Abstract
Sanitary landfill leachate treatment was evaluated using magnetite-catalyzed ozone, an upflow anaerobic sludge blanket (UASB) reactor, and microalgae, both individually and in combination, to improve biodegradability and remove organic matter, solids, metals, and nutrients. Leachates were characterized before and after each treatment, and their impacts on methanogenic activity, aerobic toxicity, and the BOD5/COD ratio were assessed. Magnetite-catalyzed ozone pretreatment enhanced biodegradability, enabling an optimal coupling point with the UASB at 40 min when the specific methanogenic activity reached 0.22 g CH4-COD/(gVSS·d). The UASB achieved COD removal rates of up to 75%, but high concentrations were maintained in the effluent with low ammoniacal nitrogen and phosphorus removal rates. Microalgae promoted nutrient removal, reducing total nitrogen and phosphorus by up to 65% and 70%, respectively, although with lower efficiency in terms of organic matter removal. Process coupling demonstrated that ozonation followed by UASB application improved anaerobic degradation, whereas the use of microalgae after biological treatment optimized the final effluent quality. Despite the improvements achieved, the final values for some parameters still exceeded the discharge limits, indicating the need for operational adjustments or additional treatments to ensure effective purification.
1. Introduction
Landfill leachate produced from sanitary landfills is an important environmental issue because of its high organic load, complex composition, and possible toxicity, which requires treatment. Landfill leachate can contain organic compounds, inorganic salts, toxic gases, halogenated hydrocarbons, and heavy metals, which can be hazardous to public health and the environment [1]. Several standard methods for treating these effluents, such as anaerobic processes, have been employed because of their efficient removal of organic matter; however, these methods have limitations in the degradation of recalcitrant compounds and nutrients, which poses a challenge for discharge compliance [2].
Landfill leachate management remains heavily dependent on energy-intensive technologies and chemical-based treatments, many of which are derived indirectly from fossil fuel consumption, thereby contributing to significant environmental impacts [3]. Traditional treatments such as coagulation–flocculation, advanced chemical oxidation, and membrane filtration typically involve high energy demands, the intensive use of chemical reagents, and the generation of large volumes of residual sludge requiring additional disposal [4,5]. Recent studies have reported that the treatment of landfill leachate through advanced oxidation processes assisted by dual-frequency ultrasound (DFUS) may require an energy consumption of approximately 9 kWh/m3, representing a significant challenge in terms of energy efficiency for large-scale applications [6]. Moreover, the production and application of chemical coagulants result in secondary pollution and resource depletion [5].
Compared to traditional approaches, the integrated treatment strategy presented in this study minimizes environmental impact by reducing reliance on external chemical reagents and limiting the production of secondary waste. Furthermore, it enhances resource recovery through methane generation and promotes effluent biodegradability, aligning with sustainable and circular economy principles [7,8,9]. In contrast, the combined use of magnetite-catalyzed ozonation, anaerobic digestion in UASB reactors, and microalgae cultivation substantially reduces chemical reagent consumption, promotes oxidative degradation with minimal catalyst dosing, and enables biological nutrient removal without external chemical addition. Anaerobic digestion enhances energy recovery, increasing the specific methanogenic activity by 57% (from 0.14 to 0.22 g CH4-COD/gVSS·d), whereas ozonation pretreatment improved aerobic biodegradability by up to 11%. Furthermore, the toxicity of the leachates was reduced by 28.6% and 33.3% in La Madera and El Guayabal, respectively. COD removal efficiencies of 70% were consistently achieved under dynamic conditions, and the treatment configuration minimized sludge production compared with traditional aerobic systems. These combined features position the proposed treatment scheme as a greener, more energy-efficient, and waste-reducing alternative for landfill leachate management.
Researchers have investigated advanced oxidation processes (AOPs) to decompose recalcitrant organic matter and enhance the biodegradability of leachates [9] in response to these challenges. Among advanced oxidation processes (AOPs), Fenton and its derivatives have shown promising results in wastewater treatment. Notably, the photo-Fenton process—an enhanced version of the conventional Fenton reaction that uses light to improve hydroxyl radical generation—has also been widely studied for its effectiveness in degrading refractory organic compounds [10]. However, operational challenges such as iron sludge generation and strict pH control limit its large-scale application, motivating the exploration of alternative catalysts such as magnetite.
Coagulation, sedimentation, and adsorption are some of the methods traditionally used to treat leachate, but they have limited effectiveness in achieving complete treatment with low costs [11], and, recently, the combined use of AOPs with biologics has provided a promising alternative process [12]. Moreover, the use of MBRs to treat leachate was found to increase leachate treatment efficiency beyond biodegradable contaminants, as MBR alone had an excellent capability to remove biodegradable organic matter, and, when combined with RO, it achieved the removal of nonbiodegradable organic matter as well [13].
While several technologies have been developed for leachate treatment, none of them alone have limitations. Biological methods, including upflow anaerobic sludge blanket (UASB) reactors, are very effective for the removal of organic matter and biogas generation but struggle with the reduction in recalcitrant pollutants and nutrients [14]. Alternatively, catalytic ozonation has been proposed to degrade refractory organics and increase leachate biodegradability, leading to better biological treatment [15]. However, these processes alone cannot achieve efficient nutrient removal, making microalgae integration an appealing alternative for nitrogen and phosphorus accumulation while improving the quality of the overall effluent [16]. Mixing treatment processes can produce synergies that enhance the performance of the entire system [17], and recent studies have corroborated this. For example, the combination of anaerobic treatment and ozonation has been shown to increase organic contaminant removal and leachate biodegradability [18]. Another way of doing this is to add them to biological processes; thus, greater nitrogen and phosphorus removal results in a more sustainable treatment scheme [16]. Conversely, the integration of catalyzed ozonation with magnetite along UASB reactors and microalgae treatment in this study represents a novel strategy to increase the removal of organic matter, heavy metals, and nutrients, thus ensuring increased efficiency of sanitary landfill leachate treatment to comply with stricter environmental requirements. This need for the integration of such processes is further prominent with recent evidence that the integration of advanced technologies, such as membranes and oxidation processes, can help reach removal efficiencies close to 99% for specific compounds [19]. Therefore, the use of multistage treatment configurations with a combination of physicochemical and biological methods becomes crucial for both optimal performance and environmental compliance.
The Fenton oxidation process has been extensively investigated owing to its potential to mineralize organic contaminants in landfill leachate. The Fenton process results in 70% chemical oxygen demand (COD) removal from raw leachate and more than 50% removal from biologically treated leachate [20]. Waste-activated sludge has a relatively high COD, which leads to a reduction in treatment cost. In addition, the coupling of Fenton oxidation with biological aeration filters (BAFs) has been confirmed to improve the decomposition efficiency of COD, ammonium, and total phosphorus [21]. Research has shown that the highest removal efficiency for COD (99%) was achieved with combined treatment processes, such as the coupling of physicochemical and biological methods, e.g., membrane bioreactors (MBRs) with nanofiltration (NF), as well as oxidation with Fenton processes and adsorption [19]. These results emphasize the necessity of integrated approaches involving advanced oxidation, biological treatment, and adsorption methods for effective leachate management.
Finally, recent research has also highlighted the role of ozone-based AOPs in improving the breakdown of organic contaminants. Ozone followed by combinations of denitrification, coagulation, and biologically activated carbon effectively removes total nitrogen, COD, and heavy metals from treated effluents that have passed stringent environmental discharge standards [22]. Moreover, COD, BOD, and ammonia nitrogen can be significantly reduced after combined physicochemical treatment with ultrafiltration and nanofiltration methods [23].
Considering these findings, the aim of the present work was to assess the performance of magnetite-catalyzed ozonation, UASB reactors, and microalgae treatment individually and together for the removal of organic, solid, metal, and nitrogenous compounds. The results will help optimize process parameters and assist in the combined/combined treatment of pollutants to achieve a better-quality final effluent.
In this context, the present study aimed to evaluate a sustainable treatment strategy for sanitary landfill leachate by integrating magnetite-catalyzed ozonation, UASB anaerobic digestion, and microalgae cultivation. Aligned with the Twelve Principles of Green Chemistry, the proposed system reduced chemical reagent consumption, enhanced energy recovery through methane production, improved leachate biodegradability, and significantly decreased contaminant concentrations and toxicity [24]. These results demonstrate the potential of this approach as a green, resource-efficient, and environmentally sustainable alternative to conventional treatment methods.
2. Results
2.1. Characteristics of the Inoculum Sludge
The characterization of the UASB (upflow anaerobic sludge blanket) inoculum sludge used in the reactor was performed to assess its intrinsic feature as a biological medium for leachate treatment. The investigation of selected parameters (COD, SVI, pH, TSS, and VSS) met essential needs by reflecting the sludge stability, biological activity, and degradation potential (Table 1). The inoculum had a high organic loading with a COD of 20,920 mg O2/L. This parameter is essential, as sludge rich in fermentative organic matter can stimulate volatile fatty acid production and methanogenesis in the system [25]. The SVI was also estimated to be 61.4 mL/g, which indicates good settling ability and, therefore, high biomass retention in the reactor [26]. This type of SVI indicates a stable floc structure that promotes process efficiency by reducing active biomass loss and enhancing cell retention time [27].

Table 1.
Characteristics of the inoculum sludge in the UASB reactor.
The sludge presented a pH of 7.41, an appropriate range for anaerobic microorganism growth. This value is paramount, as anaerobic processes must balance volatile fatty acid production and consumption to avoid inhibiting methanogenic activity [28]. The total solid composition was 21,990 mg/L of TSS and 14,733 mg/L of VSS, with VSS/TSS = 0.67. This ratio can indicate the organic part of the sludge, which has a high share of active biomass compared with inert solids. The minimum value of 0.60 is suitable for anaerobic reactors because most solids are active microorganisms critical for the biodegradation of organic matter and biogas generation [29].
