Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
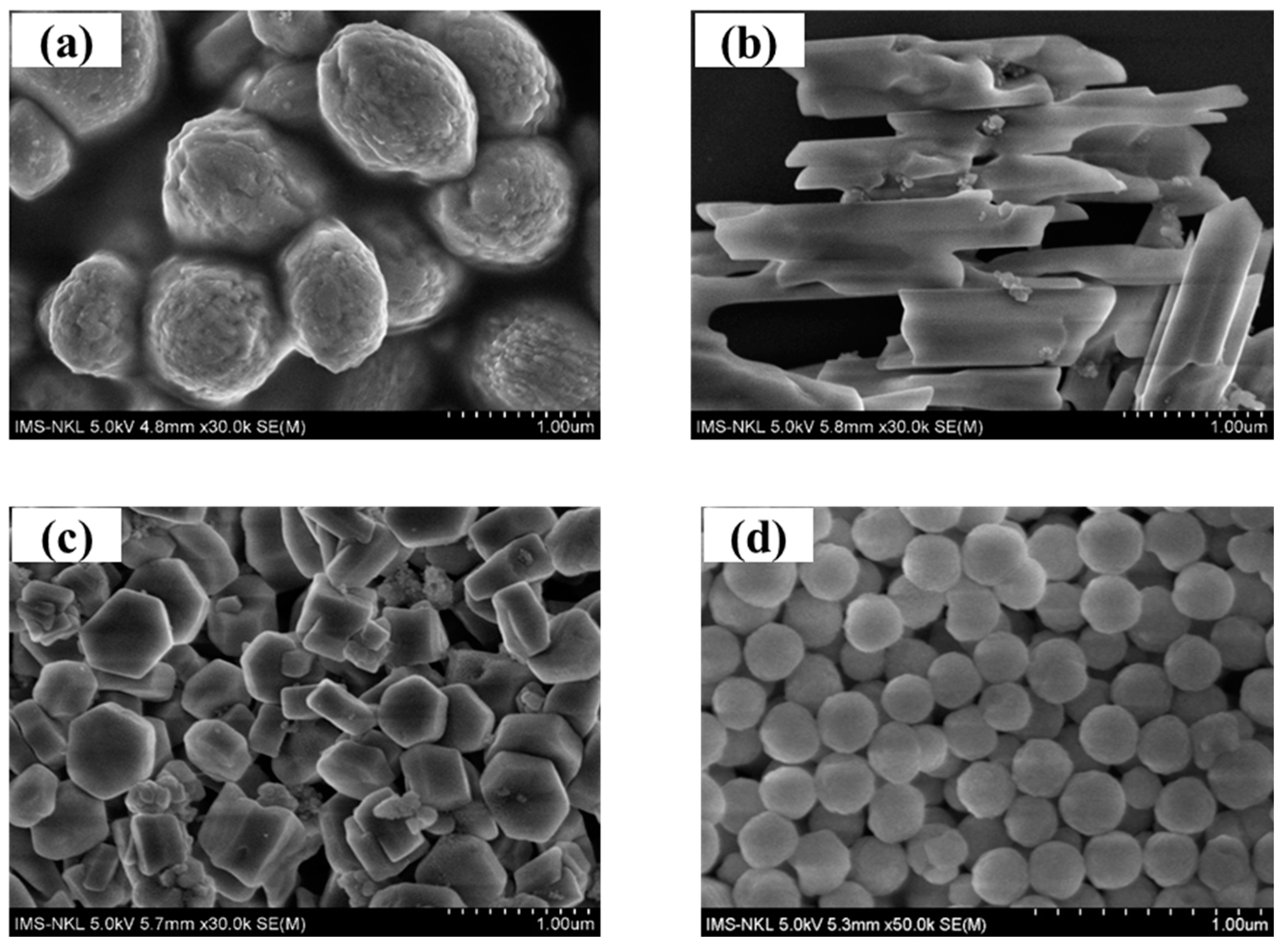
2.1. Morphological Characterization
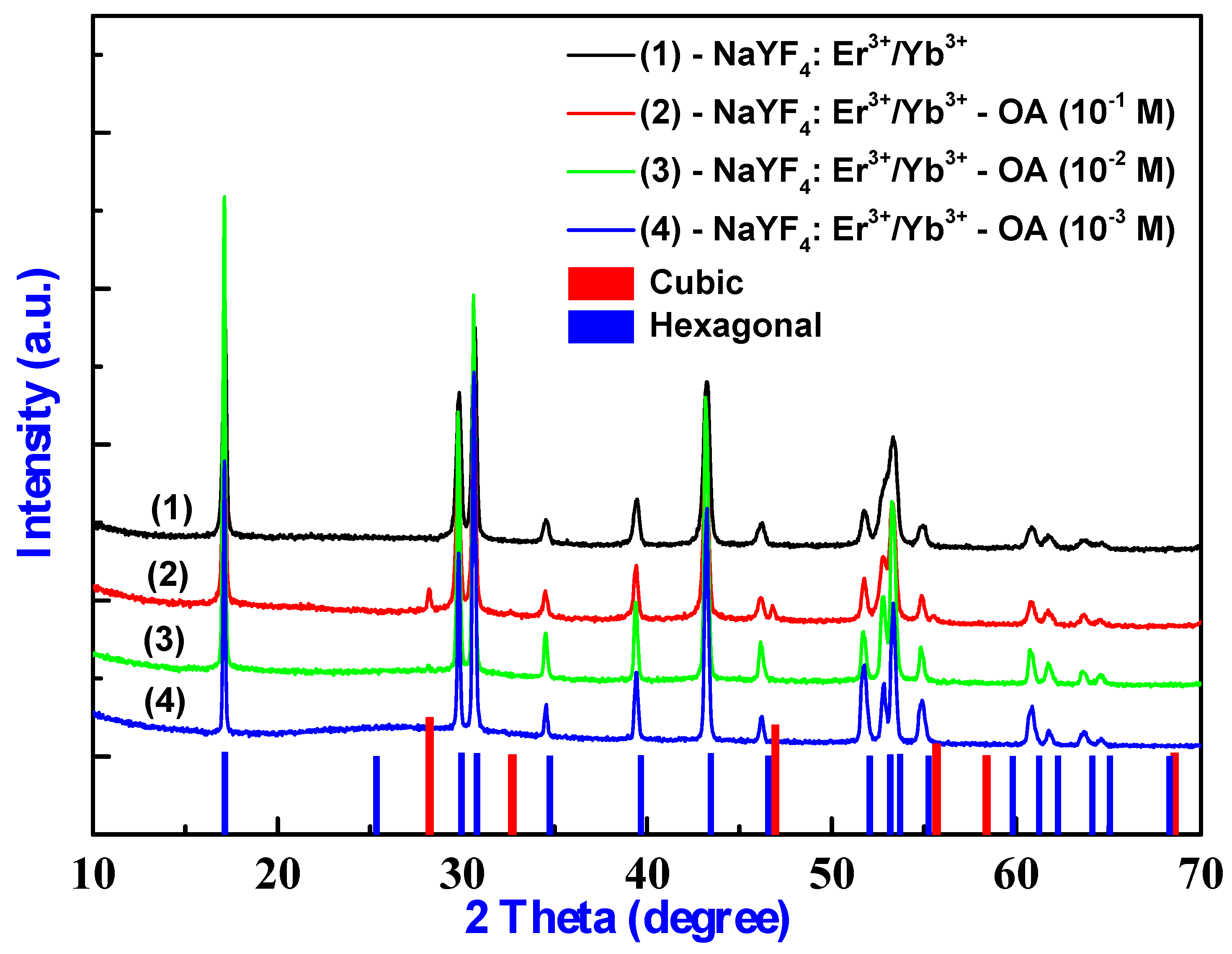
2.2. Structure Characterization
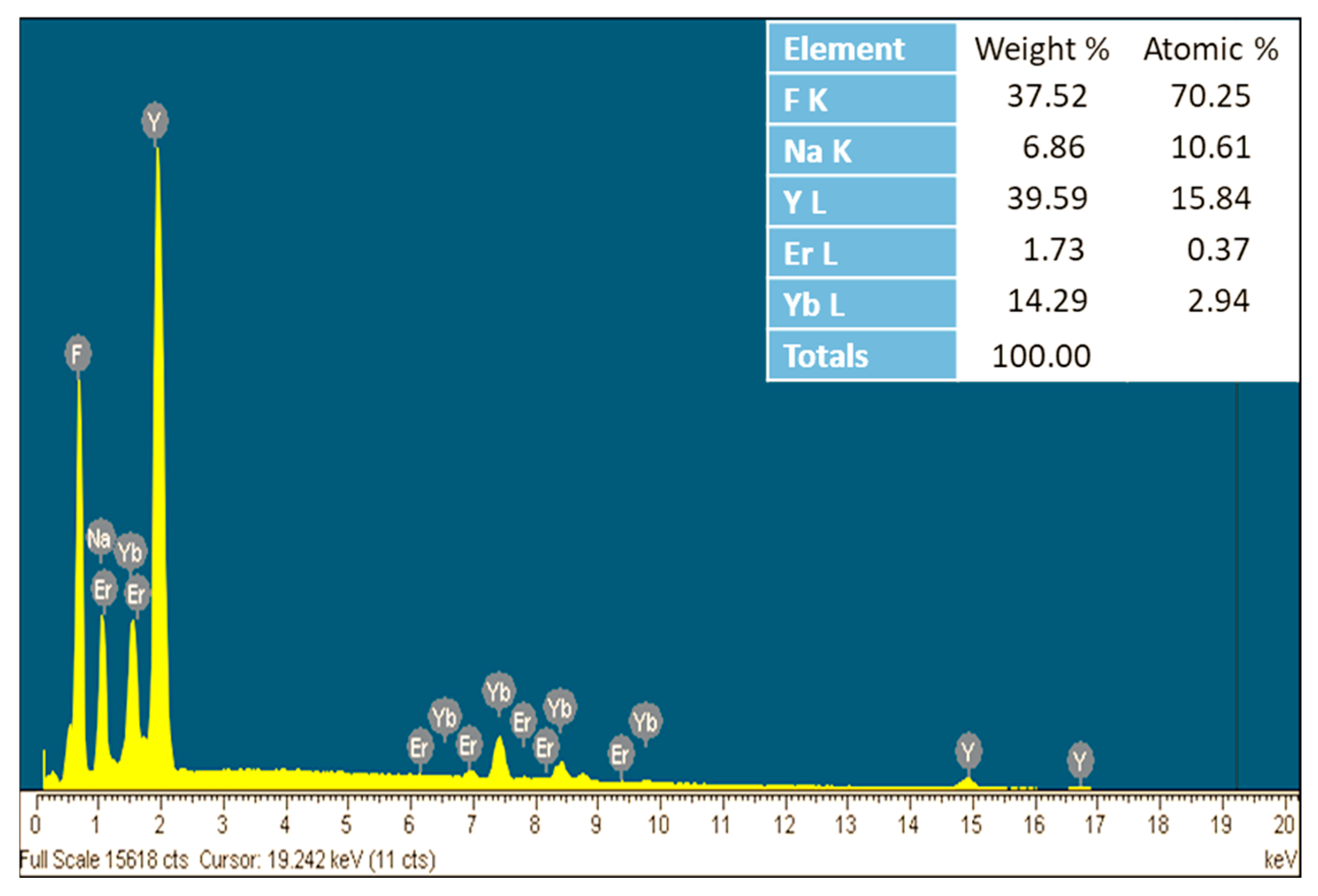
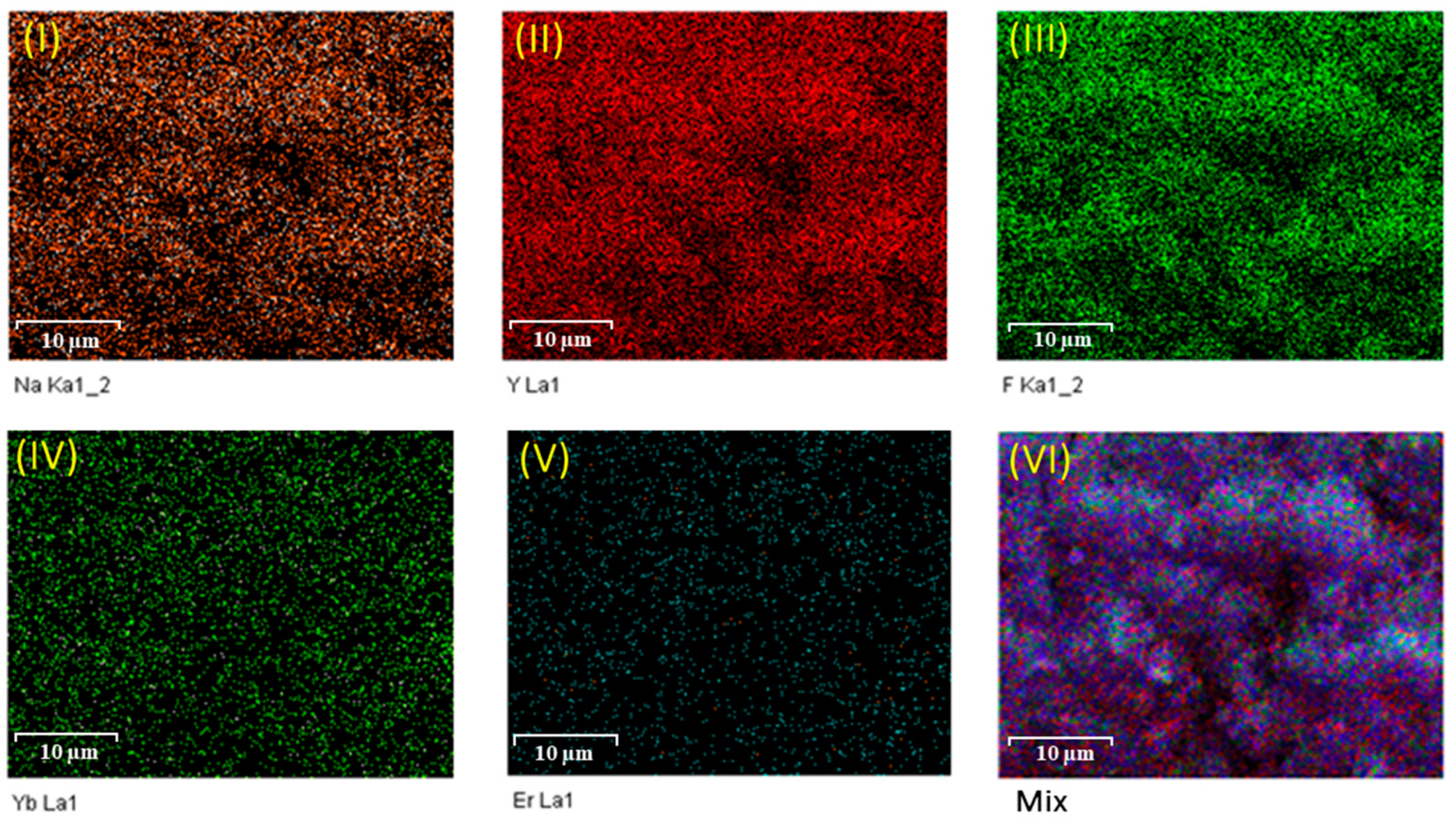
2.3. Energy Dispersive X-Ray Analysis
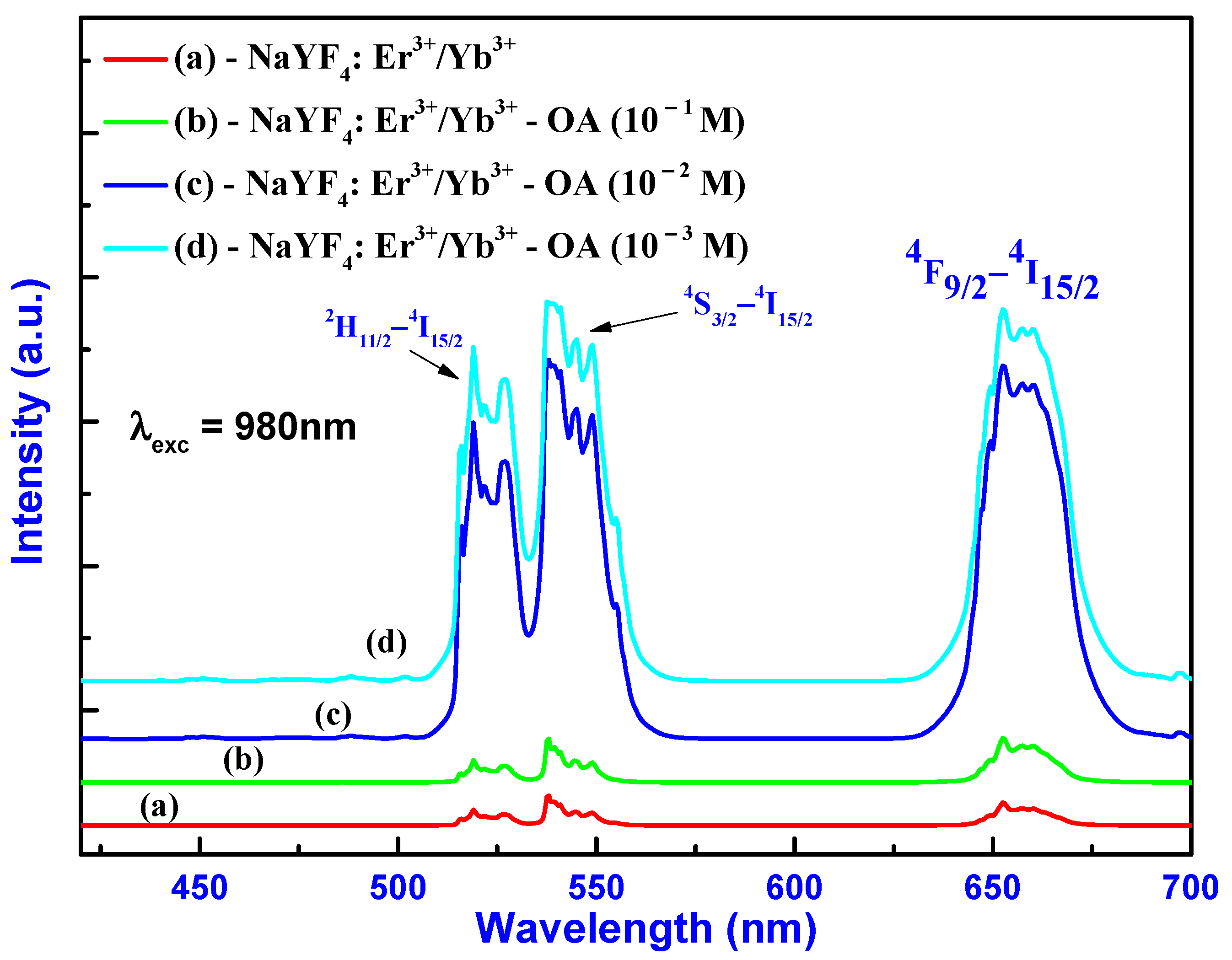
2.4. Luminescence Properties
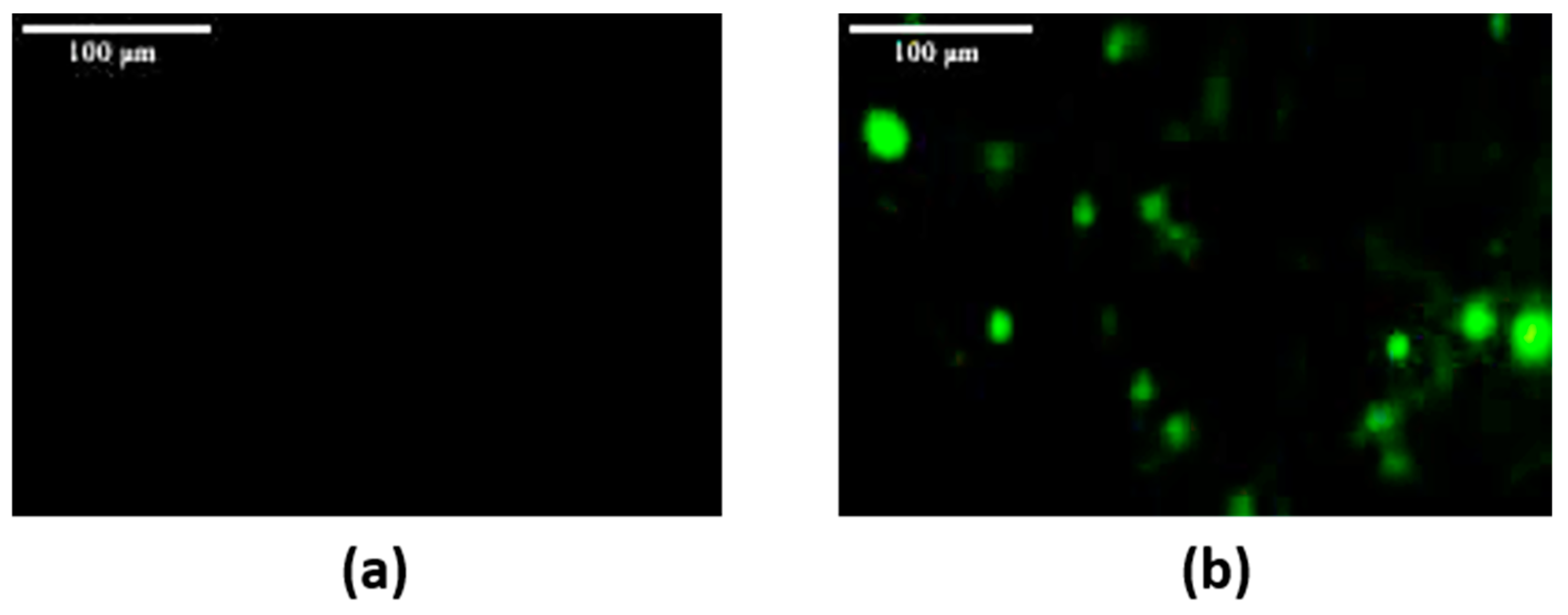
2.5. In Vitro Cellular Imaging
3. Experimental
3.1. Materials and Methods
3.2. Preparation of NaYF4: Er3+/Yb3+ Nanoparticles
3.3. Preparation of NaYF4: Er3+/Yb3+@SNGA-IgG Bio-NanoComplexes
3.4. Experiment on Incubating the Biomedical NaYF4: Er3+/Yb3+@SNGA-IgG Nanocomplex with HeLa Cervical Cancer Cells
3.4.1. In Vitro Cell Culture
3.4.2. Experiment for Labeling HeLa Cervical Cancer Cells Using the Biomedical Nanocomplex NaYF4: Er3+/Yb3+@SNGA-IgG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaoguang, Y.; Gang, C.; Yansong, Z.; Zhonghui, H. Recent advances in rare-earth elements modification of inorganic semiconductor-based photocatalysts for efficient solar energy conversion: A review. J. Rare Earths 2015, 33, 453–462. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Zhang, D.; Tripp, C.P.; Yin, S.; Qin, W. Photocatalysis of NaYF4:Yb,Er/CdSe composites under 1560 nm laser excitation. RSC Adv. 2016, 6, 8127–8133. [Google Scholar] [CrossRef]
- You, M.; Zhong, J.; Hong, Y.; Duan, Z.; Lin, M.; Xu, F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale 2015, 7, 4423–4431. [Google Scholar] [CrossRef]
- Shao, J.; Yan, J.; Xiaoguang, L.; Li, S.; Hu, T. Novel fluorescent label based on YVO4: Bi3+, Eu3+ for latent fingerprint detection. Dye. Pigment. 2019, 160, 555–562. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Yaraki, M.T.; Ahmadi, S.; Chiani, M.; Nourouzian, D. Folic acid-functionalized niosomal nanoparticles for selective dual-drug delivery into breast cancer cells: An in-vitro investigation. Adv. Powder Technol. 2020, 31, 4064–4071. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Chitta, R.P.; Resham, B.; Sujata, P.; Sujit, B.; Priyabrata, M.; Debabrata, M. Inorganic Phosphate nanorods are a novel fluorescent label in cell biology. J. Nanobiotechnol. 2006, 4, 11. [Google Scholar]
- Xu, R.; Cao, H.; Lin, D.; Yu, B.; Junle, Q. Lanthanide-doped upconversion nanoparticles for biological super-resolution fluorescence imaging. Cell Rep. Phys. Sci. 2022, 3, 100922. [Google Scholar] [CrossRef]
- Chen, G.; Ågren, H.; Ohulchanskyy, T.Y.; Prasad, P.N. Light upconverting core–shell nanostructures: Nanophotonic control for emerging applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef]
- Liang, X.; Fan, J.; Zhao, Y.; Jin, R. Core–Shell Structured NaYF4:Yb,Er Nanoparticles with Excellent Upconversion Luminescent for Targeted Drug Delivery. J. Clust. Sci. 2021, 32, 1683–1691. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, T.; Su, M.; Luo, J.; Korteweg, C.; Li, J.; Chen, Z.; Qiu, Y.; Liu, X.; Yan, M.; et al. Expression and distribution of immunoglobulin G in the normal liver, hepatocarcinoma and postpartial hepatectomy liver. Lab. Investig. 