Preparation and Photocatalytic Hydrogen Production of Pink ZnS
Abstract
1. Introduction
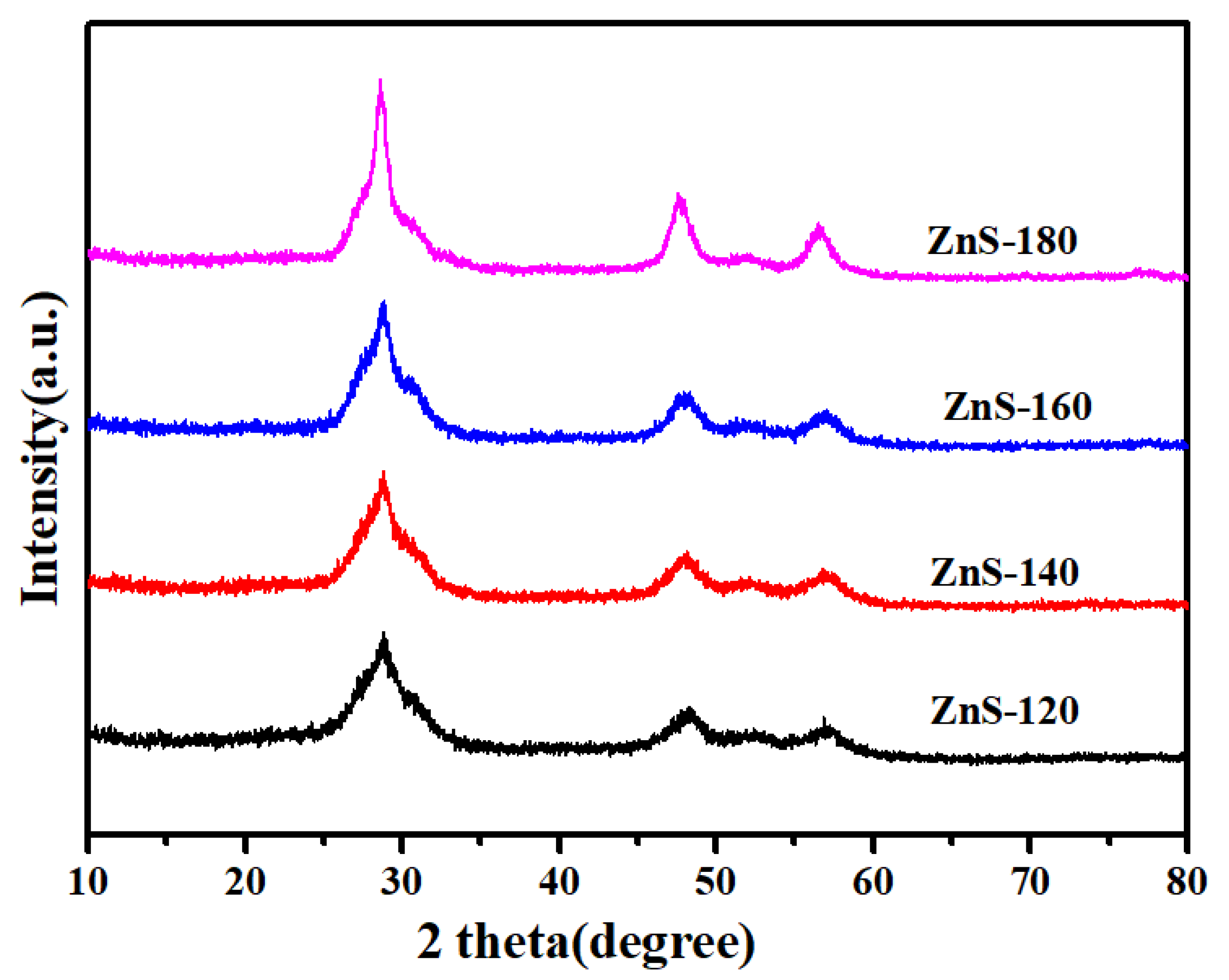
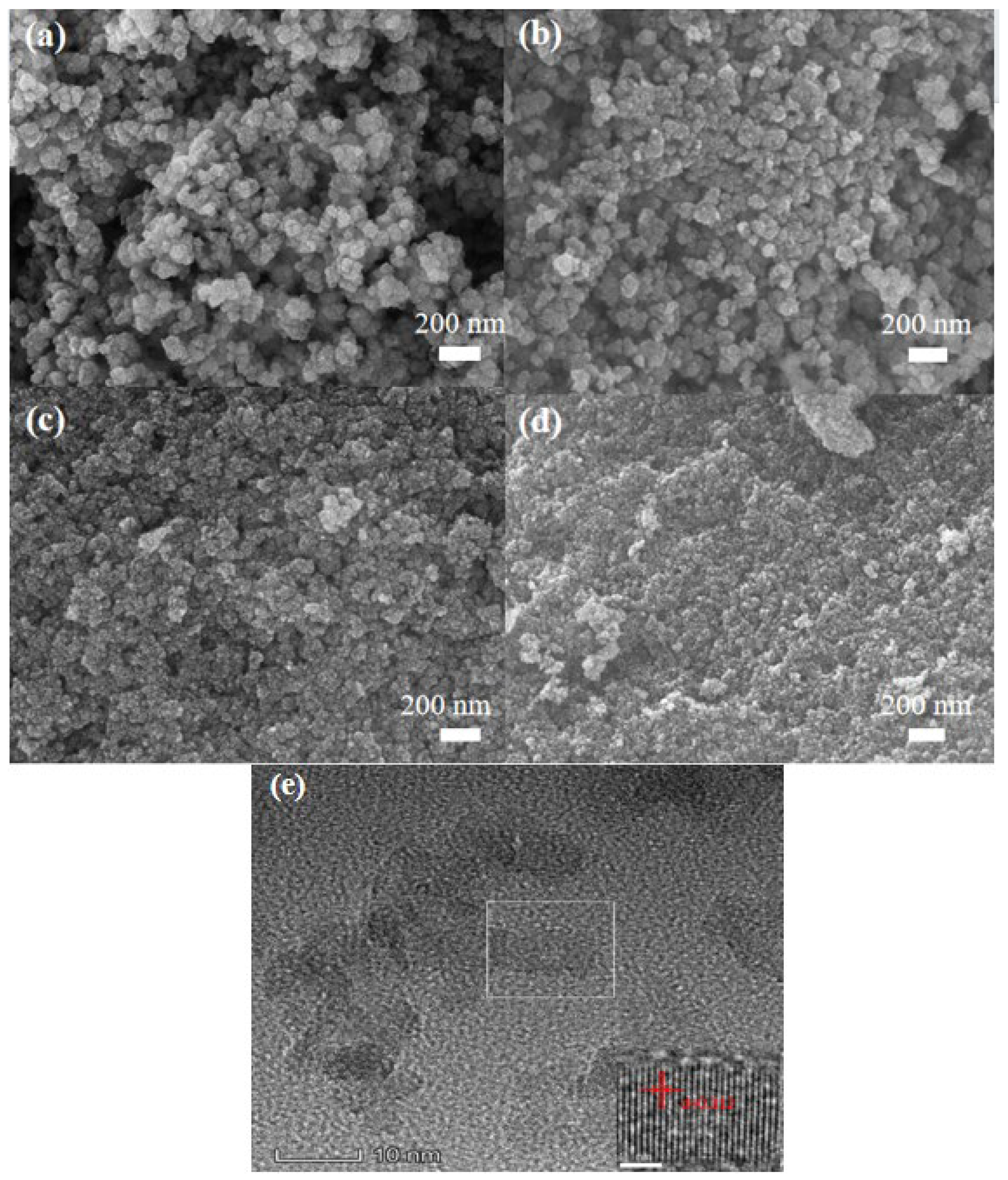
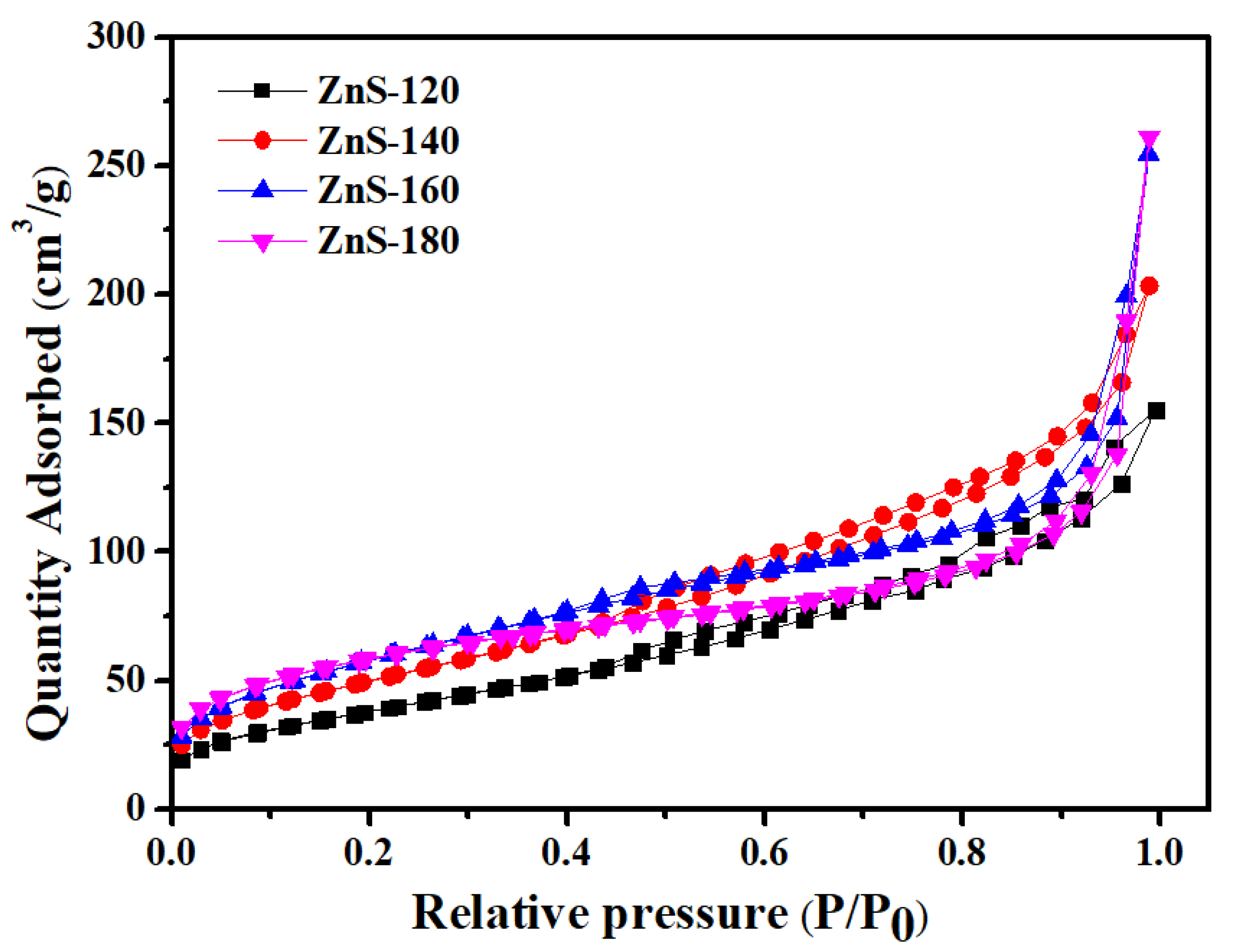
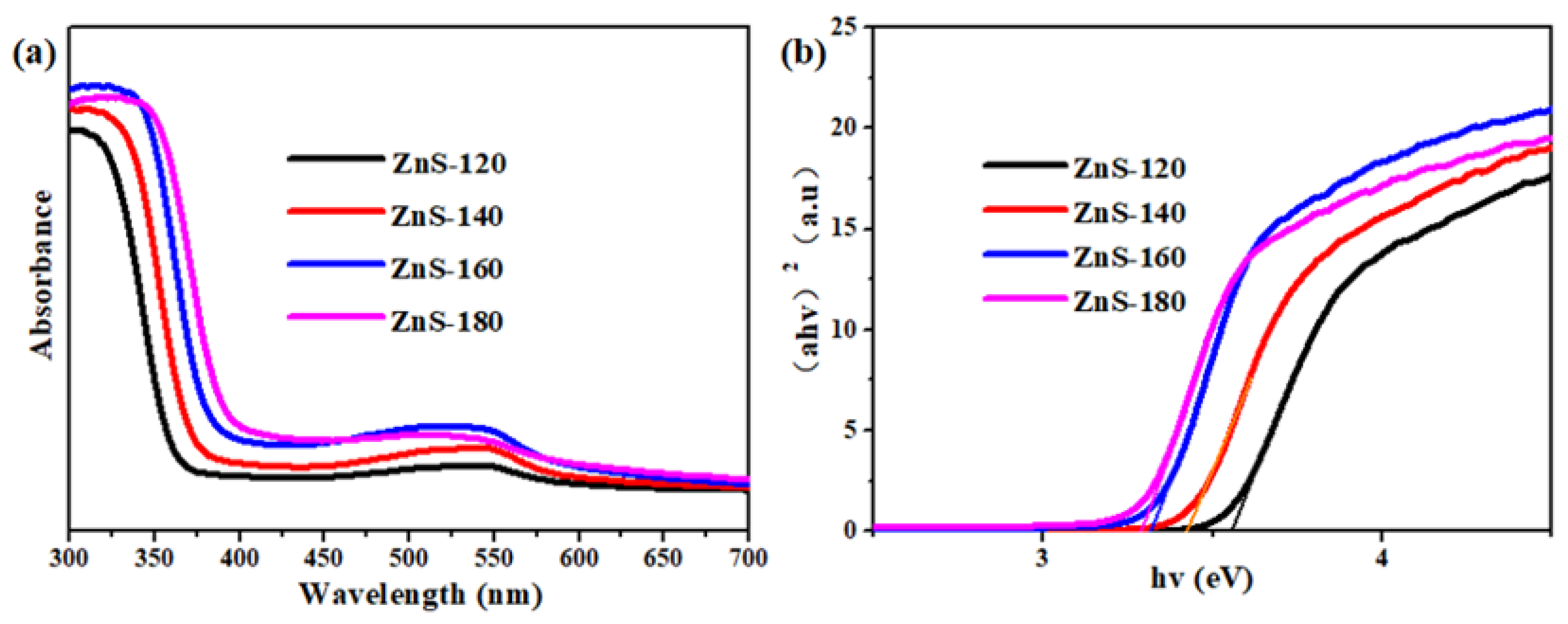
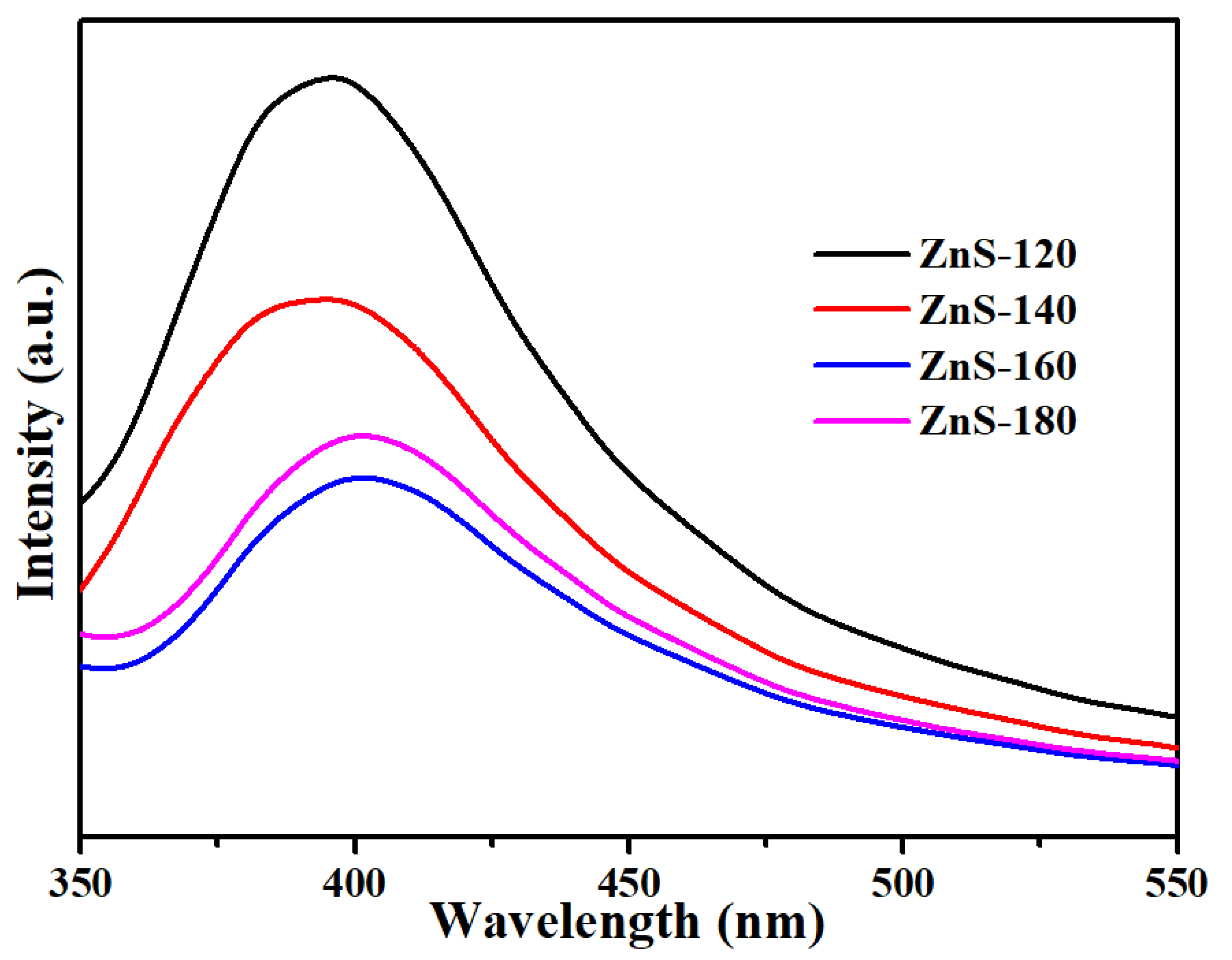
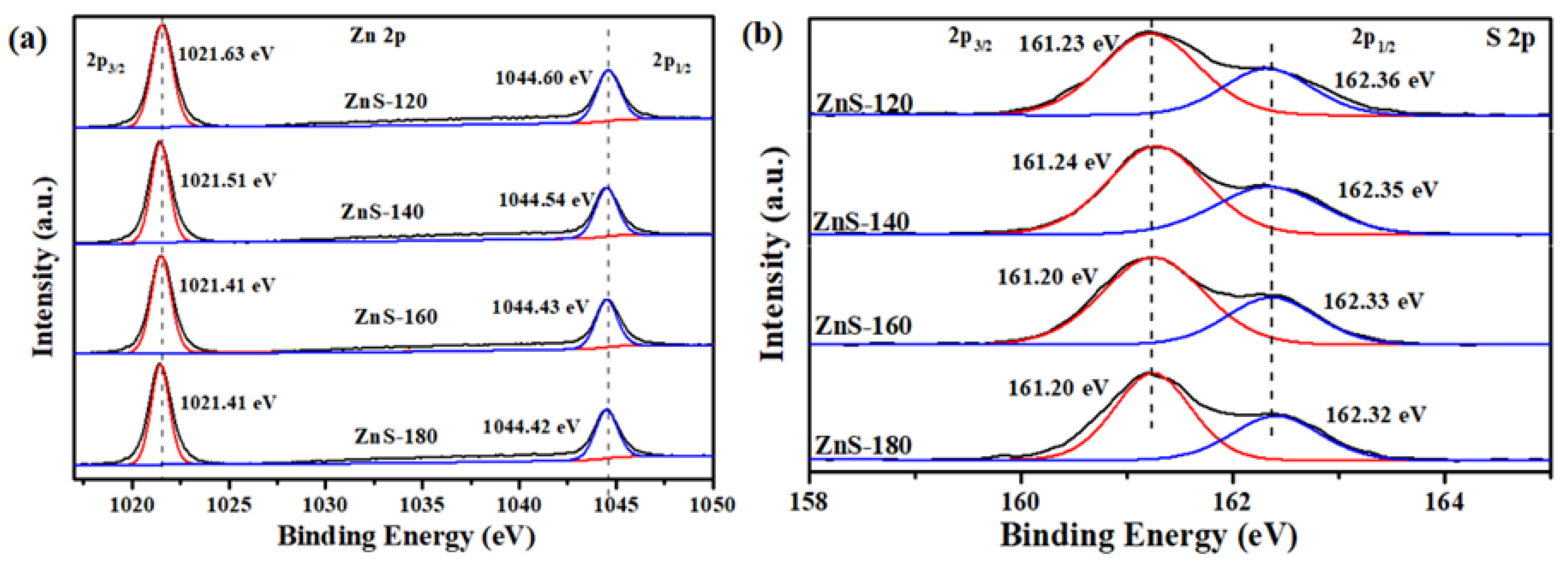
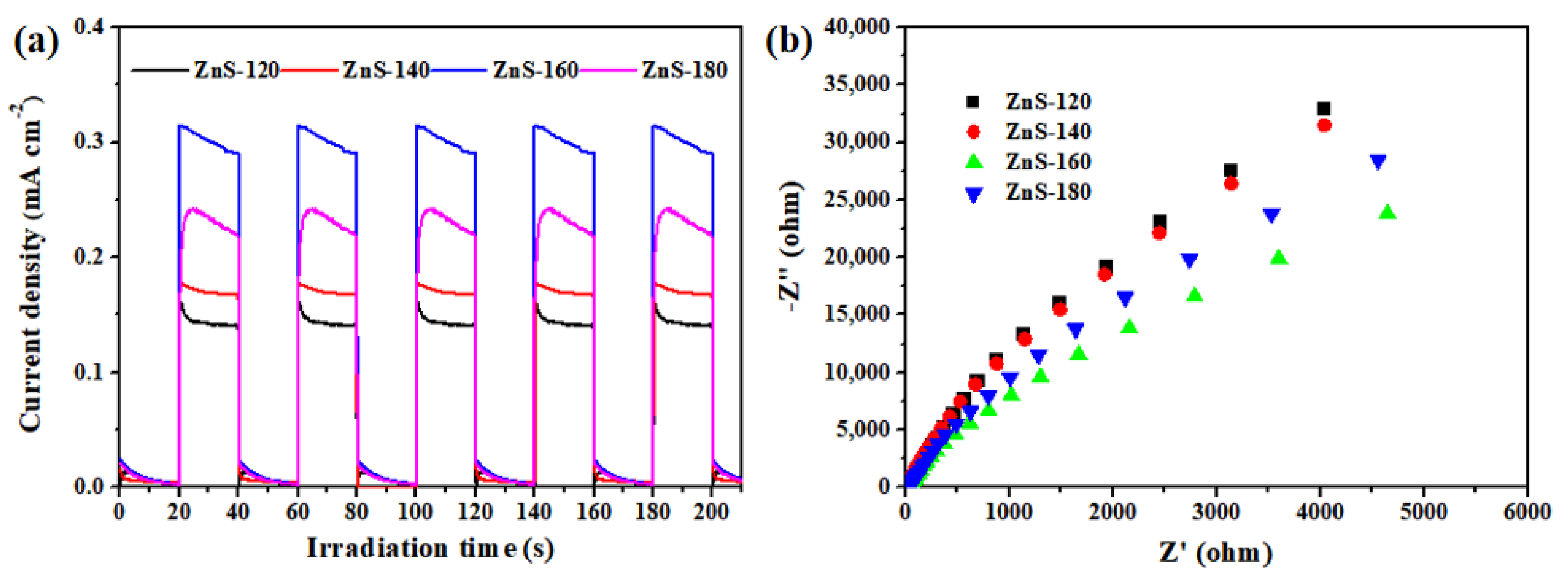
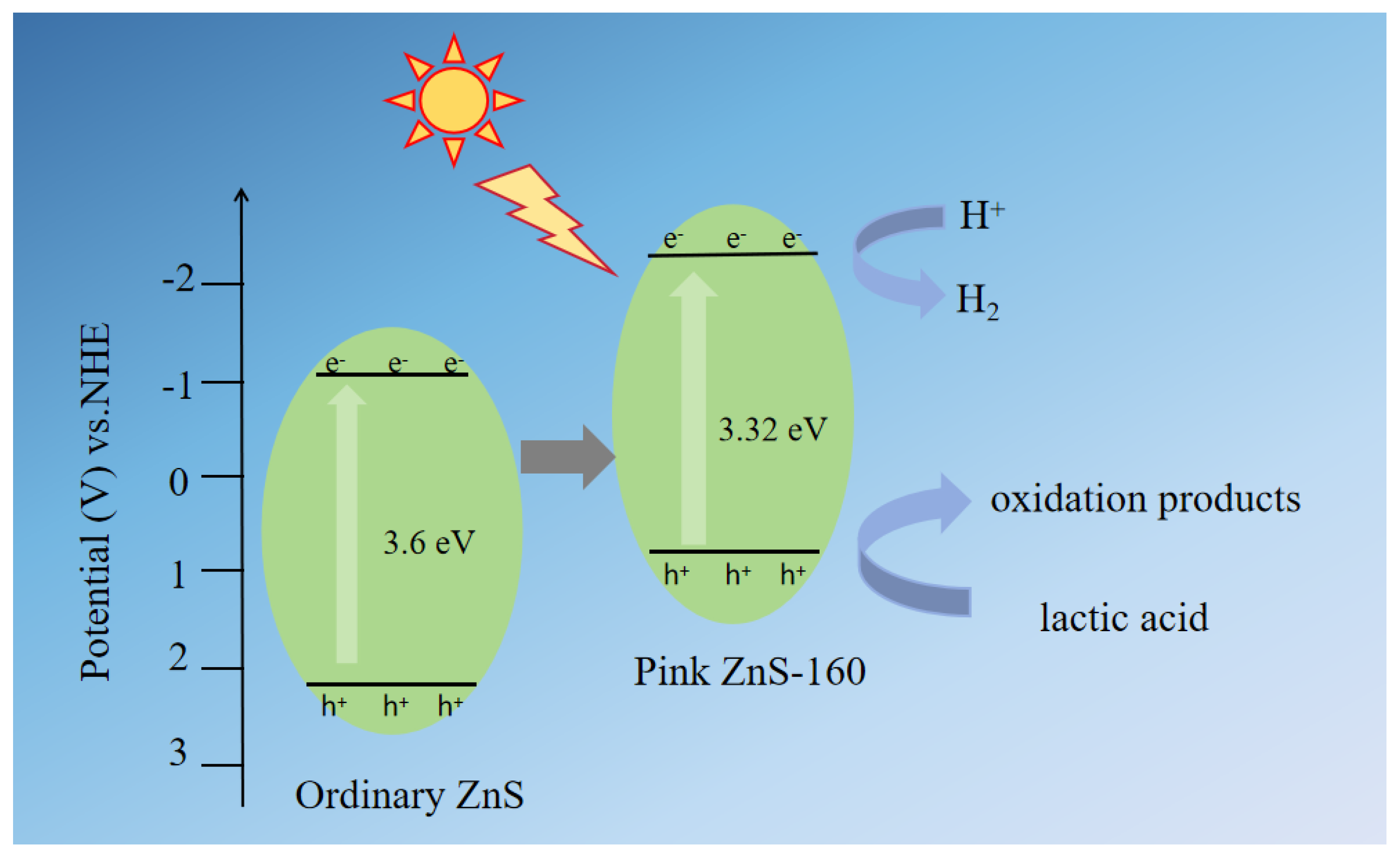
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Pink ZnS
3.3. Characterization
3.4. Photocurrent Measurements
3.5. Photocatalytic Hydrogen Production Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raza, W.; Kerketta, U.; Hwang, I.; Schmuki, P. CdS Decorated on Hierarchically Structured Single Crystal TiO2 Nanosheets for Enhanced Photoelectrochemical H2 Generation. ChemElectroChem 2022, 9, e202200706. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Alvarado, F.G.; Oh, T.H. Progress in 2D MoS2-Based Advanced Materials for Hydrogen Evolution and Energy Storage Applications. Inorganics 2025, 13, 47. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Khan, R.A.; Kim, H. Ag decorated ZnO for enhanced photocatalytic H2 generation and pollutant degradation. Int. J. Hydrogen Energy 2023, 48, 29071–29081. [Google Scholar] [CrossRef]
- Osuagwu, B.; Raza, W.; Tesler, A.B.; Schmuki, P. A drastic improvement in photocatalytic H2 production by TiO2 nanosheets grown directly on Ta2O5 substrates. Nanoscale 2021, 13, 12750–12756. [Google Scholar] [CrossRef]
- She, H.; Ma, X.; Chen, K.; Liu, H.; Huang, J.; Wang, L.; Wang, Q. Photocatalytic H2 production activity of TiO2 modified by inexpensive Cu(OH)2 cocatalyst. J. Alloys Compd. 2020, 821, 153239. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, L.; Ma, R.; Tomovska, R.; Luo, X.; Xie, X.; Su, T.; Ji, H. TiO2/BiYO3 composites for enhanced photocatalytic hydrogen production. J. Alloys Compd. 2020, 836, 155428. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, B.-J.; Jeong, S.; Jeon, K.-J.; Chung, K.-H.; Jung, S.