Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control
Abstract
1. Introduction
2. Results and Discussion
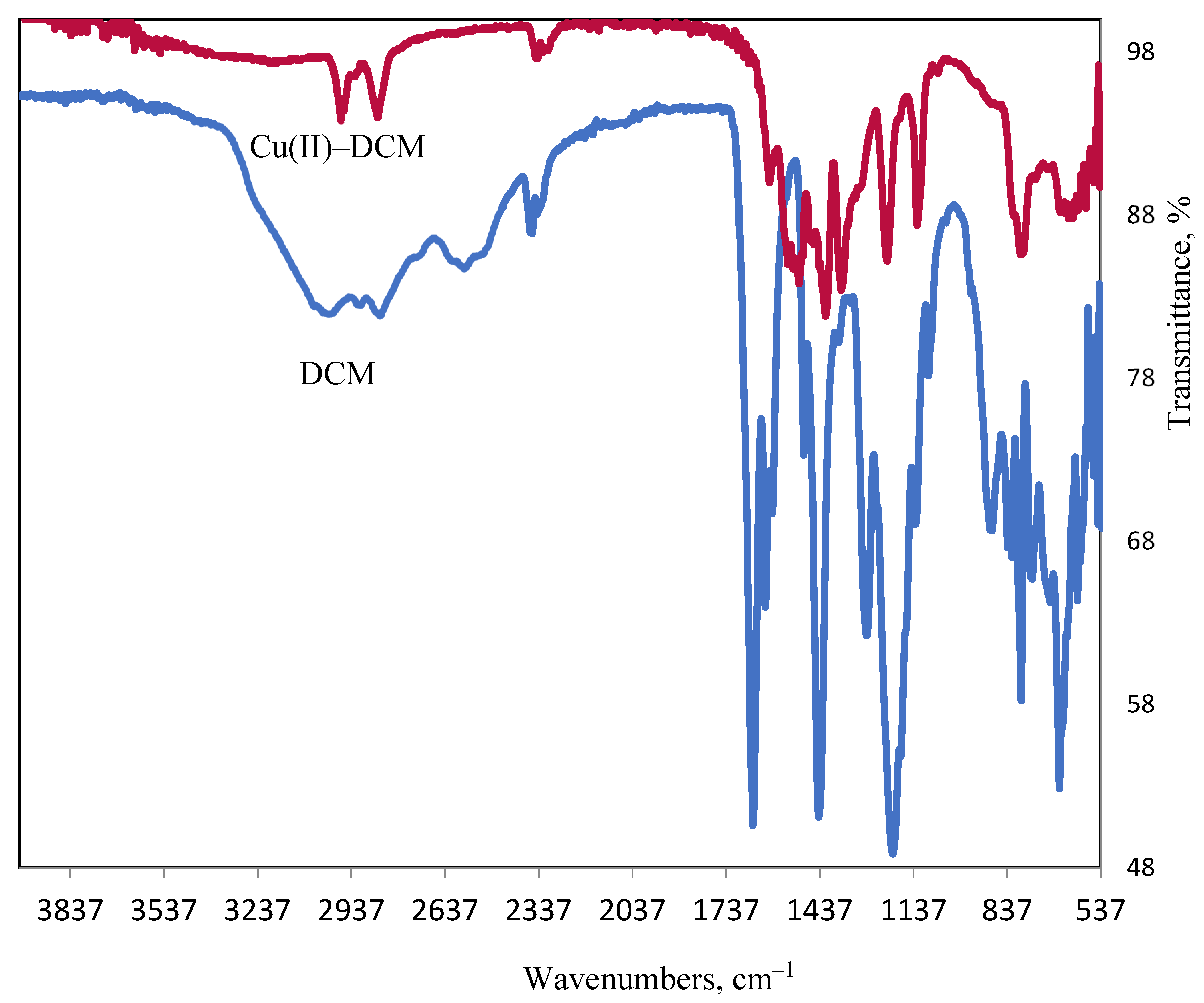
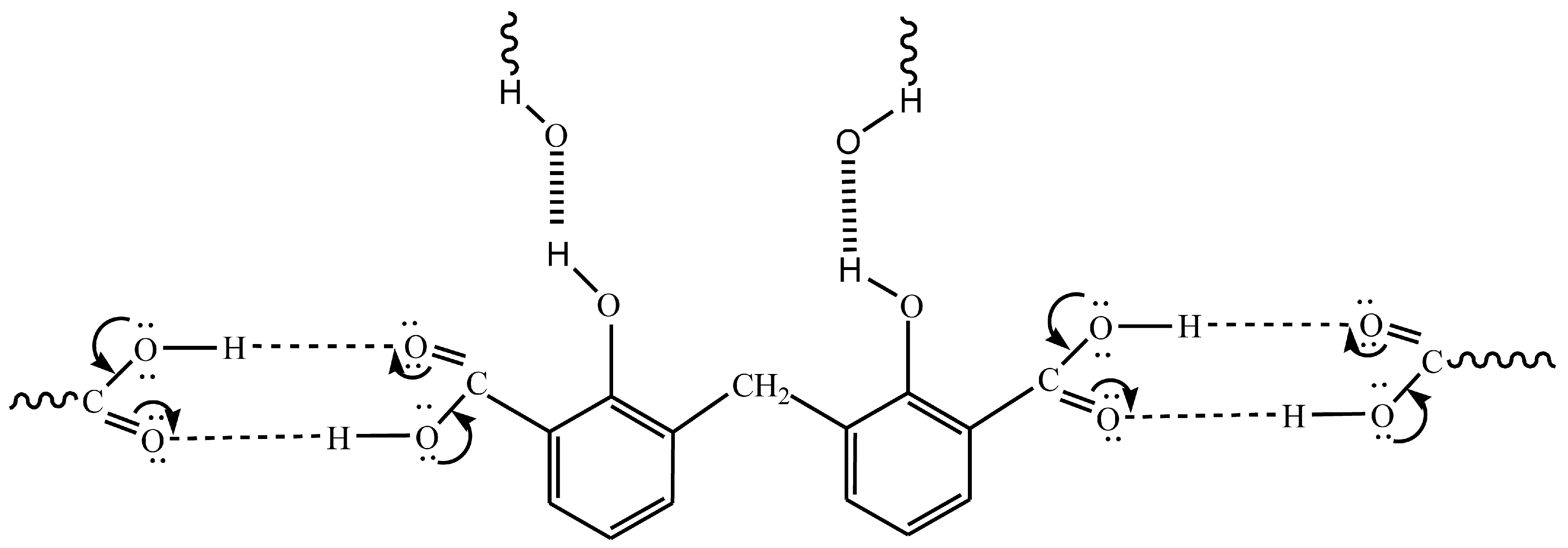
2.1. Infrared Spectroscopy
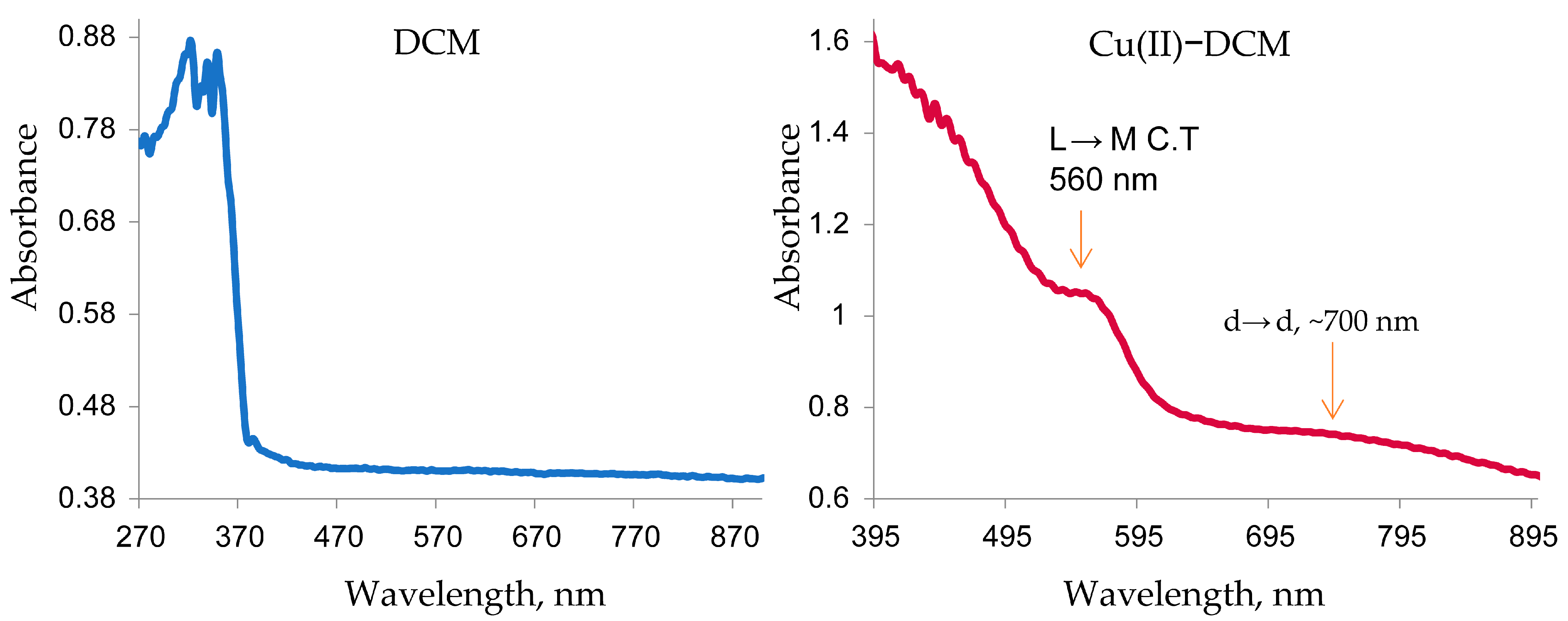
2.2. Ultraviolet–Visible Spectroscopy and Magnetic Investigation
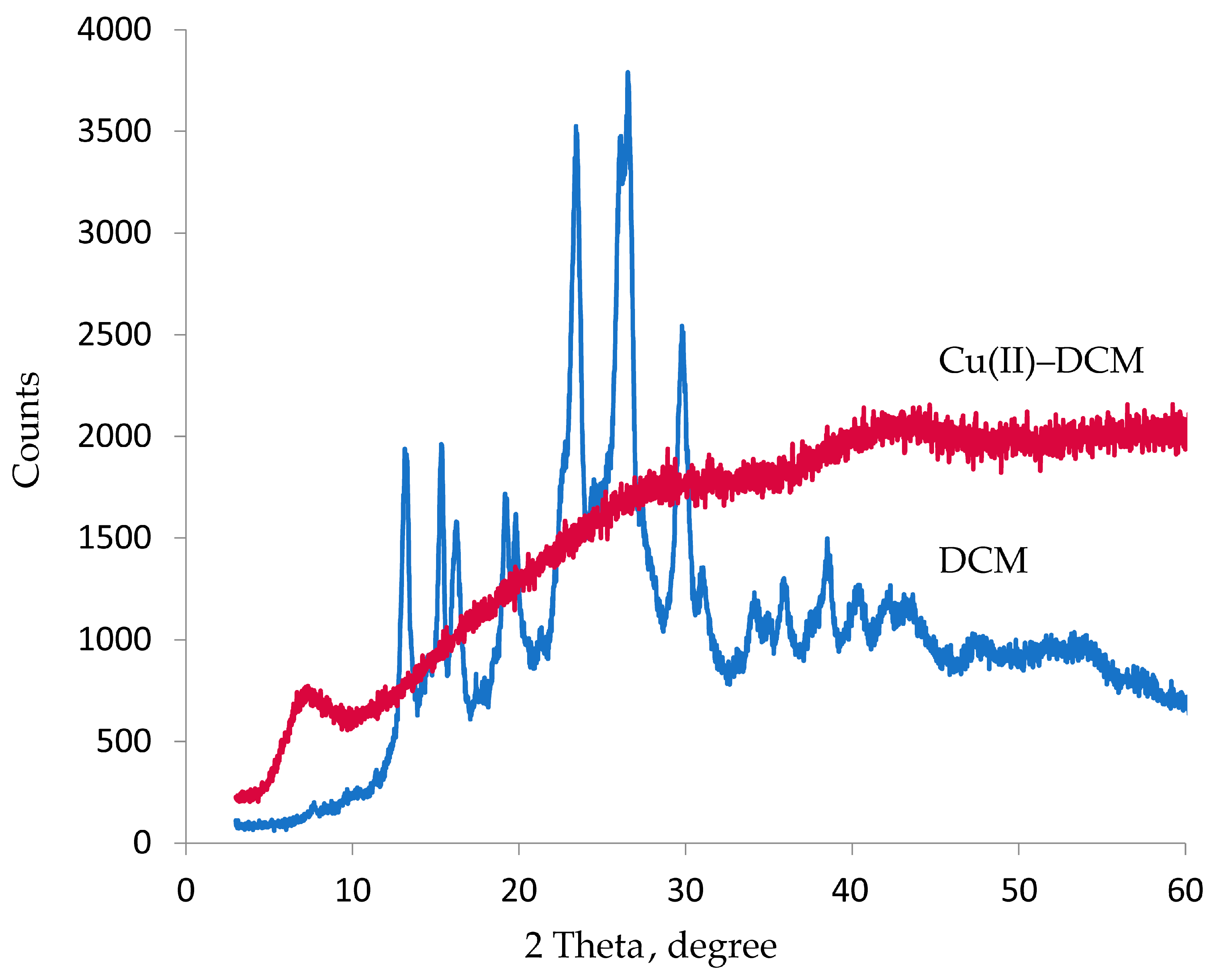
2.3. Powder XRD Studies
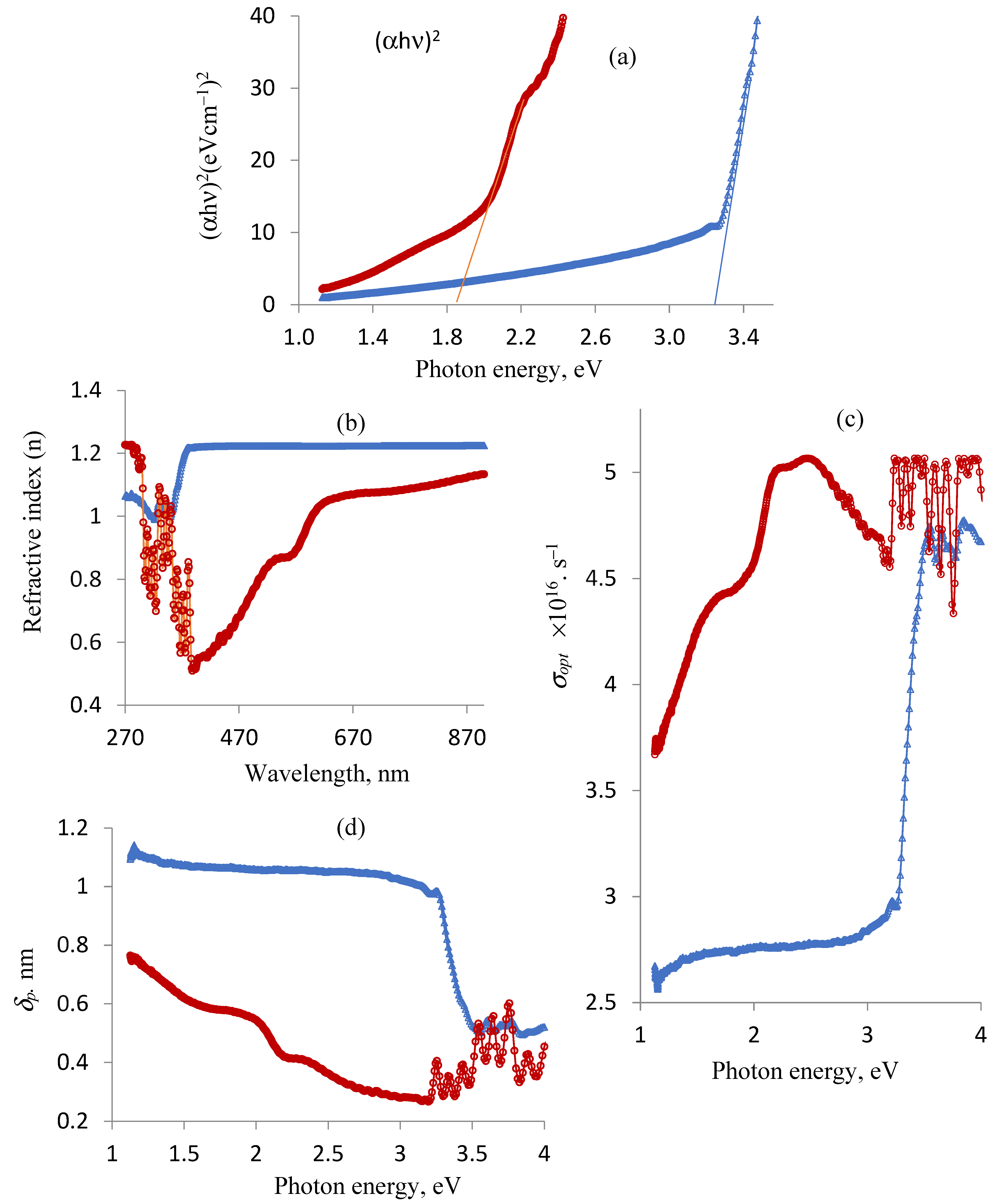
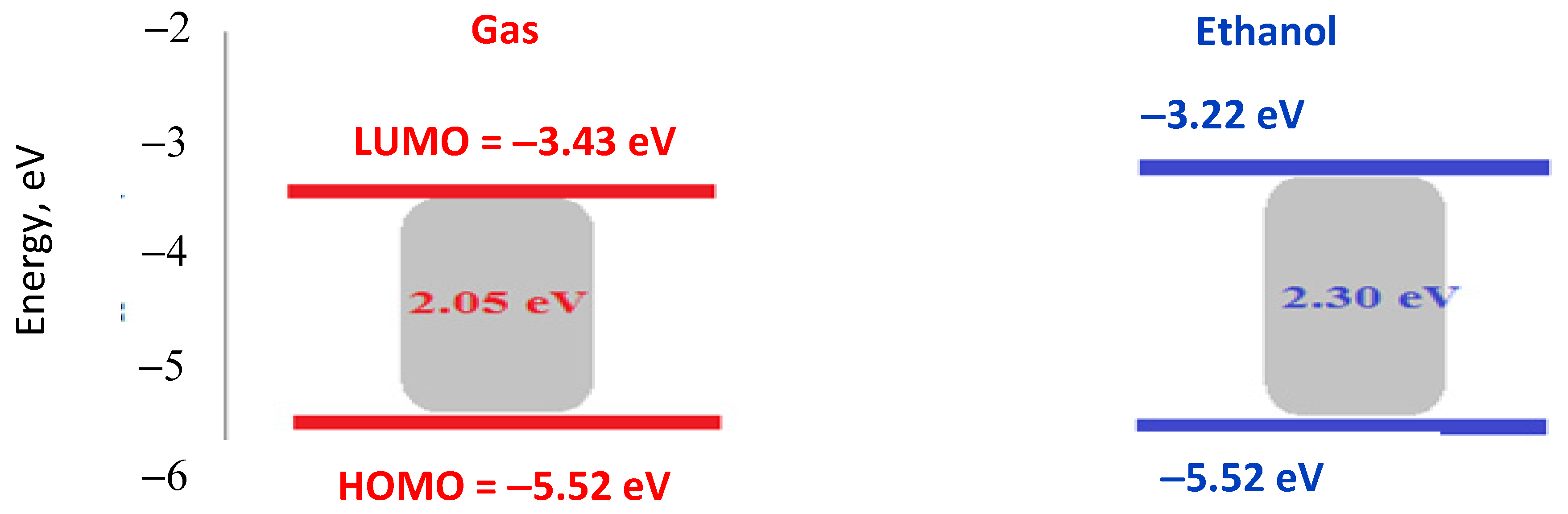
2.4. Optical Characteristics
2.5. Morphology (SEM and TEM)
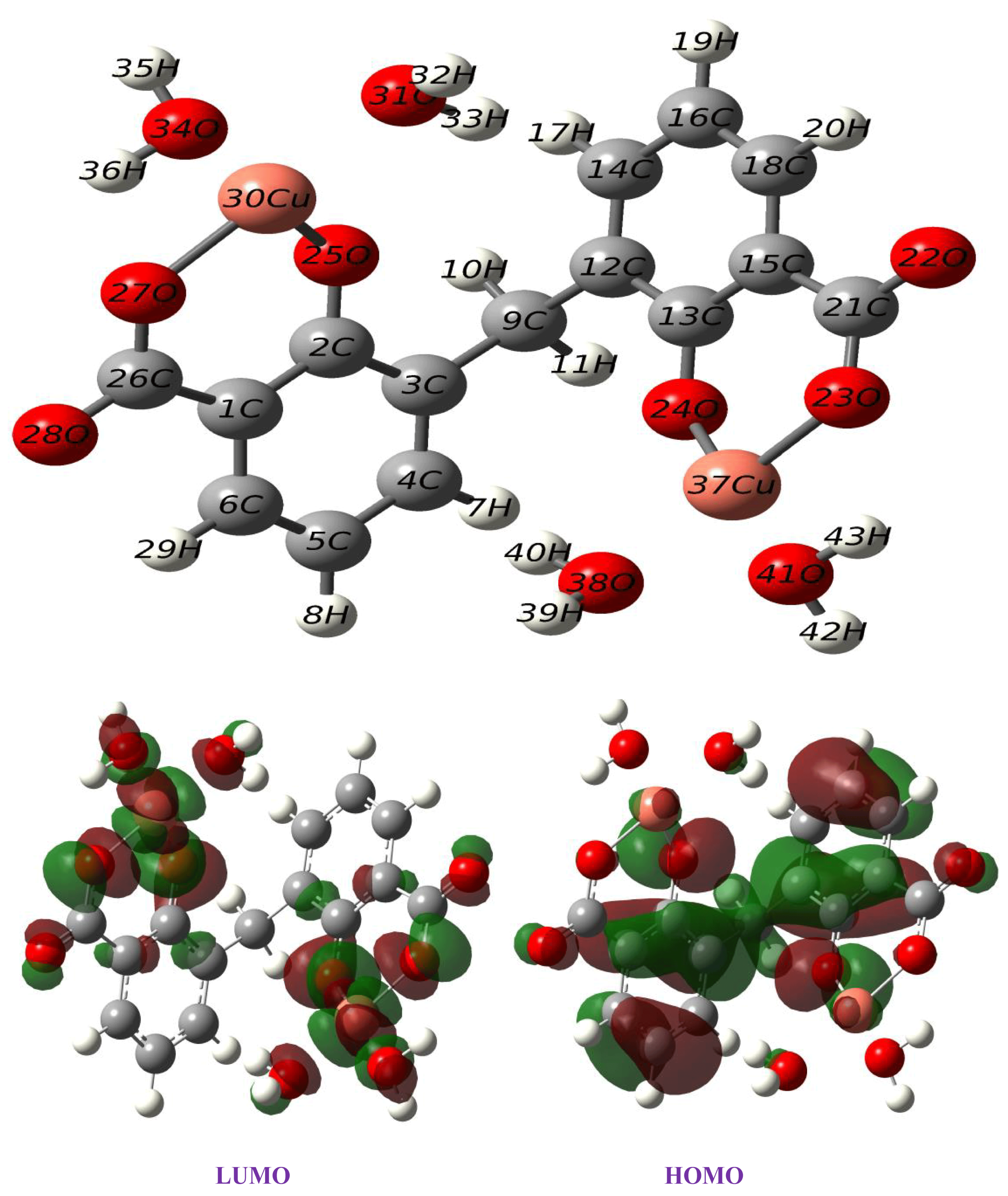
2.6. Theoretical Study
2.7. Cytotoxic Assay
2.8. Molecular Docking Simulation
3. Experimental Section
3.1. Materials and Procedures
3.2. Synthesis of Solid Compounds
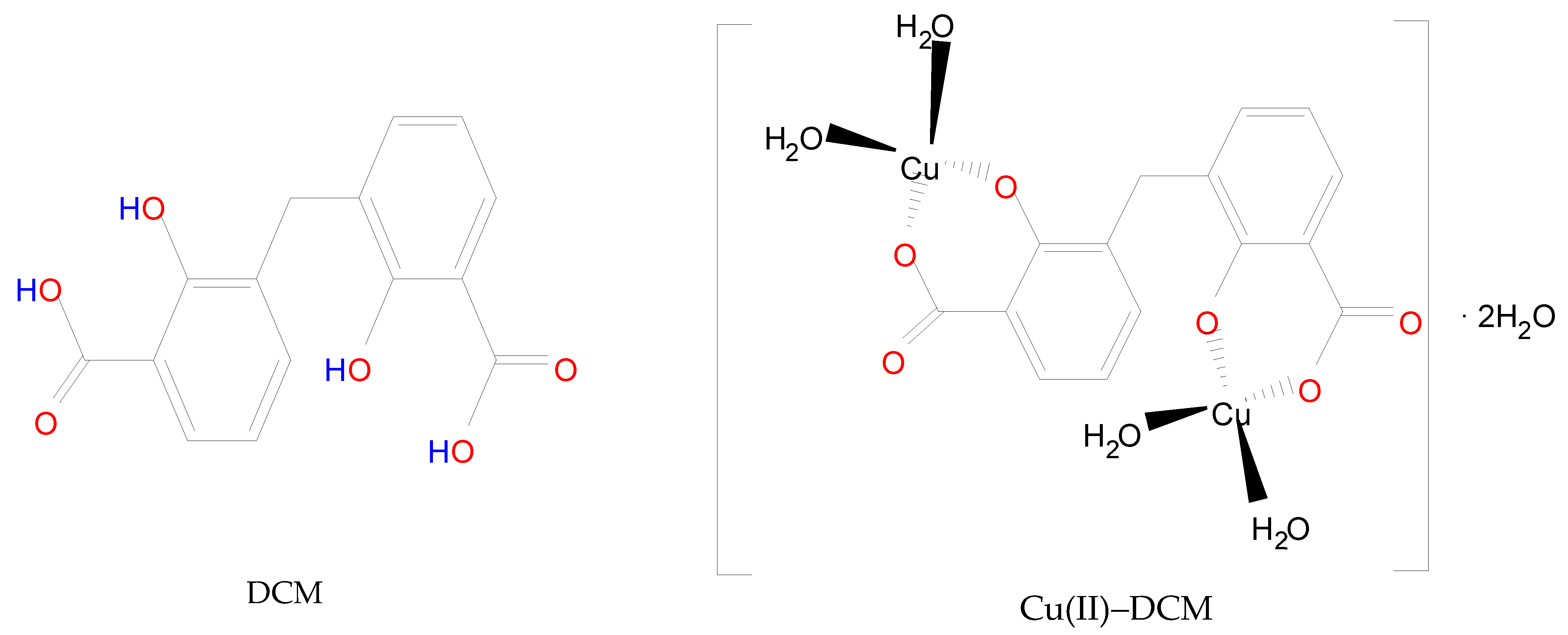
3.2.1. DCM Ligand
3.2.2. Cu(II)–DCM Complex
3.3. Cytotoxicity Evaluation
3.4. Molecular Docking (MD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parulerkar, M.H.; Bhatt, H.A.; Potnis, S.P. New Intermediates for Plastics and Coatings: 1. Preparation and Characterization of Methylene-di-salicylic-acid. J. Ind. Chem. Soc. 1972, 49, 1201–1207. [Google Scholar]
- Gao, F.; Chunji, N.; Jiazuan, N. Study on Mixed Ligand Complexes of Rare Earth with Nitrilotriacetic Acid and Amino acid. Chin. J. Appl. Chem. 1990, 3, 10–13. [Google Scholar] [CrossRef]
- Trevin, S.; Bedioui, F.; Gomez, M.G.; Charreton, C.B. Electro Polymerized Nickel Micro Cyclic Complex–Base Films Design and Elctro–catalytic Application. J. Mater. Chem. 1997, 7, 923–928. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Sherif, E.M. Methylenedisalicylic Acid as a Bio Corrosion Inhibitor for Aluminum in Concentrated Sodium Chloride Solutions. ACS Omega 2022, 7, 19193–19203. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Althobaiti, I.O.; Aljohani, M.; Gad, E.S.; Asiri, Y.M.; Hussein, O.A. Mammalian Cell Cytotoxicity, Antibacterial Activity and the Properties of Methylenebis(hydroxybenzoic acid) and Its Related Zinc(II) Complex. Crystals 2024, 14, 88. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Althobaiti, I.O.; Alenezy, E.K.; Asiri, Y.M.; Ghalab, S.; Hussein, O.A. Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex. Molecules 2024, 29, 4239. [Google Scholar] [CrossRef]
- Clemmensen, E.; Heitman, A.H.C. Methylenedisalicylic Acid and Its Reaction with Bromine and Iodine. J. Am. Chem. Soc. 1911, 33, 733–745. [Google Scholar] [CrossRef][Green Version]
- Sivapullaiah, P.V.; Soundararajan, S. Methylene di Salicylates of Rare–Earths. J. Indian Inst. Sci. 1976, 58, 289–293. [Google Scholar]
- Tobita, S.; Arakawa, M.; Tanaka, I. The Paramagnetic Metal Effect on the Ligand Localized S1. apprx. fwdarw. T1 Intersystem Crossing in the Rare–Earth–Metal Complexes with Methyl Salicylate. J. Phys. Chem. 1985, 89, 5649–5654. [Google Scholar] [CrossRef]
- Patel, R.P.; Karampurwala, A.M.; Shah, J.R. Physicochemical Studies on Square Planar Co2+, Ni2+ and Cu2+ Chelate Polymers. Macromol. Mater. Eng. 1980, 87, 87–94. [Google Scholar]
- Han, Z.X.; Wang, J.J.; Hu, H.M.; Chen, X.L.; Wu, Q.R.; Li, D.S.; Shi, Q.Z. Effects of the Size of Aromatic Chelate Ligands and d10 Metal Ions on the Structures of Dicarboxylate Complexes: From Dinuclear Molecule to Helical Chains and 2D Network. J. Mol. Struct. 2008, 891, 364–369. [Google Scholar] [CrossRef]
