1. Introduction
Zinc-ion batteries (ZIBs) have emerged as an alternative to lithium-ion batteries due to their inherent advantages, including high safety, aqueous electrolyte compatibility, and cost-effectiveness [
1,
2]. Unlike lithium-based systems, ZIBs utilize Zn metal as the anode, offering high theoretical capacity and excellent reversibility in mild aqueous electrolytes. However, the development of suitable cathode materials remains a challenge, as they must exhibit high capacity, good structural stability, and fast Zn
2+ diffusion kinetics [
3,
4,
5]. Among various cathode materials, vanadate-based compounds have shown promise due to their multi-electron redox capability, tunable structure, and favorable Zn
2+ intercalation properties [
6,
7,
8].
Particularly, metal–organic framework (MOF)-derived vanadate-based compounds as cathodes for ZIBs have attracted much attention due to their unique structural advantages, tunable composition, and high electrochemical activity, which together address many of the challenges associated with aqueous Zn-ion energy storage systems [
9,
10,
11]. MOFs, such as zeolitic imidazolate framework-8 (ZIF-8), offer highly porous, crystalline structures with large surface areas and well-defined architecture [
12,
13,
14,
15]. When used as sacrificial templates, MOFs enable the formation of nanostructured or hierarchical transition metal oxides or vanadates with controlled morphology and porosity. Upon thermal treatment, these MOFs can decompose to form vanadium-based compounds (e.g., Zn
2VO
4, V
2O
5, V
2O
3) embedded in a carbon matrix [
16]. This MOF-derived approach provides materials with enhanced electronic conductivity, structural stability, and ion-accessible pathways—features that are otherwise difficult to achieve with conventional synthesis methods.
Vanadate-based compounds are especially appealing due to their multivalent redox activity (V
3+/V
4+/V
5+), which allows them to accommodate multiple Zn
2+ ions and deliver high specific capacity [
7,
16,
17]. When derived from MOFs, these materials often inherit a porous or nanoscale architecture, which facilitates fast Zn
2+ diffusion, shortens ion/electron transport pathways, and increases the number of active sites for redox reactions. The in situ carbonization of organic ligands during annealing can also introduce N-doped carbon, which significantly improves conductivity and rate performance [
18]. Moreover, MOF-derived vanadates can often mitigate common degradation issues in ZIBs, such as the dissolution of vanadium species and structural collapse during cycling [
19]. Their robust composite structure—typically combining active vanadates with carbon—helps maintain electrode integrity and prolong cycling life.
Specifically, Chen et al. reported a composite cathode with good electrochemical stability, achieving 217 mAh g
−1 with 94.8% retention over 180 cycles at 0.1 A g
−1 and 116 mAh g
−1 after 1600 cycles at 1 A g
−1 [
20]. The improved stability was attributed to amorphous carbon’s buffering effect and enhanced conductivity. Ding et al. presented the synthesis of a high-performance cathode via MOF pyrolysis, delivering a specific capacity of 300 mAh g
−1 at 100 mA g
−1 and 240 mAh g
−1 at 2000 mA g
−1, owing to its optimized pore structure and fast ion pathways [
21]. Gong et al. demonstrated an advanced heterojunction cathode with graphene oxide coatings [
22]. V
2O
3/VN heterostructures coated with graphene oxide (V
2O
3/VN@GO) were presented as high-performance cathodes for aqueous Zn-ion batteries. The GO layer enhances conductivity, promotes oxygen vacancy formation, and suppresses vanadium dissolution. These combined effects improve ion/electron transport and reduce Zn
2+ migration barriers. As a result, the V
2O
3/VN@GO cathode delivers a high capacity of 488.1 mAh g
−1 and excellent cycling stability with 91.4% retention after 3000 cycles at 10 A g
−1.
Here, we, for the first time, fabricated a MOF-derived Zn(CN)2–V2O3–C composite cathode without binders, focusing on introducing a novel ultrathin, binder-free electrode architecture fabricated by AC electrophoretic deposition (AC-EPD) to probe the electrode–electrolyte interface with minimal interference from conductive additives or bulk electrode effects. This configuration can also bring together the complementary functionalities of its three components to enhance the electrochemical performance of aqueous ZIBs. Zn(CN)2, with its rigid three-dimensional Zn–CN–Zn framework, contributes structural stability and may help form a passivating interphase that suppresses unwanted side reactions, such as hydrogen evolution. Its porous architecture can also aid Zn2+ ion transport, while its electrochemical inactivity ensures that it acts as a stable support matrix. V2O3, on the other hand, serves as the main electrochemically active phase due to its ability to undergo multiple redox transitions (V3+/V4+/V5+), enabling high-capacity Zn2+ storage through diffusion-controlled intercalation mechanisms. The presence of carbon further improves the composite by enhancing its overall electrical conductivity, facilitating electron transport throughout the electrode, and stabilizing the electrode microstructure during cycling. The synergy among these three components results in a cathode material with improved ion and electron transport, higher rate capability, and more stable cycling behavior. Additionally, the incorporation of Zn(CN)2 and carbon into the V2O3 matrix helps mitigate structural degradation and mechanical stress during repeated Zn2+ insertion and extraction, maintaining electrode integrity.
