Hematite Nanoparticles Synthesized by Green Route: Characterization, Anticancer and Antioxidant Activities
Abstract
1. Introduction
2. Results and Discussion
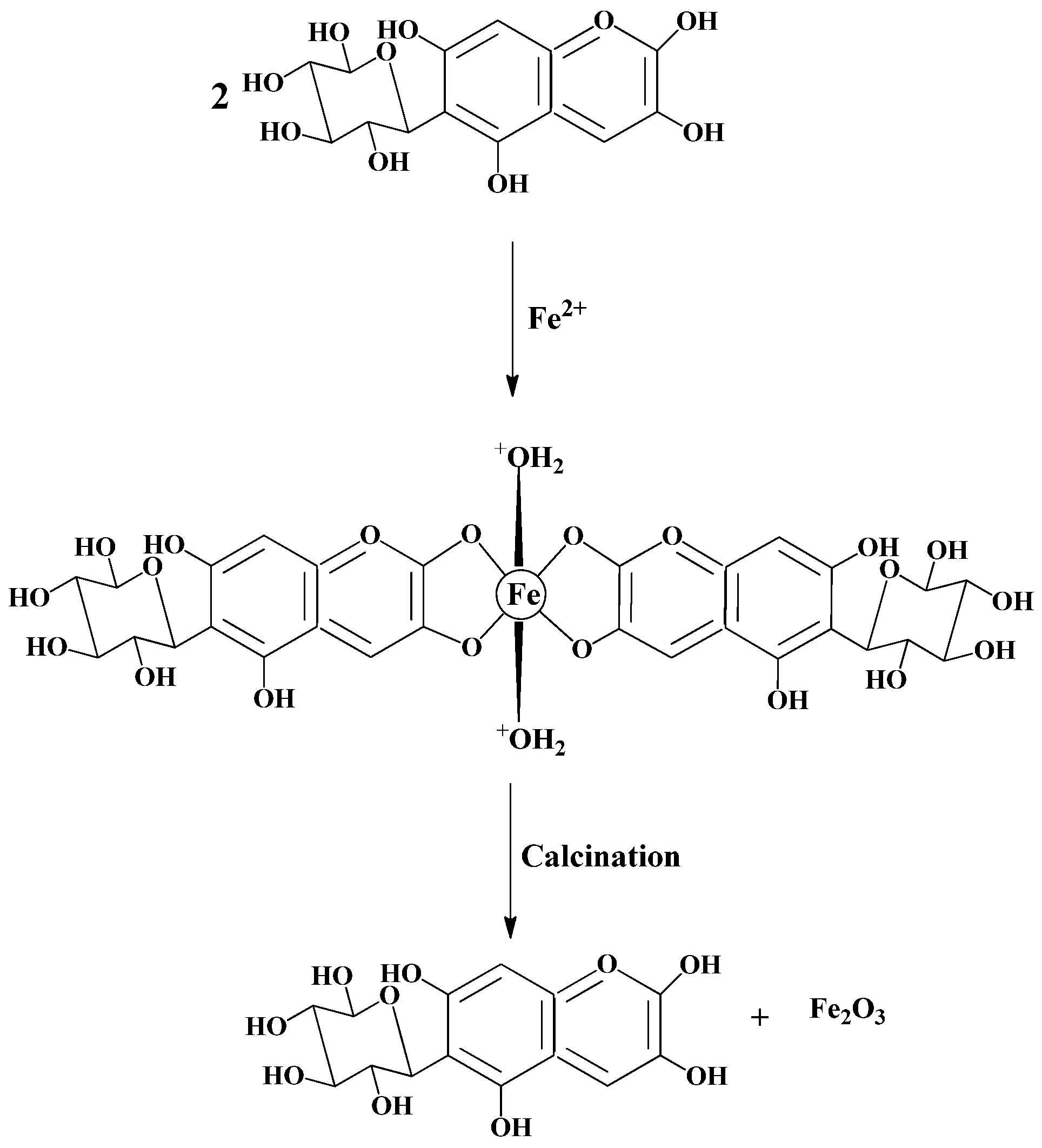
2.1. Synthesis Process
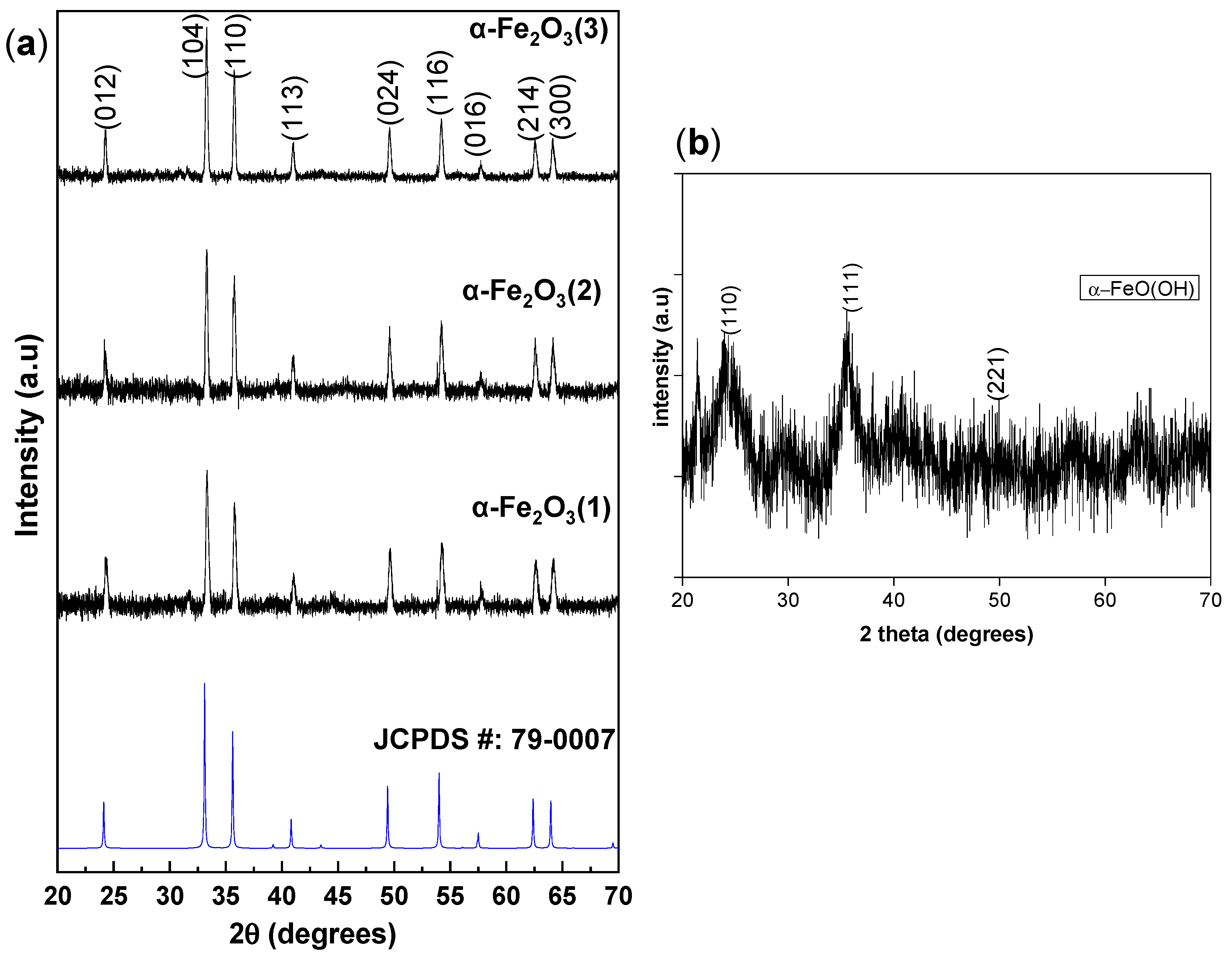
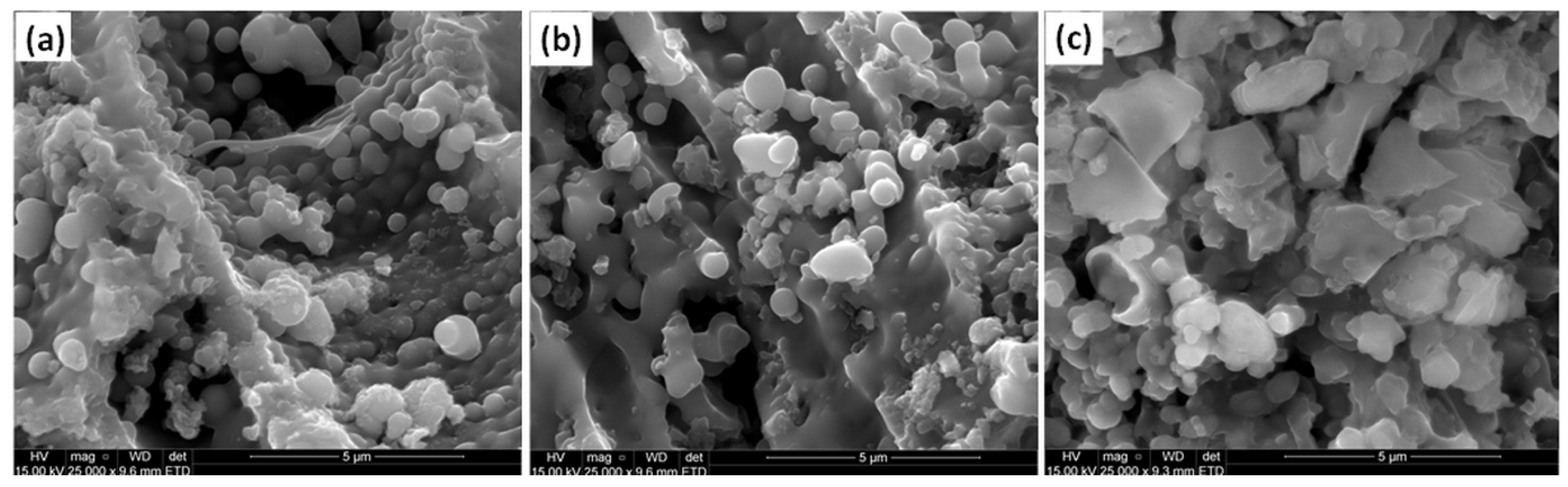
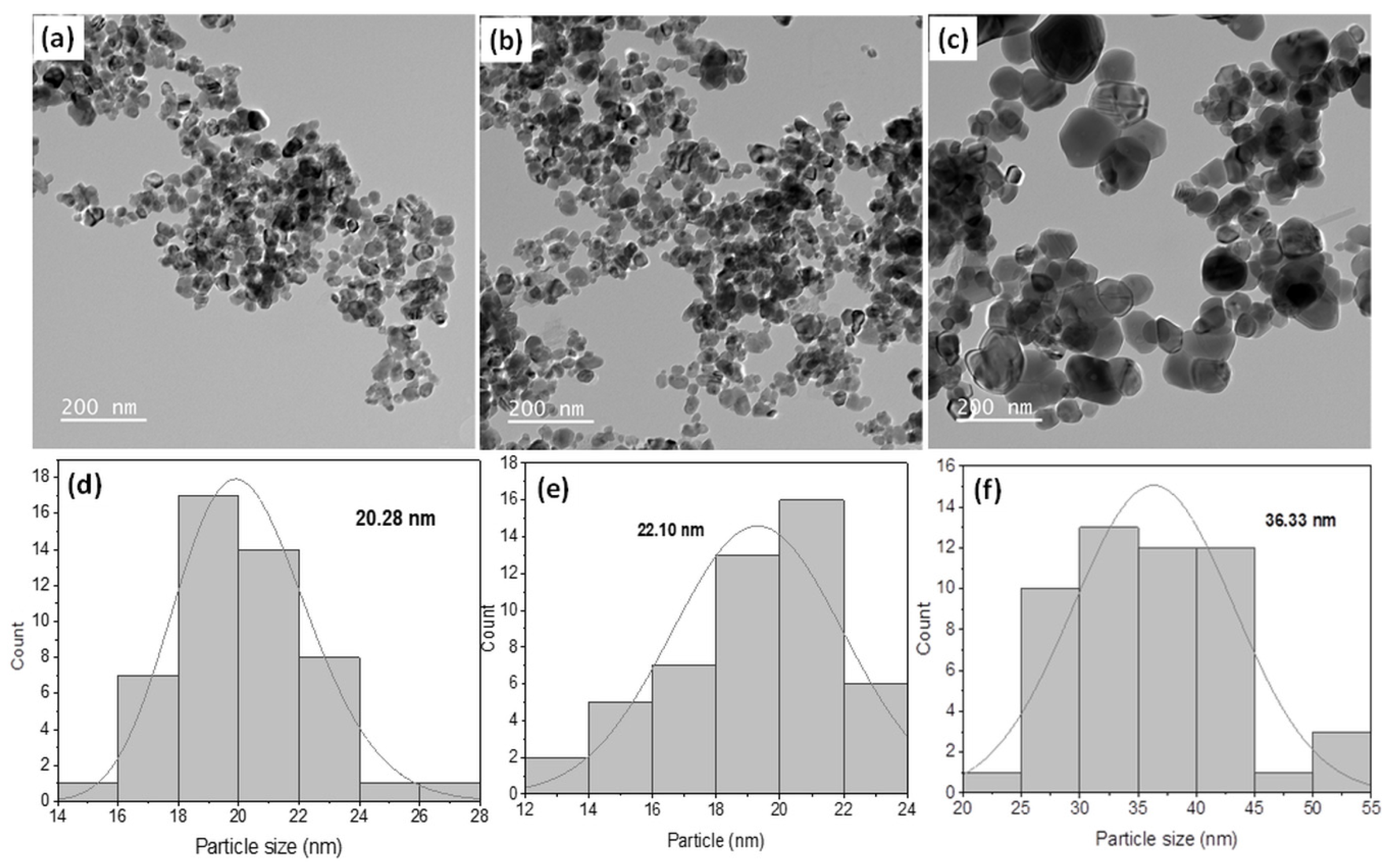
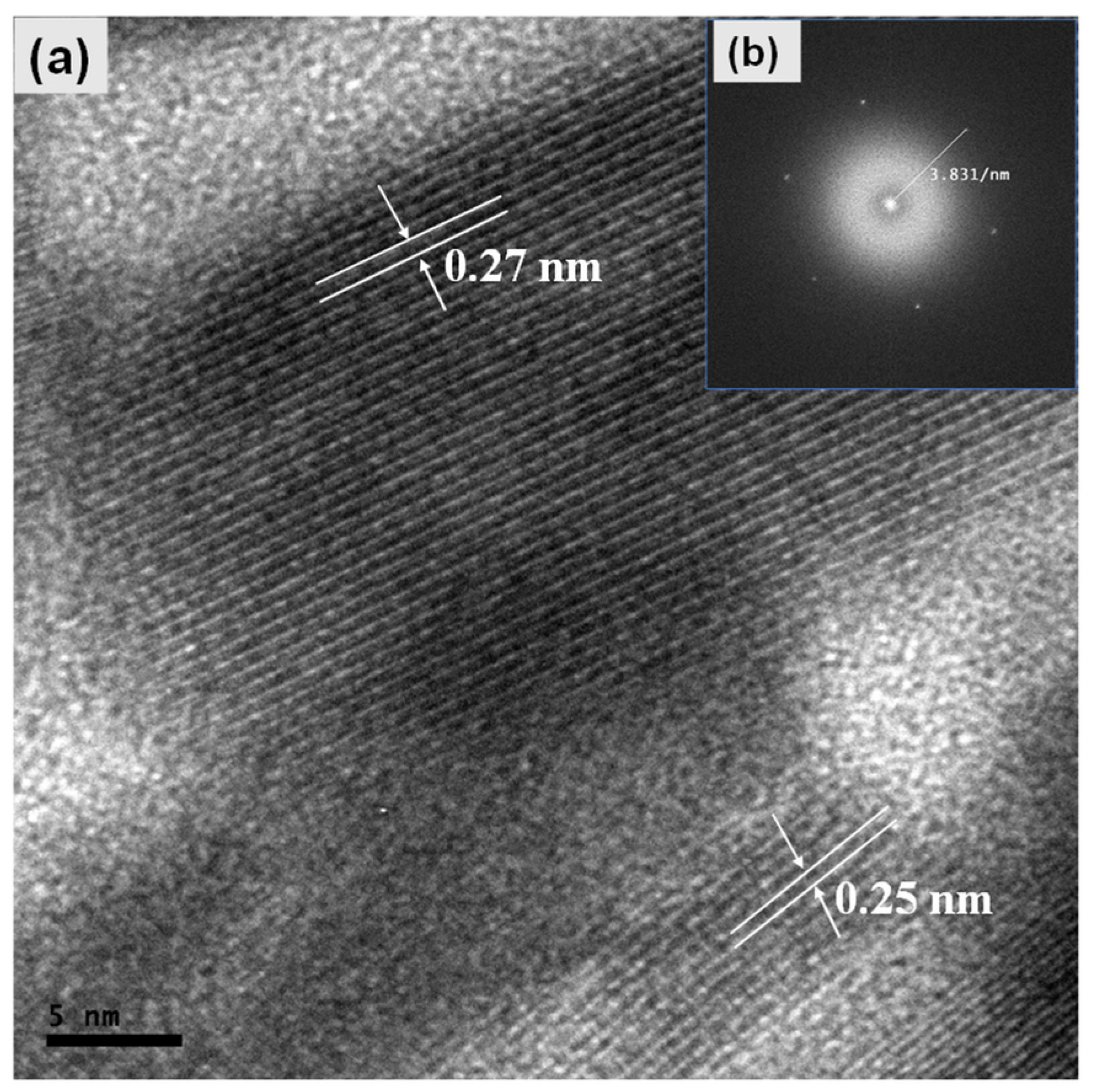
2.2. Microstructural and Morphological Properties
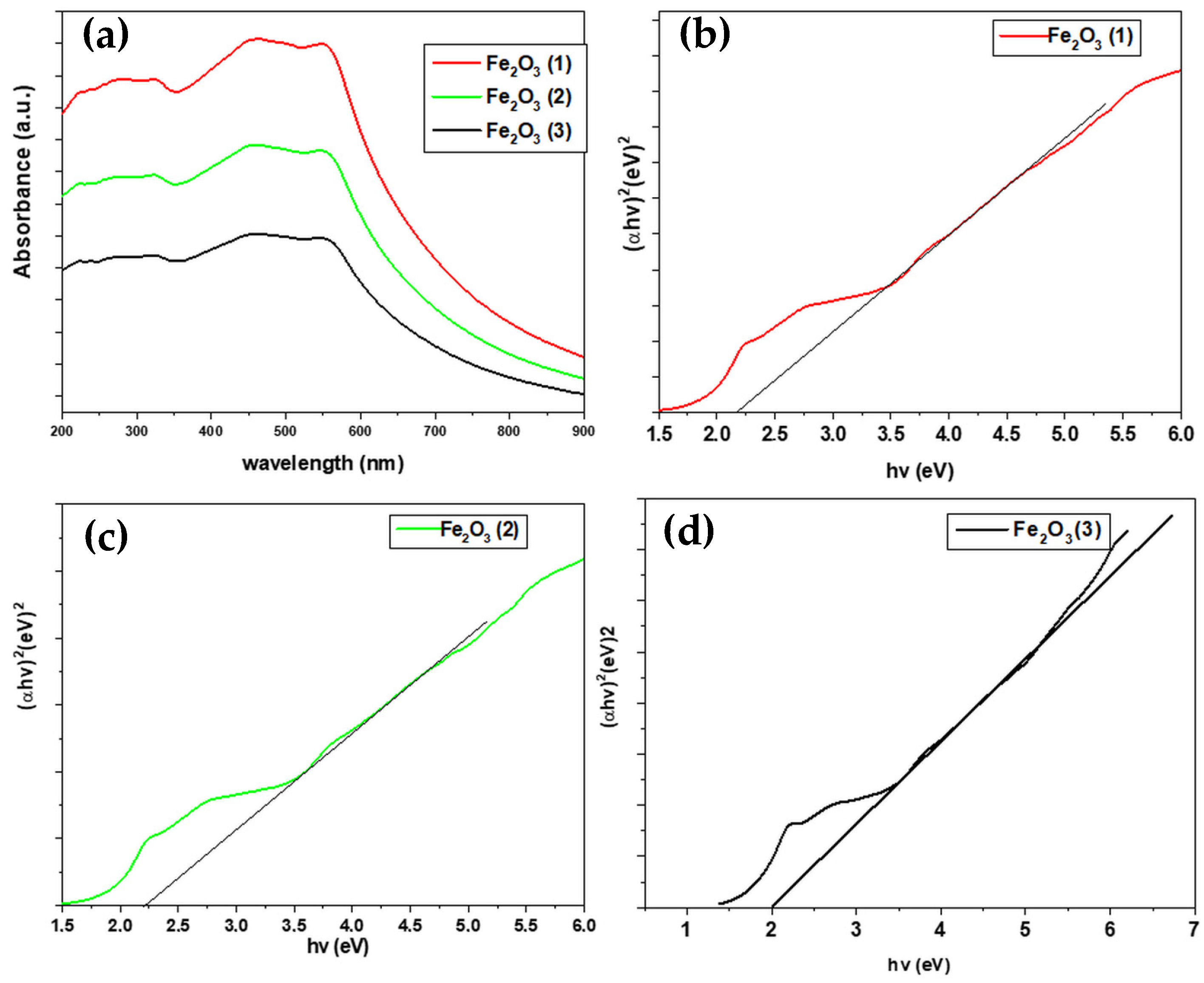
2.3. Optical Characterization
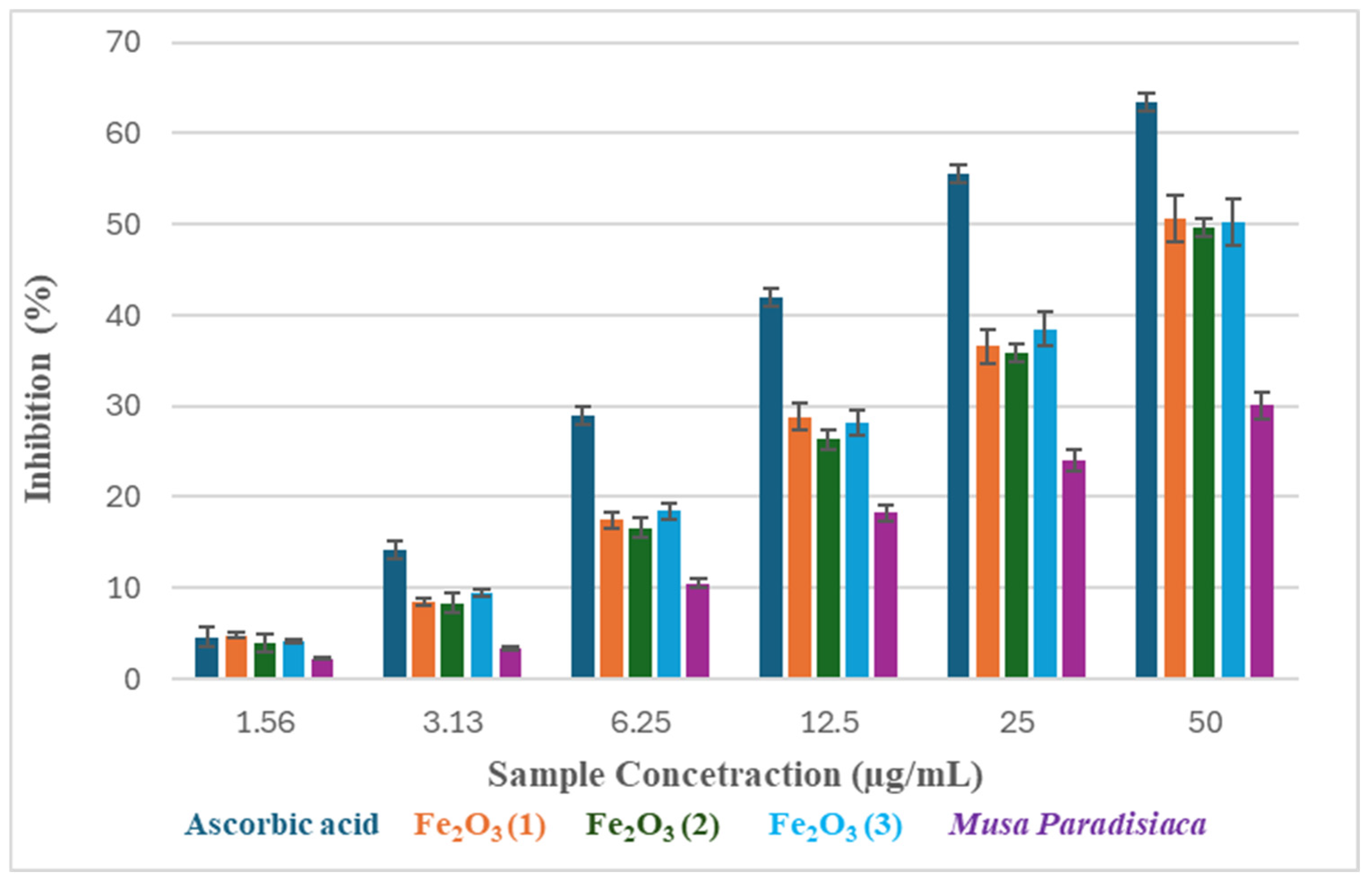
2.4. Antioxidant and Anticancer Studies
3. Materials and Methods
3.1. Chemicals
3.2. Collection and Preparation of Aqueous Plant Extract
3.3. Synthesis of α-Fe2O3 Nanoparticles
3.4. Characterization
3.5. Biological Activity
3.5.1. Antioxidant Activity Measurement Using DPPH Scavenging Assay
3.5.2. Cytotoxicity Activity Measurement Using MTT Assay of the Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begum, N.A.; Mondal, S.; Basu, S.; Laskar, R.A.; Mandal, D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf. B Biointerfaces 2009, 71, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Murad, A.; Hassan, D.; Channa, G.M.; El-Mallul, A.; Medina, D.I. Photo-catalytic and biomedical applications of one-step, plant extract-mediated green-synthesized cobalt oxide nanoparticles. Environ. Sci. Pollut. Res. 2023, 30, 20736–20745. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Joshi, N.; Pandey, D.K.; Mistry, B.G.; Singh, D.K. Metal oxide nanoparticles: Synthesis, properties, characterization, and applications. In Nanomaterials: Advances and Applications; Singh, D.K., Singh, S., Singh, P., Eds.; Springer Nature: Singapore, 2023; pp. 103–144. [Google Scholar]
- Murthy, S.; Effiong, P.; Fei, C.C. 11—Metal oxide nanoparticles in biomedical applications. In Metal Oxide Powder Technologies; Al-Douri, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–251. [Google Scholar]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide nanomaterials synthesis and applications in flexible and wearable sensors. ACS Nanosci. Au 2022, 2, 64–92. [Google Scholar] [CrossRef]
- Nowsherwan, G.A.; Ali, Q.; Ali, U.F.; Ahmad, M.; Khan, M.; Hussain, S.S. Advances in organic materials for next-generation optoelectronics: Potential and challenges. Organics 2024, 5, 520–560. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M. The greener synthesis of nanoparticles. Trends Biotechnol 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Şahin, A.; Altınsoy, Ş.; Kızılbey, K. An approach for cationic dyes removal from wastewater: Green synthesis of iron nanoparticles using Prunus avium stems extracts. Kuwait J. Sci. 2024, 51, 100226. [Google Scholar] [CrossRef]
