Abstract
Silver nanoparticles (AgNPs) have emerged as a promising antimicrobial agent in dentistry due to their distinctive physicochemical characteristics and broad-spectrum biocidal activity. For example, silver nanoparticles can be incorporated into oral hygiene products in preventive dentistry, composite resins in restorative treatment, irrigation solutions in endodontic treatment, membranes for guided tissue regeneration in periodontal treatment, acrylic resins and porcelains in prosthodontic treatment, coatings in dental implant treatment, and brackets and wires in orthodontic treatment. This paper focuses on summarizing the current knowledge on the antimicrobial use of silver nanoparticles in dentistry, highlighting their antimicrobial mechanism and potential applications in clinical treatment. The literature indicates that silver nanoparticles are a promising antimicrobial agent in dentistry. However, there are still many issues including fundamental antibacterial mechanisms that need to be completely elucidated before clinical applications.
1. Introduction
The human oral cavity is one of the important parts of the body because of its many significant functions and values. The oral cavity harbors many different habitants, including the teeth, gingiva, hard and soft palates, and tongue, which provide warm growth conditions and available nutrients for a number of colonized microbes [1]. The microbes found in the oral cavity have been defined as the oral microbiome, including bacteria, fungi, and viruses [1]. It has been demonstrated that microbial profiles differ significantly according to the oral sites, and the oral microbiome can therefore be seen as a group of site-specific microbial biofilms [2,3]. It is well known that there is a naturally symbiotic relationship between the oral microbe and the host in health. Alterations in the oral environmental conditions (such as temperature, PH, and nutrients) can do harm to the host microbe homoeostasis, hence leading to many infectious disorders including caries, periodontal diseases, pulp infections, peri-implantitis, and denture stomatitis [4]. Furthermore, growing evidence demonstrates that oral diseases play a significant role in the etiopathogenesis of various major chronic systemic conditions, including cardiovascular diseases, cerebrovascular accidents (strokes), rheumatoid arthritis, diabetes mellitus, and respiratory infections such as pneumonia [4]. This established relationship indicates the critical importance of implementing effective preventive measures and therapeutic interventions for oral infections, which could substantially reduce their contribution to the global disease burden [5]. The prevention and treatment of oral diseases remain significant global public health challenges, with mechanical biofilm removal continuing to be the primary and first-line intervention approach. Meanwhile, many systemic antibiotics have been used to meet this challenge because of their potential efficacy, such as penicillin, tetracyclines, metronidazole, and macrolides [6,7,8,9]. However, their widespread and repetitive use may cause subsequent infections due to their resistance or persistence, thus causing them to lose their effectiveness [10,11]. Many other antimicrobial agents have also been gradually developed and researched to target oral pathogens, including chlorhexidine, sodium hypochlorite, fluoride, and antimicrobial peptides (AMPs). However, a number of these studies showed that they failed to achieve expected results due to their drug resistance and some adverse side effects [9,12,13,14]. Collectively, it is essential to develop alternative strategies to treat dental disorders.
The era of nanotechnology has emerged as a prominent field [15]. Nanotechnology is the manipulation of nanometer-sized objects, which are referred to as nanoparticles. It has the potential to bring enormous changes to nano-dentistry. Nanoparticles are nanoscale objects with three external nanoscale dimensions and exhibit characteristics distinct from the corresponding bulk materials. Nanoparticles represent a promising strategy for enhancing oral health owing to their unique physicochemical properties, including ultra-small size and a high surface-to-volume ratio [16,17]. The positive charge plus increased surface area of nanoparticles makes them easier to interface with more negatively charged microbial membranes, and they can be either combined with polymers or coated onto biomaterial surfaces, thereby enhancing their antimicrobial activities [18,19]. Additionally, microorganisms are less likely to acquire resistance on metal nanoparticles than on traditional antibiotics [20]. Such findings interest researchers in the usage of different kinds of nanoparticles, especially silver nanoparticles.
This review focuses on the latest applications of silver nanoparticles in the dental field, including restorative dentistry, root canal therapy, prosthetic dentistry, periodontology, implantology, and orthodontics. In addition, we also discuss the safety issues and problems faced by AgNPs and their possible development directions in the future.
2. Antimicrobial Mechanism of AgNPs Acting on Bacteria
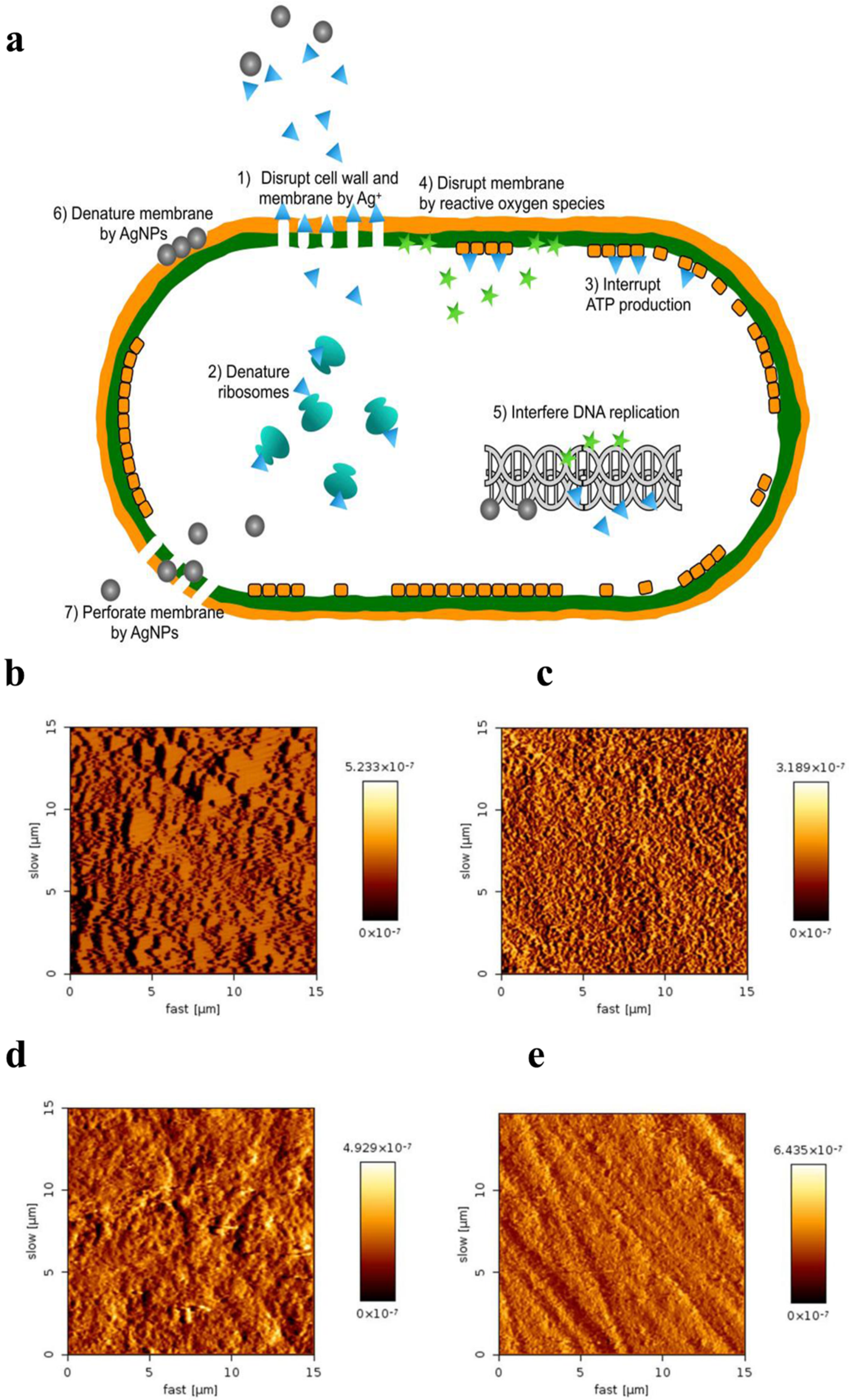
On account of their unique physical, chemical, and biological properties, AgNPs are one of the most commonly used nanoparticles in nanotechnology around the world [21]. They have been applied in diverse fields of dentistry, including restorative dentistry, root canal therapy, orthodontics, prosthetic dentistry, periodontology, and implantology. Because of their greater capacity and higher surface-area-to-volume ratio, silver ions can generate pits and perforations in the cell membrane. AgNPs have gained much attention from scientists and researchers and have shown potent antimicrobial properties [22]. As representatives of inorganic nanoparticles, AgNPs have been demonstrated in numerous studies to highly exhibit a broad spectrum of antimicrobial action on multiple Gram-positive and Gram-negative bacteria, viruses, and fungi, including all kinds of antibiotic strains [23,24,25,26]. Although their antimicrobial mechanism has been extensively investigated, the exact mode of action remains unclear. Currently, many antibacterial actions have been proposed (Figure 1a) [27,28,29]. Its antibacterial actions are mainly owed to the release of silver ions (Ag+) [30]. Free silver ions can bind to the plasma membrane and increase membrane permeability, thus causing cell leakage; silver ions can also lead to the denaturation of ribosomes and thus inhibit protein synthesis. After silver ions are uptaken into a cell, the respiratory enzymes can be deactivated, terminating adenosine triphosphate (ATP) production; Ag+ can also interrupt the electron transfer chain, hence generating reactive oxygen species (ROS) which can oxidize proteins. Silver ions and ROS can interfere with deoxyribonucleic acid, inhibiting its replication and leading to cell death.
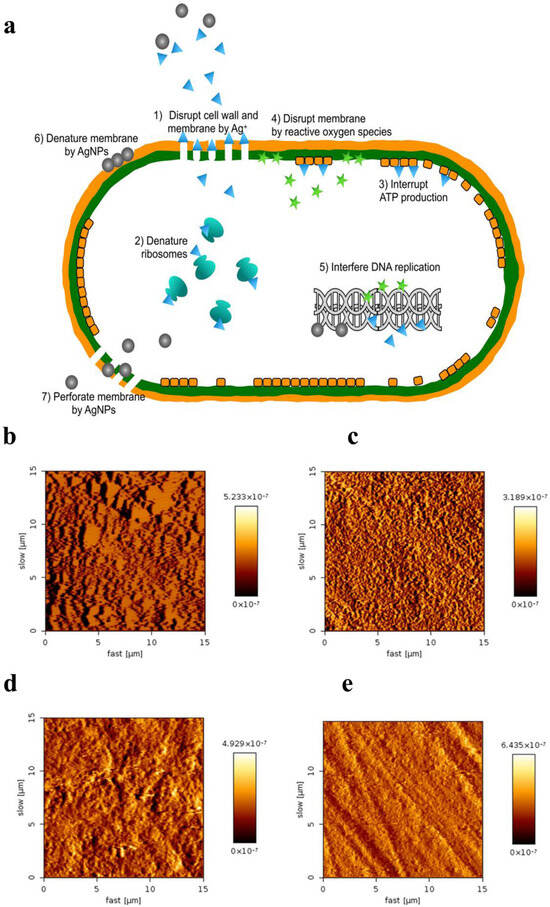
Figure 1.
Antibacterial mechanisms of AgNPs and their applications in preventive dentistry. (a) Multiple antibacterial mechanisms have been proposed for AgNPs. Reproduced with adaptation from ref. [29]. (b–e) AFM images of enamel surfaces after remineralization. Reproduced with adaptation from ref. [31]. (b) The NaF varnish group showed uniform crystals. (c) Homogenous acicular crystals were formed in n-HAP group. (d) The protective layer was observed with different sized and multi-shaped crystals in the NSF group. (e) No protective layer was observed in the control group.
3. Clinical Applications of AgNPs in Dentistry
3.1. Preventive Dentistry
Dental lesions are one of the most common disorders in the world, exerting a strong influence on 95% of the human population in various phases of their lives [32]. Different methods have been used for preventing and treating them; however, their incidence and morbidity rate remains high. With advances in nanotechnology, many studies have evaluated the antimicrobial activity of the agents containing AgNPs against cariogenic microbes, mainly against Streptococcus mutans (S. mutans) and in the prevention of dental biofilm formation, suggesting their potential as effective anticariogenic agents [33,34,35,36,37,38,39]. Thus, numerous in vitro studies [31,40,41,42,43,44,45,46,47] as well as clinical trials [48,49,50,51,52,53,54] have been carried out to evaluate the effectiveness of silver nanoparticles in preventing and arresting early-stage dental caries. A study [31] investigated the remineralization efficacy of sodium fluoride (NaF), nano-hydroxyapatite serum (n-HAP), and nano silver fluoride (NSF) using primary sound anterior teeth. Artificial incipient caries lesions created by immersing the teeth in a demineralizing solution for 72 h, followed by a 10-day pH-cycling regimen. The highest surface microhardness (SMH) values were observed in the NSF group (222.90 ± 28.79), followed sequentially by the NaF group (192.48 ± 30.96), n-HAP group (172.65 ± 21.68), and control group (114.11 ± 20.30), with statistically significant differences between groups (p < 0.05). Atomic force microscopy images showed protective layers in treated groups (Figure 1b–e). A similar study evaluated the remineralization potential of NSF for enamel though optical coherence tomography (OCT), showing that NSF is as efficient as NaF in remineralizing dental enamel [45]. In another study, to assess effectiveness of pre-treatment with NSF on secondary caries at the tooth restoration interface, forty specimens with class V cavities were prepared from 20 human premolar teeth [46]. Then, they were randomly distributed into four groups as follows: glass ionomer cement group (GIC), composite group, NSF pretreatment + GIC, and NSF pretreatment + composite, followed by a pH cycling for 14 days. The mean SMH values at enamel of NSF + GIC (287 ± 34.964) and NSF + composite (246.20 ± 17.82) were higher than GIC (230 ± 32.97) and the composite group (223.20 ± 17.96) (p < 0.05), indicating less demineralization and better surface integrity. The mean outer lesion depth at the interface was also lower in the pretreatment group (p < 0.05), showing better interface restoration integrity. A randomized controlled clinical trial was conducted to prevent untreated caries in the deciduous teeth of children from poor communities, with NSF as the treated group and saline as the control group [52]. At one week, 78% of decayed teeth in the NSF group had arrested caries, while none demonstrated this in control group. At 5 months, the NSF group showed 72.91% with arrested decay, while the control group displayed only 34%. At 12 months, in the experimental group, 65.21% of teeth exhibited arrested caries, while in the control group, only 20.88% of teeth had arrested cavities. In a similar randomized clinical trial, 130 decayed deciduous teeth were included and separated at random into two groups, with NSF as the experimental group and water as the control group [48]. On the seventh day, 81% of teeth in the experimental group displayed arrested decay, whereas none showed this in the control group (p < 0.001); At five months, the experimental group exhibited 72.7%, with 27.4% in the control group (p < 0.001). After 12 months, the NSF group showed 66.7%, while 34.7% was shown in the control group (p = 0.003). A related study demonstrated that the 600 ppm NSF solution exhibited significantly greater efficacy in arresting dental caries (72.7%; p = 0.025) compared to the 400 ppm formulation (56.5%) [54]. To determine demineralization of a pit and fissure sealant incorporating silver nanoparticles, eighty permanent molars without caries were collected in a split-mouth clinical trial [51]. After a six-month follow up, the silver nanoparticle group presented an average microleakage of 33.6%, with 30.6% in the conventional group (p = NS). The reduction in fluorescence found in the silver nanoparticle group was three times higher than the conventional group (p < 0.05), indicating that silver nanoparticles could obviously reduce dental demineralization and likely increase remineralization.
