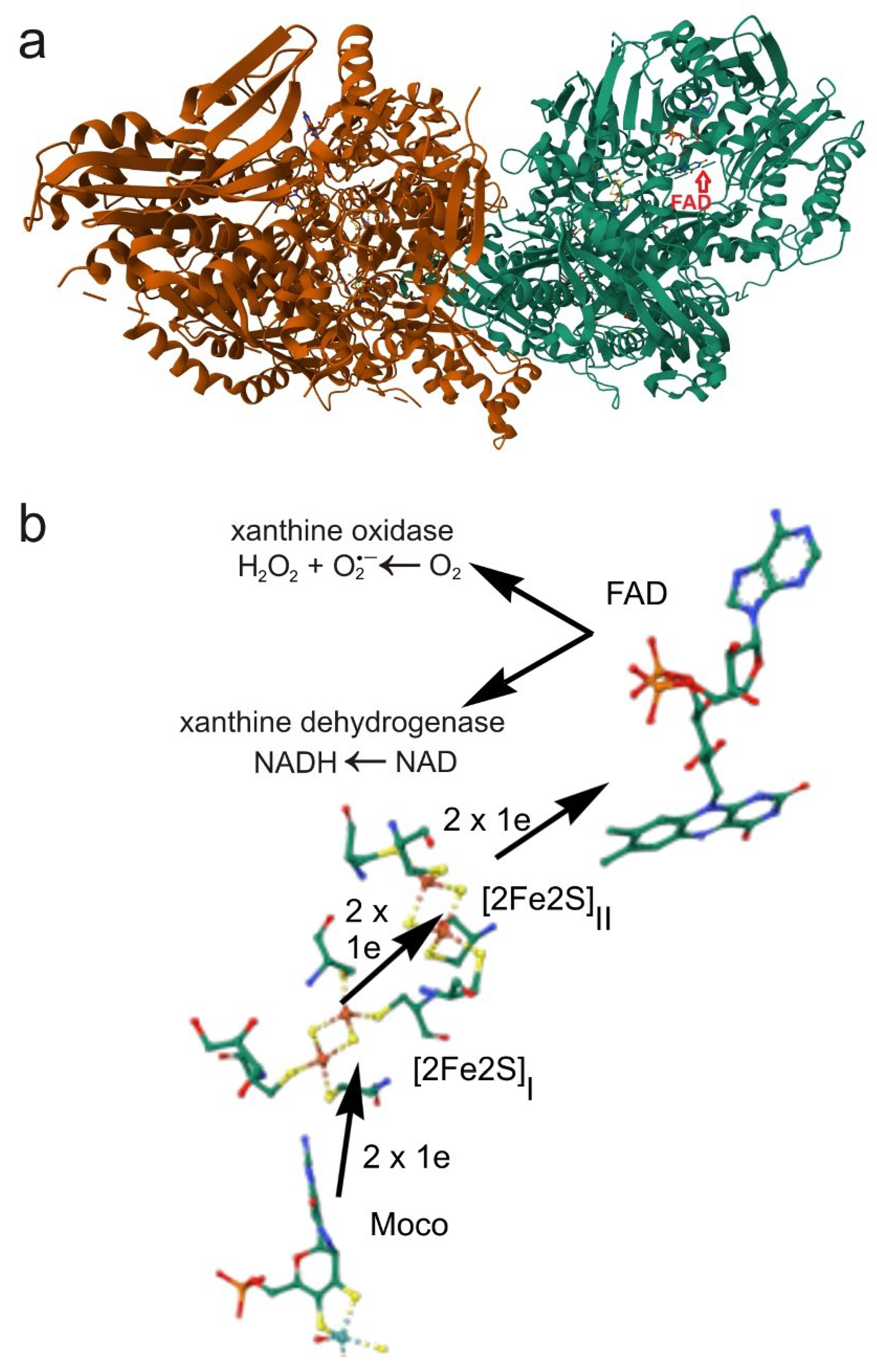
4.2. Xanthine Oxidoreductase
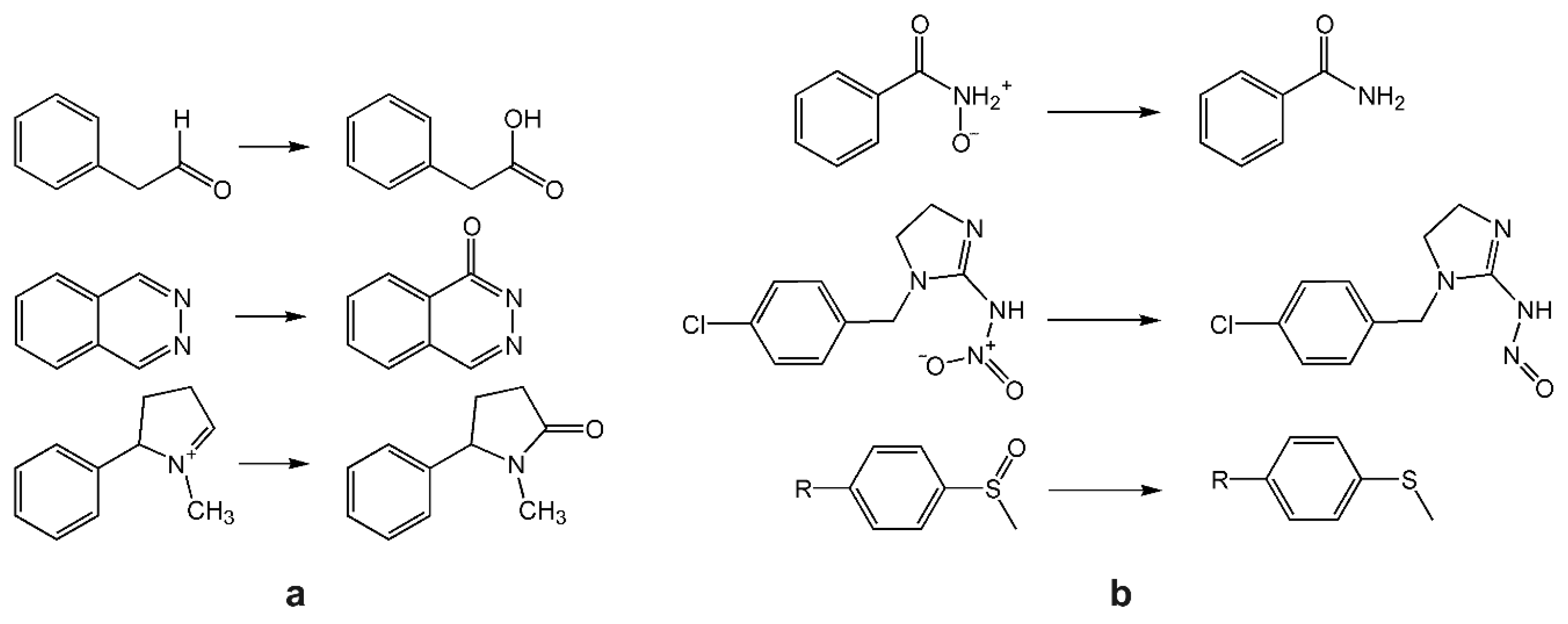
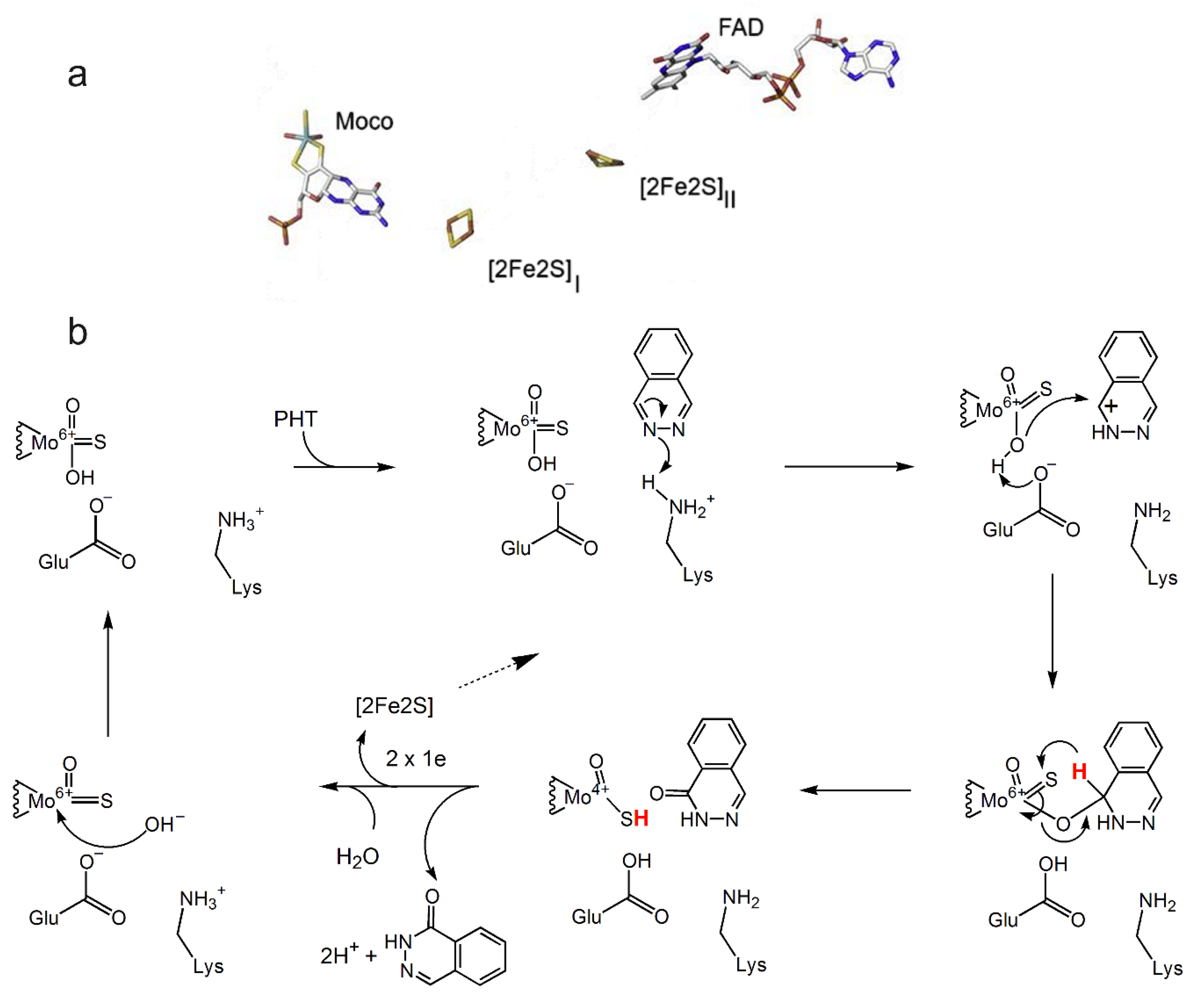
4.3. Aldehyde Oxidase
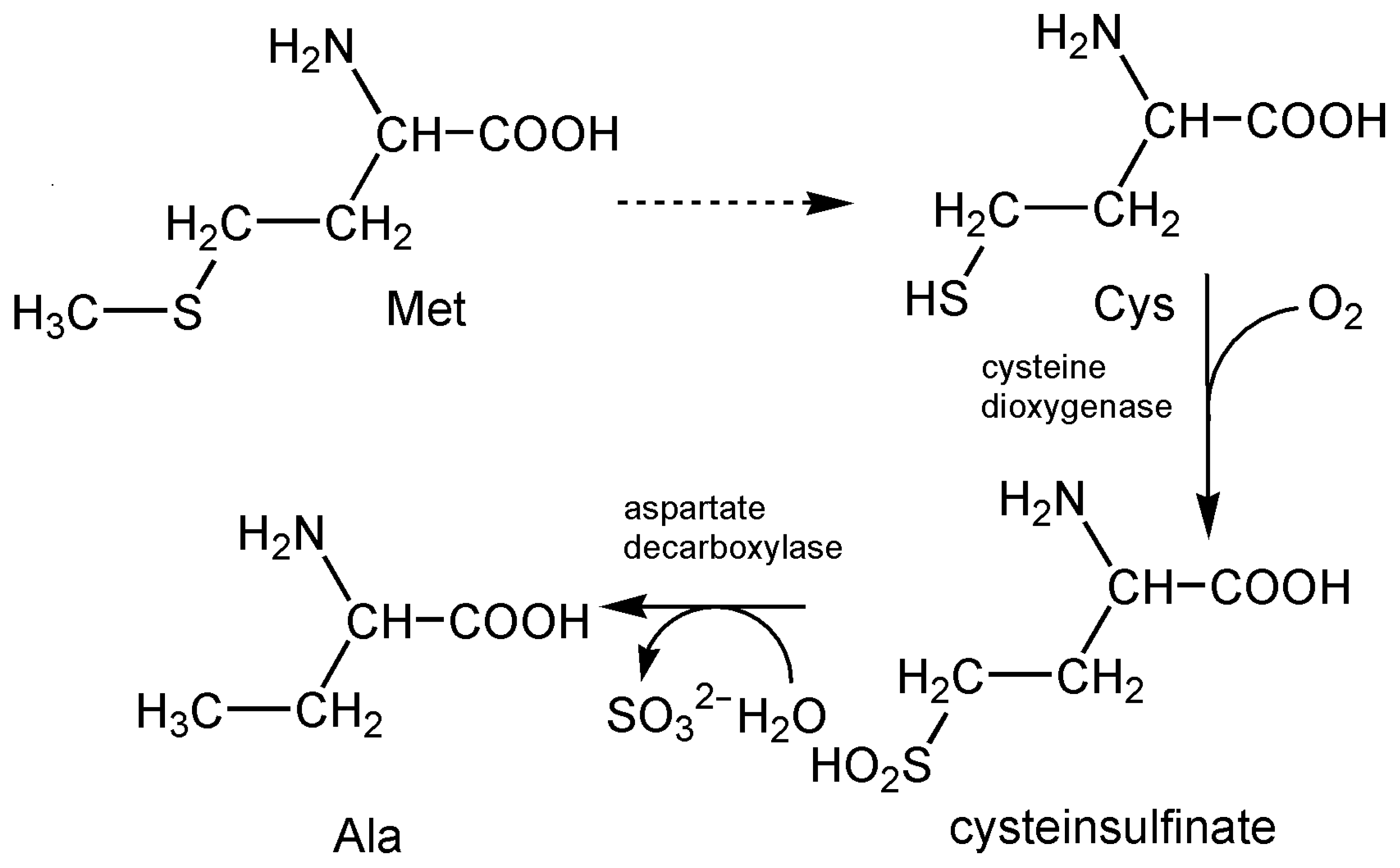
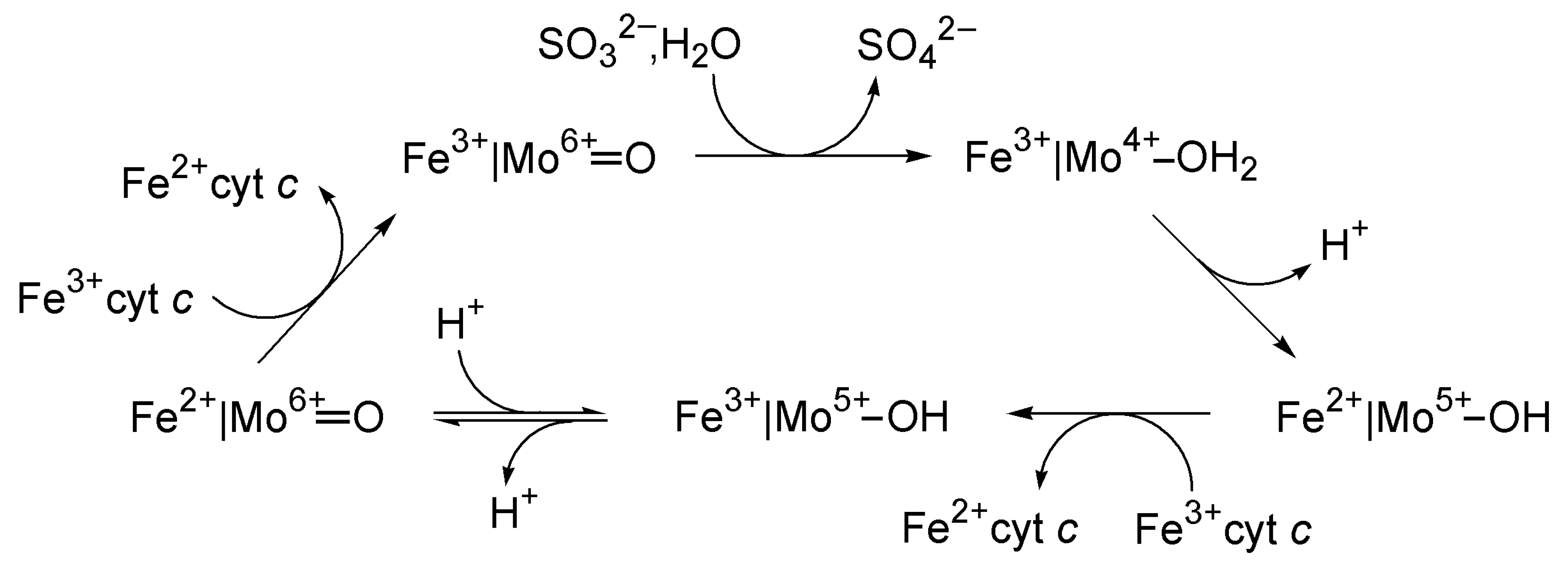
4.4. Sulfite Oxidase
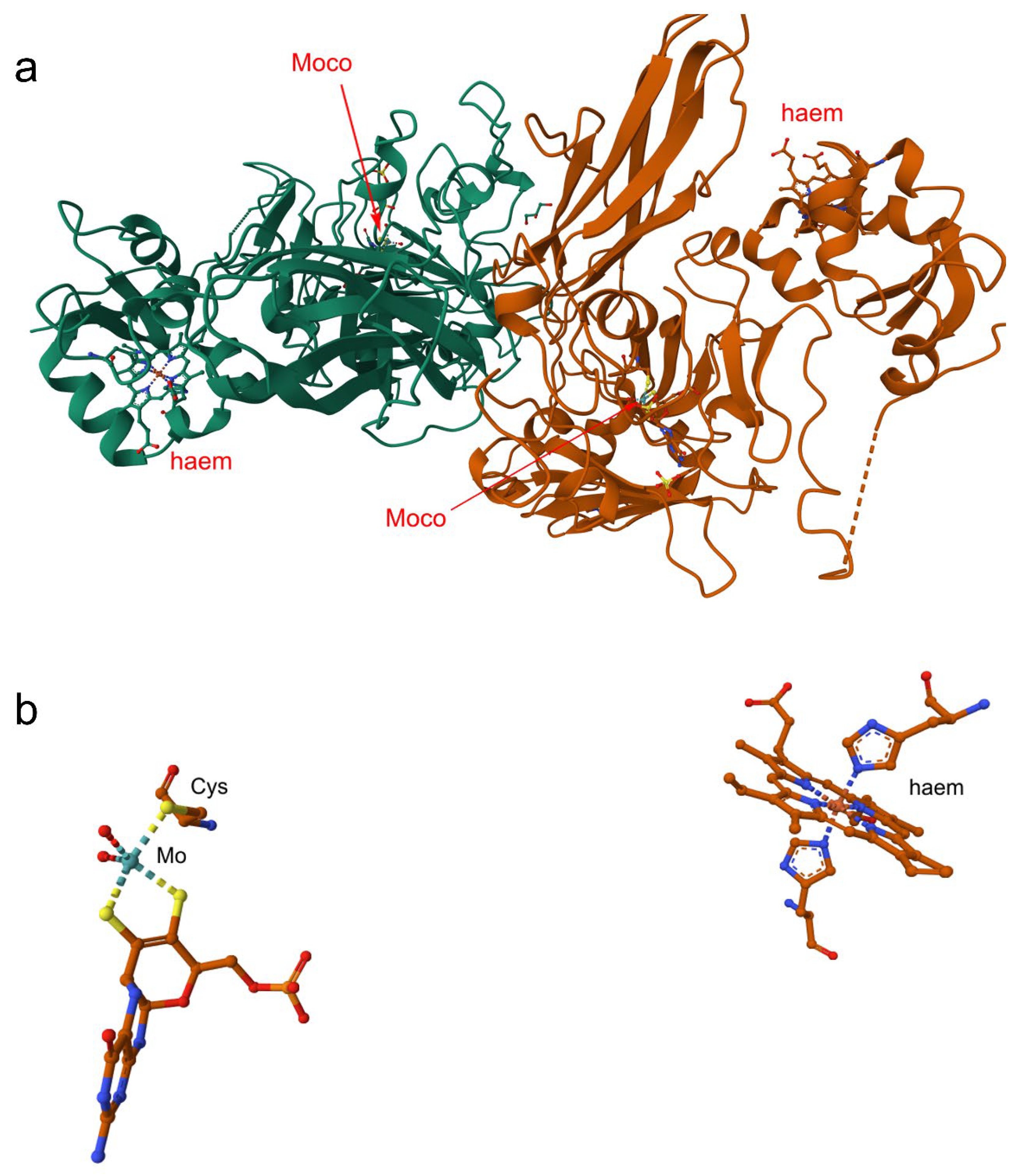
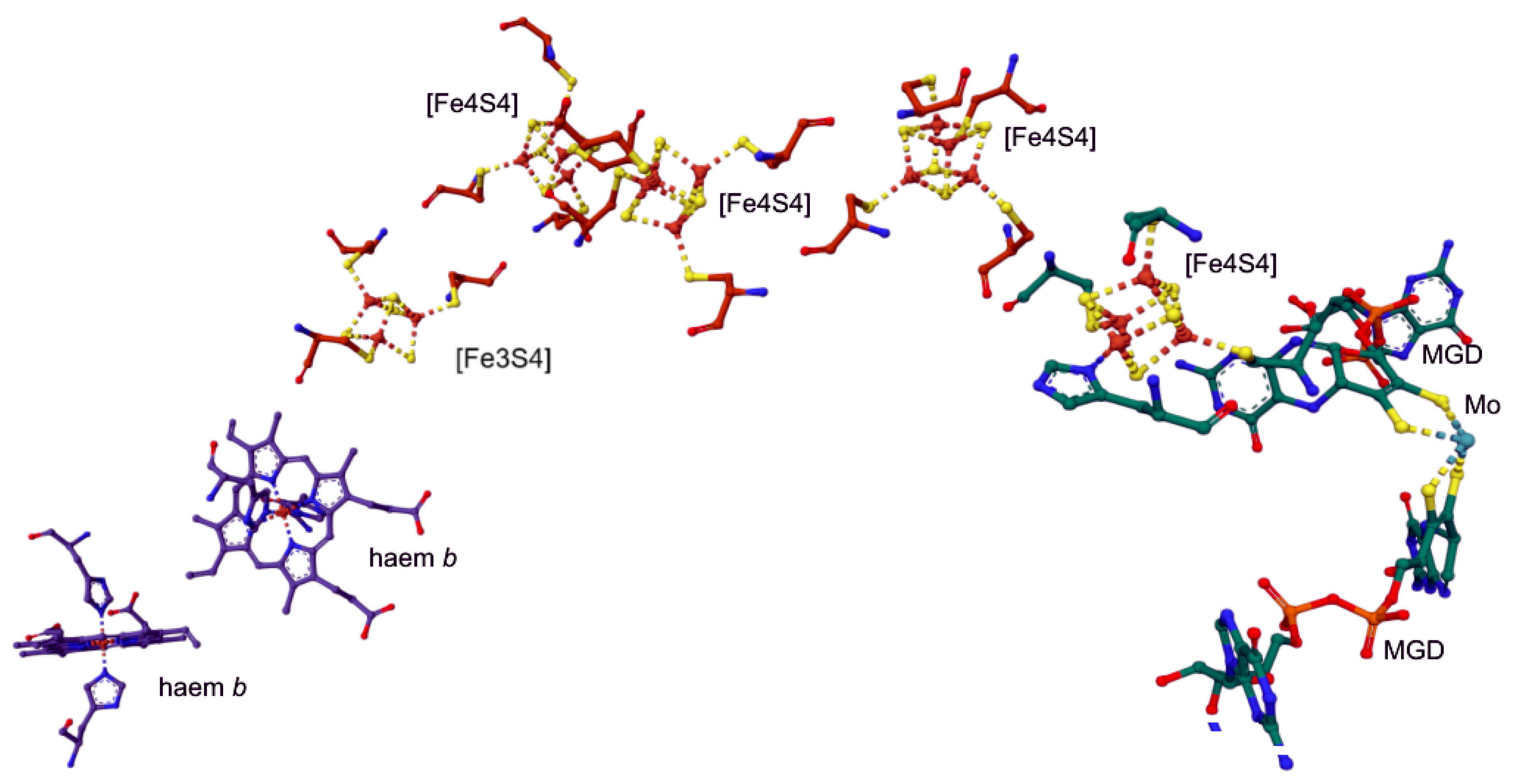
4.5. Nitrate Reductase
5. Examples of Tungsten-Dependent Enzymes
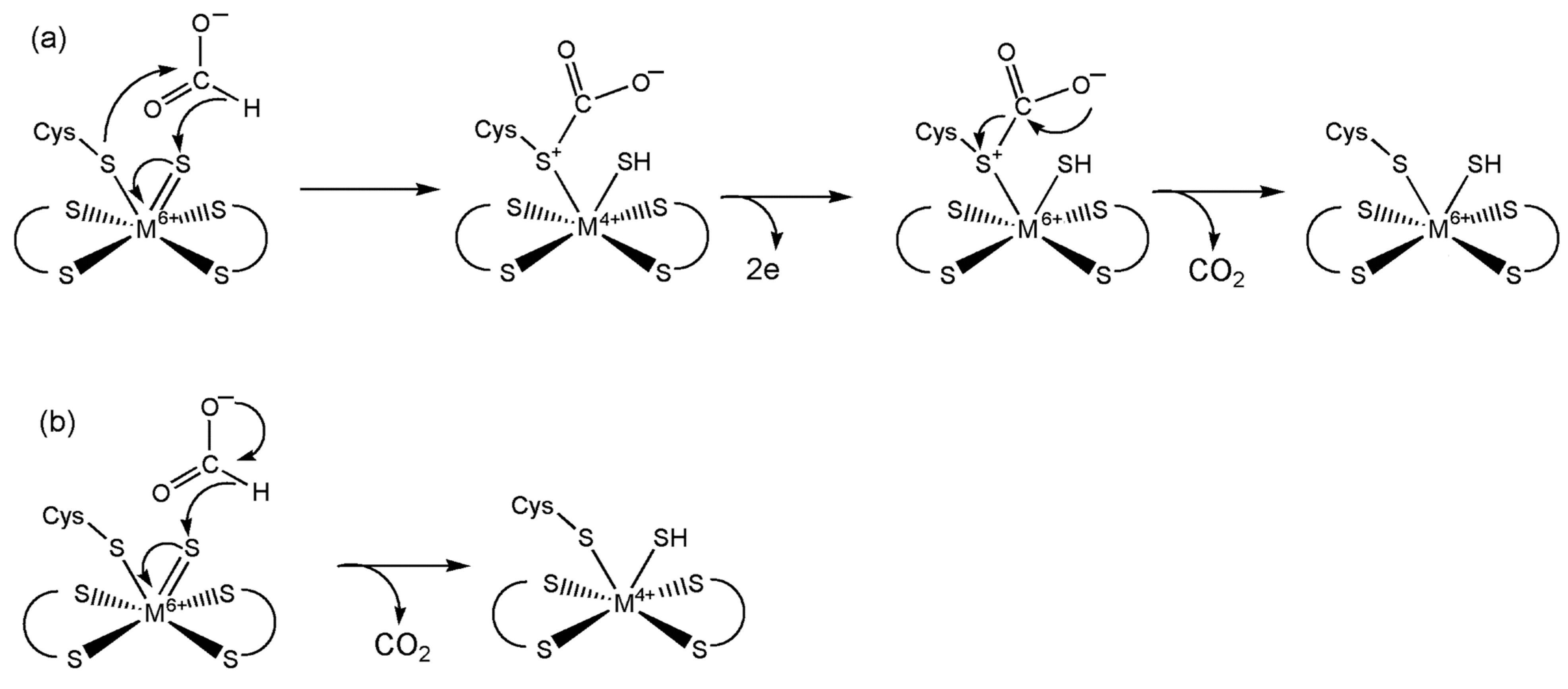
5.1. Formate Dehydrogenase
5.2. Aldehyde Oxidoreductase
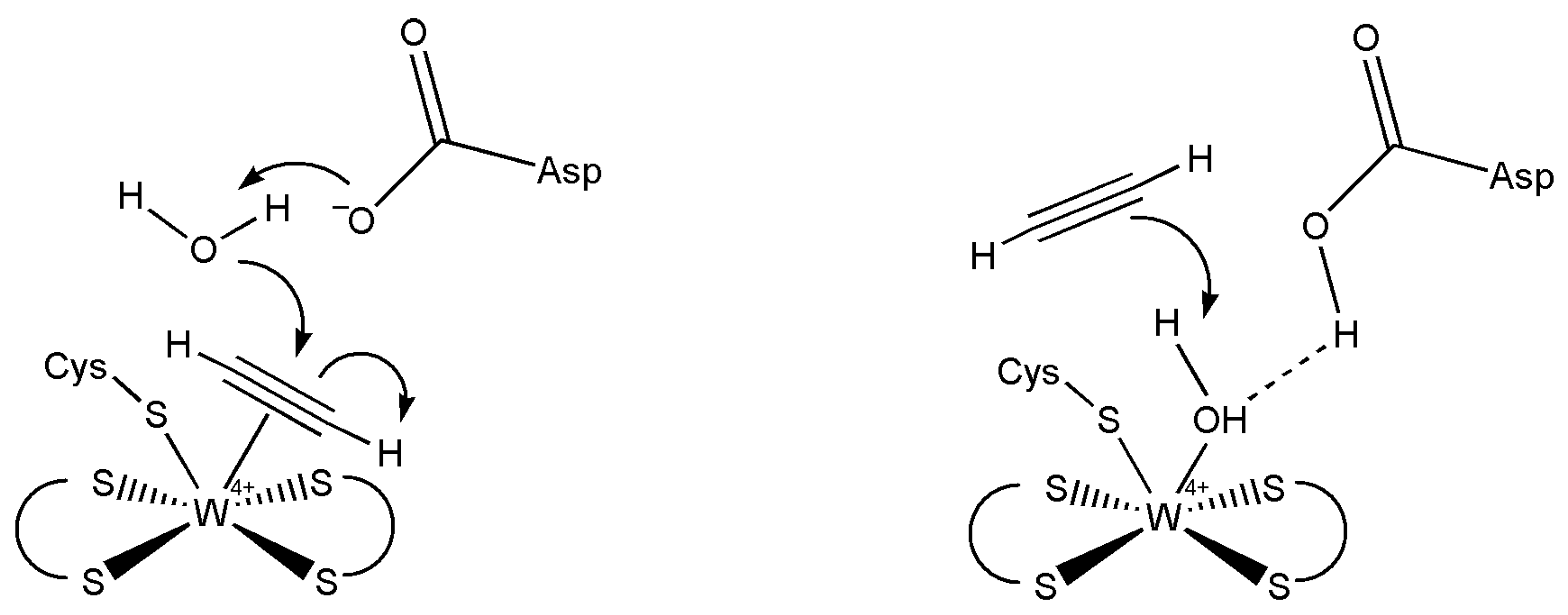
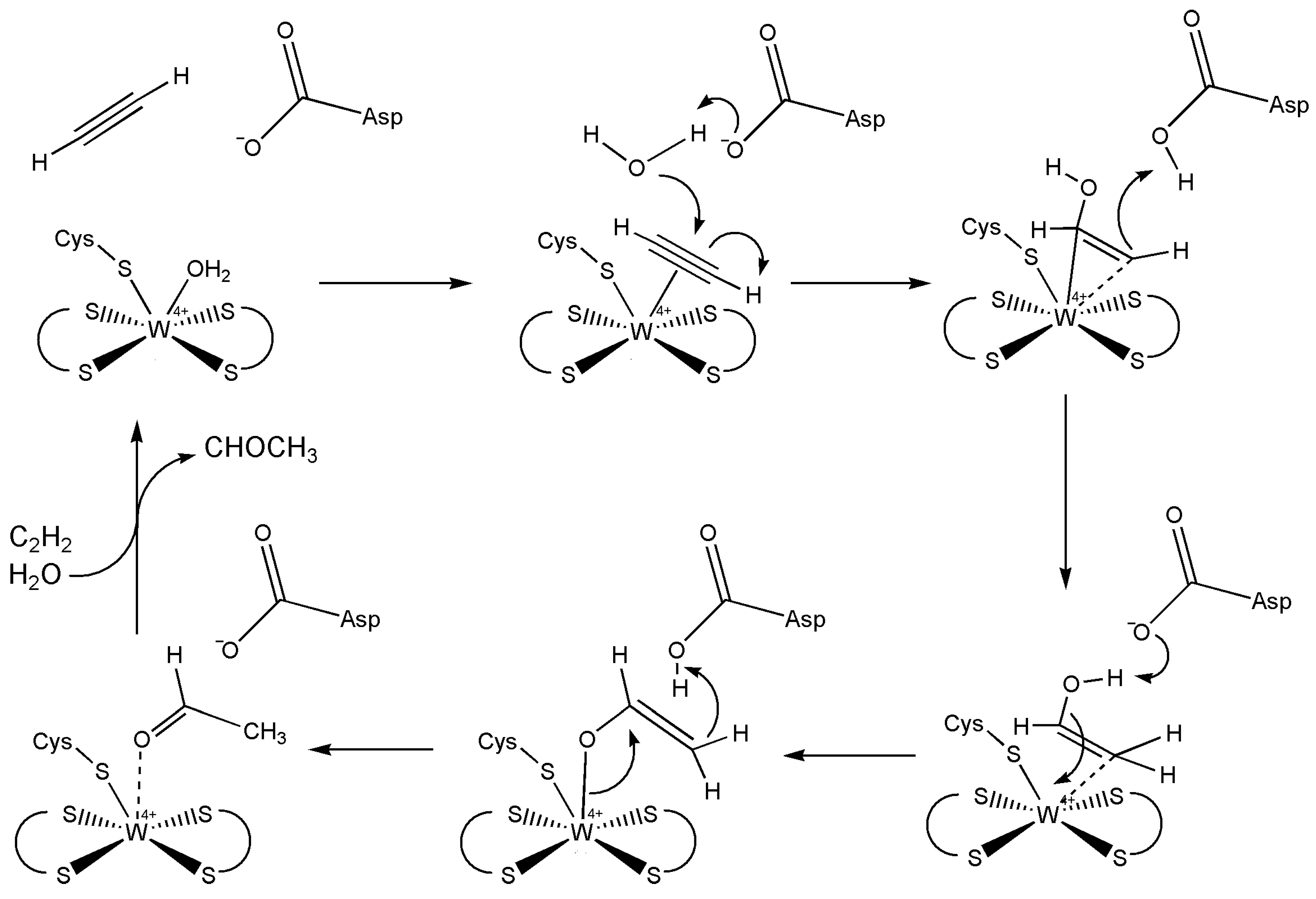
5.3. Acetylene Hydratase
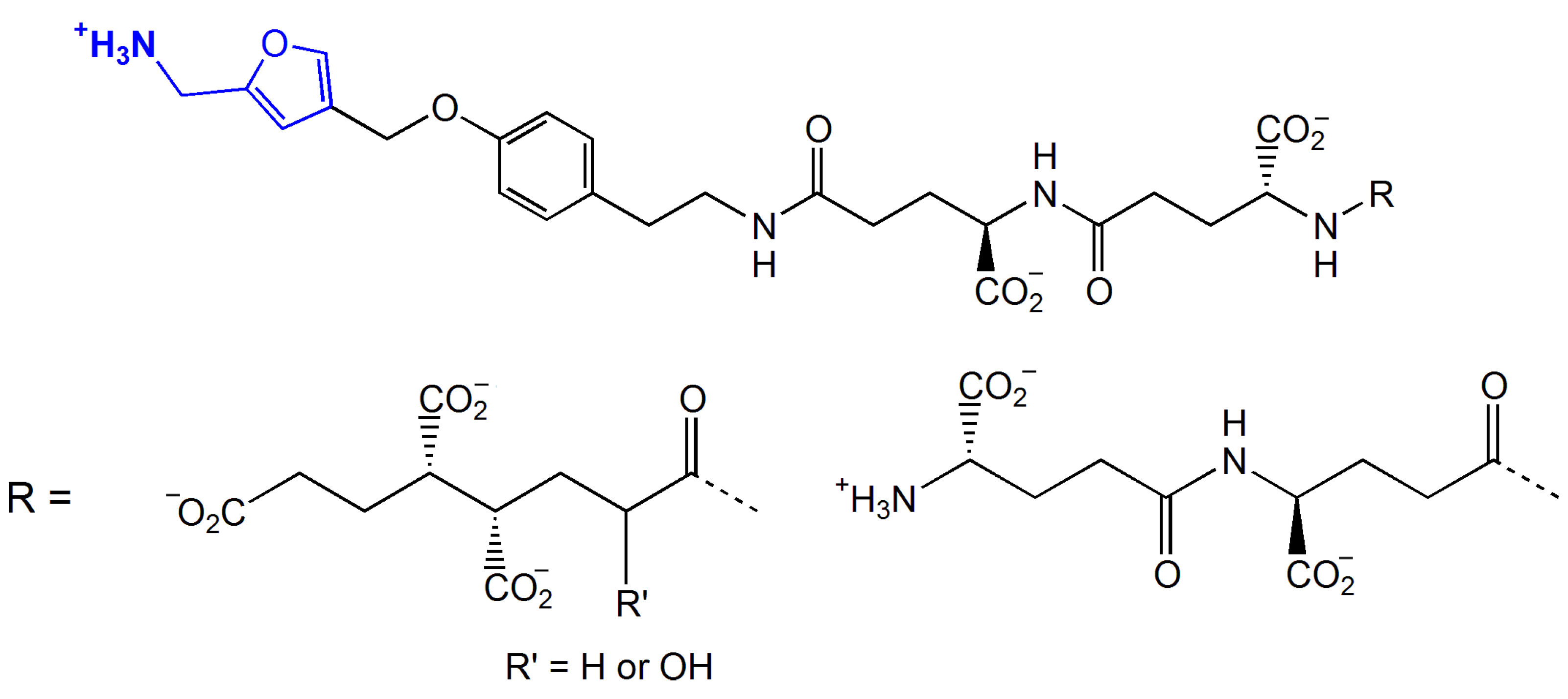
5.4. Formylmethanofuran Dehydrogenase
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marques, H.M. The Bioinorganic Chemistry of the First Row d-Block Metal Ions—An Introduction. Inorganics 2025, 13, 137. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Devi, S.; Jatav, R.S.; Dimri, U. Role of trace elements in animals: A review. Vet. World 2013, 6, 963–967. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Biol. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Wong, C.P.; Magnusson, K.R.; Sharpton, T.J.; Ho, E. Effects of zinc status on age-related T cell dysfunction and chronic inflammation. BioMetals 2021, 34, 291–301. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Kamynina, M.; Sorokin, M.; Zolotovskaia, M.; Koroleva, E.; Kremenchutckaya, K.; Gudkov, A.; Buzdin, A.; Borisov, N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines 2022, 10, 1072. [Google Scholar] [CrossRef]
- Remick, K.A.; Helmann, J.D. The elements of life: A biocentric tour of the periodic table. Adv. Microb. Physiol. 2023, 82, 1–127. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Sharma, S. Exploring the Importance of Trace Elements in Nutrition: Understanding Their Vital Role in Health and Well-being. E3S Web Conf. 2024, 509, 03016. [Google Scholar] [CrossRef]
- Hille, R. Molybdenum and tungsten in biology. Trends Biochem. Sci. 2002, 27, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Bevers, L.E.; Hagedoorn, P.-L.; Hagen, W.R. The bioinorganic chemistry of tungsten. Coord. Chem. Rev. 2009, 253, 269–290. [Google Scholar] [CrossRef]
- Maia, L.B.; Moura, I.; Moura, J.J.G. Molybdenum and Tungsten-Containing Enzymes: An Overview. In Molybdenum and Tungsten Enzymes: Biochemistry; Hille, R., Schulzke, C., Kirk, M.L., Kirk, M.L., Hille, R., Schulzke, C., Eds.; The Royal Society of Chemistry: London, UK, 2016; pp. 1–80. [Google Scholar]