- Buarki, F.; AbuHassan, H.; Al Hannan, F.; Henari, F. Green synthesis of iron oxide nanoparticles using Hibiscus rosa sinensis flowers and their antibacterial activity. J. Nanotechnol. 2022, 2022, 5474645. [Google Scholar] [CrossRef]
- Keshta, B.E.; Gemeay, A.H.; Kumar Sinha, D.; Elsharkawy, S.; Hassan, F.; Rai, N.; Arora, C. State of the art on the magnetic iron oxide nanoparticles: Synthesis, functionalization, and applications in wastewater treatment. Results Chem. 2024, 7, 101388. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Brollo, M.E.F.; Veintemillas-Verdaguer, S.; del Puerto Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Alshehri, M.A.; Saif, A.; Faridi, U.; Khasim, S.; Mohammedsaleh, Z.M.; Parveen, H.; Omer, N.; Alasmari, A.; Mukhtar, S. Green synthesis of Abutilon indicum (L) derived iron oxide (FeO) nanoparticles with excellent biological, anticancer and photocatalytic activities. Polyhedron 2024, 257, 117022. [Google Scholar] [CrossRef]
- Duan, Y.-T.; Soni, K.; Patel, D.; Choksi, H.; Sangani, C.B.; Saeed, W.S.; Ameta, K.L.; Ameta, R.K. Green synthesis of iron oxide nanoparticles using Nicotiana plumbaginifolia and their biological evaluation. J. Mol. Liq. 2024, 396, 123985. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Tringides, M.C.; Jałochowski, M.; Bauer, E. Quantum size effects in metallic nanostructures. Phys. Today 2007, 60, 50–54. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Veisi, H.; Ozturk, T.; Karmakar, B.; Tamoradi, T.; Hemmati, S. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst in Suzuki-Miyaura coupling and 4-nitrophenol reduction. Carbohydr. Polym. 2020, 235, 115966. [Google Scholar] [CrossRef]
- Tang, N.; Zhong, W.; Jiang, H.; Wu, X.; Liu, W.; Du, Y. Nanostructured magnetite (Fe3O4) thin films prepared by sol–gel method. J. Magn. Magn. Mater. 2004, 282, 92–95. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Shete, P.; Patil, R.; Tiwale, B.; Pawar, S. Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Liu, H.-L.; Ko, S.P.; Wu, J.-H.; Jung, M.-H.; Min, J.H.; Lee, J.H.; An, B.H.; Kim, Y.K. One-pot polyol synthesis of monosize PVP-coated sub-5 nm Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2007, 310, e815–e817. [Google Scholar] [CrossRef]
