Abstract
The aluminum electrolysis industry faces significant challenges due to the high consumption and environmental impact of carbon anodes, which are prone to oxidation in high-temperature and strongly oxidizing environments. This study innovatively introduces aluminum fluoride (AlF3) as an additive to enhance the oxidation resistance of carbon anodes for aluminum electrolysis. By systematically exploring microstructural evolution through SEM, XRD, Raman spectroscopy, and permeability analyses, it reveals that AlF3 inserts fluorine atoms into carbon interlayers, forming F-C bonds that reduce interlayer spacing while promoting graphitization. Simultaneously, AlF3-derived α-Al2O3 particles densify the anode and make it more compact, reaching the optimum when 7 wt.% AlF3 is doped. The bulk density of the carbon anode increased to 2.08 g/cm3, porosity decreased to 0.315, and air permeability reached a minimum of 2.3 nPm. In addition, the fluorine intercalation reduces the electrical resistance to 2.12 Ω via conductive F-C clusters. The demonstrated efficacy of AlF3 additives in enhancing the oxidation resistance and conductivity of carbon anodes suggests strong potential for industrial adoption, particularly in optimizing anode composition to reduce energy consumption.
1. Introduction
As a basic industry with high energy and resource consumption, the core process of the aluminum electrolysis industry is to produce metallic aluminum through the electrolysis of aluminum oxide (Al2O3) [1,2,3,4]. In this process, the carbon anode, as the core component of the electrolyzer, assumes the dual functions of conducting electricity and participating in electrochemical reactions [5,6,7]. However, carbon anodes are susceptible to oxidation reactions at high temperatures (about 960 °C) and strong oxidizing environments (O2, CO2, etc.) during the electrolysis process, leading to the overconsumption of anodes [8,9,10]. This not only significantly increases the production cost (the cost of carbon anodes accounts for 10–15% of the total cost of aluminum electrolysis) but also releases greenhouse gases, such as CO2, and aggravates the burden on the environment [11,12,13]. Therefore, how to improve the oxidation resistance performance of carbon anodes has become a key issue that needs to be solved in the aluminum electrolysis industry.
In recent years, academia and industry have conducted a lot of research around the improvement of the oxidation resistance performance of carbon anodes, mainly focusing on the three aspects of material optimization, additive development, and process innovation. Among them, improving the microstructure and reactivity of carbon anodes by introducing additives is a hot topic in current research [14,15,16]. Additives mainly prevent the oxidative damage of carbon anodes in a strong oxidizing environment by reducing porosity or forming a protective film on the surface of the carbon anode, enhancing the chemical stability of the carbon anode [17,18]. For example, the addition of biochar significantly enhanced the coking value, quality index (QI) content, density, viscosity, and softening point of bio pitch but had less effect on surface tension and molecular weight distribution. When the biochar addition was 9.0 wt.%, the modified bio pitch showed the best overall performance and might replace coal tar pitch (CTP) as a binder, as well as reduce the total consumption in the anode formulation and improve the oxidation resistance property of the carbon anode [19]. Aluminum powder enhanced the oxidation resistance property by enhancing the coking property of carbon anodes, increasing the degree of graphitization and reducing the porosity. The effect may originate from the catalytic effect of aluminum volume expansion on pitch pyrolysis during calcination [20]. Ren [21] et al. found that the addition of aluminum powder significantly reduced the CO2 reactivity of carbon anodes at 970 °C. With the addition of 0.8 wt.% aluminum powder, the degree of anodic oxidation and the shedding rate were reduced by 32.8% and 70%, respectively, compared with the anode without additives, and the total consumption rate was reduced to 39.28 mg·cm−2·h−1.
As an important component of the electrolyte system for aluminum electrolysis (usually forming a eutectic system with cryolite Na3AlF6), the concentration of aluminum fluoride (AlF3) directly affects the melting point, viscosity, and ion mobility of the electrolyte and the oxygen partial pressure of the electrolyte melt, thereby indirectly inhibiting the surface oxidation reaction of the carbon anode [22]. However, most of the existing studies focus on the effect of AlF3 on the physicochemical properties of the electrolyte, while the effects of AlF3 as an additive on the intrinsic oxidation resistance performance of the carbon anode still lacks systematic exploration. It is noteworthy that the evaporation loss of AlF3 will also aggravate the fluorination reaction on the carbon anode surface, resulting in the formation of CF4 and C2F6 [23]. This phenomenon indicates that changes in the content of AlF3 may further affect the oxidation behavior of carbon anodes by changing the reaction conditions at the anode–electrolyte interface [24,25]. However, the current studies on the quantitative relationship between AlF3 content and the oxidation resistance performance of carbon anodes are still insufficient, especially since the synergistic mechanism during long-term electrolysis has not been clarified.
This study systematically investigates the role of AlF3 additives in enhancing the oxidation resistance of carbon anodes for aluminum electrolysis. By incorporating varying AlF3 concentrations (1–7 wt.%), we examine its influence on the microstructural evolution through comprehensive material characterization.
