Synthesis of M-Doped MoSe2 (M = Fe, Co, Ni) via Chemical Vapor Deposition for an Electrocatalytic Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
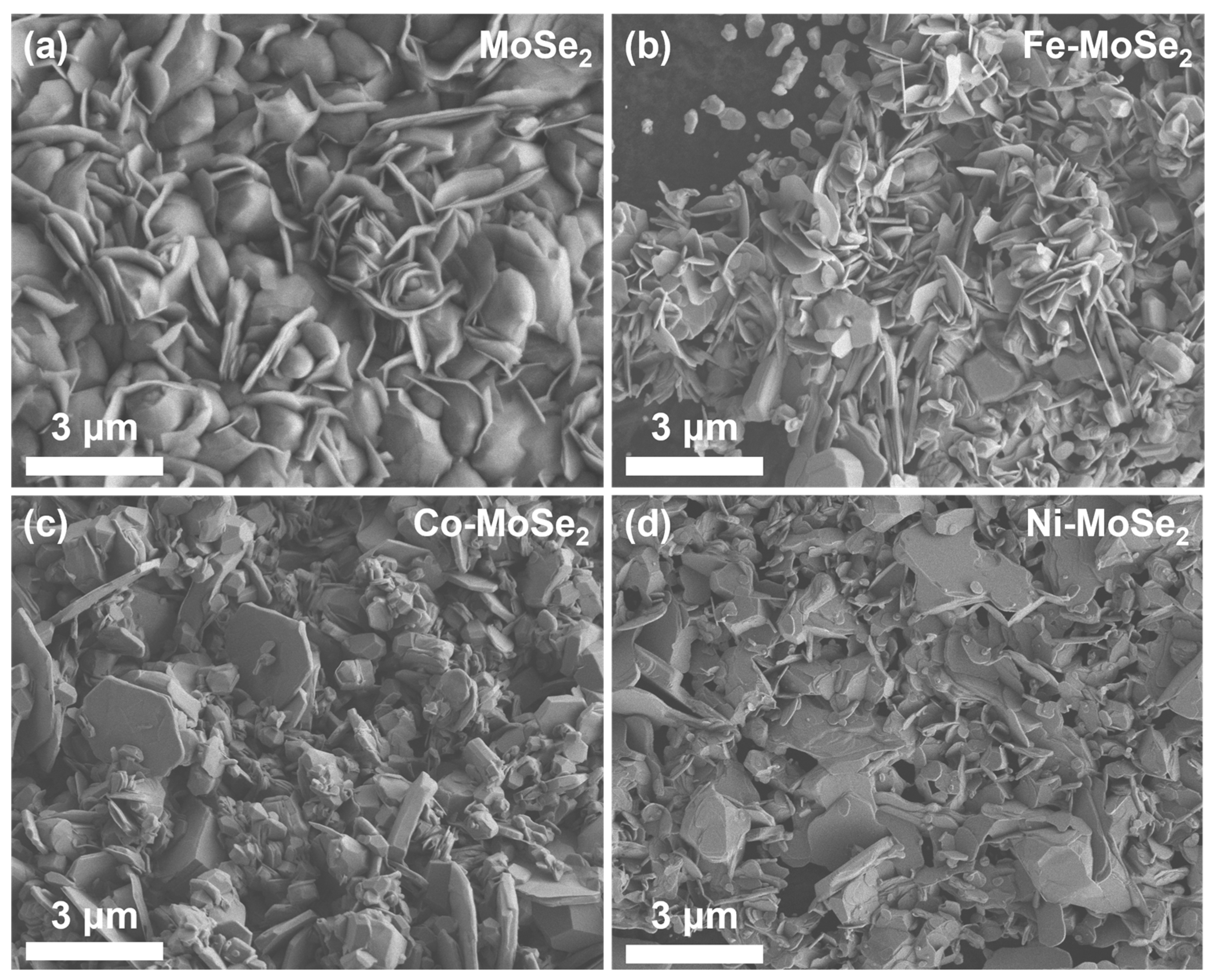
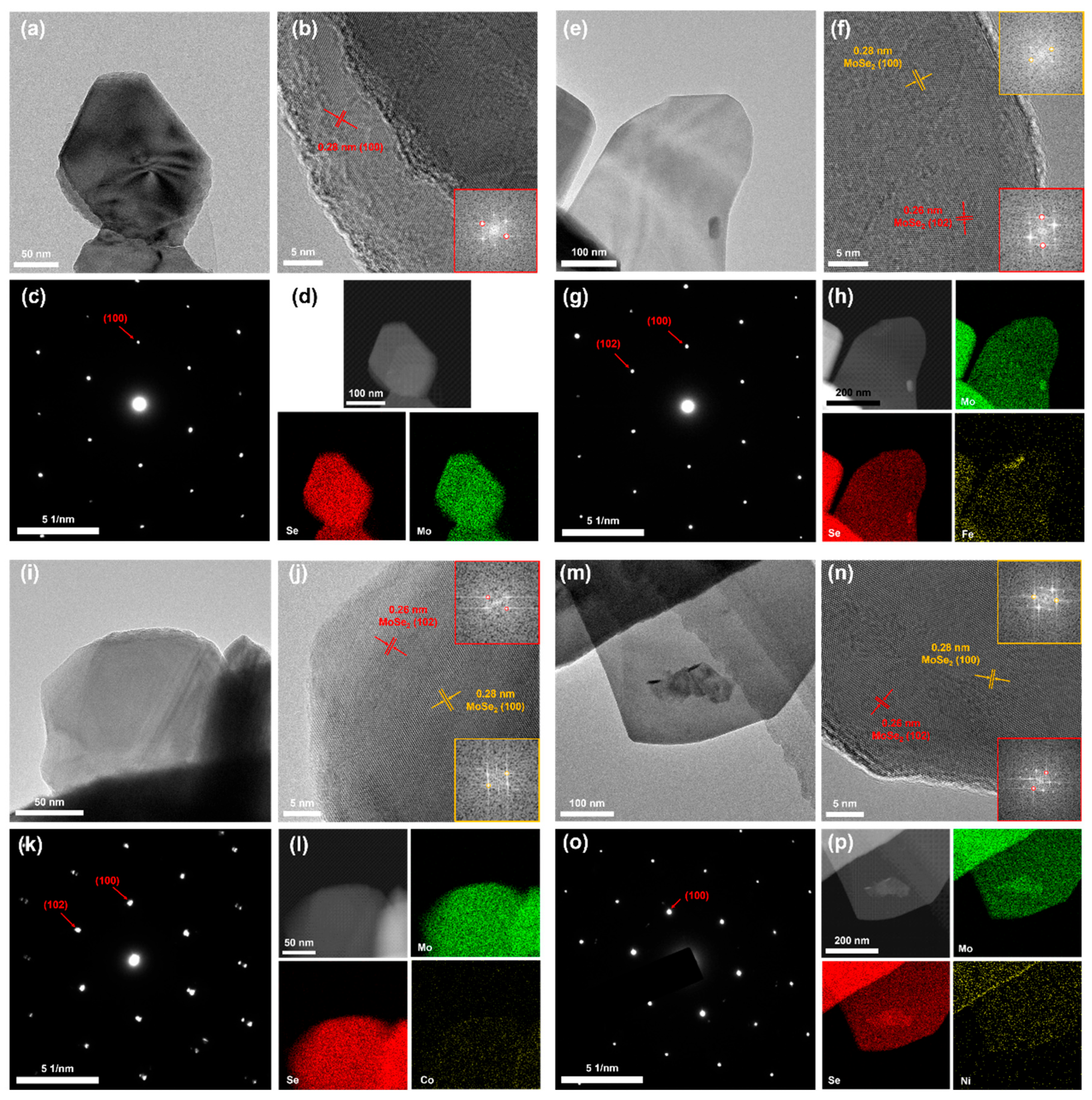
2.1. Morphology and Structure Analysis
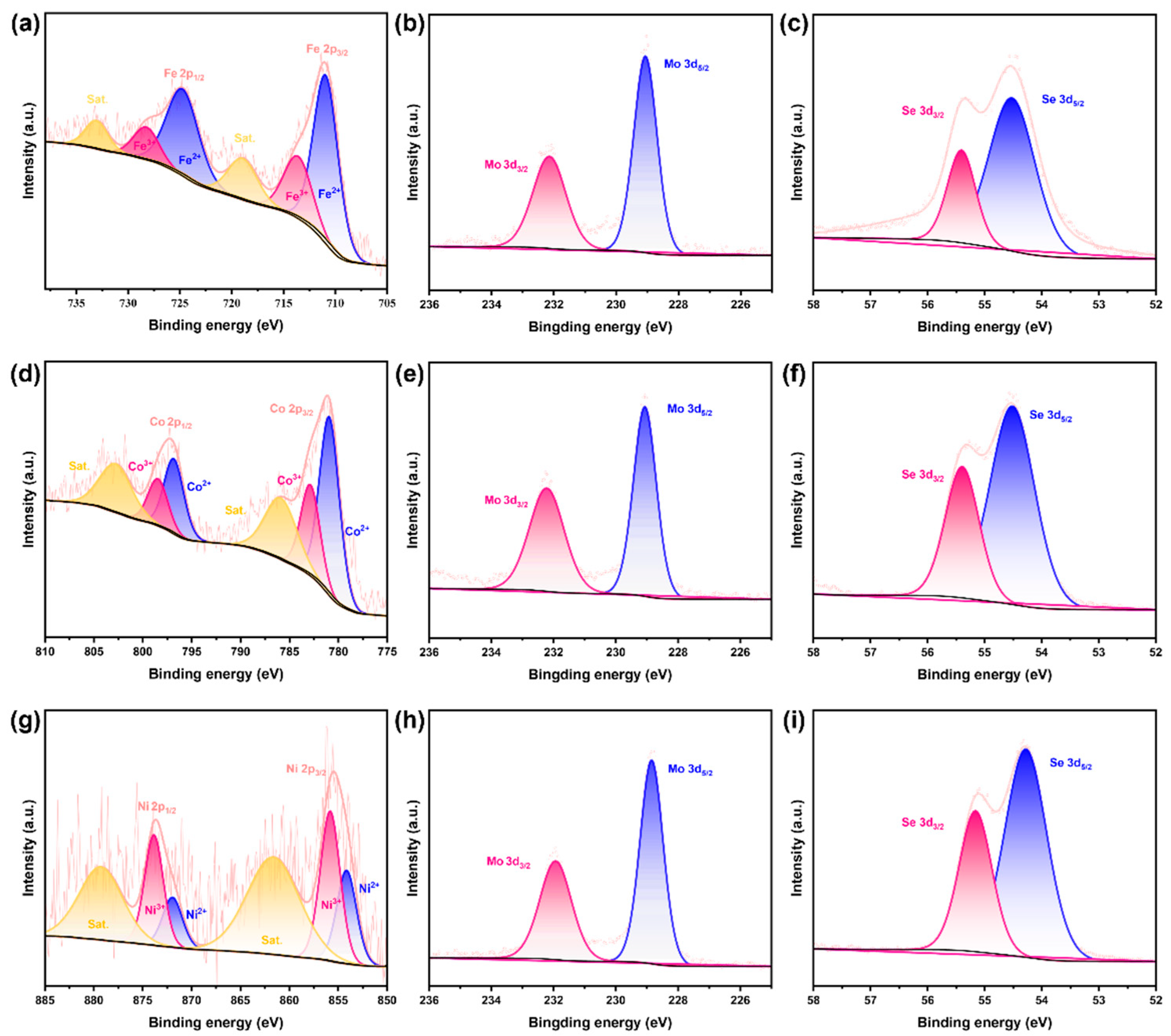
2.2. Component Analysis
2.3. Optical and Crystal Structure Analysis
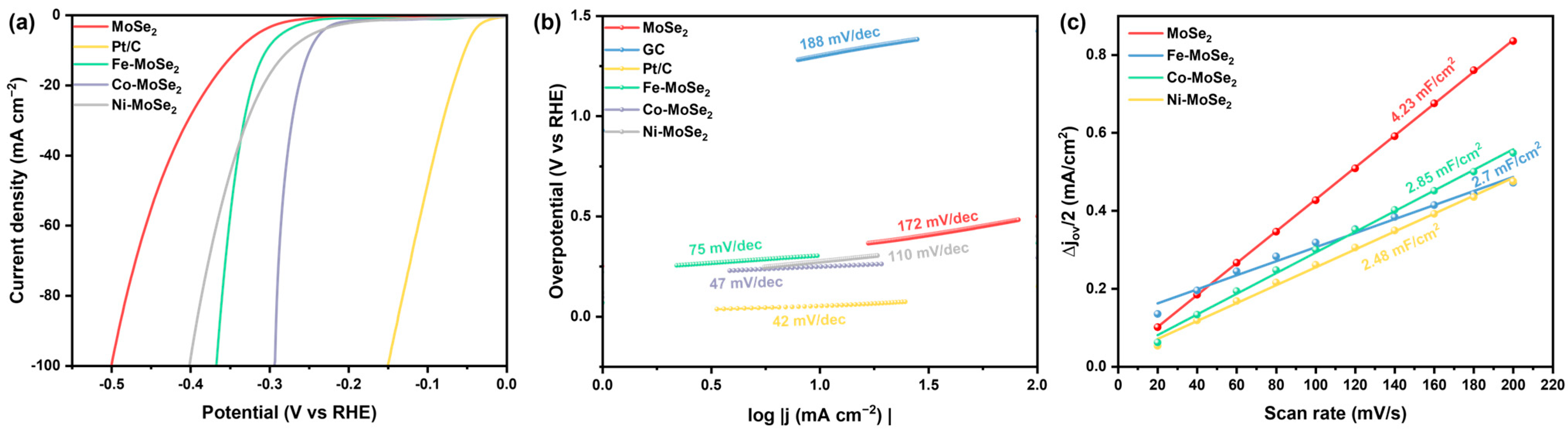
2.4. Electrochemical Characterization
| Electrocatalyst | Electrolyte | Current Density (mA cm−2) | Overpotential (mV) | Tafel Slope (mV dev−1) |
|---|---|---|---|---|
| Co-MoSe2 (this work) | 0.5 M H2SO4 | 100 | 293 | 47 |
| Fe-MoSe2 (this work) | 0.5 M H2SO4 | 100 | 367 | 75 |
| Ni-MoSe2 (this work) | 0.5 M H2SO4 | 100 | 401 | 110 |
| Ni-MoSe2 [24] | 1.0 M KOH | 50 | 455 | 96 |
| MoSe2/carbon cloth [29] | 0.5 M H2SO4 | 21 | 250 | 76 |
| 60 | 300 | |||
| Ni0.15Mo0.85Se2 [25] | 0.5 M H2SO4 | 10 | 180 | 67 |
| 90 | ~320 | |||
| MoSe2@Co [26] | 1 M KOH | 10 | 391 | 109 |
| MoSe2@Ni [26] | 1 M KOH | 10 | 367 | 227 |
| MoSe2@CoNi [26] | 1 M KOH | 10 | 378 | 170 |
| graphene/MoSe2 [28] | 0.5 M H2SO4 | 100 | ~210 | 80 |
| Ni-MoS2 [27] | 0.5 M H2SO4 | 10 | 302 | 66 |
| 35 | ~360 | |||
| Co-MoS2 [27] | 0.5 M H2SO4 | 10 | 350 | 69 |
| 35 | ~550 | |||
