Lanthanide Heterometallic MOFs with 5-Iodosophthalate Linkers: Tuning of Luminescent Properties by Varying of Metal Composition
Abstract
1. Introduction
2. Results and Discussion
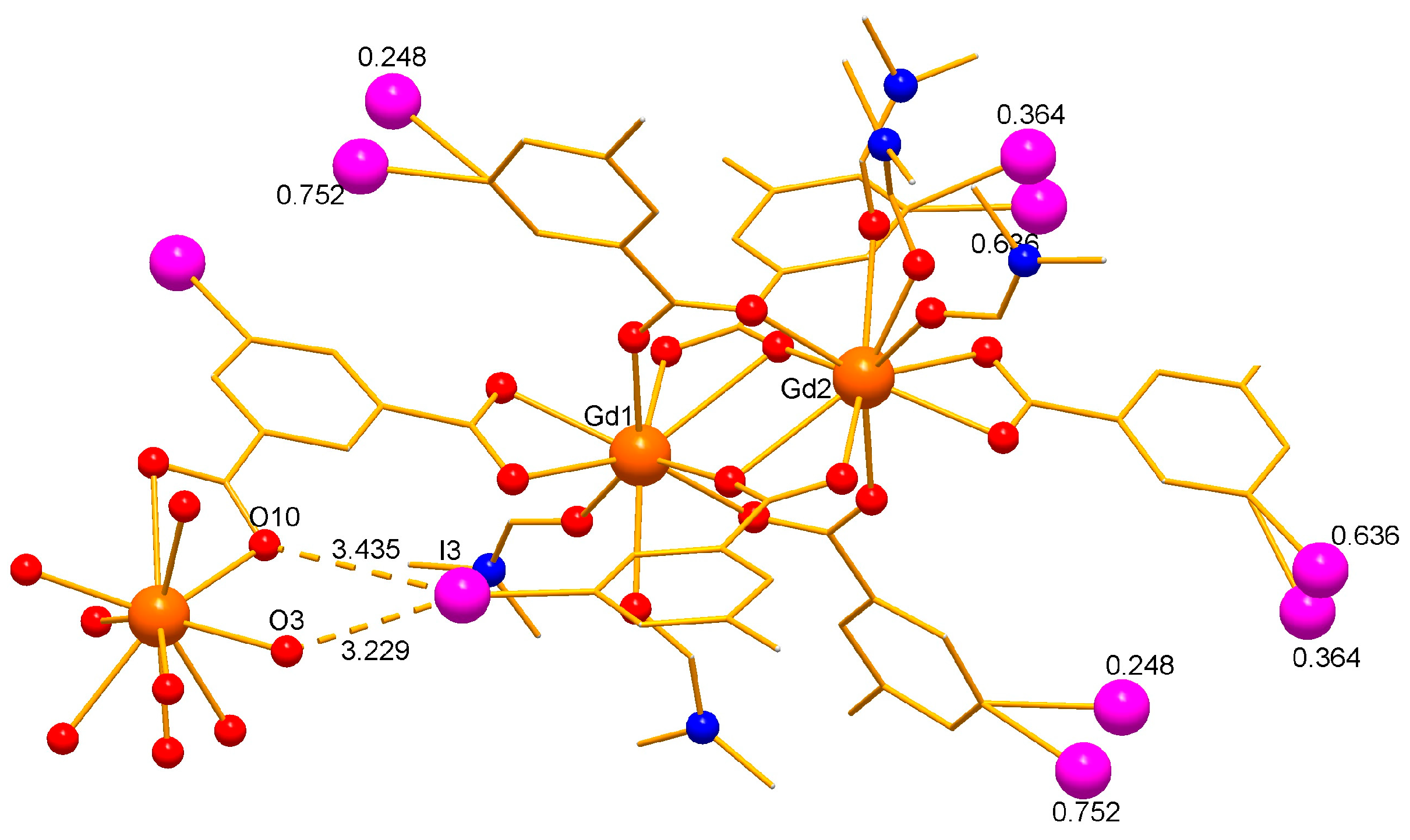
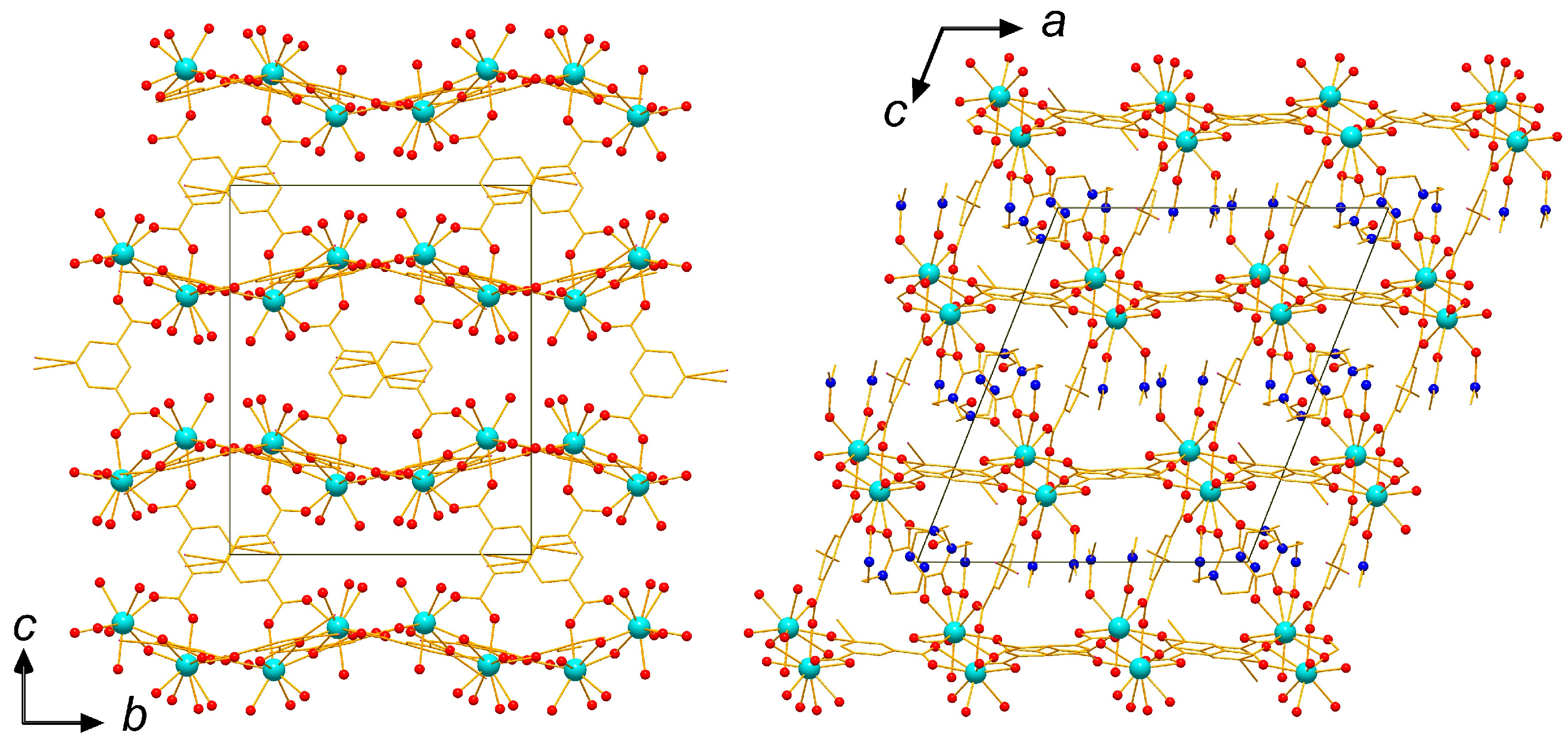
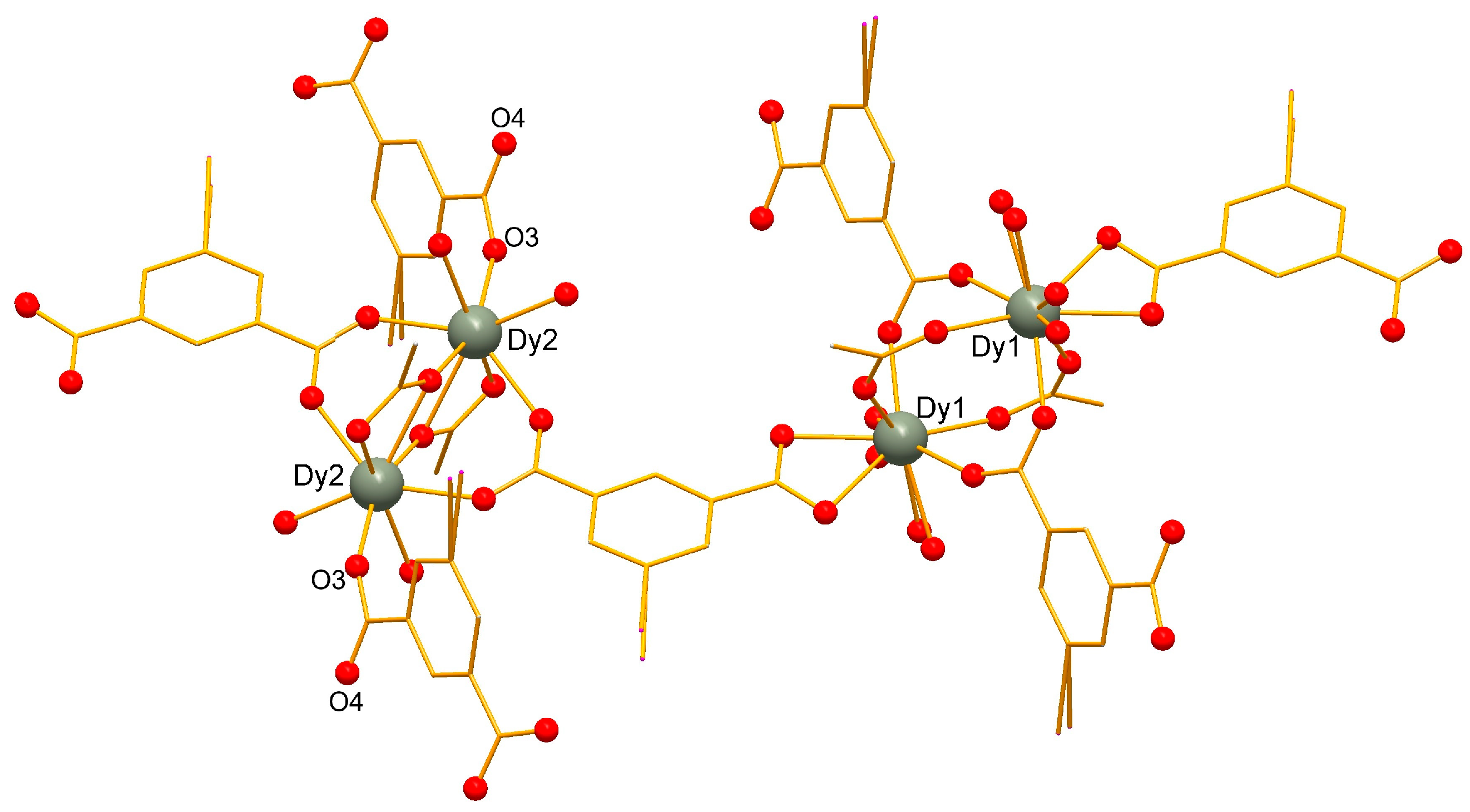
2.1. Crystal Structure of the Complexes 1 and 2
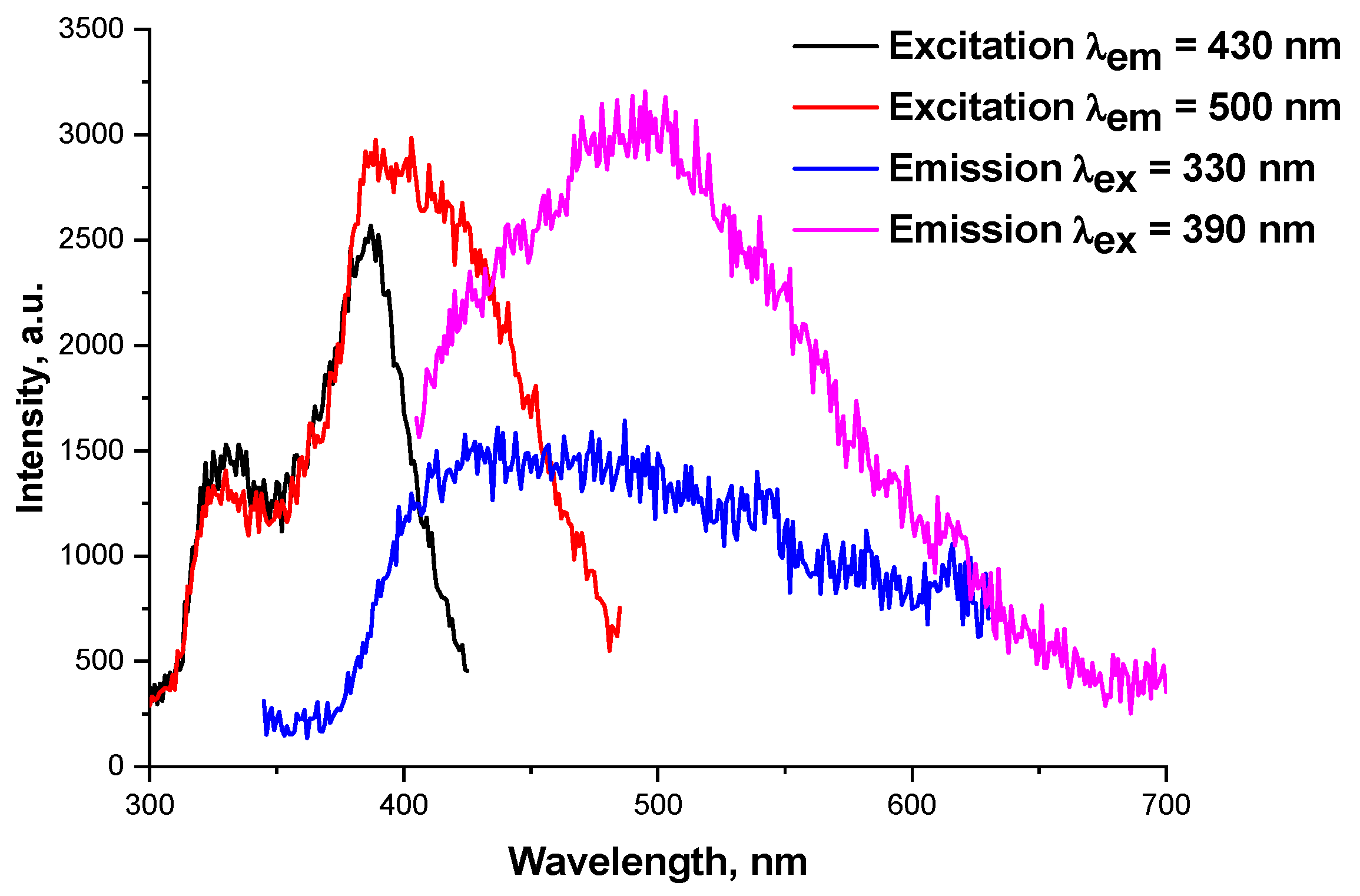
2.2. Photoluminescence Properties
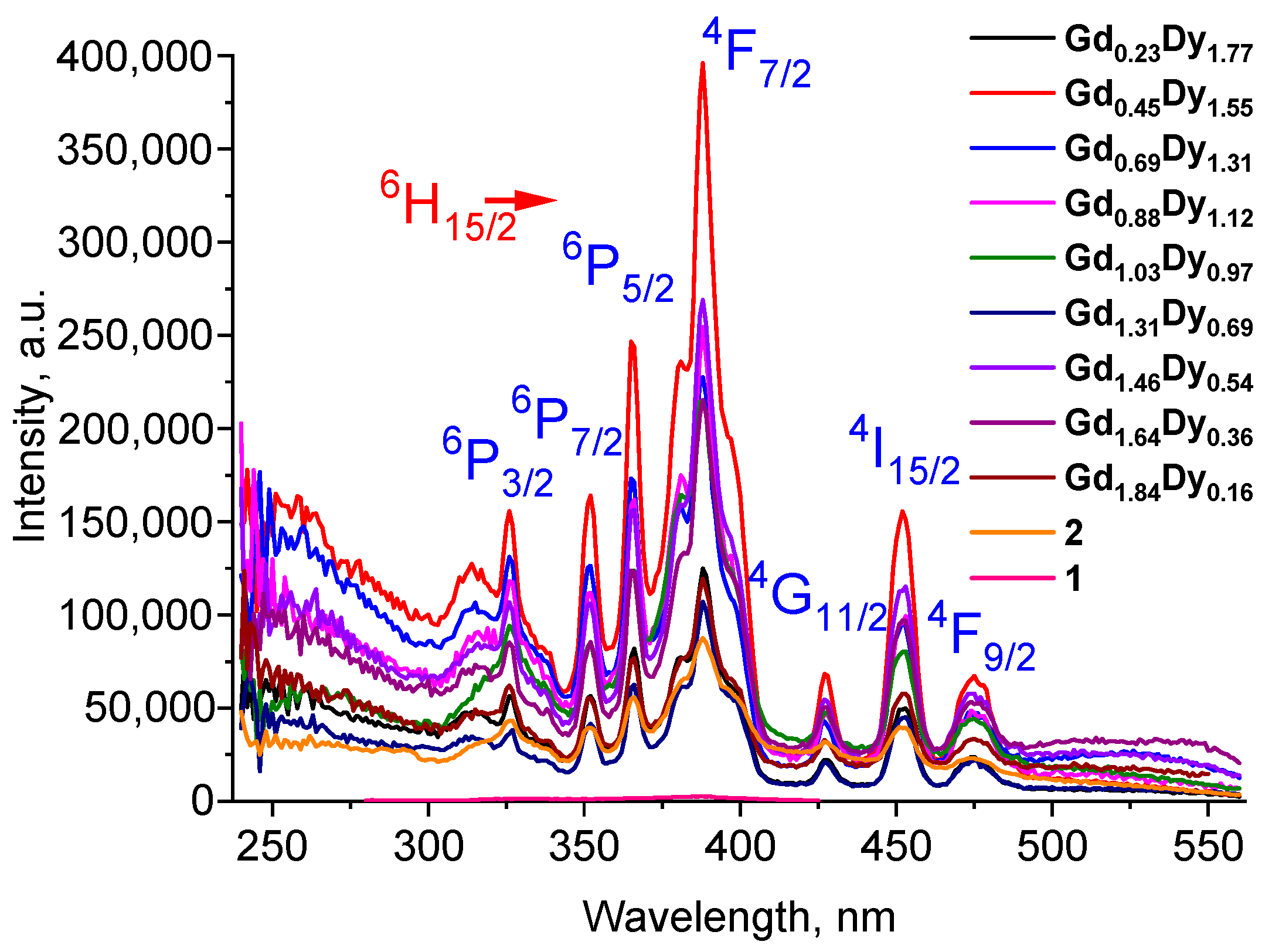
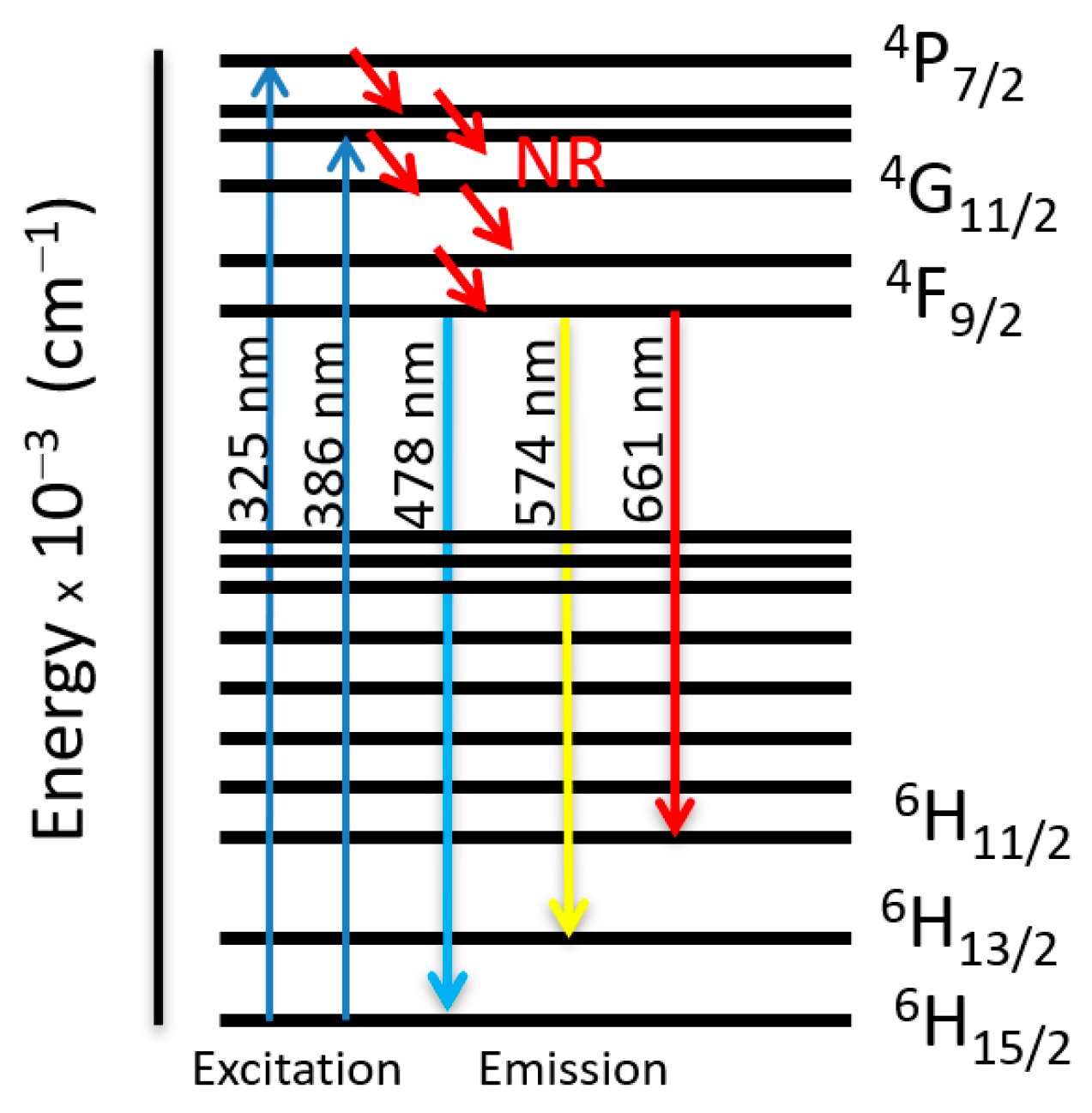
2.2.1. 1, 2 and Series of [GdxDy2−x(5-iip)3DMF2]·0.33DMF
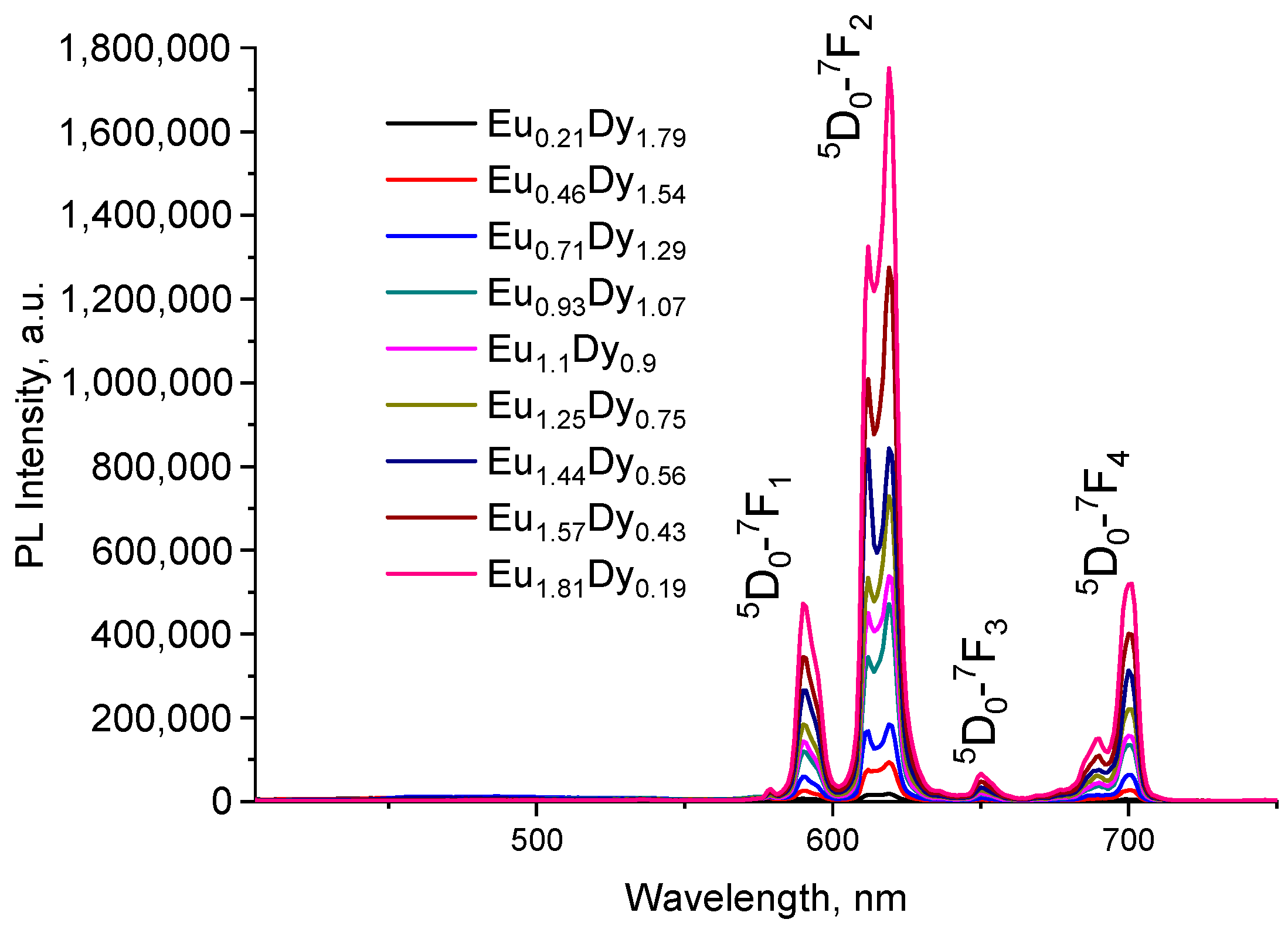
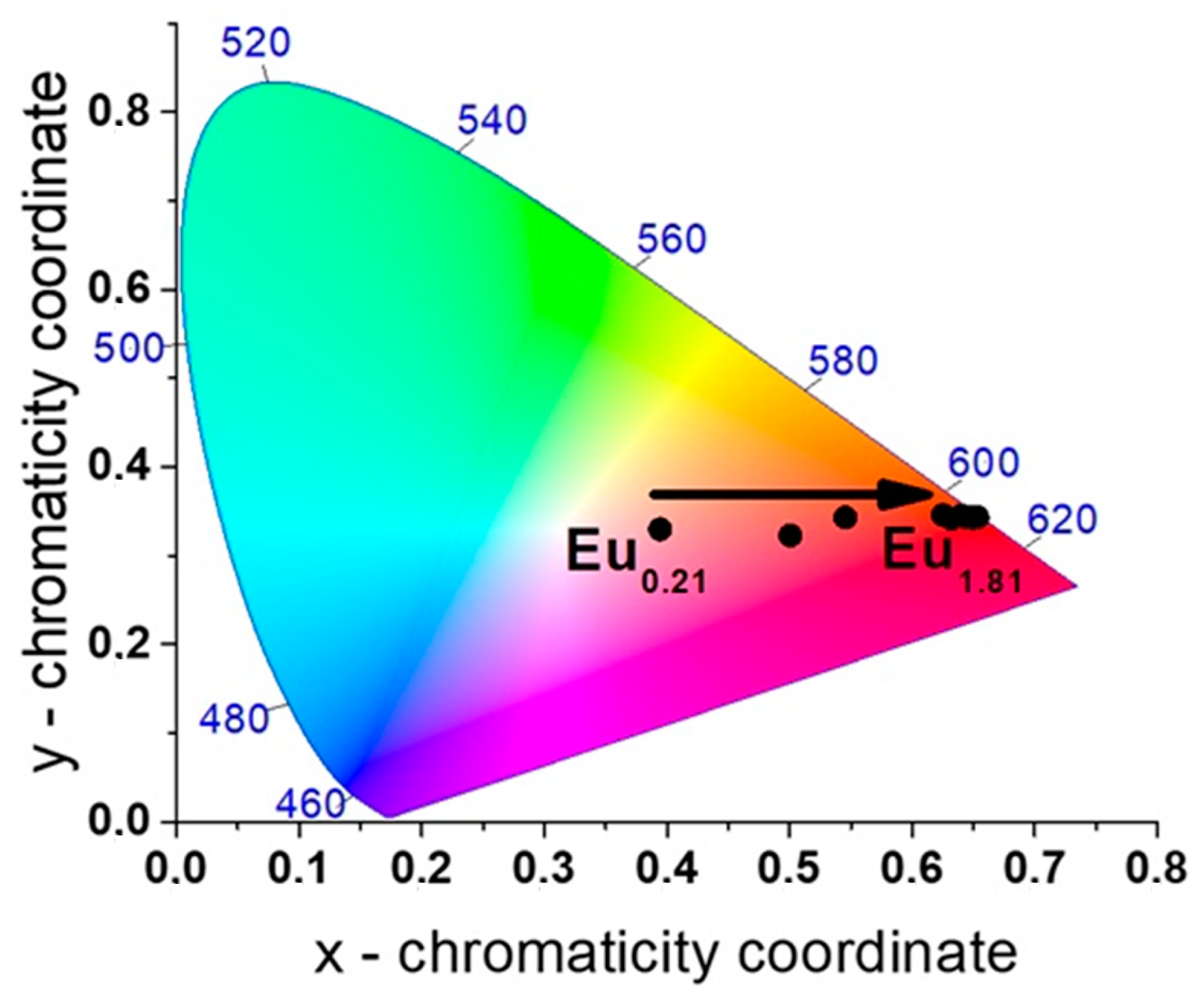
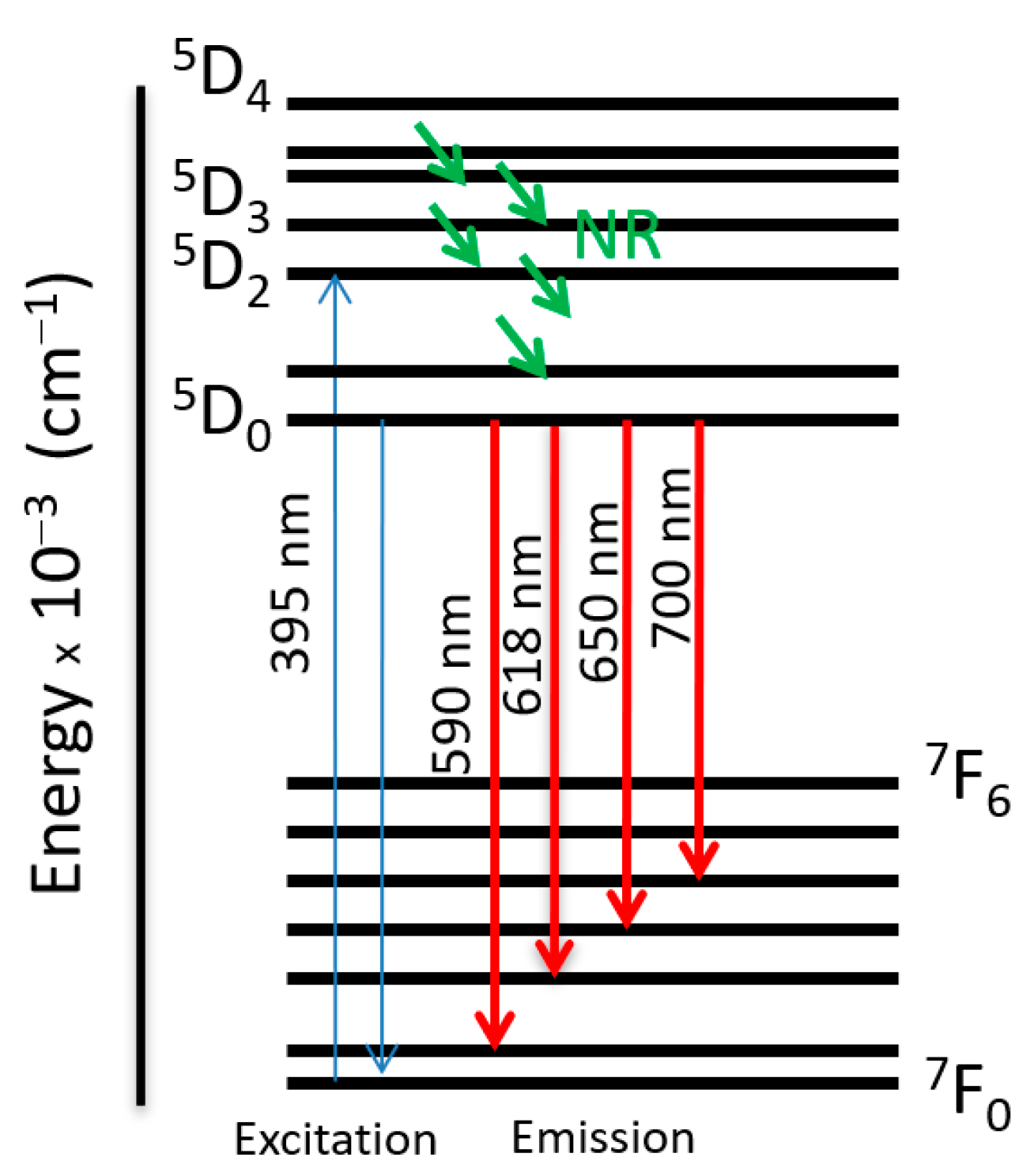
2.2.2. Series of [EuxDy2−x(5-iip)3DMF2]·0.33DMF
3. Materials and Methods
3.1. Physical Measurements
3.2. Synthesis and Characterization of MOFs
3.3. Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMF | Dimethylformamide |
| MOF | Metal-organic frameworks |
| 5-iip/5-iip2− | 5-iodoisophthalate |
| TGA | Thermogravimetric analysis |
| ICP OES | Inductively coupled plasma optical emission spectrometry |
| EDX | Energy-dispersive X-ray spectroscopy |
| CCDC | Cambridge Crystallographic Data Centre |
| XB | Halogen bonding |
| PLE | Photoluminescence excitation |
| PL | Photoluminescence |
| NR | Non-radiative |
| CIE | International Commission on Illumination |
| CCT | Correlated color temperature |
| LED | Light-emitting diode |
| W-LED | White light-emitting diode |
| CTB | Charge transfer band |
| NMR | Nuclear magnetic resonance |
| DMSO | Dimethyl sulfoxide |
References
- Gorbunova, Y.G.; Fedin, V.P.; Blatov, V.A. Metal-organic frameworks as the basis for new-generation functional materials. Russ. Chem. Rev. 2022, 91, RCR5050. [Google Scholar] [CrossRef]
- Afshariazar, F.; Morsali, A. Mixed-valence metal-organic frameworks: Concepts, opportunities, and prospects. Chem. Soc. Rev. 2024, 54, 1318–1383. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Chen, W.; Zhou, H.-C.; Morsali, A. Tuning redox activity in metal–organic frameworks: From structure to application. Coord. Chem. Rev. 2024, 517, 216004. [Google Scholar] [CrossRef]