Oral hygiene products, including toothpaste, toothbrushes, and mouthwash, are other important parts of preventive dentistry [55,56,57,58,59,60,61,62]. An in vitro comparative investigation focused on the efficacy of chitosan, AgNPs, and fluorides as antimicrobial ingredients in toothpaste formulations against S. mutans strains through an agar well diffusion array [55]. It showed that AgNPs had the highest efficacy against S. mutans followed by fluoride and chitosan. Another comparative study found that toothpaste containing silver nanoparticles had a better antimicrobial effect than gold nanoparticles; however, its effectiveness is still not as good as traditional toothpaste containing zinc citrate [59]. In 2018, an experimental dentifrice containing NSF was tested in vitro to evaluate its effects on reducing the acidogenecity and adhesion of S. mutans and preventing the demineralization of enamel [56]. All the results demonstrated that the new dentifrice containing NSF had a better antibacterial effect and similar action on enamel demineralization compared to the NaF toothpaste (p < 0.05). Twenty-four healthy people participated in a study to investigate the potential changes in the oral microbiota after toothbrushing with bristles infused with silver nanoparticles or chlorhexidine through the DNA checkerboard hybridization method [60]. After four weeks of toothbrushing, the silver group lowered the total and individual genome counts in both supragingival and subgingival biofilms, while the chlorhexidine group was ineffective in decreasing total counts in the supragingival and subgingival biofilm. But chlorhexidine indeed decreased individual genome counts in supragingival biofilm for the majority of 43 microbial species, including putative periodontal pathogens. Another in vitro study developed an ethanol-free mouthwash containing silver nanoparticles, which could be used for preventing oral infections in immunocompromised patients [61]. Results showed that there was no significant difference in antibacterial effects between ethanol-free mouthwash containing AgNPs and mouthwash containing AgNPs and ethanol (30,000 ug/mL), indicating that silver nanoparticles could be an excellent alternative for ethanol in treating immunocompromised patients.
3.2. Restorative Treatment
Dental caries, a widespread oral disease, adversely affects the health and quality of life of both children and adults [63]. Currently, resin-based composites have gained increasing popularity in dental restorations mainly due to their superior esthetics, good maneuverability, and acceptable biocompatibility [64]. However, these composites are inclined to accumulate more oral microbe and bacterial colonies beside their margins, which may eventually lead to secondary caries [65]. S. mutans are recognized as the main bacteria in cariogenic biofilms responsible for secondary caries [66,67], which are an important factor affecting the life of resin composites. Therefore, to inhibit biofilm formation and prevent microleakage, numerous attempts have been carried out to incorporate antimicrobial agents into composite resins [68,69,70,71,72,73,74,75,76,77,78,79], glass ionomer cement [80], and adhesive systems [81,82,83,84,85,86,87,88,89,90,91,92]. Among them, AgNPs have been extensively investigated as promising antimicrobial agents in dental materials.
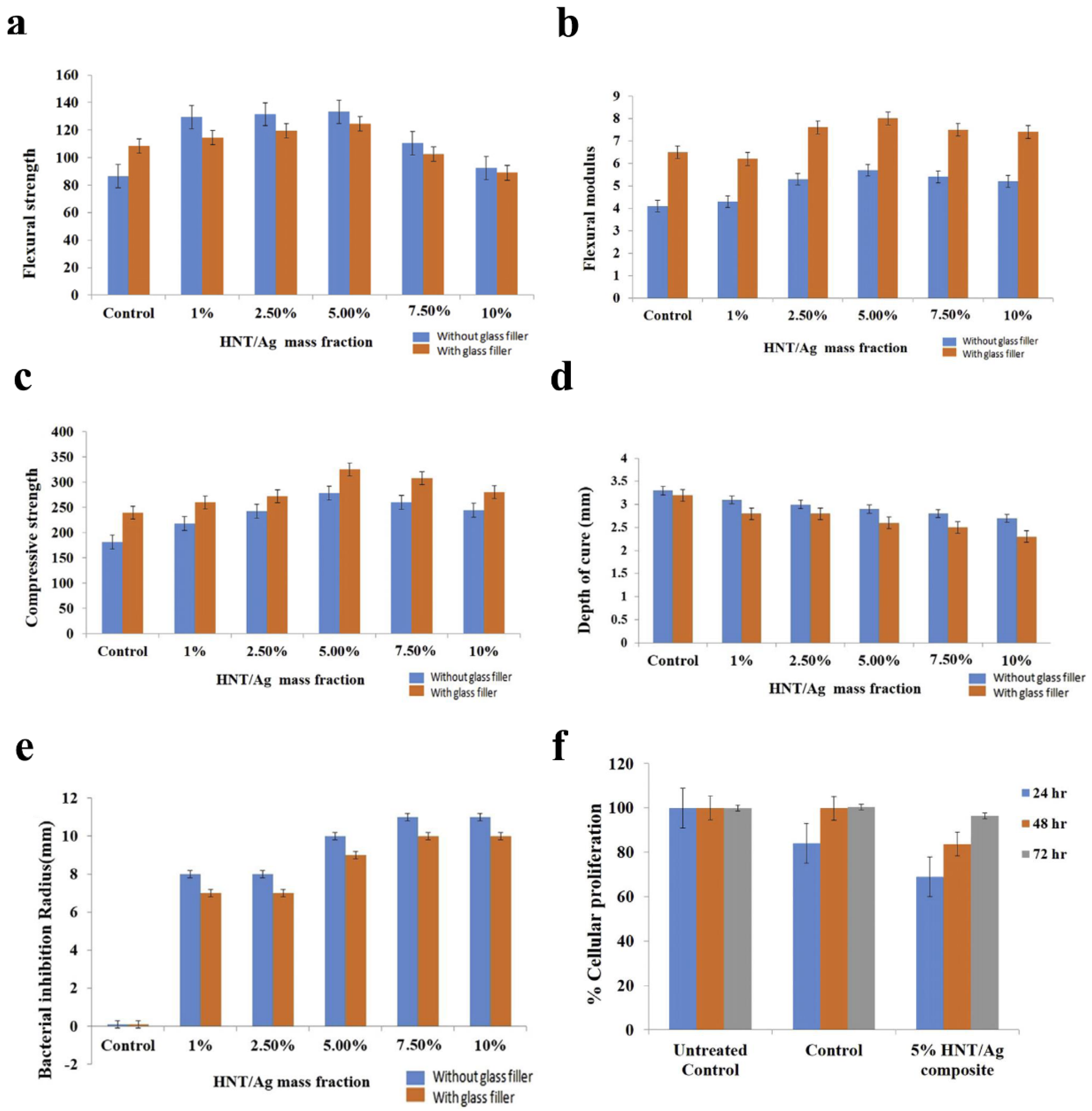
In 2010, Banot et al. [69] evaluated the efficacy of AgNP-immobilized halloysite nanotube (HNT/Ag) fillers on the physicochemical and biological properties of a new type of material containing different mass fractions (0, 1, 2.5, 5, 7.5, and 10%). Their findings indicated that the incorporating of 5% HNT/Ag into the resin composite greatly improved mechanical properties, presented satisfactory cure depth and conversion degree, exhibited obvious antibacterial action against S. mutans, and showed no distinct cytotoxicity to NIH3T3 cell lines (Figure 2a–f), evidencing that resin composite containing HNT/Ag has high value in potential dental applications. Similarly, another study (2019) [70] assessed antibacterial and mechanical properties of the new composite resin modified by Ag-doped ZnO nanoparticles. The new material was able to markedly suppress S. mutans on the surface with a slight increase in compressive strength. On account of these results, using Ag/ZnO nanoparticles could be a good choice for developing a novel potential antibacterial resin composite. Various low concentrations of AgNPs were added into the primer of a multi-purpose system; their antibacterial effect, cytotoxicity, and microtensile bond strength were evaluated by Dutra-Correa et al. (2018) [81]. It was observed that the primer containing 250 ppm AgNPs demonstrated excellent antibacterial efficacy and reliable bond strength without cytotoxicity against human dental pulp stem cells. Hence, the addition of AgNPs (250 ppm) seems promising to improve the efficacy of the conventional adhesive system.
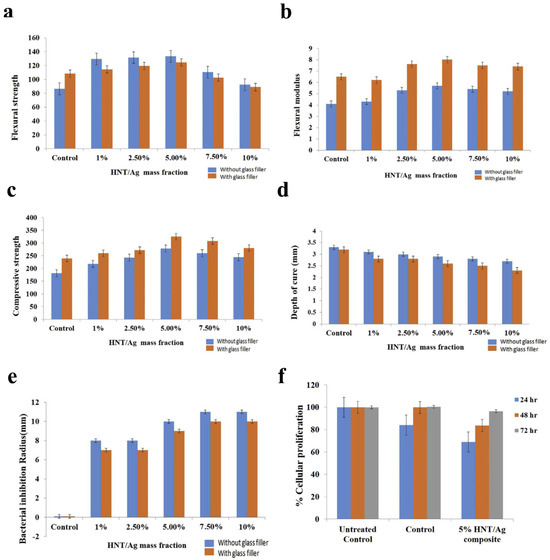
Figure 2.
Physicochemical and biological evaluation of halloysite nanotube-based resin composites immobilized with silver nanoparticles (HNT/Ag) in restorative treatment. (a) Flexural strength, (b) Flexural modulus, (c) Compressive strength, (d) Depth of cure, (e) Bacterial inhibition radius (n = 3), and (f) Cellular proliferation in the resin composite assessed using NIH-3T3 cell lines. Reproduced with adaptation from ref. [69].
The experimental data demonstrated that incorporating AgNPs into composite resins and dental adhesive systems can confer significant antimicrobial efficacy while maintaining their original physicochemical properties, biological performance, and cytocompatibility. These findings suggest that AgNP-modified restorative materials may serve as effective filler components in dental applications, potentially reducing the risk of secondary caries development through their bacteriostatic functionality.
3.3. Endodontic Treatment
Endodontic infections are inflammatory conditions of pulp associated with many various microorganisms. The goal of root canal therapy is to achieve complete eradication of microorganisms from root canal systems through a combination of mechanical and chemical debridement [93,94]. Owing to the anatomical complexity of the root canal system, the power of microorganisms to tolerate harsh environmental conditions and the intrinsic resistance of root canal biofilms, it is a great challenge to completely clean the root canal. Additionally, one of the most common bacteria related to treatment failure is Enterococcus faecalis (E. faecalis) [95]. They are able to survive without nutrients for a long time, infiltrate deeply into dentin tubules, and resist most of the root canal disinfectants and even a few antibiotics. Hence, new disinfection strategies need to be developed. Due to their antibacterial and anti-inflammatory abilities, the incorporation of nanoparticles, especially silver nanoparticles, has gained much attention and interest in the recent years [96,97]. Hou et al. (2020) employed an RNA-sequencing technique to explore the transcriptome variations in E. faecalis treated with silver nanoparticles [97]. This study demonstrated that AgNPs greatly suppressed the growth of E. faecalis by lengthening the lag period in a dose-dependent approach. Moreover, functional analyses of explored genes suggested that the two pathways related to environmental information processing and energy metabolism may be mainly involved in the suppressive effect. Since silver nanoparticles have been shown to have a destructive effect on E. faecalis, they have been evaluated as endodontic irrigants [98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117], intracanal medicaments [101,118,119,120,121,122,123], dental pulp regeneration [124,125], and root-filling materials, including modified gutta-percha [126,127,128,129,130] and incorporated mineral trioxide aggregate (MTA) [131,132,133,134,135,136,137,138].
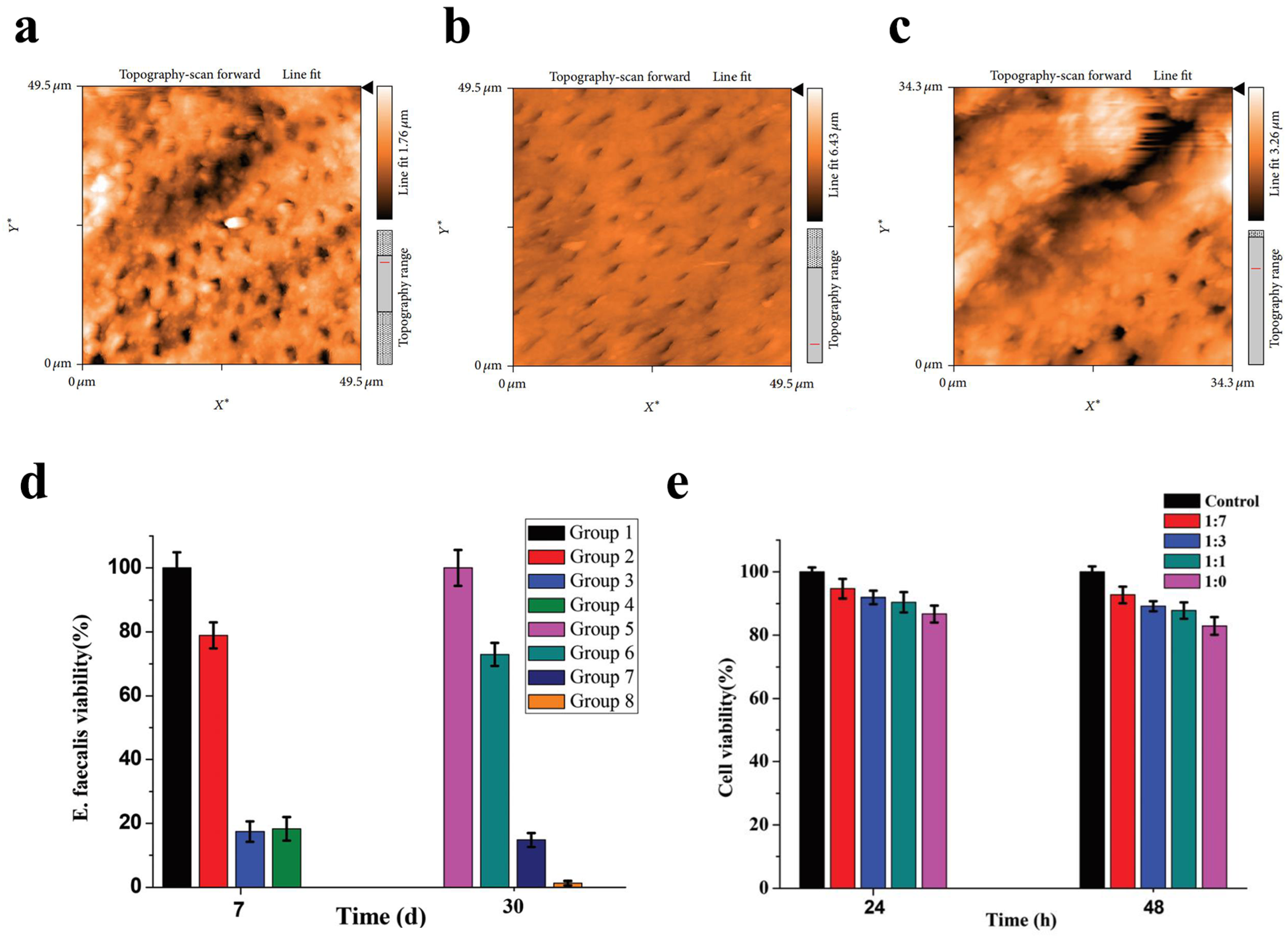
At present, sodium hypochlorite (NaOCl, 1–5%) is considered as the gold standard for the chemical cleaning of the root canal system in endodontic treatment [98]. Nevertheless, this irrigant is known for its deleterious effects on bond strength and its cytotoxicity to periapical tissues in the case of entering the peri-radicular space [139,140]. Other agents used as irrigant solutions include chlorhexidine (CHX, 2%) and ethylenediamine tetra-acetic (EDTA, 17%) [141,142]. However, both of them have a significantly lower ability to disrupt biofilms compared with sodium hypochlorite. Some studies indicated that silver nanoparticles possessed the same antibacterial effect as NaOCl [99,103,107,114,115,117]. One study demonstrated that a combination of 10-nm silver nanoparticles and 17% EDTA exhibited efficacy equivalent to sodium hypochlorite in eliminating E. faecalis, with no statistically significant difference observed between the two treatments [117]. Additionally, silver nanoparticles exhibited notable efficacy in the removal of the smear layer (Figure 3a–c). Similarly, Abbaszadegan et al. evaluated the cytotoxicity and bactericide activity of silver nanoparticles with different charges, 2.5% NaOCl, and 0.2% CHX, indicating that the positively charged silver nanoparticles were comparable to 2.5% NaOCl against E. faecalis [99]. Furthermore, compared with NaOCl and CHX, those nanoparticles presented a lower cytotoxic effect against L929 fibroblast cell lines. Moghadas et al. (2012) evaluated the antimicrobial effect of a nanosilver particle for the eradication of E. faecalis and Staphylococcus aureus (S. aureus) in comparison to 5.25% NaOCl [114]. These results found this new irrigant was equivalent to 5.25% NaOCl against E. faecalis and S. aureus [114]. However, some other studies showed contradictory results in which silver nanoparticles were not considered as better than sodium hypochlorite [98,101,102,106,112,113,116]. In 2021, Hendi investigated the antibacterial efficacy of a 940 nm diode laser with or without AgNPs against E. faecalis [112].
