Insight into the Oxygen-Sensing Mechanisms of TiO2–CeO2 Mixed Oxides Treated in a High-Energy Ball Mill: An XPS Analysis
Abstract
1. Introduction
2. Results
2.1. Particle Size Distribution (PSD) Analysis of Powders
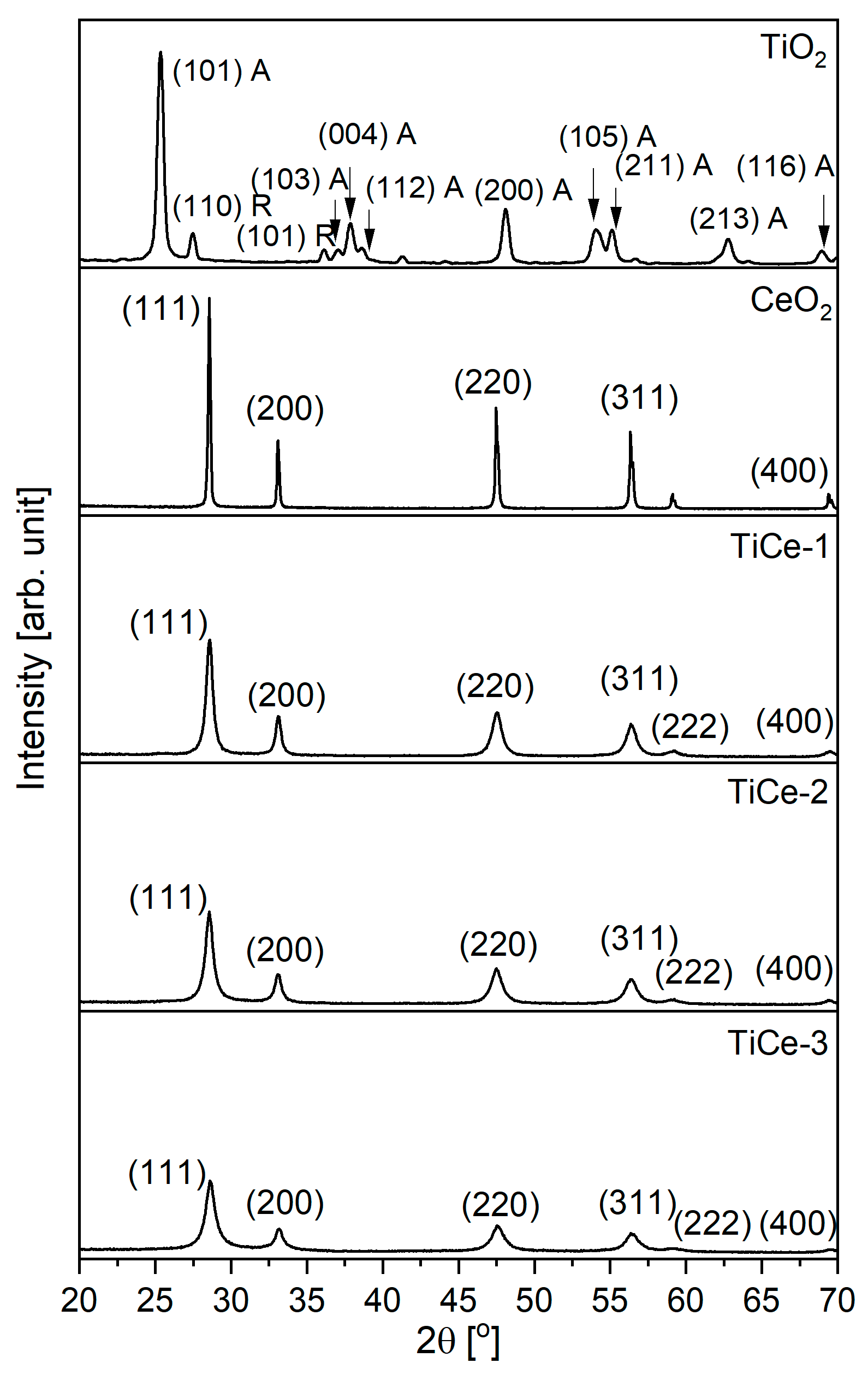
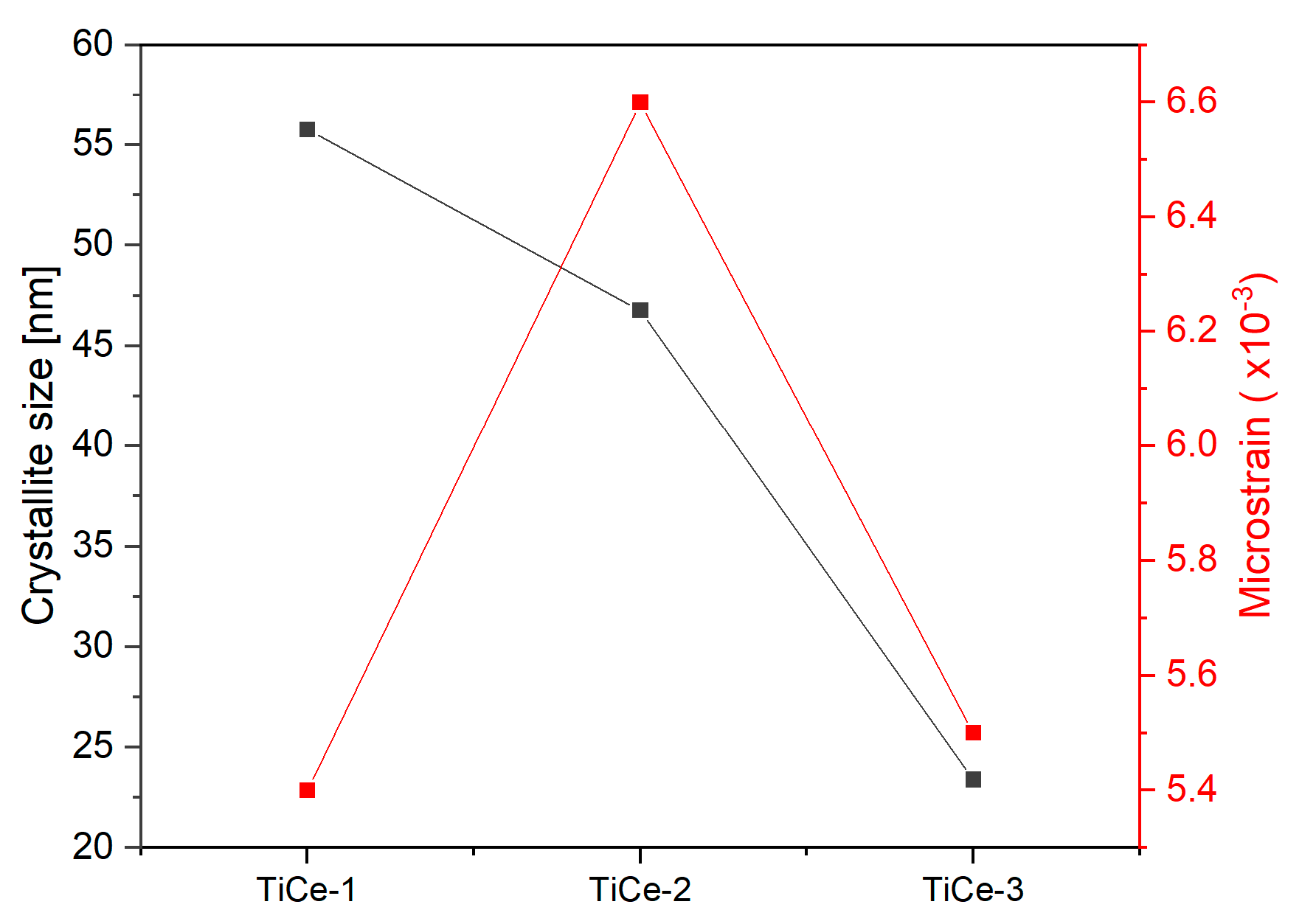
2.2. X-Ray Powder Diffraction (XRPD)
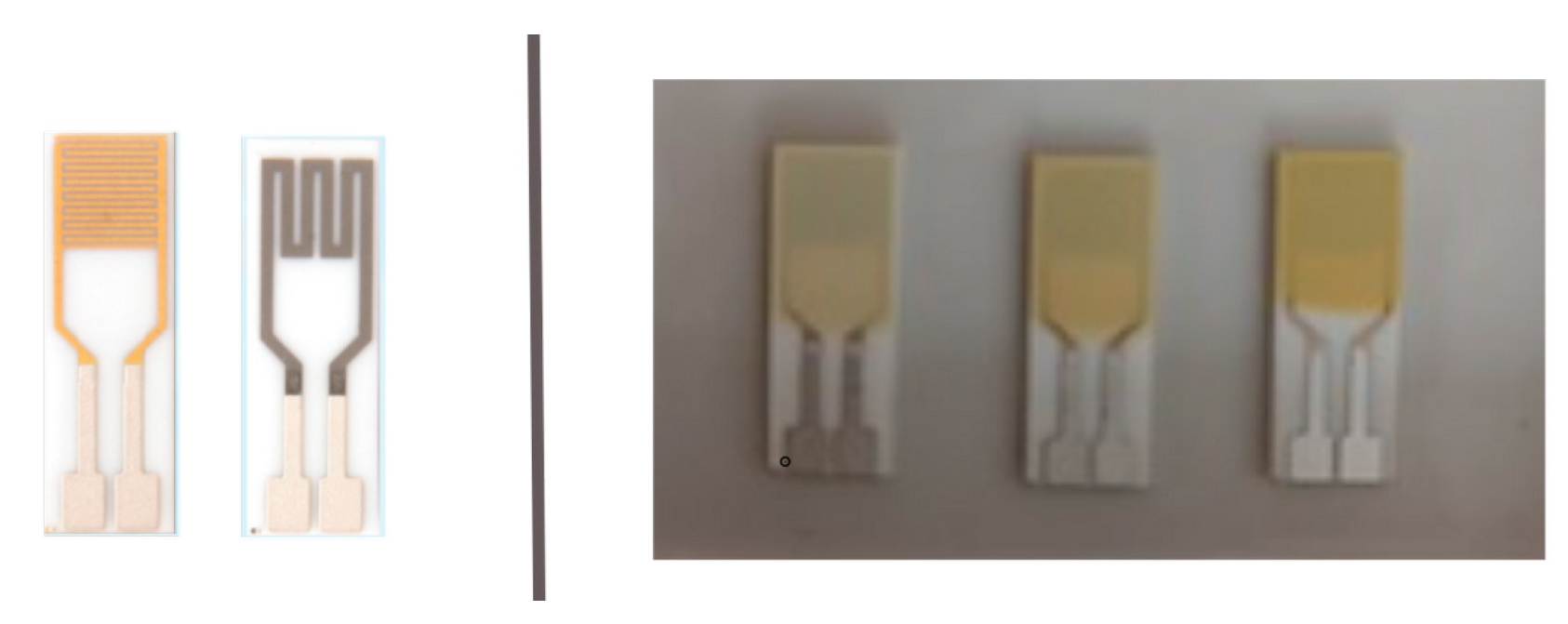
2.3. Two-Dimensional Profilometry of Sensing Layers
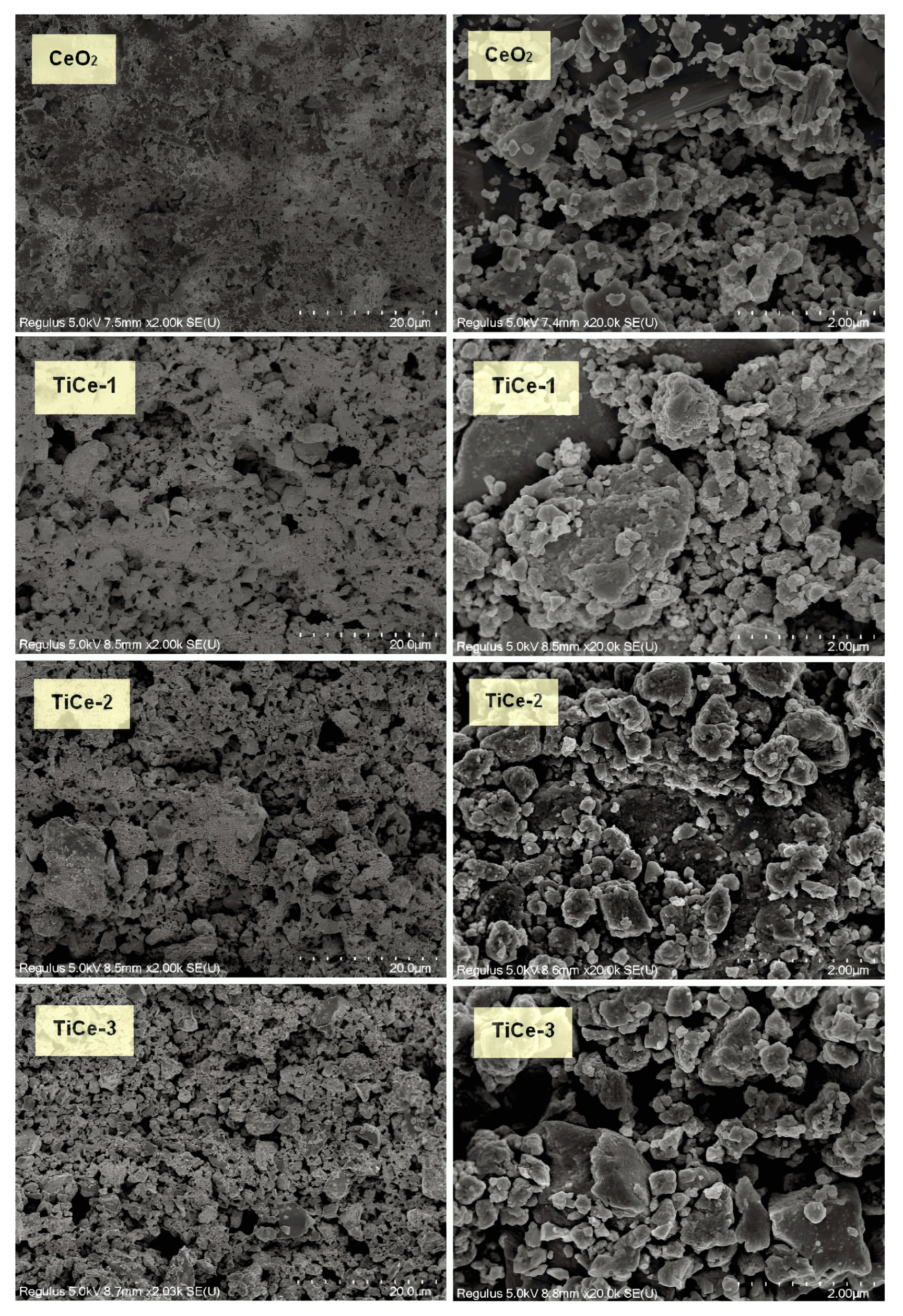
2.4. Scanning Electron Spectroscopy (SEM) and Energy Dispersive Spectroscopy (EDS) of Sensing Layer
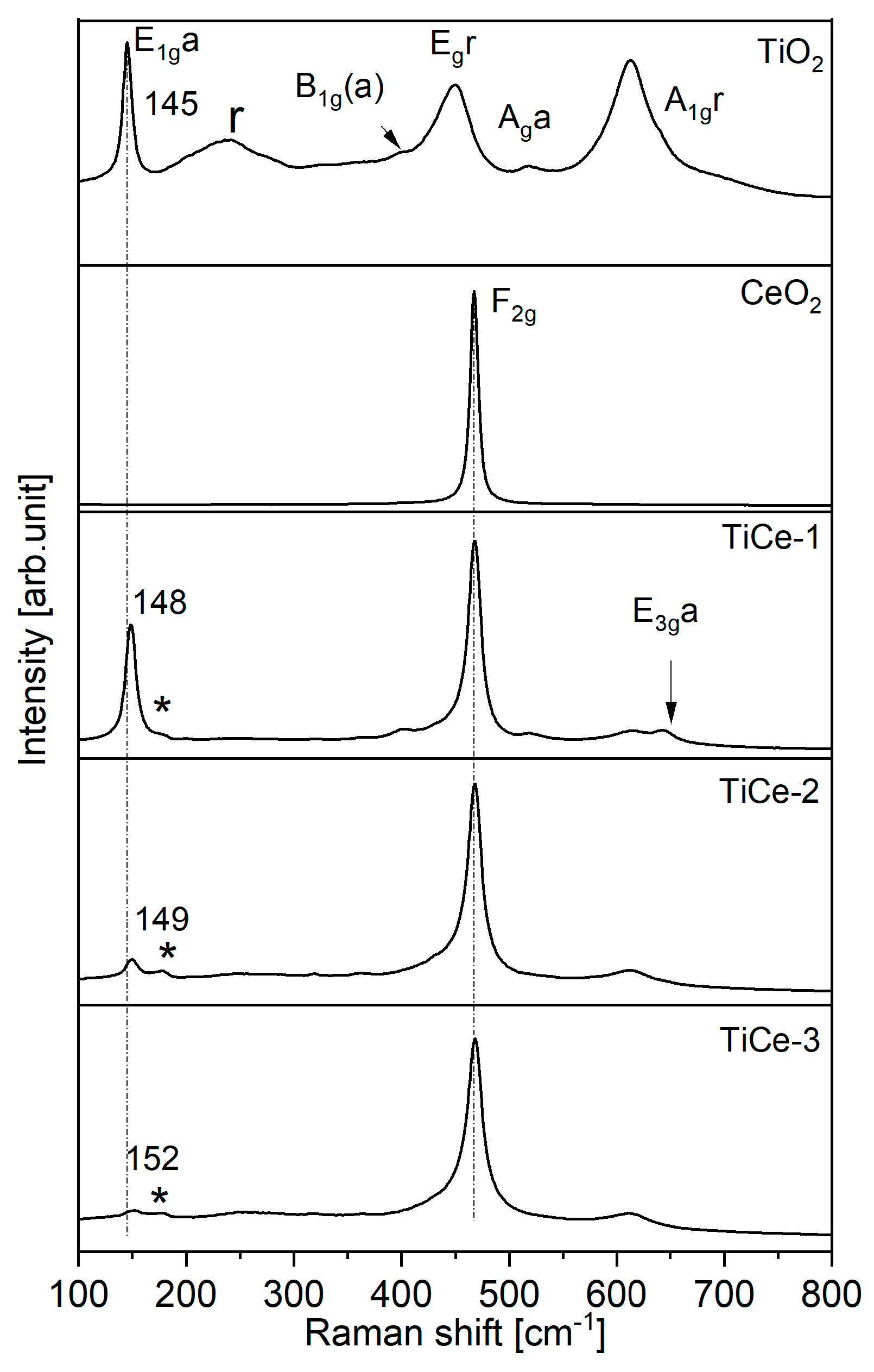
2.5. Raman Spectroscopy of Sensing Layers
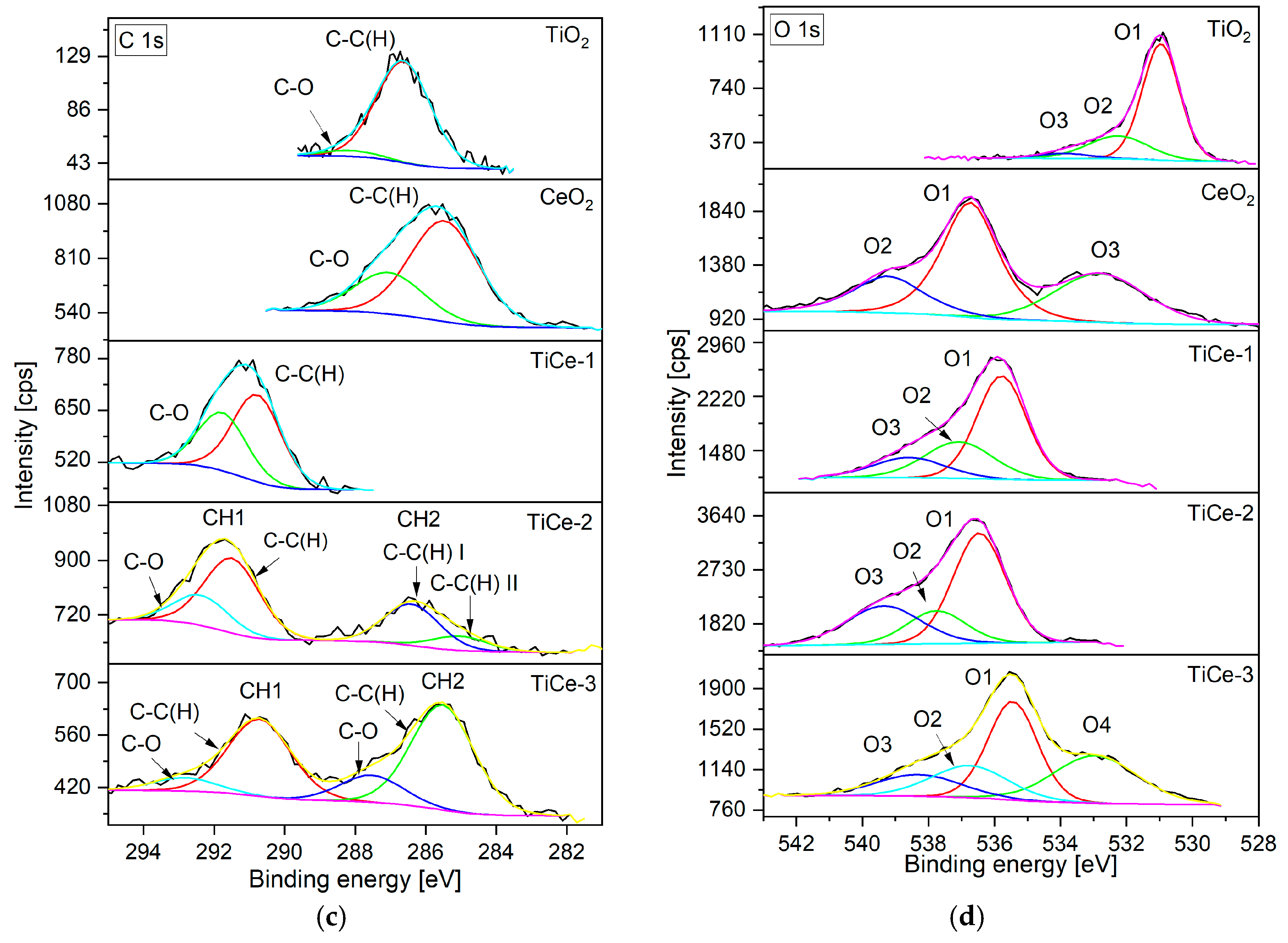
2.6. X-Ray Photoelectron Spectroscopy (XPS) of Sensing Layers
2.6.1. TiO2 Sample
2.6.2. CeO2 Sample
2.6.3. TiCe-1 Sample
2.6.4. TiCe-2 Sample
2.6.5. TiCe-3 Sample
2.6.6. XPS Quantitative Analysis
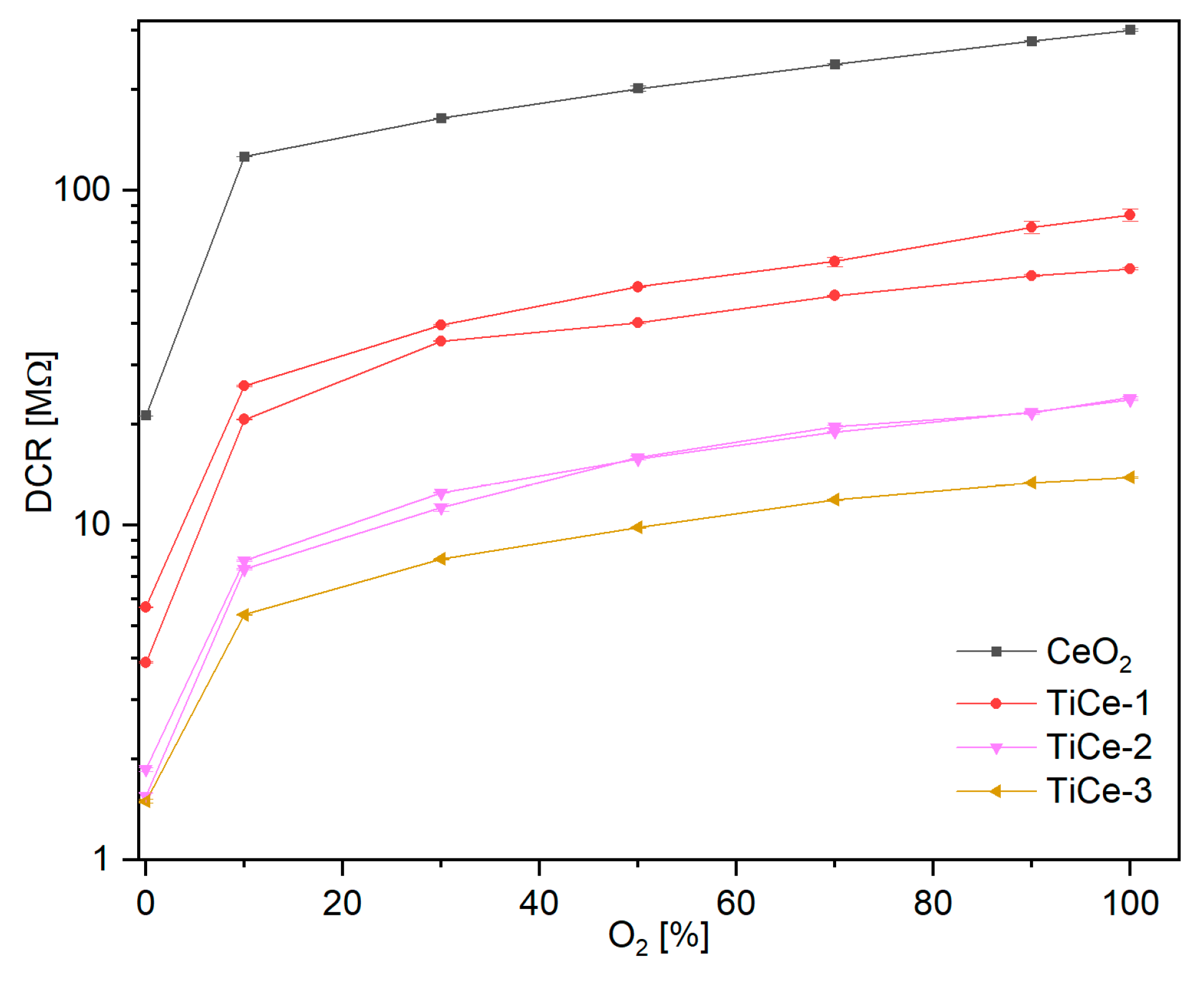
2.7. Results of Electrical Measurements
3. Discussion
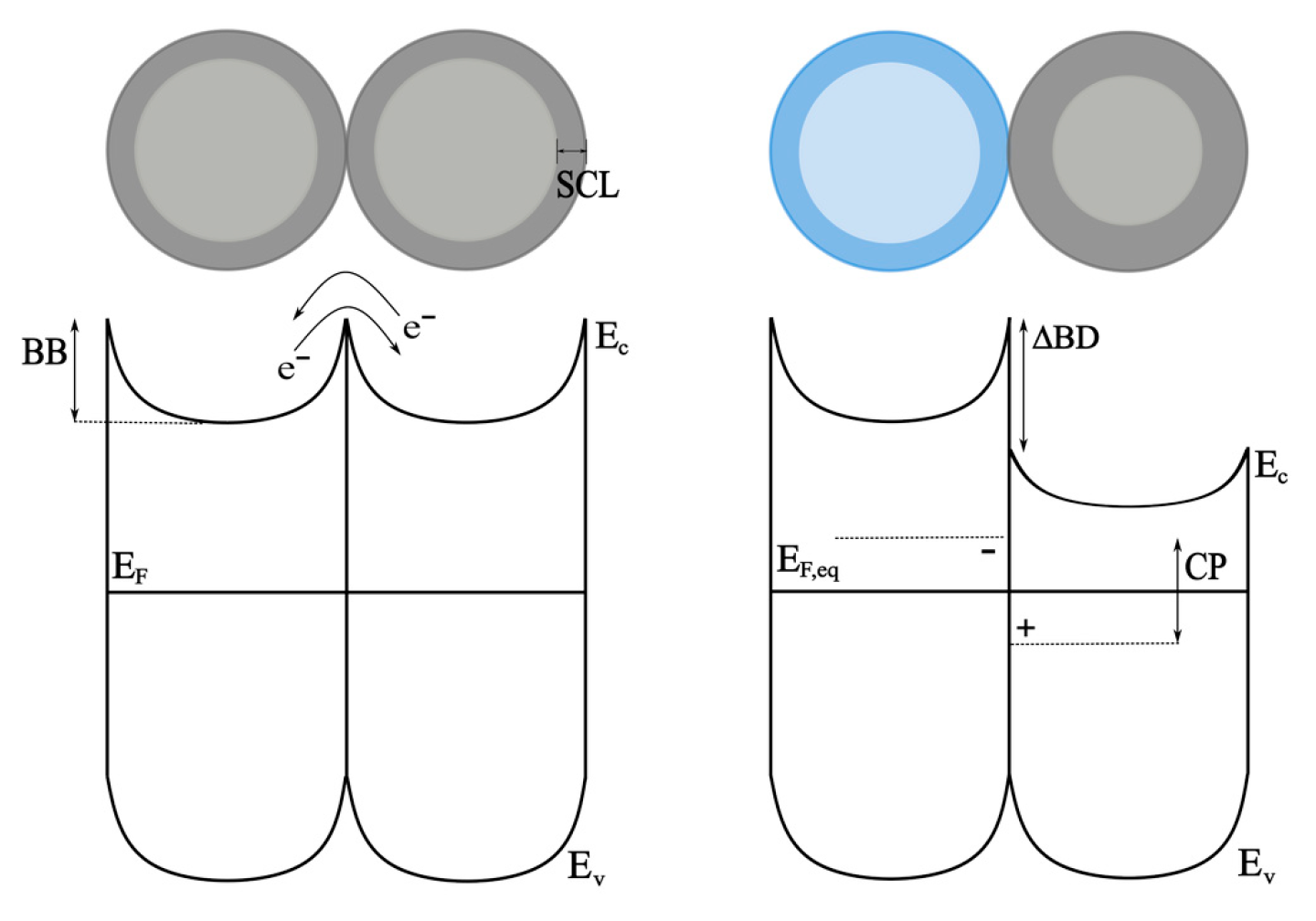
4. Materials and Methods
4.1. High-Energy Ball Milling
4.2. Paste Preparation and Sensor Fabrication
4.3. Characterization of Powders
4.4. Characterization of Sensing Material
4.5. Sensor Response Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekereci, Y.; Petrovskii, S. Global Warming Can Lead to Depletion of Oxygen by Disrupting Phytoplankton Photosynthesis: A Mathematical Modelling Approach. Geosciences 2018, 8, 201. [Google Scholar] [CrossRef]
