The Impact of Arginine Side Chains on the Mechanism of Polycondensation of Silicic Acid in Bioinspired Mineralization
Abstract
1. Introduction
2. Results
2.1. Molecular Attack Mechanism
2.2. Anion Attack Mechanism
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Silica Market Size, Share, Growth Analysis Report 2030. Available online: https://www.grandviewresearch.com/industry-analysis/silica-market (accessed on 10 March 2025).
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-08-050109-3. [Google Scholar]
- Armbrust, E.V. The Life of Diatoms in the World’s Oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Kröger, N. Silica Formation in Diatoms: The Function of Long-Chain Polyamines and Silaffins. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E. Silica Biomineralisation in Diatoms: The Model Organism Thalassiosira Pseudonana. ChemBioChem 2008, 9, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Poulsen, N. Diatoms—From Cell Wall Biogenesis to Nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification—Moving Forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Mayzel, B.; Aram, L.; Varsano, N.; Wolf, S.G.; Gal, A. Structural Evidence for Extracellular Silica Formation by Diatoms. Nat. Commun. 2021, 12, 4639. [Google Scholar] [CrossRef]
- Hildebrand, M.; Volcani, B.E.; Gassmann, W.; Schroeder, J.I. A Gene Family of Silicon Transporters. Nature 1997, 385, 688–689. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-Specific Polyamines from Diatoms Control Silica Morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Poulsen, N.; Sumper, M.; Kröger, N. Biosilica Formation in Diatoms: Characterization of Native Silaffin-2 and Its Role in Silica Morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef]
- Sumper, M. Biomimetic Patterning of Silica by Long-Chain Polyamines. Angew. Chem. Int. Ed. 2004, 43, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Lutz, K.; Gröger, C.; Sumper, M.; Brunner, E. Biomimetic Silica Formation: Analysis of the Phosphate-Induced Self-Assembly of Polyamines. Phys. Chem. Chem. Phys. 2005, 7, 2812–2815. [Google Scholar] [CrossRef] [PubMed]
- Lechner, C.C.; Becker, C.F.W. Exploring the Effect of Native and Artificial Peptide Modifications on Silaffin Induced Silica Precipitation. Chem. Sci. 2012, 3, 3500–3504. [Google Scholar] [CrossRef]