This composite structure is particularly valuable when fabricated via AC-EPD, as this method enables the formation of ultrathin, uniform films with strong particle–substrate interaction and minimal agglomeration [
23,
24,
25]. The resulting cathode not only delivers efficient energy storage performance but also serves as a robust platform for investigating interfacial charge storage processes without the effect of additives on battery performance. In this study, we utilized AC-EPD to fabricate an ultrathin Zn(CN)
2–V
2O
3–C composite cathode, enabling the precise control of film thickness; binder-free, enhanced electrolyte penetration; and improved Zn
2+ diffusion pathways. We performed scan rate-dependent CV analysis to separate the charge storage contributions from diffusion-controlled intercalation and surface-driven capacitive effects, providing insights into the advantages of ultrathin Zn(CN)
2–V
2O
3–C composite cathodes for high-performance ZIBs, and outlined strategies for further optimization.
2. Results and Discussion
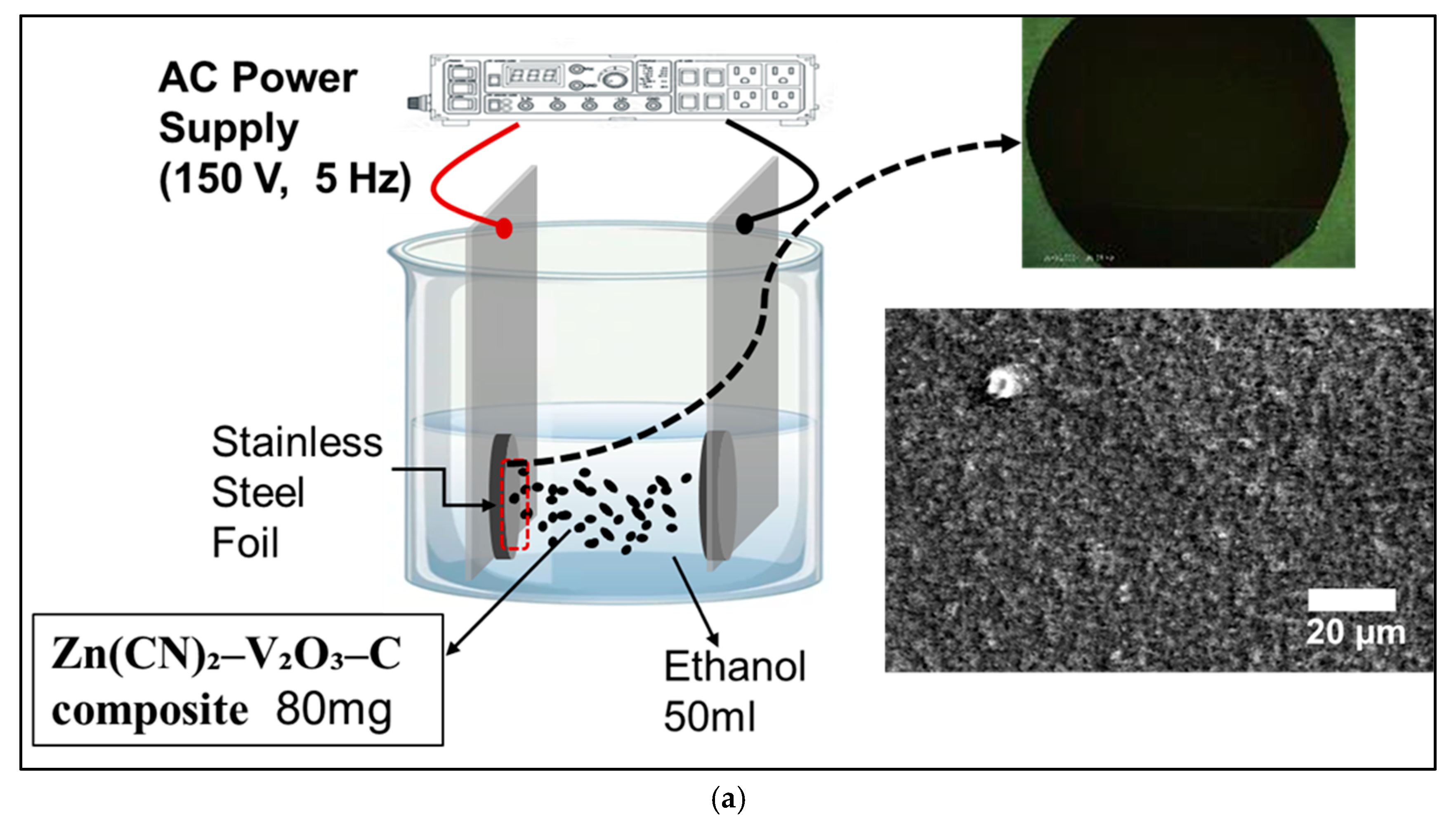
As illustrated in
Figure 1a, the AC-EPD technique was employed to fabricate a uniform cathode film approximately 300 nm thick on an SS substrate. This process was carried out by applying an alternating voltage of 150 V at a frequency of 5 Hz, ensuring even particle distribution during deposition. After the film was deposited, it underwent thermal treatment in an Ar atmosphere at 200 °C for 1 h. The use of an alternating current in the EPD process effectively prevents particle agglomeration and ensures a smooth, continuous coating with uniform thickness. This level of control over film morphology is essential for achieving reliable electrochemical performance. The annealing step further enhances the mechanical cohesion of the electrode and contributes to improved electrochemical response. The resulting thin film offers a large surface area and intimate contact with the electrolyte, facilitating efficient Zn-ion transport during charge and discharge. Overall, this fabrication strategy results in a cathode with enhanced specific capacity, better cycling behavior, and improved long-term stability in ZIB applications.