- Basnet, P.; Larsen, G.K.; Jadeja, R.P.; Hung, Y.-C.; Zhao, Y. α-Fe2O3 nanocolumns and nanorods fabricated by electron beam evaporation for visible light photocatalytic and antimicrobial applications. ACS Appl. Mater. Interfaces 2013, 5, 2085–2095. [Google Scholar] [CrossRef]
- Jana, T.; Pal, A.; Mandal, A.; Sarwar, S.; Chakrabarti, P.; Chatterjee, K. Photocatalytic and antibacterial performance of α-Fe2O3 nanostructures. ChemistrySelect 2017, 2, 3068–3077. [Google Scholar] [CrossRef]
- Al-Tememe, E.; Algalal, H.M.A.A.; Abodood, A.A.F.; Mohammed, K.A.; Khamees, E.J.; Zabibah, R.S.; Abed, A.S. Anticancer and Antimicrobial activity of PVA/Fe2O3/TiO2 hybrid nanocomposite. Int. J. Nanosci. 2022, 21, 2250018. [Google Scholar] [CrossRef]
- Yoonus, J.; Resmi, R.; Beena, B. Evaluation of antibacterial and anticancer activity of green synthesized iron oxide (α-Fe2O3) nanoparticles. Mater. Today Proc. 2021, 46, 2969–2974. [Google Scholar] [CrossRef]
- Athithan, A.S.; Jeyasundari, J.; Jacob, Y. Biological synthesis, physico-chemical characterization of undoped and Co doped α-Fe2O3 nanoparticles using tribulus terrestris leaf extract and its antidiabetic, antimicrobial applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 045003. [Google Scholar] [CrossRef]
- Ansari, M.A.; Asiri, S.M.M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 2021, 171, 44–58. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.; Deb, P. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng. C 2014, 42, 595–600. [Google Scholar] [CrossRef]
- Shams, S.; Khan, A.U.; Yuan, Q.; Ahmad, W.; Wei, Y.; Khan, Z.U.H.; Shams, S.; Ahmad, A.; Rahman, A.U.; Ullah, S. Facile and eco-benign synthesis of Au@ Fe2O3 nanocomposite: Efficient photocatalytic, antibacterial and antioxidant agent. J. Photochem. Photobiol. B Biol. 2019, 199, 111632. [Google Scholar] [CrossRef] [PubMed]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Development and characterization of multifunctional electrospun ferric oxide-gelatin-glycerol nanofibrous mat for wound dressing applications. Fibers Polym. 2016, 17, 2014–2019. [Google Scholar] [CrossRef]
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Surface modification of electrospun wound dressing material by Fe2O3 nanoparticles incorporating Lactobacillus strains for enhanced antimicrobial and antibiofilm activity. Surf. Interfaces 2022, 28, 101592. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, S.G.; Park, J.-G.; Hyeon, T. Size dependence of metal–insulator transition in stoichiometric Fe3O4 nanocrystals. Nano Lett. 2015, 15, 4337–4342. [Google Scholar] [CrossRef]
