A Pt(II) Complex with a PNN Type Ligand Dppmaphen Exhibits Selective, Reversible Vapor-Chromic Photoluminescence
Abstract
1. Introduction
2. Results and Discussion
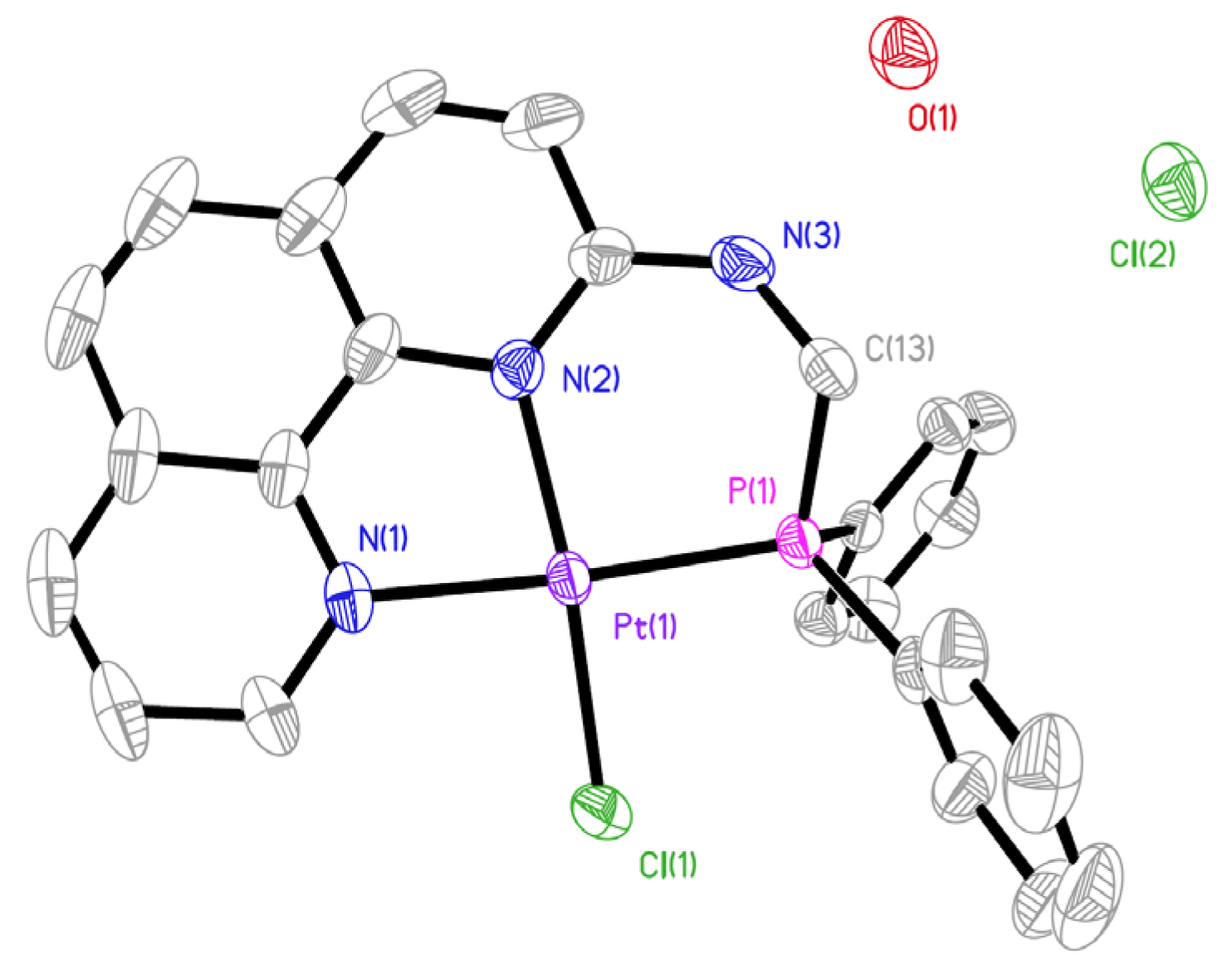
2.1. Synthesis and Characterization
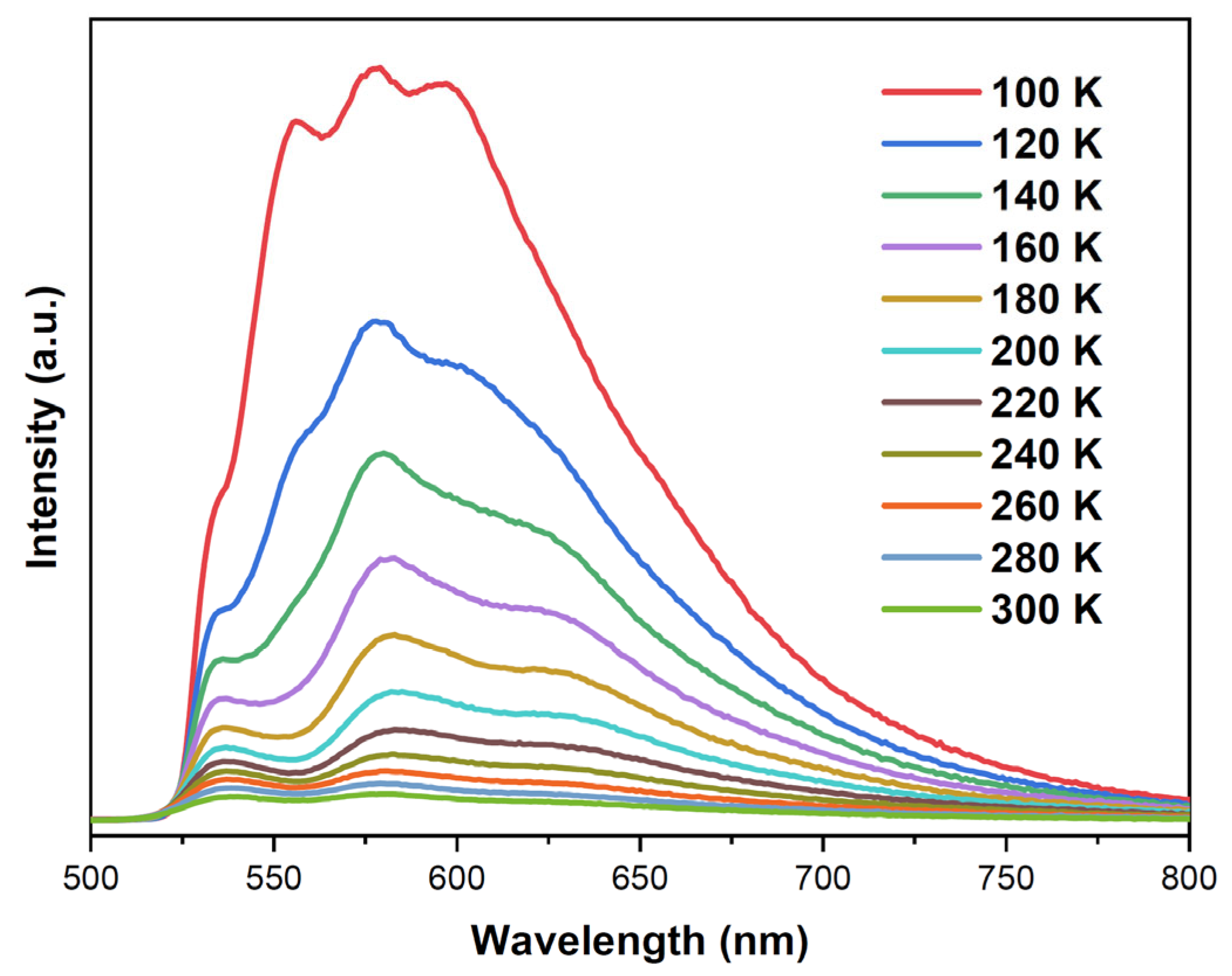
2.2. Photophysical Properties of 1
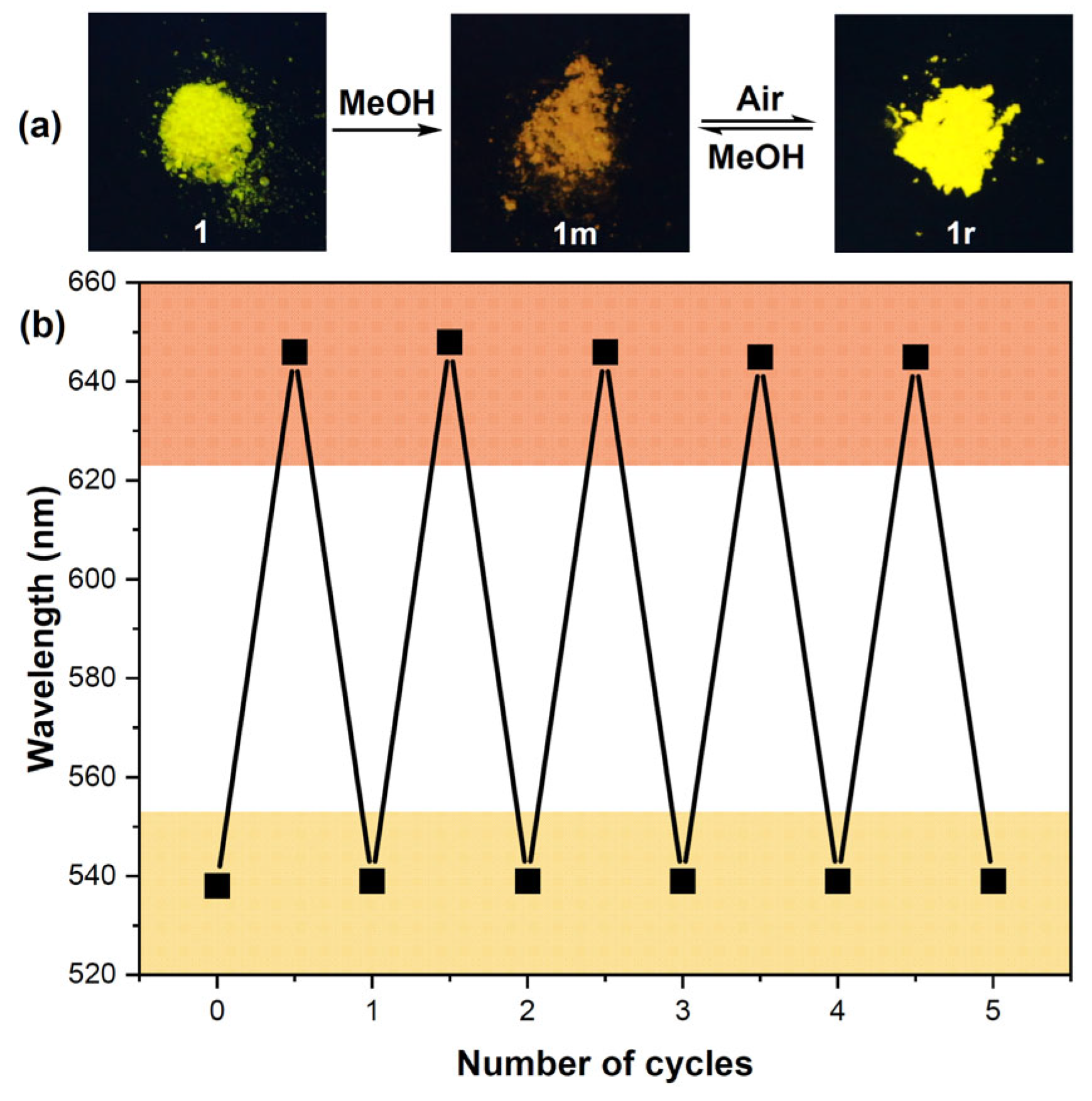
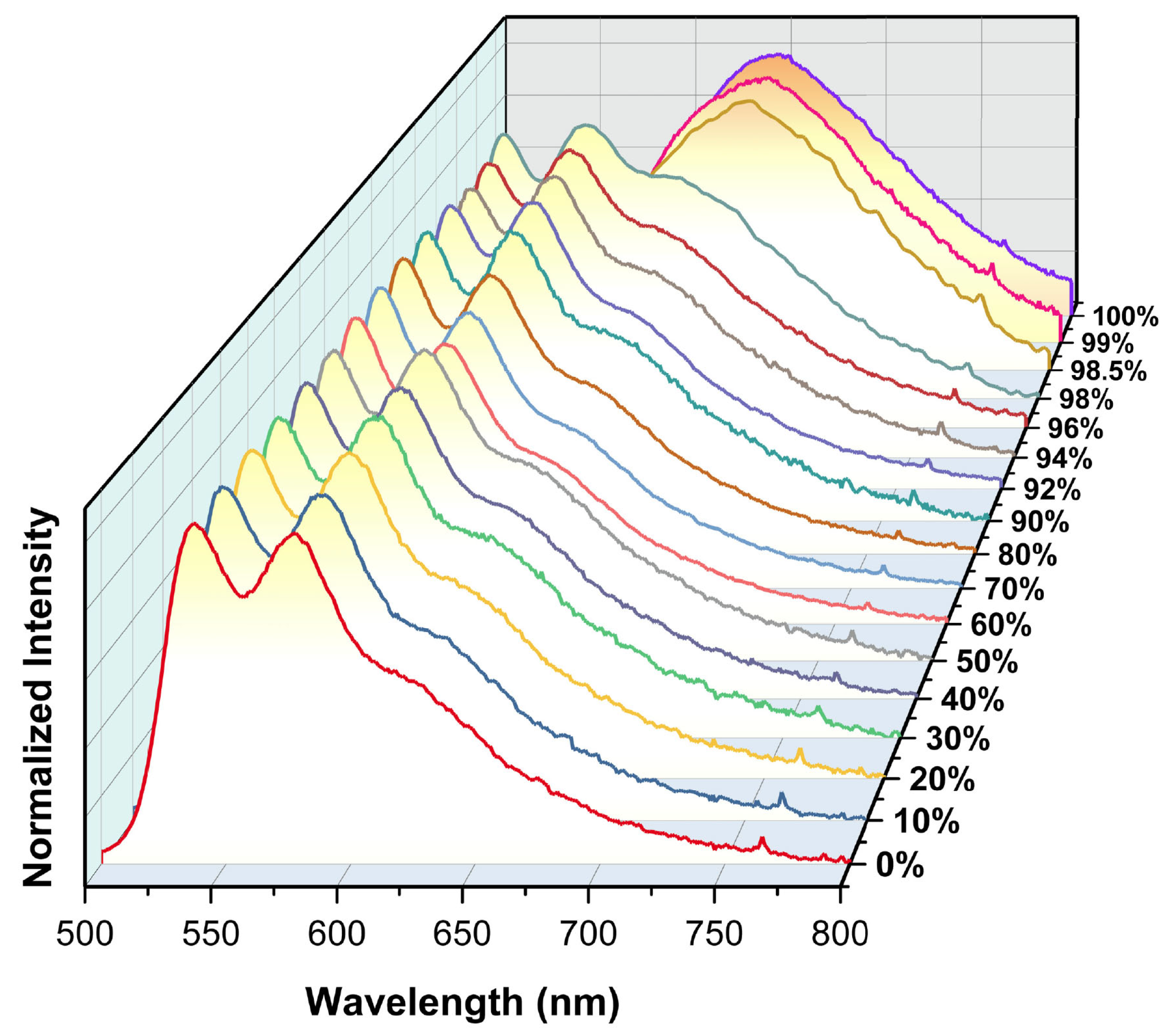
2.3. Selective PL Response of 1 Toward Small Alcohol Molecules
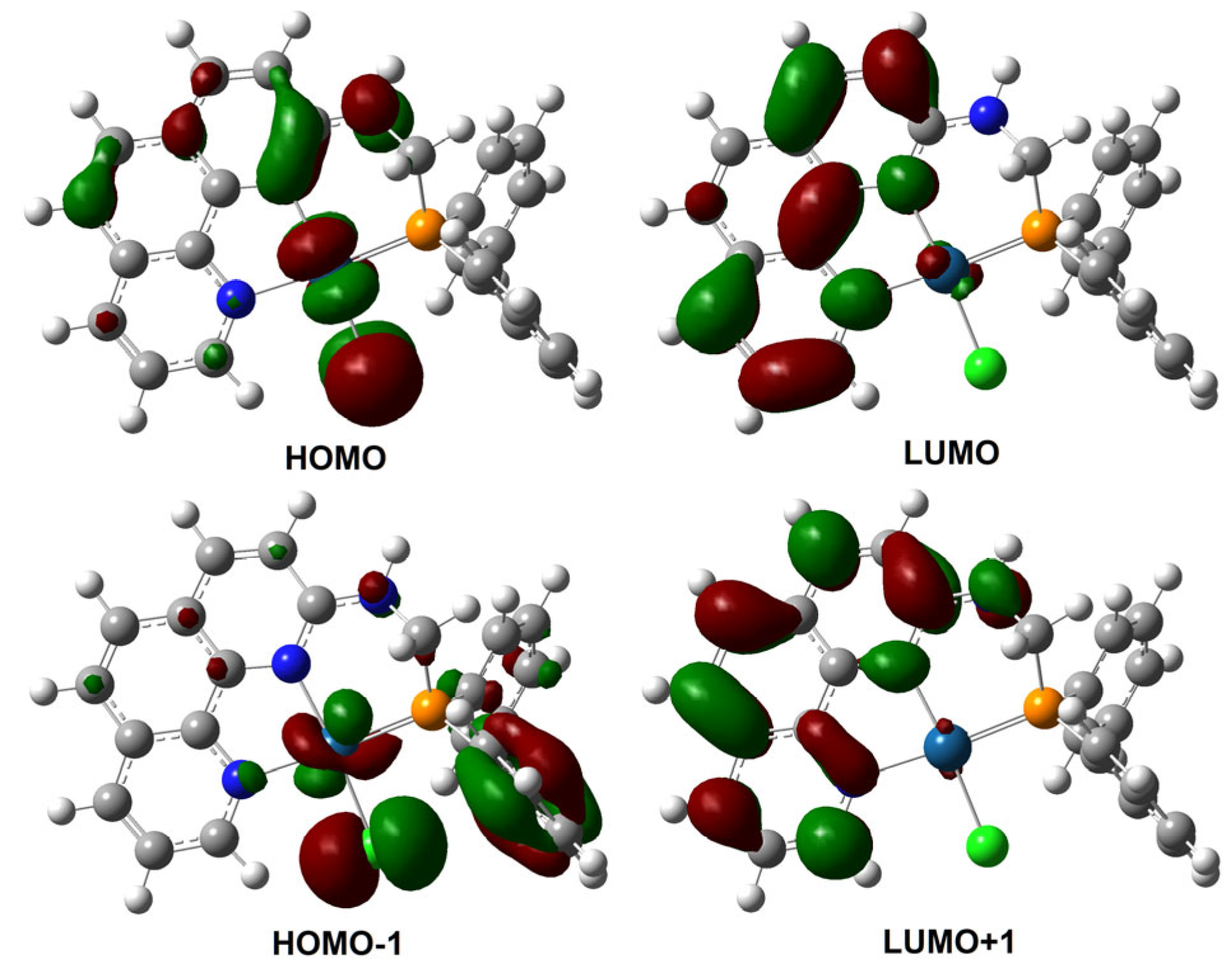
2.4. Mechanism Study on the PL Response of 1 Toward MeOH
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of [Pt(dppmaphen)Cl]Cl·H2O (1)
3.3. PL Response of 1 Toward VOC Vapors and Moisture
3.4. Detection of MeOH in Aqueous Solutions
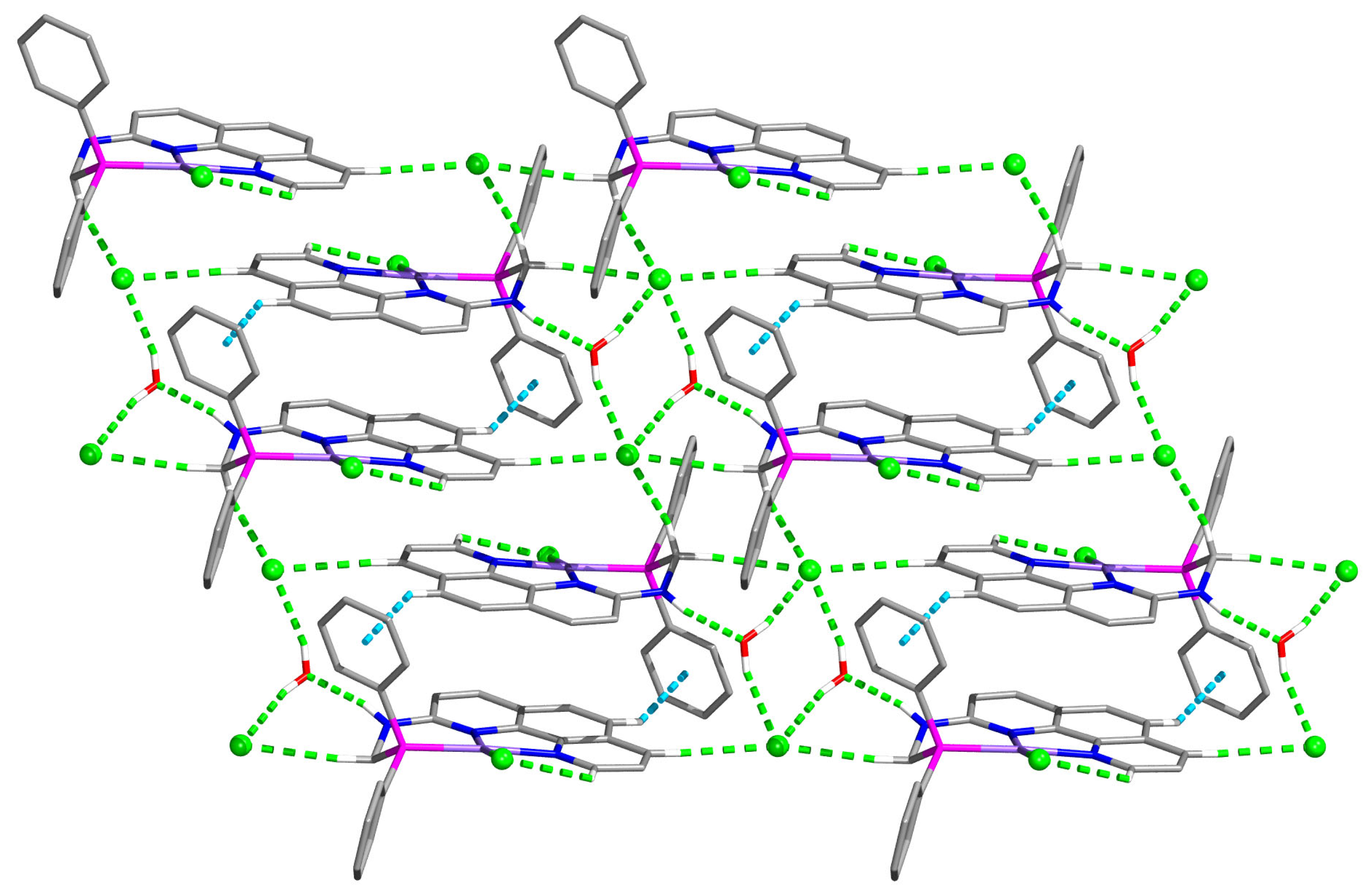
3.5. Crystallography
3.6. TD-DFT Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, Y.; Liu, C.; Xiu, J.-H.; Li, J.-Y. Photostable Fluorophenyl-Substituted Cyclometalated Platinum(II) Emitters for Monitoring of Molecular Oxygen in Real Time. Inorg. Chem. 2015, 54, 7783–7790. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, C.; Campo, J.A.; Cano, M.; Lodeiro, C. Platinum(II) Metallomesogens: New External-Stimuli-Responsive Photoluminescence Materials. Chem. Eur. J. 2016, 22, 10168–10178. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Yu, S.; Kim, S.; Nah, Y.; Son, A.; You, Y. Monocycloplatinated Solvento Complex Displays Turn-on Ratiometric Phosphorescence Responses to Histamine. Inorg. Chem. 2018, 57, 13985–13997. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Gupta, P. Luminescent terpyridine appended geminal bisazide and bistriazoles: Multinuclear Pt(II) complexes and AIPE-based DNA detection with the naked eye. Dalton Trans. 2021, 50, 10225–10236. [Google Scholar] [CrossRef]