- Huang, X.-Y.; Hu, D.-W.; Zhao, F.-J. Molybdenum: More than an essential element. J. Exp. Biol. 2021, 73, 1766–1774. [Google Scholar] [CrossRef]
- Bursakov, S.A.; Kroupin, P.Y.; Karlov, G.I.; Divashuk, M.G. Tracing the Element: The Molecular Bases of Molybdenum Homeostasis in Legumes. Agronomy 2023, 13, 2300. [Google Scholar] [CrossRef]
- Stripp, S.T.; Duffus, B.R.; Fourmond, V.; Léger, C.; Leimkühler, S.; Hirota, S.; Hu, Y.; Jasniewski, A.; Ogata, H.; Ribbe, M.W. Second and Outer Coordination Sphere Effects in Nitrogenase, Hydrogenase, Formate Dehydrogenase, and CO Dehydrogenase. Chem. Rev. 2022, 122, 11900–11973. [Google Scholar] [CrossRef]
- McGarry, J.; Mintmier, B.; Metzger, M.C.; Giri, N.C.; Britt, N.; Basu, P.; Wilcoxen, J. Insights into periplasmic nitrate reductase function under single turnover. J. Biol. Inorg. Chem. 2024, 29, 811–819. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Lu, C.; Li, H.; Guo, J. Role of molybdenum compounds in enhancing denitrification: Structure-activity relationship and the regulatory mechanisms. Chemosphere 2024, 367, 143433. [Google Scholar] [CrossRef]
- Giri, N.C.; Mintmier, B.; Radhakrishnan, M.; Mielke, J.W.; Wilcoxen, J.; Basu, P. The critical role of a conserved lysine residue in periplasmic nitrate reductase catalyzed reactions. J. Biol. Inorg. Chem. 2024, 29, 395–405. [Google Scholar] [CrossRef]
- Dash, S.; Pattanayak, S.; Jena, B.; Panda, K.M.; Singh, D.Y. Xanthine Oxidase Perspective in Human Health. Curr. Biotechnol. 2020, 9, 255–262. [Google Scholar] [CrossRef]
- Iksat, N.N.; Zhangazin, S.B.; Madirov, A.A.; Omarov, R.T. Effect of Molybdenum on The Activity of Molybdoenzymes. Eurasian J. Appl. Biotechnol. 2020, 2. [Google Scholar] [CrossRef]
- Kirk, M.L.; Hille, R. Spectroscopic Studies of Mononuclear Molybdenum Enzyme Centers. Molecules 2022, 27, 4802. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. Xanthine Oxidase—A Personal History. Molecules 2023, 28, 1921. [Google Scholar] [CrossRef] [PubMed]
- Adamus, J.P.; Ruszczyńska, A.; Wyczałkowska-Tomasik, A. Molybdenum’s Role as an Essential Element in Enzymes Catabolizing Redox Reactions: A Review. Biomolecules 2024, 14, 869. [Google Scholar] [CrossRef]
