Abstract
Energy storage devices are essential for enhancing the effectiveness and sustainability of electrical energy. Lithium-ion batteries (LIBs) are one of the most efficient energy storage solutions available. The choice of electrode materials plays a vital role in defining the performance of an energy storage device. A range of electrode materials have been developed utilizing both organic and inorganic substances. Due to their notable electrochemical characteristics, strong chemical stability, and well-established technological approaches, inorganic materials have been extensively studied to achieve high-performance devices. This review paper aims to provide a thorough and analytical review of different materials ranging from zero- to three-dimensional (3D), like quantum dots, nanotubes, and nanosheets that have been proposed for high-performance LIBs. This study also includes challenges and future pathways to address the issues with inorganic materials utilized as electrode materials for high-performance energy storage LIBs.
1. Introduction
Devices for energy storage are more and more diffusely applied across various domains, including electric power distribution, electronics, automotive, aerospace, etc. [1,2,3], for tackling the global challenges related to the finite availability of fossil fuels and to air pollution reduction.
Many different technologies have been proposed to fulfill the constraints of the required power and energy in such diversified domains. Fuel cells demonstrate significant energy density, yet they possess a relatively low power density. Supercapacitors have higher power but less energy densities and longer cycle lifetimes due to surface charge storage processes [4,5,6] whereas rechargeable batteries provide high energy densities as a result of the faradaic charge storage mechanism [7,8,9,10]. Among these last electrochemical systems, throughout the last three decades, lithium-ion batteries (LIBs) have been used as the power source for a variety of products, including electric vehicles, power tools, laptops, mobile phones, etc. As well as being lightweight, LIBs have the highest energy and power densities compared to the other battery technologies. They also have a long lifecycle and a low self-discharge rate, which makes them reliable and easy to maintain.
The three primary components of a LIB (as of any other battery) are an electrolyte and two electrodes (anode and cathode). The cathode stores ions when charging whereas the anode stores lithium ions during discharging. The presence of electrolytes in batteries enables the movement of ions between the anode and cathode, thereby ensuring efficient energy storage and release. The most commonly used liquid electrolytes employed in LIBs are Lithium salts, like LiPF6. Solid electrolytes, including those composed of ceramic or polymer, as well as gel electrolytes, represent two additional types of electrolytes. Each of these categories offers a distinct array of benefits, encompassing aspects such as safety and stability.
The electrodes significantly influence a battery’s energy storage capacity, efficiency, and overall lifespan. During the charging and discharging processes, the anode and cathode are crucial for ion mobility, directly affecting the electric battery’s overall performance. The choice of material influences ion storage capacity, conductivity, and stability during several charge-discharge cycles. Efforts are now underway to produce and use the most appropriate materials for electrode components in batteries.
Even though there have been improvements over the years, the best electrode materials for LIBs that use the intercalation mechanism have reached their peak performance. This is when lithium ions are reversibly incorporated into the structure of the host material. This implies that investigating novel materials or ion storage processes other than conventional intercalation may be necessary to achieve additional notable increases in energy capacity or efficiency. Because of this, a significant amount of attention has been focused on high-capacity conversion reaction-type cathodes, such as those containing sulfur (lithium-sulfur batteries) and oxygen (lithium-air batteries). Furthermore, safe and affordable rechargeable batteries hold great potential as workable alternatives for large electrical systems [11,12]. Promising potential for enhancing battery performance, such as cyclic stability, specific capacity, specific energy, and charge/discharge rate, has been shown for a variety of electrodes based on nanomaterials [13,14,15,16].
Nanomaterials with a significantly enhanced surface area markedly enhance ion storage and reaction speeds, increasing battery capacity and power output. Their nanoscale dimensions facilitate accelerated ion diffusion and more compact electron transport pathways, leading to enhanced efficiency. Furthermore, nanomaterials possess the potential to accommodate volumetric changes during cycling, thereby enhancing the longevity and stability of batteries. Nanoparticles can be categorized based on their size, shape, and structural morphology.
Researchers have made a lot of progress in the past few years in studying zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) nanostructures like quantum dots, nanotubes, and nanosheets as high-performance electrode materials for energy storage devices. Even though they have a lot of interesting properties, problems like material aggregation, limited electrolyte diffusion, and slow reaction kinetics limit their applicability [17,18].
More than 15,000 publications in 2024 that seek the best electrode material for high-performance LIBs are on the Google Scholar website. We conducted this search in December 2024 using the keywords anode, cathode, and electrode materials for lithium-ion batteries. Based on the literature review, this study aims to analyze the characteristics of 0D, 1D, 2D, and 3D inorganic materials applied for LIB electrodes in an organized way. It focuses on their electrochemical properties, energy and power densities, and important performance metrics like charge transfer efficiency, capacity retention, and cycle stability. The presented results offer a wide-ranging overview of the pros, cons, and trade-offs of each dimensionality by showing how structural morphology affects energy storage mechanisms. This may provide a suitable reference for researchers engaged in designing long-lasting, high-performance energy storage systems.