The optical graph and the scanning electron microscopy image in
Figure 1 display the EPD film and the surface morphology of the AC-EPD-Zn(CN)
2–V
2O
3–C composite cathode film, which exhibits an ultrathin and uniform coating across the SS substrate. The film appears dense and continuous, with a relatively smooth surface texture and minimal visible porosity or agglomeration. This indicates that the alternating current field applied during the EPD process effectively distributed the particles, ensuring homogeneous coverage. The absence of large surface defects and the consistent grain texture suggest strong particle-to-substrate adhesion and good film cohesion, both of which are critical for maintaining mechanical integrity during electrochemical cycling. The scale bar of 20 μm further confirms the fine and uniform microstructure over a wide area, consistent with the expected ultrathin film thickness (~300 nm) reported. Such morphology is ideal for ZIB applications, as it facilitates efficient Zn
2+ ion transport, maximizes electrochemical surface area, and minimizes interfacial resistance—all contributing to improved rate capability and cycling stability.
The XRD pattern in
Figure 1b confirms the presence of Zn(CN)
2 (JCPDS card no. 06-0175,
Supplementary Materials), along with V
2O
3 (JCPDS card no. 34-0187,
Supplementary Materials) and carbonaceous residues. In the Zn(CN)
2 crystal structure, each Zn
2+ ion is tetrahedrally coordinated by four cyano (–CN) ligands. These Zn-centered tetrahedra are interconnected through linear Zn–CN–Zn bridges, resulting in a three-dimensional network [
26]. The X-ray diffraction (XRD) analysis revealed distinct peaks at (012), (104), (110), (113), (202), (024), (116), (214), (300), and (0210), which are consistent with the characteristic reflections of V
2O
3. This indicates the formation of crystalline vanadium oxide phases within the sample. We found that the broad diffraction features around ~26° more accurately correspond to the (002) plane of disordered graphitic carbon, as described in JCPDS No. 41-1487 (
Supplementary Materials). These peaks may originate from the decomposition of the ZIF-8 precursor or from the residual carbon introduced during synthesis and annealing.
Upon annealing at 800 °C, the XRD pattern confirmed the presence of both V
2O
3 and carbon residues, suggesting the formation of secondary phases during thermal treatment. The X-ray photoelectron spectroscopy (XPS) analysis shown in
Supplementary Materials Figure S2 also confirms the successful formation of V