| Fe-MoS2 [27] | 0.5 M H2SO4 | 10 | 490 | 94 |
| 35 | ~700 |
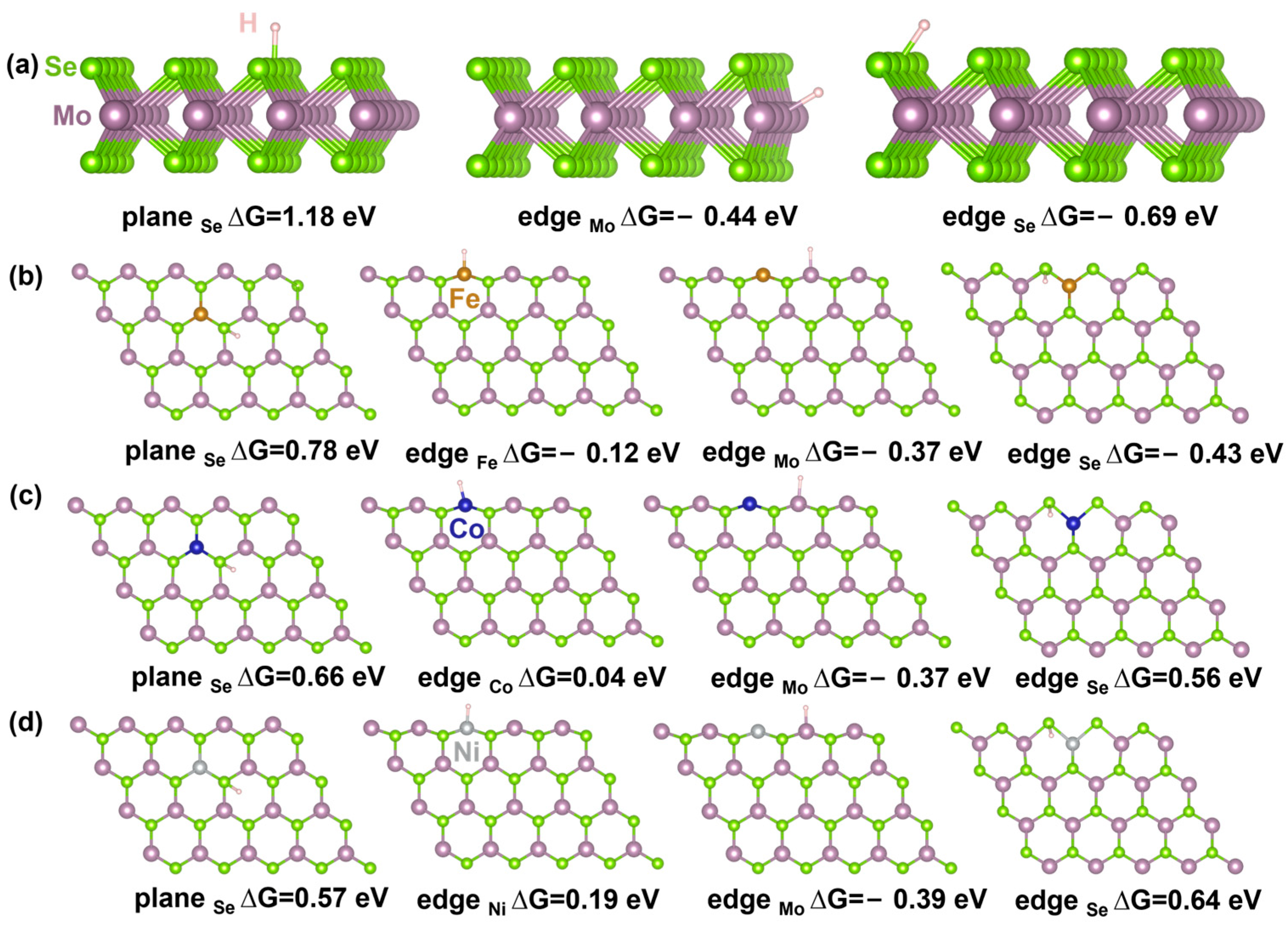
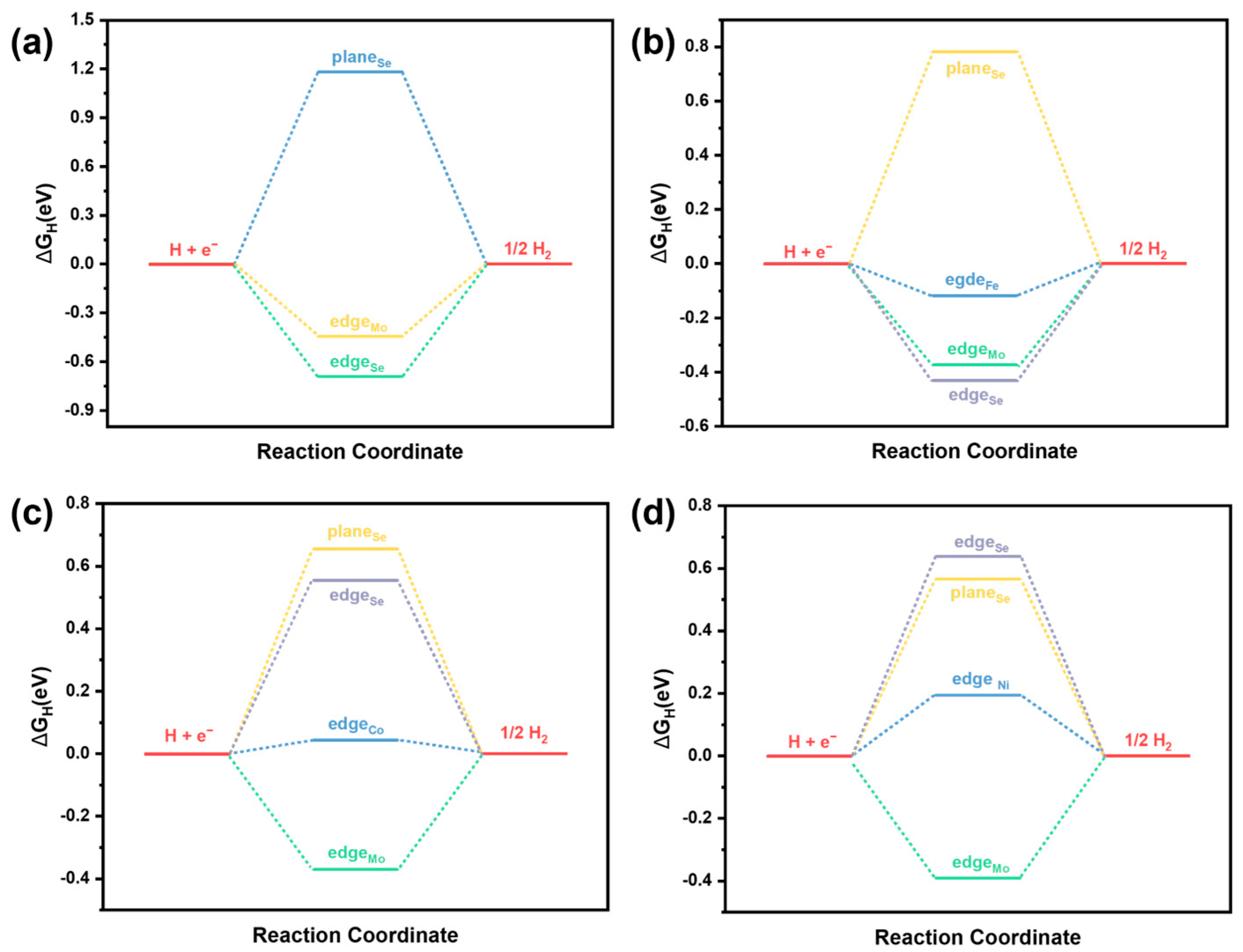
2.5. Theoretical Calculation
3. Experimental
3.1. Sample Preparation
3.2. Characterization Methods
3.3. Electrochemical Measurements
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, D.; Yun, S.; Sun, M.L.; Dang, J.E.; Zhang, Y.W.; Yuan, S.X.; Yang, G.P.; Yang, T.X.; Gao, Z.; Wang, Z.G. 1D/3D Trepang-Like N-Modified Carbon Confined Bimetal Carbides and Metal Cobalt: Boosting Electron Transfer via Dual Mott-Schottky Heterojunctions Triggered Built-In Electric Fields for Efficient Hydrogen Evolution and Tri-Iodide Reduction. Appl. Catal. B Environ. 2023, 334, 122830. [Google Scholar] [CrossRef]
- Wang, L.Q.; Hao, Y.X.; Deng, L.M.; Hu, F.; Zhao, S.; Li, L.L.; Peng, S.J. Rapid Complete Reconfiguration Induced Actual Active Species for Industrial Hydrogen Evolution Reaction. Nat. Commun. 2022, 13, 5785. [Google Scholar] [CrossRef]
- Sarwar, S.; Ali, A.; Wang, Y.F.; Ahasan, M.R.; Wang, R.G.; Adamczyk, A.J.; Zhang, X.Y. Enhancement of Hydrogen Evolution Reaction Activity Using Metal–Rich Molybdenum Sulfotelluride with Graphene Support: A Combined Experimental and Computational Study. Nano Energy 2021, 90, 106599. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Tian, H.; Bian, L.; Liu, S.Z.; Liu, Y.; Wang, Z.L. Cu-Zn-Based Alloy/Oxide Interfaces for Enhanced Electroreduction of CO2 to C2+ Products. J. Energy Chem. 2023, 83, 90–97. [Google Scholar] [CrossRef]
- Wan, C.Z.; Zhang, Z.S.; Dong, J.C.; Xu, M.J.; Pu, H.T.; Baumann, D.; Lin, Z.Y.; Wang, S.B.; Huang, J.; Shah, A.H.; et al. Amorphous Nickel Hydroxide Shell Tailors Local Chemical Environment on Platinum Surface for Alkaline Hydrogen Evolution Reaction. Nat. Mater. 2023, 22, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, X.L.; Shi, C.; Xia, G.Q.; Li, H.; Wang, P.; Di, L.B. Plasma Synthesis of Defect-Rich Flexible Carbon Cloth Decorated with PtRu Alloyed Nanoclusters for Highly Efficient pH-Universal Electrocatalytic Hydrogen Evolution. Nanoscale 2022, 14, 15942–15949. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yun, S.N.; Shi, J.; Zhang, Y.W.; Dang, J.E.; Dang, C.W.; Liu, Z.L.; Deng, Y.Y.; Yang, T.X. Defect Engineering Tuning Electron Structure of Biphasic Tungsten-Based Chalcogenide Heterostructure Improves Its Catalytic Activity for Hydrogen Evolution and Triiodide Reduction. J. Colloid Interface Sci. 2022, 625, 800–816. [Google Scholar] [CrossRef]