- Shi, X.M.; Li, M.X.; He, X.; Liu, H.-J.; Shao, M. Crystal Structures and Properties of Four Coordination Polymers Constructed from Flexible Pamoic Acid. Polyhedron 2010, 29, 2075–2080. [Google Scholar] [CrossRef]
- Zhang, L.N.; Sun, X.L.; Du, C.X.; Hou, H.W. Structural Diversity and Fluorescent Properties of New Metal–Organic Frameworks Constructed from Pamoic Acid and Different N-Donor Ligands. Polyhedron 2014, 72, 90–95. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Xiong, J.B.; Tan, Y.H.; Wang, Y.; Deng, Y.P.; Xu, Q.; Wen, H.R. Solvothermal Syntheses, Crystal Structures and Photoluminescent Properties of Four Coordination Polymers with Pamoic Acid and Pyridine mixed Ligands. Inorganica Chim. Acta 2014, 410, 82–87. [Google Scholar] [CrossRef]
- Kerraj, S.; Salah, M.; Chtita, S.; El Idrissi, M.; Belaaouad, S.; Mohammed, M.; Acharjee, N.; Komiha, N. Theoretical Study of Photovoltaic Performances of Ru, Rh and Ir Half Sandwich Complexes Containing N,N Chelating Ligands in Dye-Sensitized Solar Cells (DSSCs). DFT and TD-DFT Investigation. Comput. Theor. Chem. 2022, 1209, 113630. [Google Scholar] [CrossRef]
- Kherrouba, A.; Bensegueni, R.; Guergouri, M.; Boulkedid, A.-L.; Boutebdja, M.; Bencharif, M. Synthesis, cCystal Structures, Optical Properties, DFT and TD-DFT Studies of Ni (II) Complexes with Imine-Based ligands. J. Mol. Struct. 2022, 1247, 131351. [Google Scholar] [CrossRef]
- Patra, A.; Puschmann, H.; Manna, S.C. Bidentate Schiff Base Coordinated Square Planer Nickel(II) Complexes: Synthesis, Crystal Structure, DFT/TD-DFT Calculation and DNA/Protein binding. Polyhedron 2021, 201, 115146. [Google Scholar] [CrossRef]
- Jouypazadeh, H.; Farrokhpour, H.; Karbasizadeh, M.; Hadadzadeh, H. Water-Vapochromic Behavior of a Mononuclear Pd(II) Complex of Piroxicam: A DFT and TD-DFT study. J. Mol. Graph. Model. 2021, 102, 107773. [Google Scholar] [CrossRef]
- Brahim, H. DFT/TD-DFT Investigation on the UV–vis Absorption and Phosphorescence Spectra of Platinum(II) and Palladium(II) Complexes with Schiff-Base Ligands. J. Lumin. 2019, 210, 96–103. [Google Scholar] [CrossRef]
- Luňák, S.; Aysha, T.; Lyčka, A.; Machalický, O.; Hrdina, R. Structure and Absorption of Co(III) Azo Complex Dyes Based on Pyrrolinone esters: DFT and TD DFT Study. Chem. Phys. Lett. 2014, 608, 213–218. [Google Scholar] [CrossRef]
- Aglan, H.A.; Ahmed, H.H.; El-Toumy, S.A.; Mahmoud, N.S. Gallic Acid Against Hepatocellular Carcinoma: An Integrated Scheme of the Potential Mechanisms of Action from In Vivo Study. Tumor Biol. 2017, 39, 1010428317699127. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Imane, A.; Srinivasan, K.K.; Pathak, L.; Daoud, I. In Silico Drug-Designing Studies on Flavanoids as Anticolon Cancer Agents: Pharmacophore Mapping, Molecular Docking, and Monte Carlo Method-Based QSAR Modeling. Interdiscip. Sci. 2017, 9, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Swetha, M.; Keerthana, C.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2021, 12, 809308. [Google Scholar]
- Selvam, C.; Prabu, S.L.; Jordan, B.C.; Purushothaman, Y.; Umamaheswari, A.; Zare, M.S.H.; Thilagavathi, R. Molecular Mechanisms of Curcumin and Its Analogs in Colon Cancer Prevention and Treatment. Life Sci. 2019, 239, 117032. [Google Scholar] [CrossRef]