- Garcia, A.K.; McShea, H.; Kolaczkowski, B.; Kaçar, B. Reconstructing the evolutionary history of nitrogenases: Evidence for ancestral molybdenum-cofactor utilization. Geobiology 2020, 18, 394–411. [Google Scholar] [CrossRef]
- Burén, S.; Jiménez-Vicente, E.; Echavarri-Erasun, C.; Rubio, L.M. Biosynthesis of Nitrogenase Cofactors. Chem. Rev. 2020, 120, 4921–4968. [Google Scholar] [CrossRef]
- Watanabe, T.; Horiike, T. The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria. Biology 2021, 10, 329. [Google Scholar] [CrossRef]
- Dance, I. The binding of reducible N2 in the reaction domain of nitrogenase. Dalton Trans. 2023, 52, 2013–2026. [Google Scholar] [CrossRef]
- Threatt, S.D.; Rees, D.C. Biological nitrogen fixation in theory, practice, and reality: A perspective on the molybdenum nitrogenase system. FEBS Lett. 2023, 597, 45–58. [Google Scholar] [CrossRef]
- Einsle, O. On the Shoulders of Giants—Reaching for Nitrogenase. Molecules 2023, 28, 7959. [Google Scholar] [CrossRef]
- Dance, I. The mechanism of Mo-nitrogenase: From N2 capture to first release of NH3. Dalton Trans. 2024, 53, 19360–19377. [Google Scholar] [CrossRef] [PubMed]
- Dance, I. What triggers the coupling of proton transfer and electron transfer at the active site of nitrogenase? Dalton Trans. 2024, 53, 7996–8004. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Joyce, J.P.; Decamps, L.; Kang, L.; Bjornsson, R.; Rüdiger, O.; DeBeer, S. Investigating the Molybdenum Nitrogenase Mechanistic Cycle Using Spectroelectrochemistry. J. Am. Chem. Soc. 2025, 147, 2099–2114. [Google Scholar] [CrossRef]
- Riyaz, Z.; Khan, S.T. Nitrogen fixation by methanogenic Archaea, literature review and DNA database-based analysis; significance in face of climate change. Arch. Microbiol. 2024, 207, 6. [Google Scholar] [CrossRef] [PubMed]
- Pushie, M.J.; Cotelesage, J.J.; George, G.N. Molybdenum and tungsten oxygen transferases- structural and functional diversity within a common active site motif. Metallomics 2014, 6, 15–24. [Google Scholar] [CrossRef]
- Scott, C.; Lyons, T.W.; Bekker, A.; Shen, Y.; Poulton, S.W.; Chu, X.; Anbar, A.D. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 2008, 452, 456–459. [Google Scholar] [CrossRef]
- Rayner-Canham, G.; Grandy, J. Did molybdenum control evolution on Earth? Educ. Chem. 2011, 144–147. Available online: https://edu.rsc.org/feature/molybdenum-and-evolution/2020196.article (accessed on 19 May 2025).