2.2. Performance of the UASB Reactor
The performance of the UASB reactor was evaluated in terms of organic matter removal efficiency, as measured by COD reduction. The system exhibited progressive performance improvement during operation, reflecting microbial adaptation and process stabilization (Figure 1). In the early days of operation, the removal efficiency was limited, reaching only 20–30%. However, as the biomass acclimated to the applied organic load, COD removal steadily increased, reaching 65% in El Guayabal and 72% in La Madera by day 21. This behavior suggests proper acclimation of the microbial consortium to the reactor conditions, thereby optimizing the degradation of organic compounds in the leachates [30]. As the process continued, the system efficiency improved, achieving maximum removal values of 79% in El Guayabal and 81% in La Madera around day 39. Nevertheless, after this point, fluctuations in COD removal were observed, possibly due to variations in leachate composition and the accumulation of metabolic byproducts that temporarily affected microbial activity [31]. Ultimately, at the end of the experimental period, the removal efficiency stabilized at approximately 70% for both leachates. These findings indicate that the reactor achieved a dynamic equilibrium in terms of organic matter degradation, even under various operating conditions.
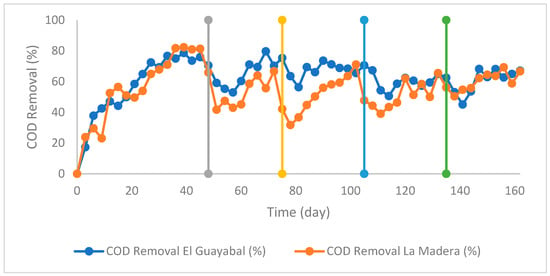
Figure 1.
Evolution of COD removal in the UASB reactor. Vertical lines indicate the transition points between the startup stages of the UASB reactor, with progressively reduced hydraulic retention times: gray (42 h), orange (36 h), blue (30 h), and green (24 h).
2.3. Performance Evaluation of Individual Treatment Processes
Given the observed variation in the chemical characteristics of leachates from the La Madera and El Guayabal sanitary landfills, these differences are likely influenced by several factors, such as the age of the landfill, composition of the waste, and environmental conditions [32]. High concentrations of organic matter, nitrogenous compounds, and suspended solids, as illustrated in Table 2, validate specialized treatments for cost-effective purification. For the organic load, the initial COD obtained was 8421.4 mg O2/L in La Madera and 6295.6 mg O2/L in El Guayabal. Following ozone and magnetite treatment, these values were reduced to 3832 mg O2/L and 1982 mg O2/L, respectively, with removal efficiencies of 54.5% (La Madera) and 68.5% (El Guayabal). This observation suggests greater effectiveness in removing organic matter in El Guayabal, potentially due to the particular composition of its leachate and the nature of the organic compounds present [33]. The total organic carbon (TOC) content also significantly decreased from 3447 mg/L to 1303 mg/L in La Madera and from 2403 mg/L to 594 mg/L in El Guayabal. In contrast, ammonium nitrogen (NH4⁺) had high concentrations (751 mg/L in La Madera and 422 mg/L in El Guayabal) throughout all the raw leachates studied. Although the values decreased because of treatment with ozone and magnetite at 600.1 mg/L and 341.6 mg/L, respectively, ammoniacal nitrogen was the compound that presented the lowest removal percentage compared with the other compounds, with efficiencies of 20.1% in La Madera and 19.1% in El Guayabal. This is consistent with previous work that demonstrated that other processes, such as nitrification-denitrification, are needed to achieve better ammonium removal [34,35,36].

Table 2.
Physicochemical characteristics of Guayabal and La Madera leachates before and after individual treatments (UASB and ozone + magnetite).
The total suspended solid concentration also significantly decreased from 5151 mg/L to 2508 mg/L in La Madera (51.3% removal) and from 8309 mg/L to 3663 mg/L in El Guayabal (55.9% removal). TSS reduction enhances treated effluent quality and reduces water turbidity to ensure possible reuse or disposal. In addition to organic matter and solids, heavy metals were another critical parameter in the analyzed leachates. In El Guayabal, the initial chromium level of 0.294 mg/L was almost eliminated after treatment (<0.050 mg/L), highlighting the effectiveness of the process in removing this metal. Conversely, mercury, detected in La Madera at an initial concentration of 0.28 mg/L, was reduced to undetectable levels (<0.001 mg/L), suggesting that the applied treatments effectively captured and precipitated these contaminants. Another noteworthy aspect is the electrical conductivity, which reflects the concentration of dissolved salts in the leachates. In El Guayabal, the initial conductivity of 18,740 µS/cm decreased after treatment but did not reach optimal levels, indicating that the applied processes did not eliminate the salt load. In La Madera, the initial conductivity was much lower (1128.6 µS/cm), suggesting a lower initial salt content.
Similarly, aerobic biodegradability is a key factor in evaluating treatment efficiency. The biodegradability of the raw leachates was 35% in La Madera and 38% in El Guayabal. After ozone and magnetite were applied, these values increased to 53% and 58%, respectively, representing improvements of 51.4% in La Madera and 52.6% in El Guayabal. This increase suggests that the applied processes transformed a considerable fraction of the recalcitrant organic compounds into more biodegradable forms, facilitating their subsequent removal through biological treatments [37]. Furthermore, aerobic toxicity, measured via the inhibition of microbial respiration, was significantly reduced. In La Madera, the initial toxicity was 63%, which decreased to 45% after treatment—a reduction of 28.6%. In El Guayabal, toxicity decreased from 57% to 38%, a reduction of 33.3%. These results indicate that the toxic compounds present in the leachates, possibly derived from heavy metals and refractory organic substances, were either removed or transformed into less harmful forms due to the applied treatments [38].
Recent results from the same research project have addressed the fate of the catalyst after use. The study confirmed that magnetite retained its catalytic activity after multiple ozonation cycles. XRD and SEM analyses showed no significant structural changes or agglomeration, suggesting high stability and reusability. Additionally, negligible iron leaching was detected, supporting the environmental safety of the process [38].
2.4. Performance of the Coupled Treatments
A comparative analysis of leachates treated by combining catalytic ozonation with magnetite and a UASB (upflow anaerobic sludge blanket) reactor revealed significant differences in the removal efficiency of organic matter, suspended solids, nutrients, and toxicity. This underscores the influence of the treatment sequence on optimizing the final effluent. A comparison of the initial values of the raw leachates with those obtained after individual and combined treatments confirmed that integrating both processes enhances contaminant elimination and improves effluent stability.
In terms of organic load, the “ozone (magnetite) + UASB” configuration resulted in a more significant reduction in COD, with final values of 495.5 mg O2/L in El Guayabal and 1034.6 mg O2/L in La Madera, whereas the “UASB + ozone (magnetite)” scheme resulted in final values of 561.4 mg O2/L and 1366.0 mg O2/L, respectively. This difference suggests that preozonation before anaerobic treatment facilitates the degradation of refractory compounds, increases the BOD5/COD ratio, and enhances the biodegradability of the effluent [30,35]. The organic matter removal efficiency in the coupled schemes exceeded that of the individual treatments, with COD reductions of 92% in the “ozone (magnetite) + UASB” scheme and 89% in the “UASB + ozone (magnetite)” scheme, confirming the synergy between the two processes (Table 3). These trends are further illustrated in Figure 2, which compares the evolution of key organic parameters (BOD5, COD, BOD5/COD ratio, and TOC) across the different treatment stages for both leachates.

Table 3.
Physicochemical and biological parameters of Guayabal and La Madera leachates after combined treatment schemes.
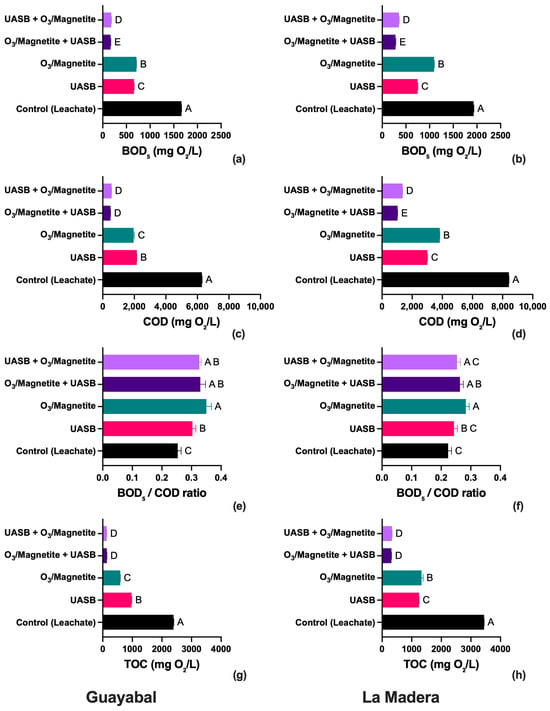
Figure 2.
Effect of combined UASB and O3/magnetite treatments on organic pollutant removal from landfill leachate at Guayabal and La Madera sites. BOD5 (a,b), COD (c,d), BOD5/COD ratio (e,f), and TOC (g,h). Uppercase letter shows ANOVA statistical significance. Same letter at the same figure shows no statistical significance. Different letter at the same figure shows statistical significance.
With respect to nitrogenous compound removal, different treatments yielded different results depending upon the order of application. The partial removal of total and ammoniacal nitrogen (361.5 mg/L and 331.4 mg/L in El Guayabal and 596.1 mg/L and 546.1 mg/L in La Madera, respectively) was reported for preozonation before the anaerobic reactor, whereas the “UASB + ozone (magnetite)” configuration resulted in greater conversion of ammonium into nitrate, attaining total nitrogen levels of 430.6 mg/L in El Guayabal and 751.3 mg/L in La Madera. With respect to solid reduction, both the TSS and VSS were reduced significantly after the combined treatment. The TSS and VSS values for the “ozone (magnetite) + UASB” arrangement in La Madera were 602 mg/L and 283 mg/L, respectively, whereas for the “UASB + ozone (magnetite)” configuration, the concentrations were 512 mg/L and 229 mg/L, respectively. These results indicate that applying ozonation as the final stage contributes to greater effluent clarification, reducing turbidity to 22 NTU in La Madera, as opposed to 52 NTU when ozonation was used before the UASB. The electrical conductivity also differed notably between the two treatment schemes, with lower values observed in the “ozone (magnetite) + UASB” configuration (3158.3 µS/cm in El Guayabal and 1055.1 µS/cm in La Madera) than in the “UASB + ozone (magnetite)” scheme (3564.8 µS/cm and 1211.4 µS/cm, respectively), suggesting that preozonation before the anaerobic process favors the removal of salts and soluble inorganic compounds.
