Mo-Dopant-Enhanced Energy Storage Performance of VS2 Microflowers as Electrode Materials for Supercapacitors
Abstract
1. Introduction
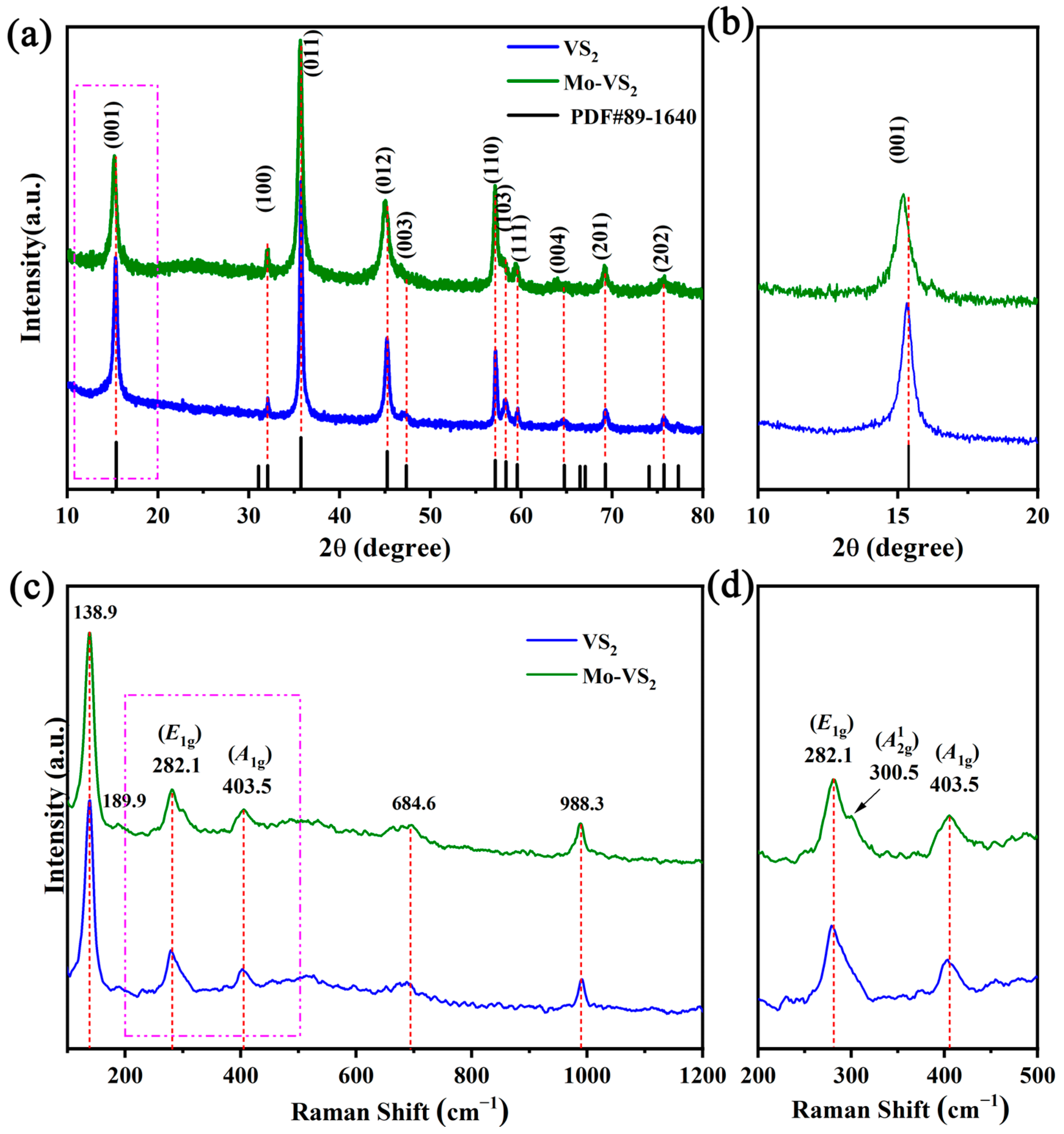
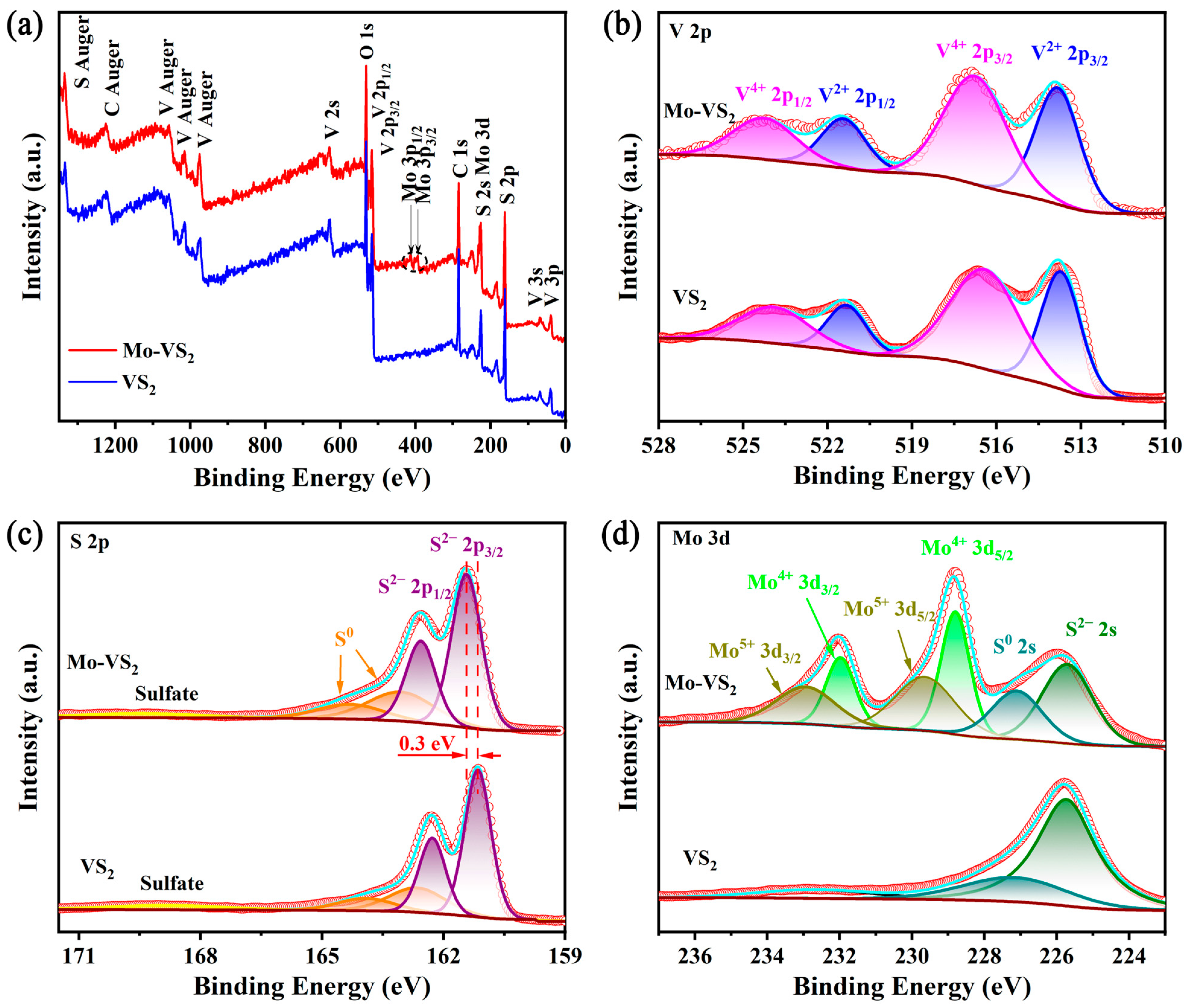
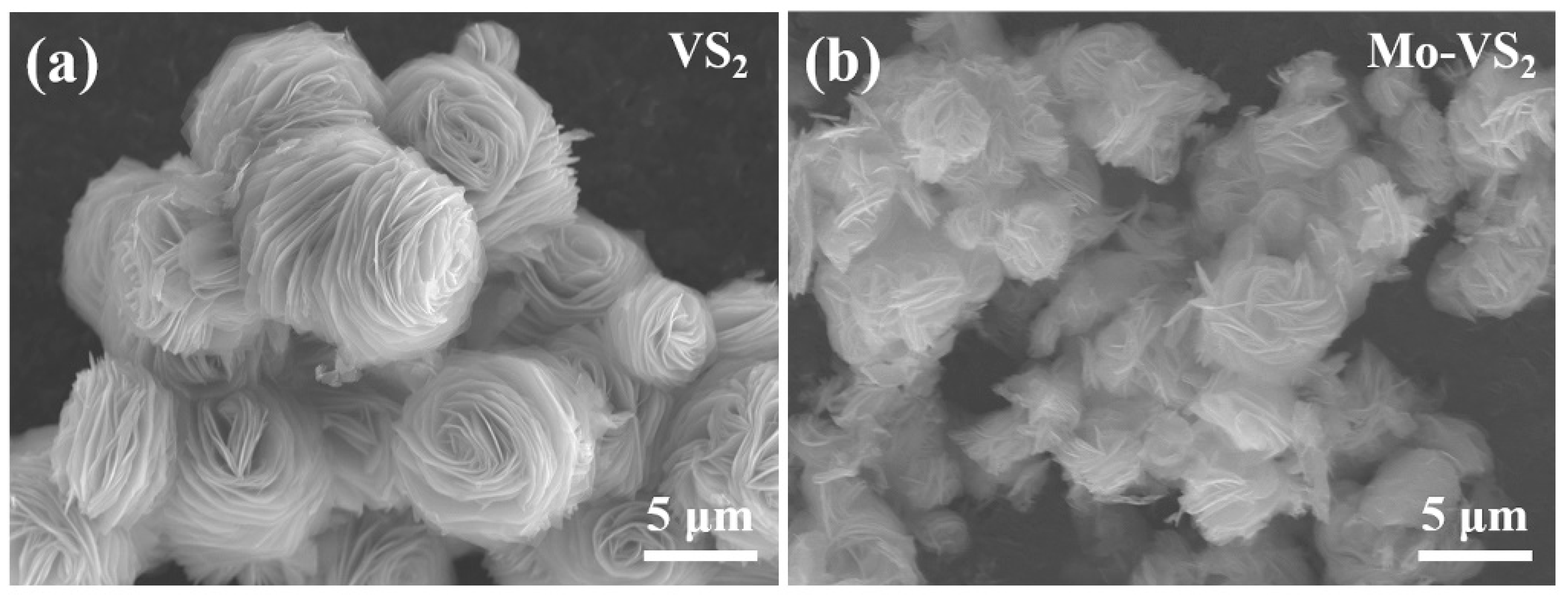
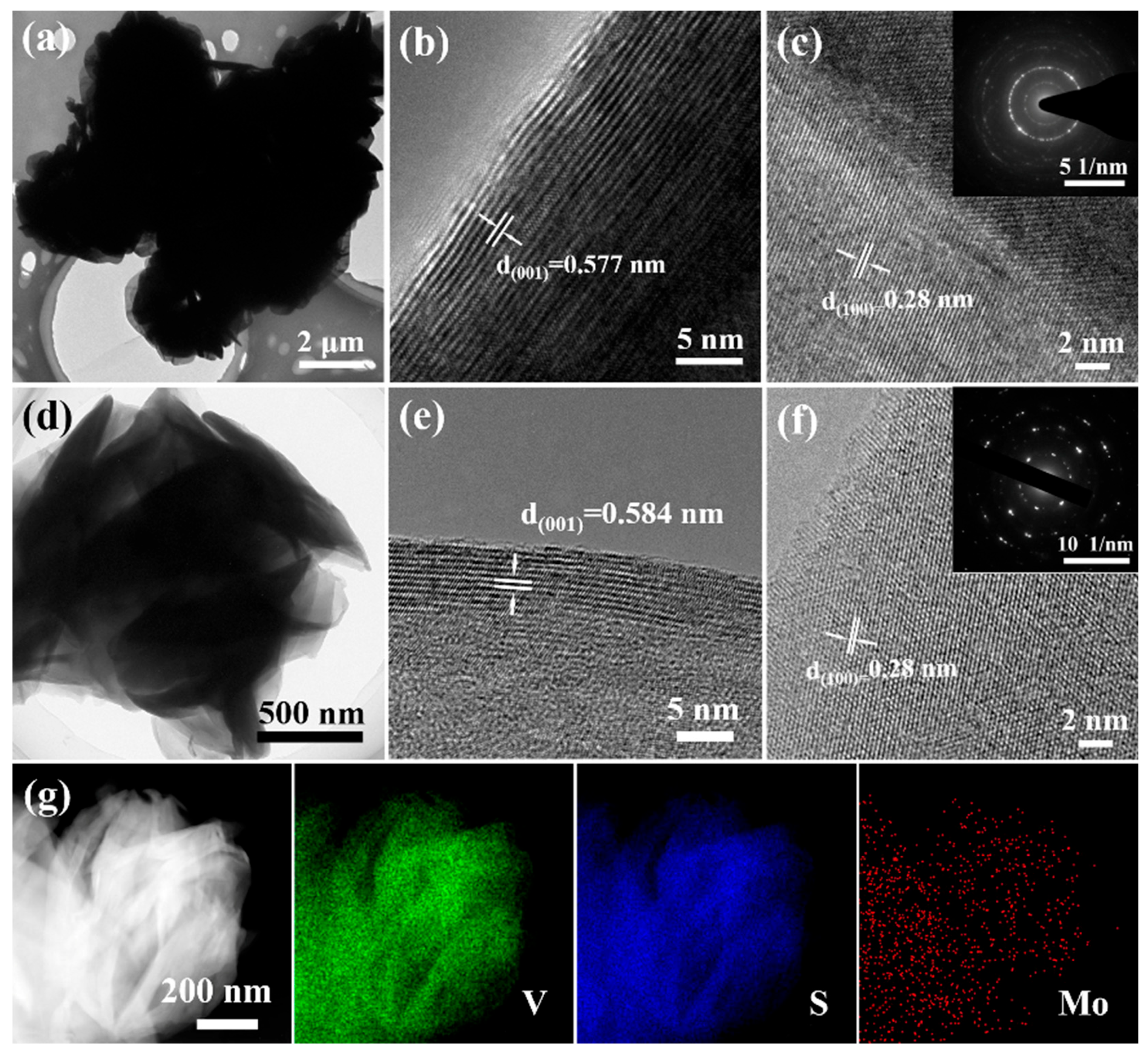
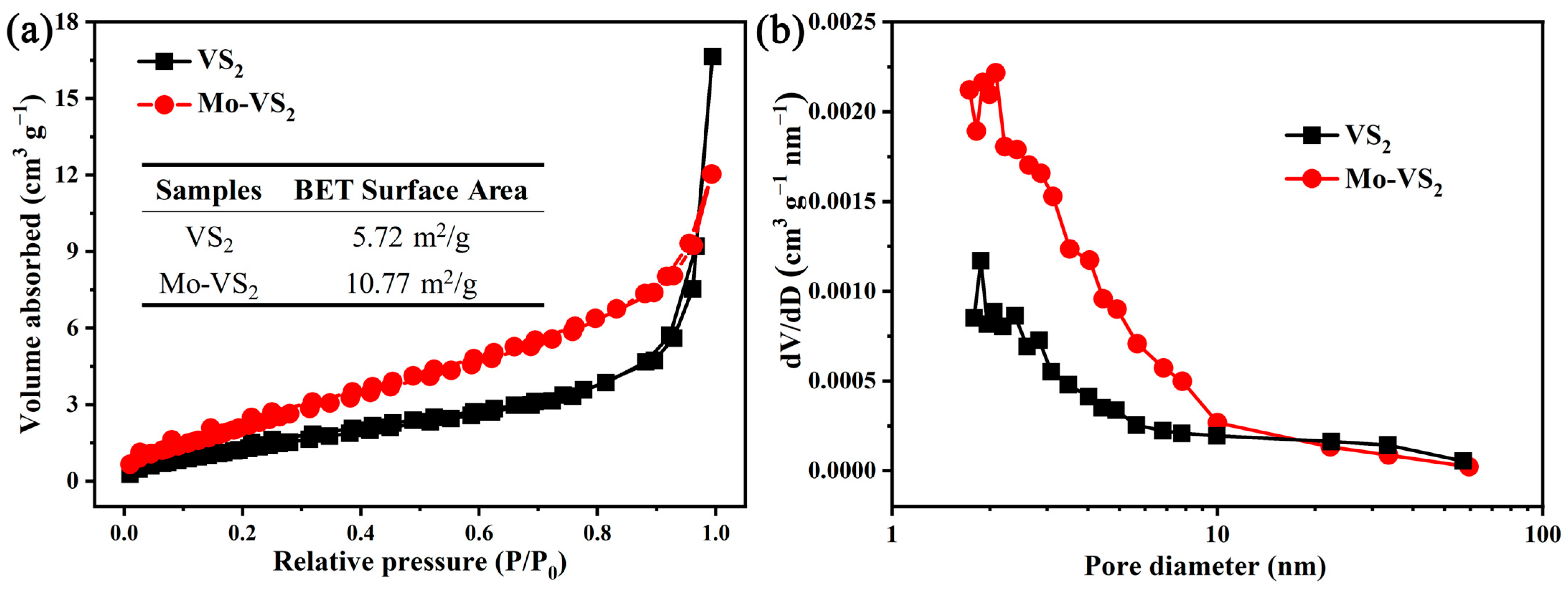
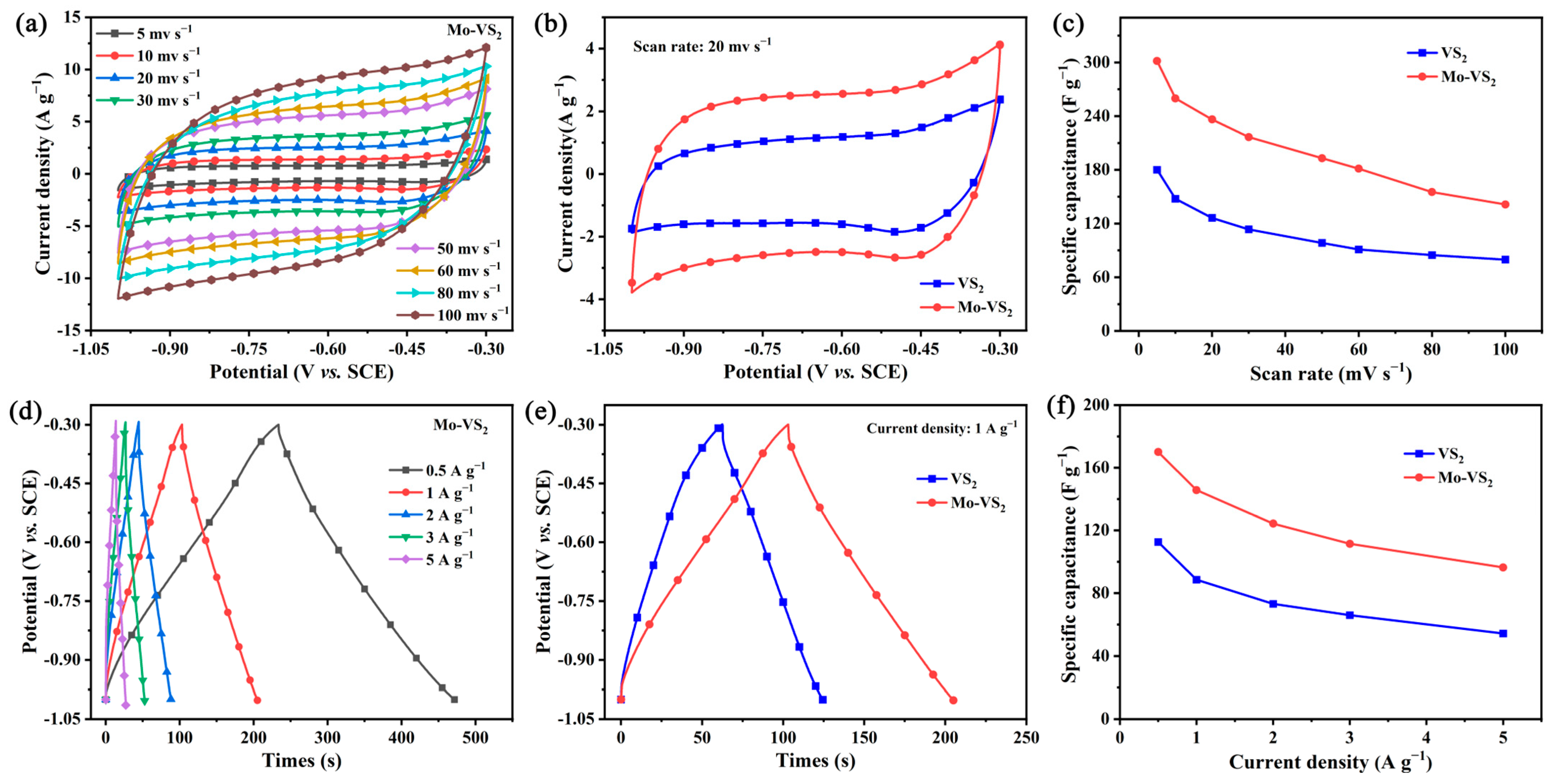
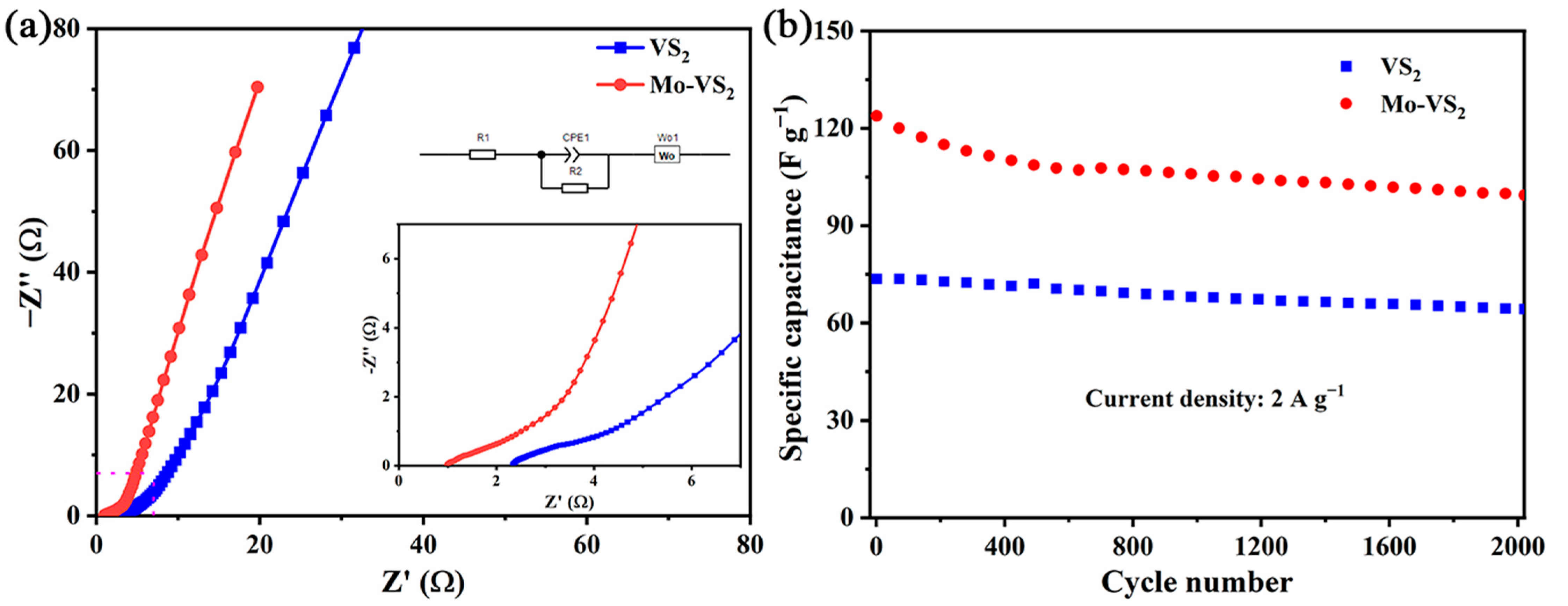
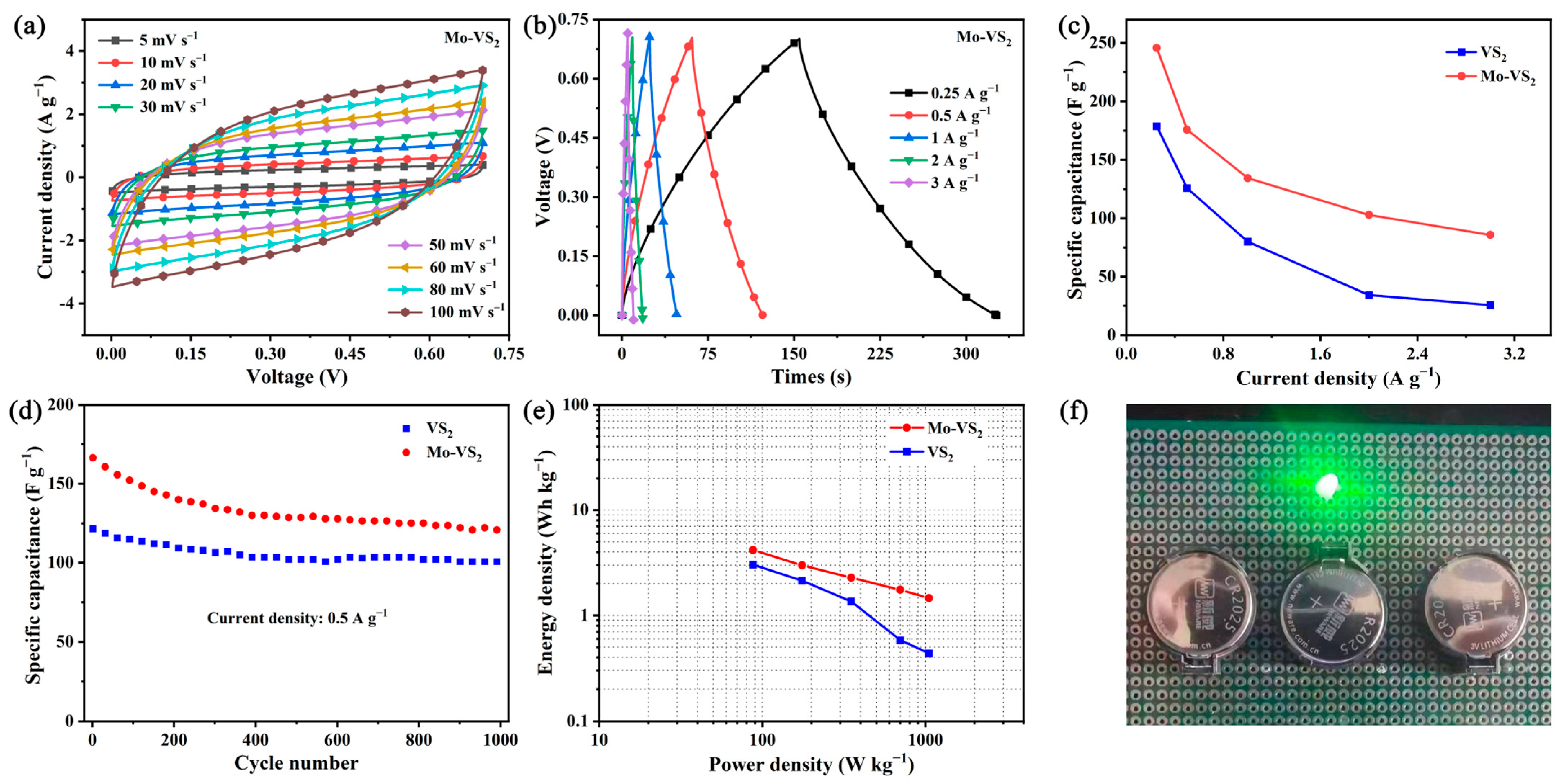
2. Results and Discussion
3. Experimental
3.1. Synthesis of the Mo-Doped VS2 and Pristine VS2 Microflowers
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef]
- Nallapristineddy, J.; Nallapristineddy, R.R.; Thomas, S.A.; Arla, S.K.; Cherusseri, J.; Prabu, S.; Pallavolu, M.R.; Joo, S.W. Hierarchical nickel molybdenum sulfide whisker nanoneedles: Binder-free in situ grown electrodes designed for enhanced supercapattery performance. J. Energy Storage 2025, 123, 116864. [Google Scholar]
- Panghal, A.; Sahoo, D.; Deepak, D.; Deshmukh, S.; Kaviraj, B.; Roy, S.S. Single-step synthesis of Ni-doped MoS2 nanostructures for symmetrical supercapacitor devices. ACS Appl. Nano Mater. 2024, 7, 5358–5371. [Google Scholar] [CrossRef]
- De, A.A.; Singh, S.; Lahiri, I. WS2@PPy heterostructured high performance supercapacitor self-powered by PVDF piezoelectric separator. J. Alloys Compd. 2023, 939, 168713. [Google Scholar]
- Yogesh, G.K.; Nandi, D.; Yeetsorn, R.; Wanchan, W.; Devi, C.; Singh, R.P.; Vasistha, A.; Kumar, M.; Koinkar, P.; Yadav, K. A machine learning approach for estimating supercapacitor performance of graphene oxide nano-ring based electrode materials. Energy Adv. 2025, 4, 119–139. [Google Scholar] [CrossRef]