- Da, P.F.; Zheng, Y.; Hu, Y.; Wu, Z.L.; Zhao, H.Y.; Wei, Y.C.; Guo, L.C.; Wang, J.J.; Wei, Y.P.; Xi, S.B.; et al. Synthesis of Bandgap-Tunable Transition Metal Sulfides through Gas-Phase Cation Exchange-Induced Topological Transformation. Angew. Chem. Int. Ed. 2023, 62, e202301802. [Google Scholar] [CrossRef]
- Chen, X.Y.; Han, Z.; Zhang, B.; Sun, B.J.; Wang, Y.; Du, Y.C.; Han, X.J.; Xu, P. Construction of Plasmonic 1T-WS2/2H-WS2/CdS Heterostructures for Enhanced Solar-Driven Hydrogen Evolution. J. Mater. Chem. A 2022, 10, 24030–24040. [Google Scholar] [CrossRef]
- Chen, S.-F.; Wu, T.-S.; Soo, Y.-L. Highly Defective Graphene Quantum Dots-Doped 1T/2H-MoS2 as an Efficient Composite Catalyst for the Hydrogen Evolution Reaction. Sci. Rep. 2023, 13, 15184. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalytic hydrogen evolution reaction: A comprehensive review. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Dai, X.P.; Du, K.L.; Li, Z.Z.; Liu, M.Z.; Ma, Y.D.; Sun, H.; Zhang, X.; Yang, Y. Co-Doped MoS2 Nanosheets with the Dominant CoMoS Phase Coated on Carbon as an Excellent Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 27242–27253. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Ning, L.; Tang, K.; Guo, Y.; You, L.; Chen, C.; Wang, G. Nickel–Iron-Layered Double Hydroxide Electrocatalyst with Nanosheets Array for High Performance of Water Splitting. Molecules 2024, 29, 2092. [Google Scholar] [CrossRef]