-C. Characteristics of hydrogen production by photocatalytic water splitting using liquid phase plasma over Ag-doped TiO2 photocatalysts. Environ. Res. 2020, 188, 109630. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D. Photocatalytic degradation of different chromophoric dyes in aqueous phase using La and Mo doped TiO2 hybrid carbon spheres. J. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Bahnemann, D. Synthesis of visible light driven TiO2 coated carbon nanospheres for degradation of dyes. Arab. J. Chem. 2019, 12, 3534–3545. [Google Scholar] [CrossRef]
- Raza, W.; Hwang, I.; Denisov, N.; Schmuki, P. Thermal Ramping Rate during Annealing of TiO2 Nanotubes Greatly Affects Performance of Photoanodes. Phys. Status Solidi A 2021, 218, 2100040. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Li, G.; Luo, D.; Liu, J.; Zhang, Y.; Wang, X.; Shui, L.; Chen, Z. Aligned sulfur-deficient ZnS1−x nanotube arrays as efficient catalyzer for high-performance lithium/sulfur batteries. Nano Energy 2021, 84, 105891. [Google Scholar] [CrossRef]
- Xiao, B.; Lv, T.; Zhao, J.; Rong, Q.; Zhang, H.; Wei, H.; He, J.; Zhang, J.; Zhang, Y.; Peng, Y.; et al. Synergistic Effect of the Surface Vacancy Defects for Promoting Photocatalytic Stability and Activity of ZnS Nanoparticles. ACS Catal. 2021, 11, 13255–13265. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Liu, R. Defect engineering of zeolite imidazole framework derived ZnS nanosheets towards enhanced visible light driven photocatalytic hydrogen production. Appl. Catal. B Environ. 2020, 278, 119265. [Google Scholar] [CrossRef]
- Gao, F.; Lei, R.; Huang, X.; Yuan, J.; Jiang, C.; Feng, W.; Zhang, L.; Liu, P. In situ etching growth of defective ZnS nanosheets anchored vertically on layered-double-hydroxide microflowers for accelerated photocatalytic activity. Appl. Catal. B Environ. 2021, 292, 120187. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Waqas, M.; Wei, Y.; Mao, D.; Qi, J.; Yang, Y.; Wang, B.; Wang, D. Multi-shelled TiO2/Fe2TiO5 heterostructured hollow microspheres for enhanced solar water oxidation. Nano Res. 2017, 10, 3920–3928. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Yu, R.; Wan, J.; Wang, D. Constructing SrTiO3–TiO2 Heterogeneous Hollow Multi-shelled Structures for Enhanced Solar Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 1422–1426. [Google Scholar] [CrossRef]