- Šedajová, V.; Nandi, D.; Langer, P.; Lo, R.; Hobza, P.; Plachá, D.; Bakandritsos, A.; Zbořil, R. Direct upcycling of highly efficient sorbents for emerging organic contaminants into high energy content supercapacitors. J. Colloid. Interface Sci. 2025, 692, 137481. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xu, L.; Guo, X.T.; Lv, T.T.; Pang, H. Vanadium sulfide based materials: Synthesis, energy storage and conversion. J. Mater. Chem. A 2020, 8, 20781–20802. [Google Scholar] [CrossRef]
- Koudahi, M.F.; Frąckowiak, E. Fast response supercapacitor based on carbon-VS2 electrodes with a wide operating voltage range. Energy Storage Mater. 2022, 49, 255–267. [Google Scholar] [CrossRef]
- Wang, H.; You, L.; Guan, Y.; Wang, H.; Ma, X.; Wang, D.; Wu, J.; Zhu, Y.; Lin, J.; Liu, J. Rational fabrication of flower-like VS2-decorated Ti3C2 MXene heterojunction nanocomposites for supercapacitance performances. Colloids Surf. A 2021, 629, 127381. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Y.; Zhang, J.; Liu, J.; Lei, M.; Zhao, C.; Lu, Q.; Wang, H.; Du, F.; Zhang, S. Solar light-driven photoelectrocatalytic and photocatalytic applications based on flower-like NV-g-C3N5@VS2 heterojunctions. New J. Chem. 2023, 47, 10973–10983. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Peng, J.; Zhang, Q.; Pei, D.; Yan, X. One-step solid-phase synthesis of binder-free molybdenum disulfide/carbon fibers paper anode for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 768, 33–41. [Google Scholar] [CrossRef]
- Pandit, B.; Karade, S.S.; Sankapal, B.R. Hexagonal VS2 anchored MWCNTs: First approach to design flexible solid-state symmetric supercapacitor device. ACS Appl. Mater. Interfaces 2017, 9, 44880–44891. [Google Scholar] [CrossRef]