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta 2006, 1763, 621–635. [Google Scholar] [CrossRef]
- Kletzin, A.; Adams, M.W.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef]
- Tse, C.; Ma, K. Growth and Metabolism of Extremophilic Microorganisms. In Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, P.H., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–46. [Google Scholar]
- Seelmann, C.S.; Willistein, M.; Heider, J.; Boll, M. Tungstoenzymes: Occurrence, Catalytic Diversity and Cofactor Synthesis. Inorganics 2020, 8, 44. [Google Scholar] [CrossRef]
- Buessecker, S.; Palmer, M.; Lai, D.; Dimapilis, J.; Mayali, X.; Mosier, D.; Jiao, J.-Y.; Colman, D.R.; Keller, L.M.; St. John, E.; et al. An essential role for tungsten in the ecology and evolution of a previously uncultivated lineage of anaerobic, thermophilic Archaea. Nat. Commun. 2022, 13, 3773. [Google Scholar] [CrossRef]
- Gutiérrez-Preciado, A.; Dede, B.; Baker, B.A.; Eme, L.; Moreira, D.; López-García, P. Extremely acidic proteomes and metabolic flexibility in bacteria and highly diversified archaea thriving in geothermal chaotropic brines. Nat. Ecol. Evol. 2024, 8, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Valdez, S.; de la Vega, F.V.; Pairazaman, O.; Castellanos, R.; Esparza, M. Hyperthermophile diversity microbes in the Calientes geothermal field, Tacna, Peru. Braz. J. Microbiol. 2023, 54, 2927–2937. [Google Scholar] [CrossRef]
- Das, U.; Das, A.; Das, A.K. Relativistic effect behind the molybdenum vs. tungsten selectivity in enzymes. Dalton Trans. 2025, 54, 8728–8744. [Google Scholar] [CrossRef] [PubMed]
- Hagen, W.R. The Development of Tungsten Biochemistry—A Personal Recollection. Molecules 2023, 28, 4017. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Das, A.; Das, A.K. Exploring the nature’s discriminating factors behind the selection of molybdoenzymes and tungstoenzymes depending on the biological environment. Coord. Chem. Rev. 2025, 523, 216290. [Google Scholar] [CrossRef]
- Laukel, M.; Chistoserdova, L.; Lidstrom, M.E.; Vorholt, J.A. The tungsten-containing formate dehydrogenase from Methylobacterium extorquens AM1: Purification and properties. Eur. J. Biochem. 2003, 270, 325–333. [Google Scholar] [CrossRef]
- Rosenbaum, F.P.; Müller, V. Moorella thermoacetica: A promising cytochrome- and quinone-containing acetogenic bacterium as platform for a CO2-based bioeconomy. Green Carbon 2023, 1, 2–13. [Google Scholar] [CrossRef]
- Boes, D.M.; Schmitz, R.A.; Hagedoorn, P.L. Tungsten containing aldehyde oxidoreductase (AOR)-family enzymes; past, present and future production strategies. Methods Enzymol. 2025, 714, 313–336. [Google Scholar] [CrossRef]
- Putumbaka, S.; Schut, G.J.; Thorgersen, M.P.; Poole, F.L.; Shao, N.; Rodionov, D.A.; Adams, M.W.W. Tungsten is utilized for lactate consumption and SCFA production by a dominant human gut microbe Eubacterium limosum. Proc. Natl. Acad. Sci. USA 2025, 122, e2411809121. [Google Scholar] [CrossRef]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The Involvement of Molybdenum in Life. Biochem. Biophys. Res. Commun. 2002, 292, 293–299. [Google Scholar] [CrossRef]
- Reimink, J.R.; Mundl-Petermeier, A.; Carlson, R.W.; Shirey, S.B.; Walker, R.J.; Pearson, D.G. Tungsten Isotope Composition of Archean Crustal Reservoirs and Implications for Terrestrial μ182W Evolution. Geochem. Geophys. Geosys. 2020, 21, e2020GC009155. [Google Scholar] [CrossRef]
- Kleine, T.; Walker, R.J. Tungsten Isotopes in Planets. Ann. Rev. Earth Planet. Sci. 2017, 45, 389–417. [Google Scholar] [CrossRef]
- Jelikić-Stankov, M.; Uskoković-Marković, S.; Holclajtner-Antunović, I.; Todorović, M.; Djurdjević, P. Compounds of Mo, V and W in biochemistry and their biomedical activity. J. Trace Elem. Med. Biol. 2007, 21, 8–16. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Moniz, T.; Silva, A.M.N.; Rangel, M. Vanadium Compounds with Antidiabetic Potential. Int. J. Molec. Sci. 2023, 24, 15675. [Google Scholar] [CrossRef]
- Dayanand, Y.; Pather, R.; Xulu, N.; Booysen, I.; Sibiya, N.; Khathi, A.; Ngubane, P. Exploring the Biological Effects of Anti-Diabetic Vanadium Compounds in the Liver, Heart and Brain. Diabetes Metab. Syndr. Obes. 2024, 17, 3267–3278. [Google Scholar] [CrossRef]
- Fuchs, V.; Cseh, K.; Hejl, M.; Vician, P.; Neuditschko, B.; Meier-Menches, S.M.; Janker, L.; Bileck, A.; Gajic, N.; Kronberger, J.; et al. Highly Cytotoxic Molybdenocenes with Strong Metabolic Effects Inhibit Tumour Growth in Mice. Chem. Eur. J. 2023, 29, e202202648. [Google Scholar] [CrossRef]
- Yamase, T. Polyoxometalates for Molecular Devices: Antitumor Activity and Luminescence. In Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity; Pope, M.T., Müller, A., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 337–358. [Google Scholar]
- Kostova, I. Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7. Molecules 2024, 29, 824. [Google Scholar] [CrossRef]
- Alterio, V.; Langella, E.; De Simone, G.; Monti, S.M. Cadmium-Containing Carbonic Anhydrase CDCA1 in Marine Diatom Thalassiosira weissflogii. Mar. Drugs 2015, 13, 1688–1697. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef]
- Tahara, N.; Lairez, O.; Endo, J.; Okada, A.; Ueda, M.; Ishii, T.; Kitano, Y.; Lee, H.E.; Russo, E.; Kubo, T. (99m) Technetium-pyrophosphate scintigraphy: A practical guide for early diagnosis of transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2022, 9, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulou, D. Technetium-99m radiochemistry for pharmaceutical applications. J. Label. Comp. Radiopharm. 2017, 60, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Gama Justi, F.V.; Araújo Matos, G.; de Sá Roriz Caminha, J.; Rodrigues Roque, C.; Muniz Carvalho, E.; Soares Campelo, M.W.; Belayev, L.; Gonzaga de França Lopes, L.; Barreto Oriá, R. The Role of Ruthenium Compounds in Neurologic Diseases: A Minireview. J. Pharm. Exp. Ther. 2022, 380, 47–53. [Google Scholar] [CrossRef]
- Aguilar-Garay, R.; Lara-Ortiz, L.F.; Campos-López, M.; Gonzalez-Rodriguez, D.E.; Gamboa-Lugo, M.M.; Mendoza-Pérez, J.A.; Anzueto-Ríos, Á.; Nicolás-Álvarez, D.E. A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents. Pharmaceuticals 2024, 17, 1134. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashmi, A.A.; Al-Bogami, A.S.; Wani, M.Y. Harnessing the power of gold: Advancements in anticancer gold complexes and their functionalized nanoparticles. J. Mater. Chem. B 2024, 12, 552–576. [Google Scholar] [CrossRef]
- van der Westhuizen, D.; Bezuidenhout, D.I.; Munro, O.Q. Cancer molecular biology and strategies for the design of cytotoxic gold(i) and gold(iii) complexes: A tutorial review. Dalton Trans. 2021, 50, 17413–17437. [Google Scholar] [CrossRef]
- Shukla, S.; Mishra, A.P. Synthesis, Structure, and Anticancerous Properties of Silver Complexes. J. Chem. 2013, 2013, 527123. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef] [PubMed]
- Nabiyeva, T.; Marschner, C.; Blom, B. Synthesis, structure and anti-cancer activity of osmium complexes bearing π-bound arene substituents and phosphane Co-Ligands: A review. Eur. J. Med. Chem. 2020, 201, 112483. [Google Scholar] [CrossRef] [PubMed]
- Matlou, M.L.; Louis, H.; Charlie, D.E.; Agwamba, E.C.; Amodu, I.O.; Tembu, V.J.; Manicum, A.-L.E. Anticancer Activities of Re(I) Tricarbonyl and Its Imidazole-Based Ligands: Insight from a Theoretical Approach. ACS Omega 2023, 8, 10242–10252. [Google Scholar] [CrossRef] [PubMed]
- Hong Enriquez, R.P.; Do, T.N. Bioavailability of Metal Ions and Evolutionary Adaptation. Life 2012, 2, 274–285. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Burgmayer, S.J.N.; Stiefel, E.I. Molybdenum enzymes, cofactors, and systems: The chemical uniqueness of molybdenum. J. Chem. Ed. 1985, 62, 943. [Google Scholar] [CrossRef]
- Leimkühler, S. Molybdenum and Ions in Living Systems. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1420–1429. [Google Scholar]
- Basolo, F.; Pearson, R.G. Mechanisms of Inorganic Reactions; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Bohme, D.K. Toward ICP-SIFT mass spectrometry and atomic cation ligation as a probe of relativistic effects—A personal journey. Mass Spectrom. Rev. 2022, 41, 593–605. [Google Scholar] [CrossRef]
- Helm, L.; Merbach, A.E. Inorganic and Bioinorganic Solvent Exchange Mechanisms. Chem. Rev. 2005, 105, 1923–1960. [Google Scholar] [CrossRef]
- van Eldik, R. Introduction: Inorganic and Bioinorganic Mechanisms. Chem. Rev. 2005, 105, 1917–1922. [Google Scholar] [CrossRef]
- Richens, D.T. Ligand Substitution Reactions at Inorganic Centers. Chem. Rev. 2005, 105, 1961–2002. [Google Scholar] [CrossRef]
- Kisker, C.; Schindelin, H.; Rees, D.C. Molybdenium-factor-containing enzymes: Structure and Mechanism. Ann. Rev. Biochem. 1997, 66, 233–267. [Google Scholar] [CrossRef]
- Rothery, R.A.; Stein, B.; Solomonson, M.; Kirk, M.L.; Weiner, J.H. Pyranopterin conformation defines the function of molybdenum and tungsten enzymes. Proc. Natl. Acad. Sci. USA 2012, 109, 14773–14778. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.R.; Marques, H.M.; Grabowski, I. Ammonia Synthesis over Transition Metal Catalysts: Reaction Mechanisms, Rate-Determining Steps, and Challenges. Int. J. Mol. Sci. 2025, 26, 4670. [Google Scholar] [CrossRef] [PubMed]
- Beller, M. Introduction: First Row Metals and Catalysis. Chem. Rev. 2019, 119, 2089. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.L.; Stein, B. Molybdenum Enzymes. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 263–293. [Google Scholar]
- Zhang, L.-H.; Mathew, S.; Hessels, J.; Reek, J.N.H.; Yu, F. Homogeneous Catalysts Based on First-Row Transition-Metals for Electrochemical Water Oxidation. ChemSusChem 2021, 14, 234–250. [Google Scholar] [CrossRef]
- Glaser, F.; Wenger, O.S. Recent progress in the development of transition-metal based photoredox catalysts. Coord. Chem. Rev. 2020, 405, 213129. [Google Scholar] [CrossRef]
- Wiebelhaus, N.J.; Cranswick, M.A.; Klein, E.L.; Lockett, L.T.; Lichtenberger, D.L.; Enemark, J.H. Metal–Sulfur Valence Orbital Interaction Energies in Metal–Dithiolene Complexes: Determination of Charge and Overlap Interaction Energies by Comparison of Core and Valence Ionization Energy Shifts. Inorg. Chem. 2011, 50, 11021–11031. [Google Scholar] [CrossRef]
- Yang, J.; Enemark, J.H.; Kirk, M.L. Metal–Dithiolene Bonding Contributions to Pyranopterin Molybdenum Enzyme Reactivity. Inorganics 2020, 8, 19. [Google Scholar] [CrossRef]
- Ryde, U.; Schulzke, C.; Starke, K. Which functional groups of the molybdopterin ligand should be considered when modeling the active sites of the molybdenum and tungsten cofactors? A density functional theory study. J. Biol. Inorg. Chem. 2009, 14, 1053–1064. [Google Scholar] [CrossRef]
- Thorpe, G.W.; Reodica, M.; Davies, M.J.; Heeren, G.; Jarolim, S.; Pillay, B.; Breitenbach, M.; Higgins, V.J.; Dawes, I.W. Superoxide radicals have a protective role during H2O2 stress. Molec. Biol. Cell 2013, 24, 2876–2884. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Molec. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Shekhova, E. Mitochondrial reactive oxygen species as major effectors of antimicrobial immunity. PLoS Pathog. 2020, 16, e1008470. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid.Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Diaz, J.M.; Plummer, S. Tight Regulation of Extracellular Superoxide Points to Its Vital Role in the Physiology of the Globally Relevant Roseobacter Clade. mBio 2019, 10, e02668-18. [Google Scholar] [CrossRef]
- Marques, H.M. Electron transfer in biological systems. J. Biol. Inorg. Chem. 2024, 29, 641–683. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Jang, S.; Imlay, J.A. Micromolar Intracellular Hydrogen Peroxide Disrupts Metabolism by Damaging Iron-Sulfur Enzymes. J. Biol. Chem. 2007, 282, 929–937. [Google Scholar] [CrossRef]
- Niks, D.; Hille, R. Molybdenum-Containing Enzymes. In Metalloproteins: Methods and Protocols; Hu, Y., Ed.; Springer: New York, NY, USA, 2019; pp. 55–63. [Google Scholar]
- Fernandes, H.S.; Teixeira, C.S.S.; Sousa, S.F.; Cerqueira, N.M.F.S.A. Formation of Unstable and very Reactive Chemical Species Catalyzed by Metalloenzymes: A Mechanistic Overview. Molecules 2019, 24, 2462. [Google Scholar] [CrossRef]
- Watson, M.P.; Weix, D.J. The Once and Future Catalysts: How the Challenges of First-Row Transition-Metal Catalysis Grew to Become Strengths. Acc. Chem. Res. 2024, 57, 2451–2452. [Google Scholar] [CrossRef]