- Lai, S.-L.; Tong, W.-Y.; Kui, S.C.F.; Chan, M.-Y.; Kwok, C.-C.; Che, C.-M. High Efficiency White Organic Light-Emitting Devices Incorporating Yellow Phosphorescent Platinum(II) Complex and Composite Blue Host. Adv. Funct. Mater. 2013, 23, 5168–5176. [Google Scholar] [CrossRef]
- Ly, K.T.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar] [CrossRef]
- Yang, X.; Jiao, B.; Dang, J.-S.; Sun, Y.; Wu, Y.; Zhou, G.; Wong, W.-Y. Achieving High-Performance Solution-Processed Orange OLEDs with the Phosphorescent Cyclometalated Trinuclear Pt(II) Complex. ACS Appl. Mater. Interfaces 2018, 10, 10227–10235. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Jiao, X.; Li, X.; Chen, Y.; Lu, Z.; Zhang, C.; Hang, X.-C.; Xu, H.; Huang, W. Planar Pt(II) Complexes for Low-Doped Excimer-Based Phosphorescent Organic Light-Emitting Diodes. Chem. Mater. 2023, 35, 5412–5419. [Google Scholar] [CrossRef]
- You, Y. Pt(II) complexes withtetradentate ligands: Toward commercially applicable blue organic electroluminescence devices. Coord. Chem. Rev. 2025, 526, 216374. [Google Scholar] [CrossRef]
- Denčić, S.M.; Poljarević, J.; Iakovic, A.M.; Marković, I.; Sabo, T.J.; Grgurić-Šipka, S. Antileukemic action of novel diamine Pt(II) halogenido complexes: Comparison of the representative novel Pt(II) with corresponding Pt(IV) complex. Chem. Biol. Drug Des. 2017, 90, 262–271. [Google Scholar] [CrossRef]
- Uruş, S.; İncesu, M.; Köşker, S.; Kurt, A.H.; Ceyhan, G. Synthesis, characterization, photoluminescence and electrochemical properties of Pt(II) and Ag (I) complexes of tetradentate aminomethylphosphine ligands and antiproliferative activities on HT-29 human colon cancer. Appl. Organometal. Chem. 2017, 31, e3550. [Google Scholar] [CrossRef]