The toxicity results indicate a reduction in the inhibition of aerobic respiration, reflecting a lower presence of toxic compounds in the treated leachates. Respiration inhibition was lower in the “ozone (magnetite) + UASB” configuration, with values of 28% in El Guayabal and 25% in La Madera. In contrast, the reverse configuration resulted in slightly more significant inhibition (32% and 30%, respectively). Finally, the specific methanogenic activity (SMA) was enhanced in the “UASB + ozone (magnetite)” configuration, reaching 0.17 g CH4-COD/(g VSS·d) in El Guayabal and 0.19 g CH4-COD/(g VSS·d) in La Madera, which suggests improved methane production efficiency from residual organic matter. These results demonstrate that combining both processes optimizes contaminant removal. However, the effectiveness varies depending on the treatment sequence, underscoring the need to adjust the operating conditions according to the specific treatment objectives.
Using BOD5, COD, TOC, and BOD5/COD ratio as indicators, the effectiveness of several treatment configurations on landfill leachate from Guayabal and La Madera was assessed (Figure 2a–h) using a one-way ANOVA followed by Dunnett’s multiple comparisons test performed using GraphPad Prism (version 10.5.0). BOD5, COD, and TOC were moderately reduced by UASB application alone; however, treatments that included O3/magnetite, either alone or in conjunction with UASB, greatly improved pollutant removal.
With final values below 500 mg O2/L and 3000 mg O2/L, respectively, the O3/magnetite + UASB and UASB + O3/magnetite treatments produced the most significant reductions in BOD5 (Figure 2a,b) and COD (Figure 2c,d) in both sites, suggesting improved oxidation and biodegradability. Interestingly, the O3/magnetite treatment by itself was less successful than the combined methods but more effective than UASB alone. Similar trends were observed in the removal of TOC (Figure 2g,h), with combined methods yielding the lowest TOC values (~1000 mg O2/L), indicating that refractory organic molecules were effectively removed.
The control and UASB-treated leachate showed lower values (≤0.2) for the BOD5/COD ratio (Figure 2e,f), which measures leachate biodegradability; however, treatments including O3/magnetite dramatically increased this ratio to >0.3. The highest ratios were found for UASB + O3/magnetite and O3/magnetite + UASB, indicating that the biodegradability of the effluent was enhanced by advanced oxidation processes, allowing for additional biological treatment.
Overall, the most successful treatment for reducing the organic load and improving biodegradability at both landfill sites was the combination of UASB and O3/magnetite, regardless of the sequence.
2.5. Experimental Coupling of Microalgal Biomass
The statistical results of the treatments performed to compare the biomass concentrations produced in the leachates of the Guayabal and Madera landfills are presented, and the results are calculated in terms of average and standard deviation. The raw leachate had a low biomass concentration of 0.213 ± 0.012 g/L in Guayabal and 0.188 ± 0.001 g/L in Madera. Afterward, the UASB treatment was applied, and the concentration ranged between 0.383 ± 0.051 g/L for Guayabal and 0.305 ± 0.009 g/L for Madera. The magnetite-assisted ozonation treatment resulted in biomass concentrations of 0.554 ± 0.018 g/L (Guayabal) and 0.431 ± 0.013 g/L (Madera). For the combined treatments, O3-UASB values of 0.724 ± 0.011 g/L were obtained in Guayabal, and 0.632 ± 0.026 g/L were obtained in Madera. Finally, the highest concentrations of biomass for the UASB-O3 treatment were 0.895 ± 0.056 g/L and 0.809 ± 0.019 g/L, respectively, in Guayabal and Madera (Figure 3).
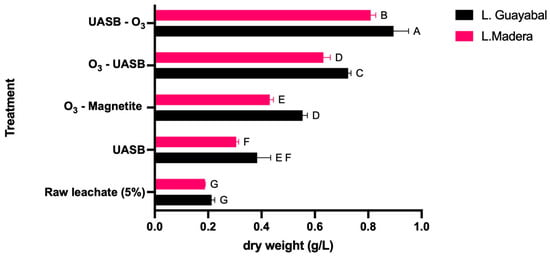
Figure 3.
Microalgal biomass production (Halochlorella sp.) as a function of leachate treatment from Guayabal and Madera. Uppercase letter shows ANOVA statistical significance. Same letter at the same figure shows no statistical significance. Different letter at the same figure shows statistical significance.
3. Discussion
The characteristics of the inoculum sludge provide a picture of its ability to be used as a leachate treatment in UASB reactors. The high chemical oxygen demand (COD) suggests that the active biomass can degrade complex organic compounds. Higher values of COD in sludge can be correlated with increased biogas production if the biodegradable fraction prevails [39]. However, when the recalcitrant organic fraction is significant, methane conversion may be compromised, thereby reducing process efficiency [40]. On the other hand, an SVI (sludge volume index) of 61.4 mL/g is an optimal value that promotes sludge settling and stability in the reactor. Reported SVI values of between 50 and 80 mL/g support a high concentration of active microorganisms without significant washout. In contrast, excessively low values may indicate sedimentation issues and reduced reactor efficiency, and excessively high values may reflect a less compact sludge structure, negatively affecting anaerobic system performance [41].
A pH of 7.41 suggests optimal conditions for methanogenic activity and overall anaerobic stability. pH values outside the range of 6.5–8.2 can impair microbial activity and reduce the efficiency of converting organic matter to methane [42]. The stable pH of the sludge indicates an adequate buffering capacity, preventing fluctuations that might compromise reactor performance. Additionally, a VSS/TSS ratio (volatile suspended solids to total suspended solids) of 0.67 confirms that the inoculum sludge contains a high concentration of active biomass relative to inert solids. Research has established that ratios between 0.6 and 0.8 reflect an optimal balance between viable microorganisms and inert solids in anaerobic reactors [43]. A lower ratio could indicate the excessive accumulation of inert material, reducing system efficiency, whereas higher values may indicate that the biomass is in a state of active growth [44].
The performance of the UASB reactor throughout the experiment reflects the interplay between microbial adaptation, the applied organic load, and the operational conditions. The increasing trend in COD removal during the startup phase aligns with previous research that emphasized the need for a stabilization period for anaerobic biomass to adapt to the complex composition of leachates [45]. This phenomenon occurs because the microbial community requires time to develop specific enzymes capable of efficiently degrading the organic compounds present [46]. A notable pattern in the results is the higher removal efficiency observed in La Madera than in El Guayabal, which may be attributed to the volumetric organic load: 8.4 kg COD/m3·d in La Madera versus 6.3 kg COD/m3·d in El Guayabal. Higher organic loads favor the proliferation of methanogenic microorganisms specialized in degrading complex compounds [47]. However, excessively high loads can cause inhibition due to the accumulation of volatile fatty acids or ammonium, possibly explaining the slight decline in efficiency observed after day 42 in both leachates [31].
Another important aspect is the stabilization of COD removal at approximately 70% by the end of the experiment. This result suggests that the reactor reached a dynamic equilibrium between producing and consuming metabolic intermediates, thereby avoiding severe inhibition. Studies have shown that the accumulation of intermediates such as propionate and butyrate can slow methanogenesis and reduce system efficiency [39]. Furthermore, regulating the hydraulic retention time (HRT) and organic load has been identified as a key strategy to avoid such imbalances and improve treatment efficiency [30]. While this study established an HRT of 24 h during the operational phase, previous research has demonstrated that reducing the HRT to lower values can compromise the COD removal efficiency because of the reduced interaction between the biomass and the organic substrate [39,48]. Additionally, implementing post-treatment systems, such as those combining aerobic reactors, has proven effective in enhancing treated effluent quality [49,50].
The analysis of the treatments applied to the leachates from the La Madera and El Guayabal landfills demonstrated how the initial composition influences the efficiency of the processes employed. In addition to contaminant reduction, the results reveal key patterns that help elucidate the interactions among waste, environmental conditions, and treatment responses. The age and composition of the leachate significantly affect treatment efficiency, explaining the variability observed in the results [39]. This variability underscores the need for combined strategies tailored to the specific nature of each leachate [49,51].
The most important insights related to the differing gradients of organic matter degradability were analyzed regarding COD, BOD, and TOC. The reductions in these parameters were most pronounced in El Guayabal, indicating that the organic matter in that leachate is more susceptible to oxidation and that complex recalcitrant fractions remain in La Madera. The BOD/COD ratios indicate that a large part of the organic matter is poorly biodegradable, which highlights the need for an additional treatability step [41] despite the results already reached with the treatments applied. Zhe et al. [52] recently demonstrated that advanced oxidation could render refractory organic compounds easier to degrade, as it increased the biodegradable fraction of the leachate. In addition, ozonation has shown reductions of 90% for COD and 85% for BOD in leachates with high organic loads [53].
The behavior of ammonium nitrogen highlights its resistance to the applied treatments. Although moderate reductions were observed, the persistence of high concentrations indicates that the processes are not entirely effective in its removal. Previous studies have noted that high ammonium concentrations can inhibit biological processes, affecting the efficiency of both anaerobic and aerobic reactors [54,55,56]. The low elimination rate of ammoniacal nitrogen suggests that these treatments must be complemented with strategies such as nitrification–denitrification or adsorption using specific materials [57,58]. In this context, research has demonstrated that ozone combined with a partial denitrification–anammox process can reduce the nitrogen concentration by up to 96%, significantly improving effluent quality [59]. Leachate treatment also reduced the phosphate concentration, indicating that the processes effectively precipitated and removed these compounds. Previous studies have shown that microbial activation and catalytic ozonation can increase the phosphate removal efficiency by up to 79% [35]. This reduction is crucial, as the presence of phosphates in water bodies can contribute to eutrophication.
Another critical parameter is conductivity, which did not decrease significantly after treatment, indicating that the concentration of dissolved salts remains high. The high conductivity suggests the presence of inorganic ions that the applied processes did not efficiently remove. Research has demonstrated that membrane filtration and electrodialysis can effectively reduce conductivity and improve final effluent quality [60]. Similarly, reducing aerobic toxicity is a key indicator of treatment effectiveness, reflecting the elimination of compounds that inhibit microbial activity. In raw leachates, high toxicity suggests the presence of heavy metals and refractory organic compounds that interfere with enzymatic activity [61,62]. Following treatment, the reduction in toxicity indicates that these compounds have been transformed into less toxic forms or removed, creating an environment more conducive to subsequent biological processes [63,64,65]. This behavior suggests that advanced oxidation not only decomposes persistent organic contaminants, but also improves the stability of the treated effluent. However, the persistence of moderate residual toxicity indicates that some compounds were not thoroughly degraded or that intermediate byproducts were formed, with effects on biological activity not yet fully characterized [20,22,64]. In this context, combining physicochemical processes with specific biological treatments, such as the use of activated sludge, may be key to mitigating residual toxicity effects [66,67].