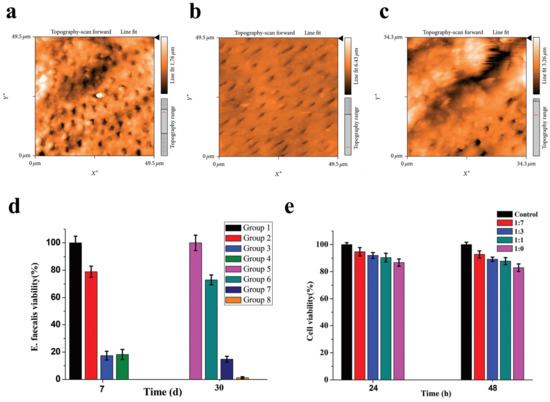
Figure 3.
Silver nanoparticles were used as a final irrigation or root canal disinfection in endodontics. AFM images demonstrating the comparative efficacy of (a) silver nanoparticles, (b) EDTA, and (c) saline solution in smear layer removal. The images demonstrate that silver nanoparticles were more effective than saline solution in removing the smear layer, though less effective than EDTA. Reproduced with adaptation from ref. [117]. (d) Viability of E. faecalis in infected root canals treated with different preparations at 7- and 30-day intervals. Group 1 and Group 5: negative control (no intracanal medication); Group 2 and Group 6: calcium hydroxide (CH) paste; Group 3 and Group 7: the cubic lyotropic liquid crystalline (LLC) precursors with 0.5% chlorhexidine (CHX); Group 4 and Group 8: the cubic LLC precursors with 0.5% CHX and 0.02% Ag-NP (mean ± S.D., n = 3). Reproduced with adaptation from ref. [120]. (e) Viability of PDLF cells following 24-h and 48-h incubation with the extracts of CHX-Ag-NP containing LLC precursor at varying dilution ratios (1:7, 1:3, 1:1, undiluted). Reproduced with adaptation from ref. [120].
Among the intracanal medicaments, calcium hydroxide (Ca(OH)2) is considered as the most commonly used medicament due to its high alkalinity that seems to have an inhibitory effect on most microorganisms [143]. However, its efficacy is not uniform across all endodontic pathogens [143]. Many researchers have suggested that silver nanoparticles as an intracanal medicament could be used as a substitute for calcium hydroxide [101,118,119,120,121]. An in vitro study concluded that the combination of silver nanoparticles with Ca(OH)2 and CHX was the best for eliminating E. faecalis biofilm [121]. In 2018, Zheng et al. performed a new intracanal disinfection based on lyotropic liquid crystal (LLC) precursor incorporation with 0.5% CHX and 0.02% AgNPs [120]. The precursor formulation demonstrated sustained bactericidal activity against E. faecalis, maintaining a ≥98.5% bacterial inactivation rate for over one month (Figure 3d). Moreover, the CCK8 assay demonstrated that the CHX-AgNPs containing the LLC precursor were safe and nontoxic (Figure 3e). Another study found that 0.02% AgNP gel as a root canal medicament had a stronger bactericidal effect against E. faecalis than calcium hydroxide (p < 0.05) [101]. However, there are also some inconsistent data about the antibacterial effect of AgNPs for root canal disinfection [122,123]. One report showed that calcium hydroxide performed better antimicrobial actions than AgNPs of aloe vera at a concentration of 500, 1000, or 2000 ug against E. faecalis, S. mutans, and Candida albicans (C. albicans) after 48 h incubation (p < 0.001). Another report also found that the antibacterial effect of Ca(OH)2 was higher than AgNPs of 2 nm alone or in combination with both materials [123]. Gutta-percha with endodontic sealers is the most widely used obturating material. However, gutta-percha has a minor antimicrobial effect. In addition, although root canal sealers exhibit some antibacterial properties, those abilities usually diminish after setting [144]. Thus, many efforts have been made to look for a new antibacterial agent [126,127,128,129,130]. Baras et al. (2019) developed a new endodontic sealant combined with dimethylaminohexadecyl methacry (DMAHDM) and AgNPs [130]. The data demonstrated that the sealer containing 5% DMAHDM and 0.15% AgNPs significantly reduced both dental biofilm formation and viability while maintaining its physical properties. In 2018, Seung et al. concluded that the addition of 2.5% DMAHDM and 0.15% AgNPs into the AH Plus sealer made it more antibacterial against E. faecalis without changing its physical properties when compared with the conventional AH Plus sealer [128]. In contrast to conventional gutta-percha, Mozayeni et al. (2017) demonstrated that nanosilver-coated gutta-percha elicited a heightened inflammatory response when implanted subcutaneously in a rat model [127]. Researchers did not find that the incorporation of silver nanoparticles into the AH Plus sealer could improve its bacterial leakage resistance property [129].
Mineral trioxide aggregate (MTA) has extensive applications in endodontics, such as direct pulp-capping, apexification, repair of furcation and lateral canal perforations, and external/internal root resorption repair [145]. Nevertheless, long solidification time, operational difficulties, and restricted antimicrobial effects have been reported as the drawbacks of MTA [146]. Therefore, it is essential to add antimicrobial agents into MTA to improve its antimicrobial properties. Based on the in vitro study, adding silver nanoparticles to MTA could significantly enhance its antimicrobial efficacy against E. faecalis, C. albicans, and Pseudomonas aeruginosa [137]. Moreover, research has reported that the combination of silver nanoparticles (100 or 200 ppm) with MTA or calcium-enriched mixture (CEM) significantly increased their antibacterial efficacy against all the tested microorganisms (p < 0.05) [132]. Researchers revealed that mixing MTA with 2% zeolite–silver–zinc (Ze-Ag-Zn) had an adverse influence on the compressive strength of MTA, although this incorporation had no cytotoxic effects [136]. Zand et al. found that the incorporation of 1% AgNPs to MTA had a similar inflammatory response in subcutaneous tissue in a rat model when compared with MTA [131]. Additionally, Fernando et al. investigated the impact of AgNPs on the physicochemical and antibacterial natures of white MTA (WMTA) and Portland cement (PC) combined with zirconium oxide (ZrO2) [138]. The incorporation of AgNPs into WMTA increased the pH and reduced the solubility and setting time. Adding AgNPs into PC/ZrO2 maintained the pH value, decreased solubility, and improved setting time and compressive strength. After 15 h of contact with an E. faecalis biofilm, WMTA/AgNPs and PC/ZrO2/AgNPs demonstrated higher levels of bacterial decline.
Collectively, although some contradictory findings have been reported, silver nanoparticles demonstrate significant potential as valuable adjuncts for cleaning of the root canal system in endodontic treatment. However, prior to clinical implementation, further studies are required to completely investigate and confirm antimicrobial, mechanical, physicochemical, and biological properties of silver nanoparticles associated with root canal materials.
3.4. Periodontal Treatment
Peridontitis, provoked by various types of microorganisms, is a prevalent global chronic inflammatory disease that can trigger the progressive failure of periodontal tissues, thereby forming a deep periodontal pocket and gingival recession. The most important pathogens associated with periodontitis are Aggregatibacter actinomycetemcomitans (A. actinomycetem), Porphyromonas gingivalis (P. gingivalis), and Fusobacterium nucleatum (F. nucleatum) [147]. Guided tissue regeneration (GTR), one of the successful clinical therapies for repairing periodontal defects, uses a barrier membrane to inhibit epithelial downgrowth and fibroblast transgrowth into a wound space, thus keeping space for periodontal tissue regeneration [148]. However, bacterial contamination of the wound site is known to markedly compromise the desired results. Thus, some reports with silver nanoparticles as potential antimicrobial agents against periodontal microorganisms have been developed [149,150,151,152,153]. An in vitro study investigated the colonization and penetration of four microorganisms on AgNP-impregnated GTR membranes [151], including S. mutans, A. actinomycetem, F. nucleatum, and P. gingivalis. In this study, three types of GTR membranes were evaluated, including a plain GTR membrane serving as the negative control (GTR-C), (2) a silver nanoparticle-incorporated GTR membrane as the experimental group (GTR-NS), and a doxycycline hydrochloride-loaded GTR membrane as the positive control (GTR-DOX). Results showed that silver nanoparticles were comparative with doxycycline in controlling bacterial adherence across all four organisms, but they were as effective as doxycycline in preventing penetration into the GTR membrane only for A. actinomycetem and P. gingivalis. Craciunescu et al. (2018) biosynthesized stable AgNPs combined with nondenatured type I collagen [152]. The results showed that AgNPs prepared in collagen gel enhanced physiochemical and biological characters, such as spherical morphology, small size (30 nm), positively charged surface, high biocompatibility with human gingival fibroblasts, a wide scope of concentrations, and better antimicrobial activity than AgNPs in citrate, indicating a greater treatment potential in periodontal disease therapy. In 2019, another similar study developed a new AgNP-modified/collagen I-coated electrospun PLGA/PCL scaffold with uncompromising physico-mechanical abilities, excellent biocompatibility, sustained antibacterial effects, and enhanced osteogenic characters, indicating their treatment potential for oral and craniofacial GTR and bone regeneration (GBR) [150]. However, an Indonesian study demonstrated that the periodontal dressing containing silver nanoparticles could provoke foreign body reaction and increase inflammation in rats by 99mTc-ciprofloxacin [154].
3.5. Prosthodontic Treatment
Denture stomatitis characterized by red focal areas is an inflammatory condition of the denture-bearing mucosa, influencing 50–70% of complete denture wearers. Its etiology is multifactorial and C. albicans is the most important etiologic agent. Denture stomatitis poses a significant clinical challenge in the field of dentistry, and silver nanoparticles as antibacterial agents can be mixed into acrylic resins [77,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172], tissue conditioners [173,174,175], impression materials [176,177], dental cements [178,179], facial prostheses [180,181], and even porcelain for crowns and bridges [182,183,184,185,186].
In 2016, the authors conducted a study to evaluate the flexural strength, impact strength, elastic modulus, and glass transition temperature (Tg) of two types of acrylic denture base resins: heat-polymerized and microwave-polymerized [162]. The incorporation of 0.8% and 1.6% AgNPs significantly reduced the flexural strength and elastic modulus of the microwave-polymerized resin, whereas their addition had little effect on the impact strength of both resin groups. Moreover, the incorporation of AgNPs decreased Tg in both denture base resins. In their study, Li et al. (2016) found that denture base resin containing 5% AgNPs exhibited an inhibitory influence on C. albicans adhesion. The average thickness and the living cell percentage of biofilm that formed on the resin specimens sequentially declined with the increase in AgNP solution concentration [161]. Sun et al. carried out an animal study (2021) in which AgNP solution (NS) was incorporated into acrylic acid and then into methyl methacrylate (MMA) and poly methyl methacrylate (PMMA) in order to prepare the NS/PMMA denture base [155]. Those data indicated that the NS/PMMA group exhibited better esthetic performance and higher antimicrobial properties against C. albicans, E. coli, and S. mutans. Meanwhile, the NS/PMMA material displayed excellent anti-aging properties and exceptional biocompatibility, with little cytotoxicity to L929 cells. A similar study by Bacali et al. in 2020 demonstrated that the PMMA resin loaded with graphene–Ag nanoparticles inhibited the bacterial growth of S. aureus, S. mutans, and E. coli in a dose-dependent manner, with minimal toxicity to human cells as well as improved flexural properties [158]. In another study (2012), compared to an unmodified group, a significantly reduced CFU of the C. albicans strain was found at the modified denture acrylic combined with AgNPs at 20% and 30% [168]. In addition, considering the minimal inhibitory concentration was 3.0 mg/L, Ag+ elution contributed little to the antifungal activity in this study (maximum 0.356 ± 0.11 mg/L). However, the poor color stability still required improvement. In a study by Nam et al. (2011), the combination of 0.1% AgNPs and tissue conditioner presented the least significant bacterial effect against S. aureus and S. mutans, and the bacterial effect on C. albicans was 0.5% [174]. There was no statistical difference between 24 h and 72 h incubation times in terms of the microbial inhibitory effect. In their study, Ginjupalli et al. indicated that the addition of silver nanoparticles into irreversible hydrocolloid impression materials produced excellent antimicrobial activity without adverse effects on physical properties (such as gel strength, permanent deformation, flow, and gel time) [176]. Meran et al. (2018) investigated the biocompatibility and antifungal behaviors of silicone facial protheses coated with silver nanoparticles in vitro [180]. Data showed that the AgNP coating was effective against the growth of C. albicans measured by ethanol production by the yeast without damaging human fibroblasts (Hs68). Uno et al. in Japan modified Noritake Super porcelain with the addition of different concentrations of AgNPs (100, 200, 500, and 1000 ppm) [186]. Results indicated that the incorporation of AgNPs significantly increased the fracture toughness and Vickers hardness of porcelain. However, the higher concentrations (500 and 1000 ppm) could cause a color change. Hashem et al. conducted a similar study and concluded that the addition of silver nanoparticles could improve the fracture strength of dental ceramic with an adverse color change [185]. Fujieda et al. used a post-indentation method to investigate the effect of AgNPs on subcritical crack growth (SCG) behaviors of porcelain [182]. Data showed that the incorporation of silver nanoparticles enhanced the fatigue parameter n, which characterized the SCG behavior and indicated that fatigue life would be extended.
3.6. Dental Implant Treatment
Titanium (Ti) implants are widely used to replace missing teeth in dentistry. Unfortunately, the infections associated with Ti implants, including peri-implant mucositis and peri-implantitis, can cause implant breakdown and additional surgical interventions [187]. In order to address these infections, AgNP coatings have been developed and proven to effectively endow implants with inherent antibacterial properties [188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219].