- Manandhar, S.; Batu, A.K.; Deveraj, A.; Shuttanandan, V.; Thevuthasan, S.; Ramana, C.V. Rapid response high temperature oxygen sensor based on titanium doped gallium oxide. Sci. Rep. 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Willett, M. Oxygen sensing for industrial safety—Evolution and new approaches. Sensors 2014, 14, 6084–6103. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensor: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2022, 20, 6694. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Nakagawa, K.; Murata, Y.; Kishida, M.; Adachi, M.; Hiro, M.; Susa, K. Formation and reaction activity of CeO2 nanoparticles of cubic structure and various shaped CeO2–TiO2 composite nanostructures. Mater. Chem. Phys. 2007, 104, 30–39. [Google Scholar] [CrossRef]
- Liu, C.; Zang, W.; Pan, J.; Qian, J.; Wang, Y.; Chen, F.; Zhou, Y.; Song, Y. Low temperature CO oxidation on cerium dioxide nanorods. Mater. Res. Express 2019, 6, 075060. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles. RSC Adv. 2014, 4, 4663–4671. [Google Scholar] [CrossRef]
- Fang, J.; Bi, X.; Si, D.; Jiang, Z.; Huang, W. Spectroscopic studies of interfacial structures of CeO2-TiO2 mixed oxides. Appl. Surf. Sci. 2007, 253, 8952–8961. [Google Scholar] [CrossRef]
- Kortocenkov, G. Metal oxides for solid state gas sensors: What determines our choice. Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. Influences of semiconductor metal oxide properties on gas sensing characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Luo, S.; Nguyen-Phan, T.; Johnston-Peck, A.C.; Barrio, L.; Sallis, S.; Arena, D.A.; Kundu, S.; Xu, W.; Piper, L.F.J.; Stach, E.A.; et al. Hierarchical heterogeneity at the CeOx-TiO2 Interface: Electronic and geometric structural influence on the photocatalytic activity of oxide on oxide nanostructures. J. Phys. Chem. C 2019, 119, 2669–2679. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.; Song, C. Characterization of structural and surface properties of nanocrystalline TiO2-CeO2 mixed oxides by XRD, XPS, TPR, and TPD. J. Phys. Chem. C 2009, 113, 14249–14257. [Google Scholar] [CrossRef]
- Zeng, Y.; Haw, K.G.; Wang, Y.; Zhang, S.; Wang, Z.; Zhong, Q.; Kawi, S. Recent progress of CeO2-TiO2 based catalysts for selective catalytic reduction of NOx by NH3. ChemCatChem 2021, 13, 491–505. [Google Scholar] [CrossRef]
- Gionco, C.; Giamello, E.; Mino, L.; Paganini, M.C. The interaction of oxygen with the surface of CeO2-TiO2 mixed systems: An example of fully reversible surface-to-molecule electron transfer. Phys. Chem. Chem. Phys. 2014, 16, 21438–21445. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the preparation method on the physicochemical properties and the CO oxidation performance of nanostructured CeO2/TiO2 Oxides. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Rožić, L.; Petrović, S.; Lončarević, D.; Grbić, B.; Radić, N.; Stojadinović, S.; Jović, V.; Lamovec, J. Influence of annealing temperature on structural, optical and photocatalytic properties of TiO2-CeO2 nanopowders. Ceram. Int. 2019, 45, 2361–2367. [Google Scholar] [CrossRef]
- Petrović, S.; Rožić, L.; Jović, V.; Stojadinović, S.; Grbić, B.; Radić, N.; Lamovec, J.; Vasilic, R. Optimization of a nanoparticle ball milling process parameters using the response surface method. Adv. Powder. Technol. 2018, 29, 2129–2139. [Google Scholar] [CrossRef]
