Variable Unidentate Ligands in Cu(I)(XXY) and Cu(I)(XYZ) Complexes—Structural Aspects
Abstract
1. Introduction
2. Results and Discussion
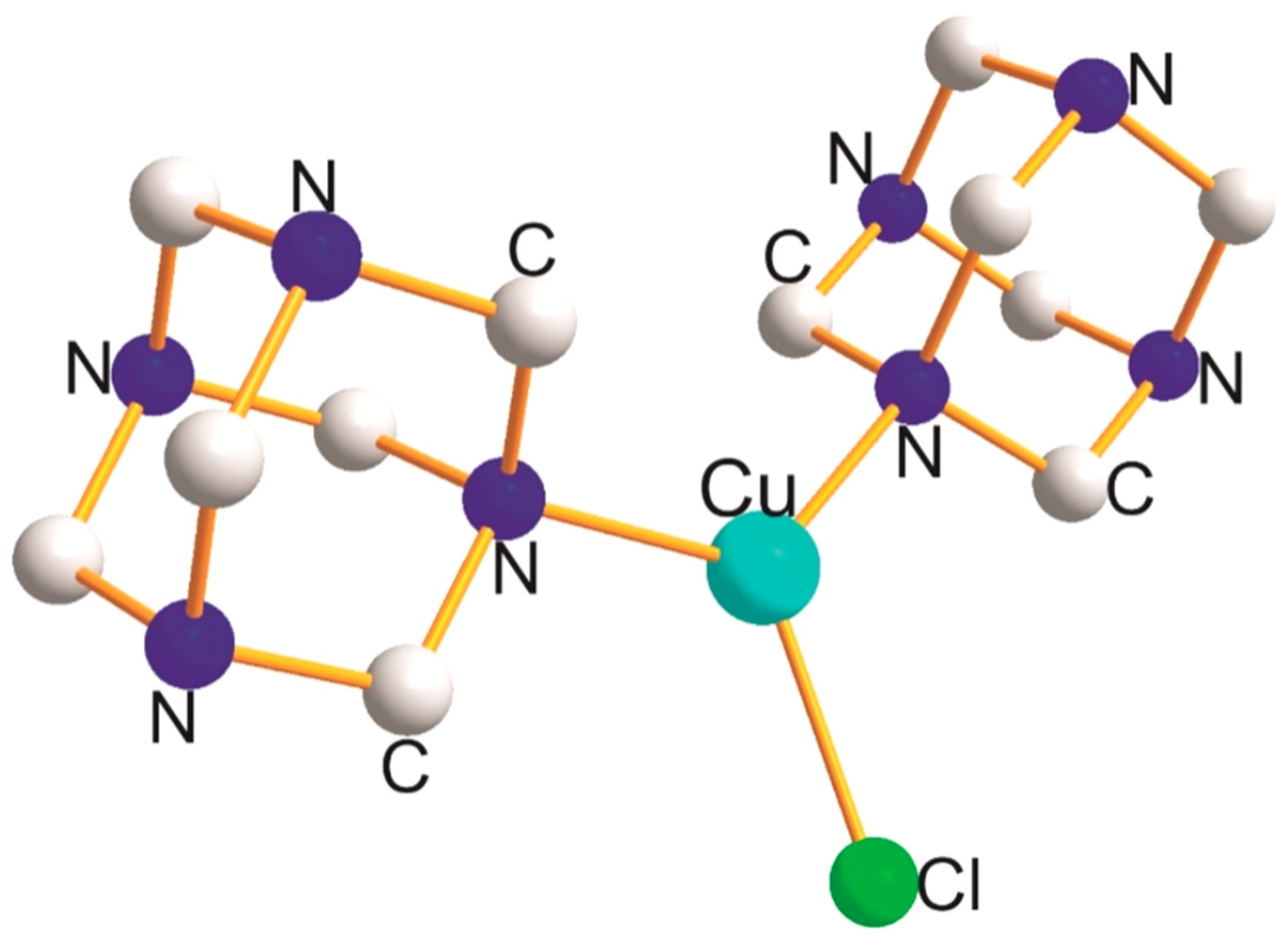
2.1. Cu(NNY) (Y = O, C, Cl, S, Br, and I); Cu(CCY) (Y = O, Cl, P, Br, and I) Derivatives
2.2. Cu(SSY), (Y = N, Cl, I), Cu(SeSeY) (Y = Cl, Br, I) Derivatives
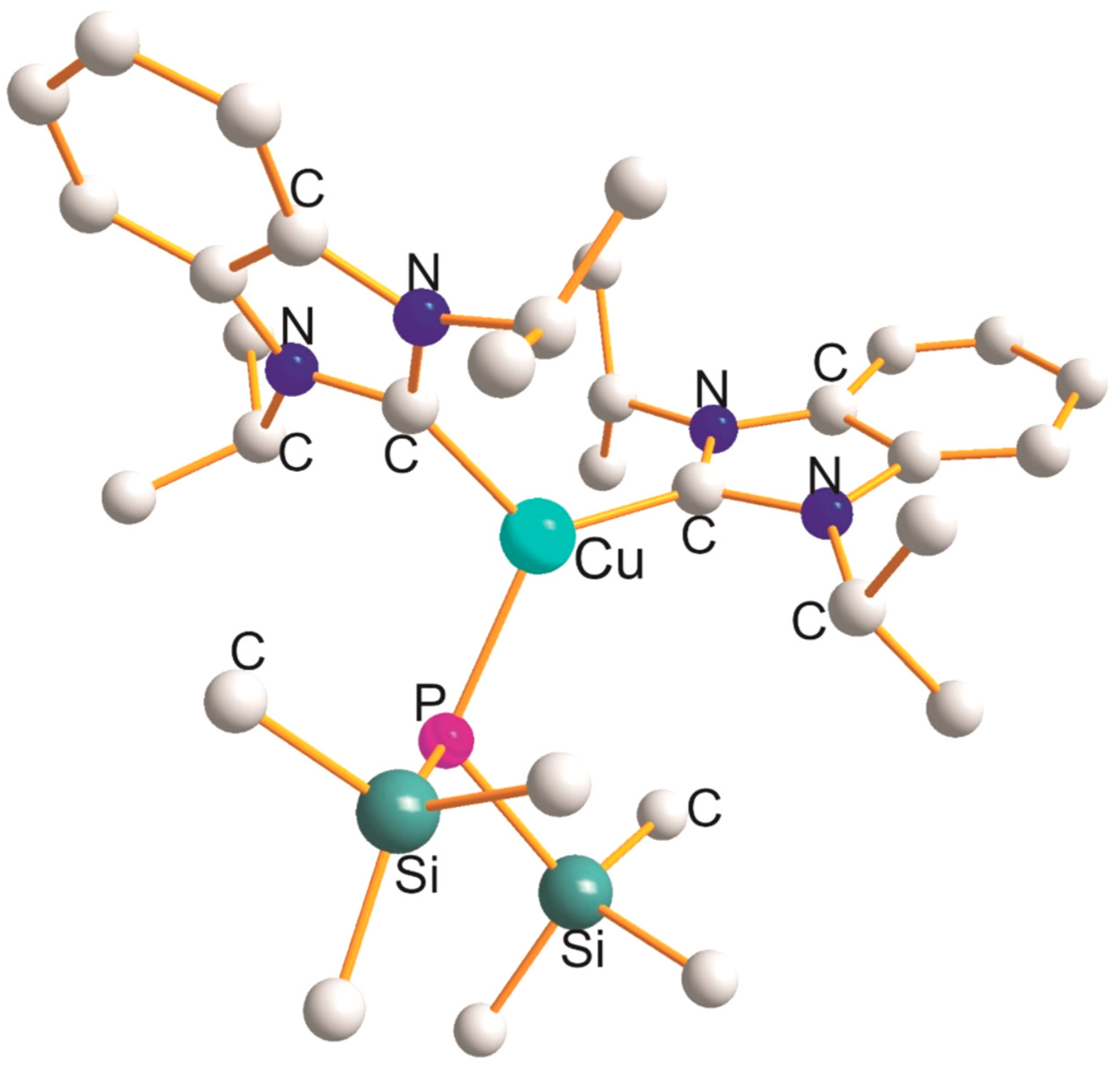
2.3. Cu(PPY) (Y = O, C, Cl, Br, and I) Derivatives
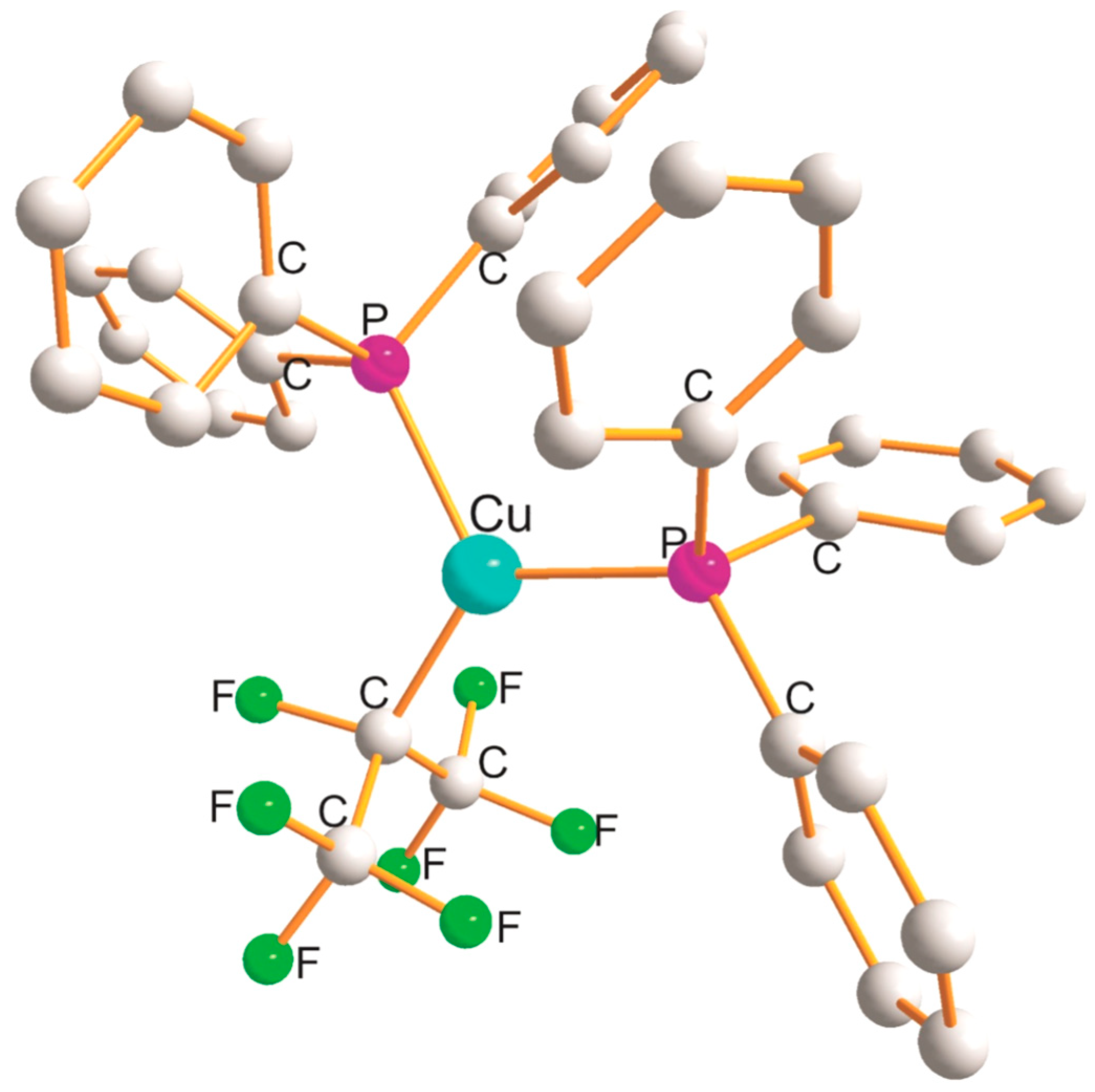
2.4. Cu(ClClY), Cu(BrBrY) (Y = N,P), and Cu(IIP) Derivatives
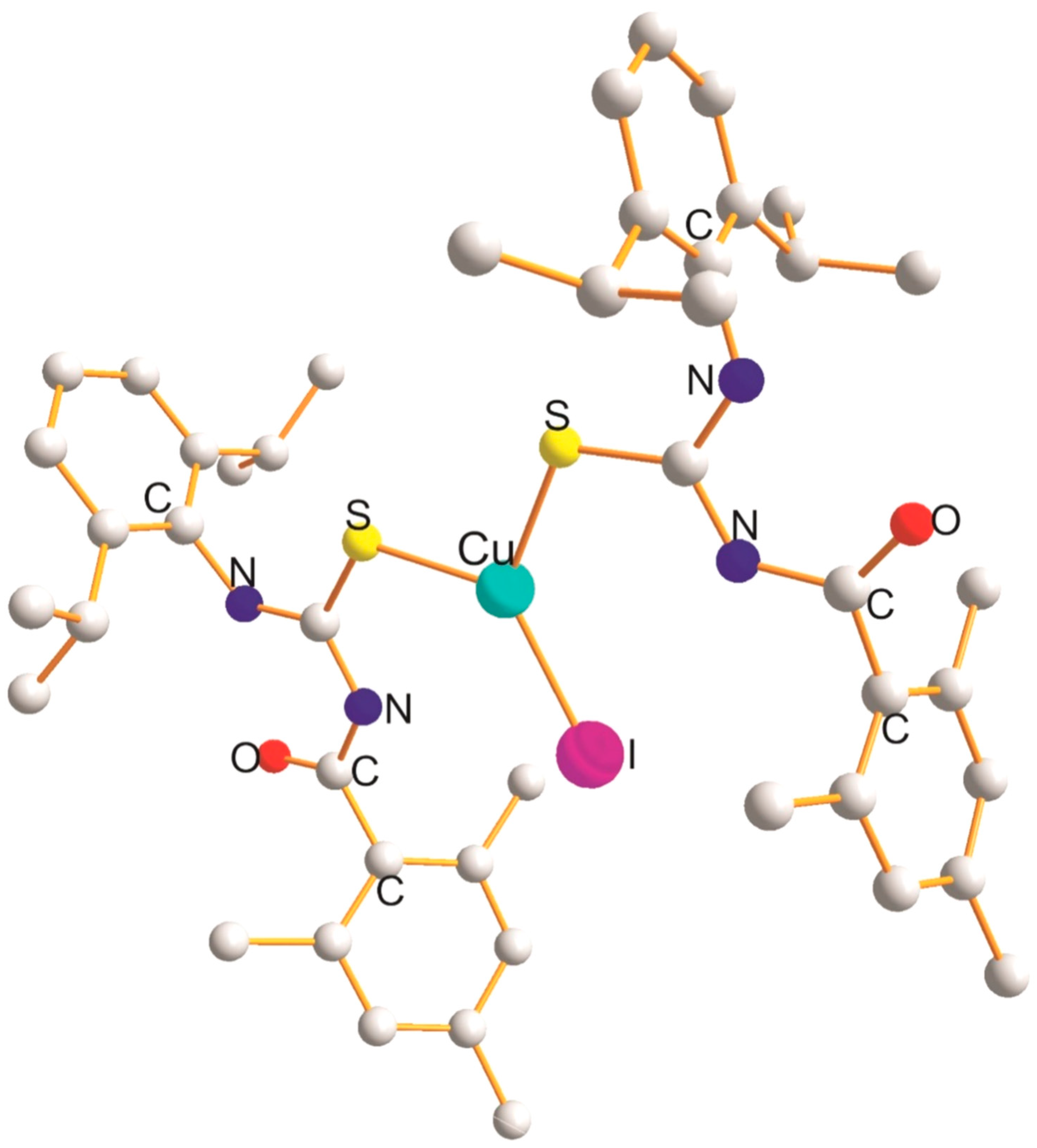
2.5. Cu(SClP), Cu(SBrP), and Cu(SIP) Derivatives
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (Me3Si)2P | (ditrimethylsilyl)phosphide |
| 2,6-But2C6H3O | 2,6-di-t-butylhexadienal |
| 2,6-Me2py | 2,6-dimethyl pyridine |
| 2-Mepy | 2-methyl pyridine |
| 2-Mequ | 2-methylimidazole |
| bpbim | 1-benzyl-2-benzimidazole |
| C3H2NO2 | cyanoacetato |
| C3H3O4 | malonato |
| C3H4N2S2 | 5-methyl-1,3,4-thiadiazole-2(3H)-thione |
| C4H5O4 | succinato |
| C4H8N2S | 1-methylimidazolidine-2-thione |
| C5H6N2 | pyridin-4-amine |
| C6H12N2S | 1-propylimidazolidine |
| C6H12N4 | (1,3,5,7-tetra-azatricyclo[3.3.1.13,7]decane) |
| C7H9N2O6 | 3,5-dinitrobenzoato |
| C9H14N2O2S | ethyl-4,6-dimethyl-2-thioxo-1,2,3,4-tetra-hydropyrimidine-5-carboxylate |
| C9H21PS | tri-isopropylphosphine sulfide |
| C10H12N2O3S | methyl ((4-methoxyphenyl)carbonothioyl)carbamate) |
| C11H22N4 | 2-t-butyl-5-(t-butylamino)-4-methyl-2,4-dihydro-3H-1,2,4-triazol-3-ylidene) |
| C11H9N | 2- phenyl pyridine |
| C12H11N | 3-methyl-2-phenylpyridine |
| C13H14N2O2S | (1-(2-methyl-4-(sulfanylidene)-3,4,5,6-tetrahydro-2H-2,6-methano-1,3,5-benzoxadiazocin-11-yl)ethan-1-one) |