2. Electrode Materials for Use in High-Performance ESD
The investigation of high-performance electrodes, electrocatalysts, and photoelectrodes stands as a pivotal element in the commercialization of these renewable energy technologies. Considering that a battery functions as a dynamic chemical reactor, enhancing its performance through the use of high-performance materials necessitates a profound understanding of the processes occurring at every level. Novel electrode materials for Lithium-ion batteries, which are renowned for their exceptional cycle stability and appealing specific capacity, have drawn a lot of interest in recent years [19]. More than 15,000 publications in 2024 that seek the best electrode material for high-performance LIBs are on the Google Scholar website. We conducted this search in December 2024 using the keywords anode, cathode, and electrode materials for lithium-ion batteries.
Typical materials for LIBs include carbon-based materials, metal oxides, conducting polymers, and different combinations of these elements as electrode components. In recent studies, anode materials for LIBs have encompassed carbon and carbon-based materials [20,21,22,23], metal oxides [24,25,26], nitrides [27,28], and carbides [29,30], along with their composites [31,32]. LiCoO2 [33], LiMnO2 [34], and transition-metal phosphates, especially olivine LiFePO4 [35], exemplify layered compounds featuring an anion close-packed or very-close-packed lattice. They have garnered significant attention in both academic inquiry and commercial applications concerning cathode materials. Other materials that are widely utilized include conducting polymers. Electrodes adopted for high-performance energy storage devices include a variety of compounds for applications, such as carbonaceous compounds like graphene and carbon nanotubes [36,37], conducting polymers like PANI [38], metal oxides like MnO2 [39], carbon nanocomposites [40,41], and metal oxide composites [42], among others. Inorganic materials hold considerable importance because of their ability to store lithium through a conversion mechanism [43]. Metals, in combination with carbon, like SnO2 [44], Mo2N [45], FeN [46], VN [47], Ni3N [48], and NbN [49], have also been proposed as electrode materials due to the elevated specific energy and power densities, superior electrical conductivity, thermal stability, and electrochemical behavior.
2.1. Organic Electrode Materials
The swift advancement of OAMs necessitates collaborative efforts across various fields, including computer science, chemistry, and materials science. Organic electrode active materials provide a number of advantages, including their low cost, low impact on the environment, sustainability, adjustable design, and exceptional electrical storage activity. We can source these materials from biomass or produce them through straightforward synthesis methods [50,51]. A wide variety of batteries are available, such as lithium-ion, sodium-ion, aluminium-ion, magnesium-ion, and proton batteries, with considerable emphasis on the investigation of organic active materials (OAMs) [52]. In contrast to the rigidity of inorganic materials, the flexibility of organic materials renders them more suitable for bendable energy storage devices. However, achieving large-scale commercialization requires addressing several challenges [53,54]. At the moment, the main problems that OAMs face in the research field are low conductivity, the ability to dissolve in electrolytes, limited working current, low specific capacity, synthesis technology, and safety.
In particular, it is crucial to consider the safety of organic electrode materials. They exhibit significantly different thermal stability, making them more susceptible to inactivation at elevated temperatures [53].
2.2. Inorganic Electrode Materials
Although inorganic materials are the subject of the majority of the present research, interest in their wider potential in energy storage is growing due to their significant benefits, which include increased surface areas, superior electrical conductivity, enhanced thermal stability, greater number of active sites, and higher capacities compared to organic compounds [54]. A substantial array of inorganic materials serve as anode or cathode materials for LIBs, with numerous reports available in the literature. The mechanism of their response enables them to be classified into three separate categories: alloying reaction (e.g., Si and Sn), intercalation reaction (e.g., Li4Ti5O12), and conversion reaction (e.g., MnO and Fe2O3) [55,56].
Silicon has a very high specific capacity of 4200 mA h g−1 due to a 4.4-electron alloying reaction. It also has a very low operational voltage of about 0.4 V compared to Li/Li+ [56]. Phosphorus (P) is an interesting anode material because it has a theoretical specific capacity of 2595 mA h g−1. This comes from the reaction of 3Li + P ↔ Li3 P. Metal oxides exhibit high experimental and theoretical specific capacities, as documented in numerous studies [57,58]. Over the past 10 years, according to data available on Google Scholar, more than 50,000 have been published on inorganic electrode materials for lithium-ion batteries. The cathode material serves as a fundamental element that directly influences the overall performance of batteries. Inorganic cathode materials have garnered significant interest, primarily due to their impressive discharge capacity. The metallic elements typically exhibit accessible oxidation states (e.g., Mn2+/Mn3+/Mn4+, V3+/V4+/V5+, and Fe2+/Fe3+/Fe4+), resulting in multi-electron reactions throughout the charge and discharge cycles. The Li2FeSiO4 (LFSO) material is cheap and good for the environment. It can help with a two-electron reaction that has a theoretical specific capacity of 332 mA h g−1 [59]. Newly discovered materials, like P14AQ/CNT nanocomposite and Triplite LiFeSO4F [60], have a lot of potential as cathodes.