- Wang, L.; Wan, J.; Zhao, Y.; Yang, N.; Wang, D. Hollow Multi-Shelled Structures of Co3O4 Dodecahedron with Unique Crystal Orientation for Enhanced Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Wan, J.; Qi, J.; Mao, D.; Yang, N.; Zhang, Q.; Gu, L.; Wang, D. Lattice Distortion in Hollow Multi-Shelled Structures for Efficient Visible-Light CO2 Reduction with a SnS2/SnO2 Junction. Angew. Chem. Int. Ed. 2019, 59, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zuo, G.; Zong, P.; Pang, H.; Ren, J.; Zeng, X.; Liu, S.; Shen, Y.; Zhou, W.; Ye, J. A rapidly room-temperature-synthesized Cd/ZnS:Cu nanocrystal photocatalyst for highly efficient solar-light-powered CO2 reduction. Appl. Catal. B Environ. 2018, 237, 68–73. [Google Scholar] [CrossRef]
- Chen, J.; Xin, F.; Qin, S.; Yin, X. Photocatalytically reducing CO2 to methyl formate in methanol over ZnS and Ni-doped ZnS photocatalysts. Chem. Eng. J. 2013, 230, 506–512. [Google Scholar] [CrossRef]
- Amaranatha Reddy, D.; Ma, R.; Choi, M.Y.; Kim, T.K. Reduced graphene oxide wrapped ZnS–Ag2S ternary composites synthesized via hydrothermal method: Applications in photocatalyst degradation of organic pollutants. Appl. Surf. Sci. 2015, 324, 725–735. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Li, Y.; Duan, J.; Zhang, G.; Hu, L.; Wang, X.; Zhang, W. Visible-light-driven photo-Fenton reactions using Zn1−1.5xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B Environ. 2020, 266, 118653. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.-B.; Wang, X.; Li, J.; Zhang, Q.; Xia, A.; Wei, S.; Wu, L.-Z.; Zhou, Y.; Patzke, G.R. Efficient photocatalytic hydrogen evolution with ligand engineered all-inorganic InP and InP/ZnS colloidal quantum dots. Nat. Commun. 2018, 9, 4009. [Google Scholar] [CrossRef]