Concerning aerobic biodegradability, the increase observed after treatment suggests that the applied processes converted a significant fraction of the recalcitrant organic matter into forms more accessible for microbial degradation. Recent studies have indicated that advanced oxidation can increase the proportion of readily biodegradable organic matter by up to 50%, facilitating its removal in subsequent biological stages [35]. However, the degree of improvement depends on the nature of the leachate compounds and the treatment operating conditions [67]. The specific methanogenic activity (SMA) is another crucial parameter when determining the potential anaerobic biodegradability of treated leachate (reduced methanogenic activity > 40%), and, hence, a negative impact on anaerobic reactor efficiency has also been reported under such high concentrations of ammonium and toxic compounds [65]. The findings of this study indicate that, although toxicity was considerably lower, certain inhibitory compounds were found to remain, which may hinder biogas production and the overall efficiency of anaerobic treatment. Therefore, the integration of specific pretreatments—such as ammonium adsorption via zeolite or pH adjustment—could optimize the conversion of organic matter into methane in anaerobic systems [26,66].
On the basis of the combined treatments of catalytic ozonation together with magnetite and a UASB (upflow anaerobic sludge blanket) reactor, their evaluation revealed that the coupling of physicochemical and biological processes could lead to increased removal of persistent contaminants as well as to process the stability of the final effluent [30]. The degradation of organic matter is one of the most significant factors in treating leachate, and the results obtained with UASB reactors have been widely published in the literature [49,68]. The chemical oxygen demand (COD) reflects the organic load of water and is an essential parameter for monitoring performance. Its reduction is of paramount importance for treatment efficiency. The application of catalytic ozonation as a pretreatment could promote the oxidization of some refractory compounds and, thus, enhance their subsequent biodegradation in UASB reactors [51,69,70]. Similar observations have been made previously by Han et al. [71], where chemical preoxidation broke complex molecules into smaller fragments, which increased the bioavailability of such biodegradable substrates for microorganisms.
On the other hand, biochemical oxygen demand (BOD5) is a parameter that indicates the biodegradable fraction of organic matter, and the relationship between COD and BOD5 can be used to elucidate the proportion of refractory compounds in the effluent. The higher BOD27/COD ratios obtained with the combination treatments indicate that preozonation before the UASB reactor increased the bioavailability and organic matter removal efficiency of the readily degradable substrates. This shift in metabolic direction has also been reported in comparable treatment systems, in which coupling physicochemical and biological processes improves final effluent stability and diminishes the occurrence of toxic metabolites. Moreover, total organic carbon (TOC) is another important parameter for estimating organic matter elimination. Research has shown that the TOC concentration is reduced after ozonation, and the chemical structure of TOC is also transformed, leading to easier degradation in anaerobic reactors. This finding is in line with prior studies that showed that advanced oxidation (POD or Sm) can convert macromolecules into more minor and more biodegradable compounds [52,72,73].
With respect to nitrogenous compounds, reducing ammonium nitrogen is a challenge in leachate treatment because of its toxicity to anaerobic microorganisms and its persistence in the system. Preozonation has been shown to favor the conversion of ammonium to nitrates and nitrites, reducing its impact on methanogenic biomass [35,74,75]. However, ozonation applied after anaerobic treatment may contribute to the additional oxidation of these compounds, thereby improving the final effluent quality [75]. Reducing the nitrogen load is critical to prevent eutrophication in receiving water bodies and to comply with environmental regulations [76]. Similarly, phosphorus is another crucial nutrient in leachate characterization, and its removal is essential for preventing the excessive growth of undesirable microorganisms in water bodies. Ozonation has proven effective in precipitating phosphates and facilitating their removal in combined treatment systems [73]. In this study, the higher phosphorus removal efficiency observed in the UASB + ozone (magnetite) configuration suggests that prior biological degradation favors subsequent precipitation and removal, which is consistent with studies demonstrating that the combination of biological and oxidative processes achieves better nutrient elimination stability [30].
Toxicity and biodegradability analyses confirmed the advantages of the combined schemes in reducing inhibitory compounds and enhancing microbial activity. Aerobic respiration inhibition decreased significantly, in agreement with studies showing that the elimination of refractory compounds through oxidative pretreatment supports the recovery of biological activity [51,73]. Moreover, aerobic biodegradability improved considerably in the combined schemes, indicating that the organic compounds were transformed into fractions more accessible to degradation by aerobic microorganisms. This increase suggests that ozonation is key in breaking complex chemical bonds, generating byproducts that are more easily assimilated by biological systems [49,77]. Furthermore, the enhanced biodegradability implies a lower content of toxic substances in the final effluent, reinforcing the viability of these combined treatments for improving water quality [35,36].
Concerning specific methanogenic activity (SMA), the UASB + ozone (magnetite) configuration demonstrated higher biogas production efficiency, suggesting that prior biological stabilization favors the conversion of organic matter into methane. This is consistent with previous studies, indicating that an initial anaerobic phase allows for the greater adaptation of the microbial consortium to reactor conditions, thereby optimizing biogas production [30]. Additionally, the lower accumulation of inhibitory compounds in this configuration suggests that the subsequent oxidation step contributes to removing toxic metabolites that could impair the activity of methanogenic archaea, thus improving overall system efficiency.
The findings demonstrate the influence of biological and physicochemical treatments on leachate biomass outputs, providing a rationale for implementing hybrid technologies in industrial effluent remediation. The use of an anaerobic sludge blanket (UASB) reactor in conjunction with ozonation, an enhanced oxidation process, can improve the deftness of compounds in leachate and promote the conversion of recalcitrant compounds into intermediaries, which could be used in subsequent metabolic processes for microbes [78]. This finding corroborates recent studies that integrated advanced oxidation systems with microbial biotechnologies to recover resources from industrial liquid wastes [79].
Although the raw leachate was diluted to 5%, the biomass values obtained under these conditions were the lowest in the present study, indicating the persistence of toxic or recalcitrant compounds that may inhibit microbial activity. It has been previously reported that leachates with lower BOD5 values and ratios of BOD5 to COD are less efficient in biological processes because they are predominantly composed of organic matter with lower biodegradability [33]. In contrast, the increase in biomass in the UASB reactor indicates that this system allowed for the anaerobic degradation of more accessible organic compounds, which promoted the growth of the microorganisms responsible for biogas generation. Research has demonstrated that UASB systems, in addition to reducing the organic load, can generate intermediate products, such as organic acids and alcohols, which are utilized in subsequent oxidative processes [30]. When magnetite-assisted ozonation was incorporated into the treatment, an additional increase in biomass was observed compared with that of the UASB alone, suggesting that ozone decomposed complex compounds into smaller and more assimilable molecules. Ozonation has been widely employed in the treatment of industrial effluents because of its ability to reduce the toxicity of persistent organic contaminants, increase the biodegradability of wastewater, and generate intermediate products such as nitrates and phosphates, which are essential for microbial metabolism [80].
The most significant increase in biomass was observed with the UASB–O3 treatment, suggesting that the preceding anaerobic degradation may have generated metabolic precursors that, upon exposure to ozone, were transformed into compounds even more accessible to microorganisms [80]. A recent study on integrating oxidation with microbial bioprocesses in industrial effluents demonstrated that prior oxidative processes could create a more favorable environment for microbial activity, particularly when the ratio between organic carbon and essential nutrients is optimized [81]. This pattern was also observed in the study by Urbina-Suarez et al. [79], in which an advanced oxidation system was integrated with microalgae cultivation to treat industrial wastewater. The authors reported that the application of hydrogen peroxide activated with bicarbonate not only improved the biodegradability of the effluent, but also promoted increased biomass synthesis in cultures of Chlorella sp., reinforcing the notion that the intermediate products generated during oxidation can act as key substrates for microbial proliferation.
Furthermore, the effectiveness of the UASB–O3–microalgae sequence may also be attributed to the selective oxidation of high-molecular-weight organic compounds into smaller and less toxic molecules that serve as direct carbon sources for algal metabolism. The partial oxidation of aromatic and humic substances can lead to the formation of low-molecular-weight carboxylic acids, aldehydes, and ketones, which are readily assimilated by microalgae [51]. Additionally, ozonation may have contributed to the release of previously complexed or particulate nutrients, such as phosphate or ammonium, increasing their bioavailability in the treated effluent. This nutrient liberation, combined with reduced toxicity and improved C:N:P balance, likely created optimal conditions for Halochlorella sp. growth [73]. These mechanisms support the hypothesis that advanced oxidation not only detoxifies, but also functionally restructures the leachate matrix, creating a more suitable environment for biological post-treatment and enhancing overall system resilience and sustainability [81].
Also, the difference in biomass generated between the leachates from Guayabal and Madera suggests that the initial composition of the leachate influences treatment efficiency. Research has reported that leachates with a higher content of readily biodegradable organic carbon favor greater microbial activity and, consequently, increased biomass production [82,83]. This may explain why, in all the evaluated treatments, the biomass values from Guayabal were greater than those from Madera, suggesting that the leachates from the latter location may contain a more significant proportion of recalcitrant compounds that adversely affect the efficiency of biological processes.
4. Materials and Methods
4.1. Leachate Sampling and Characterization
Leachate samples were collected in polyethylene containers, pretreated with nitric acid (Merck, Darmstadt, Germany), and rinsed with distilled water at the discharge points of the respective sanitary landfills using previously established methodological approaches [84]. To avoid any changes in the composition of the studied compounds, the samples were transported in insulated containers at 4 °C and, at most, 24 h after they were taken. They were analyzed as specified in the 23rd edition of the Standard Methods for the Examination of Water and Wastewater [85], and recognized sampling methods in recent studies were used [38] (Table 4).

Table 4.
Analytical methods implemented for leachate characterization.
For the determination of chemical oxygen demand (COD), the dichromate digestion method (SM 5220 D) was applied. Digestion was conducted at 150 °C for 2 h in a strongly acidic medium with potassium dichromate, using silver sulfate as a catalyst and mercuric sulfate to eliminate chloride interference. The COD concentration was then measured using UV-Vis spectrophotometry at a wavelength of 610 nm.