- Maisuls, I.; Singh, J.; Salto, I.P.; Steiner, S.T.; Kirse, T.M.; Niemann, S.; Strassert, C.A.; Faust, A. Conjugated Pt(II) Complexes as Luminescence-Switch-On Reporters Addressing the Microenvironment of Bacterial Biofilms. Inorg. Chem. 2021, 60, 11058–11069. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Ren, G.; Hou, T.; Shen, X.; Zhu, D. Synthesis, structure and anticancer activity of three platinum(II) complexes with 2-phenylpyridine derivatives. Inorg. Chem. Commun. 2021, 130, 108737. [Google Scholar] [CrossRef]
- Liu, X.; Barth, M.-C.; Cseh, K.; Kowol, C.R.; Jakupec, M.A.; Keppler, B.K.; Gibson, D.; Weigand, W. Oxoplatin-Based Pt(IV) Lipoate Complexes and Their Biological Activity. Chem. Biodivers. 2022, 19, e202200695. [Google Scholar] [CrossRef]
- Faizullin, B.A.; Khazieva, A.R.; Kholin, K.V.; Voloshina, A.D.; Lyubina, A.P.; Sapunova, A.S.; Sibgatullina, G.V.; Samigullin, D.V.; Paderina, A.V.; Grachova, E.V.; et al. pH-responsive composite nanomaterial engineered from silica nanoparticles and luminescent mitochondrion-targeted Pt(II) complex as anticancer agent. J. Mol. Liq. 2024, 399, 124381. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Gao, Q.; Peng, F.; Wang, C.; Lin, J.; Chang, X.; Zou, C.; Lu, W. Phosphorescent Zwitterionic Pt(II) N-Heterocyclic Allenylidene Complexes: Metallophilicity and Ionic Self-Assembly. Chin. J. Chem. 2021, 39, 1159–1167. [Google Scholar] [CrossRef]
- Zheng, X.; Chan, M.H.-Y.; Chan, A.K.-W.; Cao, S.; Ng, M.; Sheong, F.K.; Li, C.; Goonetilleke, E.C.; Lam, W.W.Y.; Lau, T.-C.; et al. Elucidation of the key role of Pt···Pt interactions in the directional self-assembly of platinum(II) complexes. Proc. Natl. Acad. Sci. USA 2022, 119, e2116543119. [Google Scholar] [CrossRef]
- Wong, E.K.-H.; Chan, M.H.-Y.; Tang, W.K.; Leung, M.-Y.; Yam, V.W.-W. Molecular Alignment of Alkynylplatinum(II) 2,6-Bis(benzimidazol-2yl)pyridine Double Complex Salts and the Formation of Well-Ordered Nanostructures Directed by Pt···Pt and Donor–Acceptor Interactions. J. Am. Chem. Soc. 2022, 144, 5424–5434. [Google Scholar] [CrossRef]