- Xie, Y.P.; Yu, Z.B.; Liu, G.; Ma, X.L.; Cheng, H.-M. CdS–mesoporous ZnS core–shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Energy Environ. Sci. 2014, 7, 1895–1901. [Google Scholar] [CrossRef]
- Li, P.; Luo, G.; Zhu, S.; Guo, L.; Qu, P.; He, T. Unraveling the selectivity puzzle of H2 evolution over CO2 photoreduction using ZnS nanocatalysts with phase junction. Appl. Catal. B Environ. 2020, 274, 119115. [Google Scholar] [CrossRef]
- Hong, Y.P.; Zhang, J.; Huang, F.; Zhang, J.Y.; Wang, X.; Wu, Z.C.; Lin, Z.; Yu, J.G. Enhanced visible light photocatalytic hydrogen production activity of CuS/ZnS nanoflower spheres. J. Mater. Chem. A 2015, 3, 13913–13919. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, Z.; Ren, Z.; Mao, X.; Dai, D.; Yang, X. Effect of defects on photocatalytic dissociation of methanol on TiO2(110). Chem. Sci. 2011, 2, 1980–1983. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the Relative Concentration Ratio of Bulk Defects to Surface Defects in TiO2 Nanocrystals Leads to High Photocatalytic Efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; English, N.J.; Prezhdo, O.V. Defects Are Needed for Fast Photo-Induced Electron Transfer from a Nanocrystal to a Molecule: Time-Domain Ab Initio Analysis. J. Am. Chem. Soc. 2013, 135, 18892–18900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the Relationship between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. 2008, 120, 1790–1793. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, X.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic Overall Water Splitting Promoted by an α–β phase Junction on Ga2O3. Angew. Chem. Int. Ed. 2012, 51, 13089–13092. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, Z.; Tan, S.; Ma, Z.; Mao, X.; Dai, D.; Fan, H.; Yang, X.; LaRue, J.; Cooper, R.; et al. Site-specific photocatalytic splitting of methanol on TiO2(110). Chem. Sci. 2010, 1, 575–580. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, K.; Lv, R.; Xu, Y. ZnO/ZnS–PdS core/shell nanorods: Synthesis, characterization and application for photocatalytic hydrogen production from a glycerol/water solution. Appl. Surf. Sci. 2013, 283, 732–739. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Altıokka, B. Effects of Inhibitor on ZnS Thin Films Fabricated by Electrodeposition. J. Electron. Mater. 2019, 48, 2398–2403. [Google Scholar] [CrossRef]