2O
3 in the Zn(CN)
2–V
2O
3–C composite and supports the structural assignments made from XRD. These binding energy positions are consistent with the typical values reported for the V
3+ oxidation state, which is the dominant valence state in V
2O
3. The V 2p
3/
2 peak at approximately 516 eV and the V 2p
1/
2 peak at approximately 525 eV confirm the presence of trivalent vanadium (V
3+) in the sample [
27,
28]. It also suggests that the annealing conditions effectively reduced the vanadium precursor to its trivalent form. Notably, no distinct peaks corresponding to ZnVO
4 (Zn
2VO
4) were detected. This absence may be attributed either to the partial reduction of vanadium species, favoring the formation of V
2O
3 under the annealing atmosphere, or to the poor crystallinity or amorphous nature of ZnVO
4 under the applied conditions.
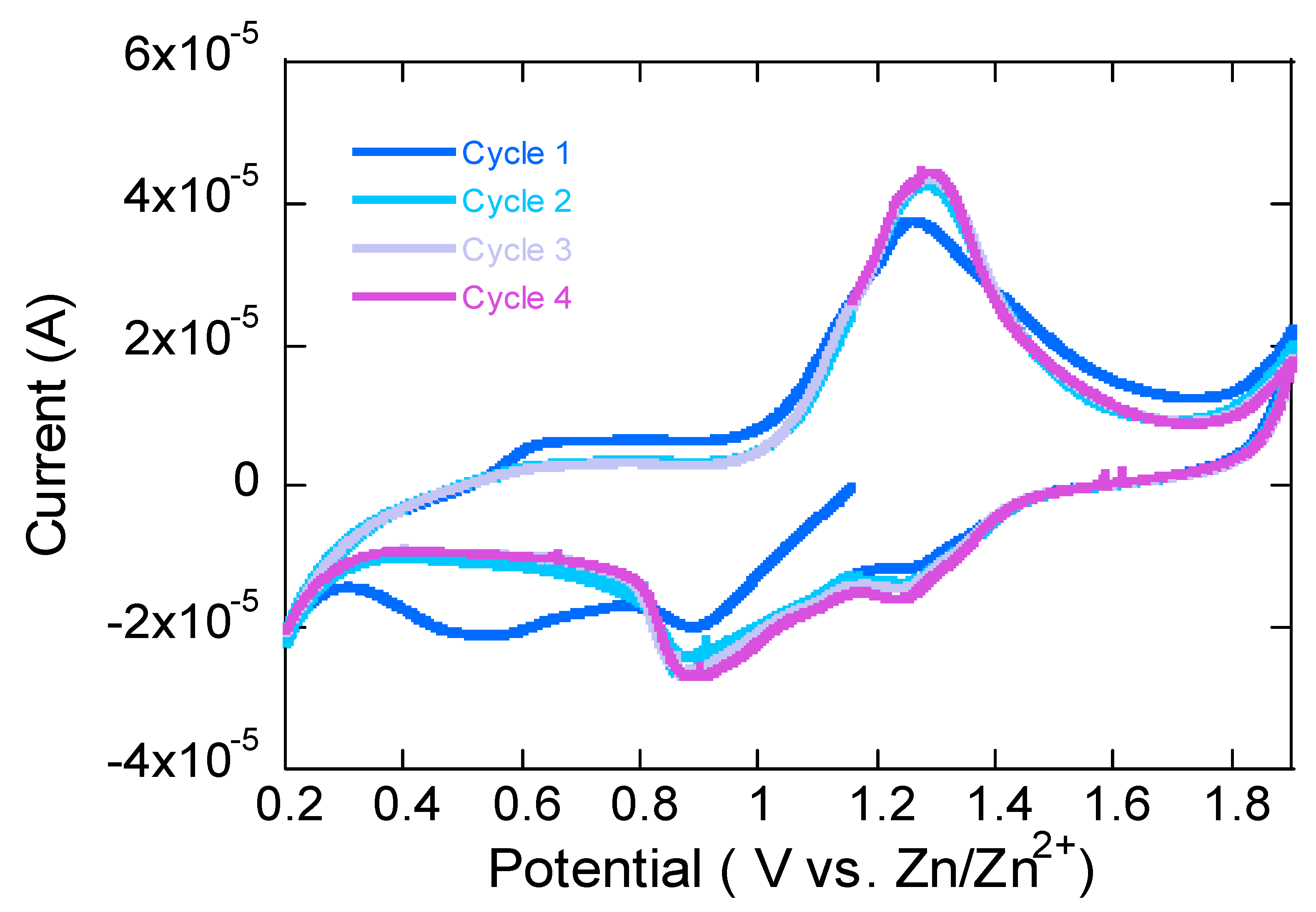
The CV plot in
Figure 2 shows distinct oxidation and reduction peaks, indicating a reversible redox reaction occurring in the Zn(CN)
2–V
2O
3–C cathode. Oxidation peaks (~1.2–1.5 V) correspond to the de-intercalation (extraction) of Zn
2+ from the Zn(CN)
2–V
2O
3–C composite cathode. Vanadium undergoes oxidation (V
3+ → V
4+ or V
4+ → V
5+), allowing Zn
2+ to leave the cathode structure. Reduction peaks (~0.7–1.0 V) represent the intercalation (insertion) of Zn
2+ back into the cathode. Vanadium is reduced (V
5+ → V
4+ or V
4+ → V
3+), incorporating Zn
2+ ions. The peak intensities remain relatively stable from Cycle 1 to Cycle 4, indicating that the Zn(CN)
2–V
2O
3–C composite has a stable Zn
2+ insertion/extraction process [
16,
29,
30].
According to the study by Ding et al., ex situ Raman spectroscopy demonstrated that both Zn
2+ ions and water molecules co-intercalate into the V
2O
3 framework during the discharge process [
21]. This co-insertion leads to the formation of hydrated zinc vanadate phases, represented by the reaction V
2O
3 +
xZn
2+ +
nH
2O + 2
xe
− → Zn
xV
2O
3·
nH
2O, alongside zinc metal deposition, Zn
2+ + 2e
− → Zn. Upon recharging, the Raman signals associated with these inserted species disappear, confirming the reversibility of the reaction. Wang et al. presents an in situ activation strategy for a V
2O
3@C microsphere cathode, where the initial tunnel-structured V
2O
3 transforms into a layered, amorphous, and oxygen-deficient Zn
0.4V
2O
5−m·nH
2O during the first charge [
31]. This new phase enables stable Zn
2+ (de-)intercalation, leading to excellent cycling stability and a high capacity of 602 mAh g
−1 over 150 cycles.