- Zhang, Q.; He, W.; Wang, Y.; Pei, D.; Zheng, X. Sonication-assisted synthesis of molybdenum disulfide aerogel for the electrode materials of supercapacitors. Nano 2019, 14, 1950055. [Google Scholar] [CrossRef]
- Pandit, B.; Bommineedi, L.K.; Sankapal, B.R. Electrochemical engineering approach of high performance solid-state flexible supercapacitor device based on chemically synthesized VS2 nanoregime structure. J. Energy Chem. 2019, 31, 79–88. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Zheng, X.; Long, B.; Ling, H.; Xie, L.; Xiang, Z.; He, W. P non-metal doping induced surface charge redistribution of VS2 for strengthening hydrogen evolution activity. Appl. Surf. Sci. 2025, 694, 162834. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, Q.; Yang, G.; Lin, J. Study of super capacitive energy storage properties of VS2 electrode materials. Appl. Surf. Sci. 2025, 688, 162453. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Princy, L.E.M.; Rathinam, Y.; Kungumadevi, L.; Ravi, G.; Al-Sehemi, A.G.; Velauthapillai, D. Rare earth-doped MoS2 for supercapacitor application. Energy Fuels 2022, 36, 6476–6482. [Google Scholar] [CrossRef]
- Saseendran, S.B.; Ashok, A.; Asha, A.S. Flexible and binder-free supercapacitor electrode with high mass loading using transition metal doped MoS2 nanostructures. J. Alloys Compd. 2023, 968, 172131. [Google Scholar] [CrossRef]
- Robertson, B.; Sapna, R.; Hareesh, K. A comprehensive review on MoSe2 nanostructures with an overview of machine learning techniques for supercapacitor applications. RSC Adv. 2024, 14, 37644–37675. [Google Scholar]