- Moulana Kareem, M.; Hari Babu, M.; Vijaya Lakshmi, G. Anticancer, antibacterial, antioxidant, and photo-catalytic activities of eco-friendly synthesized Ni nanoparticles. Inorg. Chem. Commun. 2023, 148, 110274. [Google Scholar] [CrossRef]
- Mahendran, D.; Kavi Kishor, P.; Geetha, N.; Manish, T.; Sahi, S.; Venkatachalam, P. Efficient antibacterial/biofilm, anti-cancer and photocatalytic potential of titanium dioxide nanocatalysts green synthesised using Gloriosa superba rhizome extract. J. Exp. Nanosci. 2021, 16, 11–30. [Google Scholar] [CrossRef]
- Venugopal, N.; Saiprakash, P.; Jayalakshmi, M.; Ram Reddy, Y.; Rao, M.M. A study on the effect of nanosized tin oxide on the electrochemical performance of nanosized nickel hydroxide in alkali solution. J. Exp. Nanosci. 2013, 8, 684–693. [Google Scholar] [CrossRef]
- Sagadevan, S.; Vennila, S.; Muthukrishnan, L.; Gurunathan, K.; Oh, W.C.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Jasni, A.H.; Fatimah, I. Exploring the therapeutic potentials of phyto-mediated silver nanoparticles formed via Calotropis procera (Ait.) R. Br. root extract. J. Exp. Nanosci. 2020, 15, 217–231. [Google Scholar] [CrossRef]
- Balraj, B.; Senthilkumar, N.; Potheher, I.V.; Arulmozhi, M. Characterization, antibacterial, anti-arthritic and in-vitro cytotoxic potentials of biosynthesized magnesium oxide nanomaterial. Mater. Sci. Eng. B 2018, 231, 121–127. [Google Scholar] [CrossRef]
- Sharma, R.P.; Raut, S.D.; Kadam, A.S.; Mulani, R.M.; Mane, R.S. In-vitro antibacterial and anti-biofilm efficiencies of chitosan-encapsulated zinc ferrite nanoparticles. Appl. Phys. A 2020, 126, 824. [Google Scholar] [CrossRef]
- Anu, K.; Hemalatha, J. Magnetic and electrical conductivity studies of zinc doped cobalt ferrite nanofluids. J. Mol. Liq. 2019, 284, 445–453. [Google Scholar] [CrossRef]
- Jyothish, B.; Jacob, J. Synthesis and characterization of Ni2+ and Al3+ doped zinc ferrite nanoparticles for antibacterial, antioxidant, and anticancer (MCF-7) analysis. Chem. Phys. Impact 2023, 6, 100209. [Google Scholar] [CrossRef]
- Eleazu, C.; Okafor, P. Use of unripe plantain (Musa paradisiaca) in the management of diabetes and hepatic dysfunction in streptozotocin induced diabetes in rats. Interv. Med. Appl. Sci. 2015, 7, 9–16. [Google Scholar] [CrossRef]
- Fu, J.; Tu, S.; Yi, G.; Wang, J.; Sheng, O.; Zhang, W. Plantain flour—A beneficial material for the organ and transcriptional profile of kidney of diabetic rats. J. Funct. Foods 2023, 110, 105817. [Google Scholar] [CrossRef]