- Feng, Q.L.; Mao, N.N.; Wu, J.X.; Xu, H.; Wang, C.M.; Zhang, J.; Xie, L.M. Growth of MoS2(1-x) Se2x (x = 0.41–1.00) Monolayer Alloys with Controlled Morphology by Physical Vapor Deposition. ACS Nano 2015, 9, 7450–7455. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Shen, D.Y.; Zhao, B.; Zhang, Z.C.; Zhang, H.M.; Yang, X.D.; Huang, Z.W.; Li, B.L.; Song, R.; Jin, Y.J.; Wu, R.X.; et al. Synthesis of Group VIII Magnetic Transition Metal-Doped Monolayer MoSe2. ACS Nano 2022, 16, 10623–10631. [Google Scholar] [CrossRef]
- Zimron, O.; Zilberman, T.; Kadam, S.R.; Ghosh, S.; Kolatker, S.-L.; Neyman, A.; Bar-Ziv, R.; Bar-Sadan, M. Co-Doped MoSe2 Nanoflowers as Efficient Catalysts for Electrochemical Hydrogen Evolution Reaction (HER) in Acidic and Alkaline Media. Isr. J. Chem. 2020, 60, 624–629. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, X.; Mao, H.; Ning, R.; Zheng, X.H.; Sui, J.H.; Cai, W. Nickel-doped MoSe2 nanosheets with Ni-Se bond for alkaline electrocatalytic hydrogen evolution. J. Mater. Chem. A 2023, 11, 12345–12352. [Google Scholar] [CrossRef]
- Chen, Y.P.; Huang, Y.C.; Xiong, K.Y.; Ye, X.Y.; Liu, Q.Q.; Zhuang, J.R.; Zhang, Y.F.; Zhu, J. Cooperative 3d-Metal-Doped MoSe2 Catalysts for Enhanced Electrocatalytic Hydrogen Evolution Reaction. Mol. Catal. 2024, 566, 114401. [Google Scholar] [CrossRef]