- Poulsen, N.; Scheffel, A.; Sheppard, V.C.; Chesley, P.M.; Kröger, N. Pentalysine Clusters Mediate Silica Targeting of Silaffins in Thalassiosira Pseudonana. J. Biol. Chem. 2013, 288, 20100–20109. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. A Sequence-Function Analysis of the Silica Precipitating Silaffin R5 Peptide. J. Pept. Sci. 2014, 20, 152–158. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef]
- Lenoci, L.; Camp, P.J. Self-Assembly of Peptide Scaffolds in Biosilica Formation: Computer Simulations of a Coarse-Grained Model. J. Am. Chem. Soc. 2006, 128, 10111–10117. [Google Scholar] [CrossRef]
- Lenoci, L.; Camp, P.J. Diatom Structures Templated by Phase-Separated Fluids. Langmuir 2008, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Strobl, J.; Kozak, F.; Kamalov, M.; Reichinger, D.; Kurzbach, D.; Becker, C.F. Understanding Self-Assembly of Silica-Precipitating Peptides to Control Silica Particle Morphology. Adv. Mater. 2023, 35, 2207586. [Google Scholar] [CrossRef]
- Kozak, F.; Brandis, D.; Pötzl, C.; Epasto, L.M.; Reichinger, D.; Obrist, D.; Peterlik, H.; Polyansky, A.; Zagrovic, B.; Daus, F.; et al. An Atomistic View on the Mechanism of Diatom Peptide-Guided Biomimetic Silica Formation. Adv. Sci. 2024, 11, 2401239. [Google Scholar] [CrossRef]
- Zhai, H.; Bendikov, T.; Gal, A. Phase Separation of Oppositely Charged Polymers Regulates Bioinspired Silicification. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115930. [Google Scholar] [CrossRef]
- Zhai, H.; Fan, Y.; Zhang, W.; Varsano, N.; Gal, A. Polymer-Rich Dense Phase Can Concentrate Metastable Silica Precursors and Regulate Their Mineralization. ACS Biomater. Sci. Eng. 2023, 9, 601–607. [Google Scholar] [CrossRef]
- Coradin, T.; Durupthy, O.; Livage, J. Interactions of Amino-Containing Peptides with Sodium Silicate and Colloidal Silica: A Biomimetic Approach of Silicification. Langmuir 2002, 18, 2331–2336. [Google Scholar] [CrossRef]
- Coradin, T.; Coupé, A.; Livage, J. Interactions of Bovine Serum Albumin and Lysozyme with Sodium Silicate Solutions. Colloids Surf. B Biointerfaces 2003, 29, 189–196. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Dickerson, M.B.; Sandhage, K.H.; Spain, J.C. Rapid, Room-Temperature Synthesis of Antibacterial Bionanocomposites of Lysozyme with Amorphous Silica or Titania. Small 2006, 2, 640–643. [Google Scholar] [CrossRef]
