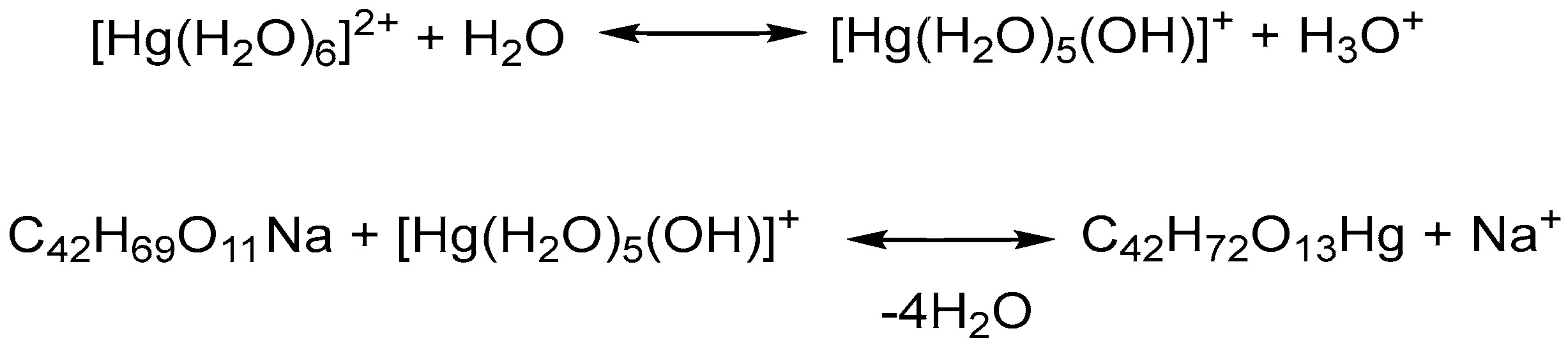Abstract
Salinomycin is a polyether ionophorous antibiotic with promising antineoplastic properties. Published studies have revealed that the compound also exerts pronounced antidotal activity against cadmium (Cd) and lead (Pb) intoxications. It has been proven that salinomycin with Cd(II) forms a coordination compound of a composition [Cd(C42H69O11)2(H2O)2] and an octahedral molecular geometry, while the coordination compound of the antibiotic with Pb(II) has a square pyramidal structure and composition [Pb(C42H69O11)(NO3)]. To date, there is no published information about the ability of salinomycin to form complexes with the mercury ion (Hg(II)). Herein, we report, for the first time, a synthetic procedure for a complex compound of salinomycin with Hg(II). The coordination compound was characterized by a variety of methods, such as elemental analysis, attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR), electrospray ionization–mass spectrometry (ESI-MS), powder X-ray diffraction, nuclear magnetic resonance spectroscopy (NMR), thermogravimetry with differential thermal analysis (TG-DTA), and thermogravimetry with mass spectrometry (TG-MS). The elemental analysis data revealed that the new compound is of the chemical composition [Hg(C42H69O11)(H2O)(OH)]. Based on the results from the spectral analyses, the most probable structure of the complex was proposed.
1. Introduction
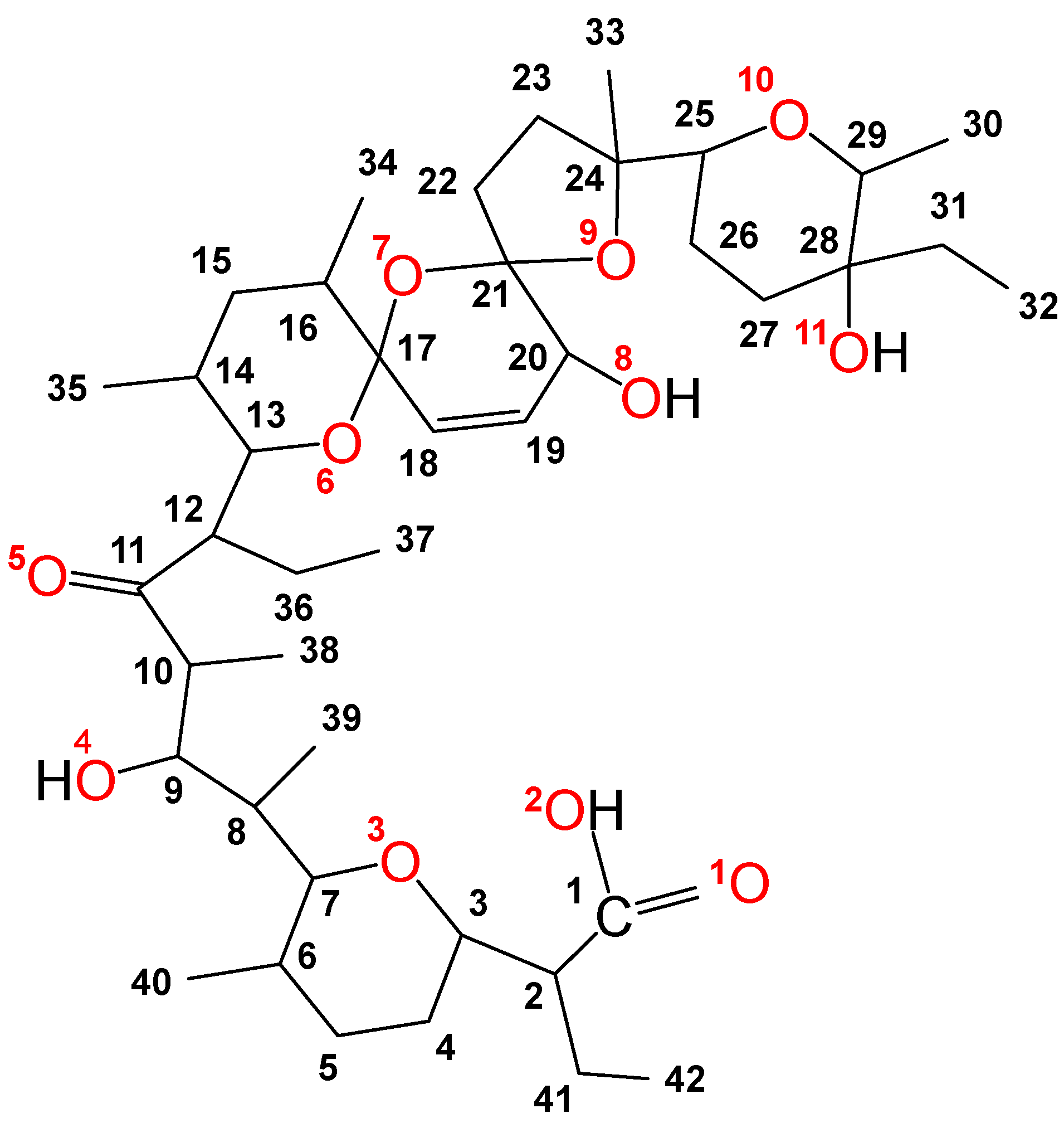
Salinomycin (C42H70O11, Figure 1) belongs to the group of natural polyether ionophorous antibiotics. It was isolated from the Streptomyces albus species by Miyazaki et al. [1]. The chemical structure of the compound comprises a unique spiroketal system, with a carboxyl group, ether, hydroxyl, and carbonyl oxygen atoms, and is essential for its broad biological activity [2,3,4,5,6,7]. In Europe, the antibiotic has been approved for the treatment of coccidiosis in chickens [8]. In 2009, Gupta et al. found that among 16,000 compounds, salinomycin exerted pronounced cytotoxicity against breast cancer stem cells responsible for metastases [9]. Since then, many literature reviews have demonstrated the potential of the antibiotic as a new antineoplastic agent [10,11,12,13,14,15,16,17,18,19].
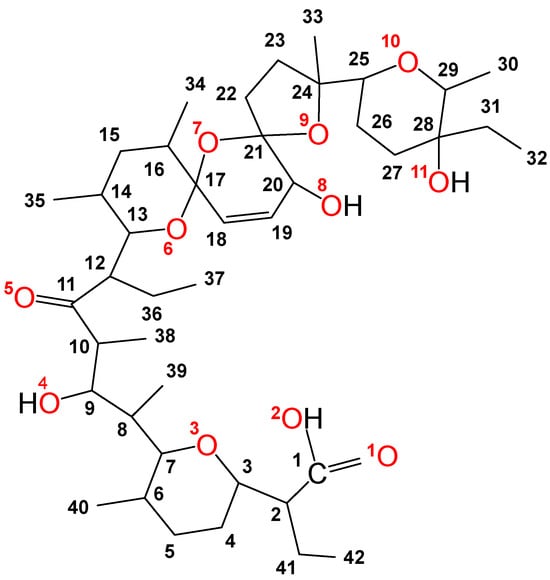
Figure 1.
Structure and numbers of carbon and oxygen atoms of salinomycin.