- Liu, T.; Xu, H.; Zhou, W.; Zhang, K.; Sun, Y. Computational Design of Single-Atom Catalysts: Cobalt-Doped MoSe2 as a High-Performance Electrocatalyst for Hydrogen Evolution. ACS Catal. 2020, 10, 12345–12356. [Google Scholar]
- Xu, C.; Peng, S.J.; Tan, C.L.; Ang, H.X.; Tan, H.T.; Zhang, H.; Yan, Q.Y. Ultrathin S-Doped MoSe2 Nanosheets for Efficient Hydrogen Evolution. J. Mater. Chem. A 2014, 2, 5597–5601. [Google Scholar] [CrossRef]
- Xiong, Q.Z.; Zhang, X.; Wang, H.J.; Liu, G.Q.; Wang, G.Z.; Zhang, H.M.; Zhao, H.J. One-Step Synthesis of Cobalt-Doped MoS2 Nanosheets as Bifunctional Electrocatalysts for Overall Water Splitting under Both Acidic and Alkaline Conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef]
- Sun, X.; Dai, J.; Guo, Y.Q.; Wu, C.Z.; Hu, F.T.; Zhao, J.Y.; Zeng, X.C.; Xie, Y. Semimetallic Molybdenum Disulfide Ultrathin Nanosheets as an Efficient Electrocatalyst for Hydrogen Evolution. Nanoscale 2014, 6, 8359–8367. [Google Scholar] [CrossRef]