- Gigli, L.; Ravera, E.; Calderone, V.; Luchinat, C. On the Mechanism of Bioinspired Formation of Inorganic Oxides: Structural Evidence of the Electrostatic Nature of the Interaction between a Mononuclear Inorganic Precursor and Lysozyme. Biomolecules 2021, 11, 43. [Google Scholar] [CrossRef]
- Pyne, P.; Mitra, R.K. Excipients Do Regulate Phase Separation in Lysozyme and Thus Also Its Hydration. J. Phys. Chem. Lett. 2022, 13, 931–938. [Google Scholar] [CrossRef]
- Bruno, F.; Gigli, L.; Ferraro, G.; Cavallo, A.; Michaelis, V.K.; Goobes, G.; Fratini, E.; Ravera, E. Lysozyme Is Sterically Trapped Within the Silica Cage in Bioinspired Silica–Lysozyme Composites: A Multi-Technique Understanding of Elusive Protein–Material Interactions. Langmuir 2022, 38, 8030–8037. [Google Scholar] [CrossRef]
- Loeb, J. The colloidal behavior of proteins. J. Gen. Physiol. 1921, 3, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Macchiagodena, M.; Fragai, M.; Gallo, A.; Pagliai, M.; Ravera, E. The Role of Lysozyme in the Formation of Bioinspired Silicon Dioxide. Chem. A Eur. J. 2024, 30, e202401249. [Google Scholar] [CrossRef] [PubMed]
- Novi Inverardi, G.; Petrolli, L.; Carnovale, F.; Bartocci, A.; Taioli, S.; Lattanzi, G. Adsorption of Silica Oligomers on Biomolecules: Structural and Dynamical Insights for Atom Probe Tomography via Classic Molecular Dynamics Simulations. Comput. Struct. Biotechnol. J. 2025, 27, 2537–2543. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Meng, C. Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation. Int. J. Mol. Sci. 2019, 20, 3037. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Feng, Z.; Meng, C. The Promoter Role of Amines in the Condensation of Silicic Acid: A First-Principles Investigation. ACS Omega 2021, 6, 22811–22819. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Becke, A.D. A New Mixing of Hartree–Fock and Local Density-functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
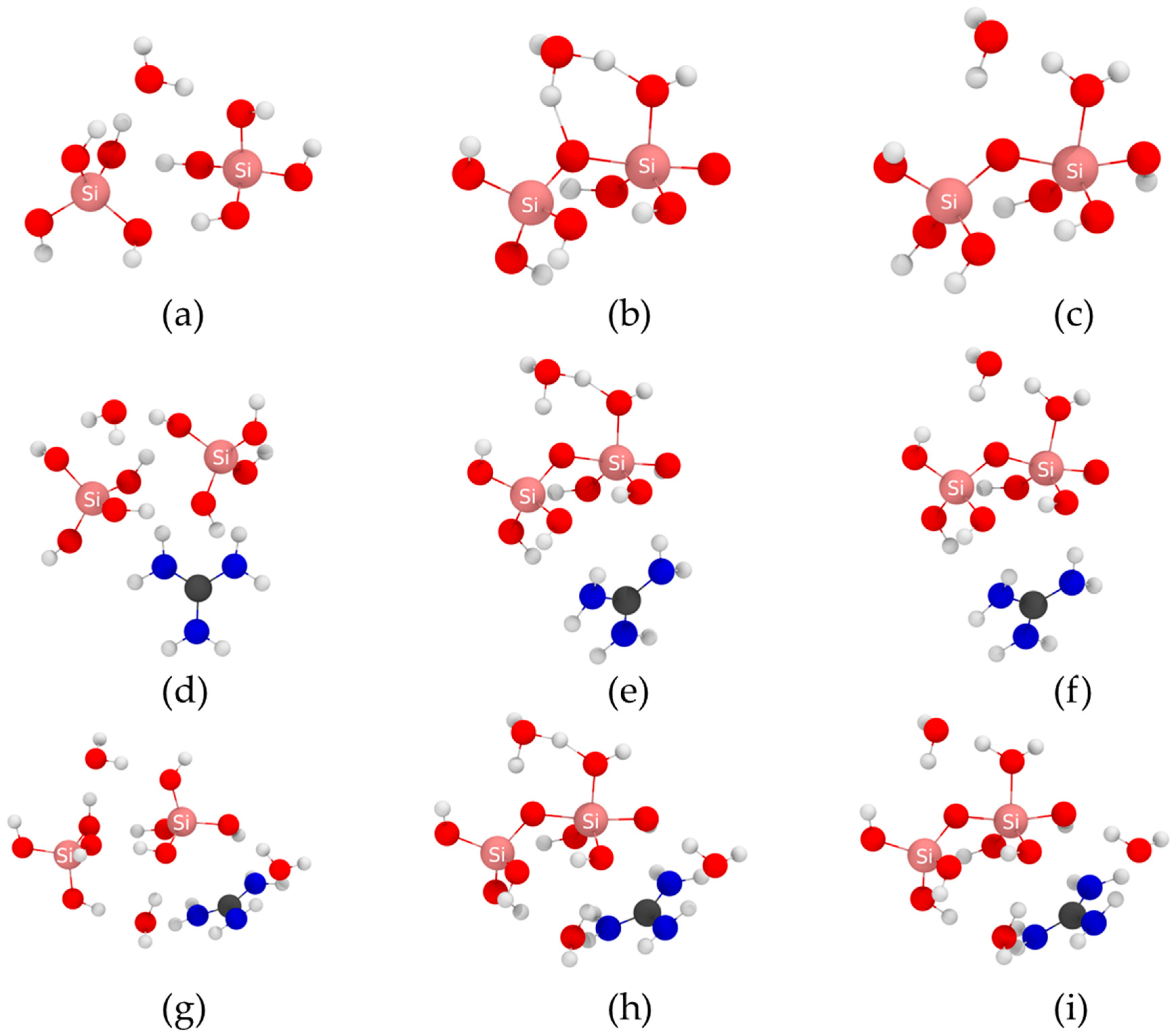
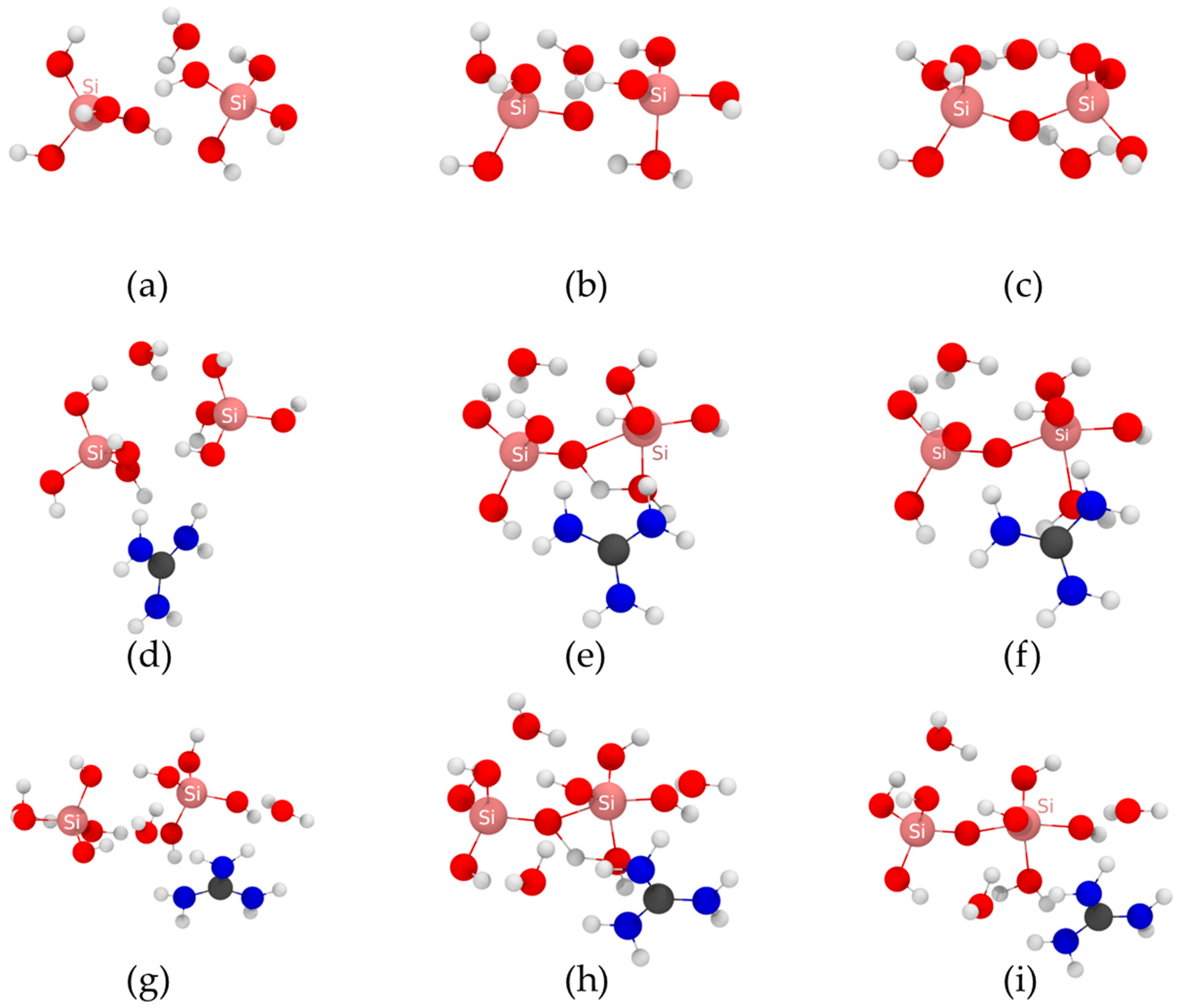
| Species | ΔEelec | ΔH | ΔG | Si-O(Nu) | O(LG)-H | O(Nu)-H |
|---|---|---|---|---|---|---|
| Unit | kcal/mol | kcal/mol | kcal/mol | Å | Å | Å |
| R | 0.00 | 0.00 | 0.00 | 3.584 | 0.990 | 1.756 |
| TS | 22.62 | 20.38 | 22.00 | 1.932 | 1.490 | 1.046 |