The coordination of salinomycin to essential metal ions has been used to enhance its biological activity and to decrease its toxicity [20,21,22]. It has been proven that the coordination compounds of salinomycin with divalent essential metal ions are of the composition [M(C42H69O11)2(H2O)2], where M is calcium (Ca), magnesium (Mg), cobalt (Co), copper (Cu), manganese (Mn), or zinc (Zn) [20,21,22]. The structure of these complexes was studied by different spectral methods such as Fourier transform infrared (FTIR) spectroscopy and mass spectrometry with fast atom bombardment (FAB-MS) [20] and with electrospray ionization (ESI-MS) [21,22]. The diamagnetic compounds were also characterized by nuclear magnetic resonance (NMR) spectroscopy, while the structure of the paramagnetic complexes of salinomycin with divalent metal ions was explored by electron paramagnetic resonance (EPR) spectroscopy. All these metal complexes had identical FTIR spectra, confirming that they were isostructural [20,21,22]. Based on the obtained spectral data and the published spectral properties and crystal structures of homonuclear metal complexes of the polyether ionophorus antibiotic monensin with the general formula [M(C36H61011)2(H2O)2] (M is a divalent metal ion) [23], it has been proposed that the metal complexes of salinomycin with essential divalent metal ions possess a distorted octahedral molecular geometry [20,21,22]. It has also been suggested that the antibiotic was coordinated to the metal center bidentately by a deprotonated carboxyl group and a terminal secondary hydroxyl group. It has been proposed that the coordination of two water molecules to the metal center stabilized the structure of the complexes by intramolecular hydrogen bonding with salinomycin [20,21,22]. The antitumor activity of the metal complexes of salinomycin with divalent metal ions was shown to depend both on the metal ion and the tested tumor cell line [20,21,22].
In contrast to the essential divalent metal ions, salinomycin forms complexes with toxic divalent metal ions with varying molecular geometries [24]. It has been demonstrated that the cadmium (Cd(II)) complex of salinomycin is of the composition [Cd(C42H69O11)2(H2O)2] with a distorted octahedral molecular geometry, similar to the structure of the complexes of salinomycin with the essential divalent metal ions [20,24]. Unlike Cd(II) disalinomycinate, the antibiotic has been coordinated to lead ion (Pb(II)) in a square pyramidal geometry [24]. The structures of Cd(II) disalinomycinate and Pb(II) salinomycinate have been proposed as the result of comprehensive studies of both compounds by FTIR spectrometry, NMR spectroscopy, and FAB-MS. It has been suggested that the salinomycinate monoanion was coordinated to Pb(II) tetradentately by carboxylate oxygen, ether oxygen atoms (O-3 and O-24), and the oxygen atom from the terminal hydroxyl group (O-28) [24]. The neutrality of the complex was achieved by a binding of nitrate ion to Pb((II)) [24]. In vivo studies have reported that salinomycin decreased the concentration of the toxic metal ions Pb(II) and Cd(II) in mice exposed to lead and cadmium intoxications and ameliorated the toxicity induced by both toxic metal ions [25,26,27]. Compared to other known chelating agents, such as deferiprone and 2,3-dimercaptosuccinic acid (DMSA), the antibiotic did not disturb the homeostasis of the essential metal ions Cu(II), iron (Fe (III)), and Zn(II) in cadmium- and lead-exposed animals [25,26,27]. Moreover, salinomycin did not induce the redistribution of the toxic metal ions in vivo.
These studies provoked our interest in the coordination chemistry of salinomycin with other toxic metal ions.
Presently, there is no available information about the ability of salinomycin to form coordination compounds with the mercury ion (Hg(II)).
Herein, we synthesized and characterized, for the first time, a metal complex of salinomycin with Hg(II).
2. Results and Discussion
2.1. Elemental Analysis
In this study, we explored, for the first time, the chemical reaction between salinomycin sodium (SalNa) (dissolved in CH3CN:CH3OH = 1:10) and an aqueous solution of Hg(II) nitrate. Published data have revealed that the hydrolysis of Hg(II) occurred through a three-step mononuclear mechanism [28]. The first stage of Hg(II) hydrolysis involved the formation of [Hg(OH)]+ species and H3O+. It was reported that the subsequent hydrolysis of the [Hg(OH)]+ ion produced Hg(OH)2 and H3O+. According to the literature data, the generation of a negatively charged [Hg(OH)3]− ion was observed only in a basic environment [28]. Another study revealed that Hg(II) is hydrated and could act as an acid, donating one or two protons [29]. Florez et al. 2023 proved the coordination of water molecules to Hg(II) [30]. The pH of our solution, measured immediately after dissolving Hg(NO3)2, was 2.2, indicating hydrolysis of Hg(II) nitrate. The elemental analysis data, presented in Table 1, revealed that the interaction of SalNa solution with the aqueous solution of Hg(II) nitrate produced a neutral mononuclear compound with a molar ratio of 1:1 between salinomycin and the metal ion. Most likely, the organic ligand reacted with [Hg(H2O)5(OH)]+, formed as a result of the first-step hydrolysis of Hg(II), as proposed in Scheme 1.

Table 1.
Elemental analysis.
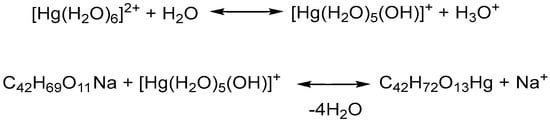
Scheme 1.
Chemical equations of the first step hydrolysis of Hg(NO3)2 and the interaction of SalNa with an aqueous solution of Hg(NO3)2.
Similar to other metal complexes of salinomycin [20,21,22,24], Hg(II) salinomycinate (SalHg) did not crystallize to a single crystal suitable for molecular geometry refinement by X-ray analysis. To obtain detailed information about the structure of the complex in the solid state and in solution, we applied attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR), electrospray ionization–mass spectrometry (ESI-MS), powder X-ray diffraction (XRD), nuclear magnetic resonance spectroscopy (NMR), thermogravimetry with differential thermal analysis (TG-DTA), and thermogravimetry with mass spectrometry (TG-MS).
2.2. ATR-FTIR Spectral Analysis
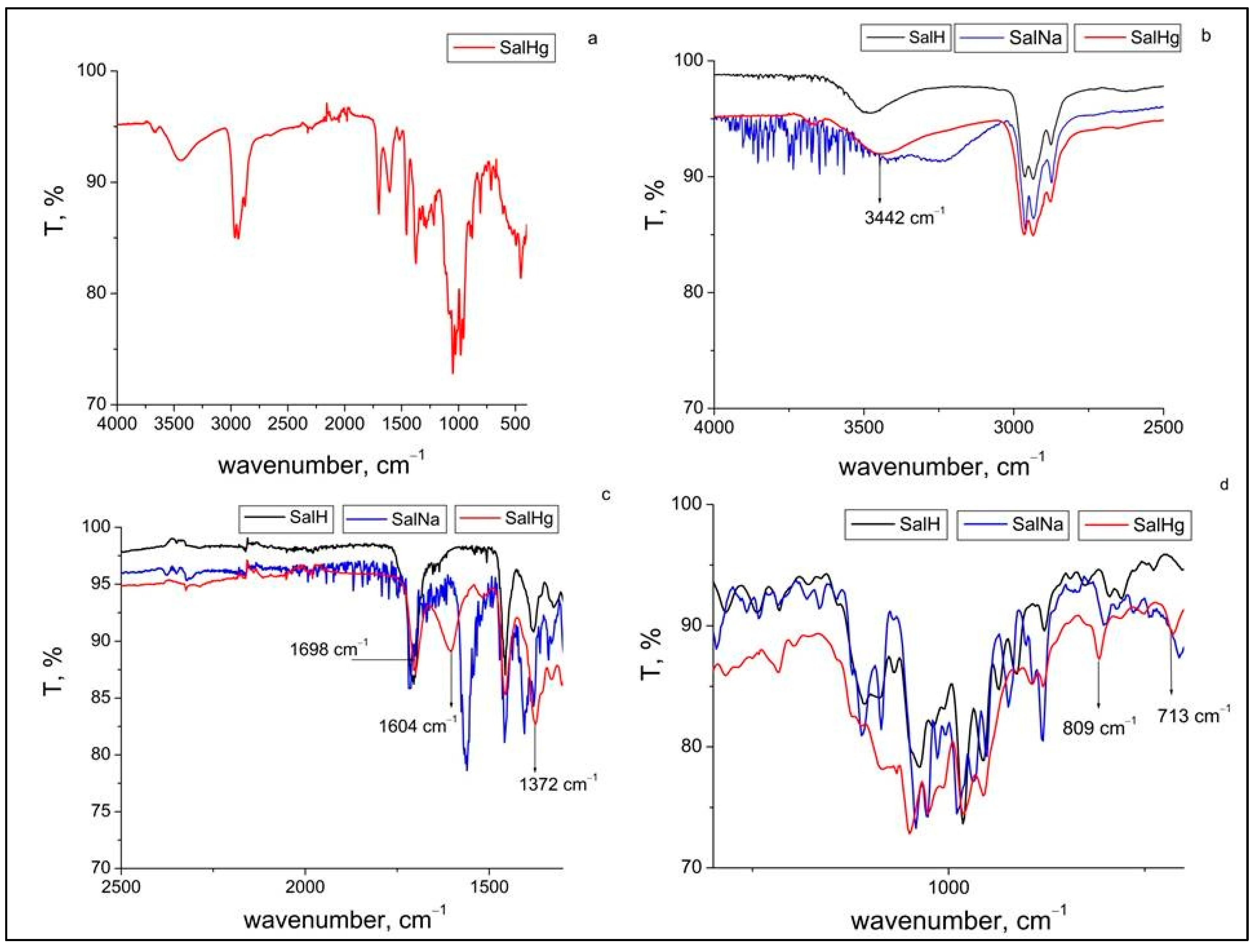