Many inorganic nano-materials have been thoroughly investigated as LIB electrode materials, including metal oxides, metal phosphides, and oxysalt nanoparticles. The tiny size of these nanoparticles enables a reduction in the collective electrode tension (mechanical stress/strain) resulting from Li insertion/removal and the diffusion channel between lithium ions. Nevertheless, a significant issue with these nanoparticles is their comparatively poor conductivity, particularly when they are in weak contact [61,62,63].
3. Nanostructured Inorganic Electrode Materials
In the sequel, the classification proposed by Pokropivny and Skorokhod for NSMs [64], encompassing 0D, 1D, 2D, and 3D NSMs, is adopted. The advancements observed in recent years indicate that NSMs possess significant potential for the innovation of new technologies aimed at meeting the enduring demand for energy. But there are still constraints on using different types of nanostructures with different properties.
Zero-dimensional (0D) nanostructures like quantum dots are strongly reactive with a quick ion exchange that is made possible by high surface-to-volume ratios, although charge transport may be hampered by their poor connectivity. Additionally, 1D materials are perfect for enhancing conductivity in energy devices because they provide continuous channels for the passage of electrons and ions. Two-dimensional materials in layered structures provide huge surface areas and high ion diffusion rates, which are essential for energy storage in batteries and supercapacitor electrodes. Additionally, 3D materials show a stability and energy storage capacity enhanced by porous structures that optimize surface area and facilitate effective molecular movement. Every dimensionality has a distinct contribution and customized morphologies are becoming more and more important for high-energy storage devices [64]. Figure 1 illustrates the diverse dimensions of categorized nanomaterials [65].
Figure 1.
Different possible dimensions of the nanomaterials.
Table 1, Shows a summary of some relevant properties of nanomaterials with different sizes (0D, 1D, 2D, and 3D) used in LIBs. This comparison underscores the possible advantages of each solution in enhancing electrochemical performance and energy storage efficiency. Due to their unique structural properties, 0D and 3D materials often have high initial capacities in some cases. However, they often face problems like poor cycling stability, agglomeration (in 0D), and inefficient ion diffusion (in 3D). These problems arise because of shape restrictions, such as particle aggregation, volume expansion, and limited access to electrolytes. In contrast, 1D and 2D materials typically offer a better balance of capacity, cycle life, and rate performance due to their optimized morphology for ion transport and structural integrity. Therefore, a material’s suitability for lithium-ion batteries depends not only on how much power it can hold but also on how well it can keep working repeatedly and in real-world situations.

Table 1.
Zero to Three Dimensional inorganic nano-materials materials with their characteristics for high-performance LIBs.
3.1. Zero-Dimensional Inorganic Electrode Materials
Zero-dimensional (0D) materials are defined as structures whose dimensions are less than 100 nm on all three sides. These include nanoparticles shaped like onions, spheres, and cages, as well as quantum dots. These structures may provide useful surfaces for electrochemical activity because of their specific dimensions [107]. Published literature extensively covers zero-dimensional electrode materials and the blending of 0D nanoparticles with other materials for use as LIB electrode materials. The electrochemical characteristics of zero-dimensional materials are highlighted, with particular attention paid to specific capacity, current density, and cyclic performance. Their usefulness and potential for use in cutting-edge energy storage systems are shown by this viewpoint.
Using co-precipitation and molten salt methods, Wang et al. created the zero-dimensional anode material Ca3Co4O9, which works very well in LIBs. The 0D polyhedron anode had a high initial charge capacity of 391.9 mA h g−1. It maintained 316.3 mA h g−1 with more than 95% Coulombic efficiency after 100 cycles. The material had a lot of surface area and ion channels that helped lithium ions (Li+) move through it. This makes the capacity 33% higher at five rates. This material proved that micro/nanostructure engineering may enhance cycle stability and rate performance [69].
Choi et al. showed that the π-conjugated molecule had better electrochemical performance. They found that the silicon quantum dot (Si QD), a bridge structure, could discharge at the value of 1957 mA h g−1 initially and keep 63% of its capacity after 100 cycles at a rate of 200 mA g−1. The π-conjugated molecules can make the electrochemical performance of the material better. They protect electrical conductivity and structural integrity from the mechanical stress that comes from the alloying process. The structure of the Si QD cluster improves stability and ion transport, which makes the cycle stability better than with Si QDs that are not clustered. By adding functional groups and altering organic structures, Si QD clusters’ electrochemical properties may be further improved. This approach has significant promise for enhancing the anode materials in high-performing lithium-ion batteries [70]. According to Chen et al. [108], there are numerous dead zones internally present during the fabrication of a material that essentially remains inactive in terms of electrochemical activity. The diffusion depth of an electrolyte is limited to approximately 20 nanometres. It has been demonstrated that quantum dot (QD) structures can offer the necessary small size and a large specific surface area with a specific pore structure, both of which are crucial for improving performance and covering all relevant zones. They synthesized NiCo2S4 quantum dots and applied them onto nickel foam. After CV tests, it was clear that the material NiCo2S4 exhibits a larger CV area compared to the CoS2 and NiS2 in the KOH electrolyte. The GCD analysis revealed the specific charge capacity (calculate the precise charge that a material can deliver or store in relation to its mass) of NiCo2S4 was 987.2 C g−1, which was greater than CoS2 and NiS2. They also constructed and tested a real two-electrode device that consists of nitrogen-doped reduced graphene oxide nanosheets used as the anode material and NiCo2S4 as the cathode material. The real device reached a value of 168.1 F g−1 specific capacitance at a low specific current of 1 A g−1. The GCD curves have almost symmetric charge-discharge branches and a small potential drop. This suggests that the capacitance is very reliable. The cyclic examination of the device demonstrated a stability level of 91.9% after 15,000 cycles. This real device demonstrated a high energy density and power density of 67.5 W h kg−1 and 850 W kg−1, respectively [108].