- Petrović, S.; Rožić, L.; Grbić, B.; Radić, N.; Stefanov, P.; Stojadinović, S.; Jović, V.; Lamovec, J. Effect of high energy ball milling on the physicochemical properties of TiO2-CeO2 mixed oxide and its photocatalytic behavior in the oxidation reaction. React. Kinet. Mech. Catal. 2019, 127, 175–186. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660. [Google Scholar] [CrossRef] [PubMed]
- Furlani, E.; Aneggi, E.; Leitenburg, C.; Maschio, S. High energy ball milling of titania and titania-ceria powder mixtures. Powder Technol. 2014, 254, 591–596. [Google Scholar] [CrossRef]
- Leonardi, M.; Villacampa, M.; Menendez, J.C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042–2064. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Frišćić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical synthesis of catalytic materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Szczesniak, B.; Choma, J.; Jaroniec, M. Recent advances in mechanochemical synthesis of mesoporous metal oxides. Mater. Adv. 2021, 2, 2510–2523. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. (Ed.) The history and necessity of mechanical alloying. In Mechanical Alloying, 2nd ed.; William Andrew Publishing: New York, NY, USA, 2015; p. 23. [Google Scholar]
- Stevanović, J.N.; Petrović, S.P.; Tadić, N.B.; Cvetanović, K.; Silva, A.G.; Radović, D.V.; Sarajlić, M. Mechanochemical synthesis of TiO2-CeO2 mixed oxides utilized as a screen-printed sensing material for oxygen sensor. Sensors 2023, 23, 1313. [Google Scholar] [CrossRef]
- Balanč, B.; Trifković, K.; Đorđević, V.; Marković, S.; Pjanović, R.; Nedović, V.; Bugarski, B. Novel resveratrol delivery systems based on alginate-sucrose and alginate-chitosan microbeads containing liposomes. Food Hydrocoll. 2016, 61, 832–842. [Google Scholar] [CrossRef]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Decker, K.D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef]