- Liu, K.-G.; Bigdeli, F.; Panjehpour, A.; Larimi, A.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Metal organic framework composites for reduction of CO2. Coord. Chem. Rev. 2023, 493, 215257. [Google Scholar] [CrossRef]
- Bigdeli, F.; Fetzer, M.N.A.; Nis, B.; Morsali, A.; Janiak, C. Coordination modulation: A way to improve the properties of metal-organic frameworks. J. Mater. Chem. A 2023, 11, 22105–22131. [Google Scholar] [CrossRef]
- Easun, T.L.; Moreau, F.; Yan, Y.; Yang, S.; Schröder, M. Structural and dynamic studies of substrate binding in porous metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 239–274. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Godfrey, H.G.W.; Da Silva, I.; Cheng, Y.; Savage, M.; Manuel, P.; Rudić, S.; Ramirez-Cuesta, A.J.; Yang, S.; Schröder, M. Direct observation of supramolecular binding of light hydrocarbons in vanadium(III) and (iv) metal-organic framework materials. Chem. Sci. 2018, 9, 3401–3408. [Google Scholar] [CrossRef]
- Gulbalkan, H.C.; Uzun, A.; Keskin, S. Evaluating CH4/N2 Separation Performances of Hundreds of Thousands of Real and Hypothetical MOFs by Harnessing Molecular Modeling and Machine Learning. ACS Appl. Mater. Interfaces 2025, 17, 17691–17702. [Google Scholar] [CrossRef]
- Gulbalkan, H.C.; Uzun, A.; Keskin, S. Assessing CO2 separation performances of IL/ZIF-8 composites using molecular features of ILs. Carbon Capture Sci. Technol. 2025, 14, 100373. [Google Scholar] [CrossRef]
- Habib, N.; Aydogdu, A.S.; Keskin, S.; Uzun, A. Selective separation of acetylene from ethylene using mixed matrix membranes with MIL-100(Fe) as a filler. J. Memb. Sci. 2025, 720, 123782. [Google Scholar] [CrossRef]
- Prasetya, N.; Gülbalkan, H.C.; Keskin, S.; Wöll, C. Boosting CO2 separation in porphyrinic MOF-based mixed matrix membranes via central metal atom integration. Carbon Capture Sci. Technol. 2024, 13, 100252. [Google Scholar] [CrossRef]