- Lin, M.H.; Cheng, C.H.; Chen, K.C.; Leem, W.T.; Wang, Y.F.; Xiao, C.Q.; Lin, C.W. Induction of ROS–Independent JNK–Activation–Mediated Apoptosis by a Novel Coumarin–Derivative, DMAC, in Human Colon Cancer Cells. Chem. Biol. Interact. 2014, 218, 42–49. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, C.; Cheng, C.; Liao, D.; Liu, J.; Chen, G. Ginger Polysaccharide UGP1 Suppressed Human Colon Cancer Growth Via p53, Bax/Bcl–2, Caspase–3 Pathways and Immunomodulation. Food Sci. Hum. Wellness 2023, 12, 467–476. [Google Scholar] [CrossRef]
- De, S.; Paul, S.; Manna, A.; Majumder, C.; Pal, K.; Casarcia, N.; Mondal, A.; Banerjee, S.; Nelson, V.K.; Ghosh, S.; et al. Phenolic Phytochemicals for Prevention and Treatment of Colorectal Cancer: A Critical Evaluation of In Vivo Studies. Cancers 2023, 15, 993. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.; Karimi, E.; Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E. Involvement of Salicylic Acid on AntiOxidant and Anticancer Properties, Anthocyanin Production Anthocyanin Production and Chalcone Synthase Activity in Ginger (Zingiber officinale Roscoe) Varieties. Int. J. Mol. Sci. 2012, 13, 14828–14844. [Google Scholar] [CrossRef]
- Kalinowska, M.; Mazur, L.; Jabłońska–Trypuć, A.; Lewandowski, W. A New Calcium 2,5–dihydroxybenzoate: Synthesis, Characterization and Antioxidant Studies and Stress Mediated Cytotoxity in MCF-7 cells. J. Saud. Chem. Soc. 2018, 22, 742–756. [Google Scholar] [CrossRef]
- Bonta, R.K. Dietary Phenolic Acids and Flavonoids as Potential Anti–Cancer Agents: Current State of the Art and Future Perspectives, Anti-Cancer Agent. Anti-Cancer Agents Med. Chem. 2020, 20, 29–48. [Google Scholar] [CrossRef]
- Kattan, S.W.; Nafie, M.S.; Elmgeed, G.A.; Alelwani, W.; Badar, M.; Tantawy, M.A. Molecular Docking, Anti-Proliferative Activity and Induction of Apoptosis in Human Liver Cancer Cells Treated with Androstane Derivatives: Implication of PI3K/AKT/mTOR Pathway. J. Steroid. Biochem. Mol. Biol. 2020, 198, 105604. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, H.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided Drug. Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Hurwitz, H.I. Targeted Inhibition of VEGF Receptor 2: An Update on Ramucirumab. Expert. Opin. Biol. Ther. 2013, 13, 1187–1196. [Google Scholar] [CrossRef]
- Kolligs, F.T. Diagnostics and Epidemiology of Colorectal Cancer. Visc. Med. 2016, 32, 158–164. [Google Scholar] [CrossRef]
- Ikwu, F.A.; Isyaku, Y.; Obadawo, B.S.; Lawal, H.A.; Ajibowu, S.A. In Silico Design and Molecular Docking Study of CDK2 Inhibitors with Potent Cytotoxic Activity Against HCT116 Colorectal Cancer Cell Line. J. Genet. Eng. Biotechnol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Bamigboye, M.O.; Clayton, H.S. Spectral, In Vitro Antiradical and Antimicrobial Assessment of Copper Complexes Containing Tridentate Schiff Base Derived from Dihydroxybenzene Functionality with Diaminoethylene Bridge. Spectrosc. Lett. 2021, 54, 212–230. [Google Scholar] [CrossRef]
- Thabet, M.S.; Ahmed, A.H. Ship–in–a–Bottle Synthesis and Physicochemical Studies on Zeolite Encapsulated Mn(II), Mn(III)–Semicarbazone Complexes: Application in the Heterogeneous hydroxylation of Benzene. J. Porous Mater. 2013, 20, 319–330. [Google Scholar] [CrossRef]
- Lal, R.A.; Basumatary, D.; Arjun, K.D.; Kumar, A. Synthesis and Spectral Characterization of Copper(II), Nickel(II) and Manganese(II) Complexes Derived from Bis(2–hydroxy–1–naphthaldehyde) Malonoyldihydrazone. Transit. Met. Chem. 2007, 32, 481–493. [Google Scholar] [CrossRef]