| C13H18N2 | 1,3-di-isopropylbenzimzadol-2-ylidene |
| C15H21BNP | (trimethylammonio(dihydro)borato)diphenylphosphine) |
| C16H18N2O2S | (isobutyl(1-naphthylcarbamothioyl)carbamate) |
| C17H20N2S | (1,3-bis(2,6-dimethylphenyl)thiourea) |
| C18H14P | ((2-chlorophenyl)(diphenylphosphine) |
| C19H15OP | (2-diphenylphophino)benzaldehyde) |
| C20H16N4 | 5-anilino-2,4-diphenyl-2,4-dihydro-3H-1,2,4-triazol-3-ylidene |
| C22H19S2P | (2-(2-phenyl-3-(2-theionyl)-4,5,6,7-tetrahydro-2H-isophosphindol-1-yl)thiophene) |
| C23H30N2OS | (N-((2,6-disopropylphenyl)carbamothioyl)-2,4,6-trimethylbenzamide) |
| C27H36N2 | (1,3-bis(2,6-bis(propan-2-yl)phenyl)-imidazol-2-ylidene) |
| C27H3N2 | (1,3-bis (2,6-di-isopropylphenyl)imidazol-2-ylidene) |
| C2H3N | acetonitrile |
| C32H57NP | (5-dicyclohexylphosphino)-1-(2,3-di-isopopylphenyl)-2,2,4,4-tetramethyl-3,4-dihydro-2H-pyrrol-1-ium) |
| C34H40N4yP | (1,3-bis(1,3-di-isopropylbenzimidzale-2-ylidene)-1,3-dihydro-2H-isophophindol-2-yl) |
| C64H78N6PS | (4-[{1,3-bis[2,6-bis(propan-2-yl)phenyl]-4-phenyl-1H-1,2,3-triazol-3-ium-5-yl}(sulfanyl)phosphenyl)-1,3-bis[2,6-bis(propan-2-yl)phenyl]-5-phenyl-1H-1,2,3 triazol-3-iumato) |
| Hmpt | methylpyruvate thiosemicarbazone |
| Imt | imidiazolidine-2-thionate |
| Me2imt | N,N′-dimethylimizadolidine-2-thicone |
| Pcy3 | tricyclohexyl phosphine |
| PMe3 | trimethylphosphine |
| PPh3 | triphenylphosphine |
| C12H11N3S | 1-phenyl-3-(2-pyridyl)-2-thiourea |
| p-tolNC | o-totylcyanide |
| Py | pyridine |
| py2SH | pyridine-2-thione |
| pymtH | pyrimidine-2-thione |
| tclH | 2-thioxohexamethyleneimine |
| Thm | thiamine |
| P(o-totyl)3 | tri(o-totyl)phosphine |
| Tuc | thiouracil |
References