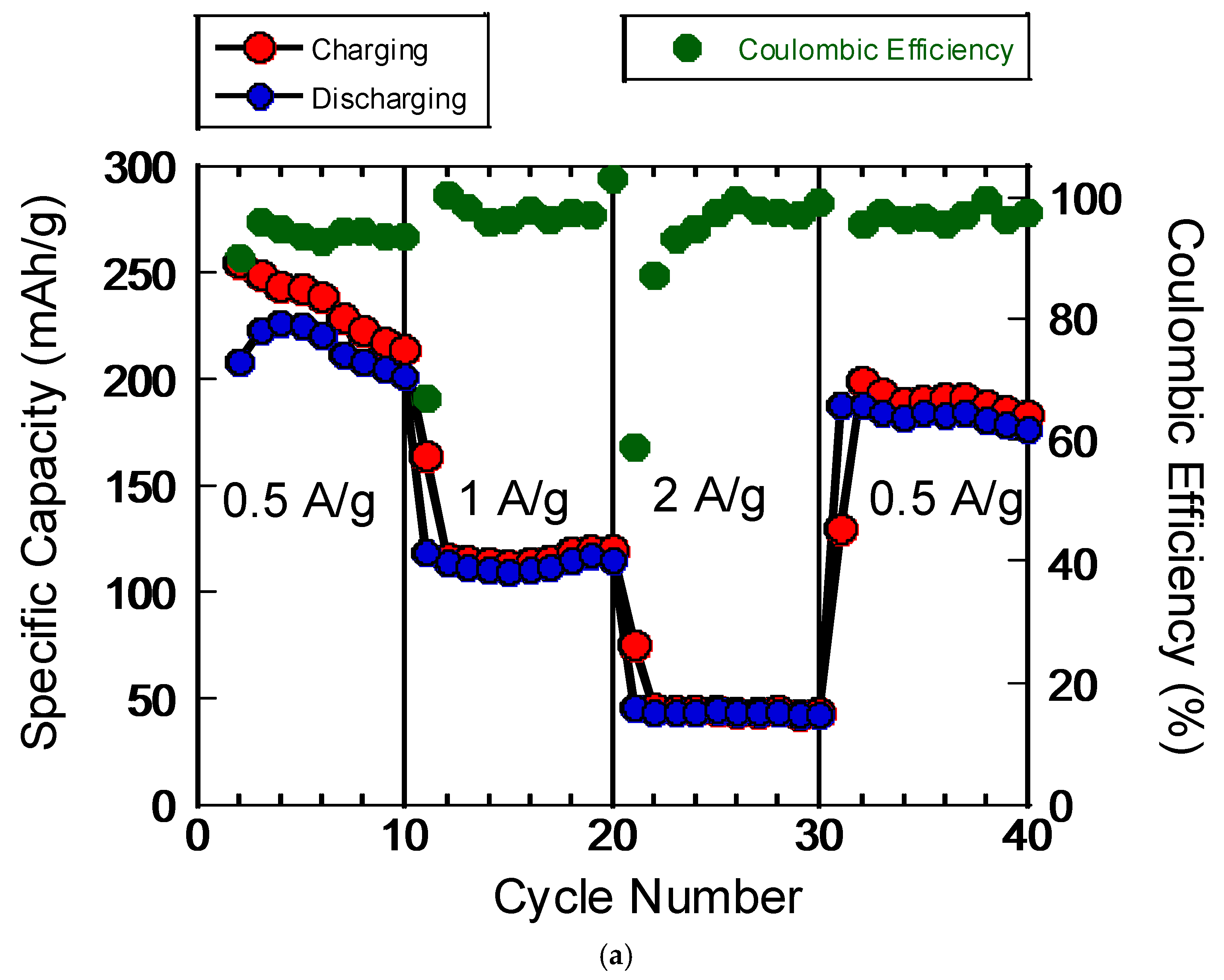
Figure 3 illustrates the electrochemical performance of the Zn(CN)
2–V
2O
3–C composite as a cathode in a ZIB, showing the cycling stability (
Figure 3a) and voltage profile during charge–discharge (
Figure 3b). The first cycle exhibits a high specific capacity (~250 mAh/g), likely due to surface-driven capacitive effects and the initial electrochemical activation of the Zn(CN)
2–V
2O
3–C composite. Such high initial capacity may involve side reactions, including electrolyte decomposition or Zn intercalation into defect sites. At 1 and 2 A/g, the charge–discharge capacity significantly decreases, stabilizing at ~50–120 mAh/g. This suggests diffusion limitations at higher currents, where Zn
2+ intercalation becomes kinetically restricted. When the current rate is reduced back to 500 mA/g, the capacity recovers, indicating the good reversibility and structural integrity of the Zn(CN)
2–V
2O
3–C composite. This behavior suggests that the Zn(CN)
2–V
2O
3–C composite maintains its electrochemical activity over cycles despite the performance drop at high rates.