- Masanta, S.; Nayak, C.; Maitra, S.; Rudra, S.; Chowdhury, D.; Raha, S.; Pradhan, M.; Satpati, B.; Pal, P.; Singha, A. Engineering multifunctionality in MoSe2 nanostructures via strategic Mn doping for electrochemical energy storage and photosensing. ACS Appl. Nano Mater. 2023, 6, 5479–5492. [Google Scholar] [CrossRef]
- Qu, Y.; Pan, H.; Kwok, C.T.; Wang, Z. Effect of doping on hydrogen evolution reaction of vanadium disulfide monolayer. Nanoscale Res. Lett. 2015, 10, 480. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Peng, J.; Dong, H.; Wang, J.; Zhao, W. Mo-dopant-strengthened basal-plane activity in VS2 for accelerating hydrogen evolution reaction. Chem. Eng. J. 2020, 396, 125227. [Google Scholar] [CrossRef]
- Feng, J.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic few-layered VS2 ultrathin nanosheets: High two-dimensional conductivity for in-plane supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef]
- Xu, X.; Ge, Y.; Wang, M.; Zhang, Z.; Dong, P.; Baines, R.; Ye, M.; Shen, J. Cobalt-doped FeSe2-RGO as highly active and stable electrocatalysts for hydrogen evolution reactions. ACS Appl. Mater. Interfaces 2016, 8, 18036–18042. [Google Scholar] [CrossRef]
- Ding, S.; Li, Z.; Dai, X.; Li, Z.; Dai, X.; Sun, C.; Meng, A. Mo-doped VS4 with interlayer-expanded and engineering sulfur vacancies as cathode for advanced magnesium storage. Chem. Eng. J. 2021, 417, 129328. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, S.; Yuan, Y.; Wei, G.; Zhao, J. Mo-doped induced amorphous phase and sulfur vacancy healing in VS4 for enhancing the storage of lithium ions. J. Mater. Chem. A 2021, 9, 1100–1109. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv. Energy. Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Wu, L.; Sun, R.; Xiong, F.; Pei, C.; Han, K.; Peng, C.; Fan, Y.; Yang, W.; An, Q.; Mai, L. A rechargeable aluminum-ion battery based on a VS2 nanosheet cathode. Phys. Chem. Chem. Phys. 2018, 20, 22563–22568. [Google Scholar] [CrossRef]