- Österberg, R. Models for copper-protein interaction based on solution and crystal structure studies. Coord. Chem. Rev. 1974, 12, 309–347. [Google Scholar] [CrossRef]
- Conry, R.C. Copper: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, S.C.; Ho, C.L.; Wong, W.L. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Li, Z.; Hou, J.T.; Wang, S.; Zhu, L.; He, X.; Shen, J. Recent advances of luminescent sensors for iron and copper: Platforms, mechanisms, and bio-applications. Coord. Chem. Rev. 2022, 469, 214695. [Google Scholar] [CrossRef]
- Soleiman-Beigi, M.; Mohammadi, M.; Kohzadi, H. An overview on copper in industrial chemistry: From ancient pigment to modern catalysis. Coord. Chem. Rev. 2025, 529, 216438. [Google Scholar] [CrossRef]
- Unavane, S.; Patil, R.; Syed, S.; Jain, H.K. Exploring the therapeutic potential of copper and cobalt complexes as anticancer agents: A comprehensive review. Transit. Met. Chem. 2025. [Google Scholar] [CrossRef]
- Pavlikov, A.Y.; Saikova, S.V.; Samoilo, A.S.; Karpov, D.V.; Novikova, S.A. Synthesis of Copper(II) Oxide Nanoparticles by Anion-Exchange Resin-Assisted Precipitation and Production of Their Stable Hydrosols. Russ. J. Inorg. Chem. 2024, 69, 265–276. [Google Scholar] [CrossRef]
- Jardine, F.H. Copper (I) Complexes. In Advances in Inorganic Chemistry and Radiochemistry; Emeléus, H.J., Sharpe, A.G., Eds.; Academic Press: Cambridge, MA, USA, 1975; Volume 17, pp. 115–163. [Google Scholar] [CrossRef]
- Babgi, B.A. Synthetic protocols and applications of copper(I) phosphine and copper(I) phosphine/diimine complexes. J. Organomet. Chem. 2021, 956, 122124. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Buzanov, G.A.; Malinina, E.A.; Kuznetsov, N.T.; Vologzhanina, A.V. Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion. Crystals 2020, 10, 389. [Google Scholar] [CrossRef]