- Baláž, M. Ball milling of eggshell waste as a green and sustainable approach. Adv. Colloid. Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, M.A.; Cadús, L.E.; Morales, M.R. Tailoring materials by high-energy ball milling: TiO2 mixtures for catalyst support application. Mater. Today Chem. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Yuesong, S.; Dahai, Z.; Bo, Y.; Songbo, N.; Shemin, Z. Synergetic catalyst of ceria and titania for selective reduction of NO. J. Rare Earths 2012, 30, 431–436. [Google Scholar] [CrossRef]
- Nath, A.K.; Jiten, C.; Chandramani Singh, K. Influence of ball milling parameters on the particle size of barium titanate nanocrystalline powders. Phys. B Condens. 2010, 405, 430–434. [Google Scholar] [CrossRef]
- Singh, P.; Abhash, A.; Yadav, B.N.; Shafeeq, M.; Singh, I.B.; Mondal, D.P. Effect of milling time on powder characteristics and mechanical performance of Ti4wt%Al alloy. Powder Technol. 2018, 342, 275–287. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Zhang, X.; Gao, M.; Pan, H. Role of particle size, grain size, microstrain and lattice distortion in improved dehydrogenation properties of the ball-milled Mg(AlH4)2. Int. J. Hydrogen Energy 2013, 38, 1460–1468. [Google Scholar] [CrossRef]
- Stevanovic, J.; Silva, A.C.; Bundaleski, N.; Vasiljevic-Radovic, D.; Sarajlic, M.; Teodoro, O.M.N.D.; Petrovic, S. Oxygen Sensitivity of Titania-Ceria Mixtures Treated in a High-Energy Ball Mill: Potential for Improved Detection. Mendeley Data, v3. Available online: https://data.mendeley.com/datasets/5wck88xj8g/3 (accessed on 17 July 2024).