2. Results and Discussion
2.1. Effect of AlF3 on Micromorphology of Carbon Anodes
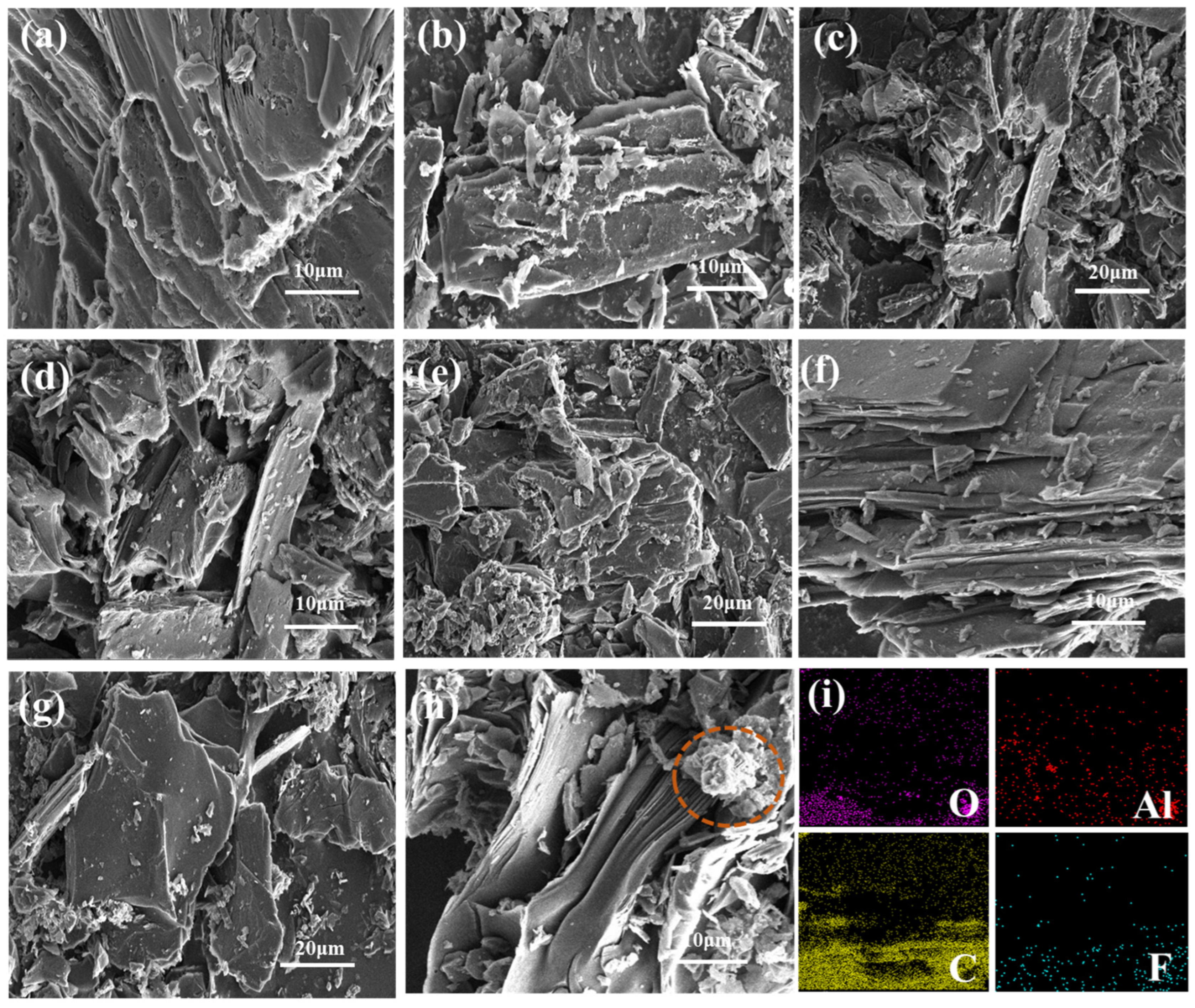
Figure 1 shows the microscopic morphology of carbon anodes doped with varying contents (1 wt.%, 2 wt.%, 5 wt.%, and 7 wt.%) of AlF3, respectively. As can be seen from Figure 1a–h, compared with the morphology of the carbon anode without doping AlF3, the layered structure of the carbon anode is more obvious with the addition of AlF3, and some residues appear on the surface of the carbon anode. When the AlF3 content was 2 wt.%, cracks appeared on the surface of the carbon anode and the bifurcation increased, which may be due to the difference in the coefficient of thermal expansion [26,27]. When the AlF3 content is increased to 5 wt.%, it can be seen that the number of carbon anode layers is organized and increased, and the surface becomes smoother. Where some localized enrichment can also be initially seen on the surface of the carbon matrix at lower magnification, as in Figure 1e,g, Figure 1i shows the swept energy spectrum analysis from the sample surface of Figure 1f, which shows that the surface contains the elements Al, O, C, and F. Among them, Al and O are distributed on the lamellar surface, which may be due to the oxidation reaction of AlF3 with carbon or oxygen in the air at high temperatures, and the resulting Al2O3 particles will adhere to the surface. The chemical equation is given in Equations (1) and (2).
C + AlF3 → CFx + Al2O3
AlF3 + O2 → Al2O3 + F2
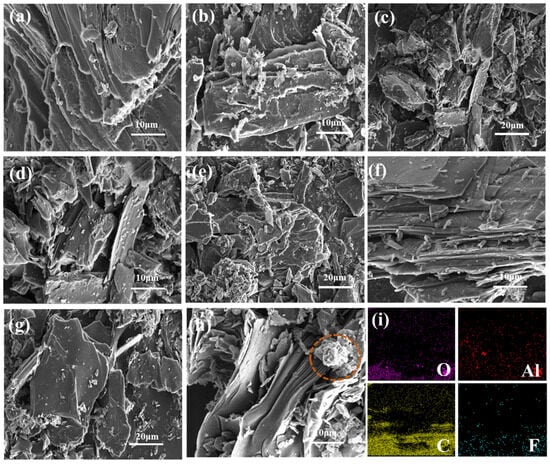
Figure 1.
SEM and EDS of carbon anode doped with varying AlF3 contents. (a) Undoped; (b) 1 wt.%; (c,d) 2 wt.%; (e,f) 5 wt.%; (g,h) 7 wt.% (with clusters); (i) EDS.
The fluorine (F) and aluminum (Al) elements are also observed between the layers from Figure 1i. This means that AlF3 has entered the interlayer of the carbon anode. When the AlF3 content reaches 7 wt.%, as shown in the red circle in Figure 1h, clusters begin to appear in the interlayer.