- Boniolo, M.; Chernev, P.; Cheah, M.H.; Heizmann, P.A.; Huang, P.; Shylin, S.I.; Salhi, N.; Hossain, M.K.; Gupta, A.K.; Messinger, J.; et al. Electronic and geometric structure effects on one-electron oxidation of first-row transition metals in the same ligand framework. Dalton Trans. 2021, 50, 660–674. [Google Scholar] [CrossRef]
- Enemark, J.H. [Moco]n, (n = 0–8): A general formalism for describing the highly covalent molybdenum cofactor of sulfite oxidase and related Mo enzymes. J. Inorg. Biochem. 2022, 231, 111801. [Google Scholar] [CrossRef] [PubMed]
- Lake, M.W.; Temple, C.A.; Rajagopalan, K.V.; Schindelin, H. The Crystal Structure of the Escherichia coliMobA Protein Provides Insight into Molybdopterin Guanine Dinucleotide Biosynthesis. J. Biol. Chem. 2000, 275, 40211–40217. [Google Scholar] [CrossRef] [PubMed]
- Spatzal, T.; Perez, K.A.; Howard, J.B.; Rees, D.C. Catalysis-dependent selenium incorporation and migration in the nitrogenase active site iron-molybdenum cofactor. eLife 2015, 4, e11620. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. The Mononuclear Molybdenum Enzymes. Chem. Rev. 1996, 96, 2757–2816. [Google Scholar] [CrossRef]
- Hille, R.; Nishino, T.; Bittner, F. Molybdenum enzymes in higher organisms. Coord. Chem. Rev. 2011, 255, 1179–1205. [Google Scholar] [CrossRef]
- Rees, D.C. Great Metalloclusters in Enzymology. Ann. Rev. Biochem. 2002, 71, 221–246. [Google Scholar] [CrossRef]
- Zimmermann, R.; Münck, E.; Brill, W.J.; Shah, V.K.; Henzl, M.T.; Rawlings, J.; Orme-Johnson, W.H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP Nature of the iron centers. Biochim. Biophs. Acta 1978, 537, 185–207. [Google Scholar] [CrossRef]
- Bjornsson, R.; Lima, F.A.; Spatzal, T.; Weyhermüller, T.; Glatzel, P.; Bill, E.; Einsle, O.; Neese, F.; DeBeer, S. Identification of a spin-coupled Mo(III) in the nitrogenase iron–molybdenum cofactor. Chem. Sci. 2014, 5, 3096–3103. [Google Scholar] [CrossRef]
- Bjornsson, R.; Neese, F.; Schrock, R.R.; Einsle, O.; DeBeer, S. The discovery of Mo(III) in FeMoco: Reuniting enzyme and model chemistry. J. Biol. Inorg. Chem. 2015, 20, 447–460. [Google Scholar] [CrossRef]
- Dance, I. Computational Investigations of the Chemical Mechanism of the Enzyme Nitrogenase. ChemBioChem 2020, 21, 1671–1709. [Google Scholar] [CrossRef]
- Maiti, B.K.; Maia, L.B.; Moura, J.J.G. Sulfide and transition metals—A partnership for life. J. Inorg. Biochem. 2022, 227, 111687. [Google Scholar] [CrossRef] [PubMed]
- Clement, B.; Struwe, M.A. The History of mARC. Molecules 2023, 28, 4713. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Romão, M.J.; Leimkühler, S.; Bolis, M.; Fratelli, M.; Coelho, C.; Santos-Silva, T.; Garattini, E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016, 90, 753–780. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, J.M.; Obach, R.S.; Dalvie, D.; Zientek, M.A. Strategies for a comprehensive understanding of metabolism by aldehyde oxidase. Expert Opin. Drug Metab. Toxicol. 2013, 9, 153–168. [Google Scholar] [CrossRef]
- Garattini, E.; Fratelli, M.; Terao, M. Mammalian aldehyde oxidases: Genetics, evolution and biochemistry. Cell Mol. Life Sci. 2008, 65, 1019–1048. [Google Scholar] [CrossRef]
- Terao, M.; Garattini, E.; Romão, M.J.; Leimkühler, S. Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes. J. Biol. Chem. 2020, 295, 5377–5389. [Google Scholar] [CrossRef]
- Beedham, C. Aldehyde oxidase; new approaches to old problems. Xenobiotica 2020, 50, 34–50. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. The History of Animal and Plant Sulfite Oxidase—A Personal View. Molecules 2023, 28, 6998. [Google Scholar] [CrossRef]
- Feng, C.; Tollin, G.; Enemark, J.H. Sulfite oxidizing enzymes. Biochim. Biophs. Acta 2007, 1774, 527–539. [Google Scholar] [CrossRef]
- Kappler, U.; Enemark, J.H. Sulfite-oxidizing enzymes. J. Biol. Inorg. Chem. 2015, 20, 253–264. [Google Scholar] [CrossRef]
- Hänsch, R.; Lang, C.; Rennenberg, H.; Mendel, R.R. Significance of Plant Sulfite Oxidase. Plant Biol. 2007, 9, 589–595. [Google Scholar] [CrossRef]
- Schwahn, B.C.; van Spronsen, F.; Misko, A.; Pavaine, J.; Holmes, V.; Spiegel, R.; Schwarz, G.; Wong, F.; Horman, A.; Pitt, J.; et al. Consensus guidelines for the diagnosis and management of isolated sulfite oxidase deficiency and molybdenum cofactor deficiencies. J. Inherit. Metab. Dis. 2024, 47, 598–623. [Google Scholar] [CrossRef] [PubMed]
- Kappler, U. Bacterial sulfite-oxidizing enzymes. Biochim. Biophys. Acta 2011, 1807, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Zhao, Z.; Li, X.; Pang, J.; Chen, J. Recent Progress and Future Perspectives on Anti-Hyperuricemic Agents. J. Med. Chem. 2024, 67, 19966–19987. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Moreira, K.L.S.; da Rocha, M.I.U.M.; da Veiga, M.L.; Santos, R.C.V.; Baldisserotto, B. Involvement of xanthine oxidase activity with oxidative and inflammatory renal damage in silver catfish experimentally infected with Streptococcus agalactiae: Interplay with reactive oxygen species and nitric oxide. Micro. Patho. 2017, 111, 1–5. [Google Scholar] [CrossRef]
- Justi, L.H.Z.; Silva, J.F.; Santana, M.S.; Laureano, H.A.; Pereira, M.E.; Oliveira, C.S.; Guiloski, I.C. Non-steroidal anti-inflammatory drugs and oxidative stress biomarkers in fish: A meta-analytic review. Toxicol. Rep. 2025, 14, 101910. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.M.; Busch, J.D.; Potting, C.; Baker, M.J.; Langer, T.; Schwarz, G. The Mitochondrial Amidoxime-reducing Component (mARC1) Is a Novel Signal-anchored Protein of the Outer Mitochondrial Membrane *. J. Biol. Chem. 2012, 287, 42795–42803. [Google Scholar] [CrossRef] [PubMed]
- Struwe, M.A.; Scheidig, A.J.; Clement, B. The mitochondrial amidoxime reducing component-from prodrug-activation mechanism to drug-metabolizing enzyme and onward to drug target. J. Biol. Chem. 2023, 299, 105306. [Google Scholar] [CrossRef]
- Miralles-Robledillo, J.M.; Torregrosa-Crespo, J.; Martínez-Espinosa, R.M.; Pire, C. DMSO Reductase Family: Phylogenetics and Applications of Extremophiles. Int. J. Mol. Sci. 2019, 20, 3349. [Google Scholar] [CrossRef]
- Calzadiaz-Ramirez, L.; Meyer, A.S. Formate dehydrogenases for CO2 utilization. Curr. Opin. Biotechnol. 2022, 73, 95–100. [Google Scholar] [CrossRef]
- Le, C.C.; Bae, M.; Kiamehr, S.; Balskus, E.P. Emerging Chemical Diversity and Potential Applications of Enzymes in the DMSO Reductase Superfamily. Annu. Rev. Biochem. 2022, 91, 475–504. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Boscari, A.; Puppo, A.; Brouquisse, R. Nitrate reductases and hemoglobins control nitrogen-fixing symbiosis by regulating nitric oxide accumulation. J. Exp. Biol. 2020, 72, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef] [PubMed]
- Besson, S.; Almeida, M.G.; Silveira, C.M. Nitrite reduction in bacteria: A comprehensive view of nitrite reductases. Coord. Chem. Rev. 2022, 464, 214560. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate Reductase Structure, Function and Regulation:: Bridging the Gap between Biochemistry and Physiology. Annu. Rev. Plant Physio.L Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Fischer, K.; Barbier, G.G.; Hecht, H.-J.; Mendel, R.R.; Campbell, W.H.; Schwarz, G. Structural Basis of Eukaryotic Nitrate Reduction: Crystal Structures of the Nitrate Reductase Active Site. Plant Cell 2005, 17, 1167–1179. [Google Scholar] [CrossRef]
- Jeoung, J.-H.; Martins, B.M.; Dobbek, H. Carbon Monoxide Dehydrogenases. In Metalloproteins: Methods and Protocols; Hu, Y., Ed.; Springer: New York, NY, USA, 2019; pp. 37–54. [Google Scholar]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef]
- Llamas, A.; Tejada-Jiménez, M.; Fernández, E.; Galván, A. Molybdenum metabolism in the alga Chlamydomonas stands at the crossroad of those in Arabidopsis and humans. Metallomics 2011, 3, 578–590. [Google Scholar] [CrossRef]
- Mendel, R.R. Cell biology of molybdenum in plants. Plant Cell Rep. 2011, 30, 1787–1797. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. Molybdenum cofactor biosynthesis in plants and humans. Coord. Chem. Rev. 2011, 255, 1145–1158. [Google Scholar] [CrossRef]
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta 2012, 1823, 1568–1579. [Google Scholar] [CrossRef]
- Weber, J.N.; Minner-Meinen, R.; Kaufholdt, D. The Mechanisms of Molybdate Distribution and Homeostasis with Special Focus on the Model Plant Arabidopsis thaliana. Molecules 2023, 29, 40. [Google Scholar] [CrossRef]
- Mendel, R.R. The Molybdenum Cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Sickerman, N.S.; Rettberg, L.A.; Lee, C.C.; Hu, Y.; Ribbe, M.W. Cluster assembly in nitrogenase. Essays Biochem. 2017, 61, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Winston, P.W. 8—Molybdenum. In Disorders of Mineral Metabolism; Bronner, F., Coburn, J.W., Eds.; Academic Press: New York, NY, USA, 1981; pp. 295–315. [Google Scholar]
- Claerhout, H.; Witters, P.; Régal, L.; Jansen, K.; Van Hoestenberghe, M.-R.; Breckpot, J.; Vermeersch, P. Isolated sulfite oxidase deficiency. J. Inherit. Metabol. Dis. 2018, 41, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Dandamudi, R.; Granadillo, J.L.; Grange, D.K.; Kakajiwala, A. Rare cause of xanthinuria: A pediatric case of molybdenum cofactor deficiency B. CEN Case Rep. 2021, 10, 378–382. [Google Scholar] [CrossRef]
- Endres, W.; Shin, Y.S.; Günther, R.; Ibel, H.; Duran, M.; Wadman, S.K. Report on a new patient with combined deficiencies of sulphite oxidase and xanthine dehydrogenase due to molybdenum cofactor deficiency. Eur. J. Pediatr. 1988, 148, 246–249. [Google Scholar] [CrossRef]
- Stiburkova, B.; Pavelcova, K.; Petru, L.; Krijt, J. Thiopurine-induced toxicity is associated with dysfunction variant of the human molybdenum cofactor sulfurase gene (xanthinuria type II). Toxicol. Appl. Pharmacol. 2018, 353, 102–108. [Google Scholar] [CrossRef]
- Dror, Y.; Stern, F. Molybdenum. In Trace Elements and Minerals in Health and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 179–207. [Google Scholar]
- Haywood, S.; Dincer, Z.; Holding, J.; Parry, N.M. Metal (molybdenum, copper) accumulation and retention in brain, pituitary and other organs of ammonium tetrathiomolybdate-treated sheep. Br. J. Nutr. 1998, 79, 329–331. [Google Scholar] [CrossRef][Green Version]
- Brewer, G.J.; Hedera, P.; Kluin, K.J.; Carlson, M.; Askari, F.; Dick, R.B.; Sitterly, J.; Fink, J.K. Treatment of Wilson Disease With Ammonium Tetrathiomolybdate: III. Initial Therapy in a Total of 55 Neurologically Affected Patients and Follow-up With Zinc Therapy. Arch. Neurol. 2003, 60, 379–385. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A.; Mbese, J.Z. Impact of Molybdenum Compounds as Anticancer Agents. Bioinorg. Chem. Appl. 2019, 2019, 6416198. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Polyoxometalates Active Against Tumors, Viruses, and Bacteria. In Biomedical Inorganic Polymers: Bioactivity and Applications of Natural and Synthetic Polymeric Inorganic Molecules; Müller, W.E.G., Wang, X., Schröder, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 65–116. [Google Scholar]
- Amend, J.P.; Shock, E.L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001, 25, 175–243. [Google Scholar] [CrossRef] [PubMed]
- Makdessi, K.; Andreesen, J.R.; Pich, A. Tungstate Uptake by a highly specific ABC transporter in Eubacterium acidaminophilum. J. Biol. Chem. 2001, 276, 24557–24564. [Google Scholar] [CrossRef] [PubMed]
- Bevers, L.E.; Hagedoorn, P.L.; Krijger, G.C.; Hagen, W.R. Tungsten transport protein A (WtpA) in Pyrococcus furiosus: The first member of a new class of tungstate and molybdate transporters. J. Bacteriol. 2006, 188, 6498–6505. [Google Scholar] [CrossRef]
- Andreesen, J.R.; Makdessi, K. Tungsten, the surprisingly positively acting heavy metal element for prokaryotes. Ann. N. Y. Acad. Sci. 2008, 1125, 215–229. [Google Scholar] [CrossRef]
- Bevers, L.E.; Schwarz, G.; Hagen, W.R. A Molecular Basis for Tungstate Selectivity in Prokaryotic ABC Transport Systems. J. Bacteriol. 2011, 193, 4999–5001. [Google Scholar] [CrossRef]
- Milojevic, T. Microbial Tungsten Assimilation. In Microbial Metabolism of Metals and Metalloids; Hurst, C.J., Ed.; Springer: Cham, Switzerland, 2022; pp. 545–561. [Google Scholar]
- Bolt, A.M.; Mann, K.K. Tungsten: An Emerging Toxicant, Alone or in Combination. Curr. Environ. Health Rep. 2016, 3, 405–415. [Google Scholar] [CrossRef]
- Maia, L.B.; Moura, J.J.G.; Moura, I. Molybdenum and tungsten-dependent formate dehydrogenases. J. Biol. Inorg. Chem. 2015, 20, 287–309. [Google Scholar] [CrossRef]
- Nissen, L.S.; Basen, M. The emerging role of aldehyde:ferredoxin oxidoreductases in microbially-catalyzed alcohol production. J. Biotechnol. 2019, 306, 105–117. [Google Scholar] [CrossRef]