For the biochemical oxygen demand (BOD5), the modified SM 5210 B method was used. Winkler bottles (300 mL) were inoculated with activated sludge from a wastewater treatment plant processing effluents from animal slaughterhouses. The samples were incubated in the dark at 20 °C for 5 days, and dissolved oxygen concentrations were measured before and after incubation using a WTW 3310 portable oxygen meter (Xylem Analytics Germany Sales GmbH, Am Achalaich 11, 82362 Weilheim, Germany).
4.2. Characterization of the Inoculum Sludge
The test inoculum sludge in the UASB reactor was sourced from the second anaerobic lagoon of a palm oil extraction plant (corozo) in Tibú, Colombia. Before use, physicochemical characterization was performed to assess its suitability for anaerobic processes. This characterization was performed under the same conditions described in Section 4.1 for the leachates, concerning pH, temperature, total suspended solids (TSS), volatile suspended solids (VSS), chemical oxygen demand (COD), and the sludge volume index (SVI). In anaerobic reactor studies [38,86], minimizing microbial degradation in transported sludge samples by collecting and storing them in airtight polyethylene containers at 15 °C before analysis is recommended. They were then moved in a container to the laboratory, where analyses were performed within 24 h of collection, ensuring the representativeness of the sample and the stability of its components.
4.3. Design and Operation of the UASB Reactor
The UASB (upflow anaerobic sludge blanket) reactor used in this study was a continuous flow design made with acrylic and had an 11.7 L working volume, 15 cm inner diameter, and 66 cm height. For operational stability, an electronic heater was installed in the intermediate section of the reactor; this heater operated to maintain a constant temperature of between 35 and 40 °C throughout the experiment. Moreover, the influent pH was buffered in the range of 6.5–7.5 to prevent conditions that could be detrimental to microbial activity and to promote the stability of the methanogenesis process. A peristaltic pump was used to feed the reactor to ensure continuous and controlled flow (Figure 4). During the startup phase, low organic loading rates were applied and gradually increased as the system stabilized. This stability was evaluated by monitoring the efficiency of organic matter removal. In contrast, the organic load was adjusted by regulating the feed flow rate and the influence of the COD concentration, which was modulated by diluting the leachate with distilled water. This controlled transition allows the microbial consortium to adapt without causing significant disturbances to the system.
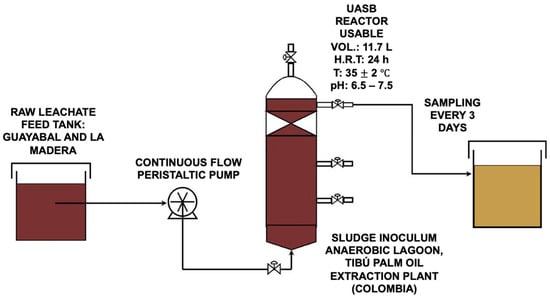
Figure 4.
Experimental setup of the UASB reactor for landfill leachate treatment.
Once the reactor could process the undiluted leachate and reach a hydraulic retention time (HRT) of 24 h—a condition recommended for leachate treatment—the operational phase commenced [30]. During this stage, the system’s performance was evaluated in terms of organic matter removal efficiency and microbiological stability (Table 5). The controlled regulation of the organic load allowed for a progressive adaptation of the anaerobic system, minimizing the risk of inhibition and ensuring efficient organic matter conversion into biogas, favoring long-term process stability. In addition, the generated gases were collected through a port at the top of the reactor, allowing for their monitoring and quantification to assess system performance.

Table 5.
Operating conditions for the UASB reactor at the El Guayabal and La Madera landfills.
4.4. Catalytic Ozonation with Magnetite
The ozonation process was carried out in a 500 mL glass reactor with a bottom gas inlet and a lateral outlet for residual gases, ensuring efficient and controlled ozone flow. Ozone was generated from pure oxygen via an O1 model unit (Pacific Ozone, Lenntech, Delfgauw, The Netherlands), with a maximum production of 6 g O3/h and a flow rate of 3.3 L/min. Ozone was introduced into the reactor to ensure proper dispersion via microbubbles generated by a diffuser with 0.45 µm orifices, resulting in a homogeneous distribution of the gas in the solution (Figure 5). The reaction was conducted at ambient temperature (20 ± 2 °C), optimizing system stability. Synthetic magnetite (Fe3O4) was used as the catalyst with a purity > 98%, an average particle size of 0.30 µm, and a surface area of 7.0 m2/g [87]. Moreover, magnetite is characterized by traces of MgO (0.9%), Mn (0.3%), TiO2 (0.25%), Zn (0.1%), Al2O3 (0.1%), and SiO2 (0.05%), in addition to many heavy metals such as Pb, Cd, As, and Hg (Alpha Chemicals, N.D.), indicating that relatively minor quantities are released into the environment and allowing its use for advanced oxidation processes. The optimal process conditions, established in previous studies, were set at a flow rate of 6 g O3/h, a flow rate of 3.3 L/h, an Fe3O4 concentration of 2.5 g/L, and a pH of 9; parameters that ensure maximum efficiency in oxidizing the contaminants present in the leachates.
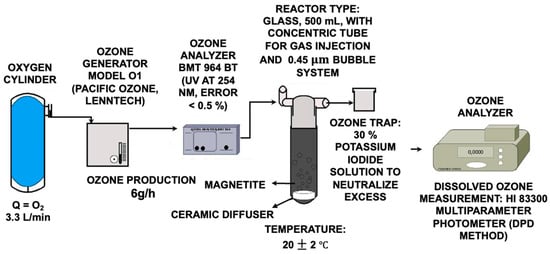
Figure 5.
Schematic of the catalytic ozonation system with magnetite.
In this study, synthetic magnetite (Fe3O4) was used, commercially sourced from Alpha Chemicals (Karachi, Pakistan). The selection of this material was based on its chemical stability and homogeneous morphology, which promote dispersion in aqueous media and surface contact in catalytic processes. Elemental characterization showed a content of 94.4% Fe3O4, along with the following minor oxides: 1.9% MgO, 1.1% TiO2, 1.0% Al2O3, 0.8% SiO2, 0.4% CaO, 0.2% Na2O, 0.1% K2O, and 0.1% MnO [38].
Magnetite (Fe3O4) acts as a heterogeneous catalyst during ozonation by promoting both radical and nonradical pathways for pollutant degradation. Its catalytic activity is attributed primarily to the generation of highly reactive hydroxyl radicals (•OH) through the decomposition of ozone, facilitated by surface hydroxyl groups and oxygen vacancies. Hydroxyl radicals possess a higher oxidation potential (2.8 V) than ozone does (2.07 V), allowing for the rapid and nonselective oxidation of recalcitrant organic compounds [88]. Furthermore, oxygen vacancies on the magnetite surface serve as active sites that enhance ozone adsorption and facilitate O–O bond cleavage, leading to the formation of surface-bound atomic oxygen (*O), a powerful nonradical oxidant with high mineralization capacity [78]. This dual mechanism—involving both •OH radicals and surface atomic oxygen—increases oxidative efficiency while minimizing ozone consumption. Consequently, the integration of magnetite significantly improves the degradation kinetics of persistent pollutants, reduces chemical reagent requirements, and enhances the overall sustainability of the ozonation process [88].
Finally, the residual gases generated were directed to a 30% potassium iodide (KI) solution to neutralize unreacted ozone, thereby preventing its release into the environment and ensuring the safe operation of the system [69].
4.5. Cultivation and Application of Halochlorella sp. for Effluent Post-Treatment
Halochlorella sp. was exposed to various leachate treatment units to evaluate the coupling of microalgal biomass to leachate treatment processes, using both untreated and previously treated leachates, through photobioreactor systems with a 2 L working volume operating under the following conditions: a 12/12 h photoperiod (light/dark), airflow rate of 6 vvm, pH of 7 ± 0.2, temperature of 28 ± 2 °C, and light intensity of 110 μmol m−2s−1. One-hundred-percent-treated leachates and untreated leachates at a concentration of 5% were the evaluated treatments to verify the differential influence of the compounds found under each treatment on biomass production (Figure 6). The biomass was quantified according to the protocol described by [79]. After 15 days of cultivation, 10 to 20 mL aliquots of the microalgal culture were collected. The samples were filtered using precombusted Whatman GF/C glass microfiber filters that were dried at 60 °C in an oven for 24 h and stored in a desiccator at 60 °C until a stable weight was achieved for at least two hours. After the filtration and drying processes were completed, the biomass concentration was calculated based on the equation, which uses the weight of the filter with the biomass.
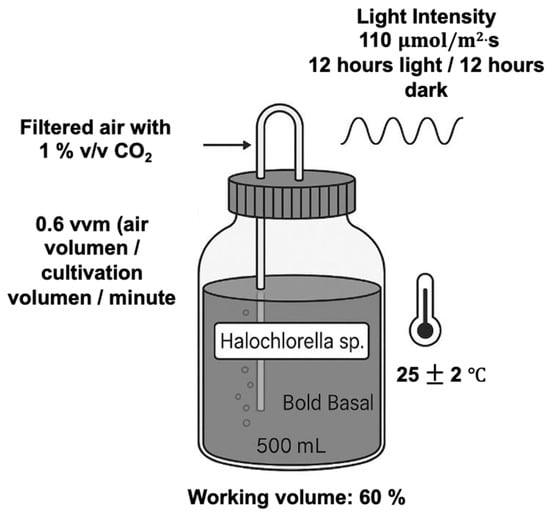
Figure 6.
Experimental setup for microalgae cultivation with Halochlorella sp.
5. Conclusions
This study demonstrated that coupling magnetite-catalyzed ozonation, UASB anaerobic digestion, and microalgae cultivation provides an effective and sustainable strategy for treating landfill leachate. The sequential integration of physicochemical and biological processes significantly enhanced contaminant removal, achieving COD reductions of up to 92%, TOC removals of up to 85%, and substantial decreases in total nitrogen and phosphorus concentrations.
Catalytic ozonation increased leachate biodegradability (BOD5/COD ratio from 0.35 to 0.58), improved specific methanogenic activity by 57%, and reduced aerobic toxicity by more than 30%. These enhancements facilitated more efficient anaerobic digestion and subsequent nutrient polishing through microalgae cultivation. Compared with conventional treatments, the proposed integrated system minimizes chemical consumption, reduces sludge production, enhances biogas recovery, and significantly decreases the residual toxicity of treated effluents, aligning with the principles of green chemistry and advancing sustainable wastewater management.