- Choroba, K.; Machura, B.; Szlapa-Kula, A.; Malecki, J.G.; Raposo, L.; Roma-Rodrigues, C.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Square Slanar Au(III), Pt(II) and Cu(II) Complexes with Quinoline-Substituted 2,2′:6′,2″-terpyridine Ligands: From In Vitro to In Vivo Biological Properties. Eur. J. Med. Chem. 2021, 218, 113404. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Moustafa, M.G. Spectroscopic, Morphology and Electrical Conductivity Studies on Co(II), Ni(II), Cu(II) and Mn(II)–oxaloyldihydrazone complexes. J. Saudi Chem. Soc. 2020, 24, 381–392. [Google Scholar] [CrossRef]
- Du, M.; Li, C.P.; Zhao, X.J.; Yu, Q. Interplay of Coordinative and Supramolecular Interactions in Engineering Unusual Crystalline Architectures of Low-Dimensional Metal–Pamoate Complexes under Co-Ligand Intervention. Cryst. Eng. Comm. 2007, 9, 1011–1028. [Google Scholar] [CrossRef]
- Joseyphus, R.S.; Nair, M.S. Synthesis, Characterization and Biological Studies of Some Co(II), Ni(II) and Cu(II) Complexes Derived from Indole–3–carboxaldehyde and Glycylglycine, as Schiff Base Ligand. Arab. J. Chem. 2010, 3, 195–204. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.-J.; Yang, B. Two novel Cr(III) Complexes [Cr(SA)2(en)]TBA and [Cr(SA)(en)2]Br: Synthesis, Characterization and Spectral Studies. Inorg. Chem. Commun. 2013, 30, 163–167. [Google Scholar] [CrossRef]
- Hayashita, T.; Higuchi, T.; Sawano, H.; Marchand, A.P.; Kumar, K.A.; Bott, S.G.; Mlinarić-Majerski, K.; Sumanovac, T.; Elkarim, N.S.; Hwang, H.S.; et al. Molecular Design of Lipophilic Disalicylic Acid Compounds with Varying Spacers for Selective Lead(II) extraction. Talanta 2000, 30, 385–396. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Streetman, B.G. Solid State Electronic Devices, 5th ed.; Prentice: Hoboken, NJ, USA, 2000. [Google Scholar]
- Elliott, R.J. Symmetry of Excitons in Cu2O. Phys. Rev. 1961, 124, 340. [Google Scholar] [CrossRef]
- Sengupta, S.K.; Pandey, O.P.; Srivastava, B.K.; Sharma, V. Trends in Structural Mechanics: Theory, Practice. Transit. Met. Chem. 1998, 23, 349–353. [Google Scholar] [CrossRef]
- Turan, N.; Gündüz, B.; Körkoca, H.; Adigüzel, R.; Çolak, N.; Buldurun, K. Study of Structure and Spectral Characteristics of the Copper(II) and Copper(II) Complexes with 5,5–dimethyl–2–(2–(3–nitrophenyl)hydrazono)cyclohexane–1,3–dione and Their Effects on Optical Properties and the Developing of the Energy Band Gap and Investigation of Antibacterial Activity. J. Mex. Chem. Soc. 2014, 58, 65–75. [Google Scholar]
- Gittleman, J.I.; Sichel, E.K.; Arie, Y. Composite Semiconductors: Selective Absorbers of Solar Energy. Sol. Energy Mater. 1979, 1, 93–104. [Google Scholar] [CrossRef]
- Belmokhtar, A.; Yahiaoui, A.; Hachemaoui, A.I.; Abdelghani, B.; Sahli, N.; Belbachir, M. A Novel Poly{(2,5–diylfuran)(benzylidene)}: A New Synthetic Approach and Electronic Properties. ISRN Phys. Chem. 2012, 2012, 781879. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Erten, H. Refractive Index Dispersion and Analysis of the Optical Constants of an Ionomer Thin Film. Opt. Appl. 2005, 4, 969–976. [Google Scholar]