The ATR-FTIR spectrum of the new complex compound is presented in Figure 2a. Figure 2b depicts a comparison of the ATR-FTIR spectrum of SalHg to the ATR-FTIR spectra of salinomycin and salinomycin sodium in the region from 4000 to 2500 cm−1. The signal observed at 3666 cm−1 in the ATR-FTIR spectrum of SalHg (Figure 2a) could be attributed to the stretching vibration of a free (not involved in hydrogen bonds) hydroxyl group [31]. The band at 3442 cm−1, assigned to the stretching vibration of the hydroxyl groups in the ATR-FTIR spectrum of SalHg, was shifted to a higher wavenumber compared to the spectrum of SalNa (blue line) and a lower wavenumber compared to the spectrum of salinomycinic acid (black line). It should be noted that the band for the stretching vibrations of the hydroxyl groups in the ATR-FTIR spectrum of SalHg was broader compared to the same band in the spectrum of SalH and could be associated with a coordinated water molecule. The band of the stretching vibration of the hydroxyl groups in the FTIR spectra of other metal complexes of salinomycin was registered at 3450 cm−1 [20,21,22]. This result is close to the wavenumber of the stretch of the hydroxyl groups, observed in the ATR-FTIR spectrum of the complex of salinomycin with Hg(II), and proves the participation of the hydroxyl group in the complexation of the ligand with the metal ion. Figure 2c demonstrates a comparison of the ATR-FTIR spectrum of SalHg to the ATR-FTIR spectra of salinomycin (SalH) and salinomycin sodium for the carbonyl and carboxyl group region. In the ATR-FTIR spectrum of SalNa, the band for the stretching vibration of the carbonyl group was registered at the highest wavenumber (1716 cm−1) compared to the spectrum of SalH (1702 cm−1) and SalHg (1698 cm−1). The small shift of this band (about 4 cm−1) in the spectrum of SalHg compared to the spectrum of SalH could be attributed to the participation of this group in hydrogen bonding. This observation corresponds well to the FTIR data of other metal (II) complexes of salinomycin [20,21,22] and confirms that the carbonyl group is not coordinated to Hg(II). The asymmetric and symmetric stretching vibrations of the carboxylate anion in the spectrum of SalHg were registered at 1604 and 1373 cm−1, respectively. Related to the ATR-FTIR spectrum of salinomycin sodium, the difference between both stretching vibrations of the carboxylate anion for SalHg was higher (231 cm−1 for SalHg versus 150 cm−1 for salinomycin sodium). Data from previous studies revealed that there are four possible binding modes of the carboxylate anion to a metal center: monodentate, chelate (bidentate), bridged, and ionic. It has been reported that the difference (Δ) between the wavenumbers of the asymmetric and symmetric stretch of the carboxylate anion could be useful for the determination of its coordination type in the metal complexes [32,33,34,35,36,37]. It has been documented that the large splitting of the stretching vibrations of the carboxylate ion (Δ > 200 cm−1) could be assigned to its monodentate coordination to the metal center [32,35,36]. Other studies supported by crystallographic data, however, demonstrated that lower values of the difference (Δ from 140 to 150 cm−1) between both stretching vibrations of the carboxylate ion can also be observed in a monodentate coordination mode of this ion to a divalent metal center [23,38]. Obviously, the splitting of the asymmetric and symmetric stretches of the monodentate-bound carboxylate ion varies in a broad range from ~140 to higher than 200 cm−1 [23,35,36,37]. The bidentate coordination mode of the carboxylate ion to a metal center has been associated with differences between the asymmetric and symmetric stretching vibrations lower than 100 cm−1 [32]. The reduced separation between both vibrations was explained by the equal contribution of both oxygens to the binding of the carboxylate ion [35]. In some cases, when the lengths of the bonds between the metal ion and carboxylate oxygens are different, the bidentate coordination mode could be associated with a large splitting of the asymmetric and symmetric stretching vibrations of the carboxylate group [37,38]. Other factors could also affect the separation of both stretching vibrations of the carboxylate group [35,37]. The observed value of 231 cm−1 in the ATR-FTIR spectrum of SalHg could be attributed to a monodentate binding mode of the carboxylate anion to Hg(II), but a bidentate coordination mode with different lengths of the bonds between mercury and carboxylate oxygens could not be completely excluded.
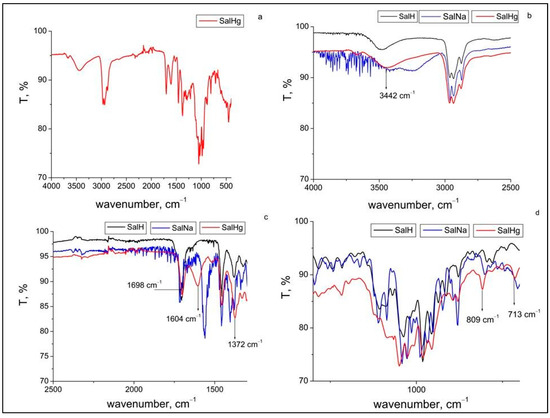
Figure 2.
ATR-FTIR spectrum of Hg(II) salinomycinate (SalHg) (a); comparison of ATR-FTIR spectra of salinomycin (SalH), SalNa, and SalHg from 4000 to 2500 cm−1 (b); comparison of ATR-FTIR spectra of SalH, SalNa, and SalHg (2500–1000 cm−1) (c); comparison of ATR-FTIR spectra of SalH, SalNa, and SalHg (fingerprint region) (d).
Several differences in the fingerprint region of the ATR-FTIR spectrum of the SalHg complex compared to the spectra of SalNa and SalH were observed (Figure 2d). The bands at 809 cm−1 and 713 cm−1 in the ATR-FTIR spectrum of SalHg were assigned to ρr(H2O) and ρw (H2O) and are indicative of a coordinated water molecule. The binding of the ligand to the metal center was also confirmed by a band at 450 cm−1 assigned to the stretching vibration of the Hg-O bond (Figure 2a).
2.3. ESI-MS Analysis
The ESI-MS spectra of SalHg, SalNa, and SalH were recorded in methanol. The mass spectral data were interpreted according to the published guidelines [39]. The peaks at 750.53 m/z and 773.39 m/z in the ESI-MS spectrum of SalHg were assigned to [C42H71O11]+ (calcd., m/z: 750.49 (100%); 751.49 (45.4%)) and [C42H70O11Na]+ (calcd. 773.48 (100%); 774.48 (45.4%)), respectively (Supplementary Information, Figure S1). Peaks at 986.09 m/z and 1985.74 m/z, which were attributed to [Hg(C42H69O11)(OH)NH4]+, (calcd., m/z (986.49 (100.0%), 984.48 (77.4%), 983.48 (56.5%), 987.49 (45.4%), 985.49 (44.1%)) and [Hg2(C42H68O11)2(CH3OH)2Na]+ (calcd. m/z, 1985.93 (100.0%), 1986.93 (93.0%), 1984.93 (73.0%), 1985.93 (67.9%), 1987.93 (64.6%), 1988.93 (60.1%), 1982.93 (56.5%), 1983.93 (52.6%), 1983.93 (43.2%), 1987.94 (42.8%), 1984.93 (40.1%)), were also detected (Supplementary Information, Figure S2). The last two peaks were not observed in the ESI-MS spectra of SalH and salinomycin sodium, which further confirmed the coordination of the ligand to the metal center. It should be noted that dimerization is not an unusual process in a gas phase, and dimeric ionic fragments were observed both in FAB-MS [38] and ESI-MS [39] spectra of coordination and organometallic compounds.