- Lin, J.; Peng, F.; Xie, M.; Xia, J.; Chang, X.; Zou, C.; Lu, W. Dicationic Diimine Pt(II) Bis(N-heterocyclic allenylidene) Complexes: Extended Pt···Pt Chains, NIR Phosphorescence, and Chromonics. Inorg. Chem. 2023, 62, 10077–10091. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Rogovoy, M.I.; Samsonenko, D.G.; Rakhmanova, M.I. Heterobimetallic PtII–AgI complex supported by diphenyl(2-pyrimidyl) phosphine: Synthesis and thermochromic photoluminescence. Inorg. Chem. Commun. 2020, 115, 107862. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Lu, J.; Shen, J.; Ma, C.; Liu, S.; Zhao, Q.; Wong, W.-Y. Phosphorescent Soft Salt Based on Platinum(II) Complexes: Photophysics, Self-Assembly, Thermochromism, and Anti-counterfeiting Application. Inorg. Chem. 2021, 60, 7510–7518. [Google Scholar] [CrossRef] [PubMed]
- Suburu, M.E.G.; Blanke, M.; Hepp, A.; Maus, O.; Schwab, D.; Doltsinis, N.L.; Zeier, W.G.; Giese, M.; Voskuhl, J.; Strassert, C.A. Pt(II) Complexes with Tetradentate CˆN*NˆC Luminophores: From Supramolecular Interactions to Temperature-Sensing Materials with Memory and Optical Readouts. Molecules 2023, 28, 7353. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Zhang, L.-Y.; Shi, L.-X.; Chen, Z.-N. Syntheses, Characterization, and Luminescence of PtII–MI (M = Cu, Ag, Au) Heterometallic Complexes by Incorporating Pt(diimine)(dithiolate) with [M2(dppm)2]2+ (dppm = Bis(diphenylphosphino)methane). Inorg. Chem. 2004, 43, 7493–7501. [Google Scholar] [CrossRef]
- Shiotsuka, M.; Ono, R.; Kurono, Y.; Asano, T.; Sakae, Y. Photoluminescence of platinum(II) diethynylphenanthroline organometallic complexes with bis-arylethynyl derivatives in solution and solid state. J. Organomet. Chem. 2019, 880, 116–123. [Google Scholar] [CrossRef]