- Mahfouz, N.; Abi Ghaida, F.; El Hajj, Y.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent Achievements on Functionalization within closo-Decahydrodecaborate [B10H10]2− Clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Corfes, P.A.F.; Marx, M.; Trose, M.; Beller, M. Heterolytic copper complexes with nitrogen and phosphorus ligands in photocatalysis: Overview and perspectives. Chem. Catal. 2021, 1, 298–338. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Peruško, S.; Phan, T.A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive copper complexes: Properties and applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef] [PubMed]
- Lazorski, M.S.; Castellano, F.N. Advances in the light conversion properties of Cu(I)-based photosensitizers. Polyhedron 2014, 82, 57–70. [Google Scholar] [CrossRef]
- Benesperi, I.; Singh, R.; Freitag, M. Copper Coordination Complexes for Energy-Relevant Applications. Energies 2020, 13, 2198. [Google Scholar] [CrossRef]
- Ravaro, L.P.; Zanoni, K.P.S.; de Camargo, A.S.S. Luminescent Copper(I) complexes as promising materials for the next generation of energy-saving OLED devices. Energy Rep. 2020, 6, 37–45. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Pérez-Álvarez, D.; Rodríguez-Argüelles, C.; Henriques, M.S.; Paixão, J.A.; Prado-Lopez, S. Synthesis, spectral characterization and X-ray crystallographic study of new copper (I) complexes. Antitumor activity in colon cancer. Polyhedron 2016, 119, 112–119. [Google Scholar] [CrossRef]
- Melník, M.; Mikušová, V.; Mikuš, P. The structural aspects of mutually trans-X-Cu(I)-X (X= OL, NL, CL, PL, SL, SeL, Cl or Br) complexes. Inorganics 2024, 12, 245. [Google Scholar] [CrossRef]