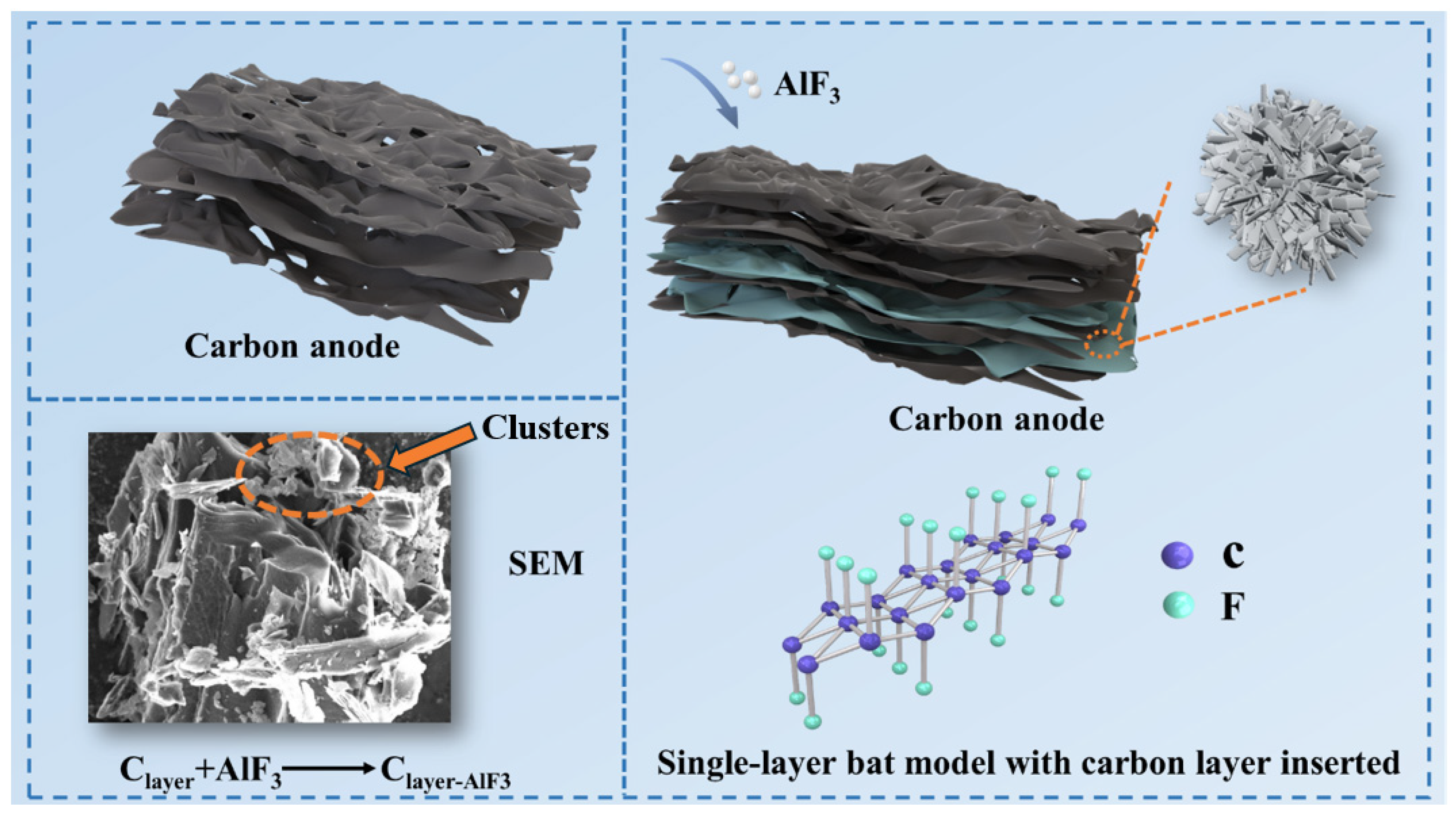
When AlF3 is added to carbon anodes, AlF3 can interact with the layered structure of carbon, especially at high temperatures, where fluoride ions in AlF3 may interact with the interlayer forces of the carbon material to insert into the carbon layers [28,29]. When a large number of F atom enters the intercalation layer and aggregates, clusters form between the layers, as shown in the red circle in Figure 2. Figure 2 also shows the single-layer ball-and-stick model of F inserted into the carbon layer. All valence bonds of the C atom in the model are involved in bonding, and there are no lone electrons or unsaturated bonds that are not involved in bonding [30,31], which improves its chemical stability and thus is favorable for the improvement of anti-oxidation properties.
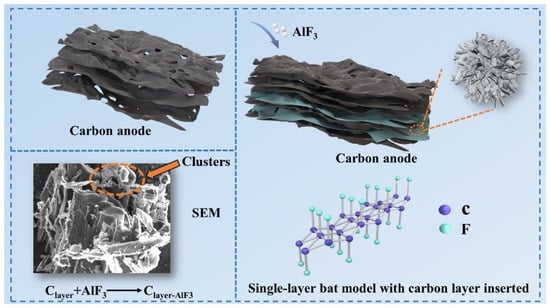
Figure 2.
Internal structure of carbon anode doped with 7 wt.% AlF3.
2.2. Effect of AlF3 on Crystalline Microstructure of Carbon Anodes
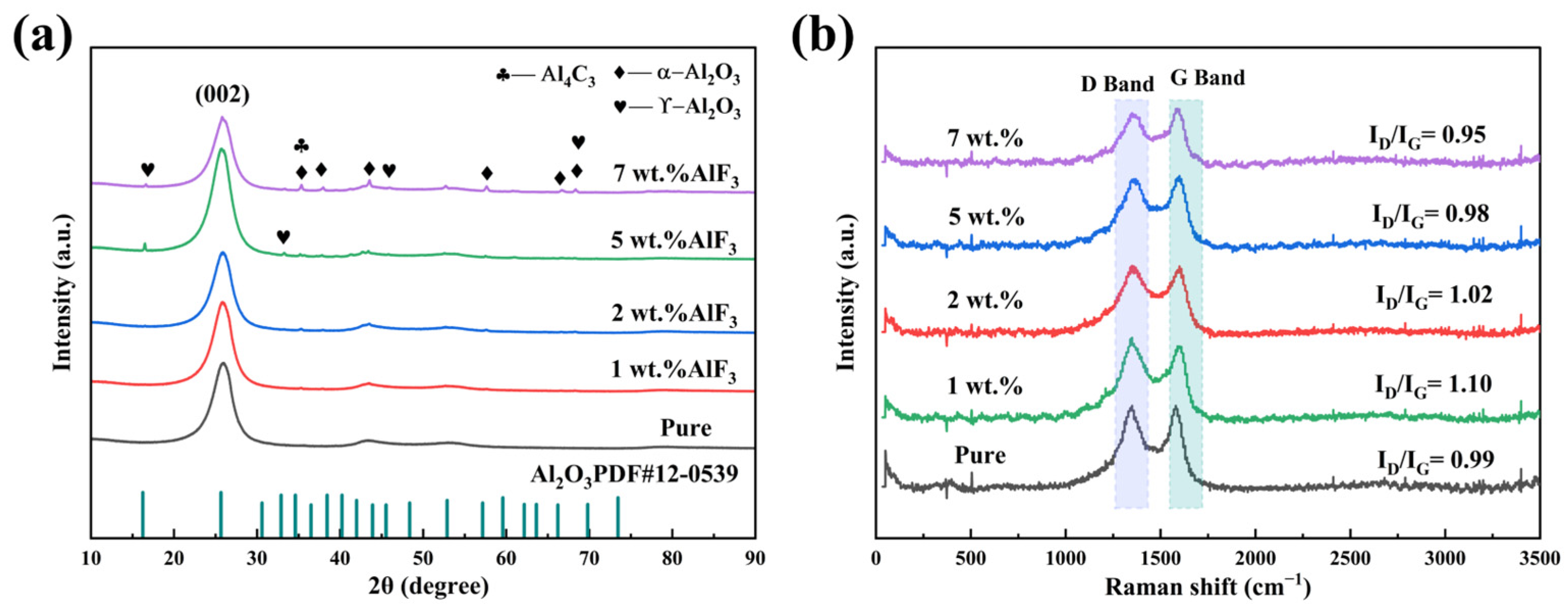
Figure 3 shows the XRD diffraction patterns of carbon anodes doped with varying AlF3 contents (1 wt.%, 2 wt.%, 5 wt.%, and 7 wt.%). As shown in Figure 3a, the presence of Al2O3 in the carbon anode is confirmed by the appearance of characteristic peaks representing the crystalline phase. Al2O3 mainly exists in the form of α-Al2O3 and γ-Al2O3, where α-Al2O3 is the most stable high-temperature phase, which is usually formed at high temperatures. Its main diffraction peaks are located at 35.1°, 43.3°, 57.4°, and 66.5°, corresponding to the crystal planes (104), (110), (113), and (116), respectively. γ-Al2O3 is a common amorphous or metastable form, which is more stable at low temperatures. Its diffraction peaks are generally around 17°, 32.2°, 45.9°, and 67.5°, corresponding to the crystal planes (111), (220), (311), and (400), respectively.