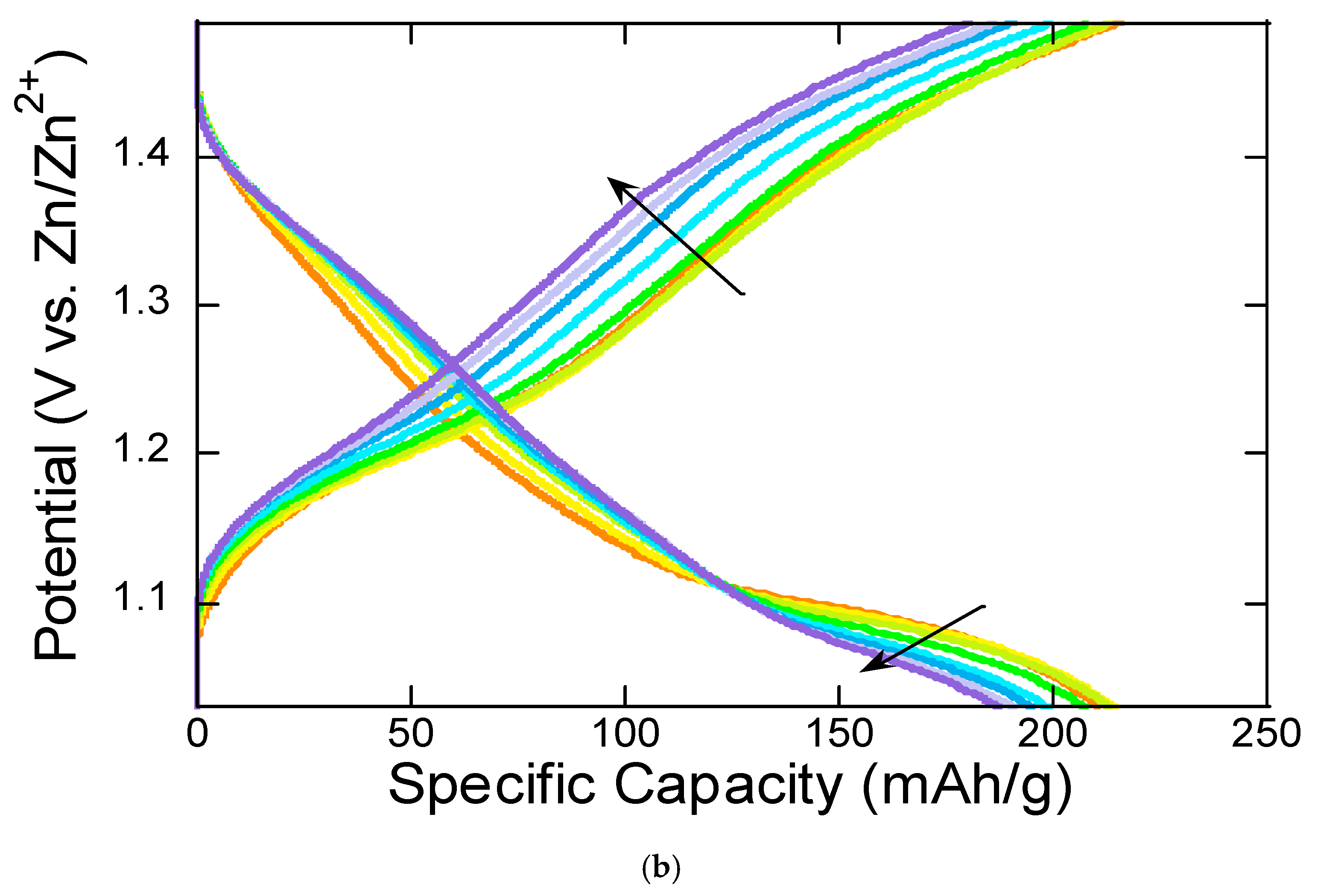
The charge–discharge curves in
Figure 3b exhibit gradual voltage slopes rather than sharp plateaus, indicating a combined diffusion-limited and pseudocapacitive behavior, which will be shown in
Figure 4. Small plateaus suggest multiple redox reactions (V
5+/V
4+ and possibly V
4+/V
3+), which is consistent with the peaks observed in the CV curves in
Figure 2. The first charge–discharge curve in
Figure 3a has a higher voltage gap, likely due to the initial activation and possible side reactions. Subsequent cycles show reduced polarization, suggesting an activation process in the material.
Under low mass loading (≈0.1 mgcm
−2), the electrode delivers high gravimetric capacities up to 120 mAh g
−1 at 0.5 Ag
−1. At a much higher loading of 5 mgcm
−2, the capacity remained similar at 120–130 mAh g
−1 under the same current densities, as shown in
Supplementary Materials Figure S3. Because AC-EPD creates uniform and well-adhered films, our electrodes exhibit nearly identical specific capacities regardless of whether the active material loading is low or high. This uniformity minimizes local thickness variations and maintains short ion diffusion paths even in thicker films. As a result, the high-loading AC-EPD cathode achieves performance on par with the low-loading electrode, highlighting AC-EPD’s ability to preserve electrochemical kinetics and active material utilization across a wide range of mass loadings.