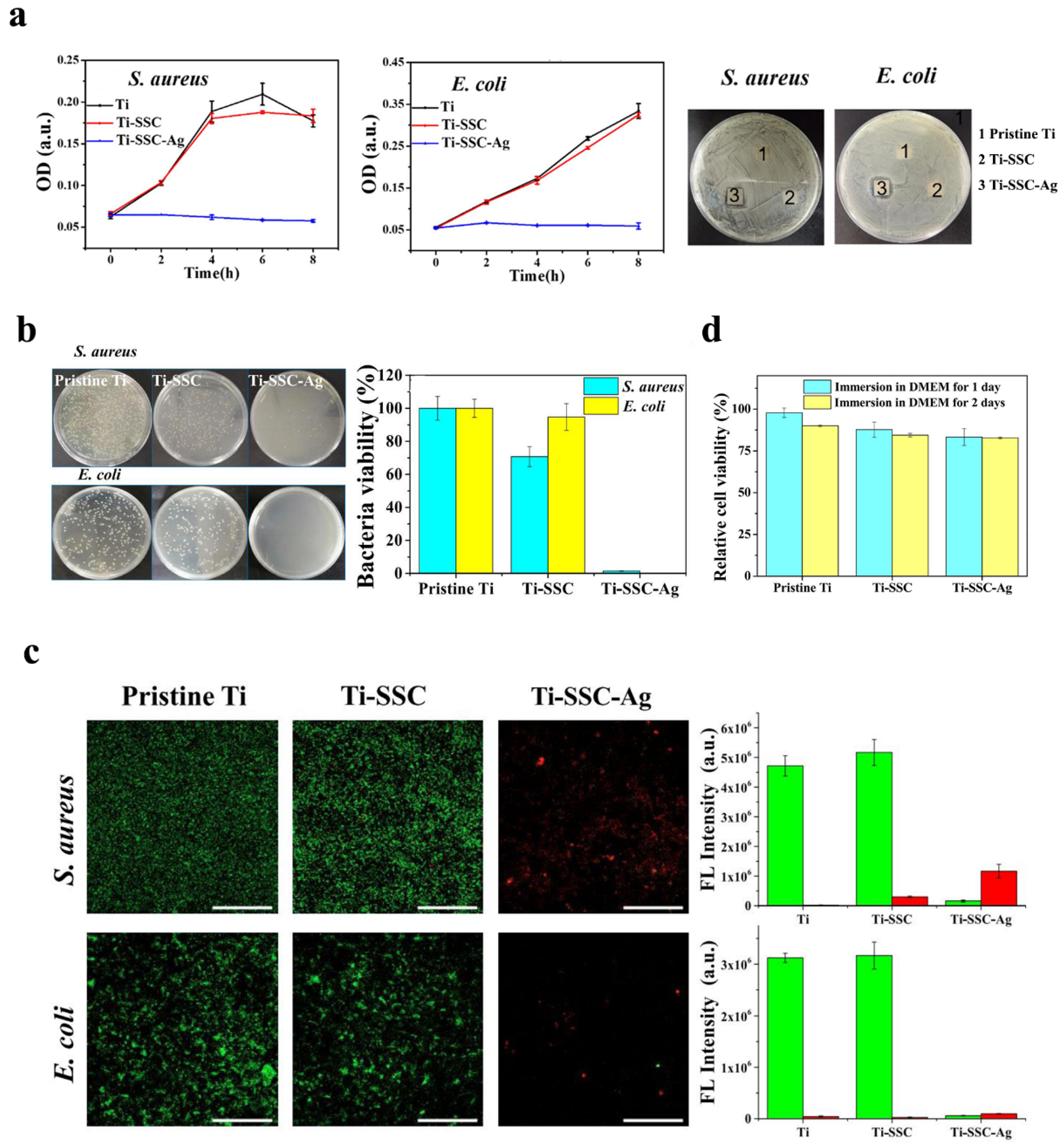
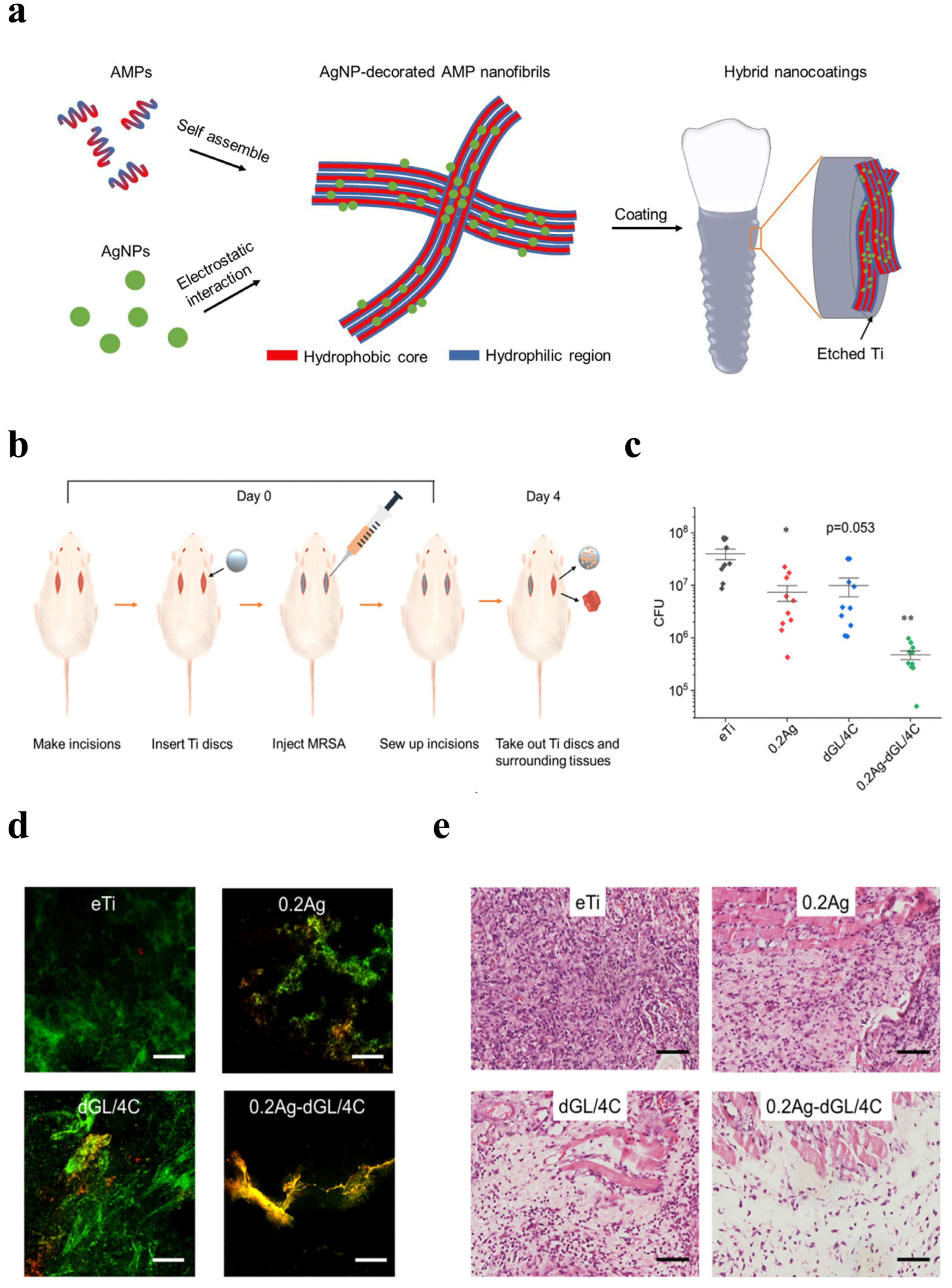
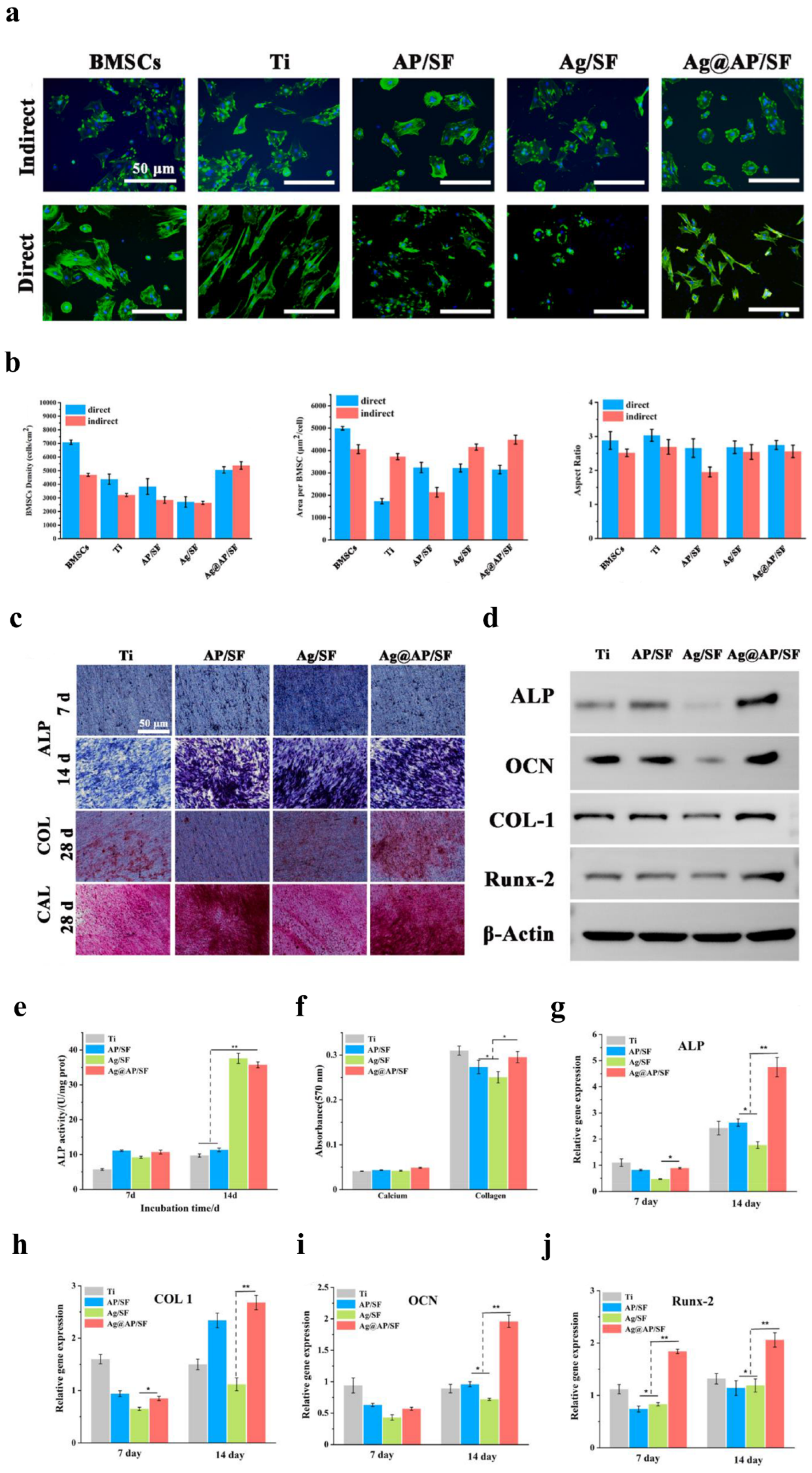
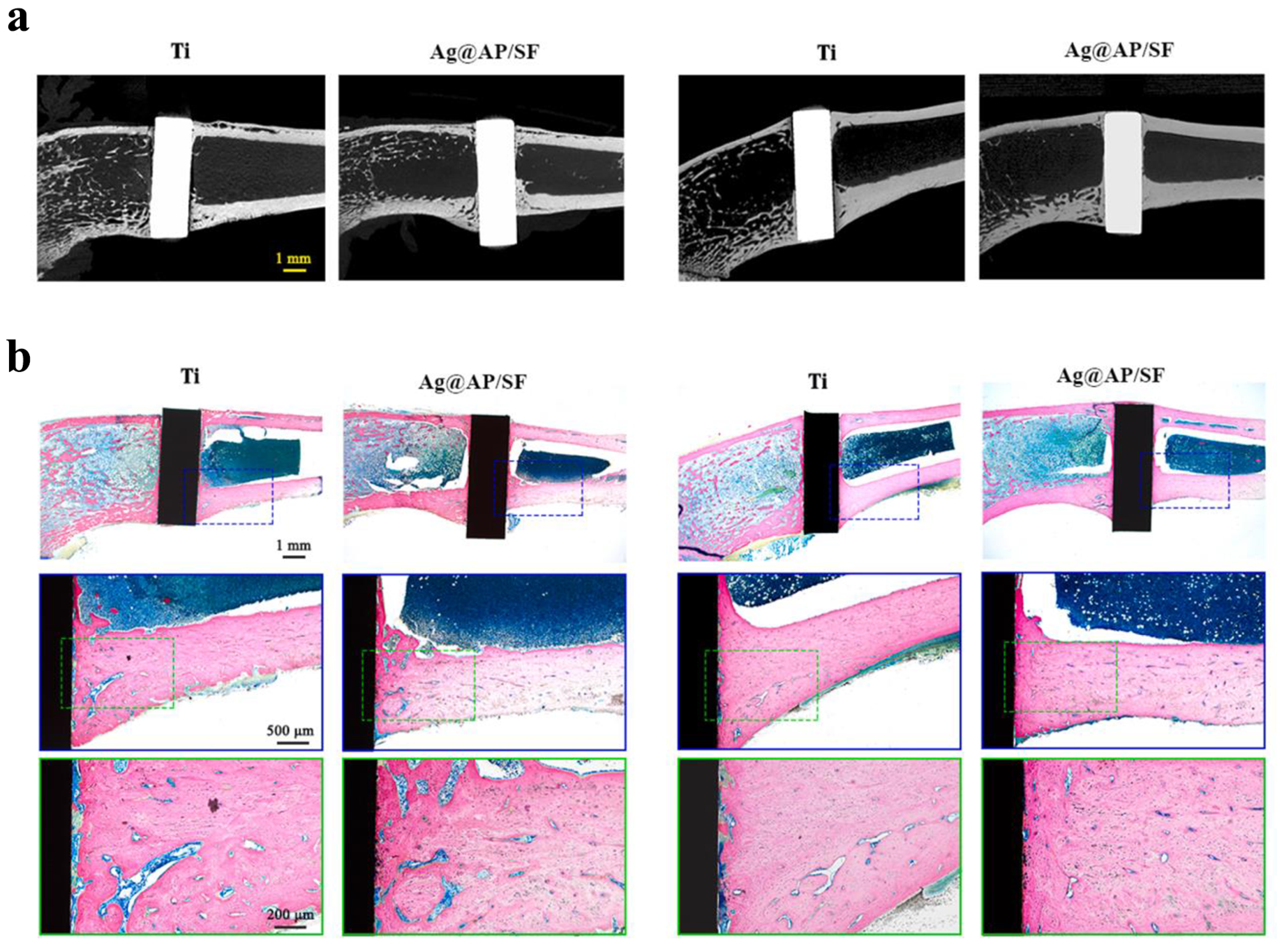
Qiang et al. (2021) fabricated Ti surfaces functionalized with catechol moiety-containing silk sericin (SSC) and AgNPs [206]. As shown in the growth curve, inhibition zone, live/dead staining assay, and quantitative measurement, the AgNP/SSC-coated Ti surface significantly prevented the adhesion of S. aureus and E. coli, as well as early formation of the biofilm (Figure 4a–c). Additionally, the cell toxicity of the c-SSC-Ag surface to L929 mouse fibroblasts was negligible (Figure 4d). Ye et al. (2022) designed a hybrid nanocoating composed of silver nanoparticles decorated with antimicrobial peptides (AMPs) and GL13K was used in this study (Figure 5a) [212]. The results demonstrated that following a 6-h incubation period, the hybrid AgNP-dGL13K coating displayed the strongest antimicrobial efficacy against methicillin-resistant S. aureus (MRSA) and P. aeruginesa. In addition, a subcutaneous infection model in rats demonstrated that more dead bacteria were found with the hybrid AgNP-dGL13K coatings and a denser neutrophilic infiltration was found around the noncoated etched Ti (eTi) (Figure 5b–e). In a study performed by Zhou et al. (2022), devising AMPs with osteogenic fragments were on the surface of AgNPs, and the silk fibroin (SF) was used to take AgNPs@AMPs which are constructed on Ti implants [215]. The 21-day antibacterial evaluation against S. aureus demonstrated that Ag@AP/SF maintained above 99% inhibitory efficacy against both planktonic and adherent bacteria throughout the testing period. Following 7- and 14-day co-culture periods with bone marrow stem cells (BMSCs), the AgNP/AMP-loaded silk fibroin (SF)-based coatings demonstrated enhanced promotion of BMSC adhesion, proliferation, and osteogenic differentiation, as shown in Figure 6a–j. Furthermore, after 4 and 8 weeks of implantation in a rat model, the Ag@AP/SF coatings not only significantly promoted new bone formation but also enhanced osseointegration at the implant interface (Figure 7a,b). In a related study, Zhou et al. (2017) developed silk fibroin (SF)-based coatings loaded with AgNPs and gentamicin (Gen) on titanium implants using polydopamine (PD)-assisted deposition and a cyclic drying process [216]. The PD-S, PD-S-Ag, and PD-S-Ag/g coatings were gained, respectively, in SF, SF/AgNP, and SF/AgNP/Gen solutions. The cell-bacteria co-culture experiment demonstrated that the PD-S-Ag/g coatings significantly enhanced MC3T3 cell proliferation while effectively inhibiting S. aureus adhesion and growth. Furthermore, AgNP/Gen-contained coatings benefited the osteogenic differentiation of MC3T3 cells, such as the production of alkaline phosphatase, secretion of collagen, and calcification. In a split-mouth randomized clinical trial by Odatsu et al. (2020), the AgNP coating applied to the healing abutments was prepared with microwave-assisted synthesis [204]. These data showed that the nano-Ag coating strongly suppressed bacterial attachment on the healing abutments without cytotoxicity on human gingival fibroblasts.