To further optimize the process, future studies should focus on integrating additional treatment stages for enhanced nitrogen removal, such as partial nitrification-denitrification systems or algal-bacterial consortia. Investigating strategies for the regeneration and reuse of magnetite catalysts and evaluating the long-term stability of the integrated system under variable leachate compositions are also recommended. Furthermore, conducting life cycle assessments (LCAs) and technoeconomic analyses will provide a more comprehensive understanding of the environmental and economic benefits of the proposed treatment scheme.
Overall, this research represents a significant contribution to the development of greener, more resource-efficient, and environmentally sustainable technologies for landfill leachate management.
Author Contributions
Conceptualization, F.M.-M., A.F.B.-S., A.Z. and D.B.-M.; methodology, L.F.R.-R. and J.B.G.-M.; software, A.F.B.-S., A.Z. and D.B.-M.; validation, D.B.-M., L.F.R.-R. and J.B.G.-M.; formal analysis, F.M.-M. and A.F.B.-S.; investigation, D.B.-M. and L.F.R.-R.; resources, F.M.-M., A.F.B.-S. and A.Z.; data curation, F.M.-M., A.F.B.-S. and D.B.-M.; writing—original draft preparation, D.B.-M.; writing—review and editing, A.Z. and J.B.G.-M.; visualization, A.F.B.-S. and A.Z.; supervision, F.M.-M.; project administration, D.B.-M.; funding acquisition, F.M.-M. and A.F.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Francisco de Paula Santander (Colombia) (FINU 011-2023), the Ministry of Science and Technology of Colombia, and the Colombian Institute of Educational Credit and Technical Studies Abroad (MINCIENCIAS-ICETEX) under the project titled “FOTOLIX” with ID 2023-0686.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our sincere gratitude to Universidad del Valle, Universidad del Llano and Universidad Francisco de Paula Santander (Colombia) for providing the equipment for this research. We also thank the Colombian Ministry of Science, Technology, and Innovation MINCIENCIAS for supporting the national Ph.D. doctorates through the Francisco José de Caldas scholarship program.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Babaei, S.; Sabour, M.R.; Moftakhari Anasori Movahed, S. Combined Landfill Leachate Treatment Methods: An Overview. Environ. Sci. Pollut. Res. 2021, 28, 59594–59607. [Google Scholar] [CrossRef]
- Li, H.; Zhou, S.; Sun, Y.; Feng, P.; Li, J. Advanced Treatment of Landfill Leachate by a New Combination Process in a Full-Scale Plant. J. Hazard. Mater. 2009, 172, 408–415. [Google Scholar] [CrossRef]
- Yao, S.; Hu, J.; Shen, D. Simulation of Landfill Leachate Treatment Systems Involving Self-Designed Disk Tube Reverse Osmosis (DTRO) Model Integrated with Mechanical Vapor Recompression (MVR). J. Water Process Eng. 2024, 60, 105124. [Google Scholar] [CrossRef]
- Liu, C.; Li, D.; Li, J.; Zhao, C.; Liu, X.; Huang, X.; Yin, F.; Shi, W.; Yu, J.; Wu, J.; et al. Treatment of Landfill Leachate by Two-Stage Electrochemical Combination Technology. J. Env. Chem. Eng. 2025, 13, 115355. [Google Scholar] [CrossRef]
- Hui, H.; Wang, Y.; Huang, C.; Wang, C.; Yang, T.; Mo, J.; Li, X.; Wang, Y.; Pei, H.; Zhang, L.; et al. Treatment of Landfill Leachate Concentrate Using an Integrated Coagulation–Fixed-Bed Electrocatalytic Reactor–Nanofiltration System. Sep. Purif. Technol. 2025, 366, 132814. [Google Scholar] [CrossRef]
- Lei, Y.; Hou, J.; Fang, C.; Tian, Y.; Naidu, R.; Zhang, J.; Zhang, X.; Zeng, Z.; Cheng, Z.; He, J.; et al. Ultrasound-Based Advanced Oxidation Processes for Landfill Leachate Treatment: Energy Consumption, Influences, Mechanisms and Perspectives. Ecotoxicol. Env. Saf. 2023, 263, 115366. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, A.; Zhang, G.; Zhang, G. Combined Coagulation-Adsorption-VUV (Persulfate)-Electrochemical Oxidation Processes for Efficient Treatment of Aged Landfill Leachate. J. Water Process Eng. 2025, 71, 107281. [Google Scholar] [CrossRef]
- Jiang, B.-C.; Tian, Y.-C.; Li, A.-M.; Han, Y.-Z.; Wu, Z.-T.; Lu, C.; Song, H.-O.; Ji, R.; Li, W.-T.; Korshin, G.V. Changes of Dissolved Organic Matter Fractions and Formation of Oxidation Byproducts during Electrochemical Treatment of Landfill Leachates: Development of Spectroscopic Indicators for Process Optimization. Water Res. 2023, 232, 119702. [Google Scholar] [CrossRef] [PubMed]
- Gripa, E.; Daflon, S.D.A.; de Almeida, R.; da Fonseca, F.V.; Campos, J.C. Landfill Leachate Treatment by High-Pressure Membranes and Advanced Oxidation Techniques with a Focus on Ecotoxicity and by-Products Management: A Review. Process Saf. Environ. Prot. 2023, 173, 747–764. [Google Scholar] [CrossRef]
- Ibrahim, I. Water treatment using perovskite materials and their applications: A comprehensive review. J. Ind. Eng. Chem. 2025, 145, 20–32. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Rasid, N.A.A.A.; Rasit, N.; Rahman Azmi, A.A.A.; Harun, M.H.C.; Yussof, W.M.H.W.; Hairom, N.H.H.; Jusoh, H.H.W.; Juahir, H.; Hamzah, S. Study of Landfill Leachate Coagulation Using Hybrid Coagulant of Copperas/Lime. Desalination Water Treat. 2023, 304, 181–189. [Google Scholar] [CrossRef]
- Ahn, W.-Y.; Kang, M.-S.; Yim, S.-K.; Choi, K.-H. Advanced Landfill Leachate Treatment Using an Integrated Membrane Process. Desalination 2002, 149, 109–114. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Lau, I.W.C.; Wang, P. Anaerobic Treatment of Hong Kong Leachate Followed by Chemical Oxidation. Water Sci. Technol. 2005, 52, 41–49. [Google Scholar] [CrossRef]
- Ismail, S.; Nasr, M.; Abdelrazek, E.; Awad, H.M.; Zhaof, S.; Meng, F.; Tawfik, A. Techno-Economic Feasibility of Energy-Saving Self-Aerated Sponge Tower Combined with up-Flow Anaerobic Sludge Blanket Reactor for Treatment of Hazardous Landfill Leachate. J. Water Process Eng. 2020, 37, 101415. [Google Scholar] [CrossRef]
- Braga, W.L.M.; de Melo, D.H.A.; de Morais, D.; Samanamud, G.R.L.; França, A.B.; Finzi Quintão, C.M.; Loures, C.C.A.; de Urzedo, A.P.F.M.; Naves, L.L.R.; de Freitas Gomes, J.H.; et al. Optimization of the Treatment of Sanitary Landfill by the Ozonization Catalyzed by Modified Nanovermiculite in a Rotating Packed Bed. J. Clean. Prod. 2020, 249, 119395. [Google Scholar] [CrossRef]
- Khen, C.; Ilmasari, D.; Yuzir, A. Development of Microalgae-Bacteria Aerobic Granular Sludge for Landfill Leachate Treatment. IOP Conf. Ser. Earth Env. Sci. 2022, 1091, 012050. [Google Scholar] [CrossRef]
- Liu, X.; Novak, J.T.; He, Z. Synergistically Coupling Membrane Electrochemical Reactor with Fenton Process to Enhance Landfill Leachate Treatment. Chemosphere 2020, 247, 125954. [Google Scholar] [CrossRef]
- Yenis Septiariva, I.; Padmi, T.; Damanhuri, E.; Helmy, Q. A Study on Municipal Leachate Treatment through a Combination of Biological Processes and Ozonation. Matec. Web Conf. 2019, 276, 06030. [Google Scholar] [CrossRef]
- Jamrah, A.; AL-Zghoul, T.M.; Al-Qodah, Z. An Extensive Analysis of Combined Processes for Landfill Leachate Treatment. Water 2024, 16, 1640. [Google Scholar] [CrossRef]
- Kanmani, S.; Dileepan, A.G.B. Treatment of Landfill Leachate Using Photocatalytic Based Advanced Oxidation Process—A Critical Review. J. Env. Manag. 2023, 345, 118794. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Gu, X.; Wang, K. Pilot Study on the Advanced Treatment of Landfill Leachate Using a Combined Coagulation, Fenton Oxidation and Biological Aerated Filter Process. Waste Manag. 2009, 29, 1354–1358. [Google Scholar] [CrossRef]
- Feng, H.; Mao, W.; Li, Y.; Wang, X.; Chen, S. Characterization of Dissolved Organic Matter during the O3-Based Advanced Oxidation of Mature Landfill Leachate with and without Biological Pre-Treatment and Operating Cost Analysis. Chemosphere 2021, 271, 129810. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Mohd, Z.; Mohd, S.; Adlan, M.N.; Hung, Y.-T. Physicochemical Treatment Processes Of Landfill Leachate. In Handbook of Environment and Waste Management; World Scientific: Singapore, 2012; pp. 819–888. [Google Scholar]
- Tan, B.; He, L.; Dai, Z.; Sun, R.; Jiang, S.; Lu, Z.; Liang, Y.; Ren, L.; Sun, S.; Zhang, Y.; et al. Review on Recent Progress of Bioremediation Strategies in Landfill Leachate—A Green Approach. J. Water Process Eng. 2022, 50, 103229. [Google Scholar] [CrossRef]
- Cardeña, R.; Buitrón, G.; Valdez-Vazquez, I.; Contreras, M. Biohydrogen Production from Cheese Whey in UASB and Packed Bed Reactors: Impact of Microbial Interactions on Productivity. Int. J. Hydrog. Energy 2025, 141, 1061–1069. [Google Scholar] [CrossRef]