- Aksu, G.O.; Keskin, S. Advancing CH4/H2 separation with covalent organic frameworks by combining molecular simulations and machine learning. J. Mater. Chem. A 2023, 11, 14788–14799. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Dudko, E.R.; Kovalenko, K.A.; Barsukova, M.O.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Metal-Organic Frameworks for Highly Selective Separation of Xylene Isomers and Single-Crystal X-ray Study of Aromatic Guest-Host Inclusion Compounds. ACS Appl. Mater. Interfaces 2021, 13, 14768–14777. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal-organic framework. Chem. Commun. 2020, 56, 8241–8244. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, X.; Liu, J.; Yu, H.; Si, X.; Ding, F.; Sun, Y. Discrimination of p-xylene from xylene isomers by precisely tuning the spacing of an interpenetrated metal-organic framework. J. Mater. Chem. A 2025, 13, 9665–9672. [Google Scholar] [CrossRef]
- Tu, T.N.; Kwon, H.T.; Scheer, M.; Kim, J. High toluene uptake at a trace concentration in a novel gallium-based metal-organic framework. J. Mater. Chem. A 2025, 13, 9479–9485. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Boissonnault, J.; Cohen, S.M. Design and synthesis of squaramide-based MOFs as efficient MOF-supported hydrogen-bonding organocatalysts. Chem. Commun. 2016, 52, 8585–8588. [Google Scholar] [CrossRef]
- Pintado-Sierra, M.; Rasero-Almansa, A.M.; Corma, A.; Iglesias, M.; Sánchez, F. Bifunctional iridium-(2-aminoterephthalate)-Zr-MOF chemoselective catalyst for the synthesis of secondary amines by one-pot three-step cascade reaction. J. Catal. 2013, 299, 137–145. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Yin, Y.; Fang, W.; Xue, H. Metal-organic framework-based sensors for the detection of toxins and foodborne pathogens. Food Control 2022, 133, 108684. [Google Scholar] [CrossRef]