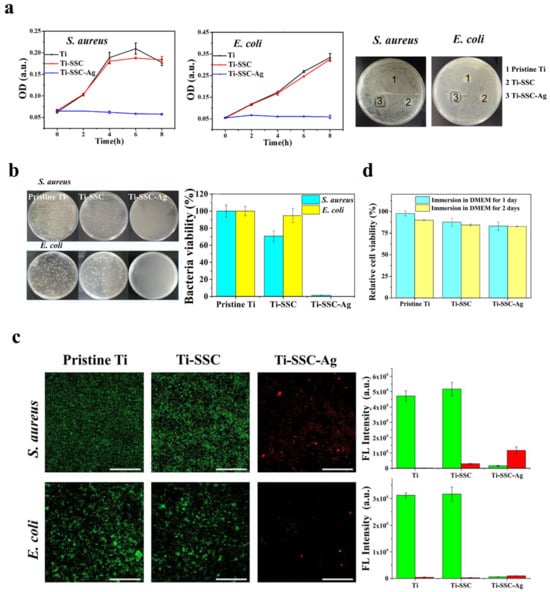
Figure 4.
Silver nanoparticles were used as antibacterial coatings in implant treatment. (a) Growth curves and inhibition zones of S. aureus and E. coli treated with the pristine Ti, Ti-SSC, and Ti-SSC-Ag surfaces. (b) Images of TSB agar plates showing bacterial colonies formed by serially diluted cultures of S. aureus and E. coli, along with quantitative analysis of viable bacterial colonization on pristine Ti, Ti-SSC, and Ti-SSC-Ag substrates. (c) CLSM micrographs showing S. aureus and E. coli biofilms stained with SYTO 9 and PI on pristine Ti, Ti-SSC, and Ti-SSC-Ag surfaces, with corresponding visualization of live (green) and dead (red) bacterial cells across the different surfaces. (d) The in vitro cytocompatibility percentages of L929 mouse fibroblast cells cultured on pristine Ti, Ti-SSC, and Ti-SSC-Ag surfaces in DMEM medium after 1-day and 2-day incubation periods. Reproduced with adaptation from ref. [206].
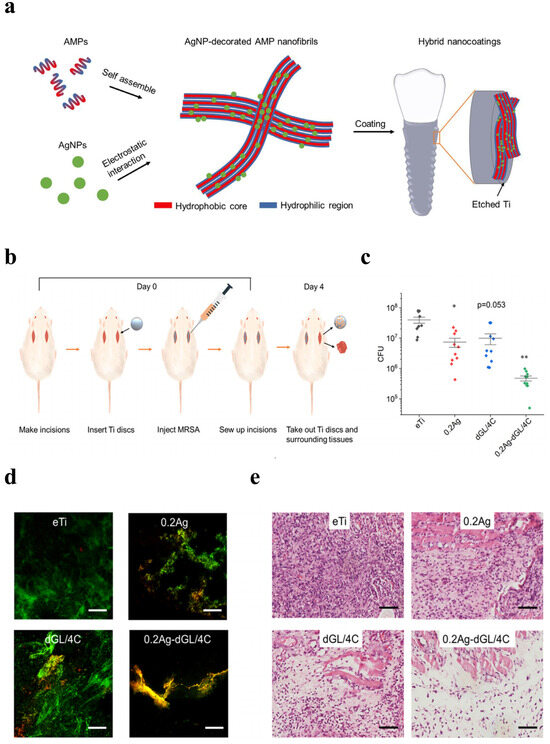
Figure 5.
Schematics of the hybrid GL13K coating on etched Ti and in vivo antimicrobial activity against MRSA. (a) Schematics of the syntheses of the AMP/AgNP nanocomposites and the hybrid nanocoating on etched Ti. (b) Schematic diagram illustrating the in vivo experimental design. (c) Colony-forming units (CFU) (n = 10 for each group) and (d) Representative CLSM images showing live/dead-stained MRSA biofilms colonizing the implanted disks. (e) Optical microscopy images of tissue sections adjacent to infected disk regions (H&E staining). (* p < 0.05; ** p < 0.01. Scale bars: 100 µm). Reproduced with adaptation from ref. [212].
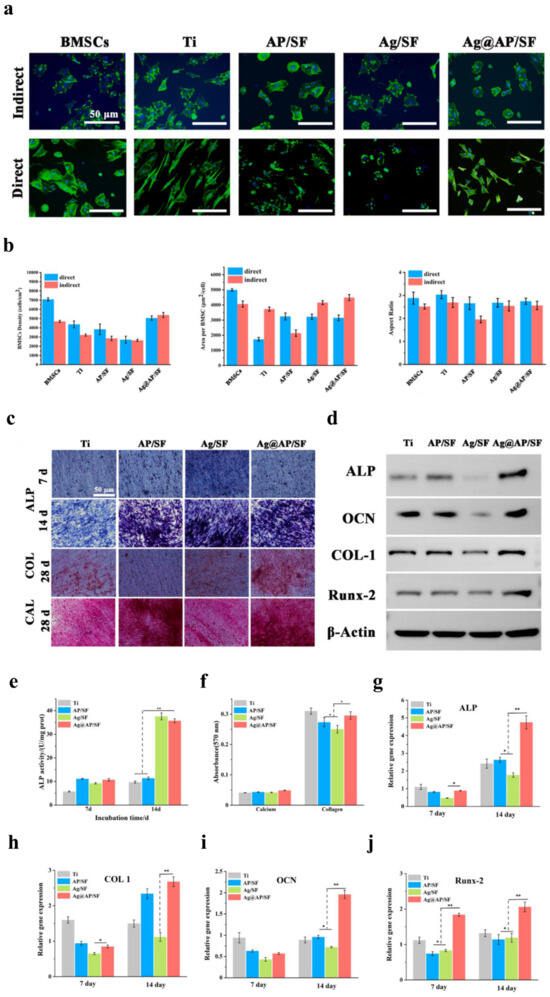
Figure 6.
In vitro osteogenic ability of BMSCs co-cultured with Ag@AP/SF coatings. (a) Fluorescent staining of BMSCs cultured under direct and indirect conditions (blue indicating DAPI-stained nuclei; green indicating Alexa Fluor 488-stained F-actin). (b) The average density, surface area, and aspect ratio of BMSCs were quantified on the sample surfaces. (c) Representative images of alkaline phosphatase (ALP) staining, Sirius Red staining, and Alizarin Red staining in BMSCs. (d) Western blotting. (e) Quantitative analysis of ALP expression levels in BMSCs on days 7 and 14. (f) Quantitative analysis of collagen secretion and calcium deposition following a 21-day period. (g–j) Osteogenic gene expression in BMSCs, including ALP, COL1, OCN, and Runx2. (* p < 0.05; ** p < 0.01). Reproduced with adaptation from ref. [215].
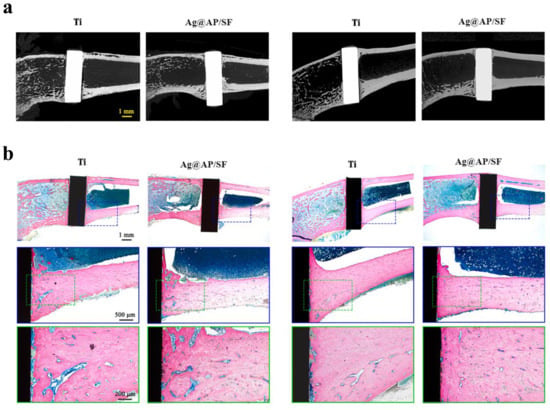
Figure 7.
In vivo osseointegration ability of Ag@AP/SF coatings implanted into rat femurs. (a) Micro-CT imaging was performed at 4- and 8-week time points following implantation. (b) Light micrographs of hard tissue sections stained with methylene blue and acid fuchsin at 4- and 8-week post-implantation time points. Reproduced with adaptation from ref. [215].
3.7. Orthodontic Treatment
White spot lesions (WSLs) represent a well-documented complication associated with fixed orthodontic treatment. The placement of fixed orthodontic devices creates a beneficial environment for the accumulation of microorganisms and formation of dental biofilm. Cariogenic microorganisms, particularly S. mutans and Lactobacilli, have been identified as primary etiological agents. If left untreated, progressive WSLs may evolve into frank caries lesions requiring restorative intervention. AgNPs have been demonstrated as antimicrobial coatings on orthodontic components (such as brackets [220,221,222,223,224,225], wires [226,227,228,229], elastomeric modules [230], micro-implants [231]) and also as antimicrobial agents for orthodontic adhesives [84,232,233,234,235,236,237,238], cements [239,240,241,242], resin-based composite and acrylic resins [243,244,245,246,247,248,249,250,251], and mouthwash [252].
Recent investigations have demonstrated the significant antimicrobial potential of AgNPs in various orthodontic applications. In a 30-day rat model study [221], AgNP-coated orthodontic brackets exhibited markedly reduced Streptococcus mutans colonization (p = 0.015) and decreased smooth surface caries incidence (p = 0.041) compared to conventional brackets. These findings align with earlier surface modification research by Lee et al. (2011), who developed nanocomposite coatings through a layer-by-layer deposition of polydopamine, functionalized poly(3,4-ethylenedioxythiophene), and AgNPs. Their SS-DO-OH-Ag and SS-DO-OH-PC-Ag coatings exhibited potent bactericidal efficacy against both E. coli and S. mutans, while demonstrating minimal cytotoxicity associated with Ag+ ion release [227]. The incorporation of AgNPs into orthodontic adhesives has shown dose-dependent antimicrobial effects. Bahador et al. (2020) identified 5% w/v as the minimum effective concentration for a significant inhibition of Streptococcus sanguinis, S. mutans, and Lactobacillus acidophilus in rat models [233]. This concentration optimization was corroborated by Moreira et al., whose AgNP-modified orthodontic adhesive maintained mechanical properties while achieving statistically significant reductions in cariogenic pathogens [241].
4. Future Perspectives and Conclusions
Extensive research highlighted in this review demonstrates that silver nanoparticles exhibit promising antimicrobial properties, positioning them as a versatile therapeutic agent with diverse applications across dental specialties. Their potential utility spans preventive care, restorative procedures, endodontic therapy, periodontal management, implantology, and orthodontic interventions.
Despite considerable progress in this field, several critical aspects remain insufficiently understood and warrant further investigation. Comprehensive studies are imperative to establish optimal parameters for silver nanoparticle (AgNP) integration, including safe dosage thresholds relative to cellular toxicity, ideal concentration ranges, and particle size specifications. Moreover, researchers should prioritize elucidating potential adverse effects associated with incorporating AgNPs into dental materials, particularly regarding chromatic stability and alterations in mechanical properties. The cytotoxic effects of AgNPs have been primarily attributed to free silver ion release, necessitating rigorous toxicological assessments to evaluate potential risks in dental applications. Current research limitations must be acknowledged that most available data originate from in vitro studies, while robust longitudinal evidence from animal models and clinical trials remains conspicuously absent. Furthermore, the fundamental antibacterial mechanisms of AgNPs have not been fully understood, demanding systematic investigations to unravel the precise molecular interactions and pathways involved.
Artificial intelligence (AI) has emerged as a transformative emerging technology for processing multidimensional information with minimal human intervention through advanced deep learning capabilities. The integration of AI with silver nanoparticles (AgNPs) has demonstrated significant potential across diverse biomedical applications, including pesticide molecular analysis within plants, the diagnosis of invasive fungal infections (IFIs), discrimination of volatile organic compounds (VOCs), and characterization of tumor suppressor genes. These interdisciplinary advances suggest substantial opportunities for implementing AI-enhanced AgNP systems in dental applications. We anticipate that the current findings will establish a valuable framework for optimizing AI-driven AgNP technologies in dentistry, potentially paving the way for innovative diagnostic tools, targeted therapeutic approaches, and enhanced biomaterial development within oral healthcare.