Wang et al. [109] created and tested a core-shell heterogeneous nanoparticle structure with a carbon shell around a LiFePO4 core. This composite demonstrated a value of 169 mA h g−1 of specific capacity at a 0.6 C discharge rate, which was almost equal to the theoretically reported values for LiFePO4. It maintained a reasonable specific capacity of 90 mA h g−1, even when discharged at a high rate of 60 C. Furthermore, the material’s cyclic ability was remarkable; after 1100 cycles at a discharge rate of 0.6, it had lost less than 5% of its specific capacity.
Oxide coatings, including MgO, Al2O3, and ZnO, have been added to LIB cathode materials to improve their performance. As a result, strain during phase transition may be reduced and the materials’ structural stability can be enhanced. In their study, Ting and colleagues showed that a 10 nm layer of nanocrystalline ZnO may improve the LiCoO2 cathode’s electrochemical performance [110]. LiCoO2, LiNiO2, and LiMn2O4 are examples of electroactive cathode materials that may have oxide coatings applied to their surfaces to minimize phase transitions, enhance structural stability, and prevent electrolyte–cathode contact. It is evident in several additional instances that the coatings could be able to enhance conductivity [111].
Another kind of electrode material utilized in energy storage batteries is transition metal dichalcogenides. When 0D inorganic materials bind with organic ones, they may function effectively. Rana et al. synthesized CNTF/MoS2, a zero-dimensional nanocomposite, for use as a battery electrode. Enhancing conductivity, diffusion, and cycle stability was the main goal. This technique uses MoS2-coated carbon nanotube fibers (CNTFs) to make composite electrodes that do not need metallic collectors or polymer additives. The electrodes’ excellent electrical conductivity and durability surpass those of conventional designs. They achieve a specific capacity of 0.7 Ah g−1 with retention rates of 108% after 50 cycles at a specific current of 0.1 Ag−1 and 89% after 250 cycles at a specific current of 1 A g−1. The flexible composite fabrics provide remarkable mechanical strength and consistent performance at varying current densities. However, CNTFs may be expensive and complicated to produce and process, which could prevent them from being widely used in certain applications. Furthermore, even though carbon nanotubes show remarkable performance metrics, issues with their long-term stability and environmental effects still need to be resolved [74].
When combined with two-dimensional materials, zero-dimensional materials may achieve remarkable results. Because of its large surface area and excellent electrical properties, which are crucial for energy storage, graphene is a popular electrode material. A significant mass of published information concerns graphene pairings with zero-dimensional inorganic materials. This 2D/0D, graphene/inorganic material combination has led to the development of several novel composite electrode material architectures [112]. These include several anode materials, like Co3O4 and SnO2, and cathode materials, including sulfur (S), LiFePO4, V2O5 [113,114,115], MoO2 [116], NiO [117], Si [118], Ge [119], and more.
Li et al. applied SnO2 onto graphene in a structurally and morphologically controlled manner via atomic layer deposition [68]. They used the amorphous SnO2 as an anode material for the real device testing. As a result, the recorded capacity was 793 mA h g−1 after 150 charge-discharge cycles. Over graphene nanosheets, Kim et al. decorated SnO2 nanoparticles and created a SnO2/graphene composite for enhancing the LIB performance [73]. In comparison to simply mixed samples, the produced composite showed better electrochemical performance. After 50 charge-discharge cycles, the material showed a specific capacity of 634 mA h g−1, which was due to the uniformly distributed symmetry of nano particles. A one-step hydrothermal method was developed by Wang et al. to synthesize graphene–SnO2 nanocomposites [71]. After 100 cycles, the generated nanocomposite showed remarkable rate capability and a 635 mA h g−1 specific capacity. SnO2 nanoparticles were combined with graphene nanosheets by Paek et al., showing noticeably improved lithium storage performance [75]. After 30 cycles, the produced 2D/0D graphene–SnO2 nanocomposite maintained a charge capacity of 570 mA h g−1.
There are a lot of inorganic materials being used as an anode material of the LIBs. A straightforward two-step production of a graphene–Fe2O3 composite using the homogeneous precipitation of FeCl3 was first reported by Zhu et al. [120]. They used a graphene composite for increasing the performance of LIBs, which showed an excellent cycle performance of 1355 and 982 mA h g−1 for discharge and charge capacities, respectively, depending on the mass of Fe2O3. There is also reported Mn₃O₄ material by Wang et al., who demonstrated that the material achieved a high specific capacity value of 810 mA h g−1 at the specific current value of 40 mA g−1. The obtained capacity value was in the range of Mn3O4’s theoretical capacity [76].