- Sun, R.; Wei, Q.; Sheng, J.; Shi, C.; An, Q.; Liu, S.; Mai, L. Novel layer-by-layer stacked VS2 nanosheets with intercalation pseudocapacitance for high-rate sodium ion charge storage. Nano Energy 2017, 35, 396–404. [Google Scholar] [CrossRef]
- Sourisseau, C.; Cavagnat, R.; Fouassier, M.; Tirado, J.L.; Morales, J. Raman study and lattice dynamics calculations of misfit layered compounds: (PbS)1.18TiS2 and (PbS)1.12VS2. J. Mol. Struct. 1995, 348, 107–110. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Wang, Z.; Zhu, J.; Wen, Z.; Zhao, X.; Zhang, X.; Xu, J.; Lu, Z. Synergistic interlayer and defect engineering in VS2 nanosheets toward efficient electrocatalytic hydrogen evolution reaction. Small 2018, 14, 1703098. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of layered Group 5 metallic transition metal dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241–14253. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Yan, C.; Zhao, P.; Hu, Y.; Kong, W.; Lin, H.; Wang, L.; Jin, Z. One-step synthesis of 2-ethylhexylamine pillared vanadium disulfide nanoflowers with ultralarge interlayer spacing for high-performance magnesium storage. Adv. Energy Mater. 2019, 9, 1900145. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Chi, X.; Lin, Y.; Chen, Z.; Ling, X.; Qiu, C.; Xu, Y.; Song, L.; Chen, W.; et al. Engineering the Electronic Structure of MoS2 Nanorods by N and Mn Dopants for Ultra-Efficient Hydrogen Production. ACS. Catal. 2018, 8, 7585–7592. [Google Scholar] [CrossRef]