- Nissen, L.S.; Moon, J.; Hitschler, L.; Basen, M. A Versatile Aldehyde: Ferredoxin Oxidoreductase from the Organic Acid Reducing Thermoanaerobacter sp. Strain X514. Int. J. Mol. Sci. 2024, 25, 1077. [Google Scholar] [CrossRef]
- Karrasch, M.; Börner, G.; Enßle, M.; Thauer, R.K. Formylmethanofuran dehydrogenase from methanogenic bacteria, a molybdoenzyme. FEBS Lett. 1989, 253, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Bertram, P.A.; Karrasch, M.; Schmitz, R.A.; Bocher, R.; Albracht, S.P.J.; Thauer, R.K. Formylmethanofuran dehydrogenases from methanogenic Archaea Substrate specificity, EPR properties and reversible inactivation by cyanide of the molybdenum or tungsten iron-sulfur proteins. Eur. J. Biochem. 1994, 220, 477–484. [Google Scholar] [CrossRef]
- Schwörer, B.; Thauer, R.K. Activities of formylmethanofuran dehydrogenase, methylenetetrahydromethanopterin dehydrogenase, methylenetetrahydromethanopterin reductase, and heterodisulfide reductase in methanogenic bacteria. Arch. Microbiol. 1991, 155, 459–465. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Krieger, R.; Ensign, S.; Kroneck, P.M.H.; Schink, B. Acetylene hydratase of Pelobacter acetylenicus. Eur. J. Biochem. 1999, 264, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.-Z.; Yu, J.-G.; Himo, F. Mechanism of tungsten-dependent acetylene hydratase from quantum chemical calculations. Proc. Natl. Acad. Sci. USA 2010, 107, 22523–22527. [Google Scholar] [CrossRef]
- Kroneck, P.M.H. Acetylene hydratase: A non-redox enzyme with tungsten and iron–sulfur centers at the active site. J. Biol. Inorg. Chem. 2016, 21, 29–38. [Google Scholar] [CrossRef]
- Cordas, C.M.; Moura, J.J.G. Molybdenum and tungsten enzymes redox properties—A brief overview. Coord. Chem. Rev. 2019, 394, 53–64. [Google Scholar] [CrossRef]
- Döring, A.; Schulzke, C. Tungsten’s redox potential is more temperature sensitive than that of molybdenum. Dalton Trans. 2010, 39, 5623–5629. [Google Scholar] [CrossRef]
- Buc, J.; Santini, C.-L.; Giordani, R.; Czjzek, M.; Wu, L.-F.; Giordano, G. Enzymatic and physiological properties of the tungsten-substituted molybdenum TMAO reductase from Escherichia coli. Mol. Microbiol. 1999, 32, 159–168. [Google Scholar] [CrossRef]
- Dridge, E.J.; Butler, C.S. Thermostable properties of the periplasmic selenate reductase from Thauera selenatis. Biochimie 2010, 92, 1268–1273. [Google Scholar] [CrossRef]
- Mesbah, N.M. Industrial Biotechnology Based on Enzymes From Extreme Environments. Front. Bioeng. Biotechnol. 2022, 10, 870083. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.; Casanueva, A.; Tuffin, M.; Cowan, D. Extremophiles in biofuel synthesis. Environ. Technol. 2010, 31, 871–888. [Google Scholar] [CrossRef]
- Mukhtar, S.; Aslam, M. Biofuel Synthesis by Extremophilic Microorganisms. In Biofuels Production—Sustainability and Advances in Microbial Bioresources; Yadav, A.N., Rastegari, A.A., Yadav, N., Gaur, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 115–138. [Google Scholar]
- Einsle, O.; Rees, D.C. Structural Enzymology of Nitrogenase Enzymes. Chem. Rev. 2020, 120, 4969–5004. [Google Scholar] [CrossRef]
- Warmack, R.A.; Rees, D.C. Nitrogenase beyond the Resting State: A Structural Perspective. Molecules 2023, 28, 7952. [Google Scholar] [CrossRef]
- Einsle, O. Catalysis and structure of nitrogenases. Curr. Opin. Struct. Biol. 2023, 83, 102719. [Google Scholar] [CrossRef]
- Rucker, H.R.; Kaçar, B. Enigmatic evolution of microbial nitrogen fixation: Insights from Earth’s past. Trends Microbiol. 2024, 32, 554–564. [Google Scholar] [CrossRef]
- Mrnjavac, N.; Degli Esposti, M.; Mizrahi, I.; Martin, W.F.; Allen, J.F. Three enzymes governed the rise of O2 on Earth. Biochim. Biophys. Acta 2024, 1865, 149495. [Google Scholar] [CrossRef]
- Eady, R.R. Structure−Function Relationships of Alternative Nitrogenases. Chem. Rev. 1996, 96, 3013–3030. [Google Scholar] [CrossRef]
- Peters, J.W.; Szilagyi, R.K. Exploring new frontiers of nitrogenase structure and mechanism. Curr. Opin. Chem. Biol. 2006, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lee, C.C.; Grosch, M.; Solomon, J.B.; Weigand, W.; Ribbe, M.W. Enzymatic Fischer–Tropsch-Type Reactions. Chem. Rev. 2023, 123, 5755–5797. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996, 96, 2983–3012. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.A.; Day, E.P.; Kent, T.A.; Orme-Johnson, W.H.; Münck, E. Mössbauer, EPR, and magnetization studies of the Azotobacter vinelandii Fe protein. Evidence for a [4Fe-4S]1+ cluster with spin S = 3/2. J. Biol. Chem. 1985, 260, 11160–11173. [Google Scholar] [CrossRef] [PubMed]
- Angove, H.C.; Yoo, S.J.; Münck, E.; Burgess, B.K. An All-ferrous State of the Fe Protein of Nitrogenase: Interaction with Nucleotides and Electron Transfer to the MoFe Protein. J. Biol. Chem. 1998, 273, 26330–26337. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; DeBeer, S. Structure, reactivity, and spectroscopy of nitrogenase-related synthetic and biological clusters. Chem. Soc. Rev. 2021, 50, 8743–8761. [Google Scholar] [CrossRef]
- Einsle, O.; Tezcan, F.A.; Andrade, S.L.A.; Schmid, B.; Yoshida, M.; Howard, J.B.; Rees, D.C. Nitrogenase MoFe-Protein at 1.16 Å Resolution: A Central Ligand in the FeMo-Cofactor. Science 2002, 297, 1696–1700. [Google Scholar] [CrossRef]
- Miller, R.W.; Eady, R.R. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase. Biochem. J. 1988, 256, 429–432. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.-Y.; Lukoyanov, D.A.; Harris, D.F.; Dean, D.R.; Raugei, S.; Hoffman, B.M. Reduction of Substrates by Nitrogenases. Chem. Rev. 2020, 120, 5082–5106. [Google Scholar] [CrossRef]
- Appia-Ayme, C.; Little, R.; Chandra, G.; de Oliveira Martins, C.; Bueno Batista, M.; Dixon, R. Interactions between paralogous bacterial enhancer-binding proteins enable metal-dependent regulation of alternative nitrogenases in Azotobacter vinelandii. Molec. Microbiol. 2022, 118, 105–124. [Google Scholar] [CrossRef]
- Harwood, C.S. Iron-Only and Vanadium Nitrogenases: Fail-Safe Enzymes or Something More? Annu. Rev. Microbiol. 2020, 74, 247–266. [Google Scholar] [CrossRef]
- Rees, D.C.; Akif Tezcan, F.; Haynes, C.A.; Walton, M.Y.; Andrade, S.; Einsle, O.; Howard, J.B. Structural basis of biological nitrogen fixation. Phil. Trans. Roy. Soc. A 2005, 363, 971–984. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, H.L.; Tezcan, F.A. Electron Transfer in Nitrogenase. Chem. Rev. 2020, 120, 5158–5193. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Danyal, K.; Shaw, S.; Lytle, A.K.; Dean, D.R.; Hoffman, B.M.; Antony, E.; Seefeldt, L.C. Electron transfer precedes ATP hydrolysis during nitrogenase catalysis. Proc. Natl. Acad. Sci. USA 2013, 110, 16414–16419. [Google Scholar] [CrossRef] [PubMed]
- Danyal, K.; Dean, D.R.; Hoffman, B.M.; Seefeldt, L.C. Electron Transfer within Nitrogenase: Evidence for a Deficit-Spending Mechanism. Biochemistry 2011, 50, 9255–9263. [Google Scholar] [CrossRef]
- Thorneley, R.N.F.; Lowe, D.J. Kinetics and Mechanism of the Nitrogenase Enzyme System. In Metal Ions in Biology: Molybdenum Enzymes; Spiro, T.G., Ed.; Wiley-Interscience: New York, NY, USA, 1985; Volume 7, pp. 221–284. [Google Scholar]
- Cao, L.; Ryde, U. Putative reaction mechanism of nitrogenase after dissociation of a sulfide ligand. J. Catal. 2020, 391, 247–259. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. Model Calculations Suggest that the Central Carbon in the FeMo-Cofactor of Nitrogenase Becomes Protonated in the Process of Nitrogen Fixation. J. Am. Chem. Soc. 2016, 138, 10485–10495. [Google Scholar] [CrossRef]
- Lukoyanov, D.; Dikanov, S.A.; Yang, Z.-Y.; Barney, B.M.; Samoilova, R.I.; Narasimhulu, K.V.; Dean, D.R.; Seefeldt, L.C.; Hoffman, B.M. ENDOR/HYSCORE Studies of the Common Intermediate Trapped during Nitrogenase Reduction of N2H2, CH3N2H, and N2H4 Support an Alternating Reaction Pathway for N2 Reduction. J. Am. Chem. Soc. 2011, 133, 11655–11664. [Google Scholar] [CrossRef]
- Raugei, S.; Seefeldt, L.C.; Hoffman, B.M. Critical computational analysis illuminates the reductive-elimination mechanism that activates nitrogenase for N2 reduction. Proc. Natl. Acad. Sci. USA 2018, 115, E10521–E10530. [Google Scholar] [CrossRef]
- Rohde, M.; Laun, K.; Zebger, I.; Stripp, S.T.; Einsle, O. Two ligand-binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle. Sci. Adv. 2021, 7, eabg4474. [Google Scholar] [CrossRef]
- Khadka, N.; Milton, R.D.; Shaw, S.; Lukoyanov, D.; Dean, D.R.; Minteer, S.D.; Raugei, S.; Hoffman, B.M.; Seefeldt, L.C. Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects. J. Am. Chem. Soc. 2017, 139, 13518–13524. [Google Scholar] [CrossRef]
- Cao, L.; Caldararu, O.; Ryde, U. Protonation and Reduction of the FeMo Cluster in Nitrogenase Studied by Quantum Mechanics/Molecular Mechanics (QM/MM) Calculations. J. Chem. Theory Comput. 2018, 14, 6653–6678. [Google Scholar] [CrossRef] [PubMed]
- Dance, I. Survey of the Geometric and Electronic Structures of the Key Hydrogenated Forms of FeMo-co, the Active Site of the Enzyme Nitrogenase: Principles of the Mechanistically Significant Coordination Chemistry. Inorganics 2019, 7, 8. [Google Scholar] [CrossRef]
- Kang, W.; Lee, C.C.; Jasniewski, A.J.; Ribbe, M.W.; Hu, Y. Structural evidence for a dynamic metallocofactor during N2 reduction by Mo-nitrogenase. Science 2020, 368, 1381–1385. [Google Scholar] [CrossRef]
- Jia, H.-P.; Quadrelli, E.A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev. 2014, 43, 547–564. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Kästner, J.; Blöchl, P.E. Ammonia Production at the FeMo Cofactor of Nitrogenase: Results from Density Functional Theory. J. Am. Chem. Soc. 2007, 129, 2998–3006. [Google Scholar] [CrossRef]
- Dance, I. The chemical mechanism of nitrogenase: Calculated details of the intramolecular mechanism for hydrogenation of η2-N2 on FeMo-co to NH3. Dalton Trans. 2008, 5977–5991. [Google Scholar] [CrossRef]
- Varley, J.B.; Wang, Y.; Chan, K.; Studt, F.; Nørskov, J.K. Mechanistic insights into nitrogen fixation by nitrogenase enzymes. Phys. Chem. Chem. Phys. 2015, 17, 29541–29547. [Google Scholar] [CrossRef]
- Rao, L.; Xu, X.; Adamo, C. Theoretical Investigation on the Role of the Central Carbon Atom and Close Protein Environment on the Nitrogen Reduction in Mo Nitrogenase. ACS Catal. 2016, 6, 1567–1577. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. The mechanism for nitrogenase including all steps. Phys. Chem. Chem. Phys. 2019, 21, 15747–15759. [Google Scholar] [CrossRef]
- Dance, I. Understanding non-reducible N2 in the mechanism of Mo–nitrogenase. Dalton Trans. 2025, 54, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.M.; Gormal, C.A.; Smith, B.E.; Lawson, D.M. Crystallographic Analysis of the MoFe Protein of Nitrogenase from a nifV Mutant of Klebsiella pneumoniae Identifies Citrate as a Ligand to the Molybdenum of Iron Molybdenum Cofactor (FeMoco). J. Biol. Chem. 2002, 277, 35263–35266. [Google Scholar] [CrossRef]
- Poole, R.K.; Hill, S. Respiratory Protection of Nitrogenase Activity in Azotobacter vinelandii -Roles of the Terminal Oxidases. Biosci. Rep. 1997, 17, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.A.; Bertrand, E.M.; Dutkiewicz, S.; Bulygin, V.V.; Moran, D.M.; Monteiro, F.M.; Follows, M.J.; Valois, F.W.; Waterbury, J.B. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc. Natl. Acad. Sci. USA 2011, 108, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Lecomte, J.T.J. The Globins of Cyanobacteria and Algae. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: New York, NY, USA, 2013; Volume 63, pp. 195–272. [Google Scholar]
- Rutten, P.J.; Poole, P.S. Oxygen regulatory mechanisms of nitrogen fixation in rhizobia. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: New York, NY, USA, 2019; Volume 75, pp. 325–389. [Google Scholar]
- Allen, J.F.; Thake, B.; Martin, W.F. Nitrogenase Inhibition Limited Oxygenation of Earth’s Proterozoic Atmosphere. Trends Plant Sci. 2019, 24, 1022–1031. [Google Scholar] [CrossRef]
- Wofford, J.D.; Bolaji, N.; Dziuba, N.; Outten, F.W.; Lindahl, P.A. Evidence that a respiratory shield in Escherichia coli protects a low-molecular-mass FeII pool from O2-dependent oxidation. J. Biol. Chem. 2019, 294, 50–62. [Google Scholar] [CrossRef]
- Cardona, T.; Sánchez-Baracaldo, P.; Rutherford, A.W.; Larkum, A.W. Early Archean origin of Photosystem II. Geobiology 2019, 17, 127–150. [Google Scholar] [CrossRef]
- Franke, P.; Freiberger, S.; Zhang, L.; Einsle, O. Conformational protection of molybdenum nitrogenase by Shethna protein II. Nature 2025, 637, 998–1004. [Google Scholar] [CrossRef]