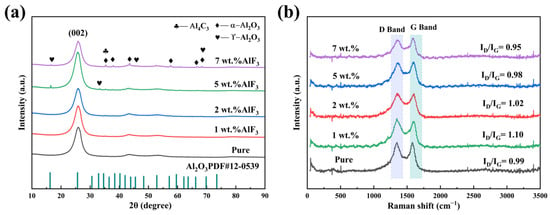
Figure 3.
(a) XRD pattern and (b) Raman pattern of carbon anode doped with varying AlF3 contents.
The microcrystalline parameters of the carbon anode were calculated using Bragg’s equation, as shown in Table 1. As the AlF3 content increases, the layer spacing (d002) increases and then decreases. At 5 wt.% AlF3 doping, the layer spacing reaches 0.3470 nm. Beyond this threshold, when the AlF3 content exceeds 5 wt.%, the crystallite size (LC) increases significantly. This is attributed to the higher AlF3 content, which enhances the intercalation density of F atoms within the carbon anode. This intercalation induces the structural reorganization or expansion of the layered structure, subsequently influencing the grain size of the carbon material [28]. At 7 wt.% AlF3 doping, the number of layers rises to 24.3459, while the layer spacing decreases to 0.3448 nm. At this doping level, the full width at half maximum (FWHM) of the diffraction peak reaches its minimum value. Additionally, as shown in Figure 3a, the XRD diffraction peaks exhibit increased intensity and sharper profiles, suggesting that higher AlF3 content improves the atomic arrangement within the carbon anode and reduces the presence of amorphous or disordered phases. This indicates enhanced crystalline ordering of the carbon anode at this concentration [32].

Table 1.
Microcrystalline parameters of carbon anode doped with varying AlF3 contents.
Raman spectroscopy analysis is shown in Figure 3b. It reveals that the ID/IG ratio initially increased and then decreased with varying AlF3 doping content. At 1 wt.% AlF3 doping, the ID/IG ratio reached 1.10, which may be attributed to the intercalation of a small amount of AlF3 disrupting the ordering of the carbon crystal lattice. This disruption likely impedes the transformation of amorphous carbon into a graphite structure. However, the ID/IG ratio decreased to 0.95 at 7 wt.% AlF3 doping content. This reduction could be linked to the incorporation of fluorine (F) into the carbon layers. The intercalation effect of AlF3 may enhance the stability of the crystal structure during graphitization, promoting the formation of larger graphite size particles at high temperatures within the carbon anode [30,33].
2.3. Effect of AlF3 on Oxidation Resistance Properties of Carbon Anodes
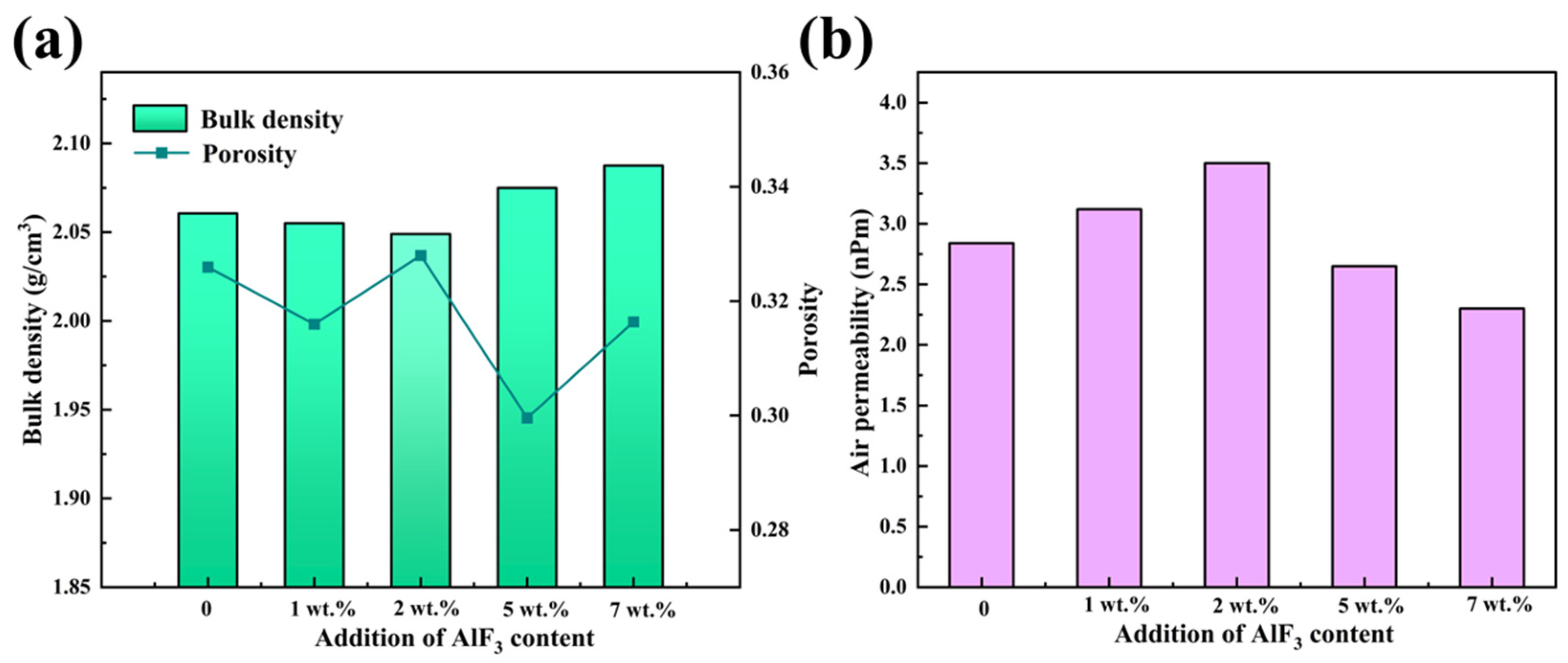
Figure 4 illustrates the bulk density and air permeability of carbon anodes doped with varying AlF3 contents (1 wt.%, 2 wt.%, 5 wt.%, and 7 wt.%). The slight decrease in bulk density observed at lower AlF3 concentrations can be attributed to the fact that small amounts of AlF3 may react with the carbon, causing volume expansion or contraction. Furthermore, the bonding between AlF3 and the carbon may not fully fill the voids, resulting in an increase in the number of localized pores. At high temperatures, AlF3 is also prone to react with moisture, oxides, or other elements in the carbon, releasing gases (e.g., fluorine gas or other volatiles). The release of these gases can cause an increase in the pores within the carbon anode, thereby reducing its bulk density.
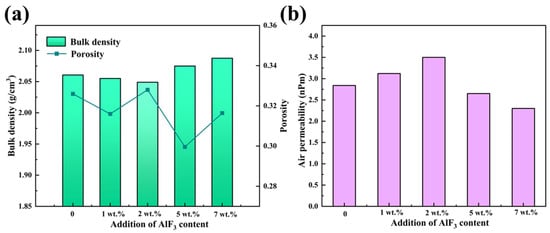
Figure 4.