- Melník, M.; Mikušová, V.; Mikuš, P. Monodentate Ligands in X-Cu(I)-Y Complexes—Structural Aspects. Inorganics 2024, 12, 279. [Google Scholar] [CrossRef]
- Melník, M.; Mikušová, V.; Mikuš, P. Homo-Chromophores in Cu(I)(XXX), (X3 = N3, C3, Cl3, S3, P3, Br3, or I3) Derivatives—Structural Aspects. Inorganics 2025, 13, 36. [Google Scholar] [CrossRef]
- Parvin, N.; Hossain, J.; George, A.; Parameswaran, P.; Khan, S. N-heterocyclic silylene stabilized monocordinated copper(i)–arene cationic complexes and their application in click chemistry. Chem. Commun. 2020, 56, 273–276. [Google Scholar] [CrossRef]
- Liske, A.; Wallbaum, L.; Holzel, T.; Foller, J.; Gernert, M.; Hupp, B.; Ganter, C.; Marian, C.M.; Steffen, A. Cu–F Interactions between Cationic Linear N-Heterocyclic Carbene Copper(I) Pyridine Complexes and Their Counterions Greatly Enhance Blue Luminescence Efficiency. Inorg. Chem. 2019, 58, 5433–5445. [Google Scholar] [CrossRef]
- Healy, P.C.; Pakawatchai, C.; White, A.H. Lewis-base adducts of Group 1B metal(I) compounds. Part 18. Stereo-chemistries and structures of the 1:1 neutral complexes of CuIX with 1,10-phenanthroline (X = I) or 2,9-dimethyl-1,10-phenanthroline (X = I, Br, or Cl). J. Chem. Soc. Dalton Trans. 1985, 2531–2539. [Google Scholar] [CrossRef]
- Vakulka, A.; Goreshnik, E. Copper(I) bromide and chloride complexes with urotropine and triethylenediamine: Synthesis, crystal structure, and Raman characterization. J. Coord. Chem. 2018, 71, 2426–2440. [Google Scholar] [CrossRef]
- Skelton, B.W. (University of Western Australia, Nedlands, Australia); Healy, P.C. (Griffith University, Brisbane, Australia). Personal Communication, 2018.