- Sen, C.; Devi, S.; Niharika, N.; Bhagat, N.; Sheikh, H.N. A 3D photoluminescent Eu(iii)-MOF sensor supported by a tetracarboxylate ligand for the sensitive and selective detection of Cd2+ and o-nitrophenol. New J. Chem. 2024, 48, 15136–15148. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a double s-hole donor: The dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Rozhkov, A.V.; Kornyakov, I.V.; Dubovtsev, A.Y.; Kukushkin, V.Y. Hexaiododiplatinate(ii) as a useful supramolecular synthon for halogen bond involving crystal engineering. Dalt. Trans. 2020, 49, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.S.; Bolotin, D.S.; Il’in, M.V. Pyrazolyliodonium platinum(II) trichloride features the highest positive electrostatic potential on the iodine atom among uncharged halogen bond donors. Inorganica Chim. Acta 2024, 573, 122335. [Google Scholar] [CrossRef]
- Sysoeva, A.A.; Novikov, A.S.; Il’in, M.V.; Bolotin, D.S. Halogen bonded associates of iodonium salts with 18-crown-6: Does structural flexibility or structural rigidity of the σ-hole donor provide efficient substrate ligation? New J. Chem. 2024, 48, 12929–12935. [Google Scholar] [CrossRef]
- Kalaj, M.; Momeni, M.R.; Bentz, K.C.; Barcus, K.S.; Palomba, J.M.; Paesani, F.; Cohen, S.M. Halogen bonding in UiO-66 frameworks promotes superior chemical warfare agent simulant degradation. Chem. Commun. 2019, 55, 3481–3484. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Kuznetsov, M.L.; Karmakar, A.; Aliyeva, V.A.; Mahmudov, K.T.; Pombeiro, A.J.L. Halogen bonding in cadmium(II) MOFs: Its influence on the structure and on the nitroaldol reaction in aqueous medium. Dalt. Trans. 2022, 51, 1019–1031. [Google Scholar] [CrossRef]
- Yang, L.-M.; Fang, G.-Y.; Ma, J.; Pushpa, R.; Ganz, E. Halogenated MOF-5 variants show new configuration, tunable band gaps and enhanced optical response in the visible and near infrared. Phys. Chem. Chem. Phys. 2016, 18, 32319–32330. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, L.A.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. A Series of Coordination Polymers Based on 4,8-Disulfo-2,6-Naphthalenedicarboxylic Acid. J. Struct. Chem. 2024, 65, 2111–2120. [Google Scholar] [CrossRef]