- Zong, H.; Yu, K.; Zhu, Z. Heterostructure nanohybrids of Ni-doped MoSe2 coupled with Ti2NTx toward efficient overall water splitting. Electrochim. Acta 2020, 353, 136598. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Wang, X.Y.; Wang, S.L.; Rui, K.; Chen, Y.P.; Yu, H.B.; Ma, J.; Dou, S.X.; Sun, W.P. Evolution Kinetics for Alkaline Water Splitting. Chem. Asian J. 2019, 14, 301–306. [Google Scholar] [CrossRef]
- Dymerska, A.; Kukułka, W.; Wenelska, K.; Mijowska, E. Two-Dimensional Molybdenum Diselenide Tuned by Bimetal Co/Ni Nanoparticles for Oxygen Evolution Reaction. ACS Omega 2020, 5, 28730–28737. [Google Scholar] [CrossRef]
- Venkatesh, P.S.; Kannan, N.; Babu, M.G.; Paulraj, G.; Jeganathan, K. Transition Metal Doped MoS2 Nanosheets for Electrocatalytic Hydrogen Evolution Reaction. Mater. Today Proc. 2022, 47, 37256–37263. [Google Scholar]
- Najafi, L.; Bellani, S.; Oropesa-Nuñez, R.; Ansaldo, A.; Prato, M.; Del Rio Castillo, A.E.; Bonaccorso, F. Engineered MoSe2-based heterostructures for efficient electrochemical hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1703212. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ren, L.; Zhang, Z.; Qi, X.; Li, H.X.; Zhong, J.X. 3D Binder-free MoSe2 Nanosheets/Carbon Cloth Electrodes for Efficient and Stable Hydrogen Evolution Prepared by Simple Electrophoresis Deposition Strategy. Sci. Rep. 2016, 6, 22516. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558(R). [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J.; Hafner, J. Theory of the Crystal Structures of Selenium and Tellurium: The Effect of Generalized-Gradient Corrections to the Local-Density Approximation. Phys. Rev. B 1994, 50, 13181–13190. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, X.; Zhang, J.; Wang, Z. Synthesis of M-Doped MoSe2 (M = Fe, Co, Ni) via Chemical Vapor Deposition for an Electrocatalytic Hydrogen Evolution Reaction. Inorganics 2025, 13, 155. https://doi.org/10.3390/inorganics13050155
Chen X, Zhang X, Zhang J, Wang Z. Synthesis of M-Doped MoSe2 (M = Fe, Co, Ni) via Chemical Vapor Deposition for an Electrocatalytic Hydrogen Evolution Reaction. Inorganics. 2025; 13(5):155. https://doi.org/10.3390/inorganics13050155
Chicago/Turabian StyleChen, Xinya, Xingchen Zhang, Jinying Zhang, and Zhiyong Wang. 2025. "Synthesis of M-Doped MoSe2 (M = Fe, Co, Ni) via Chemical Vapor Deposition for an Electrocatalytic Hydrogen Evolution Reaction" Inorganics 13, no. 5: 155. https://doi.org/10.3390/inorganics13050155
APA StyleChen, X., Zhang, X., Zhang, J., & Wang, Z. (2025). Synthesis of M-Doped MoSe2 (M = Fe, Co, Ni) via Chemical Vapor Deposition for an Electrocatalytic Hydrogen Evolution Reaction. Inorganics, 13(5), 155. https://doi.org/10.3390/inorganics13050155