- Huang, L.; Huang, S.; Zhang, Z.; Cao, L.; Xu, X.; Yan, X. Mesoionic-Carbene-Stabilized Thiophosphoryl Cation (PS+). Organometallics 2021, 40, 1190–1194. [Google Scholar] [CrossRef]
- Dyason, J.C.; Healy, P.C.; Pakawatchai, C.; Patrick, V.A.; White, A.H. Lewis base adducts of Group 11 metal compounds. 15. Structural studies of mononuclear adducts of copper(I) halides with pyridine bases of stoichiometry XCuL2,3. Inorg. Chem. 1985, 24, 1957–1960. [Google Scholar] [CrossRef]
- Zukerman-Schpector, J.; Castellane, E.E.; Oliva, G. The crystal and molecular structure of bromo bis(1-phenyl-3, 5-dimethylpyrazole)-copper(I), CuBr(pdmp)2. Inorg. Chim. Acta 1990, 175, 1–2. [Google Scholar] [CrossRef]
- Dyason, J.C.; Engelhardt, L.M.; Healy, P.C.; Kildea, J.D.; White, A.H. Lewis-Base Adducts of Group 11 Metal-Compounds. XXXIII. Structural Characterization of Two Novel ‘Trigonal Planar’ Halobis(N-Base)Copper(I) Complexes. Aust. J. Chem. 1988, 41, 335–340. [Google Scholar] [CrossRef]
- Healy, P.C.; Pakawatchai, C.; White, A.H. Lewis-base adducts of Group 1B metal(I) compounds. Part 2. Synthesis and structure of CuIL2 complexes (L = nitrogen base). J. Chem. Soc. Dalton Trans. 1983, 1917–1927. [Google Scholar] [CrossRef]
- Toth, A.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. Copper(I)-benzimidazole adducts: From mononuclear to polymeric complexes. Inorg. Chem. 1987, 26, 3897–3902. [Google Scholar] [CrossRef]
- Hernandez-Molina, R.; Gonzalez-Platas, J.; Agirretxu, A. Synthesis and structure of [CuI(3-methyl-2-phenylpiridine)2] with intermolecular stacking interactions. J. Struct. Chem. 2014, 55, 1478–1483. [Google Scholar] [CrossRef]
- Fiaschi, P.; Floriani, C.; Pasquali, M.; Chiesi-Villa, A.; Guastini, C. Copper(I)-phenoxide complexes: Synthesis and ligand-induced transformations of the copper(I)-phenoxo functionality. Inorg. Chem. 1986, 25, 462–469. [Google Scholar] [CrossRef]
- Thie, C.; Bruhn, C.; Leibold, M.; Siemeling, U. Coinage Metal Complexes of the Carbenic Tautomer of a Conjugated Mesomeric Betaine Akin to Nitron. Molecules 2017, 22, 1133. [Google Scholar] [CrossRef] [PubMed]
- Thie, C.; Hitzel, S.; Wallbaum, L.; Bruhn, C.; Siemeling, U. Coinage metal complexes of the carbenic tautomer of Nitron. J. Organomet. Chem. 2016, 821, 112–121. [Google Scholar] [CrossRef]
- Najafabadi, B.K.; Corrigan, J.F. Enhanced thermal stability of Cu–silylphosphido complexes via NHC ligation. Dalton Trans. 2015, 44, 14235–14241. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.W.; Griffith, E.A.H.; Amma, E.L. Structure of bis(2-thiouracil)chlorocopper(I) dimethylformamide solvate, a reaction product of copper(II) chloride with thiouracil. Inorg. Chem. 1976, 15, 2993–2997. [Google Scholar] [CrossRef]
- Devillanova, F.A.; Verani, G.; Battaglia, L.P.; Bonamartini Corradi, A. N,N′-dialkylsubstituted Imidazolidine-2-thionecopper(I) complexes. X-ray analysis of chloro-bis(N,N′-dimethylimidazolidine-2-thione)copper(I). Transit. Met. Chem. 1980, 5, 362–364. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Fava, G.G.; Lanfranchi, M.; Pelizzi, C.; Tarasconi, P. Synthesis, spectroscopic and structural characterization of chlorobis(methyl pyruvate thiosemicarbazone)copper(I) and chlorobis(triphenylphosphine) (methyl pyruvate thiosemicarbazone)copper(I) toluene solvate (2/1). Inorg. Chim. Acta 1991, 181, 253–262. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Fava, G.G.; Pelizzi, C.; Tarasconi, P. Synthesis and structural characterization of chloro-(triphenylphosphine)-[1-phenyl-3-(2-pyridyl)-2-thiourea]Cu(I) and chloro-bis [1-[phenyl-3-(2-pyridyl)-2-thiourea]-Cu(I). Inorg. Chim. Acta 1985, 97, 99–109. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, W.; Song, J.F.; Zhou, R.S.; Jiao, W.Z. Four new CuI/AgI-based coordination compounds containing 2-mercapto-5-methyl-1,3,4-thiadiazole: Synthesis, crystal structures and fluorescence properties. Inorg. Chim. Acta 2021, 528, 120596. [Google Scholar] [CrossRef]