- Pavlov, D.I.; Ryadun, A.A.; Fedin, V.P.; Potapov, A.S. Zinc Metal-Organic Coordination Polymer with 4,7-di(1,2,4-triazol-1-yl)-2,1,3-Benzothiadiazole and 1,4-Naphthalene Dicarboxylic Acid: Luminescent Properties and Guest–Host Charge Transfer. J. Struct. Chem. 2024, 65, 2567–2578. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Eliseev, E.A.; Samsonenko, D.G.; Fedin, V.P. Crystal Structures of Metal-Organic Frameworks Based on Magnesium Cations and Isomeric Tetrafluorobenzenedicarboxylate Ligands. J. Struct. Chem. 2024, 65, 1219–1226. [Google Scholar] [CrossRef]
- Chistyakov, A.S.; Zorina-Tikhonova, E.N.; Vologzhanina, A.V.; Kiskin, M.A.; Eremenko, I.L. 2D Coordination Polymers of Zn(II) with Diethylmalonic Acid Dianions and 4,4′-bipyridine: Synthesis and Structure. Russ. J. Coord. Chem. 2024, 50, 469–475. [Google Scholar] [CrossRef]
- Kiskin, M.A.; Konnik, O.V.; Shul’gin, V.F.; Gusev, A.N. Crystal Structure of Lanthanide Salts with 2,4-Dichlorophenoxyacetic Acid. Russ. J. Coord. Chem. 2024, 50, 476–484. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Vatolina, S.E.; Gaviko, V.S.; Litvinov, I.A.; Valieva, M.I.; Shtaitz, Y.K.; Rybakova, S.S.; Kopchuk, D.S.; Zyryanov, G.V.; Rusinov, V.L. Samarium(III) Complex Based on 5-Phenyl-2,2′-bipyridine with the Diethylenetriaminotetraacetic Acid Residue in the C6 Position: Synthesis, Crystal Structure, and Photophysical Properties. Russ. J. Coord. Chem. 2024, 50, 398–404. [Google Scholar] [CrossRef]
- Rubtsova, I.K.; Melnikov, S.N.; Shmelev, M.A.; Nikolaevskii, S.A.; Yakushev, I.A.; Voronina, J.K.; Barabanova, E.D.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Facile synthesis and structure elucidation of metal-organic frameworks with {ZnCa} and {Zn2Ca} metal cores. Mendeleev Commun. 2020, 30, 722–724. [Google Scholar] [CrossRef]
- Zaguzin, A.S.; Sukhikh, T.S.; Kolesov, B.A.; Sokolov, M.N.; Fedin, V.P.; Adonin, S.A. Iodinated vs. non-iodinated: Comparison of sorption selectivity by [Zn2(bdc)2dabco]n and superstructural 2-iodoterephtalate-based metal–organic framework. Polyhedron 2022, 212, 115587. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kalyakina, A.S.; Solodukhin, N.N.; Aslandukov, A.N. On the Structural Features of Substituted Lanthanide Benzoates. Eur. J. Inorg. Chem. 2019, 2019, 2320–2332. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Solodukhin, N.N.; Aslandukov, A.N.; Marciniak, L.; Bushmarinov, I.S.; Vashchenko, A.A.; Kuzmina, N.P. Lanthanide tetrafluorobenzoates as emitters for OLEDs: New approach for host selection. Org. Electron. 2017, 44, 85–93. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kotova, O.V.; Shchukina, E.M.; Eliseeva, S.V.; Kuz’mina, N.P. Gas-phase synthesis of terbium and lutetium carboxylates. Russ. J. Inorg. Chem. 2008, 53, 1878–1884. [Google Scholar] [CrossRef]
- Rao, X.; Huang, Q.; Yang, X.; Cui, Y.; Yang, Y.; Wu, C.; Chen, B.; Qian, G. Color tunable and white light emitting Tb3+ and Eu3+ doped lanthanide metal–organic framework materials. J. Mater. Chem. 2012, 22, 3210–3214. [Google Scholar] [CrossRef]
- Lutter, J.C.; Eliseeva, S.V.; Collet, G.; Martinić, I.; Kampf, J.W.; Schneider, B.L.; Carichner, A.; Sobilo, J.; Lerondel, S.; Petoud, S.; et al. Iodinated Metallacrowns: Toward Combined Bimodal Near-Infrared and X-Ray Contrast Imaging Agents. Chem. Eur. J. 2020, 26, 1274–1277. [Google Scholar] [CrossRef]
- Zang, S.-Q.; Fan, Y.-J.; Li, J.-B.; Hou, H.-W.; Mak, T.C.W. Halogen Bonding in the Assembly of Coordination Polymers Based on 5-Iodo-Isophthalic Acid. Cryst. Growth Des. 2011, 11, 3395–3405. [Google Scholar] [CrossRef]
- Zang, S.-Q.; Dong, M.-M.; Fan, Y.-J.; Hou, H.-W.; Mak, T.C.W. Four Cobaltic Coordination Polymers Based on 5-Iodo-Isophthalic Acid: Halogen-Related Interaction and Solvent Effect. Cryst. Growth Des. 2012, 12, 1239–1246. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, H.; Hua, Y.; Cao, H.; Zhou, B.; Duan, J.; Jin, W. Halogen bonded supramolecular porous structures with a kgm layer. CrystEngComm 2016, 18, 9227–9230. [Google Scholar] [CrossRef]
- Zaguzin, A.S.; Bondarenko, M.A.; Korobeynikov, N.A.; Usoltsev, A.N.; Fedin, V.P.; Adonin, S.A. 3D Metal-Organic Coordination Polymers of Zn(II) Based on 1,2-Bis(4-pyridyl)ethylene and Anions of Iodoterephthalic and Iodoisophthalic Acids. Russ. J. Inorg. Chem. 2024, 69, 1018–1023. [Google Scholar] [CrossRef]