- Khalid, R.; Din, M.I.; Hussain, Z. Eco-friendly synthesis of copper oxide nanomaterial by using Musa paradisiaca leaves extract and their slow pyrolysis or catalytic reduction activities. Next Nanotechnol. 2024, 6, 100041. [Google Scholar] [CrossRef]
- Yadav, P.; Manori, S.; Chamoli, P.; Raina, K.K.; Shukla, R.K. Microwave assisted green synthesis of γ-Fe2O3 nanoparticles and their application for photodegradation of ternary dye mixture. J. Mater. Sci. Mater. Electron. 2023, 34, 1065. [Google Scholar] [CrossRef]
- Al-Ruqeishi, M.S.; Mohiuddin, T.; Al-Saadi, L.K. Green synthesis of iron oxide nanorods from deciduous Omani mango tree leaves for heavy oil viscosity treatment. Arab. J. Chem. 2019, 12, 4084–4090. [Google Scholar] [CrossRef]
- Marcorius, A.; Sulaeman, U.; Afif, M.; Nurfiah, S.; Khanifudin, K.; Afifah, K. The enhanced photocatalytic properties of silver phosphate synthesized under mangosteen peel extract solution. J. Teknol. 2021, 84, 21–27. [Google Scholar] [CrossRef]
- Hamid, A.; Haq, S.; Ur Rehman, S.; Akhter, K.; Rehman, W.; Waseem, M.; Ud Din, S.; Hafeez, M.; Khan, A.; Shah, A. Calcination temperature-driven antibacterial and antioxidant activities of fumaria indica mediated copper oxide nanoparticles: Characterization. Chem. Pap. 2021, 75, 4189–4198. [Google Scholar] [CrossRef]
- Indrayana, I.; Tjuana, L.; Tuny, M.; Kurnia. Nanostructure and optical properties of Fe3O4: Effect of calcination temperature and dwelling time. J. Phys. Conf. Ser. 2019, 1341, 082044. [Google Scholar] [CrossRef]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique. J. Mater. Sci. Mater. Electron. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Archana, V.; Joseph Prince, J.; Kalainathan, S. Simple one-step leaf extract-assisted preparation of α-Fe2O3 nanoparticles, physicochemical properties, and its sunlight-driven photocatalytic activity on methylene blue dye degradation. J. Nanomater. 2021, 2021, 8570351. [Google Scholar] [CrossRef]
- Apte, S.K.; Naik, S.D.; Sonawane, R.S.; Kale, B.B.; Baeg, J.O. Synthesis of nanosize-necked structure α- and γ-Fe2O3 and its photocatalytic activity. J. Am. Ceram. Soc. 2007, 90, 412–414. [Google Scholar] [CrossRef]