- Narehood, S.M.; Cook, B.D.; Srisantitham, S.; Eng, V.H.; Shiau, A.A.; McGuire, K.L.; Britt, R.D.; Herzik, M.A.; Tezcan, F.A. Structural basis for the conformational protection of nitrogenase from O2. Nature 2025, 637, 991–997. [Google Scholar] [CrossRef]
- Rosenzweig, A.C. How a nitrogen-fixing enzyme avoids oxygen. Nature 2025, 637, 796–798. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e10. [Google Scholar] [CrossRef]
- Huynh, T.N.; Stewart, V. Purine catabolism by enterobacteria. Adv. Microb. Physiol. 2023, 82, 205–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, L.; Song, X.; Wang, J.; Li, X.; Lin, Z.; Su, T.; Liang, B.; Huang, D. Purine-Induced IFN-γ Promotes Uric Acid Production by Upregulating Xanthine Oxidoreductase Expression. Front. Immunol. 2022, 13, 773001. [Google Scholar] [CrossRef]
- Schoepp-Cothenet, B.; van Lis, R.; Philippot, P.; Magalon, A.; Russell, M.J.; Nitschke, W. The ineluctable requirement for the trans-iron elements molybdenum and/or tungsten in the origin of life. Sci. Rep. 2012, 2, 263. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lü, J.M.; Yao, Q. Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview. Med. Sci. Monit. 2016, 22, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Dolzhenko, A.V.; Mancera, R.L. Inhibitors of Xanthine Oxidase: Scaffold Diversity and Structure-Based Drug Design. ChemMedChem 2019, 14, 714–743. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsumura, T.; Ichida, K.; Okamoto, K.; Nishino, T. Human Xanthine Oxidase Changes its Substrate Specificity to Aldehyde Oxidase Type upon Mutation of Amino Acid Residues in the Active Site: Roles of Active Site Residues in Binding and Activation of Purine Substrate. J. Biochem. 2007, 141, 513–524. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Grassi, G. Beyond gout: Uric acid and cardiovascular diseases. Curr. Med. Res. Opin. 2013, 29 (Suppl. S3), 33–39. [Google Scholar] [CrossRef]
- Schuchardt, M.; Herrmann, J.; Tolle, M.; van der Giet, M. Xanthine Oxidase and its Role as Target in Cardiovascular Disease: Cardiovascular Protection by Enzyme Inhibition? Curr. Pharm. Des. 2017, 23, 3391–3404. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: A leading actor in cardiovascular disease drama. Redox Biol. 2021, 48, 102195. [Google Scholar] [CrossRef]
- Metz, S.; Thiel, W. QM/MM Studies of Xanthine Oxidase: Variations of Cofactor, Substrate, and Active-Site Glu802. J. Phys. Chem. B 2010, 114, 1506–1517. [Google Scholar] [CrossRef]
- Ribeiro, P.M.G.; Fernandes, H.S.; Maia, L.B.; Sousa, S.F.; Moura, J.J.G.; Cerqueira, N.M.F.S.A. The complete catalytic mechanism of xanthine oxidase: A computational study. Inorg. Chem. Front. 2021, 8, 405–416. [Google Scholar] [CrossRef]
- Ishikita, H.; Eger, B.T.; Okamoto, K.; Nishino, T.; Pai, E.F. Protein Conformational Gating of Enzymatic Activity in Xanthine Oxidoreductase. J. Am. Chem. Soc. 2012, 134, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Garattini, E.; Terao, M. The role of aldehyde oxidase in drug metabolism. Expert Opin. Drug Metabol. Toxicol. 2012, 8, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Pryde, D.C.; Dalvie, D.; Hu, Q.; Jones, P.; Obach, R.S.; Tran, T.-D. Aldehyde Oxidase: An Enzyme of Emerging Importance in Drug Discovery. J. Med. Chem. 2010, 53, 8441–8460. [Google Scholar] [CrossRef]
- Rendić, S.P.; Crouch, R.D.; Guengerich, F.P. Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: Review and compilation of reactions. Arch. Toxicol. 2022, 96, 2145–2246. [Google Scholar] [CrossRef]
- Ferreira, P.; Cerqueira, N.M.F.S.A.; Fernandes, P.A.; Romão, M.J.; Ramos, M.J. Catalytic Mechanism of Human Aldehyde Oxidase. ACS Catal. 2020, 10, 9276–9286. [Google Scholar] [CrossRef]
- Montefiori, M.; Jørgensen, F.S.; Olsen, L. Aldehyde Oxidase: Reaction Mechanism and Prediction of Site of Metabolism. ACS Omega 2017, 2, 4237–4244. [Google Scholar] [CrossRef]
- Coelho, C.; Mahro, M.; Trincão, J.; Carvalho, A.T.P.; Ramos, M.J.; Terao, M.; Garattini, E.; Leimkühler, S.; Romão, M.J. The First Mammalian Aldehyde Oxidase Crystal Structure: Inisights into Substrate Specificity. J. Biol. Chem. 2012, 287, 40690–40702. [Google Scholar] [CrossRef]
- Kappler, U.; Dahl, C. Enzymology and molecular biology of prokaryotic sulfite oxidation. FEMS Microbiol. Lett. 2001, 203, 1–9. [Google Scholar] [CrossRef]
- Johannes, L.; Fu, C.-Y.; Schwarz, G. Molybdenum Cofactor Deficiency in Humans. Molecules 2022, 27, 6896. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Prenatal diagnosis of molybdenum cofactor deficiency and isolated sulfite oxidase deficiency. Prenat. Diagn. 2003, 23, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Dublin, A.B.; Hald, J.K.; Wootton-Gorges, S.L. Isolated sulfite oxidase deficiency: MR imaging features. Am. J. Neuroradiol. 2002, 23, 484–485. [Google Scholar] [PubMed]
- Kohlmeier, M. Chapter 8—Amino Acids and Nitrogen Compounds. In Nutrient Metabolism, 2nd ed.; Kohlmeier, M., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 265–477. [Google Scholar]
- Hille, R. Molybdenum enzymes. Essays Biochem. 1999, 34, 125–137. [Google Scholar] [CrossRef]
- Johnson-Winters, K.; Tollin, G.; Enemark, J.H. Elucidating the Catalytic Mechanism of Sulfite Oxidizing Enzymes Using Structural, Spectroscopic, and Kinetic Analyses. Biochemistry 2010, 49, 7242–7254. [Google Scholar] [CrossRef]
- Kisker, C.; Schindelin, H.; Pacheco, A.; Wehbi, W.A.; Garrett, R.M.; Rajagopalan, K.V.; Enemark, J.H.; Rees, D.C. Molecular Basis of Sulfite Oxidase Deficiency from the Structure of Sulfite Oxidase. Cell 1997, 91, 973–983. [Google Scholar] [CrossRef]
- Astashkin, A.V.; Raitsimring, A.M.; Feng, C.; Johnson, J.L.; Rajagopalan, K.V.; Enemark, J.H. Pulsed EPR studies of nonexchangeable protons near the Mo(V) center of sulfite oxidase: Direct detection of the alpha-proton of the coordinated cysteinyl residue and structural implications for the active site. J. Am. Chem. Soc. 2002, 124, 6109–6118. [Google Scholar] [CrossRef]
- Coelho, C.; Romão, M.J. Structural and mechanistic insights on nitrate reductases. Protein Sci. 2015, 24, 1901–1911. [Google Scholar] [CrossRef]
- Moreno-Vivián, C.; Ferguson, S.J. Definition and distinction between assimilatory, dissimilatory and respiratory pathways. Mol. Microbiol. 1998, 29, 664–666. [Google Scholar] [CrossRef]
- Moir, J.W.; Wood, N.J. Nitrate and nitrite transport in bacteria. Cell. Mol. Life Sci. 2001, 58, 215–224. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Yoneyama, T.; Suzuki, A. Light-Independent Nitrogen Assimilation in Plant Leaves: Nitrate Incorporation into Glutamine, Glutamate, Aspartate, and Asparagine Traced by 15N. Plants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Kaviraj, M.; Kumar, U.; Snigdha, A.; Chatterjee, S. Nitrate reduction to ammonium: A phylogenetic, physiological, and genetic aspects in Prokaryotes and eukaryotes. Arch. Microbiol. 2024, 206, 297. [Google Scholar] [CrossRef] [PubMed]
- Hird, K.; Campeciño, J.O.; Lehnert, N.; Hegg, E.L. Recent mechanistic developments for cytochrome c nitrite reductase, the key enzyme in the dissimilatory nitrate reduction to ammonium pathway. J. Inorg. Biochem. 2024, 256, 112542. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.J.; Poole, R.K. The respiratory chains of Escherichia coli. Microbiol. Rev. 1984, 48, 222–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Stewart, V. Nitrate assimilation by bacteria. Adv. Microb. Physiol. 1998, 39, 379. [Google Scholar] [CrossRef]
- Campbell, W.H. Structure and function of eukaryotic NAD(P)H:nitrate reductase. Cell Mol. Life Sci. 2001, 58, 194–204. [Google Scholar] [CrossRef]
- Richardson, D.J.; Berks, B.C.; Russell, D.A.; Spiro, S.; Taylor, C.J. Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell Mol. Life Sci. 2001, 58, 165–178. [Google Scholar] [CrossRef]
- Cole, J.A.; Richardson, D.J. Respiration of Nitrate and Nitrite. EcoSal Plus 2008, 3, 1–28. [Google Scholar] [CrossRef]
- van Spanning, R.J.M.; Richardson, D.J.; Ferguson, S.J. Introduction to the biochemistry and molecular biology of denitrification. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–20. [Google Scholar]
- Leung, P.M.; Daebeler, A.; Chiri, E.; Hanchapola, I.; Gillett, D.L.; Schittenhelm, R.B.; Daims, H.; Greening, C. A nitrite-oxidising bacterium constitutively consumes atmospheric hydrogen. ISME J. 2022, 16, 2213–2219. [Google Scholar] [CrossRef]
- Islam, Z.F.; Welsh, C.; Bayly, K.; Grinter, R.; Southam, G.; Gagen, E.J.; Greening, C. A widely distributed hydrogenase oxidises atmospheric H2 during bacterial growth. ISME J. 2020, 14, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jung, H.-C.; Oh, H.-M.; Choi, B.G.; Lee, H.S.; Kang, S.G. NADP+ or CO2 reduction by frhAGB-encoded hydrogenase through interaction with formate dehydrogenase 3 in the hyperthermophilic archaeon Thermococcus onnurineus NA1. Appl. Environ. Microbiol. 2023, 89, e01474-01423. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.J.; Bonnefoy, V.; Newman, D.K. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011, 19, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Shergill, J.K.; Lu, W.-P.; Wood, A.P. Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Van Leeuwenhoek 1997, 71, 95–107. [Google Scholar] [CrossRef]
- Shaikh, Z.; Ali, A. Nitrate assimilation: Cross talk between plants and soil. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: New York, NY, USA, 2021; pp. 135–151. [Google Scholar]
- Salas, A.; Cabrera, J.J.; Jiménez-Leiva, A.; Mesa, S.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Bacterial nitric oxide metabolism: Recent insights in rhizobia. Adv. Microb. Physiol. 2021, 78, 259–315. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Rocha, B.S.; Laranjinha, J. Nitrate from diet might fuel gut microbiota metabolism: Minding the gap between redox signaling and inter-kingdom communication. Free Radic. Biol. Med. 2020, 149, 37–43. [Google Scholar] [CrossRef]
- White, S.A.; Christofferson, A.J.; Grainger, A.I.; Day, M.A.; Jarrom, D.; Graziano, A.E.; Searle, P.F.; Hyde, E.I. The 3D-structure, kinetics and dynamics of the E. coli nitroreductase NfsA with NADP+ provide glimpses of its catalytic mechanism. FEBS Lett. 2022, 596, 2425–2440. [Google Scholar] [CrossRef]
- Bertero, M.G.; Rothery, R.A.; Palak, M.; Hou, C.; Lim, D.; Blasco, F.; Weiner, J.H.; Strynadka, N.C.J. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat. Struct. Mol. Biol. 2003, 10, 681–687. [Google Scholar] [CrossRef]
- Elliott, S.J.; Hoke, K.R.; Heffron, K.; Palak, M.; Rothery, R.A.; Weiner, J.H.; Armstrong, F.A. Voltammetric studies of the catalytic mechanism of the respiratory nitrate reductase from Escherichia coli: How nitrate reduction and inhibition depend on the oxidation state of the active site. Biochemistry 2004, 43, 799–807. [Google Scholar] [CrossRef]
- Cerqueira, N.M.; Gonzalez, P.J.; Brondino, C.D.; Romão, M.J.; Romão, C.C.; Moura, I.; Moura, J.J. The effect of the sixth sulfur ligand in the catalytic mechanism of periplasmic nitrate reductase. J. Comput. Chem. 2009, 30, 2466–2484. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, I.; Moura, J.J.G. Carbon Dioxide Utilisation—The Formate Route. In Enzymes for Solving Humankind’s Problems: Natural and Artificial Systems in Health, Agriculture, Environment and Energy; Moura, J.J.G., Moura, I., Maia, L.B., Eds.; Springer: Cham, Switzerland, 2021; pp. 29–81. [Google Scholar]
- Leimkühler, S. Metal-Containing Formate Dehydrogenases, a Personal View. Molecules 2023, 28, 5338. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Taketa, M.; Sowa, K.; Kano, K.; Higuchi, Y.; Ogata, H. Structure and function relationship of formate dehydrogenases: An overview of recent progress. IUCrJ 2023, 10, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.F.; Sistu, H. Formate and hydrogen in hydrothermal vents and their use by extremely thermophilic methanogens and heterotrophs. Front. Microbiol. 2023, 14, 1093018. [Google Scholar] [CrossRef]
- Niks, D.; Hille, R. Molybdenum- and tungsten-containing formate dehydrogenases and formylmethanofuran dehydrogenases: Structure, mechanism, and cofactor insertion. Protein Sci. 2019, 28, 111–122. [Google Scholar] [CrossRef]
- Hartmann, T.; Schwanhold, N.; Leimkühler, S. Assembly and catalysis of molybdenum or tungsten-containing formate dehydrogenases from bacteria. Biochim. Biophs. Acta 2015, 1854, 1090–1100. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar Padhi, S. Harnessing Ruthenium and Copper Catalysts for Formate Dehydrogenase Reactions. Chem. Rec. 2024, 24, e202400172. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. (Eds.) Global Warming of 1.5 °C.; Intergovernmental Panel on Climate Change (IPCC): Cambridge University Press: Cambridge, UK, 2018; p. 616. [Google Scholar]