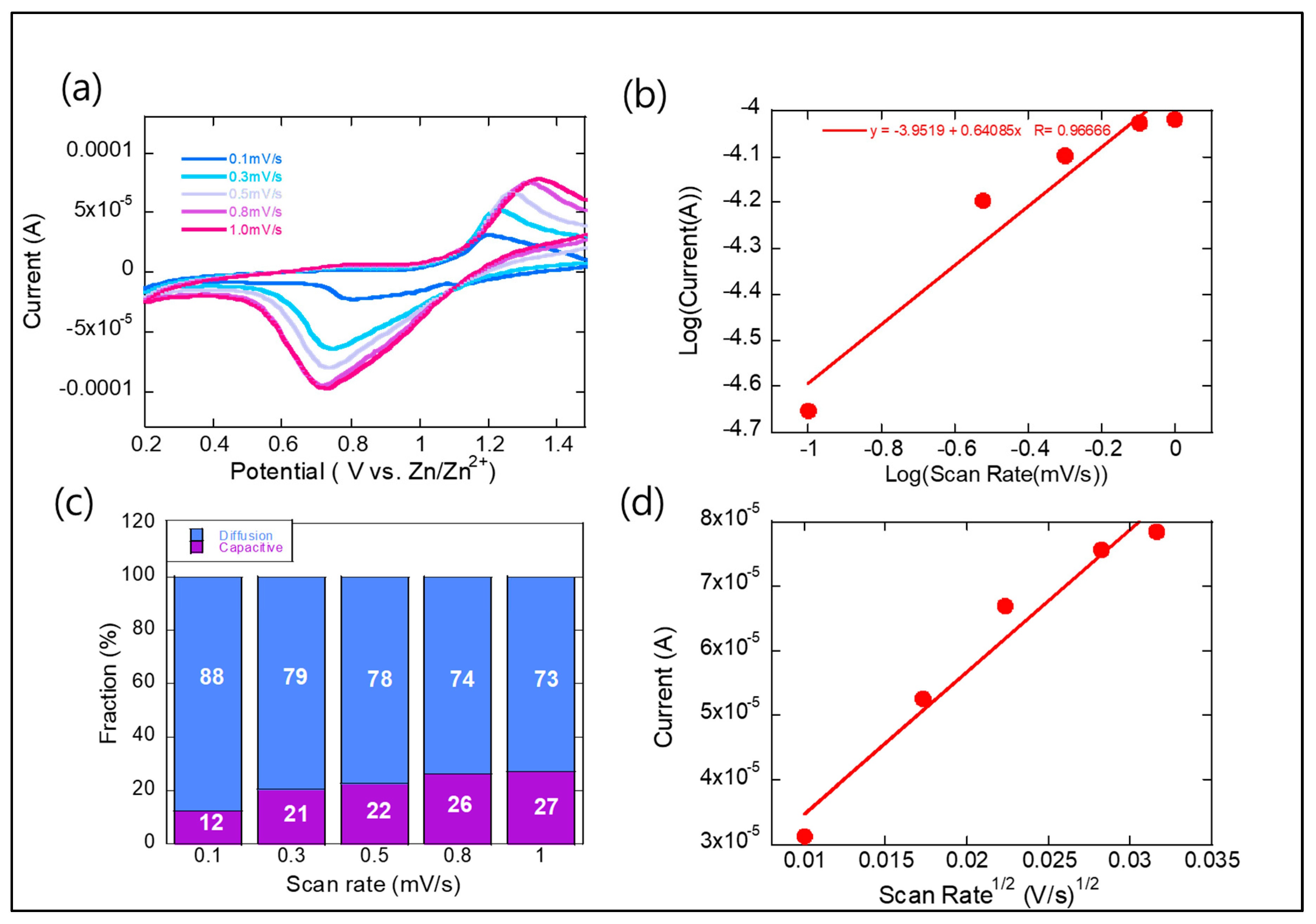
Figure 4 shows scan rate-dependent CV curves and the contribution of capacitive vs. diffusion-controlled charge storage for the Zn(CN)
2–V
2O
3–C composite cathode in a ZIB. The CV measurements in
Figure 4a revealed a
b-value of 0.64 from the cathodic peak (0.7 V), confirming a hybrid charge storage mechanism, as estimated in
Figure 4b. The
b-value, derived from scan rate-dependent CV curves, serves as a quantitative indicator of the dominant charge storage mechanism in battery electrodes. It is determined based on the power-law relationship between the peak current (
ip) and the scan rate (
v), which can be expressed as
ip = avb. In this equation,
a and
b are empirical constants. To determine the
b-value experimentally, the equation is typically linearized by taking the logarithm of both sides, resulting in log(
ip) = log(
a) +
blog(
v). A plot of log(
ip) versus log(
v) yields a straight line, where the slope corresponds to the
b-value. The magnitude of the
b-value provides insight into the nature of the charge storage mechanism. A
b-value of 0.5 indicates a diffusion-controlled process, typical of faradaic intercalation, where ions like Zn
2+ diffuse into the bulk of the electrode material. In contrast, a
b-value of 1.0 reflects a capacitive process, where charge is stored through surface or near-surface redox reactions—commonly referred to as pseudocapacitive behavior. When the
b-value falls between 0.5 and 1.0, it suggests a hybrid mechanism, where both diffusion-controlled intercalation and capacitive processes contribute to the overall charge storage. A
b-value of 0.64 indicates a hybrid charge storage mechanism where both Zn
2+ intercalation (diffusion-limited process) and surface-controlled pseudocapacitive effects contribute to energy storage. This means that while the Zn(CN)
2–V
2O
3–C composite stores charge predominantly via Zn
2+ intercalation into the bulk of the cathode, a substantial fraction is capacitive, leading to improved power capability. Faster charging/discharging is feasible due to enhanced surface-driven redox reactions. The
b-value being closer to 0.5 than 1.0 suggests that Zn
2+ diffusion within the bulk is still the dominant mechanism.
The scan rate-dependent CV analysis was used to distinguish between capacitive and diffusion-controlled charge storage mechanisms, as shown in
Figure 4c. The current response at a given potential was described by the equation
i(V) = k1ν + k2ν1⁄2, where
k1ν represents the capacitive (surface-controlled) contribution, and
k2ν1/2 corresponds to the diffusion-controlled process. To quantify each contribution, the equation was rearranged as
i(V) ⁄ ν1⁄2 =
k1ν1⁄2 + k2, allowing for the separation of the two processes by plotting current versus scan rate and extracting
k1 and
k2 from the slope and intercept. As the scan rate increased from 0.1 to 1.0 mV/s, the proportion of the capacitive contribution increased noticeably, as shown in
Figure 4c.