2.4. Powder X-Ray Diffraction
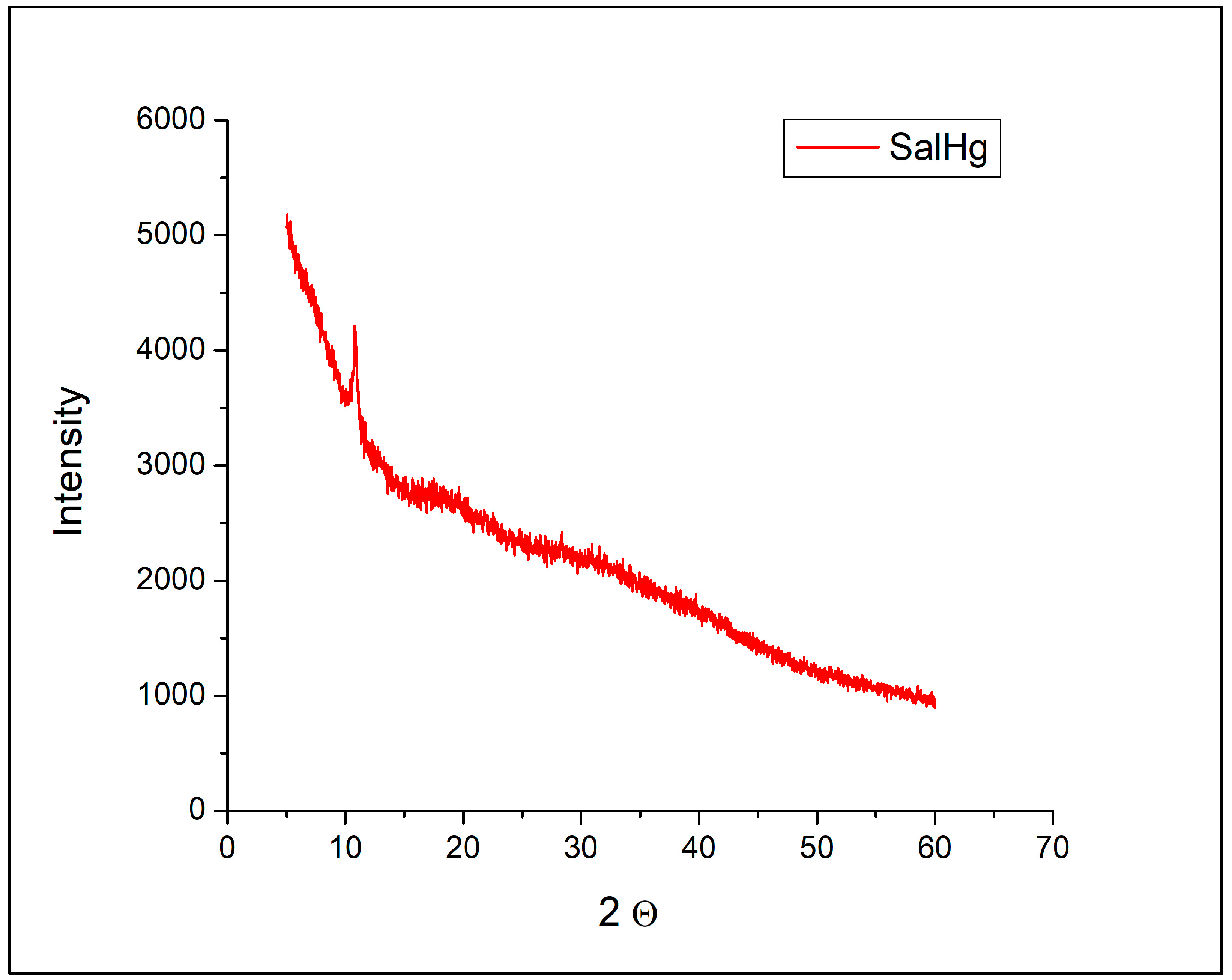
Powder diffractograms of SalH and SalNa were reported in our previous study [40]. The diffraction pattern of SalH was presented by two broad peaks at 2Θ = 8.8° and 14.2°. The diffractogram of SalNa consisted of many sharp and intense signals [40].
No peaks that could be assigned to SalH or SalNa were observed in the diffractogram of SalHg (Figure 3). The diffraction pattern of the complex consisted of only one sharp peak at 2Θ = 10.81°.
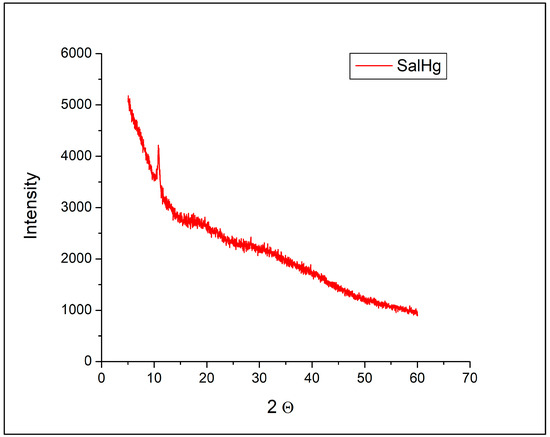
Figure 3.
Powder X-ray diffractogram of SalHg.
According to published data, the powder X-ray diffractogram of crystalline sodium nitrate was characterized by many sharp and intense peaks in the region from 30° to 50° [41]. Such signals were not detected in the diffractogram of SalHg (Figure 3). Figure 3 revealed that the isolated compound did not contain measurable amounts of sodium nitrate, SalH, or SalNa.
2.5. NMR Studies
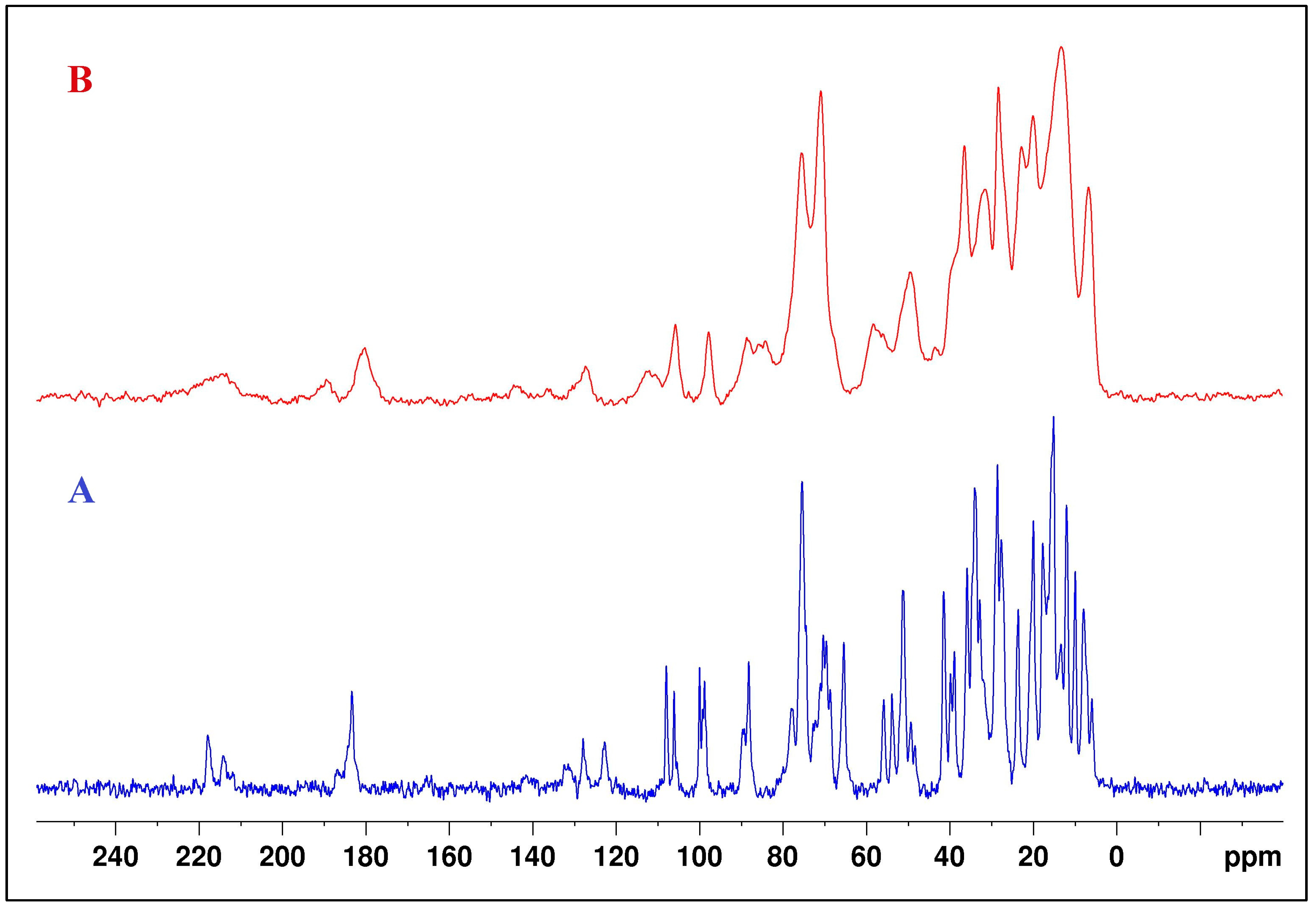
NMR 13C spectra of the compounds in CDCl3 are presented in Table S1, Supplementary Information. The assignment of the peaks for SalH and SalNa was conducted based on the published data for the 13C NMR spectra of both compounds in CDCl3 [42,43]. There was good agreement between our results and the published values for the shifts of the carbon atoms. It was interesting to observe that the peaks assigned to C-1, C-17, C-18, C-19, and C-21 carbon atoms were not registered in the NMR spectrum of SalHg. The peaks, marked with an asterisk in Table 1, were of very low intensity. The absence of peaks in the 13C NMR spectra of SalHg could be attributed to the insufficient solubility of the compound in CDCl3. Small alterations in the chemical shifts for the other carbon atoms in the isolated compound compared to the NMR 13C spectra of salinomycin and salinomycin sodium were observed. Most likely, these changes are related to different conformations of the ligand in the structure of Hg(II) salinomycinate.