- Paul, T.C.; Podder, J. Synthesis and Characterization of Zn–incorporated TiO2 Thin Films: Impact of Crystallite Size on X-Ray Line Broadening and Bandgap Tuning. Appl. Phys. A 2019, 125, 818. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Montoro-Bustos, A.R.; Pereira-Reyes, R.; Paniagua, S.A.; Vega-Baudrit, J.R. Novel Pathway for the Sonochemical Synthesis of Silver Nanoparticles with Near-Spherical Shape and High Stability in Aqueous Media. Sci. Rep. 2022, 12, 882. [Google Scholar] [CrossRef]
- Min, H.S. The Influence of Concentration on the Formation of Chemical Bath Deposited Copper Tin Sulphide Thin Films: SEM and EDX Studies. J. Chem. Eng. Res. Updates 2022, 9, 22–29. [Google Scholar]
- Ejidike, I.P.; Direm, A.; Parlak, C.; Adeniyi, A.A.; Ata, M.A.; Eze, M.O.; Hollett, J.W.; Clayton, H.S. Spectroscopic Characterization, DFT Calculations, in Vitro Pharmacological Potentials, and Molecular Docking Studies of N,N,O-Schiff Base and Its Trivalent Metal Complexes. Chem. Phys. Impact 2024, 8, 100549. [Google Scholar] [CrossRef]
- Tabares-Mendoza, C.; Guadarrama, P. Predicting the Catalytic Efficiency by Quantum-Chemical Descriptors: Theoretical Study of Pincer Metallic Complexes Involved in the Catalytic Heck Reaction. J. Organomet. Chem. 2006, 691, 2978–2986. [Google Scholar] [CrossRef]
- Saren, D.; Das, S.; Paul, A.; Tat, S.S.; Santra, M.K.; Si, T.K.; Puschmann, H.; Manna, S.C. Tris Chelated Meridional Isomers of Co(III) Complexes: Synthesis, Crystal Structure, Protein Binding, Cytotoxicity Studies and DFT/TDDFT Calculation. Inorg. Chim. Acta 2023, 550, 121423. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Bahadori, M.; Moghadam, M.; Ashfaq, M.; Munawar, K.S.; Tahir, M.N. Pd(II) and Ni(II) Complexes Containing ONNO Tetradentate Schiff Base Ligand: Synthesis, Crystal Structure, Spectral Characterization, Theoretical Studies, and Use of PdL as an Efficient Homogeneous Catalyst for Suzuki–Miyaura Cross-Coupling Reaction. Polyhedron 2022, 213, 115622. [Google Scholar] [CrossRef]
- Ahmed, A.H. N,N’–Bis[2–hydroxynaphthylidene]/[2–methoxybenzylidene]amino]oxamides and Their Divalent Manganese Complexes: Isolation, Spectral Characterization, Morphology, Antibacterial and Cytotoxicity Against Leukemia Cells. Open Chem. 2020, 18, 426–437. [Google Scholar] [CrossRef]
- Varga, I.; Luburić, D.B.; Kolanovic, B.S.; Varenina, I.K.; Bilandžić, N. Salicylic Acid-a Medicine with Various Healing Properties. Vet. Stanica 2018, 49, 413–422. [Google Scholar]
- Wiśniewska, J.; Klasik-Ciszewska, S.; Duda-Grychtoł, K. Salicylic Acid and Its Use in Cosmetology. Aesth. Cosmetol. Med. 2023, 12, 91–95. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully Automatic Flexible Molecular Docking Using a Molecular Similarity-Based Search Engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Jin, W. Mechanism of Glycitein in the Treatment of Colon Cancer Based on Network Pharmacology and Molecular Docking. Lifestyle Genom. 2023, 16, 1–10. [Google Scholar] [CrossRef]
- Vogel, A.I. A Text Book of Quantitative Inorganic Analysis; Longmans: London, UK, 1961. [Google Scholar]
- Ahmed, A.H.; Thabet, M.S. Metallo–Hydrazone Complexes Immobilized in ZeoliteY: Synthesis, Identification and Acidviolet–1 Degradation. J. Mol. Struct. 2011, 1006, 527–535. [Google Scholar] [CrossRef]