- Charinpanitkul, T.; Chanagul, A.; Dutta, J.; Rungsardthong, U.; Tanthapanichakoon, W. Effects of cosurfactant on ZnS nanoparticle synthesis in microemulsion. Sci. Technol. Adv. Mater. 2005, 6, 266–271. [Google Scholar] [CrossRef]
- Yu, L.; Ruan, H.; Zheng, Y.; Li, D. A facile solvothermal method to produce ZnS quantum dots-decorated graphene nanosheets with superior photoactivity. Nanotechnology 2013, 24, 375601. [Google Scholar] [CrossRef] [PubMed]
- Goktas, A.; Tumbul, A.; Aslan, F. A new approach to growth of chemically depositable different ZnS nanostructures. J. Sol-Gel Sci. Technol. 2019, 90, 487–497. [Google Scholar] [CrossRef]
- Shanmugam, N.; Cholan, S.; Kannadasan, N.; Sathishkumar, K.; Viruthagiri, G. Effect of polyvinylpyrrolidone as capping agent on Ce3+ doped flowerlike ZnS nanostructure. Solid State Sci. 2014, 28, 55–60. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Hrubaru, M.; Onwudiwe, D.C.; Hosten, E. Synthesis and properties of ZnS nanoparticles by solvothermal and pyrolysis routes using the Zn dithiocarbamate complex as novel single source precursor. J. Sulfur Chem. 2015, 37, 37–47. [Google Scholar] [CrossRef]
- Sabaghi, V.; Davar, F.; Fereshteh, Z. ZnS nanoparticles prepared via simple reflux and hydrothermal method: Optical and photocatalytic properties. Ceram. Int. 2018, 44, 7545–7556. [Google Scholar] [CrossRef]
- Jadraque, M.; Evtushenko, A.B.; Ávila-Brande, D.; López-Arias, M.; Loriot, V.; Shukhov, Y.G.; Kibis, L.S.; Bulgakov, A.V.; Martín, M. Co-Doped ZnS Clusters and Nanostructures Produced by Pulsed Laser Ablation. J. Phys. Chem. C 2013, 117, 5416–5423. [Google Scholar] [CrossRef]