Bulk density and porosity (a) and air permeability (b) of carbon anode doped with varying AlF3 contents.
However, combined with XRD analysis, it can be seen from Figure 4a that with the increase of AlF3 doping content, the bulk density of the carbon anode is improved due to the formation of stable α-Al2O3 [34,35]. In addition, AlF3 itself has a high density (2.5–3.0 g/cm3), which can fill the voids inside the carbon anode through intercalation, thereby improving the density of the carbon anode. Compared with the density of an ordinary carbon anode, which is 1.5–1.8 g/cm3, when the carbon anode contains 7 wt.% AlF3, the bulk density reaches 2.08 g/cm3 and the porosity is 0.315.
According to the permeability test results, when the content of AlF3 is 7 wt.%, the permeability reaches the minimum value of 2.3 nPm, as shown in Figure 4b. This is due to the fact that the higher AlF3 content leads to the formation of a large number of stable α-Al2O3 phase particles, which improves the surface densification of the carbon anode and reduces the air permeability. In addition, the high content of AlF3 forms a saturated F-C structure in the intercalation layer [36], which improves the internal stability of the carbon anode and enhances its oxidation resistance.
2.4. Effect of AlF3 on Resistance Properties of Carbon Anodes
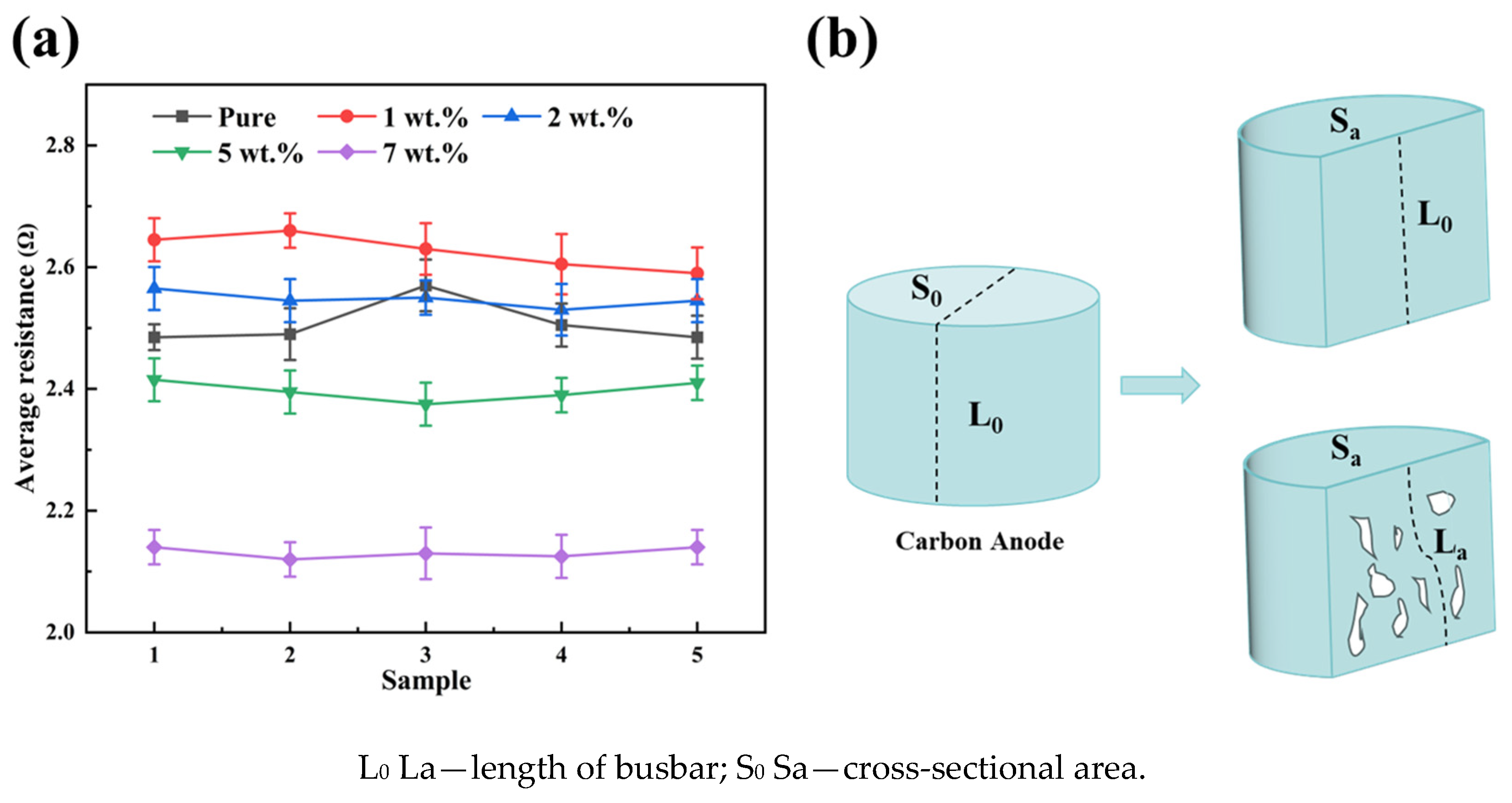
Figure 5a shows the average resistance values of carbon anodes doped with varying AlF3 contents (1 wt.%, 2 wt.%, 5 wt.%, and 7 wt.%). With the increase in AlF3 content, the average resistance value of the carbon anode increases and then decreases. When the content of AlF3 is 1 wt.%, the average resistance value reaches the maximum value (2.65 Ω). This is due to the fact that AlF3 dispersion in the carbon anode affects the arrangement and binding of carbon, thus increasing the barrier for the carbon anode current to pass through, leading to a rise in resistance. AlF3 in the carbon anode also does not interact strongly enough with C and may not be able to sufficiently form a conductive path. And there are more disordered carbon regions or defects in the carbon anode, resulting in limited electron conduction and high resistance.
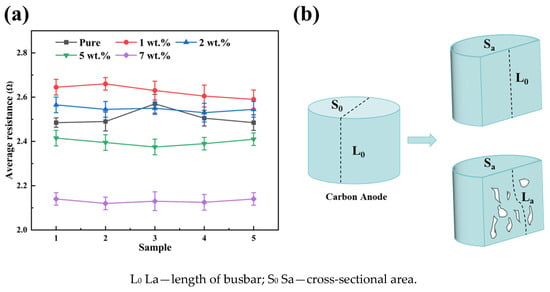
Figure 5.
(a) Average resistance of carbon anode doped with varying AlF3 contents and (b) schematic diagram of effect of pores on resistance of carbon anodes.