- Xie, M.; Yu, M.; Liu, C.; Zhao, Y.; Li, Z.; Li, S.; Jiang, F.; Chen, L.; Hong, M. Deep-red to near-infrared emission of binuclear platinum(II) complexes by breaking hydrogen bonds. J. Mater. Chem. C 2022, 10, 16100–16107. [Google Scholar] [CrossRef]
- Kui, S.C.F.; Chui, S.S.-Y.; Che, C.-M.; Zhu, N. Structures, Photoluminescence, and Reversible Vapoluminescence Properties of Neutral Platinum(II) Complexes Containing Extended π-Conjugated Cyclometalated Ligands. J. Am. Chem. Soc. 2006, 128, 8297–8309. [Google Scholar] [CrossRef]
- Quan, J.; Chen, Z.-H.; Zhang, X.; Wang, J.-Y.; Zhang, L.-Y.; Chen, Z.-N. Geometrically isomeric Pt2Ag2 acetylide complexes of 2,6-bis(diphenylphosphino)pyridine: Luminescent and vapochromic properties. Inorg. Chem. Front. 2021, 8, 2323–2332. [Google Scholar] [CrossRef]
- Shiotsuka, M.; Hanada, T.; Ogihara, M.; Okada, M.; Mizuno, M. Characteristic vapochromic detection of platinum(II) 3,8-bis-(2-triisopropylsilylethynyl)-phenanthroline organometallic complexes with bis-arylethynyl derivatives. J. Organomet. Chem. 2023, 122631, 987–988. [Google Scholar] [CrossRef]
- Yu, A.; Jiang, W.; Shen, Y.; Zhang, Y. Binuclear Pt(II) Complexes with NĈN Ligands: Synthesis, Photophysical Properties, and Vapochromic Responses. Cryst. Growth Des. 2025, 25, 1872–1879. [Google Scholar] [CrossRef]
- Cuerva, C.; Campo, J.A.; Cano, M.; Arredondo, B.; Romero, B.; Otón, E.; Otón, J.M. Bis(pyridylpyrazolate)platinum(II): A mechanochromic complex useful as a dopant for colour-tunable polymer OLEDs. New J. Chem. 2015, 39, 8467–8473. [Google Scholar] [CrossRef]