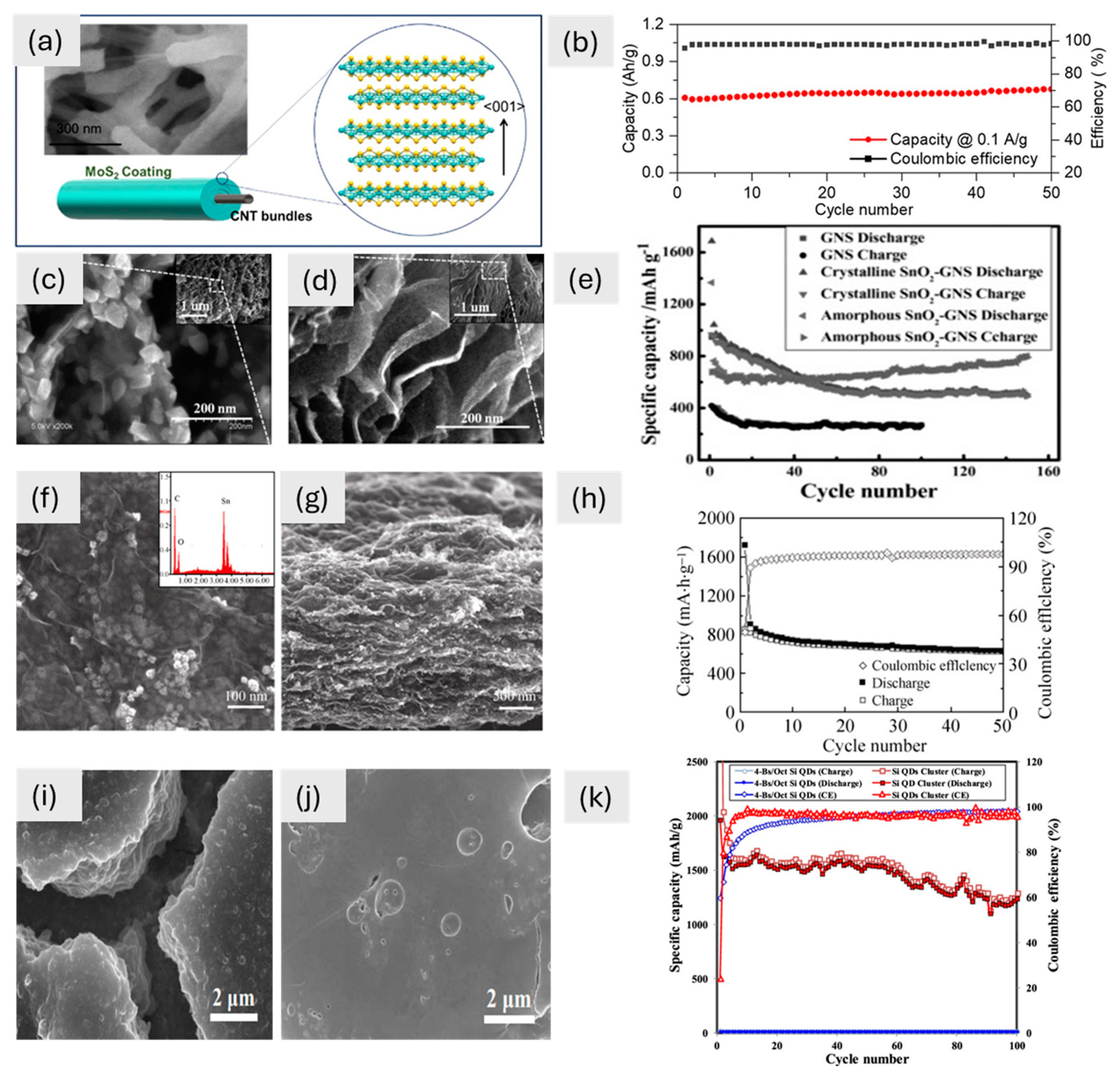
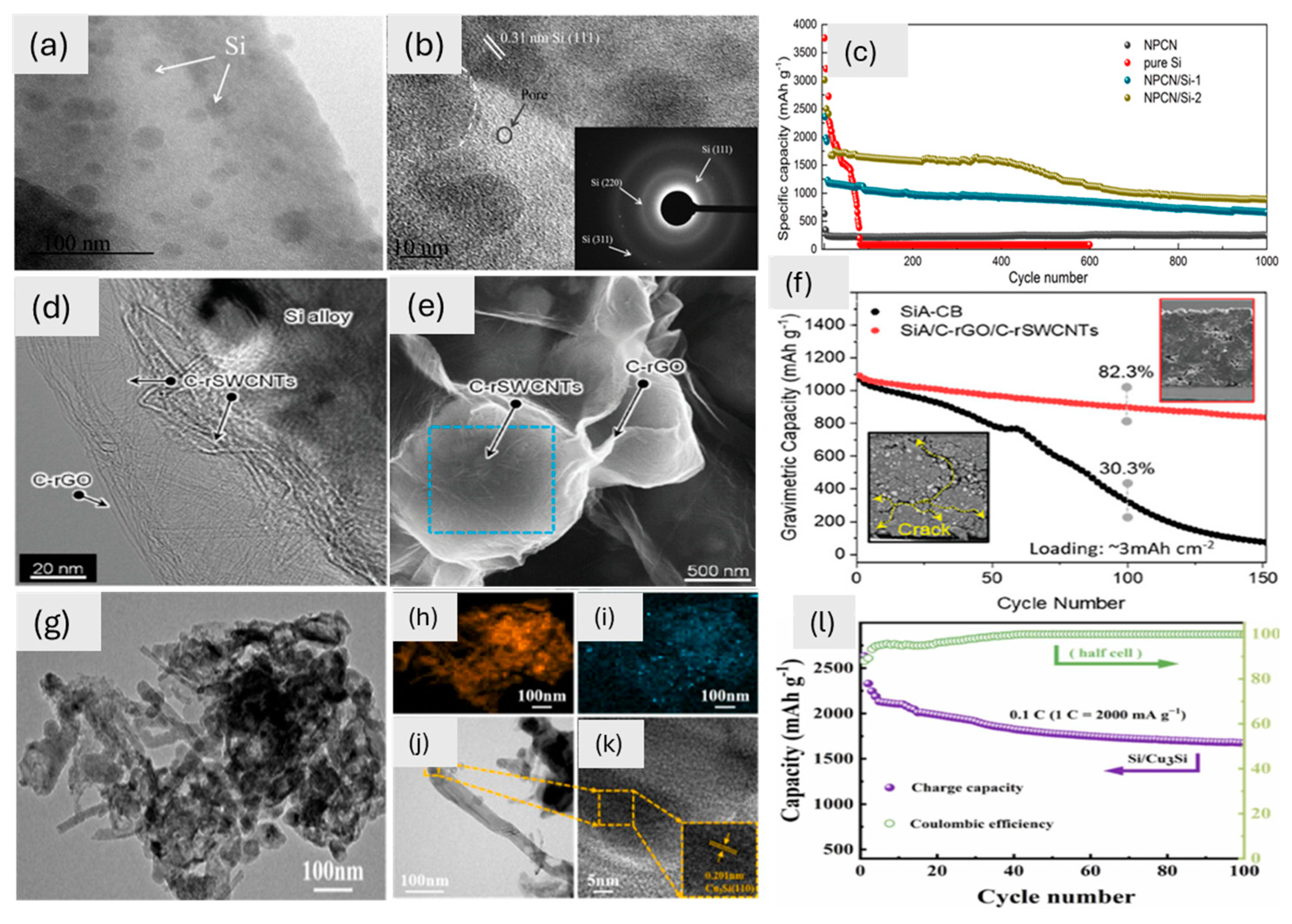
In storage applications, the morphology of the material is very important and may impart, unlike electrochemical behaviour. Figure 2 shows various 0D materials: SEM images highlight unique morphologies whereas cycling tests lead to different performances. The MoS2 coating, with its nanoscale thickness (7–80 nm), exhibits 0D characteristics when analyzed independently but integrates into a 1D CNT-based hybrid system, forming a continuous conformal layer (Figure 2a). The large surface area of 0D MoS2 helps ions move around better and the structural stability of 1D CNTs helps electrons move around better. This makes for a high capacity and great cycling performance (Figure 2b). Similarly, the amorphous SnO2-GNS composite, with its 0D morphology of 3–5 nm nanoparticles, maximizes the electrolyte contact area, improving ion transport and capacity (Figure 2d). The isotropic structure of this battery responds well to changes in volume during cycling. This keeps the structure stable and gives it a high capacity of 793 mA h g⁻1 (Figure 2e). This makes it better than larger crystalline SnO2 nanoparticles (30–40 nm), which have trouble adapting their structure (Figure 2c). The SnO2/graphene composite has even more of the benefits of a 0D shape. The main SnO2 nanoparticles are about 4 nm wide and spread out evenly on the graphene (Figure 2f). This makes it easier for ions to move through and for electrochemical reactions to happen. These small particles stick together to make bigger structures (about 14 nm), which keeps the capacity and stability in check (Figure 2g). After 50 cycles, they have a reversible capacity of 634 mA h g⁻1 and a 98% coulombic efficiency (Figure 2h). Finally, Si quantum dots (Si QDs) have a 0D shape that gives lithium ions a lot of surface area to interact with. The π-conjugated molecules around the material act as a buffer layer, reducing the mechanical stress caused by changes in volume and making it easier for electrons and Li⁺ to move around (Figure 2i,j). This is what lets the material keep about 63% of its capacity (1232 mA h g⁻1) after 100 cycles (Figure 2k). These studies collectively emphasize how 0D morphologies—whether as independent nanoparticles, integrated into hybrid systems or combined with secondary structures—play a pivotal role in maximizing electrochemical performance by enhancing surface area, ion transport, and structural stability. Due to their small size, 0D materials often generate a lot of aggregation and poor connectivity, which limits the routes that ions and electrons may take. To make matters worse, when 0D materials are often cycled (charge/discharge), they may lose their stability.