At an AlF3 content of 7 wt.%, the average resistance decreases to 2.12 Ω. This reduction is attributed to the high AlF3 concentration, which significantly enhances the sintering and bonding of carbon particles, fostering closer interparticle connections and forming a more efficient conductive pathway [37]. As illustrated in Figure 5b, the increased density of the carbon anode reduces pore space, shortening the effective path length for current flow and consequently lowering resistance [13]. Furthermore, the elevated AlF3 content ensures a relatively uniform distribution within the carbon anode, improving ion diffusion. The resulting F-C structure intercalates between carbon layers [38], forming clusters that serve as conductive bridges. This process reduces defects in the carbon material, enhances the ordering of the crystal lattice, and ultimately improves overall conductivity.
3. Materials and Methods
3.1. Experimental Materials and Preparation
Calcined coke (MC Zhenjiang Anode Solutions Co., Ltd., Zhenjiang, China) as the raw material and coal pitch (MC Zhenjiang Anode Solutions Co., Ltd., Zhenjiang, China) as the binder were used to prepare the carbon anode through the steps of batching, mixing and kneading, molding, and sintering. The ratio of calcined coke was coarse coke/medium coke/fine coke/powder = 26:31:9:34. The particle sizes of coarse coke, medium coke, fine coke, and powder were 3–6 mm, 1–3 mm, 0.074–1 mm, and less than 0.074 mm, respectively. The amount of powdered coal pitch added through a 60-mesh (0.25 mm) sieve was 15.3 wt.% of the total raw material. Different contents (1 wt.%, 2 wt.%, 5 wt.%, and 7 wt.%) of AlF3 were taken as additives for mixing and kneading with the raw material.
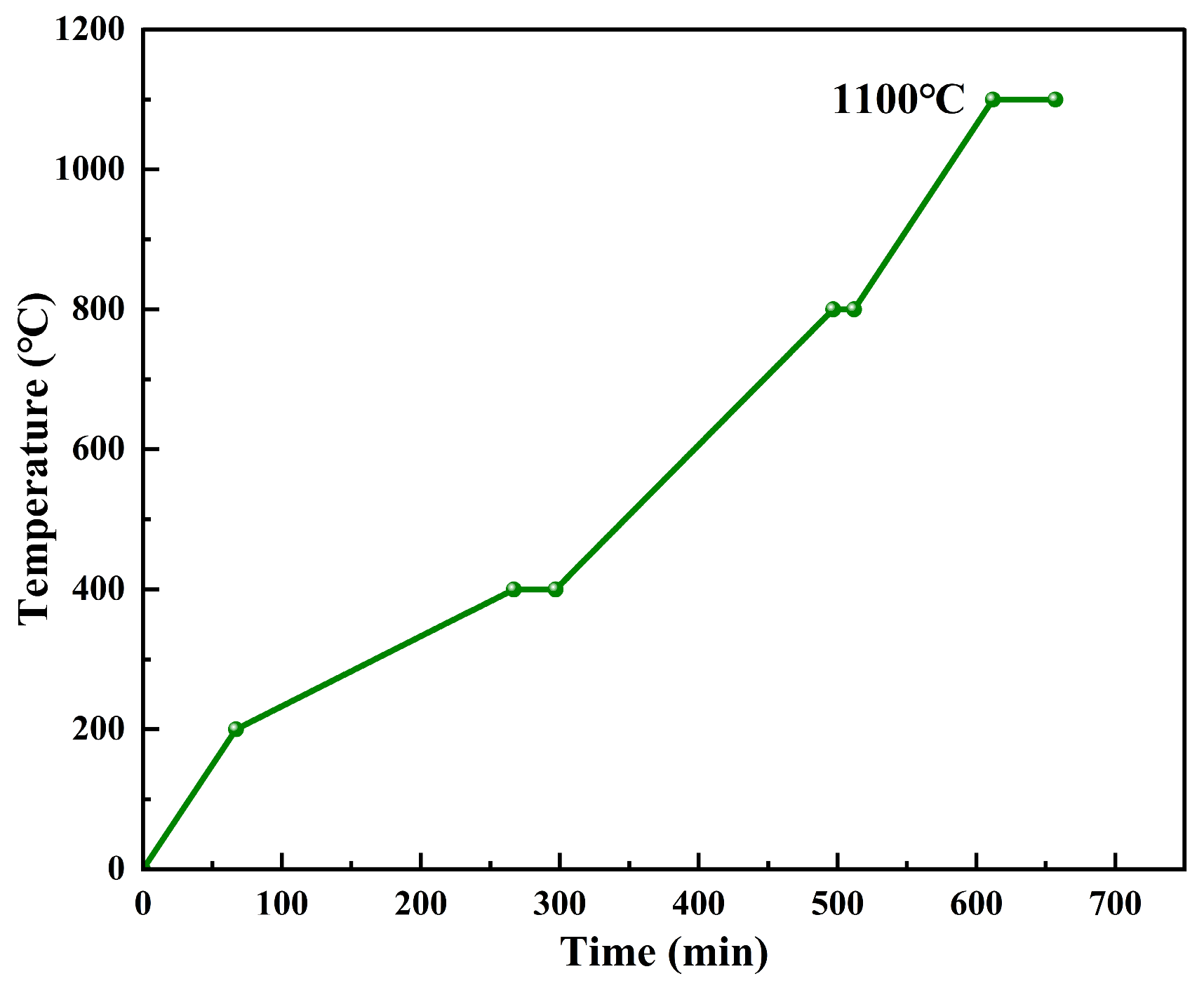
The calcined coke and coal pitch were dried and mixed at 120 °C for 30 min to form a mixture. A total of 65 g of the mixture was weighed and placed into a mold preheated to 130 °C. The mixture was then molded into green anodes with dimensions Φ50 × 20 mm using a hydraulic press. The green anode was placed in a crucible, which was buried in the metallurgical coke((MC Zhenjiang Anode Solutions Co., Ltd., Zhenjiang, China) to ensure that the carbon anode was completely isolated from the atmosphere. The sintering temperature and keeping time were carried out according to Figure 6. As shown in the figure, the temperature was rapidly raised to 200 °C and then slowly rose to 400 °C at a heating rate of 1 °C/min. After 30 min of heat preservation, the temperature was heated to 800 °C at 2 °C/min. After 15 min of heat preservation, the temperature was heated to 1100 °C for 30 min.
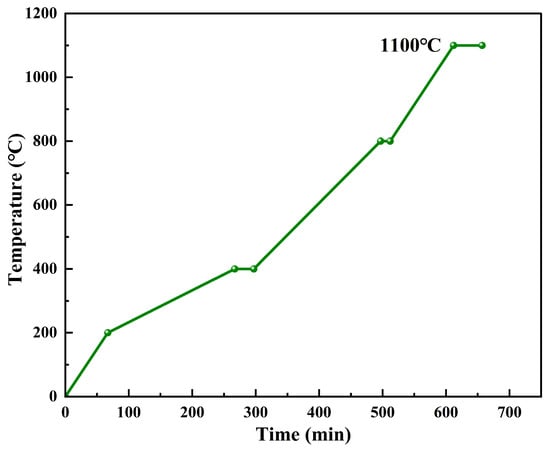
Figure 6.