- Lien, C.-Y.; Hsu, Y.-F.; Liu, Y.-H.; Peng, S.-M.; Shinmyozu, T.; Yang, J.-S. Steric Engineering of Cyclometalated Pt(II) Complexes toward High-Contrast Monomer-Excimer-Based Mechanochromic and Vapochromic Luminescence. Inorg. Chem. 2020, 59, 11584–11594. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Junquera, M.; Lara, R.; Lalinde, E.; Moreno, M.T. Isomerism, aggregation-induced emission and mechanochromism of isocyanide cycloplatinated(II) complexes. J. Mater. Chem. C 2020, 8, 7221–7233. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Zhang, H.-H.; Qi, X.-W.; Sun, S.-S.; Zhang, D.-S.; Han, L.-Z.; Zhang, X.-P.; Shi, Z.-F. Mechanochromic luminescence properties of fluoro-substituted pinene-containing cyclometalated platinum(II) complexes with multiple triplet excited states. Dalton Trans. 2021, 50, 8938–8946. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yamamoto, N.; Shigeta, Y.; Yoshida, M.; Kato, M. Two-way vapochromism of a luminescent platinum(II) complex with phosphonic-acidfunctionalized bipyridine ligand. Dalton Trans. 2018, 47, 1548–1556. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, J.; Ma, J.-Q.; Zheng, W.; Chen, L.-J.; Sun, B.; Li, C.; Hu, B.-W.; Tan, H.; Li, X.; et al. Vapochromic Behavior of a Chair-Shaped Supramolecular Metallacycle with Ultra-Stability. J. Am. Chem. Soc. 2016, 138, 738–741. [Google Scholar] [CrossRef]
- Yan, J.-J.; Wu, Y.; Zhai, W.; Yang, N.; Li, H.-X.; Yang, W.; Lu, C.; Young, D.J.; Ren, Z.-G. A Multiple Stimuli–Responsive Ag/P/S Complex Showing Solvochromic and Mechanochromic Photoluminescence. Molecules 2023, 28, 5513. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Y.; Cui, L.; Feng, M.; Young, D.J.; Li, H.-X.; He, X.; Lu, C.; Ren, Z.-G. Aggregation-Induced Emission Phosphorescence Featured Au-Ag Coordination Polymer with a Diphosphine N-Heterocyclic Carbene Ligand for Highly Sensitive Detection of Cr(VI). Inorg. Chem. 2024, 63, 14415–14424. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Hu, S.; Young, D.J.; Lu, C.; Li, H.-X.; Ren, Z.-G. A photoluminescent Au(I)/Ag(I)/PNN coordination complex for relatively rapid and reversible alcohol sensing. Dalton Trans. 2021, 50, 6773–6777. [Google Scholar] [CrossRef]