- Hung, C.M.; Hoa, N.D.; Van Duy, N.; Van Toan, N.; Le, D.T.T.; Van Hieu, N. Synthesis and gas-sensing characteristics of α-Fe2O3 hollow balls. J. Sci. Adv. Mater. Devices 2016, 1, 45–50. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Ma, S.Y.; Jin, W.X.; Yang, H.M.; Chen, H.; Ge, Q.; Ma, L. Hydrothermal synthesis of monodisperse α-Fe2O3 hollow microspheroids and their high gas-sensing properties. J. Alloys Compd. 2017, 705, 427–437. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Zhuang, J.; Wang, X. Morphology-controlled synthesis of hematite nanocrystals and their facet effects on gas-sensing properties. Inorg. Chem. 2011, 50, 10143–10151. [Google Scholar] [CrossRef]
- Deepa, P.R.; Vandhana, S.; Jayanthi, U.; Krishnakumar, S. Therapeutic and toxicologic evaluation of anti-lipogenic agents in cancer cells compared with non-neoplastic cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 494–503. [Google Scholar] [CrossRef]
- Tahir, M.; Fakhar-e-Alam, M.; Atif, M.; Mustafa, G.; Ali, Z. Investigation of optical, electrical and magnetic properties of hematite α-Fe2O3 nanoparticles via sol-gel and co-precipitation method. J. King Saud Univ.-Sci. 2023, 35, 102695. [Google Scholar] [CrossRef]
- Raja, K.; Mary Jaculine, M.; Jose, M.; Verma, S.; Prince, A.A.M.; Ilangovan, K.; Sethusankar, K.; Jerome Das, S. Sol–gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlattices Microstruct. 2015, 86, 306–312. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Ogunjinmi, O.E.; Ogunjinmi, S.O.; Alayande, S.O.; Nwoke, E.O.; Adedosu, T.A. Evaluation of antioxidant activities of Celosia trigyna (Linn) extracts african extinction vegetable. Science 2020, 8, 102–106. [Google Scholar]
- Adegbola, P.; Fadahunsi, O.S.; Alabi, A.A. Comparative antioxidant study of ripe and unripe plantain and the qualitative assessment of some food oil extracts. Ann. Food Sci. Technol. 2018, 19, 758–765. [Google Scholar]
- Adenike, A.A.; Adegbola, P.; Fadahunsi, O.S. Antioxidant property and GCMS profile of oil extracted from Cocos nucifera using a fermentation method. BioTechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2019, 100, 349–358. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Sañudo-Barajas, J.; Vélez De La Rocha, R.; González-Aguilar, G.; Bello-Perez, L.A. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound. CyTA-J. Food 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Arun, K.; Persia, F.; Aswathy, P.; Chandran, J.; Sajeev, M.; Jayamurthy, P.; Nisha, P. Plantain peel-a potential source of antioxidant dietary fibre for developing functional cookies. J. Food Sci. Technol. 2015, 52, 6355–6364. [Google Scholar] [CrossRef]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, G.; Zhou, Z.; Sun, S.; Wang, Q.; Yan, S.; Li, H.; Tian, J.; Guo, J.; Zhou, B. Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts. J. Phys. Chem. B 2005, 109, 8774–8778. [Google Scholar] [CrossRef]