Solid-state NMR 13C spectra of SalNa and SalHg were also recorded. The 13C CPMAS NMR spectra are presented in Figure 4. The results for the assigned peaks in 13C CPMAS NMR spectra of both compounds are given in Table S2, Supplementary Information. Literature data for the peaks in the 13C CPMAS NMR spectrum of SalH were also presented. Due to the amorphous character of the SalHg, many of the carbon signals overlapped and appeared as broad peaks in the 13C CPMAS NMR spectrum. However, the peaks for the carboxyl and carbonyl C atoms were clearly established. The signal for the carbon atom of the carboxyl group in the 13C CPMAS NMR spectrum of SalHg had a higher frequency than the signal for the corresponding atom in the SalH spectrum [21] and a lower frequency compared to the same signal in the SalNa spectrum.
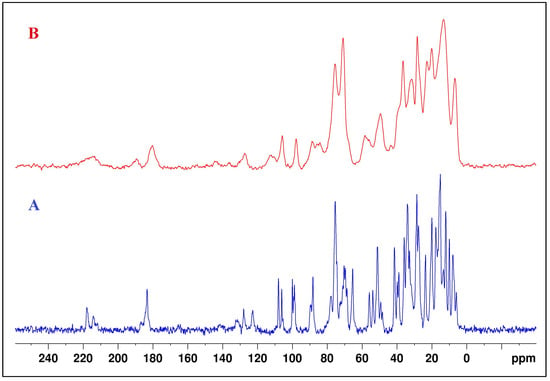
Figure 4.
The 13C CPMAS NMR spectra of SalNa (A) and SalHg (B).
2.6. TG-DTA and TG-MS Analysis
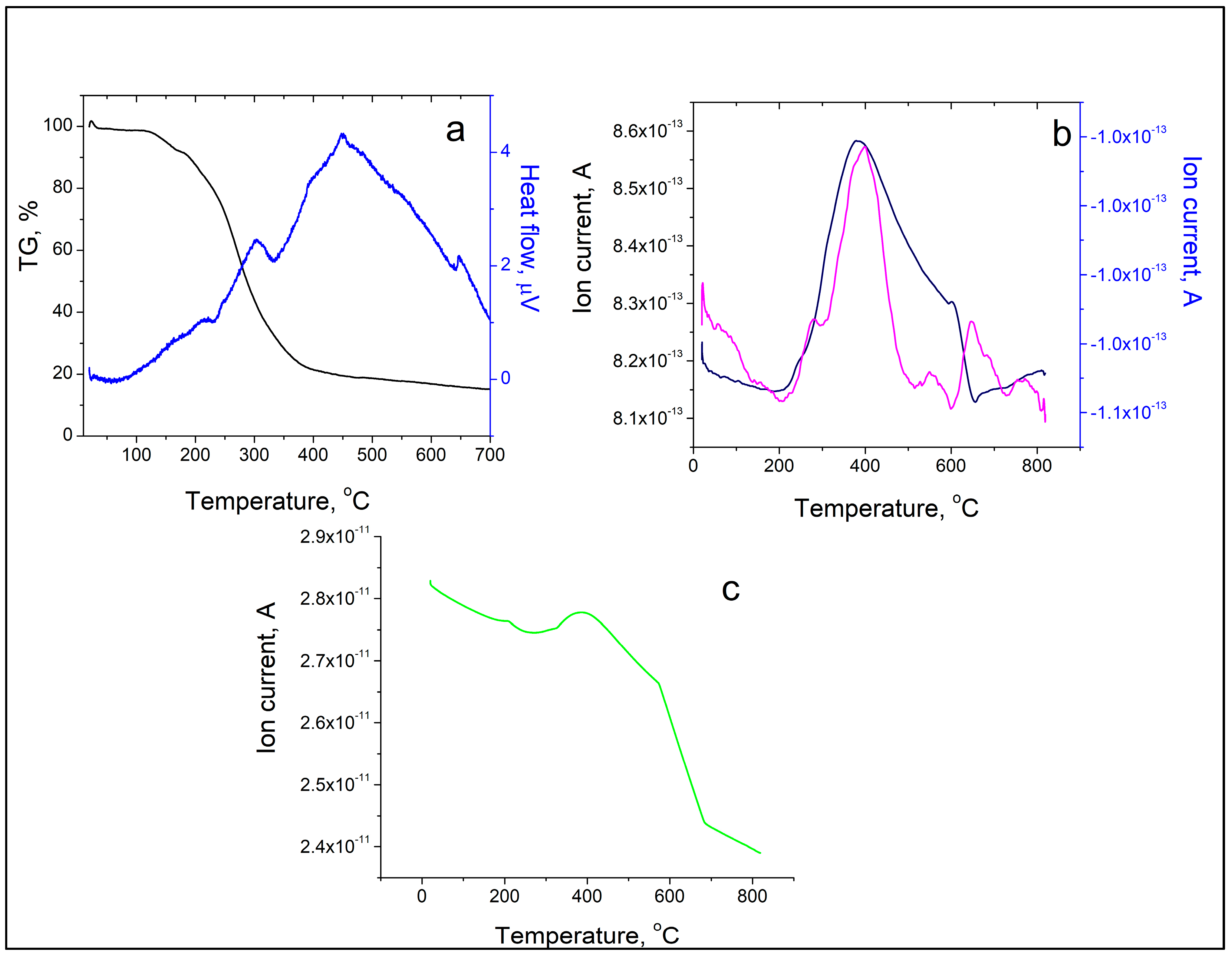
The thermal decomposition of the new metal complex started at 170 °C and was accompanied by three endothermic peaks (at 232.72, 332.74, and 642.49 °C) and one exothermic event at 448.95 °C (Figure 5a). The results from TG-MS of SalHg are presented in Figure 5b,c. The N2 peak with a maximum at 400 °C is due to the presence of a small amount of nitrogen-containing impurities in the complex compound (Figure 5c). Compared to other metal complexes of salinomycin, the thermal stability of SalHg was either similar [22] or higher [44]. It should be noted that the thermal decomposition of another metal complex of salinomycin with a divalent metal ion ([Mn(C42H69O11)2(H2O)2]) was accompanied by two peaks for CO2 release at 220 °C and 460 °C, respectively (unpublished results). In our study, the maximum CO2 loss was observed at 400 °C. The observed differences in the TG-MS spectrum of SalHg compared to the TG-MS of [Mn(C42H69O11)2(H2O)2] could be attributed to the different molecular geometry of both complexes. TG-MS studies confirmed that no lattice water was present in SalHg (Figure 5b).
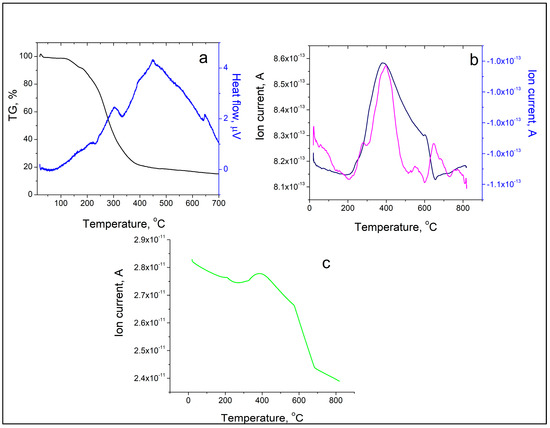
Figure 5.
TG/DTA/MS analysis of SalHg. (a) TG curve (black line), DTA curve (blue line). (b) CO2 release (pink line), water release (dark blue). (c) N2 release (green line).