- Palve, A.M. Deposition of Zinc Sulfide Thin Films from Zinc(II) Thiosemicarbazones as Single Molecular Precursors Using Aerosol Assisted Chemical Vapor Deposition Technique. Front. Mater. 2019, 6, 46. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, G.Y.; Hu, X.Y.; Shi, Q.F.; Qiao, T.; Yang, Y. Phase-controlled synthesis of ZnS nanocrystallites by mild solvothermal decomposition of an air-stable single-source molecular precursor. J. Cryst. Growth 2005, 284, 554–560. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Du, Z.N.; Li, K.W.; Zhang, M. Size-controlled hydrothermal synthesis of SnS2 nanoparticles with high performance in visible light-driven photocatalytic degradation of aqueous methyl orange. Sep. Purif. Technol. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tang, J.Y.; Hu, X.Y. Controllable synthesis and magnetic properties of pure hematite and maghemite nanocrystals from a molecular precursor. J. Alloys Compd. 2008, 462, 24–28. [Google Scholar] [CrossRef]
- Bai, J.; Sun, J.; Zhu, X.; Liu, J.; Zhang, H.; Yin, X.B.; Liu, L. Enhancement of Solar-Driven Photocatalytic Activity of BiOI Nanosheets through Predominant Exposed High Energy Facets and Vacancy Engineering. Small 2020, 16, e1904783. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Fei, G.T.; Huang, J.Y.; Xia, K.; Wang, B. Controllable hydrothermal synthesis of hollow ZnS nanospheres: Morphological evolution mechanism and photocatalytic performance. Ceram. Int. 2024, 50, 1556–1563. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Wang, C.-C. Hydrothermally derived zinc sulfide sphere-decorated titanium dioxide flower-like composites and their enhanced ethanol gas-sensing performance. J. Alloys Compd. 2018, 730, 333–341. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Liu, Y.; Song, Z.; Sun, Y.; Fang, J.; Liu, Z. Synthesis of ZnS nanoparticles via hydrothermal process assisted by microemulsion technique. J. Alloys Compd. 2009, 486, L40–L43. [Google Scholar] [CrossRef]
- Park, S.-J.; Song, J.-H. Glycothermally Synthesized Self-aggregated ZnS Spherical Particles for Methyl Orange Photodecomposition. Korean J. Met. Mater. 2021, 59, 732–740. [Google Scholar] [CrossRef]