- Drago, R.S. Physical Methods in Inorganic Chemistry, 1st ed.; Affiliated East–West Press Pvt. Ltd.: Darya Ganj, New Delhi, India, 2012. [Google Scholar]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non–Crystalline Materials, 2nd ed.; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M.; Omran, A.A. Copper(II)–Oxaloyldihydrazone Complexes: Physico–Chemical Studies; Energy Band Gap and Inhibition Evaluation of Free Oxaloyldihydrazones Toward the Corrosion of Copper metal in Acidic Medium. Arab. J. Chem. 2019, 12, 4287–4302. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Time-Dependent Density Functional Theory for Molecules in Liquid Solutions. J. Chem. Phys. 2001, 115, 4708–4717. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Haque, R.A.; Iqbal, M.A.; Khadeer, M.B. Design, Synthesis and Structural Studies of Meta-Xylyl Linked Bis-Benzimidazolium Salts: Potential Anticancer Agents Against Human Colon Cancer. Chem. Cent. J. 2012, 6, 68. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P. 3-Hydrazinoisatin-Based Benzenesulfonamides as Novel Carbonic Anhydrase Inhibitors Endowed with Anticancer Activity: Synthesis, in Vitro Biological Evaluation and in Silico Insights. Eur. J. Med. Chem. 2019, 184, 111768. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-rafted-poly(vinylpyridine) as Basic Catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Albqmi, M.; Elkanzi, N.A.A.; Ali, A.M.; Abdou, A. Design, Characterization, and DFT Exploration of New Mononuclear Fe(III) and Co(II) Complexes Based on Isatin-hydrazone Derivative: Anti-Inflammatory Profiling and Molecular Docking Insights. J. Mol. Struct. 2025, 1319, 139494. [Google Scholar] [CrossRef]
- Mert, S.; Demir, Y.; Sert, Y.; Kasımoğulları, R.; Gülçin, İ. Synthesis, Biological Evaluation and Molecular Docking of Novel Pyrazole Derivatives as Multitarget Acetylcholinesterase and Carbonic Anhydrase Inhibitors. J. Mol. Struct. 2025, 1319, 139472. [Google Scholar] [CrossRef]
- Mustafa, G.; Younas, S.; Mahrosh, H.S.; Albeshr, M.F.; Bhat, E.A. Molecular Docking and Simulation-Binding Analysis of Plant Phytochemicals with the Hepatocellular Carcinoma Targets Epidermal Growth Factor Receptor and Caspase-9. Molecules 2023, 28, 3583. [Google Scholar] [CrossRef]
- Coumar, M.S. Molecular Docking for Computer-Aided Drug Design: Fundamentals, Techniques, Resources and Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Wang, S. Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Curr. Issues Mol. Biol. 2023, 45, 6564–6582. [Google Scholar] [CrossRef]






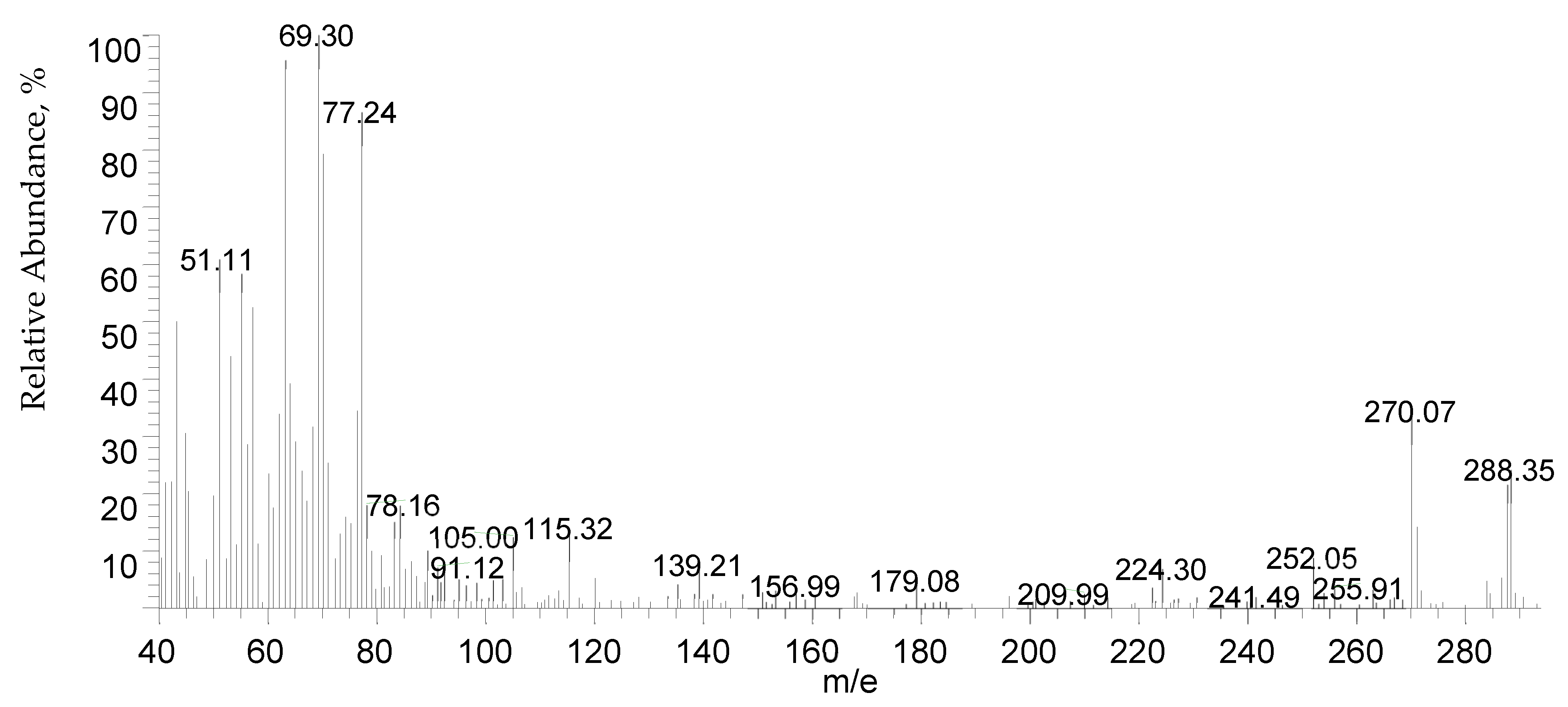
| Compound | XRD Data | |||
|---|---|---|---|---|
| Angle (d-Value) | θ° | β (rad) | D (nm) | |
| DCM | 13.21° (6.699 Å), 15.32° (5.778 Å), 16.24°(5.454 Å), 19.17° (4.626 Å), 19.80° (4.481 Å), 22.70° (3.915 Å), 23.42° (3.795 Å), 26.52° (3.358 Å), 29.79° (2.997 Å), 30.99° (2.883 Å), 34.08° (2.629 Å), 35.86° (2.502 Å), 38.49° (2.337 Å), 40.30° (2.236 Å), 42.07° (2.146 Å) | 23.47 | 0.011 | 14.40 |
| Cu(II)–DCM | 7.32° (12.068 Å) | - | - | - |
| Optimized Geometrical Parameter | Cu(II)–DCM |
|---|---|
| Bond distance, Å | 1.43 (C1-C2)(C2-C3), 1.39(C3-C4)(C5-C6), 1.40(C4-C5)(C6-C1), 1.32 (C2-O25), 1.87(O25-Cu30), 1.88(Cu30-O27), 2.03(Cu30-O31), 2.06(Cu30-O34), 1.31(O27-C26), 1.24(C26-O28), 1.51(C3-C9). |
| Angle (°) | 119.0(C1-C2-C3), 121.7(C3-C4-C5), 124.3(C1-C2-O25), 96.3(O25-Cu30-O27), 88.8(O25-Cu30-O31), 90.0(O31-Cu30-O34), 85.0(O34-Cu30-O27), 119.7(O27-C26-C1). |
| Dihedral angle (°) | 57.5(C2-C3-C9-C12), −2.09(C1-C26-O27-Cu30), 2.09(C9-C3-C2-O25) |