2.7. Proposed Structure of SalHg
The structure of salinomycin sodium has been refined in a solid state by single-crystal X-ray analysis and in solution by NMR spectroscopy [45]. It has been proven that in a solid state, salinomycin sodium exists as two conformers with distorted trigonal and distorted tetragonal bipyramid molecular geometries, respectively [45]. In both conformers, the organic ligand is coordinated to the metal center in a tetradentate coordination mode by carboxyl, carbonyl, and two ether oxygen atoms (corresponding to oxygen numbers 2, 5, 9, and 10 in Figure 1) [45]. The coordination of one and two water molecules in both conformers, correspondingly, has also been reported. The NMR structure in CDCl3 has been found to be similar to the solid-state structure of both conformers. Therefore, in our study, we selected this solvent to explore, by NMR spectroscopy, the structure of our new compound in solution. In the NMR spectra of metal complexes of salinomycin with divalent toxic metal ions, recorded in acetonitrile-d3, the signals of the carboxyl and carbonyl carbon atoms have been registered at a higher frequency compared to the same signals in the NMR spectrum of salinomycinic acid and at a lower frequency compared to the NMR spectrum of salinomycin sodium, respectively [24]. The alteration of the chemical shift for the carboxyl carbon atom in the NMR spectra of the metal complexes compared to the NMR spectrum of salinomycin sodium has been related to the coordination of the carboxylate ion to the divalent metal center [24]. The lower chemical shift for the carbonyl carbon atom in the NMR spectra of the metal complexes of salinomycin compared to the NMR spectrum of salinomycin sodium has been associated with the breaking of the chemical bond between the sodium ion and the carbonyl oxygen atom [24]. In the 13C CPMAS NMR spectrum of SalHg, the signal for the carboxyl atom was observed at a lower frequency compared to the NMR spectrum of salinomycin sodium. Related to the CPMAS NMR spectrum of SalH [21], in the 13C CPMAS NMR spectrum of SalHg, a shift (Δ = 2.6 pm) of the signal of the carboxyl carbon atom to a higher frequency was registered (Table S2, Supplementary Information) [21]. This result is in good agreement with the results from the ATR-FTIR analysis and confirms the coordination of the carboxylate ion to the metal center. It should be noted that the band assigned to the stretching vibration of the carbonyl group in the spectrum of SalHg was not significantly shifted compared to the ATR-FTIR spectrum of salinomycin. Therefore, it could be concluded that this group did not participate in the binding of the ligand to Hg(II).
To date, there is only one published X-ray structure of a homonuclear Hg(II) complex of a polyether ionophorous antibiotic [38]. It has been demonstrated that the polyether ionophorous antibiotic monensin forms with Hg(II), a homonuclear metal complex of the composition [Hg(C36H60O11)(H2O)] [38]. Similar to salinomycin, the structure of monensin consists of one carboxyl group, hydroxyl groups, and ether oxygen atoms. The crystallographic data confirmed that monensin was coordinated in a tetradentate coordination mode to Hg(II) by a bidentately bound carboxylate ion, a deprotonated secondary hydroxyl group, and a primary hydroxyl group. The ether oxygen atoms were not involved in the binding of the ligand to the metal center. The study proved the coordination of one water molecule to the divalent metal center and its location in the cavity of the organic ligand.
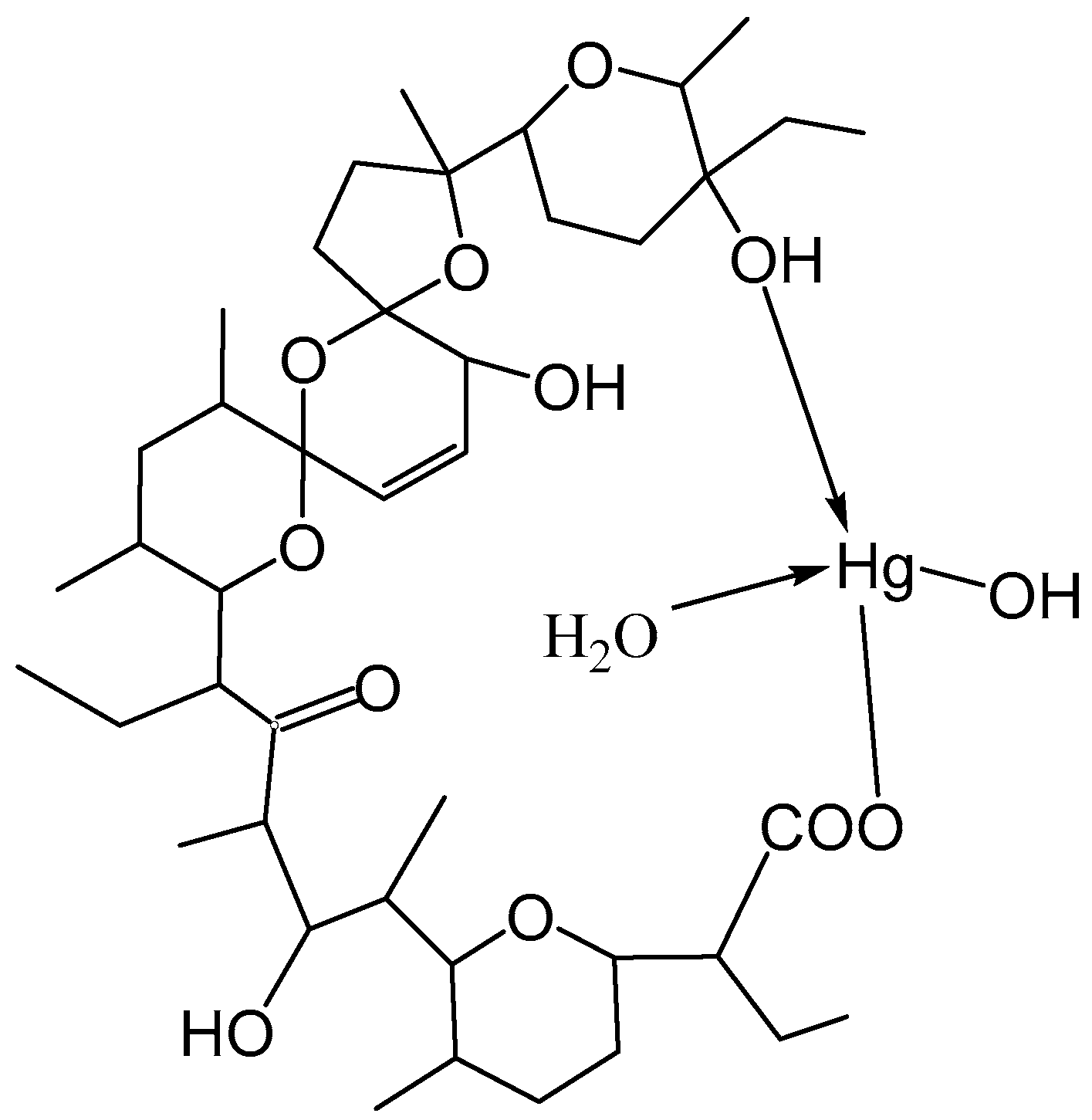
Based on all the results in this study and the published crystallographic data for the structure of [Hg(C36H60O11)(H2O)], it could be suggested that salinomycin is coordinated to Hg(II) by a terminal deprotonated carboxylate group and a terminal hydroxyl group (Figure 6). The neutrality of the complex was achieved by the binding of the hydroxide anion to the metal center. Most likely, the coordination of one water molecule to Hg(II) stabilized the structure of the complex by intramolecular hydrogen bonding with the organic ligand. Coordination of the terminal hydroxyl group of salinomycin to divalent metal ions has also been reported for other metal complexes of salinomycin [20,21,22,24].
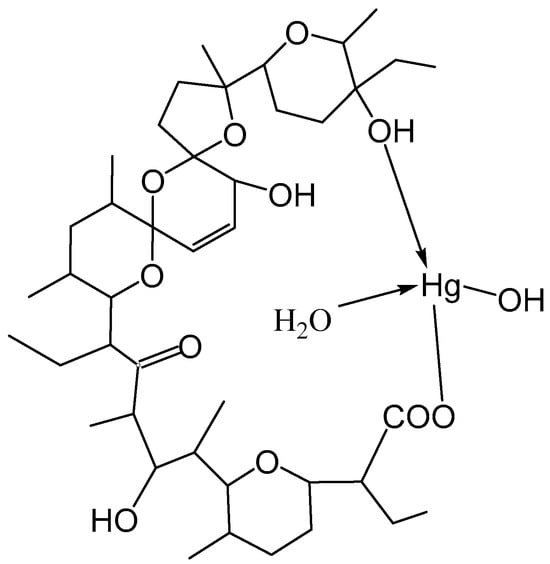
Figure 6.
Proposed structure of [Hg(C42H69O11)(H2O)(OH)].
3. Materials and Methods
3.1. Chemicals
Salinomycin sodium (C42H69O11Na; SalNa) with high purity (>95%) was gifted by Biovet Ltd. (Peshtera, Bulgaria). Organic solvents (CH3CN, CH3OH, DMSO) and Hg(NO3)2 of analytical grade were supplied by Fisher Scientific (Loughborough, UK).