Funding
We gratefully acknowledge the financial support from the Youth Talent Support Program of the China Association for Science and Technology (YESS20220554) and the Youth Talent Support Program of the Beijing Association for Science and Technology (BYESS2024297).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000 2006, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Maddi, A.; Scannapieco, F.A. Oral biofilms, oral and periodontal infections, and systemic disease. Am. J. Dent. 2013, 26, 249–254. [Google Scholar]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31 (Suppl. S1), 3–23. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, W.; Fan, M.; Tong, Z.; Kuang, R.; Jiang, W.; Ni, L. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides 2014, 52, 61–67. [Google Scholar] [CrossRef]
- Vohra, F.; Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Ghanem, A.; Sergis, K.; Javed, F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagnosis Photodyn. Ther. 2016, 13, 139–147. [Google Scholar] [CrossRef]
- Alaki, S.M.; Burt, B.A.; Garetz, S.L. The association between antibiotics usage in early childhood and early childhood caries. Pediatr. Dent. 2009, 31, 31–37. [Google Scholar]
- Wang, W.; Tao, R.; Tong, Z.; Ding, Y.; Kuang, R.; Zhai, S.; Liu, J.; Ni, L. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides 2012, 33, 212–219. [Google Scholar] [CrossRef]
- Godreuil, S.; Leban, N.; Padilla, A.; Hamel, R.; Luplertlop, N.; Chauffour, A.; Vittecoq, M.; Hoh, F.; Thomas, F.; Sougakoff, W.; et al. Aedesin: Structure and antimicrobial activity against multidrug resistant bacterial strains. PLoS ONE 2014, 9, e105441. [Google Scholar] [CrossRef]
- Tanwir, F.; Khiyani, F. Antibiotic resistance: A global concern. J. Coll. Physicians Surg. Pak. 2011, 21, 127–129. [Google Scholar] [PubMed]
- Liao, Y.; Chen, J.; Brandt, B.W.; Zhu, Y.; Li, J.; van Loveren, C.; Deng, D.M. Identification and functional analysis of genome mutations in a fluoride-resistant Streptococcus mutans strain. PLoS ONE 2015, 10, e0122630. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, T.M.; Szkaradkiewicz, A.K. Chlorhexidine—Pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar] [PubMed]
- Saafan, A.; Zaazou, M.H.; Sallam, M.K.; Mosallam, O.; El Danaf, H.A. Assessment of Photodynamic Therapy and Nanoparticles Effects on Caries Models. Open Access Maced. J. Med. Sci. 2018, 6, 1289–1295. [Google Scholar] [CrossRef]
- Saravana, K.R.; Vijayalakshmi, R. Nanotechnology in dentistry. Indian J. Dent. Res. 2006, 17, 62–65. [Google Scholar]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef]
- Hannig, M.; Kriener, L.; Hoth-Hannig, W.; Becker-Willinger, C.; Schmidt, H. Influence of nanocomposite surface coating on biofilm formation in situ. J. Nanosci. Nanotechnol. 2007, 7, 4642–4648. [Google Scholar] [CrossRef]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Skora, B.; Krajewska, U.; Nowak, A.; Dziedzic, A.; Barylyak, A.; Kus-Liskiewicz, M. Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 2021, 11, 13451. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Adam, R.Z.; Khan, S.B. Antimicrobial efficacy of silver nanoparticles against Candida albicans: A systematic review protocol. PLoS ONE 2021, 16, e0245811. [Google Scholar] [CrossRef]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Nozari, A.; Ajami, S.; Rafiei, A.; Niazi, E. Impact of Nano Hydroxyapatite, Nano Silver Fluoride and Sodium Fluoride Varnish on Primary Teeth Enamel Remineralization: An In Vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC97–ZC100. [Google Scholar] [PubMed]
- Smith, D.J. Dental caries vaccines: Prospects and concerns. Expert Rev. Vaccines 2010, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Haghgoo, R.; Saderi, H.; Eskandari, M.; Haghshenas, H.; Rezvani, M. Evaluation of the antimicrobial effect of conventional and nanosilver-containing varnishes on oral streptococci. J. Dent. 2014, 15, 57–62. [Google Scholar]
- Targino, A.G.; Flores, M.A.; dos Santos Junior, V.E.; de Godoy Bene Bezerra, F.; de Luna Freire, H.; Galembeck, A.; Rosenblatt, A. An innovative approach to treating dental decay in children. A new anti-caries agent. J. Mater. Sci. Mater. Med. 2014, 25, 2041–2047. [Google Scholar] [CrossRef]
- Freire, P.L.L.; Albuquerque, A.J.R.; Sampaio, F.C.; Galembeck, A.; Flores, M.A.P.; Stamford, T.C.M.; Rosenblatt, A. AgNPs: The New Allies Against, S. mutans Biofilm—A Pilot Clinical Trial and Microbiological Assay. Braz. Dent. J. 2017, 28, 417–422. [Google Scholar] [CrossRef]
- Schwass, D.R.; Lyons, K.M.; Love, R.; Tompkins, G.R.; Meledandri, C.J. Antimicrobial Activity of a Colloidal AgNP Suspension Demonstrated In Vitro against Monoculture Biofilms: Toward a Novel Tooth Disinfectant for Treating Dental Caries. Adv. Dent. Res. 2018, 29, 117–123. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, Q.; Lu, S.; Fu, Y.; Yu, D.; Zhao, W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2018, 27, e20180042. [Google Scholar] [CrossRef]
- Espindola-Castro, L.F.; Rosenblatt, A.; Galembeck, A.; Monteiro, G. Dentin Staining Caused by Nano-silver Fluoride: A Comparative Study. Oper. Dent. 2020, 45, 435–441. [Google Scholar] [CrossRef]
- Soekanto, S.A.; Fadillah, F.; Nuraisiya, P.; Gultom, F.E.R.R.Y.; Sarwono, A.T. The potential of several fluoride-based varnishes as remineralization agents: Morphological studies, dentin surface hardness, and crystallinity tests. Int. J. Appl. Pharm. 2017, 9, 60–66. [Google Scholar] [CrossRef]
- Soekanto, S.A.; Rosithahakiki, N.; Sastradipura, D.F.S.; Sahlan, M. Comparison of the potency of several fluoride-based varnishes as an anticariogenic on calcium, phosphate, and fluoride ion levels. Int. J. Appl. Pharm. 2017, 9, 55–59. [Google Scholar] [CrossRef]
- Scarpelli, B.B.; Punhagui, M.F.; Hoeppner, M.G.; Almeida, R.S.C.; Juliani, F.A.; Guiraldo, R.D.; Berger, S.B. In Vitro Evaluation of the Remineralizing Potential and Antimicrobial Activity of a Cariostatic Agent with Silver Nanoparticles. Braz. Dent. J. 2017, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liang, K.; Weir, M.D.; Cheng, L.; Liu, H.; Zhou, X.; Ding, Y.; Xu, H.H.K. Combining Bioactive Multifunctional Dental Composite with PAMAM for Root Dentin Remineralization. Materials 2017, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz, M.; Sonmez, I.S. Comparison of Remineralising Potential of Nano Silver Fluoride, Silver Diamine Fluoride and Sodium Fluoride Varnish on Artificial Caries: An In Vitro Study. Oral Health Prev. Dent. 2019, 17, 469–477. [Google Scholar]
- Silva, A.V.C.S.; Teixeira, J.A.; Júnior, P.C.M.; Lima, M.G.S.; Mota, C.C.B.O.; Lins, E.C.C.C.; Pereira, J.R.D.; Gomes, A.S.L.G.; Targino, A.G.R.T.; Rosenblatt, A. Remineralizing Potential of Nano-Silver-Fluoride for Tooth Enamel: An Optical Coherence Tomography Analysis. Pesqui. Bras. Odontopediatria Clín. Integr. 2019, 19, e4002. [Google Scholar] [CrossRef]
- Nanda, K.J.; Naik, S. An In-Vitro Comparative Evaluation of Pre-treatment with Nano-Silver Fluoride on Inhibiting Secondary Caries at Tooth Restoration Interface. Cureus 2020, 12, e7934. [Google Scholar] [CrossRef]
- Vieira Costa e Silva, A.; Teixeira, J.A.; Mota, C.C.; Clayton Cabral Correia Lins, E.; Correia de Melo Júnior, P.; de Souza Lima, M.G.; Arnaud, M.; Galembeck, A.; Targino Gadelha, A.; Pereira, J.R.D.; et al. In vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol. Rev. 2018, 7, 509–520. [Google Scholar] [CrossRef]
- Santos, V.E., Jr.; Vasconcelos Filho, A.; Targino, A.G.; Flores, M.A.; Galembeck, A.; Caldas, A.F., Jr.; Rosenblatt, A. A new “silver-bullet” to treat caries in children—Nano silver fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951. [Google Scholar] [CrossRef]
- Burns, J.; Hollands, K. Nano Silver Fluoride for preventing caries. Evid. Based Dent. 2015, 16, 8–9. [Google Scholar] [CrossRef]
- Butrón-Téllez Girón, C.; Mariel-Cárdenas, J.; Pierdant-Pérez, M.; Hernández-Sierra, J.F.; Morales-Sánchez, J.E.; Ruiz, F. Effectiveness of a combined silver nanoparticles/fluoride varnish in dental remineralization in children: In vivo study. Superf. Vacío 2017, 30, 21–24. [Google Scholar] [CrossRef]
- Salas-Lopez, E.K.; Pierdant-Perez, M.; Hernandez-Sierra, J.F.; Ruiz, F.; Mandeville, P.; Pozos-Guillen, A.J. Effect of Silver Nanoparticle-Added Pit and Fissure Sealant in the Prevention of Dental Caries in Children. J. Clin. Pediatr. Dent. 2017, 41, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Nagireddy, V.R.; Reddy, D.; Kondamadugu, S.; Puppala, N.; Mareddy, A.; Chris, A. Nanosilver Fluoride—A Paradigm Shift for Arrest in Dental Caries in Primary Teeth of Schoolchildren: A Randomized Controlled Clinical Trial. Int. J. Clin. Pediatr. Dent. 2019, 12, 484–490. [Google Scholar] [PubMed]
- Tirupathi, S.; Svsg, N.; Rajasekhar, S.; Nuvvula, S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 2019, 11, e105–e112. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.; Junior, P.C.; Lima, M.G.; e Silva, A.V.; Araujo, J.T.; Gallembeck, A.; de Franca Caldas Junior, A.; Rosenblatt, A. Nano-silver Fluoride at Higher Concentration for Caries Arrest in Primary Molars: A Randomized Controlled Trial. Int. J. Clin. Pediatr. Dent. 2021, 14, 207–211. [Google Scholar] [CrossRef]
- Ahmed, F.; Prashanth, S.T.; Sindhu, K.; Nayak, A.; Chaturvedi, S. Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 46–54. [Google Scholar] [CrossRef]
- Teixeira, J.A.; Silva, A.; Dos Santos Junior, V.E.; de Melo Junior, P.C.; Arnaud, M.; Lima, M.G.; Flores, M.A.P.; Stamford, T.C.M.; Dias Pereira, J.R.; Ribeiro Targino, A.G.; et al. Effects of a New Nano-Silver Fluoride-Containing Dentifrice on Demineralization of Enamel and Streptococcus mutans Adhesion and Acidogenicity. Int. J. Dent. 2018, 2018, 1351925. [Google Scholar] [CrossRef]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. The release of silver nanoparticles from commercial toothbrushes. J. Hazard. Mater. 2017, 322, 270–275. [Google Scholar] [CrossRef]
- Baygin, O.; Tuzuner, T.; Yilmaz, N.; Aksoy, S. Short-term antibacterial efficacy of a new silver nanoparticle-containing toothbrush. J. Pak. Med. Assoc. 2017, 67, 818–819. [Google Scholar]
- Junevicius, J.; Zilinskas, J.; Cesaitis, K.; Cesaitiene, G.; Gleiznys, D.; Mazeliene, Z. Antimicrobial activity of silver and gold in toothpastes: A comparative analysis. Stomatologija 2015, 17, 9–12. [Google Scholar]
- do Nascimento, C.; Paulo, D.F.; Pita, M.S.; Pedrazzi, V.; de Albuquerque Junior, R.F. Microbial diversity of the supra- and subgingival biofilm of healthy individuals after brushing with chlorhexidine- or silver-coated toothbrush bristles. Can. J. Microbiol. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Abadi, M.F.; Mehrabian, S.; Asghari, B.; Namvar, A.E.; Ezzatifar, F.; Lari, A.R. Silver nanoparticles as active ingredient used for alcohol-free mouthwash. GMS Hyg. Infect. Control 2013, 8, Doc05. [Google Scholar] [PubMed]
- Ahmed, O.A.K.; Sibuyi, N.R.S.; Fadaka, A.O.; Maboza, E.; Olivier, A.; Madiehe, A.M.; Meyer, M.; Geerts, G. Prospects of Using Gum Arabic Silver Nanoparticles in Toothpaste to Prevent Dental Caries. Pharmaceutics 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Kassebaum, N.J.; Bernabe, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite-state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef]
- Caufield, P.W.; Schon, C.N.; Saraithong, P.; Li, Y.; Argimon, S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015, 94, 110S–118S. [Google Scholar] [CrossRef]
- Takahashi, N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Favaro, J.C.; de Mello Peixoto, Y.C.T.; Geha, O.; Dias, F.A.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength? Restor. Dent. Endod. 2021, 46, e7. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Marangoni, V.S.; de Abreu Bernardi, A.C.; de Souza Rastelli, A.N.; Hernandes, A.C. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 391–401. [Google Scholar] [CrossRef]
- Ai, M.; Du, Z.; Zhu, S.; Geng, H.; Zhang, X.; Cai, Q.; Yang, X. Composite resin reinforced with silver nanoparticles-laden hydroxyapatite nanowires for dental application. Dent. Mater. 2017, 33, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Gallorini, M.; Di Giulio, M.; Guarnieri, S.; Mariggio, M.A.; Traini, T.; Di Pietro, R.; Cellini, L.; Marsich, E.; Sancilio, S. Adhesion of human gingival fibroblasts/Streptococcus mitis co-culture on the nanocomposite system Chitlac-nAg. J. Mater. Sci. Mater. Med. 2016, 27, 88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Xu, S.M.; Zhou, X. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef]
- Durner, J.; Stojanovic, M.; Urcan, E.; Hickel, R.; Reichl, F.X. Influence of silver nano-particles on monomer elution from light-cured composites. Dent. Mater. 2011, 27, 631–636. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohashi, S.; Aono, M.; Kokubo, T.; Yamada, I.; Yamauchi, J. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent. Mater. 1996, 12, 227–229. [Google Scholar] [CrossRef]
- Arif, W.; Rana, N.F.; Saleem, I.; Tanweer, T.; Khan, M.J.; Alshareef, S.A.; Sheikh, H.M.; Alaryani, F.S.; Al-Kattan, M.O.; Alatawi, H.A.; et al. Antibacterial Activity of Dental Composite with Ciprofloxacin Loaded Silver Nanoparticles. Molecules 2022, 27, 7182. [Google Scholar] [CrossRef]
- Azhar, S.; Rana, N.F.; Kashif, A.S.; Tanweer, T.; Shafique, I.; Menaa, F. DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites. Polymers 2022, 14, 3143. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Chen, C.; Hu, R.; Chen, J.; Rogachev, A.V.; Jiang, X.; Liu, X.; Yang, J. Tricalcium silicate enhanced by silver nanoparticles-bacterial cellulose for dental restoration. Int. J. Biol. Macromol. 2025, 307, 141862. [Google Scholar] [CrossRef]
- Imran, M.; Mallick, R.; Vadlamani, R.; Dhar, A. Assessment of the Antimicrobial Efficacy and Mechanical Properties of Glass Ionomer Cement (GIC) Incorporating Silver Nanoparticles in Varying Concentrations for Pediatric Dental Applications. J. Pharm. Bioallied Sci. 2024, 16, S3689–S3691. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.; de Cara, S.; Diniz, I.M.A.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Imazato, S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J. Dent. 2013, 41, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.; Zhou, X.; Xu, H.H. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef]
- Zhang, K.; Li, F.; Imazato, S.; Cheng, L.; Liu, H.; Arola, D.D.; Bai, Y.; Xu, H.H. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 929–938. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef]
- Zhang, K.; Melo, M.A.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef]
- Aguiar, J.D.; Pedrosa, M.D.S.; Toma, S.H.; Araki, K.; Marques, M.M.; Medeiros, I.S. Antibacterial effect, cytotoxicity, and bond strength of a modified dental adhesive containing silver nanoparticles. Odontology 2023, 111, 420–427. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Deng, J.; Nie, R.; Meng, X. Antibacterial one-step self-etching dental adhesive with silver nanoparticles synthesized in situ. J. Dent. 2023, 129, 104411. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Ekrikaya, S.; Ildiz, N.; Yusufbeyoglu, S.; Ocsoy, I. Evaluation of the antibacterial activity of dental adhesive containing biogenic silver nanoparticles decorated nanographene oxide nanocomposites (Ag@nGO NCs) and effect on bond strength to dentine. Odontology 2024, 112, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.C.; Gomes, B.P.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J. Endod. 2001, 27, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Jeansonne, M.J.; White, R.R. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J. Endod. 1994, 20, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef]