- Lima, V.D.O.; Barros, V.G.D.; Duda, R.M.; Oliveira, R.A. de Anaerobic Digestion of Vinasse and Water Treatment Plant Sludge Increases Methane Production and Stability of UASB Reactors. J. Env. Manag. 2023, 327, 116451. [Google Scholar] [CrossRef]
- Jiraprasertwong, A.; Seneesrisakul, K.; Pornmai, K.; Chavadej, S. High Methanogenic Activity of a Three-Stage UASB in Relation to the Granular Sludge Formation. Sci. Total Environ. 2020, 724, 138145. [Google Scholar] [CrossRef] [PubMed]
- López-Gutiérrez, I.; Montiel-Corona, V.; Calderón-Soto, L.F.; Palomo-Briones, R.; Méndez-Acosta, H.O.; Razo-Flores, E.; Ontiveros-Valencia, A.; Alatriste-Mondragón, F. Evaluation of the Continuous Methane Production from an Enzymatic Agave Bagasse Hydrolysate in Suspended (CSTR) and Granular Biomass Systems (UASB). Fuel 2021, 304, 121406. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Yi, X.; Zhao, Y.; Jin, F.; Chen, L.; Hua, D. Study of Two-Phase Anaerobic Digestion of Corn Stover: Focusing on the Conversion of Volatile Fatty Acids and Microbial Characteristics in UASB Reactor. Ind. Crops Prod. 2021, 160, 113097. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Zhang, Y.; Zhan, Y.; Liu, J.-Q.; Yan, C.-C.; Zhang, Y.; Zhang, H.-L. Synergistic Enhancement of UASB Reactor for Leachate Treatment Using Fe2O3 Nanomodified Pumice and Ozone Oxidation. Chem. Eng. J. 2024, 497, 154891. [Google Scholar] [CrossRef]
- Fulazzaky, M.A.; Yuzir, A.; Messer, T.; Sofyan, A. Methanogenesis Kinetics of Organic Matter of the Leachate in an Up-Flow Anaerobic Sludge Blanket Reactor. Waste Manage Bull. 2024, 2, 1–10. [Google Scholar] [CrossRef]
- Clemente, E.; Domingues, E.; Quinta-Ferreira, R.M.; Leitão, A.; Martins, R.C. European and African Landfilling Practices: An Overview on MSW Management, Leachate Characterization and Treatment Technologies. J. Water Process Eng. 2024, 66, 105931. [Google Scholar] [CrossRef]
- Asaithambi, P.; Govindarajan, R.; Busier Yesuf, M.; Selvakumar, P.; Alemayehu, E. Enhanced Treatment of Landfill Leachate Wastewater Using Sono(US)-Ozone(O3)–Electrocoagulation(EC) Process: Role of Process Parameters on Color, COD and Electrical Energy Consumption. Process Saf. Environ. Prot. 2020, 142, 212–218. [Google Scholar] [CrossRef]
- Ricky, R.; Shanthakumar, S.; Gothandam, K.M. A Pilot-Scale Study of the Integrated Phycoremediation-Photolytic Ozonation Based Municipal Solid Waste Leachate Treatment Process. J. Env. Manag. 2022, 323, 116237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Gao, B.; Sillanpää, M. Landfill Leachate Treatment In-Depth by Bio-Chemical Strategy: Microbial Activation and Catalytic Ozonation Mechanism. Chem. Eng. J. 2022, 444, 136464. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, Z.; Song, B.; Li, Q. Treatment of Landfill Leachate MBR Effluent by Ozone Combined with a Semi-Aerobic Aged Refuse Bioreactor. J. Water Process Eng. 2025, 69, 106625. [Google Scholar] [CrossRef]
- Becerra, D.; Soto, J.; Villamizar, S.; Machuca-Martínez, F.; Ramírez, L. Alternative for the Treatment of Leachates Generated in a Landfill of Norte de Santander–Colombia, by Means of the Coupling of a Photocatalytic and Biological Aerobic Process. Top. Catal. 2020, 63, 1336–1349. [Google Scholar] [CrossRef]
- Becerra-Moreno, D.; Machuca-Martínez, F.; Ramírez-Rios, L.F.; García-Martínez, J.B.; Barajas-Solano, A.F. Synergistic Degradation of Organic Contaminants in Landfill Leachates Using Catalytic Ozonation with Magnetite. Science 2025, 7, 31. [Google Scholar] [CrossRef]
- Xiao, J.; Qaisar, M.; Zhu, X.; Li, W.; Zhang, K.; Liang, N.; Feng, H.; Cai, J. Increasing Methane Production in an Anaerobic Membrane Bioreactor for Treating Landfill Leachate: Impact of Organic Concentration and HRT. J. Env. Manag. 2024, 367, 122061. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Li, W.; Liu, X.; Lv, L.; Gao, W.; Liu, X.; Sun, L.; Liang, J.; Zhang, G.; et al. Enhanced Anaerobic Digestion of Landfill Leachate Based on a Novel Redox Mediator: Synergistic Mechanism of Enhancing Extracellular Electron Transfer. Chem. Eng. J. 2024, 490, 151649. [Google Scholar] [CrossRef]
- Kayan, I.; Oz, N.A.; Kantar, C. Comparison of Treatability of Four Different Chlorophenol-Containing Wastewater by Pyrite-Fenton Process Combined with Aerobic Biodegradation: Role of Sludge Acclimation. J. Env. Manag. 2021, 279, 111781. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Ma, H. The Key Regulative Parameters in Pilot-Scale IC Reactor for Effective Incineration Landfill Leachate Treatment: Focus on the Process Performance and Microbial Community. J. Water Process Eng. 2023, 51, 103322. [Google Scholar] [CrossRef]
- Li, L.; Kong, Z.; Xue, Y.; Wang, T.; Kato, H.; Li, Y.-Y. A Comparative Long-Term Operation Using up-Flow Anaerobic Sludge Blanket (UASB) and Anaerobic Membrane Bioreactor (AnMBR) for the Upgrading of Anaerobic Treatment of N,N-Dimethylformamide-Containing Wastewater. Sci. Total Environ. 2020, 699, 134370. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, A.; Shim, H.; Wang, D.; Cheng, S.; Wang, Y.; Li, M. Effect of Ozone Pretreatment on Biogranulation with Partial Nitritation—Anammox Two Stages for Nitrogen Removal from Mature Landfill Leachate. J. Env. Manag. 2022, 317, 115470. [Google Scholar] [CrossRef]
- Zou, X.; Mohammed, A.; Gao, M.; Liu, Y. Mature Landfill Leachate Treatment Using Granular Sludge-Based Reactor (GSR) via Nitritation/Denitritation: Process Startup and Optimization. Sci. Total Environ. 2022, 844, 157078. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, R.; Yao, H.; Hu, Z.-H.; Wang, Y.; Yuan, S.; Wang, W. Start-up of Partial Nitrification-Anammox (PN/A) Process Treating Piggery Wastewater. Desalination Water Treat. 2020, 180, 156–163. [Google Scholar] [CrossRef]
- Pinpatthanapong, K.; Puengpraput, T.; Phattarapattamawong, S.; Phalakornkule, C.; Panichnumsin, P.; Boonapatcharoen, N.; Paensiri, P.; Malila, K.; Ponata, N.; Ngamcharoen, T.; et al. Effect of Propionate-Cultured Sludge Augmentation on Methane Production from Upflow Anaerobic Sludge Blanket Systems Treating Fresh Landfill Leachate. Sci. Total Environ. 2023, 881, 163434. [Google Scholar] [CrossRef]
- Trisakti, B.; Sidabutar, R.; Irvan; Adriani, L.; Manurung, J.F.; Simbolon, D.K.; Alexander, V.; Takriff, M.S.; Daimon, H. Effect of Hydraulic Retention Time and Effluent Recycle Ratio on Biogas Production from POME Using UASB-HCPB Fermentor Assisted with Ultrafiltration Membrane at Mesophilic Condition. S. Afr. J. Chem. Eng. 2024, 50, 209–222. [Google Scholar] [CrossRef]
- Tian, Z.; Li, G.; Bai, M.; Hou, X.; Li, X.; Zhao, C.; Zhu, Q.; Du, C.; Li, M.; Liu, W.; et al. Microbial Mechanisms of Refractory Organics Degradation in Old Landfill Leachate by a Combined Process of UASB-A/O-USSB. Sci. Total Environ. 2022, 848, 157737. [Google Scholar] [CrossRef]
- Han, Y.; Guo, J.; Liu, T.; Wang, N.; Su, Z.; Wang, S.; Ma, X.; Wang, J.; Zhao, F. Iron-carbon Micro-Electrolysis and Anaerobic-Aerobic Activated Sludge Integrated Process for Treating Landfill Leachate: Process Optimization, Stability, and Mechanism. J. Water Process Eng. 2025, 69, 106704. [Google Scholar] [CrossRef]
- Babu Ponnusami, A.; Sinha, S.; Ashokan, H.; V Paul, M.; Hariharan, S.P.; Arun, J.; Gopinath, K.P.; Hoang Le, Q.; Pugazhendhi, A. Advanced Oxidation Process (AOP) Combined Biological Process for Wastewater Treatment: A Review on Advancements, Feasibility and Practicability of Combined Techniques. Env. Res. 2023, 237, 116944. [Google Scholar] [CrossRef]
- Zhe, J.; He, H.; Yi, Z.; Guo, Z.; Xu, H.; Huang, B.; Pan, X. Mechanism and Molecular Level Insight of Refractory Dissolved Organic Matter in Landfill Leachate Treated by Electroflocculation Coupled with Ozone. Sep. Purif. Technol. 2025, 356, 129812. [Google Scholar] [CrossRef]
- Bao, M.; Fang, F.; Luo, X.; Li, Q. Transformation Characteristics of Dissolved Organic Matter in Landfill Leachate Nanofiltrated Concentrate during Ozonation-Based Advanced Oxidation Systems. J. Env. Chem. Eng. 2024, 12, 112076. [Google Scholar] [CrossRef]
- Wu, L.; Yin, J.; Zhang, Y.; Luo, A.; Tian, Y.; Liu, Y.; Peng, Y. Nitrogen Removal and Carbon Reduction of Mature Landfill Leachate under Extremely Low Dissolved Oxygen Conditions by Simultaneous Partial Nitrification Anammox and Denitrification. Bioresour. Technol. 2024, 401, 130704. [Google Scholar] [CrossRef]
- Lin, X.; Xu, L.; Xiong, L.; Wang, X.; He, Y.; Chen, H.; Zhang, W.; Xue, G. Mechanistic Insight into Assimilation Capture: Implication for High-Efficiency Nitrogen Removal from Mature Landfill Leachate. Chem. Eng. J. 2024, 498, 155395. [Google Scholar] [CrossRef]