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of iron oxide nanoparticles by mechanical milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Venkataraman, K.S.; Narayanan, K.S. Energetics of collision between grinding media in ball mills and mechanochemical effects. Powder Technol. 1998, 96, 190–201. [Google Scholar] [CrossRef]
- Rajamani, R.K.; Songfack, P.; Mishra, B.K. Impact energy spectra of tumbling mills. Powder Technol. 2000, 108, 116–121. [Google Scholar] [CrossRef]
- Dhara, S.; Giri, P.K. Size Dependent anisotropic strain and optical properties of strained Si nanocrystals. J. Nanosci. Nanotechnol. 2011, 11, 9215–9221. [Google Scholar] [CrossRef] [PubMed]
- Torres-Romero, A.; Cajero-Juárez, M.; Nuñez-Anita, R.E.; Contreras-García, M.E. Ceria-doped titania nanoparticles as drug delivery system. J. Nanosci. Nanotechnol. 2020, 20, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-Ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1956; pp. 259–295. [Google Scholar]
- Vieira, G.B.; Jose, H.J.; Peterson, M.; Baldissarelli, V.Z.; Alvarez, P.; Moreira, R.F.P.M.M. CeO2/TiO2 nanostructures enhance adsorption and photocatalytic degradation of organic compounds in aqueous suspension. J. Photochem. Photobiol. A 2018, 352, 325–336. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.C. Structural characterization of CeO2-TiO2 and V2O5/CeO2-TiO2 catalysts by Raman and XPS techniques. J. Phys. Chem. B 2003, 107, 5162–5167. [Google Scholar] [CrossRef]
- Baidya, T.; Gayen, A.; Hedge, M.S.; Ravishankar, N.; Dupont, L. Enhanced reducibility of Ce1x2212;xTixO2 compared to that of CeO2 and higher redox catalytic activity of Ce1x2212;xx2212;yTixPtyO2x2212;δ compared to that of Ce1−xPtxO2−δ. J. Phys. Chem. B 2006, 110, 5262–5272. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, F.; Liu, Y.; Guo, M.; Cheng, C.; Hou, J.; Miao, Y. Ti4+-incorporated fluorite-structured high-entropy oxide (Ce,Hf,Y,Pr,Gd)O2−δ: Optimizing preparation and CMAS corrosion behavior. J. Adv. Ceram. 2022, 11, 1801–1814. [Google Scholar] [CrossRef]
- Ramshanker, N.; Ganapathi, K.L.; Bhat, M.S.; Mohan, S. RF sputtered CeO2 thin films-based oxygen sensors. IEEE Sens. J. 2019, 19, 10821–10828. [Google Scholar] [CrossRef]
- Sakai, G.; Baik, N.S.; Miura, N.; Yamazoe, N. Gas sensing properties of tin oxide thin film fabricated from hydrothermally treated nanoparticles: Dependence of CO and H2 response of film thickness. Sens. Actuators B Chem. 2001, 77, 116–121. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Deka, K.; Nath, N.; Saikia, B.K.; Deb, P. Kinetic analysis of ceria nanoparticle catalyzed efficient biomass pyrolysis for obtaining high-quality bio-oil. J. Therm. Anal. Calorim. 2017, 130, 1875–1883. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Fleury, P.A.; Damen, T.C. Raman spectra of TiO2, MgF2, ZnF2, FeF2, and MnF2. Phys. Rev. 1967, 154, 522. [Google Scholar] [CrossRef]
- Robert, T.D.; Laude, L.D.; Geskin, V.M.; Lazzaroni, R.; Gouttebaron, R. Micro-Raman spectroscopy study of surface transformations induced by excimer laser irradiation of TiO2. Thin Solid Film. 2003, 440, 268–277. [Google Scholar] [CrossRef]
- Hardcastle, F.; Ishihara, H.; Sharma, R.; Boris, A.S. Photoelectroactivity and Raman spectroscopy of anodized titania (TiO2) photoactive water-splitting catalysts as a function of oxygen-annealing temperature. J. Mater. Chem. 2011, 21, 6337–6345. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.C. Structural characterization of CeO2-MO2 (M = Si4+, Ti4+, and Zr4+) mixed oxides by Raman spectroscopy, X-ray photoelectron spectroscopy, and other techniques. J. Phys. Chem. B 2003, 107, 11475–11484. [Google Scholar] [CrossRef]
- Siuzdak, K.; Sawczak, M.; Klein, M.; Nowaczyk, G.; Jurgac, S.; Ceniana, A. Preparation of platinum modified titanium dioxide nanoparticles with the use of laser ablation in water. Phys. Chem. Chem. Phys. 2014, 16, 15199–15206. [Google Scholar] [CrossRef]
- Nicol, M.; Fong, M.Y. Raman spectrum and polymorphism of titanium dioxide at high pressures. J. Chem. Phys. 1971, 54, 3167–3170. [Google Scholar] [CrossRef]
- Gajović, A.; Stubičar, M.; Ivanda, M.; Furić, K. Raman spectroscopy of ball-milled TiO2. J. Mol. Struct. 2001, 563–564, 315–320. [Google Scholar] [CrossRef]
- Begin-Colin, S.; Girot, T.; Mocellin, A.; Le Caër, G. Kinetics of formation of nanocrystalline TiO2 II by high energy ball-milling of anatase TiO2. Nanostruct. Mater. 1999, 12, 195–198. [Google Scholar] [CrossRef]