- Mehrvarzfar, P.; Saghiri, M.A.; Asatourian, A.; Fekrazad, R.; Karamifar, K.; Eslami, G.; Dadresanfar, B. Additive effect of a diode laser on the antibacterial activity of 2.5% NaOCl, 2% CHX and MTAD against Enterococcus faecalis contaminating root canals: An in vitro study. J. Oral Sci. 2011, 53, 355–360. [Google Scholar] [CrossRef]
- Hou, X.; Fu, H.; Han, Y.; Xue, Y.; Li, C. Analysis of Transcriptome in Enterococcus faecalis Treated with Silver Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 1046–1055. [Google Scholar] [CrossRef]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Nabavizadeh, M.; Gholami, A.; Aleyasin, Z.S.; Dorostkar, S.; Saliminasab, M.; Ghasemi, Y.; Hemmateenejad, B.; Sharghi, H. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: A promising disinfectant in root canal treatment. Int. Endod. J. 2015, 48, 790–800. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Andrade, G.M.; Tanomaru-Filho, M.; Rodrigues, E.M.; Gomes-Cornelio, A.L.; Faria, G.; Bernardi, M.I.B.; Guerreiro-Tanomaru, J.M. Cytotoxicity, genotoxicity and antibacterial activity of poly(vinyl alcohol)-coated silver nanoparticles and farnesol as irrigating solutions. Arch. Oral Biol. 2017, 84, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, M.; Ghahramani, Y.; Abbaszadegan, A.; Jamshidzadeh, A.; Jenabi, P.; Makarempour, A. In Vivo Biocompatibility of an Ionic Liquid-protected Silver Nanoparticle Solution as Root Canal Irrigant. Iran. Endod. J. 2018, 13, 293–298. [Google Scholar]
- Mishra, P.; Tyagi, S. Surface analysis of gutta percha after disinfecting with sodium hypochlorite and silver nanoparticles by atomic force microscopy: An in vitro study. Dent. Res. J. 2018, 15, 242–247. [Google Scholar] [CrossRef]
- Rodrigues, C.T.; de Andrade, F.B.; de Vasconcelos, L.; Midena, R.Z.; Pereira, T.C.; Kuga, M.C.; Duarte, M.A.H.; Bernardineli, N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2018, 51, 901–911. [Google Scholar] [CrossRef]
- Charannya, S.; Duraivel, D.; Padminee, K.; Poorni, S.; Nishanthine, C.; Srinivasan, M.R. Comparative Evaluation of Antimicrobial Efficacy of Silver Nanoparticles and 2% Chlorhexidine Gluconate When Used Alone and in Combination Assessed Using Agar Diffusion Method: An In vitro Study. Contemp. Clin. Dent. 2018, 9, S204–S209. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Evaluation of Antibacterial Efficacy of Fungal-Derived Silver Nanoparticles against Enterococcus faecalis. Contemp. Clin. Dent. 2018, 9, 45–48. [Google Scholar] [CrossRef]
- Suzuki, T.Y.U.; Gallego, J.; Assuncao, W.G.; Briso, A.L.F.; Dos Santos, P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS ONE 2019, 14, e0217750. [Google Scholar] [CrossRef]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The Effect of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Final Irrigation Solutions on the Fracture Resistance of Root-Filled Teeth. Clin. Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef]
- Afkhami, F.; Ahmadi, P.; Chiniforush, N.; Sooratgar, A. Effect of different activations of silver nanoparticle irrigants on the elimination of Enterococcus faecalis. Clin. Oral Investig. 2021, 25, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Hendi, S.S.; Shiri, M.; Poormoradi, B.; Alikhani, M.Y.; Afshar, S.; Farmani, A. Antibacterial Effects of a 940 nm Diode Laser With/Without Silver Nanoparticles Against Enterococcus faecalis. J. Lasers Med. Sci. 2021, 12, e73. [Google Scholar] [CrossRef] [PubMed]
- Hendi, S.S.; Amiri, N.; Poormoradi, B.; Alikhani, M.Y.; Afshar, S.; Farhadian, M. Antibacterial Effects of Erbium Chromium Laser along with/without Silver Nanoparticles in Root Canals Infected by Enterococcus faecalis. Int. J. Dent. 2021, 2021, 6659146. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, L.; Shahmoradi, M.; Narimani, T. Antimicrobial activity of a new nanobased endodontic irrigation solution: In vitro study. Dent. Hypotheses 2012, 3, 142–146. [Google Scholar] [CrossRef]
- Lotfi, M.; Vosoughhosseini, S.; Ranjkesh, B.; Khani, S.; Saghiri, M.; Zand, V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr. J. Biotechnol. 2011, 10, 6799–6803. [Google Scholar]
- Rodriguez-Chang, S.; Ramirez-Mora, T.; Valle-Bourrouet, G.; Rojas-Campos, N.; Chavarria-Bolanos, D.; Montero-Aguilar, M. Antibacterial Efficacy of a Dispersion of Silver Nanoparticles in Citrate Medium for the Treatment of E. faecalis: An In Vitro Study. Odovtos Int. J. Dent. Sci. 2016, 18, 99–107. [Google Scholar] [CrossRef]
- Gonzalez-Luna, P.; Martinez-Castanon, G.; Zavala-Alonso, N.; Patino-Marin, N.; Nino-Martinez, N.; Moran-Martinez, J.; Ramirez-Gonzalez, J. Bactericide Effect of Silver Nanoparticles as a Final Irrigation Agent in Endodontics on Enterococcus faecalis: An Ex Vivo Study. J. Nanomater. 2016, 2016, 7597295. [Google Scholar] [CrossRef]
- Javidi, M.; Afkhami, F.; Zarei, M.; Ghazvini, K.; Rajabi, O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J. 2014, 40, 61–65. [Google Scholar] [CrossRef]
- Fan, W.; Wu, D.; Tay, F.R.; Ma, T.; Wu, Y.; Fan, B. Effects of adsorbed and templated nanosilver in mesoporous calcium-silicate nanoparticles on inhibition of bacteria colonization of dentin. Int. J. Nanomed. 2014, 9, 5217–5230. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, X.; Chen, J.; Feng, D.; Mei, L.; Huang, Y.; Quan, G.; Zhu, C.; Singh, V.; Ran, H.; et al. A liquid crystalline precursor incorporating chlorhexidine acetate and silver nanoparticles for root canal disinfection. Biomater. Sci. 2018, 6, 596–603. [Google Scholar] [CrossRef]
- Nayyar, P.; Sethi, A.; Thakur, D.; Khullar, S.; Gayati, S.; Adarsh, K. Antibacterial Effect of Silver Nanoparticle Gel as an Intracanal Medicament in Combination with Other Medicaments against Enterococcus faecalis: An In vitro Study. J. Pharm. Bioallied Sci. 2021, 13, S408–S411. [Google Scholar] [CrossRef] [PubMed]
- Haripriya, S.A.P. Antimicrobial efficacy of silver nanoparticles of Aloe vera. J. Adv. Pharm. Educ. Res. 2017, 7, 163–167. [Google Scholar]
- Alabdulmohsen, Z.A.; Saad, A.Y. Antibacterial effect of silver nanoparticles against Enterococcus faecalis. Saudi Endod. J. 2017, 7, 29–35. [Google Scholar]
- He, Y.; Zhang, Y.; Hu, F.; Chen, M.; Wang, B.; Li, Y.; Xu, H.; Dong, N.; Zhang, C.; Hu, Y.; et al. Photosensitive Hydrogels Encapsulating DPSCs and AgNPs for Dental Pulp Regeneration. Int. Dent. J. 2024, 74, 836–846. [Google Scholar] [CrossRef]
- Mahmoud, A.; Moussa, S.; El Backly, R.; El-Gendy, R. Investigating the residual effect of silver nanoparticles gel as an intra-canal medicament on dental pulp stromal cells. BMC Oral Health 2022, 22, 545. [Google Scholar] [CrossRef]
- Shantiaee, Y.; Maziar, F.; Dianat, O.; Mahjour, F. Comparing microleakage in root canals obturated with nanosilver coated gutta-percha to standard gutta-percha by two different methods. Iran. Endod. J. 2011, 6, 140–145. [Google Scholar]
- Mozayeni, M.A.; Dianat, O.; Tahvildari, S.; Mozayani, M.; Paymanpour, P. Subcutaneous Reaction of Rat Tissues to Nanosilver Coated Gutta-Percha. Iran. Endod. J. 2017, 12, 157–161. [Google Scholar]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Afkhami, F.; Nasri, S.; Valizadeh, S. Bacterial leakage assessment in root canals sealed with AH Plus sealer modified with silver nanoparticles. BMC Oral Health 2021, 21, 577. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Weir, M.D.; Xie, X.; Bai, Y.; Xu, H.H.K. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent. Mater. 2019, 35, 1117–1129. [Google Scholar] [CrossRef]
- Zand, V.; Lotfi, M.; Aghbali, A.; Mesgariabbasi, M.; Janani, M.; Mokhtari, H.; Tehranchi, P.; Pakdel, S.M. Tissue Reaction and Biocompatibility of Implanted Mineral Trioxide Aggregate with Silver Nanoparticles in a Rat Model. Iran. Endod. J. 2016, 11, 13–16. [Google Scholar] [PubMed]
- Jonaidi-Jafari, N.; Izadi, M.; Javidi, P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J. Clin. Exp. Dent. 2016, 8, e22–e26. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y. Characterization and antimicrobial efficacy of Portland cement impregnated with silver nanoparticles. J. Adv. Prosthodont. 2017, 9, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bahador, A.; Pourakbari, B.; Bolhari, B.; Hashemi, F.B. In vitro evaluation of the antimicrobial activity of nanosilver-mineral trioxide aggregate against frequent anaerobic oral pathogens by a membrane-enclosed immersion test. Biomed. J. 2015, 38, 77–83. [Google Scholar]
- Sheethal Dsouza, T.; Shetty, A.; Dsouza, N. Evaluation of pH, Calcium Ion Release, and Dimensional Stability of an Experimental Silver Nanoparticle-Incorporated Calcium Silicate-Based Cement. Bioinorg. Chem. Appl. 2021, 2021, 3919543. [Google Scholar] [CrossRef]
- Samiei, M.; Ghasemi, N.; Asl-Aminabadi, N.; Divband, B.; Golparvar-Dashti, Y.; Shirazi, S. Zeolite-silver-zinc nanoparticles: Biocompatibility and their effect on the compressive strength of mineral trioxide aggregate. J. Clin. Exp. Dent. 2017, 9, e356–e360. [Google Scholar] [CrossRef]
- Samiei, M.; Aghazadeh, M.; Lotfi, M.; Shakoei, S.; Aghazadeh, Z.; Vahid Pakdel, S.M. Antimicrobial Efficacy of Mineral Trioxide Aggregate with and without Silver Nanoparticles. Iran. Endod. J. 2013, 8, 166–170. [Google Scholar]
- Vazquez-Garcia, F.; Tanomaru-Filho, M.; Chavez-Andrade, G.M.; Bosso-Martelo, R.; Basso-Bernardi, M.I.; Guerreiro-Tanomaru, J.M. Effect of Silver Nanoparticles on Physicochemical and Antibacterial Properties of Calcium Silicate Cements. Braz. Dent. J. 2016, 27, 508–514. [Google Scholar] [CrossRef]
- Oyarzun, A.; Cordero, A.M.; Whittle, M. Immunohistochemical evaluation of the effects of sodium hypochlorite on dentin collagen and glycosaminoglycans. J. Endod. 2002, 28, 152–156. [Google Scholar] [CrossRef]
- Pashley, E.L.; Birdsong, N.L.; Bowman, K.; Pashley, D.H. Cytotoxic effects of NaOCl on vital tissue. J. Endod. 1985, 11, 525–528. [Google Scholar] [CrossRef]
- de Almeida, J.; Hoogenkamp, M.; Felippe, W.T.; Crielaard, W.; van der Waal, S.V. Effectiveness of EDTA and Modified Salt Solution to Detach and Kill Cells from Enterococcus faecalis Biofilm. J. Endod. 2016, 42, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Thoma, K.; Zehnder, M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J. Endod. 2004, 30, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Ferraz, C.C.; Vianna, M.E.; Rosalen, P.L.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. In vitro antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz. Dent. J. 2002, 13, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kayaoglu, G.; Erten, H.; Alacam, T.; Orstavik, D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int. Endod. J. 2005, 38, 483–488. [Google Scholar] [CrossRef]
- Torabinejad, M. Clinical applications of mineral trioxide aggregate. Alpha Omegan 2004, 97, 23–31. [Google Scholar] [CrossRef]
- Leal, F.; De-Deus, G.; Brandao, C.; Luna, A.; Souza, E.; Fidel, S. Similar sealability between bioceramic putty ready-to-use repair cement and white MTA. Braz. Dent. J. 2013, 24, 362–366. [Google Scholar] [CrossRef]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 2013, 62, 95–162. [Google Scholar] [CrossRef]
- Sam, G.; Pillai, B.R. Evolution of Barrier Membranes in Periodontal Regeneration—“Are the third Generation Membranes really here?”. J. Clin. Diagn. Res. 2014, 8, ZE14–ZE17. [Google Scholar] [CrossRef]
- Pandey, A.; Yang, T.S.; Yang, T.I.; Belem, W.F.; Teng, N.C.; Chen, I.W.; Huang, C.S.; Kareiva, A.; Yang, J.C. An Insight into Nano Silver Fluoride-Coated Silk Fibroin Bioinspired Membrane Properties for Guided Tissue Regeneration. Polymers 2021, 13, 2659. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Rani, S.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Nagarajan, S.; Naveen, A. Evaluation of the Antibacterial Effect of Silver Nanoparticles on Guided Tissue Regeneration Membrane Colonization—An in Vitro Study. J. Int. Acad. Periodontol. 2015, 17, 66–76. [Google Scholar] [PubMed]
- Craciunescu, O.; Seciu, A.M.; Manoiu, V.S.; Trif, M.; Moisei, M.; Nicu, A.L.; Zarnescu, O. Biosynthesis of silver nanoparticles in collagen gel improves their medical use in periodontitis treatment. Part. Sci. Technol. 2018, 37, 757–763. [Google Scholar] [CrossRef]
- Habiboallah, G.; Mahdi, Z.; Majid, Z.; Nasroallah, S.; Taghavi, A.M.; Forouzanfar, A.; Arjmand, N. Enhancement of Gingival Wound Healing by Local Application of Silver Nanoparticles Periodontal Dressing Following Surgery: A Histological Assessment in Animal Model. Mod. Res. Inflamm. 2014, 3, 128–138. [Google Scholar] [CrossRef]
- Budhi, C.P.; Rizky, J.S.; Isa, M.; Iim, H.; Eva, M.W.; Nunung, R.; Indra, M. Evaluation of Silver Nanoparticles Addition in Periodontal Dressing for Wound Tissue Healing By 99mTc-ciprofloxacin. J. Young Pharm. 2019, 11, 17–20. [Google Scholar]
- Sun, J.; Wang, L.; Wang, J.; Li, Y.; Zhou, X.; Guo, X.; Zhang, T.; Guo, H. Characterization and evaluation of a novel silver nanoparticles-loaded polymethyl methacrylate denture base: In vitro and in vivo animal study. Dent. Mater. J. 2021, 40, 1100–1108. [Google Scholar] [CrossRef]
- Bacali, C.; Carpa, R.; Buduru, S.; Moldovan, M.L.; Baldea, I.; Constantin, A.; Moldovan, M.; Prodan, D.; Dascalu Rusu, L.M.; Lucaciu, O.; et al. Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials 2021, 11, 1643. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Flores-Arriaga, J.C.; Serrano-Diaz, P.N.; Gonzalez-Garcia, I.A.; Viveros-Garcia, J.C.; Villanueva-Vilchis, M.D.C.; Villanueva-Sanchez, F.G.; Garcia-Contreras, R.; Arenas-Arrocena, M.C. Antifungal biomaterial for reducing infections caused by Candida albicans in edentulous patients. Gac. Med. Mex. 2021, 157, 422–427. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- Jo, J.K.; El-Fiqi, A.; Lee, J.H.; Kim, D.A.; Kim, H.W.; Lee, H.H. Rechargeable microbial anti-adhesive polymethyl methacrylate incorporating silver sulfadiazine-loaded mesoporous silica nanocarriers. Dent. Mater. 2017, 33, e361–e372. [Google Scholar] [CrossRef]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, A.; Sahin, O.; Kurkcuoglu, I.; Dede, D.O.; Ozdemir, T.; Hazer, B. Silver nanoparticle incorporation effect on mechanical and thermal properties of denture base acrylic resins. J. Appl. Oral Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Mahross, H.Z.; Baroudi, K. Effect of silver nanoparticles incorporation on viscoelastic properties of acrylic resin denture base material. Eur. J. Dent. 2015, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, T.; Hamedi, R.F.; Ezzati, B. Does Addition of Silver Nanoparticles to Denture Base Resin Increase Its Thermal Conductivity? J. Dent. Sch. 2014, 32, 139–144. [Google Scholar]
- Suganya, S.; Ahila, S.C.; Kumar, B.M.; Kumar, M.V. Evaluation and comparison of anti-Candida effect of heat cure polymethylmethacrylate resin enforced with silver nanoparticles and conventional heat cure resins: An in vitro study. Indian J. Dent. Res. 2014, 25, 204–207. [Google Scholar] [CrossRef]
- Hamedi-Rad, F.; Ghaffari, T.; Rezaii, F.; Ramazani, A. Effect of nanosilver on thermal and mechanical properties of acrylic base complete dentures. J. Dent. 2014, 11, 495–505. [Google Scholar]
- Ghaffari, T.; Hamedirad, F.; Ezzati, B. In Vitro Comparison of Compressive and Tensile Strengths ofAcrylic Resins Reinforced by Silver Nanoparticles at 2% and 0.2% Concentrations. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 204–209. [Google Scholar]
- Nam, K.Y.; Lee, C.H.; Lee, C.J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, e413–e419. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; de Camargo, E.R.; Filho, A.C.; Barbosa, D.B. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. J. Prosthodont. 2012, 21, 7–15. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Mendieta, I.; Nunez-Anita, R.E.; Cajero-Juarez, M.; Castano, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar]