- Catalano, V.J.; Bennett, B.L.; Noll, B.C. Pt(0) and Pd(0) based metallocryptands: Metallophilic hosts for Pb(II) ion. Chem. Commun. 2000, 1413–1414. [Google Scholar] [CrossRef]
- Catalano, V.J.; Malwitz, M.A.; Noll, B.C. A linearly coordinated Hg(0) trapped in a gold(I) metallocryptand cage. Chem. Commun. 2001, 581–582. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Klyushova, L.S.; Sadykov, E.H.; Rakhmanova, M.I.; Sukhikh, T.S. Coinage metal(I) clusters supported by a 1,10-phenanthroline-phosphine: Orange-to-NIR phosphorescence, metallophilic interactions and enhanced cytotoxicity. Dalton Trans. 2024, 53, 18027–18036. [Google Scholar] [CrossRef] [PubMed]
- Büchner, R.; Field, J.S.; Haines, R.J.; Ledwaba, L.P.; McGuire, R.; McMillin, D.R.; Munro, O.Q. Synthesis, crystal structure and solid state photoluminescence of [Pt(trpy)(C≡CPh)]SbF6 (trpy=2,2′:6′,2″-terpyridine). Inorg. Chim. Acta 2007, 360, 1633–1638. [Google Scholar] [CrossRef]
- Maladeniya, C.; Darshani, T.; Samarakoon, S.R.; Fronczek, F.R.; Sameera, W.M.C.; Perera, I.C.; Perera, T. Biological Evaluation of Platinum(II) Sulfonamido Complexes: Synthesis, Characterization, Cytotoxicity, and Biological Imaging. Bioinorg. Chem. Appl. 2022, 2022, 7821284. [Google Scholar] [CrossRef]
- Soto, M.A.; Kandel, R.; MacLachlan, M.J. Chromic Platinum Complexes Containing Multidentate Ligands. Eur. J. Inorg. Chem. 2021, 2021, 894–906. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, Y.; Liu, S.; Zhang, J. Synthesis, structure and tri-stimuli-responsive luminescent switching properties of a bis(σ-acetylide) platinum(II) complex. J. Organomet. Chem. 2024, 1004, 122948. [Google Scholar] [CrossRef]