- Johnson, M.K.; Rees, D.C.; Adams, M.W. Tungstoenzymes. Chem. Rev. 1996, 96, 2817–2840. [Google Scholar] [CrossRef]
- Moura, J.J.; Brondino, C.D.; Trincão, J.; Romão, M.J. Mo and W bis-MGD enzymes: Nitrate reductases and formate dehydrogenases. J. Biol. Inorg. Chem. 2004, 9, 791–799. [Google Scholar] [CrossRef]
- Roy, R.; Adams, M.W. Tungsten-dependent aldehyde oxidoreductase: A new family of enzymes containing the pterin cofactor. Met. Ions Biol. Syst. 2002, 39, 673–697. [Google Scholar]
- L’Vov N, P.; Nosikov, A.N.; Antipov, A.N. Tungsten-containing enzymes. Biochemistry 2002, 67, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, U.; Das, A.K. Relativistic effects on the chemical bonding properties of the heavier elements and their compounds. Coord. Chem. Rev. 2023, 479, 215000. [Google Scholar] [CrossRef]
- Tenderholt, A.L.; Szilagyi, R.K.; Holm, R.H.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Sulfur K-edge XAS of WVO vs. MoVO bis(dithiolene) complexes: Contributions of relativistic effects to electronic structure and reactivity of tungsten enzymes. J. Inorg. Biochem. 2007, 101, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.S. Relativistic Effects and the Chemistry of the Heaviest Main-Group Elements. J. Chem. Ed. 2005, 82, 1721. [Google Scholar] [CrossRef]
- Pyykko, P. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Rivas, M.G.; González, P.J.; Brondino, C.D.; Moura, J.J.; Moura, I. EPR characterization of the molybdenum(V) forms of formate dehydrogenase from Desulfovibrio desulfuricans ATCC 27774 upon formate reduction. J. Inorg. Biochem. 2007, 101, 1617–1622. [Google Scholar] [CrossRef]
- Duffus, B.R.; Schrapers, P.; Schuth, N.; Mebs, S.; Dau, H.; Leimkühler, S.; Haumann, M. Anion Binding and Oxidative Modification at the Molybdenum Cofactor of Formate Dehydrogenase from Rhodobacter capsulatus Studied by X-ray Absorption Spectroscopy. Inorg. Chem. 2020, 59, 214–225. [Google Scholar] [CrossRef]
- Dong, G.; Ryde, U. Reaction mechanism of formate dehydrogenase studied by computational methods. J. Biol. Inorg. Chem. 2018, 23, 1243–1254. [Google Scholar] [CrossRef]
- Niks, D.; Hille, R. Reductive activation of CO2 by formate dehydrogenases. Methods Enzymol. 2018, 613, 277–295. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Niks, D.; Hakopian, S.; Canchola, A.; Lin, Y.-H.; Hille, R. Mechanism of Action of Formate Dehydrogenases. J. Am. Chem. Soc. 2024, 146, 28601–28604. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Khosraneh, M.; Bandaru, S.S.M.; Schulzke, C.; Leimkühler, S. The Mechanism of Metal-Containing Formate Dehydrogenases Revisited: The Formation of Bicarbonate as Product Intermediate Provides Evidence for an Oxygen Atom Transfer Mechanism. Molecules 2023, 28, 1537. [Google Scholar] [CrossRef] [PubMed]
- Radon, C.; Mittelstädt, G.; Duffus, B.R.; Bürger, J.; Hartmann, T.; Mielke, T.; Teutloff, C.; Leimkühler, S.; Wendler, P. Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase. Nat. Commun. 2020, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.; Mota, C.; Mourato, C.; Domingos, R.M.; Santos, M.F.A.; Gesto, D.; Guigliarelli, B.; Santos-Silva, T.; Romão, M.J.; Cardoso Pereira, I.A. Toward the Mechanistic Understanding of Enzymatic CO2 Reduction. ACS Catal. 2020, 10, 3844–3856. [Google Scholar] [CrossRef]
- Verhees, C.H.; Kengen, S.W.; Tuininga, J.E.; Schut, G.J.; Adams, M.W.; De Vos, W.M.; Van Der Oost, J. The unique features of glycolytic pathways in Archaea. Biochem. J. 2003, 375, 231–246. [Google Scholar] [CrossRef]
- Roy, R.; Menon, A.L.; Adams, M.W. Aldehyde oxidoreductases from Pyrococcus furiosus. Methods Enzymol. 2001, 331, 132–144. [Google Scholar] [CrossRef]
- Kengen, S.W.M. ‘Pyrococcus furiosus, 30 years on’. Microb. Biotechnol. 2017, 10, 1441–1444. [Google Scholar] [CrossRef]
- Bevers, L.E.; Bol, E.; Hagedoorn, P.-L.; Hagen, W.R. WOR5, a Novel Tungsten-Containing Aldehyde Oxidoreductase from Pyrococcus furiosus with a Broad Substrate Specificity. J. Bacteriol. 2005, 187, 7056–7061. [Google Scholar] [CrossRef]
- Winiarska, A.; Ramírez-Amador, F.; Hege, D.; Gemmecker, Y.; Prinz, S.; Hochberg, G.; Heider, J.; Szaleniec, M.; Schuller, J.M. A bacterial tungsten-containing aldehyde oxidoreductase forms an enzymatic decorated protein nanowire. Sci. Adv. 2023, 9, eadg6689. [Google Scholar] [CrossRef]
- Rauh, D.; Graentzdoerffer, A.; Granderath, K.; Andreesen, J.R.; Pich, A. Tungsten-containing aldehyde oxidoreductase of Eubacterium acidaminophilum. Eur. J. Biochem. 2004, 271, 212–219. [Google Scholar] [CrossRef]
- Huber, C.; Caldeira, J.; Jongejan, J.A.; Simon, H. Further characterization of two different, reversible aldehyde oxidoreductases from Clostridium formicoaceticum, one containing tungsten and the other molybdenum. Arch. Microbiol. 1994, 162, 303–309. [Google Scholar] [CrossRef]
- Schut, G.J.; Thorgersen, M.P.; Poole, F.L.; Haja, D.K.; Putumbaka, S.; Adams, M.W.W. Tungsten enzymes play a role in detoxifying food and antimicrobial aldehydes in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2109008118. [Google Scholar] [CrossRef] [PubMed]
- Sevcenco, A.-M.; Bevers, L.E.; Pinkse, M.W.H.; Krijger, G.C.; Wolterbeek, H.T.; Verhaert, P.D.E.M.; Hagen, W.R.; Hagedoorn, P.-L. Molybdenum Incorporation in Tungsten Aldehyde Oxidoreductase Enzymes from Pyrococcus furiosus. J. Bacteriol. 2010, 192, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, P.; Hege, D.; Winiarska, A.; Gemmecker, Y.; Szaleniec, M.; Heider, J.; Bernhardt, P.V. Electrocatalytic Aldehyde Oxidation by a Tungsten Dependent Aldehyde Oxidoreductase from Aromatoleum Aromaticum. Chem. Eur. J. 2023, 29, e202203072. [Google Scholar] [CrossRef]
- Winiarska, A.; Hege, D.; Gemmecker, Y.; Kryściak-Czerwenka, J.; Seubert, A.; Heider, J.; Szaleniec, M. Tungsten Enzyme Using Hydrogen as an Electron Donor to Reduce Carboxylic Acids and NAD+. ACS Catal. 2022, 12, 8707–8717. [Google Scholar] [CrossRef]
- Hagedoorn, P.-L. Steady-state kinetics of the tungsten containing aldehyde: Ferredoxin oxidoreductases from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biotechnol. 2019, 306, 142–148. [Google Scholar] [CrossRef]
- Chan, M.K.; Mukund, S.; Kletzin, A.; Adams, M.W.W.; Rees, D.C. Structure of a Hyperthermophilic Tungstopterin Enzyme, Aldehyde Ferredoxin Oxidoreductase. Science 1995, 267, 1463–1469. [Google Scholar] [CrossRef]
- Arndt, F.; Schmitt, G.; Winiarska, A.; Saft, M.; Seubert, A.; Kahnt, J.; Heider, J. Characterization of an Aldehyde Oxidoreductase From the Mesophilic Bacterium Aromatoleum aromaticum EbN1, a Member of a New Subfamily of Tungsten-Containing Enzymes. Front. Microbiol. 2019, 10, 71. [Google Scholar] [CrossRef]
- ten Brink, F. Living on Acetylene. A Primordial Energy Source. In The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment; Kroneck, P.M.H., Torres, M.E.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–35. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Abbasian, F.; Lockington, R.; Megharaj, M.; Naidu, R. A Review on the Genetics of Aliphatic and Aromatic Hydrocarbon Degradation. Appl. Biochem. Biotechnol. 2016, 178, 224–250. [Google Scholar] [CrossRef]
- Stewart, W.D.; Fitzgerald, G.P.; Burris, R.H. In situ studies on N2 fixation using the acetylene reduction technique. Proc. Natl. Acad. Sci. USA 1967, 58, 2071–2078. [Google Scholar] [CrossRef]
- Oremland, R.S. Got acetylene: A personal research retrospective. FEMS Microbes 2021, 2, xtab009. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.W.; Strohmaier, F.E.; Oremland, R.S. Acetylene as a substrate in the development of primordial bacterial communities. Orig. Life Evol. Biosph. 1988, 18, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.M.; Schink, B. Purification and characterization of acetylene hydratase of Pelobacter acetylenicus, a tungsten iron-sulfur protein. J. Bacteriol. 1995, 177, 5767–5772. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, G.B.; Ullmann, G.M.; Messerschmidt, A.; Schink, B.; Kroneck, P.M.H.; Einsle, O. Structure of the non-redox-active tungsten/[4Fe:4S] enzyme acetylene hydratase. Proc. Natl. Acad. Sci. USA 2007, 104, 3073–3077. [Google Scholar] [CrossRef]
- tenBrink, F.; Schink, B.; Kroneck, P.M.H. Exploring the Active Site of the Tungsten, Iron-Sulfur Enzyme Acetylene Hydratase. J. Bacteriol. 2011, 193, 1229–1236. [Google Scholar] [CrossRef]
- Vidovič, C.; Peschel, L.M.; Buchsteiner, M.; Belaj, F.; Mösch-Zanetti, N.C. Structural Mimics of Acetylene Hydratase: Tungsten Complexes Capable of Intramolecular Nucleophilic Attack on Acetylene. Chem. Eur. J. 2019, 25, 14267–14272. [Google Scholar] [CrossRef]
- Liao, R.-Z.; Thiel, W. Comparison of QM-Only and QM/MM Models for the Mechanism of Tungsten-Dependent Acetylene Hydratase. J. Chem. Theory Comp. 2012, 8, 3793–3803. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Liao, R.-Z.; Ding, W.-J.; Yu, J.-G.; Liu, R.-Z. Theoretical investigation of the first-shell mechanism of acetylene hydration catalyzed by a biomimetic tungsten complex. J. Biol. Inorg. Chem. 2011, 16, 745–752. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Thauer, R.K. The active species of ‘CO2’ utilized by formylmethanofuran dehydrogenase from methanogenic Archaea. Eur. J. Biochem. 1997, 248, 919–924. [Google Scholar] [CrossRef]
- Lemaire, O.N.; Wagner, T. All-in-One CO2 Capture and Transformation: Lessons from Formylmethanofuran Dehydrogenases. Acc. Chem. Res. 2024, 57, 3512–3523. [Google Scholar] [CrossRef]
- Sahin, S.; Lemaire, O.N.; Belhamri, M.; Kurth, J.M.; Welte, C.U.; Wagner, T.; Milton, R.D. Bioelectrocatalytic CO2 Reduction by Mo-Dependent Formylmethanofuran Dehydrogenase. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311981. [Google Scholar] [CrossRef] [PubMed]
- Mand, T.D.; Metcalf, W.W. Energy Conservation and Hydrogenase Function in Methanogenic Archaea, in Particular the Genus Methanosarcina. Microbiol. Mol. Bio.L Rev. 2019, 83, e00020-19. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Morel, F.M.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Saito, M.A.; George, G.N.; Pickering, I.J.; Prince, R.C.; Morel, F.M.M. A cadmium enzyme from a marine diatom. Nature 2005, 435, 42. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Jeffrey, P.D.; Shi, Y.; Morel, F.M.M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Baranu, B.S.; Lawrence, E. Nitrogen Bacteria Associated with Bioremediation Soil and their Ability to Degrade Hydrocarbon. Int. J. Curr. Microbiol. App. Sci. 2022, 11, 306–314. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Li, D.; Peng, C. Nitrogen-fixing cyanobacteria have the potential to improve nitrogen use efficiency through the reduction of ammonia volatilization in red soil paddy fields. Soil Tillage Res. 2022, 217, 105274. [Google Scholar] [CrossRef]
- Coelho, F.C.; Cerchiaro, G.; Araújo, S.E.S.; Daher, J.P.L.; Cardoso, S.A.; Coelho, G.F.; Guimarães, A.G. Is There a Connection between the Metabolism of Copper, Sulfur, and Molybdenum in Alzheimer’s Disease? New Insights on Disease Etiology. Int. J. Mol. Sci. 2022, 23, 7935. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, N.; Karimi-Nazari, E.; Yaghoubi, F.; Zarei, S.; Azadmanesh, F.; Reza, J.Z.; Sargazi, S. Molybdenum Cofactor Biology and Disorders Related to Its Deficiency; A Review Study. J. Nutr. Food Secur. 2019, 4, 206–217. [Google Scholar] [CrossRef]
- McGlynn, S.E.; Boyd, E.S.; Peters, J.W.; Orphan, V.J. Classifying the metal dependence of uncharacterized nitrogenases. Front. Microbiol. 2013, 3, 419. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Freeze frame: Cracking molecular motion. Time-resolved cryo-electron microscopy. Nature 2025, 642, 827–829. [Google Scholar] [CrossRef]

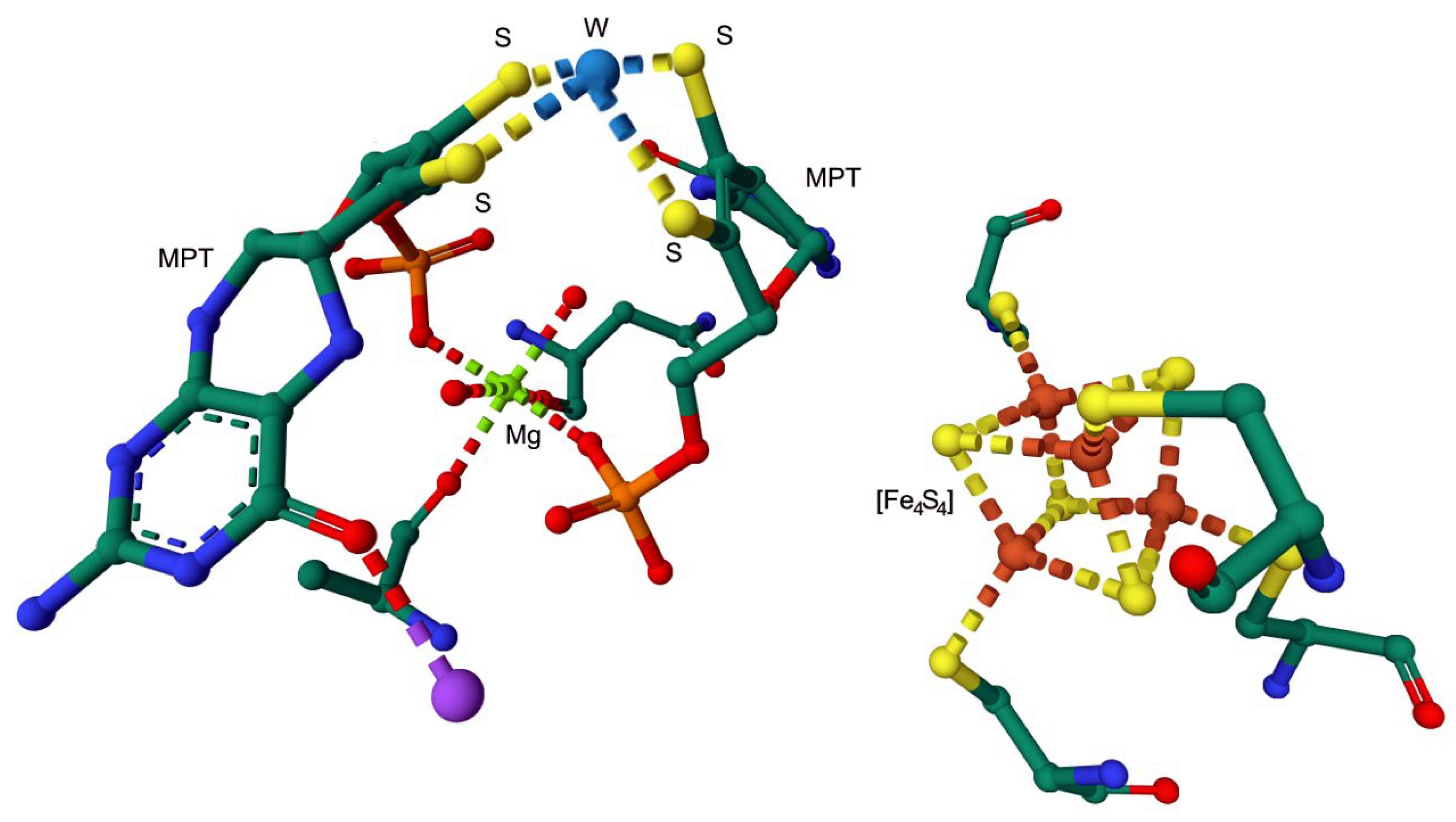
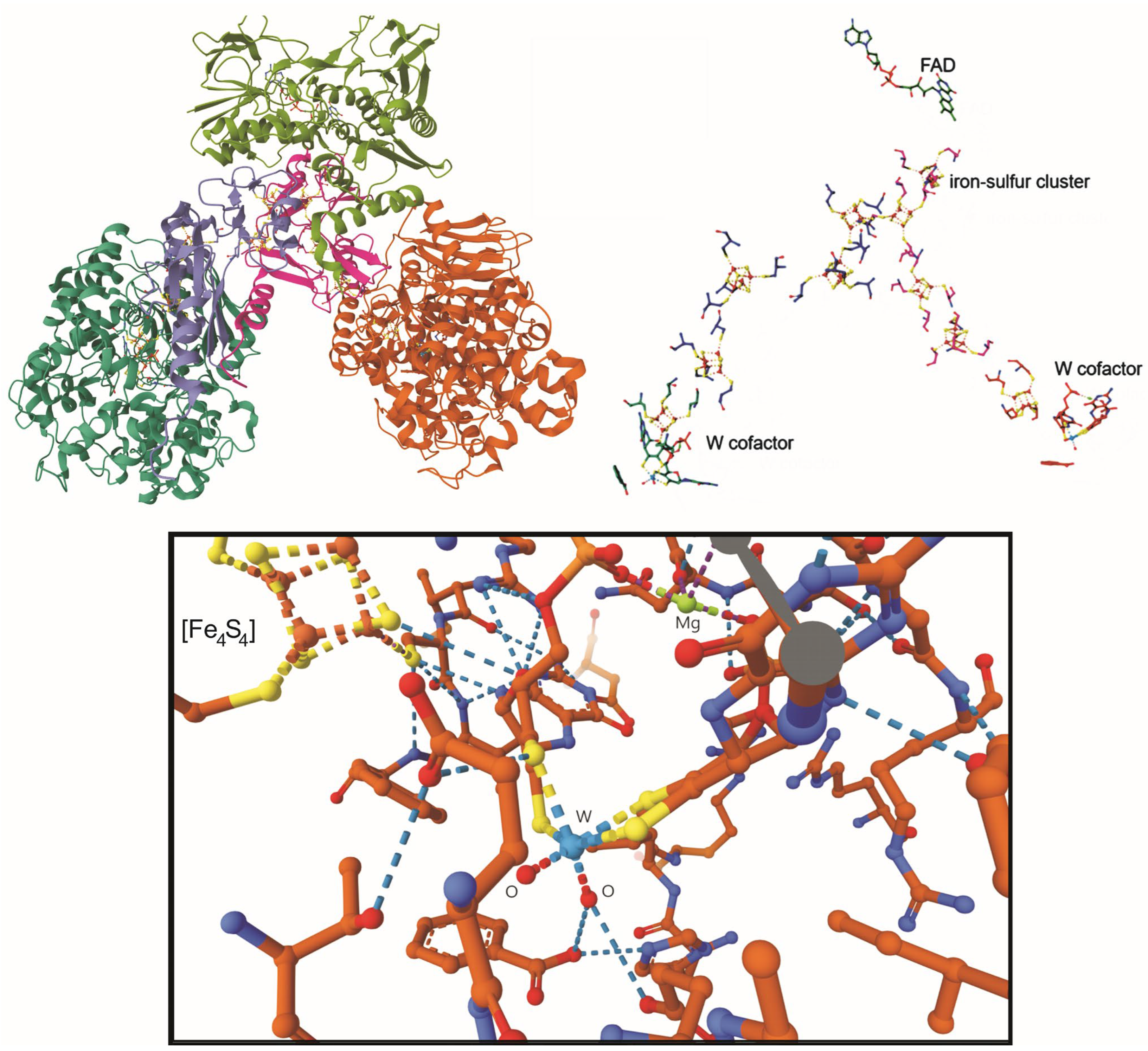
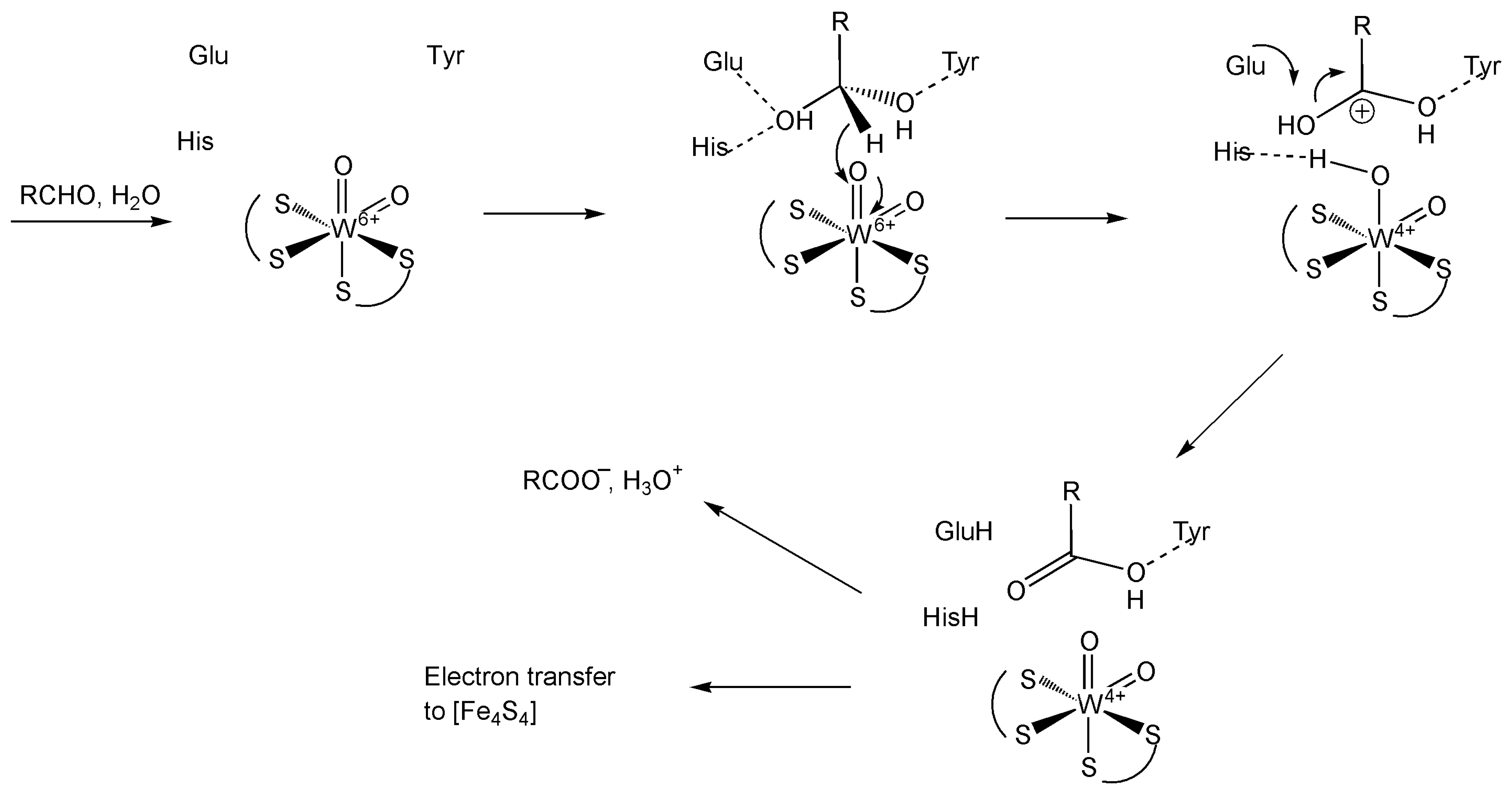
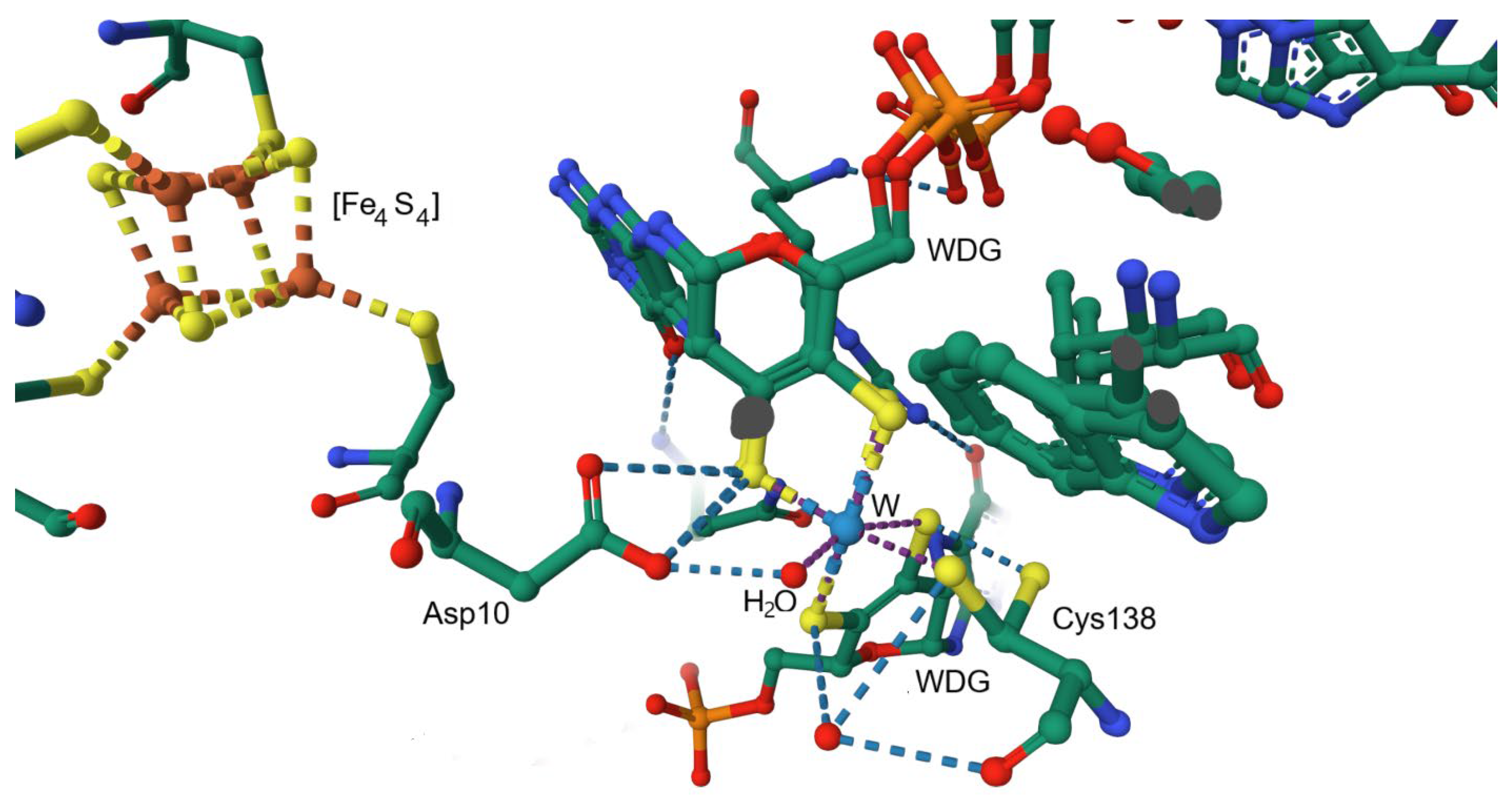
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, H.M. An Introduction to the Role of Molybdenum and Tungsten in Biology. Inorganics 2025, 13, 219. https://doi.org/10.3390/inorganics13070219
Marques HM. An Introduction to the Role of Molybdenum and Tungsten in Biology. Inorganics. 2025; 13(7):219. https://doi.org/10.3390/inorganics13070219
Chicago/Turabian StyleMarques, Helder M. 2025. "An Introduction to the Role of Molybdenum and Tungsten in Biology" Inorganics 13, no. 7: 219. https://doi.org/10.3390/inorganics13070219
APA StyleMarques, H. M. (2025). An Introduction to the Role of Molybdenum and Tungsten in Biology. Inorganics, 13(7), 219. https://doi.org/10.3390/inorganics13070219