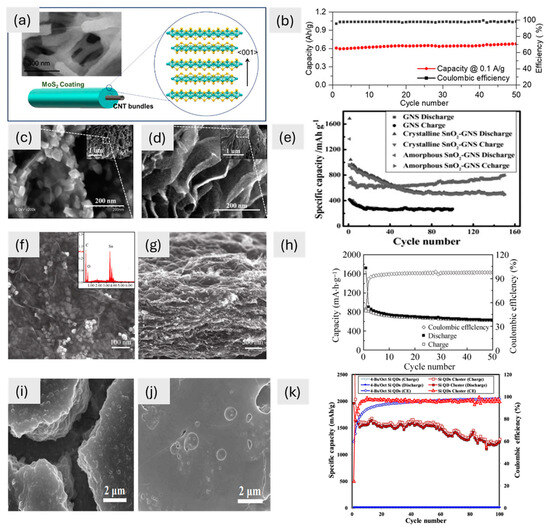
Figure 2.
(a) Field emission SEM image of the CNTF/MoS2 composite and a schematic picture of the MoS2 coating around the CNTF bundles, with an inset showing a magnification; (b) cyclic performance of composite at 0.1 A g−1 [74]. Copyright © 2021 American Chemical Society. SEM images of (c) crystalline SnO2-GNS, (d) amorphous SnO2-GNS, (e) cycle performance at different rates but the same potential window of 0.01–3.00 V for GNS (100 mA g−1) and for crystalline and amorphous SnO2-GNS (400 mA g−1) [68]. Copyright © 2012 Wiley. (f) SnO2/graphene SEM image at 100 nm, with an inset of the energy spectrum, (g) SEM image (500 nm) of the composite, and (h) cyclic performance of SnO2/graphene at a rate of 100 mA g−1 [73]. Copyright © 2010 Springer. SEM images of (i) 4-Bs/Oct Si QDs and (j) Si QD cluster and (k) galvanostatic cycling of Si QD cluster [70]. Copyright © 2020 American Chemical Society.
3.2. One-Dimensional Inorganic Electrode Materials
Unlike 0D nanostructures, 1D nanomaterials allow for effective charge transfer along their micro-scale axis by a direct conduit. Owing to their unique benefits, 1D nanostructures have been largely investigated for possible application in electrochemical energy storage systems [21,121,122,123,124,125,126]. With a lot of ongoing research, these kinds of materials are being employed commercially for high-performance energy storage systems. We have chosen to present 1D inorganic materials based on their electrochemical performance related to their energy outputs. This approach highlights their potential as high-performance electrode materials for energy storage applications.
Yan et al. successfully coated SnO2 nanowires with amorphous MnO2 through the straightforward method of immersing them in a hot KMnO4 solution [127]. The material showed a high value of specific capacitance (637 F g−1) at a scan rate of 2 mV s−1. This material also performed well in terms of energy density and power density; the reported values are 35.4 Wh kg⁻1 and 25 kW kg⁻1, respectively. A one-dimensional nanostructured composite of manganese dioxide and polypyrrole was synthesized by Li et al. [128]. Following 500 cycles (charging/discharging) in an electrolyte, the composite demonstrated a specific capacitance of 320 Fg−1 within the potential range of 0.4–0.6 V. In another published paper by Chen et al. [129], a capacity of 185 mA h g−1 is reported for nanotubes composed of LiCoO2, which is greater than the typical value of 140 mA h g−1; for such material at higher cycle rates, its capacity quickly decreases.
Plenty of polyanionic compounds have been studied on a large scale due to their high theoretical specific capacities and stability, i.e., LiFePO4, LiMnPO4, Li3V2(PO4)3, and others [130,131,132]. LiFePO4, LiMnPO4, and Li3V2(PO4)3 can be made to work better with other types of nanomaterials by studying their one-dimensional morphologies. This is a revolutionary way to make lithium-ion batteries work better, even in hybrid materials, as shown in Figure 3. These structures greatly lower internal resistance by making continuous paths for ionic and electronic conduction. They also make it easier for lithium ions to move around quickly, which is important for high-rate capability. This shape also provides mechanical integrity, which effectively reduces problems related to volume expansion and contraction during cycling. This makes sure that the battery lasts a long time and works reliably. Ultimately, the strategic balance of a high surface area with superior conductivity not only optimizes electrochemical performance but also paves the way for next-generation energy storage solutions that meet the demands of modern technology.