Synthesis of Complex of Salinomycin with Hg(II)
To synthesize the Hg(II)–salinomycin complex, 0.1041 g of Hg(II) nitrate (0.3 mmol) was dissolved in water (3 mL). A solution of salinomycin sodium was prepared by dissolving 0.1150 g (0.15 mmol) of the compound in 11 mL mixed solvent (CH3CN:CH3OH = 1:10). The aqueous solution of Hg(II) nitrate (C = 0.1 M) was added dropwise to the solution of the ligand. The reactants were stirred at ambient temperature for 30 min. The solvents were evaporated, and the solid phase was isolated by filtration. The pale yellow precipitate was washed with water and dried over P2O5. Yield: 105 mg, 68%. The complex is sparingly soluble in water and partially soluble in organic solvents (DMSO, CHCl3, CH5CN, CH3OH).
3.2. Methods
3.2.1. Elemental Analysis
Elemental analysis was conducted at Mikroanalytisches Laboratorium of the University of Vienna, as described in detail [34]. Briefly, an EA 3000 CHNS-O instrument (Eurovector Srl, Pavia, Italy) was used for C/H/N/S-analysis. Oxygen determination was performed with a high-temperature pyrolysis oven HT 1500 (Fa. Hekatech, Wegberg, Germany). Two duplicate analyses run on two different days confirmed the results. The reproducibility of the results was in line with typical method uncertainty. The elemental analysis was performed according to the guidelines of Kandioller et al. 2021 [46].
To map the content of heavier elements, one equivalent of the sample was characterized by XRF analysis. An Epsilon 1.0 instrument (Malvern Panalytical, Malvern, UK) was equipped with an Ag-X-ray tube. No significant content of any element with Z > 12 (Mg), except mercury, was detected. The quantitative evaluation was performed using the instrument software Epsilon 3. The algorithm applied used the empirical organic composition as determined by elemental micro-analysis to fit the signals. As we had no specific calibration, the uncertainty of the resulting Hg content was estimated to be ±2.5 wt-%.
As the total elements determined added up to a total of about 96 wt-% and minor nitrogen content was determined with high reproducibility, we prepared two aqueous extracts of the material that were filtered by a 0.4 µm membrane filter and characterized by capillary ion electrophoresis. The CE 7100 (Agilent Technologies, Santa Clara, CA, USA) was equipped with a TraceDec contactless conductivity detector (Innovative Sensor Technologies, GmbH, Strasshof, Austria). Anions and cations were both determined using the same buffer solution (30 mM N-morpholino-ethane-sulphonic acid/30 mM L-cystine, pH = 6.0) in different analytical runs. The absence of Na, K, Ca, Mg, and ammonium was proven at a level of 0.05 wt-%. Also, no small anions, such as chloride, nitrate, sulphate, or trifluoroacetate, were detected at levels above 0.1 wt-%.
The sample solutions prepared for ion analysis contained 25 to 30 µM.L−1 of the target compound. Thus, it is expected that any sodium salt or complex dissociates to release Na+(aq) with a high yield. Trifluoroacetate is known to form rather stable ion pairs. However, it can be detected with a yield of 100 ± 2% under those conditions.
3.2.2. Attenuated Total Reflectance–Fourier Transform Spectroscopy
ATR-FTIR spectral analyses were conducted on an IRAffinity-1 spectrophotometer (Shimadzu Co., Kyoto, Japan).
3.2.3. Electrospray Ionization–Mass Spectrometry (ESI-MS)
ESI-MS analyses were conducted on a Waters Micromass ZQ2000 Single Quadrupole mass spectrometer (Waters, Milford, MA, USA), positive mode, in the range of 0–2000 m/z.
3.2.4. Powder X-Ray Diffraction (XRD)
A PANalytical Empyrean X-ray diffractometer (Malvern Panalytical, Malvern, UK) with CuKα radiation (λ = 0.15418 nm), functioning at 40 kV, 30 mA, was used for powder X-ray analysis of the new complex. The measurement conditions were the same as those published in our previous study [40]. The limit of quantification of the method for crystalline solids was 1%.
3.2.5. Nuclear Magnetic Spectroscopy
The 1H and 13C NMR spectra of SalH, SalNa, and SalHg in CDCl3 were recorded on a Bruker Avance III HD spectrometer (Billerica, MA, USA) equipped with a double resonance broadband probe-head with working frequencies of 500.13 MHz for the proton and 125.8 MHz for 13C and a temperature of 298.0 ± 0.1K. A 2.5 mm CP/MAS cross-polarization probe-head with double resonance 1H/X was used to record the solid-state NMR spectra. α-glycine was used as an external reference. The chemical shift for the carboxyl carbon atom signal was set to 176.0 ppm.
3.2.6. Thermogravimetric Analysis with Differential Thermal Analysis (TG-DTA) and Mass Spectrometry Thermogravimetric Measurements (TG−MS)
TG-DTA and TG-MS analyses were performed on a Setaram Labsys Evo 1600 (25–600 °C) (Caluire-et-Cuire, France), with a heating rate of 10 K/min in an argon atmosphere. The detection of water and CO2 was performed with the Omnistar GSD 301 O2 mass spectrometer, Pfeiffer Vacuum (Gottingen, Germany).
4. Conclusions
In this study, we reported, for the first time, a synthetic procedure for a new complex of salinomycin with Hg(II). The compound was characterized by a variety of methods. Based on the spectroscopic data and elemental analysis data, it could be proposed that the ligand is coordinated with Hg(II), a deprotonated carboxyl group, and a terminal hydroxyl group. The hydroxide ion, bound to Hg(II), secured the neutrality of the complex. Further studies are needed to elucidate the potential of salinomycin as an antidote for the treatment of Hg(II) poisoning.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13070220/s1, Figure S1: ESI-MS spectrum of SalHg from 670 to 915 m/z; Figure S2: ESI-MS of SalHg from 880 to 2000 m/z; Table S1: 13C NMR spectral data of SalH, SalNa, SalHg. Spectra were recorded in CDCl3; Table S2: 13C CPMAS NMR spectral data for SalH, SalNa, and SalHg.
Author Contributions
Conceptualization, J.I.; Data curation, J.T. and N.B.; Formal analysis, J.I., I.P.-M., J.T., N.B. and I.G.; Funding acquisition, I.G.; Investigation, J.I., I.P.-M., J.T. and N.B.; Methodology, J.I., J.T. and N.B.; Project administration, I.G.; Resources, P.D. and I.G.; Validation, J.I., J.T. and N.B.; Visualization, J.I. and N.B.; Writing—original draft, J.I.; Writing—review and editing, J.I., I.P.-M., J.T., N.B., P.D. and I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no. BG-RRP-2.004-0008-C01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or in the Supplementary Materials.
Acknowledgments
The authors thank Vladimir Gelev and Nicol Georgieva from the Faculty of Chemistry and Pharmacy, Sofia University “St. Kliment Ohridski”, and the service staff from the Mass Spectrometry Centre (MSC) of the University of Vienna for the excellent assistance with ESI-MS analysis. The assistance of Georgy Avdeev and Elzhana Encheva from the Institute of Physical Chemistry, BAS, with powder X-ray diffraction analyses is acknowledged. The authors thank Mary McAllister for her work in reviewing and editing the manuscript.