- Dong, M.M.; Zhu, D.Y.; Zhao, Y.; Li, X.Y.; Zang, S.Q. A two-fold interpenetrated diamondlike 3D metal-organic Cd(II) complex based on 5-Iodo-isophthalic acid and 1,3-Bi(4-pyridyl)propane. Z. Naturforsch. Sect. B J. Chem. Sci. 2012, 67, 499–503. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Zaguzin, A.S.; Spiridonova, D.V.; Novikov, A.S.; Rakhmanova, M.I.; Zherebtsov, D.A.; Fedin, V.P.; Adonin, S.A. Two-dimensional ZnII coordination polymer based on 5-iodoisophthalate: Synthesis, crystal and electronic structure. Russ. Chem. Bull. 2023, 72, 177–183. [Google Scholar] [CrossRef]
- Lee, R.L.; Romero, J.; Hernández-Andrés, J. Calculating correlated color temperatures across the entire gamut of daylight and skylight chromaticities. Appl. Opt. 1999, 38, 5703–5709. [Google Scholar]
- Schubert, E.F. Light-Emitting Diodes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–422. ISBN 9780521865388. [Google Scholar]
- Krishnaiah, K.V.; Kumar, K.U.; Jayasankar, C.K. Spectroscopic properties of Dy3+-doped oxyfluoride glasses for white light emitting diodes. Mater. Express 2013, 3, 61–70. [Google Scholar] [CrossRef]
- Vijaya, N.; Upendra Kumar, K.; Jayasankar, C.K. Dy3+-doped zinc fluorophosphate glasses for white luminescence applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 113, 145–153. [Google Scholar] [CrossRef]
- Wang, H.; Deng, T.; Cai, C. Fluorous bis(oxazolines) ligand: Synthesis and application in Kabachnik-Fields reaction. J. Fluor. Chem. 2014, 168, 144–150. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




| Ratio of GdCl3·6H2O:Dy(NO3)3·5H2O | Amount of GdCl3·6H2O, mg (mmol) | Amount of Dy(NO3)3·5H2O, mg (mmol) |
|---|---|---|
| 0.2:1.8 | 6.8 (0.018) | 72.1 (0.164) |
| 0.4:1.6 | 13.6 (0.037) | 64.1 (0.146) |
| 0.6:1.4 | 20.4 (0.054) | 56.1 (0.128) |
| 0.8:1.2 | 27.2 (0.073) | 48.1 (0.109) |
| 1.0:1.0 | 33.9 (0.091) | 40.0 (0.091) |
| 1.2:0.8 | 40.7 (0.109) | 32.0 (0.073) |
| 1.4:0.6 | 47.5 (0.128) | 24.0 (0.055) |
| 1.6:0.4 | 54.3 (0.146) | 16.0 (0.036) |
| 1.8:0.2 | 61.1 (0.164) | 8.0 (0.018) |