- Asimeng, B.O.; Nyankson, E.; Efavi, J.K.; Nii Amarkai, A.; Manu, G.P.; Tiburu, E. Characterization and inhibitory effects of magnetic iron oxide nanoparticles synthesized from plant extracts on HeLa cells. Int. J. Biomater. 2020, 2020, 2630735. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Elemike, E.E.; Nna, P.J.; Ikenweke, C.; Onwudiwe, D.; Omotade, E.T.; Singh, M. Synthesis, characterization, anti-cancer and antimicrobial studies of iron oxide nanoparticles mediated by Terminalia catappa (Indian almond) leaf extract. Inorg. Chem. Commun. 2023, 155, 111048. [Google Scholar] [CrossRef]
- Liu, X.; Shan, K.; Shao, X.; Shi, X.; He, Y.; Liu, Z.; Jacob, J.A.; Deng, L. Nanotoxic effects of silver nanoparticles on normal HEK-293 cells in comparison to cancerous HeLa cell line. Int. J. Nanomed. 2021, 16, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Bethu, M.S.; Netala, V.R.; Domdi, L.; Tartte, V.; Janapala, V.R. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: An insight into the mechanism. Artif. Cells Nanomed. Biotechnol. 2018, 46, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.A.; Thomas, A.; Revi, N.; Ramakrishna, B.; Rengan, A.K. Iron oxide nanoparticles for theranostic applications—Recent advances. J. Drug Deliv. Sci. Technol. 2022, 70, 103196. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Wan, L.; Yan, S.; Wang, X.; Li, Z.; Zou, Z. Solvothermal synthesis of monodisperse iron oxides with various morphologies and their applications in removal of Cr (VI). CrystEngComm 2011, 13, 2727–2733. [Google Scholar] [CrossRef]
- Zamani, M.; Delfani, A.M.; Jabbari, M. Scavenging performance and antioxidant activity of γ-alumina nanoparticles towards DPPH free radical: Spectroscopic and DFT-D studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 288–299. [Google Scholar] [CrossRef]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Saiyed, T.A.; Adeyemi, J.O.; Singh, M.; Okafor, S.N.; Onwudiwe, D.C. Synthesis, characterization, and biological evaluation of some metal complexes containing N and S donor atoms. Results Chem. 2023, 6, 101052. [Google Scholar] [CrossRef]

| Test Samples | Sample Concentrations (µg/mL) | IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 1.56 | 3.13 | 6.25 | 12.5 | 25 | 50 | ||
| Ascorbic acid | 4.62 ± 0.022 | 14.16 ± 0.030 | 28.95 ± 0.021 | 42.04 ± 0.020 | 55.51 ± 0.052 | 63.50 ± 0.044 | 30.17 |