- Kassaee, M.; Akhavan, A.; Sheikh, N.; Sodagar, A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. J. Appl. Polym. Sci. 2008, 110, 1699–1703. [Google Scholar] [CrossRef]
- Ortiz-Magdaleno, M.; Sanchez-Vargas, L.; Gardea-Contreras, D.; Campos-Ibarra, V.; Pozos-Guillen, A.; Marquez-Preciado, R. Antibiofilm properties of silver nanoparticles incorporated into polymethyl methacrylate used for dental applications. Biomed. Mater. Eng. 2023, 34, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Kasperski, J.; Barszczewska-Rybarek, I.; Zmudzki, J. Sorption, solubility, bond strength and hardness of denture soft lining incorporated with silver nanoparticles. Int. J. Mol. Sci. 2012, 14, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J. Adv. Prosthodont. 2011, 3, 20–24. [Google Scholar] [CrossRef]
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Zmudzki, J.; Krol, W.; Lukaszczyk, J. Antifungal activity of denture soft lining material modified by silver nanoparticles-a pilot study. Int. J. Mol. Sci. 2011, 12, 4735–4744. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Alla, R.K.; Tellapragada, C.; Gupta, L.; Upadhy Perampalli, N. Antimicrobial activity and properties of irreversible hydrocolloid impression materials incorporated with silver nanoparticles. J. Prosthet. Dent. 2016, 115, 722–728. [Google Scholar] [CrossRef]
- Singer, L.; Beuter, L.; Karacic, S.; Bierbaum, G.; Karacic, J.; Bourauel, C. Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control. Gels 2024, 10, 600. [Google Scholar] [CrossRef]
- Sundeep, D.; Vijaya Kumar, T.; Rao, P.S.S.; Ravikumar, R.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef]
- Magalhaes, A.P.R.; Santos, L.B.; Lopes, L.G.; Estrela, C.R.d.A.; Carlos Estrela, E.M.T.; Bakuzis, A.F.; Cardoso, P.C.; Carriao, M.S. Nanosilver Application in Dental Cements. Int. Sch. Res. Netw. 2012, 2012, 365438. [Google Scholar] [CrossRef]
- Meran, Z.; Besinis, A.; De Peralta, T.; Handy, R.D. Antifungal properties and biocompatibility of silver nanoparticle coatings on silicone maxillofacial prostheses in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1038–1051. [Google Scholar] [CrossRef]
- de Castro, D.T.; do Nascimento, C.; Alves, O.L.; de Souza Santos, E.; Agnelli, J.A.M.; Dos Reis, A.C. Analysis of the oral microbiome on the surface of modified dental polymers. Arch. Oral Biol. 2018, 93, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Kamemizu, H.; Wakamatsu, N.; Doi, Y. Effects of dental porcelain containing silver nanoparticles on static fatigue. Dent. Mater. J. 2013, 32, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Nonogaki, R.; Fujieda, T.; Ishigami, H.; Kurachi, M.; Kamemizu, H.; Wakamatsu, N.; Doi, Y. Toughening of CAD/CAM all-ceramic crowns by staining slurry. Dent. Mater. J. 2012, 31, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Addition of platinum and silver nanoparticles to toughen dental porcelain. Dent. Mater. J. 2012, 31, 711–716. [Google Scholar] [CrossRef]
- Hashem, R.M.M.; Mohsen, C.A.; Abu-Eittah, M.R. Effect of silver nanoparticles and silver hydroxyapatite nanoparticles on color and fracture strength of dental ceramic. Mater. Sci. Med. 2015, 61, 2–7. [Google Scholar]
- Uno, M.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Effects of adding silver nanoparticles on the toughening of dental porcelain. J. Prosthet. Dent. 2013, 109, 241–247. [Google Scholar] [CrossRef]
- Ma, M.; Kazemzadeh-Narbat, M.; Hui, Y.; Lu, S.; Ding, C.; Chen, D.D.; Hancock, R.E.; Wang, R. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J. Biomed. Mater. Res. A 2012, 100, 278–285. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. A 2015, 103, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silverhydroxyapatite composite coatings through two-stage electrochemical synthesis. Surf. Coat. Technol. 2016, 301, 13–19. [Google Scholar] [CrossRef]
- Della Valle, C.; Visai, L.; Santin, M.; Cigada, A.; Candiani, G.; Pezzoli, D.; Arciola, C.R.; Imbriani, M.; Chiesa, R. A novel antibacterial modification treatment of titanium capable to improve osseointegration. Int. J. Artif. Organs 2012, 35, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Durgalakshmi, D.; Balakumar, S.; Raja, C.A.; George, R.P.; Mudali, U.K. Structural, Morphological and Antibacterial Investigation of Ag-Impregnated Sol-Gel-Derived 45S5 NanoBioglass Systems. J. Nanosci. Nanotechnol. 2015, 15, 4285–4295. [Google Scholar] [CrossRef]
- Flores, C.Y.; Diaz, C.; Rubert, A.; Benitez, G.A.; Moreno, M.S.; Fernandez Lorenzo de Mele, M.A.; Salvarezza, R.C.; Schilardi, P.L.; Vericat, C. Spontaneous adsorption of silver nanoparticles on Ti/TiO2 surfaces. Antibacterial effect on Pseudomonas aeruginosa. J. Colloid Interface Sci. 2010, 350, 402–408. [Google Scholar] [CrossRef]
- Flores, C.Y.; Minan, A.G.; Grillo, C.A.; Salvarezza, R.C.; Vericat, C.; Schilardi, P.L. Citrate-capped silver nanoparticles showing good bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces 2013, 5, 3149–3159. [Google Scholar] [CrossRef]
- Heng Li, H.u.a.n.g.; Yin Yu, C.h.a.n.g.; Meng Cheng, L.a.i.; Cai Rong, L.i.n.; Chih Ho, L.a.i.; Shieh, T.M. Antibacterial TaN-Ag coatings on titanium dental implants. Surf. Coat. Technol. 2010, 205, 1636–1641. [Google Scholar] [CrossRef]
- Lampe, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombradi, Z.; Hajdu, P.; Hegedus, V.; Racz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2011, 8, 529–539. [Google Scholar] [CrossRef]
- Marsich, E.; Travan, A.; Donati, I.; Turco, G.; Kulkova, J.; Moritz, N.; Aro, H.T.; Crosera, M.; Paoletti, S. Biological responses of silver-coated thermosets: An in vitro and in vivo study. Acta Biomater. 2013, 9, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Igai, F.; Tamaki, R.; Tortamano Neto, P.; Nakamae, A.E.; Mori, M. Use of Silver Nanoparticles Reduces Internal Contamination of External Hexagon Implants by Candida albicans. Braz. Dent. J. 2015, 26, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Odatsu, T.; Kuroshima, S.; Sato, M.; Takase, K.; Valanezhad, A.; Naito, M.; Sawase, T. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics 2020, 9, 347. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Li, W. Antibacterial TiO2 Coating Incorporating Silver Nanoparticles by Microarc Oxidation and Ion Implantation. J. Nanomater. 2013, 2013, 542878. [Google Scholar] [CrossRef]
- Qiang, W.P.; He, X.D.; Zhang, K.; Cheng, Y.F.; Lu, Z.S.; Li, C.M.; Kang, E.T.; Xia, Q.Y.; Xu, L.Q. Mussel Adhesive Mimetic Silk Sericin Prepared by Enzymatic Oxidation for the Construction of Antibacterial Coatings. ACS Biomater. Sci. Eng. 2021, 7, 3379–3388. [Google Scholar] [CrossRef]
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653–664. [Google Scholar]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110210. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Barakat, N.A.; Kanjwal, M.A.; Nirmala, R.; Lee, J.H.; Kim, H.; Kim, H.Y. Electrospun titanium dioxide nanofibers containing hydroxyapatite and silver nanoparticles as future implant materials. J. Mater. Sci. Mater. Med. 2010, 21, 2551–2559. [Google Scholar] [CrossRef]
- Svensson, S.; Suska, F.; Emanuelsson, L.; Palmquist, A.; Norlindh, B.; Trobos, M.; Backros, H.; Persson, L.; Rydja, G.; Ohrlander, M.; et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine 2013, 9, 1048–1056. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Wang, D.; Liu, H.; Boughton, R.I. In situ fabrication of silver nanoparticle-filled hydrogen titanate nanotube layer on metallic titanium surface for bacteriostatic and biocompatible implantation. Int. J. Nanomed. 2013, 8, 2903–2916. [Google Scholar]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverria, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Zhou, W.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.; Yu, S.; Zheng, Y. Biomimetic AgNPs@antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, Y.; Li, M.; Cheng, Y.; Zheng, Y. Bioinspired and Biomimetic AgNPs/Gentamicin-Embedded Silk Fibroin Coatings for Robust Antibacterial and Osteogenetic Applications. ACS Appl. Mater. Interfaces 2017, 9, 25830–25846. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef]
- Senthil, R.; Cakir, S. Nano apatite growth on demineralized bone matrix capped with curcumin and silver nanoparticles: Dental implant mechanical stability and optimal cell growth analysis. J. Oral Biosci. 2024, 66, 232–240. [Google Scholar] [CrossRef]
- Yu, C.; Yu, Y.; Lu, Y.; Quan, K.; Mao, Z.; Zheng, Y.; Qin, L.; Xia, D. UiO-66/AgNPs Coating for Dental Implants in Preventing Bacterial Infections. J. Dent. Res. 2024, 103, 516–525. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and Antimicrobial Properties of Silver Nanoparticles against Streptococcus mutans on Brackets and Wires Used for Orthodontic Treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Metin-Gursoy, G.; Taner, L.; Akca, G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017, 39, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, T.; Arash, V.; Rabiee, S.M.; Rajabnia, R.; Pourzare, A.; Rakhshan, V. Antimicrobial effect, frictional resistance, and surface roughness of stainless steel orthodontic brackets coated with nanofilms of silver and titanium oxide: A preliminary study. Microsc. Res. Tech. 2017, 80, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Metin-Gursoy, G.; Taner, L.; Baris, E. Biocompatibility of nanosilver-coated orthodontic brackets: An in vivo study. Prog. Orthod. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Al-Thomali, Y. Shear bond strength of orthodontic brackets after adding silver nanoparticles to a nano-bond adhesive at different thermal cycles and cyclic loading—An in vitro study. J. Orthod. Sci. 2022, 11, 28. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 24. [Google Scholar] [CrossRef]
- Nafarrate-Valdez, R.A.; Martinez-Martinez, R.E.; Zaragoza-Contreras, E.A.; Ayala-Herrera, J.L.; Dominguez-Perez, R.A.; Reyes-Lopez, S.Y.; Donohue-Cornejo, A.; Cuevas-Gonzalez, J.C.; Loyola-Rodriguez, J.P.; Espinosa-Cristobal, L.F. Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina 2022, 58, 877. [Google Scholar] [CrossRef]
- Lee, B.S.; Lin, Y.C.; Hsu, W.C.; Hou, C.H.; Shyue, J.J.; Hsiao, S.Y.; Wu, P.J.; Lee, Y.T.; Luo, S.C. Engineering Antifouling and Antibacterial Stainless Steel for Orthodontic Appliances through Layer-by-Layer Deposition of Nanocomposite Coatings. ACS Appl. Bio Mater. 2020, 3, 486–494. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kandasamy, R.; Sivaraman, U.S.; Nandkumar, M.A.; Nair, P.D. Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J. Med. Res. 2016, 144, 580–586. [Google Scholar] [CrossRef]
- Mhaske, A.R.; Shetty, P.C.; Bhat, N.S.; Ramachandra, C.S.; Laxmikanth, S.M.; Nagarahalli, K.; Tekale, P.D. Antiadherent and antibacterial properties of stainless steel and NiTi orthodontic wires coated with silver against Lactobacillus acidophilus—An in vitro study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef]
- Hernandez-Gomora, A.E.; Lara-Carrillo, E.; Robles-Navarro, J.B.; Scougall-Vilchis, R.J.; Hernandez-Lopez, S.; Medina-Solis, C.E.; Morales-Luckie, R.A. Biosynthesis of Silver Nanoparticles on Orthodontic Elastomeric Modules: Evaluation of Mechanical and Antibacterial Properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef]
- Venugopal, A.; Muthuchamy, N.; Tejani, H.; Gopalan, A.I.; Lee, K.P.; Lee, H.J.; Kyung, H.M. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J. Orthod. 2017, 47, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Bahador, A.; Ayatollahi, B.; Akhavan, A.; Pourhajibagher, M.; Kharazifard, M.J.; Sodagar, A. Antimicrobial Efficacy of Silver Nanoparticles Incorporated in an Orthodontic Adhesive: An Animal Study. Front. Dent. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Akhavan, A.; Hashemi, E.; Arab, S.; Pourhajibagher, M.; Sodagar, K.; Kharrazifard, M.J.; Bahador, A. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog. Orthod. 2016, 17, 40. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.; Garcia, I.M.; Arthur, R.A.; Samuel, S.M.; Collares, F.M. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef]
- Blocher, S.; Frankenberger, R.; Hellak, A.; Schauseil, M.; Roggendorf, M.J.; Korbmacher-Steiner, H.M. Effect on enamel shear bond strength of adding microsilver and nanosilver particles to the primer of an orthodontic adhesive. BMC Oral Health 2015, 15, 42. [Google Scholar] [CrossRef]
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H.K. Novel Dental Cement to Combat Biofilms and Reduce Acids for Orthodontic Applications to Avoid Enamel Demineralization. Materials 2016, 9, 413. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Weir, M.D.; Bai, Y.; Xu, H.H. Antibacterial and protein-repellent orthodontic cement to combat biofilms and white spot lesions. J. Dent. 2015, 43, 1529–1538. [Google Scholar] [CrossRef]
- Moreira, D.M.; Oei, J.; Rawls, H.R.; Wagner, J.; Chu, L.; Li, Y.; Zhang, W.; Whang, K. A novel antimicrobial orthodontic band cement with in situ-generated silver nanoparticles. Angle Orthod. 2015, 85, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.C.O.; Nunes, L.P.; Batista, E.S.; Viana, M.M.; Rodrigues, M.C.; Bueno-Silva, B.; Roscoe, M.G. Experimental composite containing silicon dioxide-coated silver nanoparticles for orthodontic bonding: Antimicrobial activity and shear bond strength. Dent. Press. J. Orthod. 2022, 27, e222116. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Usefi Mashoof, R.; Khanizadeh, S.; Ghaderi, E.; Farhadian, M.; Miresmaeili, A. Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs. those wearing conventional retainers: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 155–160. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Pourakbari, B.; Bahador, A. Effects of Baseplates of Orthodontic Appliances with in situ generated Silver Nanoparticles on Cariogenic Bacteria: A Randomized, Double-blind Cross-over Clinical Trial. J. Contemp. Dent. Pract. 2015, 16, 291–298. [Google Scholar] [CrossRef]
- Mazumder, J.A.; Khatoon, N.; Batra, P.; Sardar, M. Biosynthesized silver nanoparticles for orthodontic applications. Adv. Sci. Eng. Med. 2018, 10, 1169–1173. [Google Scholar] [CrossRef]
- Mirhashemi, A.; Bahador, A.; Sodagar, A.; Pourhajibagher, M.; Amiri, A.; Gholamrezayi, E. Evaluation of antimicrobial properties of nano-silver particles used in orthodontics fixed retainer composites: An experimental in-vitro study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 87–93. [Google Scholar] [CrossRef]
- Najafi, H.Z.; Azadeh, N.; Motamedifar, M. Evaluation of the Preventive Effect of Composites Containing Silver and TiO2 Nanoparticles on Demineralization around Orthodontic Brackets. J. Contemp. Dent. Pract. 2020, 21, 874–879. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Tay, L.Y. Antibacterial and white spot lesions preventive effect of an orthodontic resin modified with silver-nanoparticles. J. Clin. Exp. Dent. 2021, 13, e685–e691. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Tay, L.Y. Effect of an orthodontic resin modified with silver-nanoparticles on enamel color change. J. Clin. Exp. Dent. 2022, 14, e241–e246. [Google Scholar] [CrossRef]
- Santoso, J.; Purbiati, M.; Krisnawati. Antibacterial activity of silver nanoparticles on fixed retainer adhesive toward treponema denticola. Int. J. Appl. Pharm. 2019, 11, 198–200. [Google Scholar] [CrossRef]
- Ali, A.; Ismail, H.; Amin, K. Effect of nanosilver mouthwash on prevention of white spot lesions in patients undergoing fixed orthodontic treatment—A randomized double-blind clinical trial. J. Dent. Sci. 2022, 17, 249–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).