- Aulakh, J.K.; Lobana, T.S.; Sood, H.; Arora, D.S.; Garcia-Santos, I.; Hundal, G.; Kaur, M.; Smolenski, V.A.; Jasinski, J.P. Variable coordination and C–S bond cleavage activity of N-substituted imidazolidine-2-thiones towards copper: Synthesis, spectroscopy, structures, ESI-mass and antimicrobial studies. Dalton Trans. 2017, 46, 1324–1339. [Google Scholar] [CrossRef]
- Kuzovlev, A.S.; Savinkina, E.V.; Chernyshev, V.V.; Grigoriev, M.S.; Volov, A.N. Copper and palladium complexes with substituted pyrimidine-2-thiones and 2-thiouracils: Syntheses, spectral characterization, and X-ray crystallographic study. J. Coord. Chem. 2016, 69, 508–521. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, D.P.; Pratap, S.; Marverti, G.; Butcher, R.J. Copper(I) complexes of N-(2/4 methoxy/2-chloro-4-nitro)phenyl-N′ (methoxycarbonyl)thiocarbamides as potential anticancer agents: Synthesis, crystal structure, in vitro cytotoxicity and DNA damage studies. Polyhedron 2019, 170, 431–439. [Google Scholar] [CrossRef]
- Kuzovlev, A.S.; Volkova, D.A.; Parfenova, I.V.; Kulakov, I.V.; Shkirdova, A.O.; Zamilatskov, I.A.; Chernyshev, V.V.; Rybakov, V.B.; Tyurin, V.S.; Fefilov, N.N.; et al. Copper(i) halide and palladium(ii) chloride complexes of 4-thioxo [1,3,5]oxadiazocines: Synthesis, structure and antibacterial activity. New J. Chem. 2020, 44, 7865–7875. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, D.P.; Marverti, G.; Butcher, R.J.; Pratap, S. Monodentate Coordination of N,N′-Disubstituted Thiocarbamide Ligands: Syntheses, Structural Analyses, In Vitro Cytotoxicity and DNA Damage Studies of Cu(I) Complexes. ChemistrySelect 2018, 3, 3675–3679. [Google Scholar] [CrossRef]
- Barman, M.K.; Sinha, A.K.; Nembenna, S. An efficient and recyclable thiourea-supported copper(i) chloride catalyst for azide–alkyne cycloaddition reactions. Green Chem. 2016, 18, 2534–2541. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S.Y.; Li, H.P.; Yang, Y.; Roesky, H.W. Synthesis and Characterization of Copper Complexes with the N-(2,6-Diisopropylphenyl)-N′-acylthiourea Ligands. Eur. J. Inorg. Chem. 2017, 2017, 1406–1413. [Google Scholar] [CrossRef]
- Jones, P.G. (Braunschweig University of Technology, Braunschweig, Germany); Hrib, C.G. (Braunschweig University of Technology, Braunschweig, Germany); du Mont, W.W. (Braunschweig University of Technology, Braunschweig, Germany); Daniliuc, C.G. (Universitat Munster, Munster, Germany). Personal Communication, 2015.
- Restivo, R.J.; Costin, A.; Ferguson, G.; Carty, A.J.; Perchlorato, Nitrato, and Acetylacetonato Complexes of Copper(1). The Crystal and Molecular Structure of Perchloratobis(tricyclohexylphosphine)Copper. Can. J. Chem. 1975, 53, 1949. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Khandelwal, B.; Reibenspies, J.H. Intramolecular and intermolecular hydrogen bonding in triphenylphosphine derivatives of copper(I) carboxylates, (Ph3P)2CuO2C(CH2)nCOOH. Role of copper(I) in the decarboxylation of malonic acid and its derivatives. Inorg. Chem. 1994, 33, 531–537. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Longridge, E.M.; Khandelwal, B.; Klausmeyer, K.K.; Reibenspies, J.H. Role of the Metal Center in the Homogeneous Catalytic Decarboxylation of Select Carboxylic Acids. Copper(I) and Zinc(II) Derivatives of Cyanoacetate. J. Am. Chem. Soc. 1995, 117, 318–328. [Google Scholar] [CrossRef]
- Mauro, A.E.; Porta, C.C.; De Simone, C.A.; Zukerman-Schpector, J.; Catellano, E.E. Synthesis and structural studies of (3,5-dinitrobenzoate)bis(triphenylphosphine)copper(I). Polyhedron 1993, 12, 1141–1143. [Google Scholar] [CrossRef]
- Andrella, N.O.; Liu, K.; Gabidullin, B.; Vasiliu, M.; Dixon, D.A.; Baker, T.R. Metal Heptafluoroisopropyl (M-hfip) Complexes for Use as hfip Transfer Agents. Organometallics 2018, 37, 422–432. [Google Scholar] [CrossRef]
- Skelton, B.W. (University of Western Australia, Nedlands, Australia); Pettinari, G. (Universita di Camerino, Camerino, Italy). Personal Communication, 2018.
- Healy, P.C. (University of Western Australia, Nedlands, Australia); Noack, C.L. (Boston University, Boston, MA, USA); Williams, M.L. (Northwestern University, Evanston, IL, USA). Personal Communication, 2016.