| P | 19.49 | 19.72 | 19.84 | 1.805 | 1.866 | 0.984 |
| R-G | 0.00 | 0.00 | 0.00 | 3.476 | 1.005 | 1.645 |
| TS-G | 19.37 | 17.45 | 19.86 | 1.886 | 1.534 | 1.034 |
| P-G | 17.37 | 17.87 | 18.98 | 1.796 | 1.908 | 0.982 |
| R-GW | 0.00 | 0.00 | 0.00 | 3.428 | 1.005 | 1.628 |
| TS-GW | 16.31 | 14.23 | 16.47 | 1.853 | 1.563 | 1.027 |
| P-GW | 14.70 | 15.00 | 16.07 | 1.785 | 1.873 | 0.984 |
| R-GW+ | 0.00 | 0.00 | 0.00 | 3.302 | 1.006 | 1.607 |
| TS-GW+ | 15.56 | 14.52 | 17.19 | 1.850 | 1.757 | 1.002 |
| P-GW+ | 11.91 | 12.27 | 13.67 | 1.758 | 2.351 | 1.065 |
| Species | ΔEelec | ΔH | ΔG | Si-O(Nu) | O(LG)-H | O(Nu)-H |
|---|---|---|---|---|---|---|
| Unit | kcal/mol | kcal/mol | kcal/mol | Å | Å | Å |
| R | 0.00 | 0.00 | 0.00 | 3.572 | 0.993 | 1.698 |
| TS | 28.48 | 27.87 | 29.85 | 2.204 | 1.565 | 1.021 |
| P | 2.30 | 1.92 | 1.83 | 1.669 | 2.883 | 0.973 |
| R-G | 0.00 | 0.00 | 0.00 | 3.761 | 0.975 | 3.274 |
| TS-G | 27.33 | 24.78 | 26.49 | 1.967 | 1.260 | 1.162 |
| P-G | 17.25 | 17.83 | 17.90 | 1.726 | 2.315 | 0.974 |
| R-GW | 0.00 | 0.00 | 0.00 | 4.589 | 0.983 | 1.769 |
| TS-GW | 25.36 | 23.26 | 25.23 | 1.929 | 1.239 | 1.183 |
| P-GW | 16.31 | 17.19 | 20.25 | 1.727 | 2.142 | 0.976 |
| R-GW+ | 0.00 | 0.00 | 0.00 | 3.521 | 0.989 | 1.683 |
| TS-GW+ | 27.51 | 24.84 | 25.38 | 1.931 | 1.233 | 1.184 |
| P-GW+ | −1.29 | −1.75 | −1.93 | 1.666 | 1.828 | 0.979 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanuza, J.; Ravera, E. The Impact of Arginine Side Chains on the Mechanism of Polycondensation of Silicic Acid in Bioinspired Mineralization. Inorganics 2025, 13, 206. https://doi.org/10.3390/inorganics13060206
Lanuza J, Ravera E. The Impact of Arginine Side Chains on the Mechanism of Polycondensation of Silicic Acid in Bioinspired Mineralization. Inorganics. 2025; 13(6):206. https://doi.org/10.3390/inorganics13060206
Chicago/Turabian StyleLanuza, Jose, and Enrico Ravera. 2025. "The Impact of Arginine Side Chains on the Mechanism of Polycondensation of Silicic Acid in Bioinspired Mineralization" Inorganics 13, no. 6: 206. https://doi.org/10.3390/inorganics13060206
APA StyleLanuza, J., & Ravera, E. (2025). The Impact of Arginine Side Chains on the Mechanism of Polycondensation of Silicic Acid in Bioinspired Mineralization. Inorganics, 13(6), 206. https://doi.org/10.3390/inorganics13060206