2014, 94, 1283–1295. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef]
- Feng, W.; Zhu, X.; Li, F. Recent advances in the optimization and functionalization of upconversion nanomaterials for in vivo bioapplications. NPG Asia Mater. 2013, 5, e75. [Google Scholar] [CrossRef]
- Oleksa, V.; Macková, H.; Engstová, H.; Patsula, V.; Shapoval, O.; Velychkivska, N.; Ježek, P.; Horák, D. Poly (N,N-di met hyl acr yla mid e)-coated upconverting NaYF4:Yb,Er@ NaYF4:Nd core–shell nanoparticles for fluorescent labeling of carcinoma cells. Sci. Rep. 2021, 11, 21373. [Google Scholar] [CrossRef]
- Doronkina, A.A.; Kochubey, V.I.; Maksutova, A.V.; Pravdin, A.B.; Mylnikov, A.M.; Navolokin, N.A.; Yanina, I.Y. NaYF4: Yb,Er Upconversion Nanoparticles for Imaging: Effect on Red Blood Cells. Photonics 2023, 10, 1386. [Google Scholar] [CrossRef]
- Skaptsov, A.A.; Ustalkov, S.O.; Mohammed, A.H.; Zakharevich, A.M.; Kozyrev, A.A.; Sagaidachnaya, E.A.; Kochubey, V.I. Application of Upconversion Luminescence of NaYF4:Yb,Er Nanoparticles to Study Protein Coagulation Dynamics. Opt. Spectrosc. 2020, 128, 952–958. [Google Scholar] [CrossRef]
- Hua, L.; Xiaobo, S.; Xia, L.; Lingbo, Z. Size-tunable β-NaYF4:Yb/Er up-converting nanoparticles with a strong green emission synthesized by thermal decomposition. Opt. Mater. 2020, 108, 110144. [Google Scholar] [CrossRef]
- Takahashi, N.; Gubarevich, A.; Sakurai, J.; Hata, S.; Tsuge, T.; Kitamoto, Y.; Yamazaki, Y.; Odawara, O.; Wada, H. Preparation and Optical Properties of Rare Earth Doped Y2O3 Nanoparticles Synthesized by Thermal Decomposition with Oleic Acid. Adv. Mater. Res. 2011, 332–334, 1974–1978. [Google Scholar] [CrossRef]
- Gunaseelan, M.; Yamini, S.; Kumar, G.A.; Senthilselvan, J. Highly efficient upconversion luminescence in hexagonal NaYF4:Yb3+, Er3+ nanocrystals synthesized by a novel reverse microemulsion method. Opt. Mater. 2018, 75, 174–186. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Xu, W.; Dong, B.; Xu, S.; Chen, B.; Yu, W.; Zhang, S. Phase transition, size control and color tuning of NaREF4:Yb3+, Er3+ (RE = Y, Lu) nanocrystals. Nanoscale 2013, 5, 3412–3420. [Google Scholar] [CrossRef]
- Ding, M.; Yin, S.; Ni, Y.; Lu, C.; Chen, D.; Zhong, J.; Ji, Z.; Xu, Z. Controlled synthesis of β-NaYF4:Yb3+/Er3+ microstructures with morphology-and size dependent up conversion luminescence. Ceram. Int. 2015, 41, 7411–7420. [Google Scholar] [CrossRef]
- Xie, J.; Hu, W.; Tian, D.; Wei, Y.; Zheng, G.; Huang, L.; Liang, E. Selective growth and upconversion photoluminescence of Y-based fluorides: From NaYF4: Yb/Er to YF3: Yb/Er crystals. Nanotechnology 2020, 31, 505605. [Google Scholar] [CrossRef]
- Stauffer, M.T. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Capeletti, L.B., Zimnoch, J.H., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Li, Q.; Yuan, J.; Tu, L.; Ning, L.; Zhang, H. Internal OH− induced cascade quenching of upconversion luminescence in NaYF4:Yb/Er nanocrystals. Light Sci. Appl. 2021, 10, 105. [Google Scholar] [CrossRef]