Sintering process.
3.2. Characterization Method
The micromorphology of the carbon anodes was observed with a field emission scanning electron microscope (NovaNanoSEM450, FEI, Portland, OR, USA), the air permeability of the carbon anode samples was characterized by an air permeability tester (TD-ISPC-27, Beijing Tongde Entrepreneurship Technology Co., Ltd., Beijing, China), and their phase structure and crystallinity were analyzed using an X-ray diffractometer (D8 Advance, BRUKER-AXS, Karlsruhe, Germany). The layer spacing, layer stacking height, and number of stacked layers of carbon anodes were calculated by Bragg’s Equations (3), (4), and (5), respectively [39,40,41].
where λ is the X-ray wavelength (0.15418 nm); θ002 denotes the diffraction angle (°) corresponding to the maximum diffraction peak of the (002) crystalline surface and d002 characterizes the spacing of the laminae (nm); LC is the height of the lamellae stacking (nm); and N denotes the number of stacked layers.
The internal structure of carbon anodes was analyzed by a laser microscopy Raman spectrometer (Thermo Fisher DXR, Scientific, Waltham, MA, USA), typically using ID/IG to characterize the degree of graphitization of the carbon material. And the samples were treated in a vacuum drying oven (DZF-6020, Shanghai Jiecheng Experimental Instrument Co., Ltd., Shanghai, China); then, the bulk density and porosity of the carbon anodes were determined by the Archimedes drainage method, in which the bulk density was calculated according to Equation (6) and the porosity was calculated according to Equation (7).
where m1 represents the dry weight when the mass is weighed, m2 represents the suspended weight after submerging the sample in water for 30 min, and m3 refers to the wet weight weighed directly after removing it from the water.
The electrical resistance of carbon anodes was tested by using a multimeter. The resistance of the specimens was tested five times, and the average value was taken as the average resistance of the specimens.
4. Conclusions
This study demonstrates that AlF3 as an additive enhances the oxidation resistance of carbon anodes by improving their microstructure, bulk density, and crystallinity. At 7 wt.% AlF3 doping, optimal performance of the AlF3-modified carbon anode was achieved, including increased bulk density (2.08 g/cm3), reduced porosity (0.315), and minimized air permeability (2.3 nPm). The intercalation of fluorine into carbon layers promoted graphitization, stabilized the crystal structure, and lowered electrical resistance (2.12 Ω) by forming conductive pathways. This innovative approach exhibits remarkable potential for industrial implementation due to its demonstrated ability to significantly improve oxidation resistance and electrochemical performance. Particularly noteworthy is its capacity to optimize anode composition, leading to substantial reductions in energy consumption. These advantages collectively present an environmentally favorable solution for mitigating the ecological footprint of aluminum electrolysis processes.
Author Contributions
Methodology, F.B.; Validation, Y.Z. and J.Y.; Investigation, G.X., F.B. and Y.Z.; Resources, J.Y.; Data curation, F.B.; Writing—original draft, G.X.; Writing—review & editing, C.C.; Supervision, Y.D.; Project administration, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 52465005), the funder was Youming Zhang. The research was also funded by MC Zhenjiang Anode Solutions Co., Ltd. (HX20221031), and the funder was Guifang Xu.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Yonggang Ding and Jianhua Yin were employed by the MC Zhenjiang Anode Solutions Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fang, X.; Fei, X.; Wang, K.; Fang, T.; Chen, R. A novel SVD-UKFNN algorithm for predicting current efficiency of aluminum electrolysis. Sci. Rep. 2025, 15, 9173. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Irshad, A.; Margenot, A.; Zamanian, K.; Li, N.; Ullah, S.; Kuzyakov, Y. Inorganic carbon is overlooked in global soil carbon research: A bibliometric analysis. Geoderma 2024, 443, 116831. [Google Scholar] [CrossRef]
- Liang, Z. Selecting the proper material for a grain loss sensor based on DEM simulation and structure optimization to improve monitoring ability. Precis. Agric. 2021, 22, 1120–1133. [Google Scholar] [CrossRef]
- Zhou, C.; Okonkwo, C.E.; Inyinbor, A.A.; Yagoub, A.E.A.; Olaniran, A.F. Ultrasound, infrared and its assisted technology, a promising tool in physical food processing: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2023, 63, 1587–1611. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.J.; Li, G.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Guan, W.; Guo, W.; Chen, F.; Han, X.; Wang, H.; Sun, W.; Zhao, Q.; Jia, D.; Wei, X.; Zhu, Q. Multi-Span Greenhouse Energy Saving by External Insulation: System Design and Implementation. Agriculture 2024, 14, 281. [Google Scholar] [CrossRef]
- Kallummal, M.; Gupta, A.K.; Khosla, S. Estimating Carbon Emission Intensity of Energy Intensive Firms: A Firm-Level Analyses. In FDI, MSMEs, Digitalization, and Green Industrialization: Challenges, Opportunities and Policy Lessons for India; Springer: Singapore, 2025; pp. 251–276. [Google Scholar]
- Kvande, H.; Saevarsdottir, G.; Welch, B. Decarbonizing the primary aluminum industry: Opportunities and challenges. Light Met. Age 2023, 81, 38–45. [Google Scholar]
- Zhang, Y.J.; He, P.Y.; Chen, H.; Liu, L.C. Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst. Materials 2018, 11, 1773. [Google Scholar] [CrossRef]
- Han, J.; Zhu, W.; Chen, C. Identifying Emissions Reduction Opportunities in International Bilateral Emissions Trading Systems to Achieve China’s Energy Sector NDCs. Int. J. Environ. Res. Public Health 2023, 20, 1332. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Chahardeh, A.; Mollaabbasi, R.; Picard, D.; Taghavi, S.M.; Alamdari, H. Discrete Element Method Modeling for the Failure Analysis of Dry Mono-Size Coke Aggregates. Materials 2021, 14, 2174. [Google Scholar] [CrossRef]
- Jiang, P.; Tian, Y.; Wang, B.; Guo, C. Design and analysis of centrifugal compressor in carbon dioxide heat pump system. Sci. Rep. 2024, 14, 5286. [Google Scholar] [CrossRef]
- Tkac, M.; Foosnæs, T.; Øye, H.A. Effect of vacuum vibroforming on porosity development during anode baking. Light Met. 2007, 2007, 885–890. [Google Scholar] [CrossRef]