- Gyakwaa, F.; Aula, M.; Alatarvas, T.; Vuolio, T.; Shu, Q.; Huttula, M.; Fabritius, T. Application of Raman spectroscopy for characterizing synthetic non-metallic inclusions consisting of calcium sulphide and oxides. Appl. Sci. 2020, 10, 2113. [Google Scholar] [CrossRef]
- Ellouzi, I.; Oualid, H.A. Efficient and eco-friendly mechanical milling preparation of anatase/rutile TiO2-glucose composite with energy gap enhancement. Proceedings 2019, 3, 3. [Google Scholar] [CrossRef]
- Takacs, L.; McHenry, J.S. Temperature of the milling balls in shaker and planetary mills. J. Mater. Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- López, T.; Rojas, F.; Alexander-Katz, R.; Galindo, F.; Balankin, A.; Buljan, A. Porosity, structural and fractal study of sol-gel TiO2-CeO2 mixed oxides. J. Solid. State Chem. 2004, 177, 1873–1885. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, R.; Chen, L.; Du, J.; Tao, C.; Yang, F.; Dong, L. Selective catalytic reduction of NOx by NH3 over CeO2 supported on TiO2: Comparison of anatase, brookite, and rutile. Appl. Catal. B Environ. 2017, 208, 82–93. [Google Scholar] [CrossRef]
- Carter, J.H.; Shah, P.M.; Nowicka, E.; Freakley, S.J.; Morgan, D.J.; Golunski, S.; Hutchings, G.J. Enhanced activity and stability of gold/ceria-titania for the low temperature water-gas shift reaction. Front. Chem. Sec. Catal. Photocatal. 2019, 7, 443. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of structural transformations in nanophase titanium dioxide by Raman spectroscopy. Appl. Phys. A 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Rezaee, M.; Khoie, S.M.M.; Liu, K.H. The role of brookite in mechanical activation of anatase-to-rutile transformation of nanocrystalline TiO2: An XRD and Raman spectroscopy investigation. CrystEngComm 2011, 13, 5055–5061. [Google Scholar] [CrossRef]
- Dhara, S.; Giri, P.K. Size-dependent visible absorption and fast photoluminescence decay dynamics from freestanding strained silicon nanocrystals. Nanoscale Res. Lett. 2011, 6, 320. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, H.; Zhao, W.; Luan, Z.; Tian, X.; Tan, C.; Huang, Y. Coexistence of doping and strain to tune electronic and optical properties of GaN monolayer. Superlattices Microstruct. 2019, 130, 93–102. [Google Scholar] [CrossRef]
- Swamy, V. Size-dependent modifications of the first-order Raman spectra of nanostructured rutile TiO2. Phys. Rev. B 2008, 77, 195414. [Google Scholar] [CrossRef]
- Stamenković, T.; Bundaleski, N.; Barudžija, T.; Validžić, I.; Lojpur, V. XPS study of iodine and tin-doped Sb2S3 nanostructures affected by non-uniform charging. Appl. Surf. Sci. 2021, 567, 150822. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley Interscience: Chichester, UK, 1992. [Google Scholar]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G.J. Satellite Structure in the X-Ray Photoelectron Spectra of some Binary and Mixed Oxides of Lanthanum and Cerium. J. Chem. Soc. Dalton Trans. 1967, 17, 1686–1698. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifácio, V.D.B.; Varma, R.S.; Nogueira, I.D.; Bundaleski, N.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst—Applications in multicomponent reactions under benign condition. Green. Chem. 2013, 15, 1226–1231. [Google Scholar] [CrossRef]
- Han, Z.K.; Zhang, L.; Liu, M.; Ganduglia-Pirovano, M.V. The structure of oxygen vacancies in the near-surface of reduced CeO2 (111) under strain. Front. Chem. 2019, 7, 436. [Google Scholar] [CrossRef]
- Riemer, D.E. The Theoretical Fundamentals of the Screen Printing Process. Microelectron. Int. 1989, 6, 8–17. [Google Scholar] [CrossRef]
- Averardi, A.; Cola, C.; Zeltmann, S.E.; Gupta, N. Effect of particle size distribution on the packing ofpowder beds: A critical discussion relevant to additive manufacturing. Mater. Today Commun. 2018, 24, 75–78. [Google Scholar] [CrossRef]
- Spierings, A.B.; Herres, N.; Levy, G. Influence of the particle size distribution on surface quality andmechanical properties in AM steel parts. Rapid Prototyp. J. 2011, 17, 195–202. [Google Scholar] [CrossRef]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. Atom. Data Nucl. Data 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Effective Attenuation Length Database; Version 1.3, SRD 82; National Institute of Standards and Technology, NIST: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Seah, M.P.; Gilmore, I.S. Quantitative AES. VIII: Analysis of auger electron intensities from elementaldata in a digital auger database. Surf. Interface Anal. 1998, 26, 908–929. [Google Scholar] [CrossRef]