- Zaki, M.I.; Fouad, N.E.; Mekhemer, G.A.H.; Jagadale, T.C.; Ogale, S.B. TiO2 nanoparticle size dependence of porosity, adsorption and catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 195–200. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, H.; Jiang, L.; Liu, Z.; Qu, J.; Zhang, C.; Jia, X.; Chen, K. Heterostructured WO3/RGO/protonated g-C3N4 three-layer nanosheets for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 496, 143639. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Alharethy, F.; Kim, H. Designing of Ag decorated ZnO catalyst for on-demand and sustainable H2 production at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131757. [Google Scholar] [CrossRef]
- Raza, W.; Faisal, S.M.; Owais, M.; Bahnemann, D.; Muneer, M. Facile fabrication of highly efficient modified ZnO photocatalyst with enhanced photocatalytic, antibacterial and anticancer activity. RSC Adv. 2016, 6, 78335–78350. [Google Scholar] [CrossRef]
- Kurnia, F.; Ng, Y.H.; Amal, R.; Valanoor, N.; Hart, J.N. Defect engineering of ZnS thin films for photoelectrochemical water-splitting under visible light. Sol. Energy Mater. Sol. Cells 2016, 153, 179–185. [Google Scholar] [CrossRef]
- Raza, W.; Tesler, A.B.; Mazare, A.; Tomanec, O.; Kment, S.; Schmuki, P. Pt Single Atoms as Co-Catalysts on CdS-Sensitized Single-Crystalline TiO2 Nanoflakes for Enhanced Visible Light Photocatalytic H2 Generation. ChemCatChem 2023, 15, e202300327. [Google Scholar] [CrossRef]
- Osuagwu, B.; Raza, W.; Tesler, A.B.; Schmuki, P. Facile Approach of Direct Sulfidation of FTO to Form Vertically Aligned SnS2 Nanoflake Photoanodes for Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 8395–8400. [Google Scholar] [CrossRef]
- Raza, W.; Tesler, A.B.; Mazare, A.; Schmuki, P. Solar Light-Induced Photoelectrochemical H2 Generation Over Hierarchical TiO2 Nanotube Arrays Decorated with CdS Nanoparticles. J. Electrochem. Soc. 2024, 171, 066506. [Google Scholar] [CrossRef]