| No. | Energy (eV) | Wavelength (nm) | Osc. Strength | Major Contribs. |
|---|---|---|---|---|
| 1 | 1.16 | 1064.1 | 0.0013 | H-1(B)→L + 1(B)(28%), HOMO(B)→LUMO(B)(62%). |
| 2 | 1.18 | 1050.7 | 0.0032 | H-1(B)→LUMO(B)(35%), HOMO(B)→L + 1(B)(55%). |
| 3 | 1.81 | 685.4 | 0.0122 | H-1(B)→LUMO(B)(61%), HOMO(B)→L + 1(B) (38%). |
| 4 | 1.82 | 679.5 | 0.0021 | H-1(B)→L + 1(B)(67%), HOMO(B)→LUMO(B) (32%) |
| 7 | 2.20 | 562.0 | 0.0084 | H-11(B)→L + 1(B) (17%), H-10(B)→LUMO(B) (18%). |
| 8 | 2.21 | 561.6 | 0.0089 | H-11(B)→LUMO(B) (18%), H-10(B)→L + 1(B) (16%). |
| Compound | Receptor | Binding Affinity(kcal/mol) | Interacting Amino Acids | Docking Parameters |
|---|---|---|---|---|
| L: 3,3′-dicarboxy-2,2′-dihydroxydiphenylmethane acid (DCM) | 6GUE (implicated in the pathophysiology of CRC) | −7.2 | Phe: 82; Glu: 81; His: 84; and Asn: 136 | Exhaustiveness = 20; the center coordinates of the grid box are x = −29.066, y = −9.632, z = −0.1268; the dimensions of the grid box (Ǻ) are x = 149.187, y = 124.808, and z = 142.086. |
| Complex: (Cu(II)–DCM) | −9.3 | Val-29, Glu-81, Phe-82, Lys-142, and Ser-247 | Exhaustiveness = 20; the center coordinates of the grid box are x = −26.0915, y = −6.129, and z = 20.136; the dimensions of the grid box (Ǻ) are x = 126.936, y = 117.523, and z = 117.620. |
| Analysis | Appliance/Description |
| The melting point | The melting point in an open capillary tube was ascertained without correction using the George, Griffin melting point device. |
| C and H% | The elemental analysis for carbon and hydrogen was performed on Perkin Elmer 2400 at the Micro-Analytical Unit of Cairo University, Egypt. |
| Cu content | The Cu(II) content was determined by complexometric titration using EDTA in a hexamine buffer solution at pH = 6 [65]. |
| IR spectra | The IR spectra were recorded using the ATR technique on Thermo Scientific’s iS50 FT–IR spectrometer (Thermo Scientific company, Göteborg–Sweden) at Jouf University, KSA. |
| Electronic spectra | The Nujol mull method was used in the 200–1100 nm region using a Perkin–Elmer lambda 35 UV–Vis spectrophotometer (Perkin-Elmer company, Überlingen–Germany) at Al Azhar University, Egypt. |
| 1H-NMR | A Bruker spectrometer (400 MHz) was used in dimethylsulfoxide–d6 at room temperature. Tetramethylsilane (TMS) was employed as the reference, and chemical shifts were provided in δ (ppm). The analysis was performed at the Regional Center of Mycology and Biotechnology, Cairo, Egypt. |
| Mass spectroscopy | The Thermo Scientific GCMS model ISQ was selected to confirm the molecular weight expectation using the direct inlet method. |
| Magnetic susceptibility | The magnetic susceptibility balance of the Johnson Metthy product (manufactured by Sherwood Scientific in Cambridge, UK) was used to determine the effective magnetic moment µeff at room temperature [66,67]. For this purpose, the relation applied to determine the mass magnetic susceptibility (χg) was as follows: where R = the balance reading for the sample in the tube, Ro = the balance reading for the empty tube, l = the sample length in cm, W2 = the weight of the sample and tube in gm, W1 = the weight of the tube empty in gm, and χg = the mass magnetic susceptibility in c.g.s. |
| XRD | Using Germany’s Bruker Co. D8 Discover XRD analyzer (Bruker company, Bremen, Germany), the crystallinity of DCM and the Cu(II)–DCM complex was examined at 3 to 80 degrees using Cu target K radiation (1.54 Å, 40 kV and 40 mA). |