Conflicts of Interest
The author Peter Dorkov was employed by BIOVET JSC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. BIOVET JSC provided the material salinomycin sodium (SalNa) for this research. However, the company was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
References
- Miyazaki, Y.; Shibuya, M.; Sugawara, H.; Kawaguchi, O.; Hirose, C.; Nagatsu, J.; Esumi, S. Salinomycin, a new polyether antibiotic. J. Antibiot. 1974, 27, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Westley, J.W. Polyether antibiotics: Versatile carboxylic acid ionophores produced by Streptomyces. Adv. Appl. Microbiol. 1977, 22, 177–223. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wong, E.T.; Fonkem, E.; Hsieh, T.C.; Wu, J.M.; Wu, E. Salinomycin: A novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 2013, 20, 4095–4101. [Google Scholar] [CrossRef]
- Antoszczak, M.; Steverding, D.; Huczyński, A. Anti-parasitic activity of polyether ionophores. Eur. J. Med. Chem. 2019, 166, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Shin, J.S.; Yoon, Y.S.; Go, Y.Y.; Lee, H.W.; Kwon, O.S.; Park, S.; Park, M.S.; Kim, M. Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function. J. Virol. 2018, 92, e01441-18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Huang, X.; Zhai, R.; Ma, Y.; Xu, A.; Zhang, P.; Yang, Q. In Vitro Antiviral Activities of Salinomycin on Porcine Epidemic Diarrhea Virus. Viruses 2021, 13, 580. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Chen, S.; Lai, R.; Zheng, C.; Lu, J.; Jiang, X.; He, F.; Yang, C.; Li, K.; et al. Salinomycin alleviates osteoarthritis progression via inhibiting Wnt/β-catenin signaling. Int. Immunopharmacol. 2022, 112, 109225. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Kolar, B.; et al. Safety and efficacy of Sacox® microGranulate (salinomycin sodium) for chickens for fattening and chickens reared for laying. EFSA J. 2017, 15, e04670. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores-promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef]
- Naujokat, C.; Steinhart, R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol. 2012, 2012, 950658. [Google Scholar] [CrossRef]
- Antoszczak, M.; Huczyński, A. Anticancer Activity of Polyether Ionophore-Salinomycin. Anticancer Agents Med. Chem. 2015, 15, 575–591. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Kaysiewicz, J.; Markowska, J.; Huczyński, A. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg. Med. Chem. Lett. 2019, 29, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumour Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Qaed, E.; Zhang, J.; Song, Y.; Wu, R.; Bu, X.; Wang, Q.; Tang, Z. Salinomycin, as an autophagy modulator-- a new avenue to anticancer: A review. J. Exp. Clin. Cancer Res. 2018, 37, 26. [Google Scholar] [CrossRef]
- Antoszczak, M. A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur. J. Med. Chem. 2019, 166, 48–64. [Google Scholar] [CrossRef]
- Magrath, J.W.; Kim, Y. Salinomycin’s potential to eliminate glioblastoma stem cells and treat glioblastoma multiforme (Review). Int. J. Oncol. 2017, 51, 753–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tefas, L.R.; Barbălată, C.; Tefas, C.; Tomuță, I. Salinomycin-Based Drug Delivery Systems: Overcoming the Hurdles in Cancer Therapy. Pharmaceutics 2021, 13, 1120. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Zhorova, R.; Momekov, G.; Simova, S.; Stoyanova, R.; Zhecheva, E.; Ivanova, S.; Mitewa, M. Synthesis, spectral properties, antibacterial and antitumor activity of salinomycin complexes with Co(II), Ni(II), Cu(II) and Zn(II) transition metal ions. J. Chem. Chem. Eng. 2012, 6, 551–562. [Google Scholar]
- Pantcheva, I.; Petkov, N.; Simova, S.; Zhorova, R.; Dorkov, P. 4 Alkaline-earth metal(II) complexes of salinomycin—Spectral properties and antibacterial activity. In Biochemical and Environmental Applications; Ramasami, P., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022; Volume 2, pp. 65–78. [Google Scholar] [CrossRef]
- Pashkunova-Martic, I.; Kukeva, R.; Stoyanova, R.; Pantcheva, I.; Dorkov, P.; Friske, J.; Hejl, M.; Jakupec, M.; Hohagen, M.; Legin, A.; et al. Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties. Pharmaceutics 2022, 14, 2319. [Google Scholar] [CrossRef]
- Pantcheva, I.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W. First solid state alkaline-earth complexes of monensic acid A (MonH): Crystal structure of [M(Mon)2(H2O)2] (M = Mg, Ca), spectral properties and cytotoxicity against aerobic Gram-positive bacteria. Biometals 2010, 3, 59–70. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Mitewa, M.; Simova, S.; Tanabe, M.; Osakada, K. Cd(II) and Pb(II) complexes of the polyether ionophorous antibiotic salinomycin. Chem. Cent. J. 2011, 5, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanova, J.; Petrova, E.; Kamenova, K.; Gluhcheva, Y. Comparative effects of meso-2,3-dimercaptosuccinic acid, monensin, and salinomycin on cadmium-induced brain dysfunction in cadmium-intoxicated mice. Interdiscip. Toxicol. 2017, 10, 107–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanova, J.; Kamenova, K.; Petrova, E.; Vladov, I.; Gluhcheva, Y.; Dorkov, P. Comparative study on the effects of salinomycin, monensin and meso-2,3-dimercaptosuccinic acid on the concentrations of lead, calcium, copper, iron and zinc in lungs and heart in lead-exposed mice. J. Trace Elements Med. Biol. 2019, 58, 126429. [Google Scholar] [CrossRef]
- Gluhcheva, Y.; Pashkunova-Martic, I.; Schaier, M.; Vladov, I.; Stoykova, S.; Petrova, E.; Pavlova, E.; Dorkov, P.; Helbich, T.H.; Keppler, B.; et al. Comparative effects of deferiprone and salinomycin on lead-induced disturbance in the homeostasis of intrarenal essential elements in mice. Int. J. Mol. Sci. 2022, 23, 4368. [Google Scholar] [CrossRef] [PubMed]
- Ugrina, M.; Čeru, T.; Nuić, I.; Trgo, M. Comparative Study of Mercury(II) Removal from Aqueous Solutions onto Natural and Iron-Modified Clinoptilolite Rich Zeolite. Processes 2020, 8, 1523. [Google Scholar] [CrossRef]
- Hietanen, S.; Sillen, L.G. Studies on the hydrolysis of metal ions. II. The hydrolysis of the mercury (II) ion Hg2+. Acta Chim. Scand. 1952, 6, 747–758. [Google Scholar] [CrossRef]
- Florez, E.; Zapata-Escobar, A.D.; Ferraro, F.; Becerra, C.I.; Chamorro, Y.; Maldonado, A.F. Coordination of Mercury(II) in Water Promoted over Hydrolysis in Solvated Clusters [Hg(H2O)1–6](aq)2+: Insights from Relativistic Effects and Free Energy Analysis. J. Phys. Chem. A 2023, 127, 8032–8049. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectroscopy of Inorganic and Coordination Compounds, 5th ed.; Wiley: Toronto, ON, Canada, 1997. [Google Scholar]
- Nara, M.; Tanokura, M. Infrared spectroscopic study of the metal-coordination structures of calcium-binding proteins. Biochem. Biophys. Res. Commun. 2008, 369, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.C.; da Silva, G.; Franks, G.V. Modeling the IR spectra of aqueous metal carboxylate complexes: Correlation between bonding geometry and stretching mode wavenumber shifts. Chemistry 2015, 21, 6801–6805. [Google Scholar] [CrossRef]
- Justi, M.; Puggina de Freitas, M.; Silla, J.M.; Nunes, C.A.; Silva, C.A. Molecular structure features and fast identification of chemical properties of metal carboxylate complexes by FTIR and partial least square regression. J. Mol. Struct. 2021, 1237, 130405. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J.; Deacon, R.; Phillips, G. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W. Crystal structures and spectral properties of new Cd(II) and Hg(II) complexes of monensic acid with different coordination modes of the ligand. Open Chem. 2010, 8, 852–860. [Google Scholar] [CrossRef]
- McIndoe, J.S.; Vikse, K.L. Assigning the ESI mass spectra of organometallic and coordination compounds. J. Mass. Spectrom. 2019, 54, 466–479. [Google Scholar] [CrossRef]
- Ivanova, J.; Kukeva, R.; Stoyanova, R.; Zhivkova, T.; Abudalleh, A.; Dyakova, L.; Alexandrova, R.; Pashkunova-Martic, I.; Theiner, J.; Dorkov, P.; et al. New Iron(III)-Containing Composite of Salinomycinic Acid with Antitumor Activity—Synthesis and Characterization. Inorganics 2024, 12, 206. [Google Scholar] [CrossRef]
- Kumar, T.V.; Chary, A.S.; Bhardwaj, S.; Awasthi, A.M.; Reddy, S.N. Dielectric Relaxation, Ionic Conduction and Complex Impedance Studies on NaNo3 Fast Ion Conductor. Int. J. Mater. Sci. Appl. 2013, 2, 173–178. [Google Scholar] [CrossRef]
- Thompsett, S.J. A Study of the Effect of Structure and Conformation on the Transport Properties of Ionophores Using NMR. Ph.D. Thesis, University of St Andrews, St Andrews, Scotland, 1992. [Google Scholar]
- Seto, H.; Miyazaki, Y.; Fujita, K.-I.; Otake, N. Studies on the ionophorous antibiotics. X. The assignments of 13C-NMR spectrum of salinomycin. Tetrahedron Lett. 1977, 28, 2417–2420. [Google Scholar] [CrossRef]
- Petkov, N.; Pantcheva, I.; Ivanova, A.; Stoyanova, R.; Kukeva, R.; Alexandrova, R.; Abudalleh, A.; Dorkov, P. Novel Cerium(IV) Coordination Compounds of Monensin and Salinomycin. Molecules 2023, 28, 4676. [Google Scholar] [CrossRef] [PubMed]
- Paulus, E.F.; Kurz, M.; Matter, H.; Vértesy, L. Solid-State and Solution Structure of the Salinomycin–Sodium Complex: Stabilization of Different Conformers for an Ionophore in Different Environments. J. Am. Chem. Soc. 1998, 120, 8209–8221. [Google Scholar] [CrossRef]
- Kandioller, W.; Theiner, J.; Keppler, B.K.; Kowol, C.R. Elemental analysis: An important purity control but prone to manipulations. Inorg. Chem. Front. 2021, 9, 412–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).