- Liu, S.; Cai, C.; Sun, F.; Ma, M.; An, T.; Chen, C. Advanced Nitrogen Removal of Landfill Leachate Treatment with Anammox Process: A Critical Review. J. Water Process Eng. 2024, 58, 104756. [Google Scholar] [CrossRef]
- Xiong, L.; Li, X.; Li, J.; Zhang, Q.; Zhang, L.; Wu, Y.; Peng, Y. Efficient Nitrogen Removal from Real Municipal Wastewater and Mature Landfill Leachate Using Partial Nitrification-Simultaneous Anammox and Partial Denitrification Process. Water Res. 2024, 251, 121088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Zhou, S.; Fan, J.; Huang, L.; Deng, Z.; Zhang, C.; Wang, X. Improving Stability and Nitrogen Removal Performance of Pilot-Scale Autotrophic Process for Mature Landfill Leachate Treatment Utilizing in-Situ Organics. Bioresour. Technol. 2023, 381, 129118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zou, L.; Jiang, M.; Li, Y.-Y.; Liu, J. Ozone Pretreatment Combined with Partial Denitrification-Anammox Process for Efficient Nitrogen Removal from Nanofiltration Concentrate of Landfill Leachate. Chem. Eng. J. 2023, 471, 144641. [Google Scholar] [CrossRef]
- Yang, H.-R.; Li, B.; Zhang, C.-Q.; Yang, J.-C.; Zheng, Y.-M.; Younas, M.; Jiang, Y.-H.; Yuan, Z.-H. Bipolar Membrane Electrodialysis for Sustainable Utilization of Inorganic Salts from the Reverse Osmosis Concentration of Real Landfill Leachate. Sep. Purif. Technol. 2023, 308, 122898. [Google Scholar] [CrossRef]
- Jolaosho, T.L. Characterization of Potentially Toxic Elements in Leachates from Active and Closed Landfills in Nigeria and Their Effects on Groundwater Systems Using Spatial, Indexical, Chemometric and Health Risk Techniques. Chemosphere 2024, 369, 143678. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhu, P.; Ma, Y. Interaction and Coexistence Characteristics of Dissolved Organic Matter and Toxic Metals with Per- and Polyfluoroalkyl Substances in Landfill Leachate. Env. Res. 2024, 260, 119680. [Google Scholar] [CrossRef] [PubMed]
- Amancio Frutuoso, F.K.; da Silva, V.E.P.S.G.; Silva, T.F.C.V.; Vilar, V.J.P.; Bezerra dos Santos, A. Solids Retention Time (SRT) Control in the Co-Treatment of Leachate with Domestic Sewage in Aerobic Granular Sludge Systems: Impacts on System Performance, Operational Stability, and Bioresource Production. Bioresour. Technol. 2025, 415, 131664. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.A.T.; Dantas, E.R.B.; Lopes, W.D.S.; Leite, V.D.; Sousa, J.T.D.; Lopes, W.S. Toxicity Assessment of Sanitary Landfill Leachate before and after Fenton Treatment Process. Sci. Total Environ. 2023, 893, 164870. [Google Scholar] [CrossRef] [PubMed]
- Kalčíková, G.; Tratar Pirc, E.; Žgajnar Gotvajn, A. Aerobic and Anaerobic Biodegradation Potential of Leachate from Old Active Landfill. Desalin. Water Treat. 2016, 57, 8619–8625. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, V.; Ormeci, B.; Hussain, A.; Cheng, L.; Venkiteshwaran, K. Anaerobic–Aerobic Treatment of Wastewater and Leachate: A Review of Process Integration, System Design, Performance and Associated Energy Revenue. J. Env. Manag. 2023, 327, 116898. [Google Scholar] [CrossRef]
- Castillo-Suárez, L.A.; Lugo-Lugo, V.; Linares-Hernández, I.; Martínez-Miranda, V.; Esparza-Soto, M.; Mier-Quiroga, M. de los Á. Biodegradability Index Enhancement of Landfill Leachates Using a Solar Galvanic-Fenton and Galvanic-Fenton System Coupled to an Anaerobic–Aerobic Bioreactor. Sol. Energy 2019, 188, 989–1001. [Google Scholar] [CrossRef]
- Lakshmi, A.A.; Raj, S.A. Natural Gum Based Polymers as Additives in Enhancing the Startup of UASB Reactor Treating Municipal Sewage—Comparative Study. Desalin. Water Treat. 2022, 252, 89–97. [Google Scholar] [CrossRef]
- Duan, Y.; Gao, B.; Liu, J.; Wang, X.; Sillanpää, M. Treatment of Landfill Leachate in the MBR and Catalytic Ozonation Coupled System Based on Mn Ni Catalyst: Efficiency and Bio/Chemical Mechanism. J. Water Process Eng. 2024, 66, 106033. [Google Scholar] [CrossRef]
- Bai, F.; Liu, S.; Zhang, Y.; Ma, J. Effective and Mechanistic Insights into Reverse Osmosis Concentrate of Landfill Leachate Treatment Using Coagulation-Catalytic Ozonation-Bioaugmentation-Based AnMBR. Chem. Eng. J. 2023, 463, 142430. [Google Scholar] [CrossRef]
- Han, S.; Wang, W.; Xu, Z.; Qi, L. Research and Perspective of Heterogeneous Catalytic Oxidation by Ozone in the Removal of Pollutants from Industrial Wastewater: A Review. J. Ind. Eng. Chem. 2025, 147, 20–38. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, H.; Wang, T.; Zhou, J.; Zhu, Y.; Hou, J. Advanced Treatment of Landfill Leachate by Catalytic Ozonation with MnCeOx/γ-Al2O3 Catalyst. Surf. Interfaces 2024, 46, 104113. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; He, X.; Yang, Y.; Yang, X.; Van Hulle, S.W.H. Comparison of Macro and Micro-Pollutants Abatement from Biotreated Landfill Leachate by Single Ozonation, O3/H2O2, and Catalytic Ozonation Processes. Chem. Eng. J. 2023, 452, 139503. [Google Scholar] [CrossRef]
- An, W.; Xiao, S.; Liu, H.; Ma, L. UV Irradiation Enhanced Removal of Ammonia Nitrogen and Mineralization of Typical Organic Pollutants of High-Chlorine Wastewater in Catalytic Ozonation. J. Env. Chem. Eng. 2025, 13, 115395. [Google Scholar] [CrossRef]
- Goli, T.; Ahmadi, K.; Pagilla, K.R.; Marchand, E.A. Dissolved Organic Nitrogen Removal from Reclaimed Water by Enhanced Coagulation, Ozonation, and Biodegradation. J. Water Process Eng. 2023, 55, 104212. [Google Scholar] [CrossRef]
- Guerrero-Brotons, M.; Gómez, R.; Álvarez-Rogel, J.; Sánchez-Monedero, M.Á.; Arce, M.I. The Effect of Leaf Leachates Addition on Denitrification in Subsurface Flow Constructed Wetlands Is Shaped by the Bed Substrate Type. J. Water Process Eng. 2024, 68, 106360. [Google Scholar] [CrossRef]
- Mu, S.; Chen, X.; Song, B.; Wu, C.; Li, Q. Enhanced Performance and Mechanism of the Combined Process of Ozonation and a Semiaerobic Aged Refuse Biofilter for Mature Landfill Leachate Treatment. Chemosphere 2022, 308, 136432. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R.; Omran, N.R. Development of an Efficient Catalyst from Magnetite Ore: Characterization and Catalytic Potential in the Ozonation of Water Toxic Contaminants. Appl. Catal. A Gen. 2012, 445–446, 42–49. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Salcedo-Pabón, C.J.; Contreras-Ropero, J.E.; López-Barrera, G.L.; García-Martínez, J.B.; Barajas-Solano, A.F.; Machuca-Martínez, F. Biotechnological Strategy for Tannery Wastewater Treatment: Bicarbonate/H2O2 Oxidation Integrated with Microalgae Cultivation. Case Stud. Chem. Environ. Eng. 2025, 11, 101060. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, N.-Q.; Ren, H.-Y. Ozone Oxidation of Actual Waste Leachate Coupled with Culture of Microalgae for Efficient Lipid Production under Different Temperatures. Water Res. 2025, 277, 123305. [Google Scholar] [CrossRef]
- Sari, M.M.; Septiariva, I.Y.; Prayogo, W.; Helmy, Q.; Suryawan, I.W.K. Effects of Detention Time and Ozone Dosage on Organic Content Removal and Biodegradability Index in High Salinity Leachate. Desalin. Water Treat. 2023, 284, 81–86. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, Z.; Yang, L.; Lai, L.; Xiao, H.; Ding, Y.; Luo, X. Enhancing Nitrogen Removal in Mature Landfill Leachate by Mixed Microalgae through Elimination of Inhibiting Factors. Sci. Total Environ. 2022, 828, 154530. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Wang, Z.; Liu, Z.; Xiong, Z.; Dai, H.; Yang, L.; Liu, Y.; Yang, J.; Geng, Y.; Hu, M.; et al. A New Microalgal Negative Carbon Technology for Landfill Leachate Treatment: Simultaneous Removal of Nitrogen and Phosphorus. Sci. Total Environ. 2024, 948, 174779. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Wei, H.; Zhang, J. Nitric Acid Rain Decreases Soil Bacterial Diversity and Alters Bacterial Community Structure in Farmland Soils. Agronomy 2024, 14, 971. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association. Water Environment Federation Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Li, K.; Yun, J.; Zhang, H.; Yu, Z. Full-Scale Anaerobic Reactor Samples Would Be More Suitable than Lab-Scale Anaerobic Reactor and Natural Samples to Inoculate the Wheat Straw Batch Anaerobic Digesters. Bioresour. Technol. 2019, 293, 122040. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Yin, J.; Xiang, Y.; Yuan, Y.; Zhang, J.; He, C.; Zhang, H.; Xiong, Z.; Lai, B. Critical Role of Oxygen Vacancies in Catalytic Ozonation: Non-Radical Pathways and Enhanced Hydroxyl Groups. Chem. Eng. J. 2024, 500, 156952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).