- Lewis, S.E.; De Boer, J.R.; Gole, J.L.; Hesketh, P.J. Sensitive, selective, and analytical improvements to a porous silicon gas sensor. Sens. Actuators B Chem. 2005, 110, 54–65. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Proposal of contact potential promoted oxide semiconductor gas sensor. Sens. Actuators B Chem. 2013, 187, 162–167. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Review: Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Basic approach to the transducer function of oxide semiconductor gas sensors. Sens. Actuators B Chem. 2011, 160, 1352–1362. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Péchy, P.; Grätze, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitized solar cells. Prog. Photovolt. Res. Appl. 2017, 15, 603–612. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef]
- Teodoro, O.M.N.D.; Silva, J.A.M.C.; Moutinho, A.M.C. Multitechnique surface analysis system: Apparatus description. Vacuum 1995, 46, 1205–1209. [Google Scholar] [CrossRef]
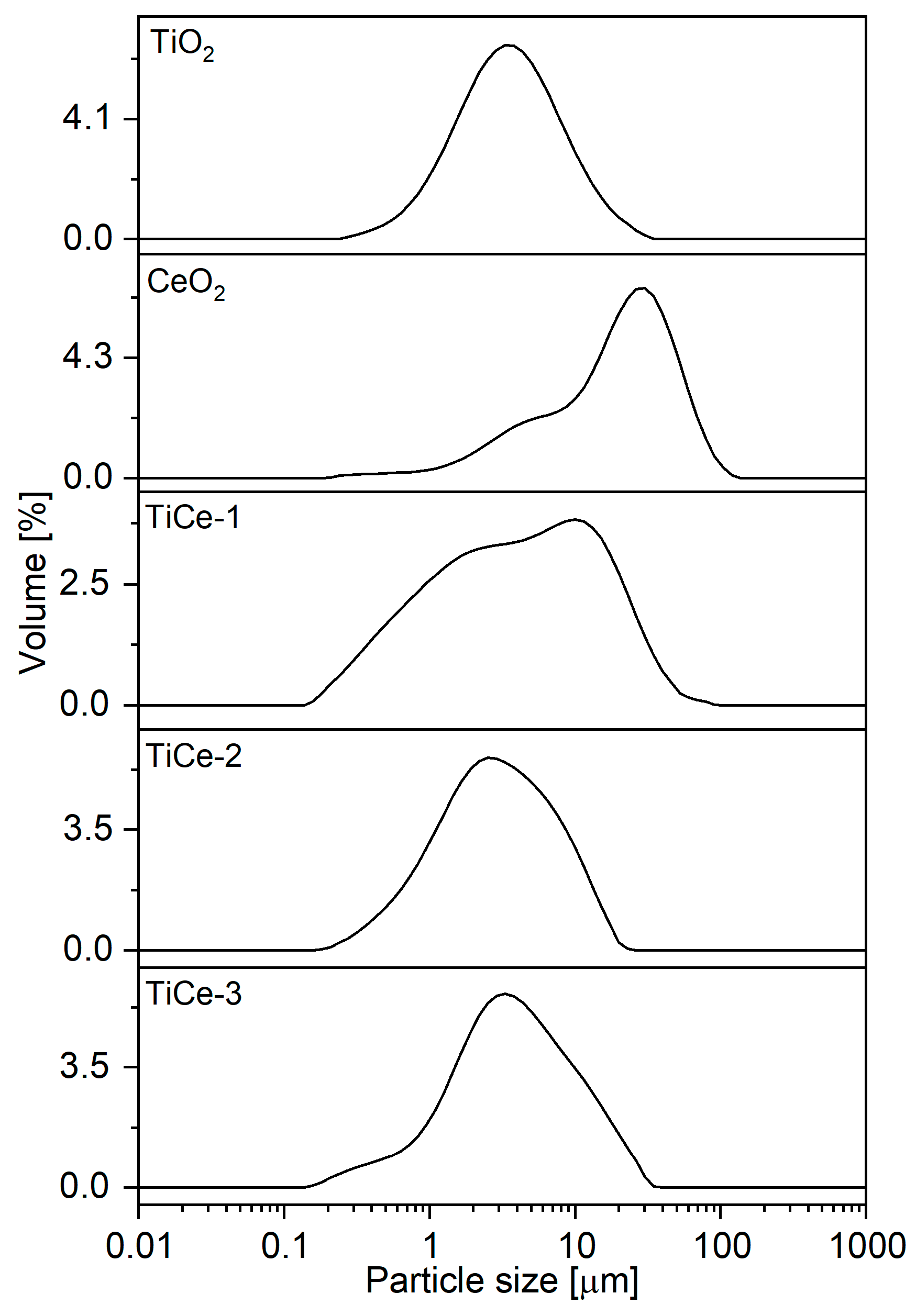


| Sample | d10 [µm] | d50 [µm] | d90 [µm] | Span |
|---|---|---|---|---|
| TiO2 | 1.25 | 3.70 | 10.61 | 2.53 |
| CeO2 | 3.80 | 22.58 | 55.81 | 2.30 |
| TiCe-1 | 0.67 | 4.46 | 21.06 | 4.57 |
| TiCe-2 | 0.75 | 2.64 | 8.53 | 2.94 |
| TiCe-3 | 0.90 | 3.375 | 12.01 | 3.29 |
| Sample | Peak Position [°] | d-Spacing [Å] |
|---|---|---|
| CeO2 | 28.55 | 3.124 |
| TiCe-1 | 28.54 | 3.125 |
| TiCe-2 | 28.53 | 3.126 |
| TiCe-3 | 28.61 | 3.117 |
| Sample | Ra [nm] | Rq [nm] | Thickness [μm] |
|---|---|---|---|
| TiCe-1 | 660 | 820 | ≈7.7 |
| TiCe-2 | 543 | 752 | ≈4.4 |
| TiCe-3 | 562 | 711 | ≈4.7 |
| Sample | Weight (%) | Ti:Ce Weight Ratio Expected/Calculated | ||||
|---|---|---|---|---|---|---|
| Ti | Ce | O | C | Al | ||
| TiCe-1 | 11.00 | 61.93 | 20.79 | 5.99 | 0.29 | 0.25/0.18 |
| TiCe-2 | 11.23 | 64.71 | 17.77 | 5.96 | 0.33 | 0.25/0.17 |
| TiCe-3 | 10.44 | 59.83 | 21.28 | 8.12 | 0.33 | 0.25/0.17 |
| Spectrum | Ia–Ir |
|---|---|
| TiO2 | 1.12 |
| TiCe-1 | 5.49 |
| TiCe-2 | 1.46 |
| TiCe-3 | 1.12 |
| Ti 2p | Ce 3d | C 1s | O 1s | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3/2 | v | v′ | v″ | v‴ | C-C(H) | C-O | O1 | O2 | O3 | O4 | |
| TiO2 | 460.1 | / | / | / | / | 286.6 | 288.1 | 531.0 | 532.3 | 533.9 | / |
| CeO2 | / | 889.5 | 891.9 | 896.3 | 905.5 | 285.5 | 287.0 | 536.7 | 539.2 | 532.8 | / |
| TiCe-1 | 464.7 | 888.6 | 890.6 | 895.4 | 904.6 | 290.8 | 291.8 | 535.8 | 537.1 | 538.6 | / |
| TiCe-2 | 465.3 | 889.2 | 891.2 | 896.0 | 905.2 | 291.5 (CH1) 286.4 (CH2 I) 285.0 (CH2 II) | 292.4 (CH1) | 536.5 | 537.8 | 539.3 | / |
| TiCe-3 | 464.4 | 888.4 | 890.6 | 895.2 | 907.0 | 290.7 (CH1) 285.5 (CH2) | 292.8 (CH1) 287.5 (CH2) | 535.4 | 536.7 | 538.3 | 533.0 |
| Sample | θTi:θCe [%] | d [nm] | O:Ti–O:Ce |
|---|---|---|---|
| TiO2 | 1.15 | O:Ti = 1.98 | |
| CeO2 | 2.73 | O:Ce = 2.52 | |
| TiCe-1 | 43:57 | 1.51 | O:Ce = 2.65 |
| TiCe-2 | 31.7:68.3 | 2.42 | O:Ce = 2.72 |
| TiCe-3 | 34.5:65.5 | 2.15 | O:Ce = 2.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevanović, J.N.; Silva, A.G.; Bundaleski, N.; Vasiljević-Radović, D.; Sarajlić, M.; Teodoro, O.M.N.D.; Petrović, S.P. Insight into the Oxygen-Sensing Mechanisms of TiO2–CeO2 Mixed Oxides Treated in a High-Energy Ball Mill: An XPS Analysis. Inorganics 2025, 13, 159. https://doi.org/10.3390/inorganics13050159
Stevanović JN, Silva AG, Bundaleski N, Vasiljević-Radović D, Sarajlić M, Teodoro OMND, Petrović SP. Insight into the Oxygen-Sensing Mechanisms of TiO2–CeO2 Mixed Oxides Treated in a High-Energy Ball Mill: An XPS Analysis. Inorganics. 2025; 13(5):159. https://doi.org/10.3390/inorganics13050159
Chicago/Turabian StyleStevanović, Jelena N., Ana G. Silva, Nenad Bundaleski, Dana Vasiljević-Radović, Milija Sarajlić, Orlando M. N. D. Teodoro, and Srđan P. Petrović. 2025. "Insight into the Oxygen-Sensing Mechanisms of TiO2–CeO2 Mixed Oxides Treated in a High-Energy Ball Mill: An XPS Analysis" Inorganics 13, no. 5: 159. https://doi.org/10.3390/inorganics13050159
APA StyleStevanović, J. N., Silva, A. G., Bundaleski, N., Vasiljević-Radović, D., Sarajlić, M., Teodoro, O. M. N. D., & Petrović, S. P. (2025). Insight into the Oxygen-Sensing Mechanisms of TiO2–CeO2 Mixed Oxides Treated in a High-Energy Ball Mill: An XPS Analysis. Inorganics, 13(5), 159. https://doi.org/10.3390/inorganics13050159