| Fe2O3 (1) | 4.82 ± 0.042 | 8.44 ± 0.023 | 17.48 ± 0.041 | 28.80 ± 0.025 | 36.56 ± 0.051 | 50.63 ± 0.310 | 46.84 |
| Fe2O3 (2) | 3.95 ± 0.039 | 8.36 ± 0.074 | 16.63 ± 0.052 | 26.30 ± 0.055 | 35.80 ± 0.031 | 49.63 ± 0.640 | 46.14 |
| Fe2O3 (3) | 4.11 ± 0.026 | 9.46 ± 0.033 | 18.43 ± 0.034 | 28.20 ± 0.055 | 38.49 ± 0.034 | 50.23 ± 0.065 | 47.04 |
| Plantain peels | 2.21 ± 0.018 | 3.34 ± 0.040 | 10.52 ± 0.024 | 18.29 ± 0.033 | 24.09 ± 0.074 | 30.11 ± 0.047 | 79.26 |
| Cell Lines | Test Samples | Sample Concentration (µg/mL) | IC50 (µg/mL) | |||
|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 100 | |||
| HEK 293 | 5-FU | 72.14 ± 0.062 | 53.54 ± 0.033 | 44.30 ± 0.061 | 32.23 ±0.054 | 47.64 |
| Fe2O3 (1) | 55.23 ± 0.041 | 42.34 ± 0.052 | 35.22 ± 0.062 | 24.51 ± 0.055 | 11.93 | |
| Fe2O3 (2) | 48.24 ± 0.014 | 34.64 ± 0.024 | 26.21 ± 0.061 | 20.10 ± 0.046 | 17.03 | |
| Fe2O3 (3) | 42.34 ± 0.015 | 23.72 ± 0.056 | 18.35 ± 0.032 | 13.15 ± 0.054 | 48.40 | |
| Plantain peel | 65.65 ± 0.043 | 52.33 ± 0.023 | 23.52 ± 0.036 | 14.40 ± 0.060 | 26.68 | |
| HeLa | 5-FU | 80.40 ± 0.20 | 71.94 ± 0.060 | 58.85± 0.32 | 45.45 ± 0.040 | 83.39 |
| Fe2O3 (1) | 65.45 ± 0.012 | 54.24 ± 0.052 | 38.34 ± 0.041 | 25.11 ± 0.061 | 36.56 | |
| Fe2O3 (2) | 53.43 ± 0.042 | 38.28 ± 0.032 | 22.64 ± 0.054 | 16.54 ± 0.015 | 0.84 | |
| Fe2O3 (3) | 58.32 ± 0.062 | 44.51 ± 0.021 | 30.02 ± 0.015 | 21.02 ± 0.055 | 16.63 | |
| Plantain peel | 76.63 ± 0.032 | 68.47 ± 0.071 | 52.52 ± 0.028 | 18.62 ± 0.071 | 52.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzine, S.; Ferjani, H.; Ogunjinmi, O.E.; Onwudiwe, D.C. Hematite Nanoparticles Synthesized by Green Route: Characterization, Anticancer and Antioxidant Activities. Inorganics 2025, 13, 167. https://doi.org/10.3390/inorganics13050167
Ezzine S, Ferjani H, Ogunjinmi OE, Onwudiwe DC. Hematite Nanoparticles Synthesized by Green Route: Characterization, Anticancer and Antioxidant Activities. Inorganics. 2025; 13(5):167. https://doi.org/10.3390/inorganics13050167
Chicago/Turabian StyleEzzine, Safa, Hela Ferjani, Oluwasayo E. Ogunjinmi, and Damian C. Onwudiwe. 2025. "Hematite Nanoparticles Synthesized by Green Route: Characterization, Anticancer and Antioxidant Activities" Inorganics 13, no. 5: 167. https://doi.org/10.3390/inorganics13050167
APA StyleEzzine, S., Ferjani, H., Ogunjinmi, O. E., & Onwudiwe, D. C. (2025). Hematite Nanoparticles Synthesized by Green Route: Characterization, Anticancer and Antioxidant Activities. Inorganics, 13(5), 167. https://doi.org/10.3390/inorganics13050167