- Alfonzo, S.; Gonzalez, S.; Higuera-Padilla, A.R.; Vidal, A.; Fernandez, M.; Taylor, P.; Urdanibia, I.; Reiber, A.; Otero, Y.; Castro, W. A new complex of copper-phosphole. Synthesis, characterization and evaluation of biological activity. Inorg. Chim. Acta 2016, 453, 538–546. [Google Scholar] [CrossRef]
- Mousa, M.E.; Braese, J.; Marquardt, C.; Seidl, M.; Scheer, M. The Coordination Chemistry of the Phosphanylborane (C6H5)2PBH2·N(CH3)3 towards Copper(I) Salts. Eur. J. Inorg. Chem. 2020, 26, 2501–2505. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Dyason, J.A.; Healy, P.C.; Engelhardt, L.M.; Pakawatchai, C.; White, A.H. Lewis-base adducts of Group 11 metal(I) compounds. Part 27. Solid-state phosphorus-31 cross-polarization magic-angle spinning nuclear magnetic resonance, far-infrared, and structural studies on the mononuclear 2:1 adducts of triphenylphosphine with copper(I) and gold(I) halides. J. Chem. Soc. Dalton Trans. 1987, 1089–1097. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Engelhardt, L.M.; Healy, P.C.; Kildea, J.D.; Papasergio, R.L.; White, A.H. Lewis-base adducts of Group 11 metal(I) compounds. 32. Steric effects in the 2:1 adducts of (2-methylphenyl)diphenylphosphine with copper(I) halides. Inorg. Chem. 1987, 26, 3533–3538. [Google Scholar] [CrossRef]
- Davis, P.H.; Belford, R.L.; Paul, I.C. Crystal and molecular structure of bromobis (triphenylphosphine)copper(I) hemibenzenate. Inorg. Chem. 1973, 12, 213–218. [Google Scholar] [CrossRef]
- Aguirrechu-Comeron, A.; Hernandez-Molina, R.; Gonzalez-Platas, J. Structure of Two New Compounds of Copper(I) Iodide with N-Donor and P-Donor Ligands. J. Struct. Chem. 2018, 59, 943–948. [Google Scholar] [CrossRef]
- Cramer, R.E.; Maynard, R.B.; Evangelista, R.S. Synthesis and crystal and molecular structure of a copper(I) complex of vitamin B1, dichloro(thiamin)copper. J. Am. Chem. Soc. 1984, 106, 111–116. [Google Scholar] [CrossRef]
- Chong, C.C.; Rao, B.; Ganguly, R.; Li, Y.; Kinjo, R. Bis(N-heterocyclic olefin) Derivative: An Efficient Precursor for Isophosphindolylium Species. Inorg. Chem. 2017, 56, 8608–8614. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, Y.; Haldon, E.; Goddard, R.; Bill, E.; Thiel, W.; Alcarazo, M. α-Radical Phosphines: Synthesis, Structure, and Reactivity. Angew. Chem.-Int. Ed. 2017, 56, 8790–8794. [Google Scholar] [CrossRef]
- Hadjikakou, S.K.; Aslanidis, P.; Karagiannidis, P.; Aubry, A.; Skoulika, S. Copper(I) complexes with tri-o-tolylphosphine and heterocyclic thione ligands. Crystal structures of [(pyrimidine-2-thione)(tri-o-tolylphosphine)copper(I) chloride] and [(pyridine-2-thione)(tri-o-tolylphosphine)copper(I) iodide]. Inorg. Chim. Acta 1992, 193, 129–135. [Google Scholar] [CrossRef]

| Cu(XXY) | (X-Cu-X)° | (X-Cu-Y)°2 | Ʃ (a + b)° |
|---|---|---|---|
| Cu(IIY) | I-Cu-I | (I-Cu-P)2 | 3.2 |
| (Y = P) | 118.6 (−1.4) a | 120.9 (+1.8) b | |
| Cu(ClClY) | Cl-Cl-Cl | (Cl-Cu-Y)2 | 6.1 |
| (Y = N, P) | 117.9 (−2.1) a | 122.0 (+4.0) b | |
| Cu(SSY) | S-Cu-S | (S-Cu-Y)2 | 6.5 |
| (Y = N, Cl, I) | 117.1 (−2.9) a | 121.8 (+3.6) b | |
| Cu(PPY) | P-Cu-P | (P-Cu-Y)2 | 8.9 |
| (Y = O, L, Cl, Br, I) | 126.2 (+6.2) b | 119.0 (−2.0) a | |
| Cu(BrBrY) | Br-Cu-Br | (Br-Cu-Y)2 | 8.2 |
| (Y = N, P) | 115.9 (−4.1) a | 122.4 (+4.8) b | |
| Cu(NNY) | N-Cu-N | (N-Cu-Y)2 | 16.9 |
| (Y = O, L, Cl, S, Br, I) | 126.9 (+6.9) b | 115.0 (−10) a | |
| Cu(CCY) | C-Cu-C | (C-Cu-Y)2 | 19.8 |
| (Y = O, Cl, P, Br, I | 129.8 (+9.8) b | 115.0 (−10) a | |
| Cu(SeSeY) | Se-Cu-Se | (Se-Cu-Y)2 | 25.5 |
| (Y = Cl, Br, I) | 102.8 (−16.7) a | 128.9 (+8.9) b |
| Cu(SIP) | Cu(SClP)° | Cu(SBrP)° |
|---|---|---|
| 119.4 (−0.6)°(S-Cu-I) | 117.8 (−2.2)°(S-Cu-Cl) | 113.4 (−6.6) (S-Cu-Br) |
| 118.2 (−1.8)°(I-Cu-P) | 114.7 (−5.3)°(Cl-Cu-P) | 116.9 (−3.1)°(Br-Cu-P) |
| 120.7 (+0.7)°(S-Cu-P) | 126.8 (+6.8)°(S-Cu-P) | 125.8 (+5.8)°(S-Cu-P) |
| Ʃ3.1° | Ʃ14.3° | Ʃ15.5° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melník, M.; Mikušová, V.; Mikuš, P. Variable Unidentate Ligands in Cu(I)(XXY) and Cu(I)(XYZ) Complexes—Structural Aspects. Inorganics 2025, 13, 182. https://doi.org/10.3390/inorganics13060182
Melník M, Mikušová V, Mikuš P. Variable Unidentate Ligands in Cu(I)(XXY) and Cu(I)(XYZ) Complexes—Structural Aspects. Inorganics. 2025; 13(6):182. https://doi.org/10.3390/inorganics13060182
Chicago/Turabian StyleMelník, Milan, Veronika Mikušová, and Peter Mikuš. 2025. "Variable Unidentate Ligands in Cu(I)(XXY) and Cu(I)(XYZ) Complexes—Structural Aspects" Inorganics 13, no. 6: 182. https://doi.org/10.3390/inorganics13060182
APA StyleMelník, M., Mikušová, V., & Mikuš, P. (2025). Variable Unidentate Ligands in Cu(I)(XXY) and Cu(I)(XYZ) Complexes—Structural Aspects. Inorganics, 13(6), 182. https://doi.org/10.3390/inorganics13060182