The Zn-ion diffusion coefficient in the ZIB was estimated using CV data at various scan rates, based on the Randles–Sevcik equation:
Here,
Ip is the peak current,
ν is the scan rate, and
DZn is the Zn-ion diffusion coefficient. Other constants include
n (number of electrons),
F (Faraday constant),
A (electrode area),
CZn (initial Zn
2+ concentration),
R (gas constant), and
T (temperature). By plotting
Ip versus
ν1/2, as shown in
Figure 4d, the diffusion coefficients were calculated to be 5.1 × 10
−11 cm
2/s for reduction. These values indicate efficient Zn-ion transport within the ultrathin cathode in the ZIB.
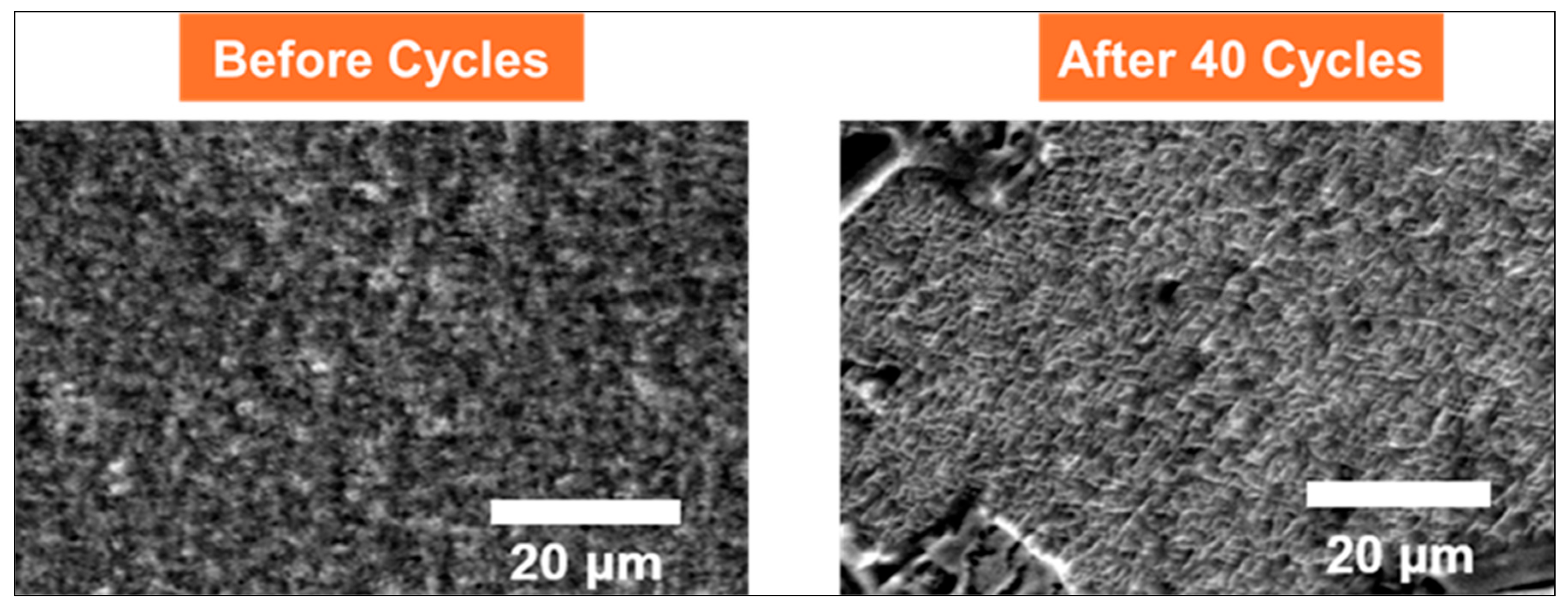
The morphological evolution of the Zn(CN)
2–V
2O
3–C composite cathode is shown in
Figure 5, as observed by SEM before and after 40 cycles. Initially, the electrode surface appears smooth, compact, and uniform, characteristic of a well-deposited ultrathin film with excellent structural integrity. After 40 cycles, the surface develops a more textured, wrinkled morphology with a mesh-like appearance, indicating structural rearrangement likely induced by repeated Zn
2+ intercalation and water co-insertion. Despite these changes, the film remains continuous, with no evidence of cracking or delamination. This preservation of structural coherence highlights the mechanical resilience of the ultrathin electrode architecture and underscores its viability as a stable platform for probing electrode–electrolyte interfacial behavior in Zn-ion batteries.
The Zn(CN)2–V2O3–C composite cathode enhances Zn-ion battery performance by combining the strengths of its components. Zn(CN)2 provides structural stability and may form a protective interphase that suppresses side reactions, while its porous framework supports Zn2+ transport. V2O3 acts as an active material, enabling high-capacity storage through multivalent redox reactions. Carbon improves conductivity and helps maintain electrode integrity during cycling, resulting in a stable, high-performance composite. The Zn(CN)2–V2O3–C composite cathode offers enhanced ion/electron transport, improved rate capability, and stable cycling. Zn(CN)2 and carbon help reduce structural stress during Zn2+ insertion, maintaining electrode integrity. Fabrication via AC-EPD ensures ultrathin, uniform films with strong adhesion, boosting performance and enabling interfacial charge storage studies. This composite presents a promising strategy for high-performance and durable Zn-ion batteries.