- Puentes-Prado, E.; Garcia, C.R.; Oliva, J.; Galindo, R.; Bernal-Alvarado, J.J.; Diaz-Torres, L.A.; Gomez-Solis, C. Enhancing the solar photocatalytic hydrogen generation of ZnS films by UV radiation treatment. Int. J. Hydrogen Energy 2020, 45, 12308–12317. [Google Scholar] [CrossRef]
- Yao, Z.; Hou, X.; He, Y.; Li, D.; Jiang, Z. Hydrothermal synthesis of Ni-doped ZnS solid solution photocatalysts for photocatalytic H2 production. Res. Chem. Intermed. 2019, 45, 4927–4940. [Google Scholar] [CrossRef]
- Tie, L.; Sun, R.; Jiang, H.; Liu, Y.; Xia, Y.; Li, Y.-Y.; Chen, H.; Yu, C.; Dong, S.; Sun, J.; et al. Facile fabrication of N-doped ZnS nanomaterials for efficient photocatalytic performance of organic pollutant removal and H2 production. J. Alloys Compd. 2019, 807, 151670. [Google Scholar] [CrossRef]
- Ren, H.; Ye, K.; Chen, H.; Wang, F.; Hu, Y.; Shi, Q.; Yu, H.; Lv, R.; Chen, M. ZnO@ZnS core–shell nanorods with homologous heterogeneous interface to enhance photocatalytic hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129844. [Google Scholar] [CrossRef]
- Bolatov, A.; Manjovelo, A.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Uralbekov, B.; Schneider, R. Ternary ZnS/ZnO/Graphitic Carbon Nitride Heterojunction for Photocatalytic Hydrogen Production. Materials 2024, 17, 4877. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Lu, Y.; Ma, T.; Liu, H.; Zhang, J. Preparation and Photocatalytic Hydrogen Production of Pink ZnS. Inorganics 2025, 13, 166. https://doi.org/10.3390/inorganics13050166
Gao S, Lu Y, Ma T, Liu H, Zhang J. Preparation and Photocatalytic Hydrogen Production of Pink ZnS. Inorganics. 2025; 13(5):166. https://doi.org/10.3390/inorganics13050166
Chicago/Turabian StyleGao, Shangjie, Yongxin Lu, Teng Ma, Haixia Liu, and Jie Zhang. 2025. "Preparation and Photocatalytic Hydrogen Production of Pink ZnS" Inorganics 13, no. 5: 166. https://doi.org/10.3390/inorganics13050166
APA StyleGao, S., Lu, Y., Ma, T., Liu, H., & Zhang, J. (2025). Preparation and Photocatalytic Hydrogen Production of Pink ZnS. Inorganics, 13(5), 166. https://doi.org/10.3390/inorganics13050166