| Morphology | A Quanta FEG 250 scanning electron microscope (FEI Company, Hillsboro, OR, USA) was used to obtain surface images of the copper complex where SEM stubs were used to mount the samples. For TEM investigation, a drop of solution was placed on the carbon-coated copper grids, and they were then left to dry out at room temperature by evaporation. A JEOL GEM–1010 transmission electron microscope was used to generate electron micrographs at 80 kV. |
| Optical band gap, HOMO (Highest Occupied Molecular Orbital), and LUMO (Lowest Unoccupied Molecular Orbital) forms | The Tauc’s equation, αhυ = A(hυ − Eg)m, was applied to estimate the energy gap (Eg) [68,69], where m is equal to 1/2 for direct transitions (electron–photon interacion) and 2 for indirect transitions (electron–photon–phonon interaction), respectively, and A is an energy-independent constant. The Chem. Draw Professional 16 program was used to identify the MM2 (molecular mechanics) and MMFF94 (molecular mechanics force field) energies, HOMO and LUMO forms, and optimal lowest energy structure for the compounds. The following particular Chem3D settings are utilized to minimize energy: (1) MM2 minimization (frame interval = 10, step interval = 2, target temperature = 300 K, minimum RMS gradient = 0.01) and (2) MMFF94 minimization (minimum RMS gradient = 0.100, maximum number of iterations = 500; Vander Vaals: cut off parameter = 10; electrostatic: dielectric exponent = 1, dielectric constant = 1). |
| DFT | DFT calculations were performed using the Gaussian 09 (G09) software package [70] by using the (B3LYP) level of theory [71]. A 6–31 g (d, p) basis set was used for C, H, and O atoms, and effective core potentials plus the Double Zeta (LanL2DZ) were employed for the Cu atom [72] for the present calculation. The ground-state geometric structure of the Cu(II)–DCM complex was fully optimized at the B3LYP level. Vibrational frequency calculations were conducted to confirm that the optimized geometry corresponds to a local minimum, indicated by exclusively positive eigenvalues. Vertical electronic excitations were then determined using TD-DFT (time-dependent density functional theory) [73] with the B3LYP functional in ethanol, modeled through CPCM [74]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.H.; Althobaiti, I.O.; Soliman, K.A.; Asiri, Y.M.; Alenezy, E.K.; Alrashdi, S.; Gad, E.S. Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control. Inorganics 2025, 13, 151. https://doi.org/10.3390/inorganics13050151
Ahmed AH, Althobaiti IO, Soliman KA, Asiri YM, Alenezy EK, Alrashdi S, Gad ES. Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control. Inorganics. 2025; 13(5):151. https://doi.org/10.3390/inorganics13050151
Chicago/Turabian StyleAhmed, Ayman H., Ibrahim O. Althobaiti, Kamal A. Soliman, Yazeed M. Asiri, Ebtsam K. Alenezy, Saad Alrashdi, and Ehab S. Gad. 2025. "Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control" Inorganics 13, no. 5: 151. https://doi.org/10.3390/inorganics13050151
APA StyleAhmed, A. H., Althobaiti, I. O., Soliman, K. A., Asiri, Y. M., Alenezy, E. K., Alrashdi, S., & Gad, E. S. (2025). Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control. Inorganics, 13(5), 151. https://doi.org/10.3390/inorganics13050151