- Huang, H.; Huang, W.; Yang, Z.; Huang, J.; Lin, J.; Liu, W.; Liu, Y. Strongly coupled MoS2 nanoflake–carbon nanotube nanocomposite as an excellent electrocatalyst for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 1558–1566. [Google Scholar] [CrossRef]
- Rasamani, K.D.; Alimohammadi, F.; Sun, Y. Interlayer-expanded MoS2. Mater. Today 2017, 20, 83–91. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy. Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B 2020, 277, 119171. [Google Scholar] [CrossRef]
- Rout, C.S.; Khare, R.; Kashid, R.V.; Joag, D.S.; More, M.A.; Lanzillo, N.A.; Washington, M.; Nayak, S.K.; Late, D.J. Metallic few-layer flowerlike VS2 Nanosheets as field emitters. Eur. J. Inorg. Chem. 2014, 2014, 5331–5336. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.X.; Li, H.H.; Su, L.W.; Wei, J.P.; Zhou, Z. Mesoporous slit-structured NiO for high-performance pseudocapacitors. Phys. Chem. Chem. Phys. 2012, 14, 11048–11052. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Bartholomew, C.H.; Woodfield, B.F. Improved calculations of pore size distribution for relatively large, irregular slit-shaped mesopore structure. Microporous Mesoporous Mater. 2014, 184, 112–121. [Google Scholar] [CrossRef]
- Singal, S.; Joshi, A.; Singh, G.; Sharma, R.K. Dual approach of antimonene hybridization and hierarchical structuration to expose more active sites for improved charge storage characteristics of VS4. J. Power Sources 2020, 475, 228669. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Dong, Y.; Chen, L.; Chen, L.; He, W. Reactant conversion–intercalation strategy toward interlayer-expanded MoS2 microflowers with superior supercapacitor performance. Dalton Trans. 2023, 52, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yuan, Z.; Xu, C.