- Paderina, A.; Slavova, S.; Tupikina, E.; Snetkov, D.; Grachova, E. Aggregation Game: Changing Solid-State Emission Using Different Counterions in Monoalkynylphosphonium Pt(II) Complexes. Inorg. Chem. 2024, 63, 17548–17560. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Zhang, M.; Chen, X.-R.; Lu, C.; Young, D.J.; Ren, Z.-G.; Lang, J.-P. Cobalt(II) and Nickel(II) Complexes of a PNN Type Ligand as Photoenhanced Electrocatalysts for the Hydrogen Evolution Reaction. Inorg. Chem. 2020, 59, 1038–1045. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL-2016; University Göttingen: Göttingen, Germany, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, J.; Young, D.J.; Li, H.-X.; Lu, Y.; Ren, Z.-G. A Pt(II) Complex with a PNN Type Ligand Dppmaphen Exhibits Selective, Reversible Vapor-Chromic Photoluminescence. Inorganics 2025, 13, 170. https://doi.org/10.3390/inorganics13050170
Hu Y, Wang J, Young DJ, Li H-X, Lu Y, Ren Z-G. A Pt(II) Complex with a PNN Type Ligand Dppmaphen Exhibits Selective, Reversible Vapor-Chromic Photoluminescence. Inorganics. 2025; 13(5):170. https://doi.org/10.3390/inorganics13050170
Chicago/Turabian StyleHu, Yuanyuan, Jiangyue Wang, David James Young, Hong-Xi Li, Yuxin Lu, and Zhi-Gang Ren. 2025. "A Pt(II) Complex with a PNN Type Ligand Dppmaphen Exhibits Selective, Reversible Vapor-Chromic Photoluminescence" Inorganics 13, no. 5: 170. https://doi.org/10.3390/inorganics13050170
APA StyleHu, Y., Wang, J., Young, D. J., Li, H.-X., Lu, Y., & Ren, Z.-G. (2025). A Pt(II) Complex with a PNN Type Ligand Dppmaphen Exhibits Selective, Reversible Vapor-Chromic Photoluminescence. Inorganics, 13(5), 170. https://doi.org/10.3390/inorganics13050170