| Ratio of EuCl3·6H2O:Dy(NO3)3·5H2O | Amount of EuCl3·6H2O, mg (mmol) | Amount of Dy(NO3)3·5H2O, mg (mmol) |
|---|---|---|
| 0.2:1.8 | 6.7 (0.018) | 72.1 (0.164) |
| 0.4:1.6 | 13.4 (0.037) | 64.1 (0.146) |
| 0.6:1.4 | 20.1 (0.055) | 56.1 (0.128) |
| 0.8:1.2 | 26.8 (0.073) | 48.1 (0.109) |
| 1.0:1.0 | 33.5 (0.091) | 40.0 (0.091) |
| 1.2:0.8 | 40.2 (0.110) | 32.0 (0.073) |
| 1.4:0.6 | 46.8 (0.128) | 24.0 (0.055) |
| 1.6:0.4 | 53.5 (0.146) | 16.0 (0.036) |
| 1.8:0.2 | 60.2 (0.164) | 8.0 (0.018) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarenko, M.A.; Redkin, R.V.; Pilyukova, E.A.; Korobeynikov, N.A.; Zaguzin, A.S.; Rakhmanova, M.I.; Guselnikova, T.Y.; Maksimovsky, E.A.; Fedin, V.P.; Adonin, S.A. Lanthanide Heterometallic MOFs with 5-Iodosophthalate Linkers: Tuning of Luminescent Properties by Varying of Metal Composition. Inorganics 2025, 13, 154. https://doi.org/10.3390/inorganics13050154
Bondarenko MA, Redkin RV, Pilyukova EA, Korobeynikov NA, Zaguzin AS, Rakhmanova MI, Guselnikova TY, Maksimovsky EA, Fedin VP, Adonin SA. Lanthanide Heterometallic MOFs with 5-Iodosophthalate Linkers: Tuning of Luminescent Properties by Varying of Metal Composition. Inorganics. 2025; 13(5):154. https://doi.org/10.3390/inorganics13050154
Chicago/Turabian StyleBondarenko, Mikhail A., Roman V. Redkin, Elizaveta A. Pilyukova, Nikita A. Korobeynikov, Alexander S. Zaguzin, Marianna I. Rakhmanova, Tatiana Ya. Guselnikova, Evgeny A. Maksimovsky, Vladimir P. Fedin, and Sergey A. Adonin. 2025. "Lanthanide Heterometallic MOFs with 5-Iodosophthalate Linkers: Tuning of Luminescent Properties by Varying of Metal Composition" Inorganics 13, no. 5: 154. https://doi.org/10.3390/inorganics13050154
APA StyleBondarenko, M. A., Redkin, R. V., Pilyukova, E. A., Korobeynikov, N. A., Zaguzin, A. S., Rakhmanova, M. I., Guselnikova, T. Y., Maksimovsky, E. A., Fedin, V. P., & Adonin, S. A. (2025). Lanthanide Heterometallic MOFs with 5-Iodosophthalate Linkers: Tuning of Luminescent Properties by Varying of Metal Composition. Inorganics, 13(5), 154. https://doi.org/10.3390/inorganics13050154