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. Construction of mesoporous Cu-doped Co9S8 rectangular nanotube arrays for high energy density all-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 5333–5343. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Yin, H.; Zhao, H.; Wang, D.; Tang, Z. Growth of polypyrrole ultrathin films on MoS2 monolayers as high-performance supercapacitor electrodes. Adv. Mater. 2015, 27, 1117–1123. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Z.; Zhao, X.; Chen, X.; Liu, Y. Green needle coke-derived porous carbon for high-performance symmetric supercapacitor. J. Power Sources 2021, 494, 229770. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Li, M.; Yin, Y.; Li, J.; Li, G.; Wang, W.; Peng, W.; Cao, F.; Guo, Y. Porous carbon for high-energy density symmetrical supercapacitor and lithium-ion hybrid electrochemical capacitors. Chem. Eng. J. 2019, 375, 122020. [Google Scholar] [CrossRef]
- Thakur, A.K.; Choudhary, R.B.; Majumder, M.; Gupta, G.; Shelke, M. Enhanced electrochemical performance of polypyrrole coated MoS2 nanocomposites as electrode material for supercapacitor application. J. Electroanal. Chem. 2016, 782, 278–287. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Cheng, J.; Yuan, D.; Ni, W.; Guan, Q.; Gao, L.; Wang, B. Fiber-shaped solid-state supercapacitors based on molybdenum disulfide nanosheets for a self-powered photodetecting system. Nano Energy 2016, 21, 228–237. [Google Scholar] [CrossRef]