- Huong, T.T.; Phuong, H.T.; Vinh, L.T.; Khuyen, H.T.; Thao, D.T.; Tuyen, L.D.; Anh, T.K.; Minh, L.Q. Upconversion NaYF4:Yb3+/Er3+@silica-TPGS bio-nano complexes: Synthesis, characterization, and in vitro test for labelling cancer cells. J. Phys. Chem. B 2021, 125, 9768–9775. [Google Scholar] [CrossRef]
- Liu, S.; De, G.; Xu, Y.; Wang, X.; Liu, Y.; Cheng, C.; Wang, J. Size, phase-controlled synthesis, the nucleation and growth mechanisms of NaYF4: Yb/Er nanocrystals. J. Rare Earths 2018, 36, 1060–1066. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Huong, T.T.; Vinh, L.T.; Khuyen, H.T.; Tuyen, L.D.; Van, N.D.; Thao, D.T.; Phuong, H.T. Synthesis and in vitro testing of YVO4:Eu3+@silica- NH-GDA-IgG bio-nano complexes for labelling MCF-7 breast cancer cells. Molecules 2023, 28, 280. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong, H.T.; Vinh, L.T.; Cong, T.Q.; Tien, T.Q.; Van, N.D.; Hong Ha, V.T.; Phan, V.N.; Hoi, L.T.; Thang, P.D.; Thao, D.T.; et al. Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications. Inorganics 2025, 13, 140. https://doi.org/10.3390/inorganics13050140
Phuong HT, Vinh LT, Cong TQ, Tien TQ, Van ND, Hong Ha VT, Phan VN, Hoi LT, Thang PD, Thao DT, et al. Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications. Inorganics. 2025; 13(5):140. https://doi.org/10.3390/inorganics13050140
Chicago/Turabian StylePhuong, Ha Thi, Le Thi Vinh, Tong Quang Cong, Tran Quoc Tien, Nguyen Duc Van, Vu Thi Hong Ha, Vu Ngoc Phan, Le Thi Hoi, Pham Duc Thang, Do Thi Thao, and et al. 2025. "Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications" Inorganics 13, no. 5: 140. https://doi.org/10.3390/inorganics13050140
APA StylePhuong, H. T., Vinh, L. T., Cong, T. Q., Tien, T. Q., Van, N. D., Hong Ha, V. T., Phan, V. N., Hoi, L. T., Thang, P. D., Thao, D. T., & Huong, T. T. (2025). Optimized NaYF4: Er3+/Yb3+ Upconversion Nanocomplexes via Oleic Acid for Biomedical Applications. Inorganics, 13(5), 140. https://doi.org/10.3390/inorganics13050140