- Morozov, Y.A.; Yalunin, V.S. Inert anode technology in the concept of green aluminum metallurgy. RUDN J. Eng. Res. 2022, 23, 15–22. [Google Scholar]
- Amara, B.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D.; Côté, J.; Gilbert, A. Partial Replacement of Petroleum Coke with Modified Biocoke During Production of Anodes Used in the Aluminum Industry: Effect of Additive Type. Appl. Sci. 2022, 12, 3426. [Google Scholar] [CrossRef]
- Gao, Z.; Cao, X.; Peng, J.; Li, Y.; Wang, Y.; Di, Y. Effects of vacuum-distilled carbon residue on the reactivity of carbon anode. Chin. J. Eng. 2024, 46, 230–238. [Google Scholar] [CrossRef]
- Lu, Y.; Hussein, A.; Lauzon-Gauthier, J.; Ollevier, T.; Alamdari, H. Biochar as an additive to modify biopitch binder for carbon anodes. ACS Sustain. Chem. Eng. 2021, 9, 12406–12414. [Google Scholar] [CrossRef]
- Altharan, Y.M.; Shamsudin, S.; Al-Alimi, S.; Saif, Y.; Zhou, W. A review on solid-state recycling of aluminum machining chips and their morphology effect on recycled part quality. Heliyon 2024, 10, e34433. [Google Scholar] [CrossRef]
- Ren, Y.J.; Sun, Z.; Quan, G.G. Effect of Aluminium Powder Additives upon CO2 Reactivity of Electrolytic Aluminium Carbon Anode with Calcined Anthracite. Adv. Mater. Res. 2014, 852, 44–50. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Hou, D.; Zhao, W.; Li, X.; Qu, T.; Zhu, Q. Research on the preparation parameters and basic properties of premelted calcium aluminate slag prepared from secondary aluminum dross. Materials 2021, 14, 5855. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Preger, Y.; Burroughs, H.; Sun, C.; Ohodnicki, P.R. Fiber optic sensing technologies for battery management systems and energy storage applications. Sensors 2021, 21, 1397. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Qu, X.D.; Zhou, J.M. Coupled heat/mass-balance model for analyzing correlation between excess AlF3 concentration and aluminum electrolyte temperature. Trans. Nonferrous Met. Soc. China 2009, 19, 724–729. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, T.; Xiong, Z.; Yuan, L.; Hong, H.; Gao, R.; Bao, Y. Oxidation affects dye binding of myofibrillar proteins via alteration in net charges mediated by a reduction in isoelectric point. Food Res. Int. 2023, 163, 112204. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Cui, Q.; Gu, S.; Liu, K.; Cai, Y.; Su, Y.; Zhang, D.; Ouyang, Q. Effect of reinforcement volume fraction and T6 heat treatment on microstructure, thermal and mechanical properties of mesophase pitch-based carbon fiber reinforced aluminum matrix composites. Mater. Sci. Eng. A 2022, 834, 142469. [Google Scholar] [CrossRef]
- Hulse, K.L. Understanding the Relationships Between Raw Material Characteristics, Formulation and Processing Parameters in the Manufacture of Green Carbon Anodes; R & D Carbon Limited: Granges, Switzerland, 2000. [Google Scholar]
- Sun, Y.; Wei, X.; Zhang, W.; Wang, Z.; Jiang, J.; Liu, F.; Liu, B.; Han, W. Carbon-Interacted AlF3 Clusters as Robust Catalyst for Dehydrofluorination Reaction with Enhanced Undercoordination and Stability. ACS Catal. 2024, 14, 2358–2368. [Google Scholar] [CrossRef]
- Guo, C.; Yang, H.; Naveed, A.; Nuli, Y.; Yang, J.; Cao, Y.; Yang, H.; Wang, J. AlF3-Modified carbon nanofibers as a multifunctional 3D interlayer for stable lithium metal anodes. Chem. Commun. 2018, 54, 8347–8350. [Google Scholar] [CrossRef]
- Yoshida, K.; Sugawara, Y.; Saitoh, M.; Matsumoto, K.; Hagiwara, R.; Matsuo, Y.; Kuwabara, A.; Ukyo, Y.; Ikuhara, Y. Microscopic characterization of the C–F bonds in fluorine—Graphite intercalation compounds. J. Power Sources 2020, 445, 227320. [Google Scholar] [CrossRef]
- Ahrweiler, E.; Schoetz, M.D.; Singh, G.; Bindschaedler, Q.P.; Sorroche, A.; Schoenebeck, F. Triply Selective & Sequential Diversification at Csp3: Expansion of Alkyl Germane Reactivity for C−C & C−Heteroatom Bond Formation. Angew. Chem. 2024, 136, e202401545. [Google Scholar] [CrossRef]
- Rodríguez, S.J.; Candia, A.E.; Stankovic, I.; Passeggi Jr, M.C.; Ruano, G.D. Study of in-plane and interlayer interactions during aluminum fluoride intercalation in graphite: Implications for the development of rechargeable batteries. ACS Appl. Nano Mater. 2023, 6, 16977–16985. [Google Scholar] [CrossRef]
- Luo, K.; Wang, D.; Chen, D.; Zhong, Y.; Zheng, Z.; Wang, G.; Liu, Y.; Zhong, B.; Wu, Z.; Guo, X. Solid electrolyte interphase composition regulation via coating AlF3 for a high-performance hard carbon anode in sodium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 8242–8251. [Google Scholar] [CrossRef]
- Riello, D.; Zetterström, C.; Parr, C.; Braulio, M.; Moreira, M.; Gallo, J.; Pandolfelli, V. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram. Int. 2016, 42, 9804–9814. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhu, L.; Qi, H.; Fu, M. Effect of hydrated aluminium fluoride on phase transformation and morphology evolution of alumina. J. Aust. Ceram. Soc. 2025, 61, 321–331. [Google Scholar] [CrossRef]
- Wang, T.; Zang, X.; Wang, X.; Gu, X.; Shao, Q.; Cao, N. Recent advances in fluorine-doped/fluorinated carbon-based materials for supercapacitors. Energy Storage Mater. 2020, 30, 367–384. [Google Scholar] [CrossRef]
- Wei, X.; Jia, Z.; Wang, C.; Yu, H.; Wu, S.; Liu, B.; Han, W.; Lu, C. Under-coordinated AlF3 clusters confined in carbon matrix with robust sintering resistance for dehydrofluorination of hydrofluorocarbons. Chem. Eng. J. 2022, 431, 134178. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Zhang, Y.; Wu, S.; Jamil, S.; Niu, Y.; Qin, L.; Xu, M. In Situ Generation of AlF3 in Nanoporous Carbon to Enable Cathode–Electrolyte Interface Construction for Stable Li–Se Batteries. ACS Appl. Nano Mater. 2023, 6, 5414–5421. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.H.; Kim, W.S.; Roh, J.S. The Effect of the Heating Rate during Carbonization on the Porosity, Strength, and Electrical Resistivity of Graphite Blocks Using Phenolic Resin as a Binder. Materials 2022, 15, 3259. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H. Graphene-like BN@ SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mariod, A.A.; Wiliam, T. Rapid determination of antioxidant compounds and antioxidant activity of Sudanese Karkade (Hibiscus sabdariffa L.) using near infrared spectroscopy. Food Anal. Methods 2016, 9, 1228–1236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).