- Ali, B.A.; Omar, A.M.A.; Khalil, A.S.G.; Allam, N.K. Untapped Potential of Polymorph MoS2: Tuned Cationic Intercalation for High-Performance Symmetric Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 33955–33965. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Barzegar, F.; Mirghni, A.A.; Khaleed, A.A.; Masikhwa, T.M.; Manyala, N. Examination of high-porosity activated carbon obtained from dehydration of white sugar for electrochemical capacitor applications. ACS Sustain. Chem. Eng. 2018, 7, 537–546. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, Q.; Sun, Y. Superior Capacitive Performance Enabled by Edge-Oriented and Interlayer-Expanded MoS2 Nanosheets Anchored on Reduced Graphene Oxide Sheets. Ind. Eng. Chem. Res. 2018, 57, 4571–4576. [Google Scholar] [CrossRef]
- Nandana, A.B.; Rakhi, R.B. Exploring Vanadium Disulfide (VS2) Nanosheets as High Efficiency Supercapacitor Electrodes. Energy Technol. 2025, 13, 2402153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zheng, X.; Xie, L.; Xiang, Z.; He, W. Mo-Dopant-Enhanced Energy Storage Performance of VS2 Microflowers as Electrode Materials for Supercapacitors. Inorganics 2025, 13, 199. https://doi.org/10.3390/inorganics13060199
Wang J, Zheng X, Xie L, Xiang Z, He W. Mo-Dopant-Enhanced Energy Storage Performance of VS2 Microflowers as Electrode Materials for Supercapacitors. Inorganics. 2025; 13(6):199. https://doi.org/10.3390/inorganics13060199
Chicago/Turabian StyleWang, Jingwei, Xuejun Zheng, Long Xie, Zhenhua Xiang, and Wenyuan He. 2025. "Mo-Dopant-Enhanced Energy Storage Performance of VS2 Microflowers as Electrode Materials for Supercapacitors" Inorganics 13, no. 6: 199. https://doi.org/10.3390/inorganics13060199
APA StyleWang, J., Zheng, X., Xie, L., Xiang, Z., & He, W. (2025). Mo-Dopant-Enhanced Energy Storage Performance of VS2 Microflowers as Electrode Materials for Supercapacitors. Inorganics, 13(6), 199. https://doi.org/10.3390/inorganics13060199