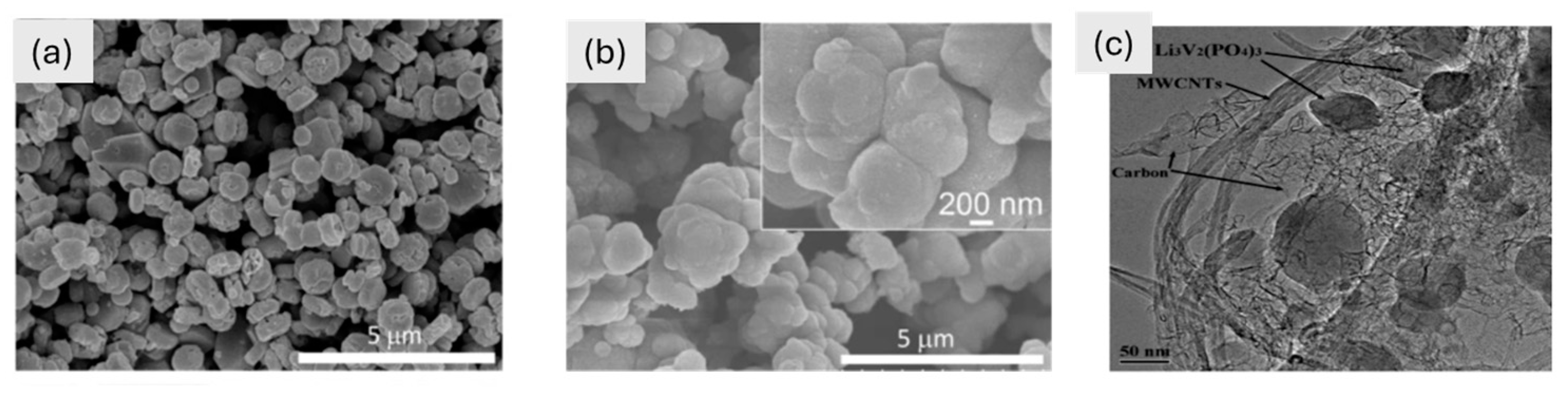
Figure 3.
(a–c) SEM images of (a) LiFePO4 and (b) LiMn0.1Fe0.9PO4 at two different magnifications [133]. Copyright © 2021 scientific reports. (c) TEM image of the composite Li3V2(PO4)3/(C + MWCNTs) at 50 nm [134]. Copyright © 2011 ScienceDirect.
A hierarchical-structure Li3V2(PO4)3/C mesoporous nanowire was fabricated by Wei et al., which included uniformly implanted Li3V2(PO4)3 nanocrystals that maintained close contact with conductive carbon scaffolds [130]. Compared to regular LVP particles covered in carbon, these mesoporous nanowires showed better performance in terms of rate and cycle. The mesoporous nanowires retained 80% of their capacity even after 3000 cycles.
Nanofiber nanorod-structured Fe2O3-C was created by Kang and colleagues for use as electrode material in high-performance energy storage devices [81]. These nanofibers consist of nanosized hollow Fe2O3 spheres evenly distributed throughout the amorphous carbon nanofiber matrix. The improved rate and cycling performance were a consequence of the combination of the carbon matrix’s continuous electron transport and the hollow nanospheres’ ability to accept the volume shift that happens during cycling. This material shows a value of 812 mA h g−1 reversible capacity at a specific current of 1.0 A g−1 after 300 charge-discharge cycles.
Theoretically, silicon is another promising candidate for use in energy storage devices with high-capacity values of 4200 mA h g−1. However, using Si material in lithium-ion batteries presents several challenges, such as the large volume variation (>300%) during lithiation/delithiation processes (removal and insertion of lithium-ions during charge-discharge), rapid capacity loss, and unstable SEI (Solid Electrolyte Interphase) layers. Furthermore, since Si is a semiconductor, its electronic conductivity is rather low. New research has found that mesoporous carbon nanofibers mixed with silicon nanoparticles or silicon nanofibers themselves work better for cycling and rate than silicon nanowires or particles in bulk. Hollow Si nanotubes may be used to achieve high performance [135]. Cui et al. presented the electrochemical characteristics of silicon nanowires [136]. These silicon nanowires have achieved a value of 1000 mA h g−1 and, even after 1000 charge-discharge cycles, they perform very well. Wu et al. [137] created a double-walled Si-SiOx nanotube structure. During lithiation, a stable outer SEI layer formed, enabling the expansion of the inner Si into the hollow region. Cycling performance was better with this SEI layer than with Si nanowires and nanotubes because it was thin and stable. Even after 6000 cycles and when tested at a high rate of 12, the capacity remained unchanged.
Wei et al. optimized Li1.2Ni2.5B2 for use as an anode material for high-performance LIBs. This material shows outstanding stability during 500 charge-discharge cycles. Additionally, this anode material showed an excellent reversible capacity of 350 mA h g−1 at a current of 0.1 A g−1. The results show that the 1D Ni/B structure is very promising as a commercially available LIB anode with the ability to charge quickly [80]. Dawei et al. presented the findings of a quasi-1D hexagonal chalcogenide perovskite material, Sr8Ti7S21. The theoretical results of density functional theory showed that Sr8Ti7S21 forms strong bonds with lithium polysulfides (LiPS). The effective sulfur host may circumvent the constraints. Consequently, S@Sr8Ti7S21-based cathodes show a remarkable cycling stability of a 1315 mA h g−1 capacity over 400 cycles at 0.2 [16].
Woosung et al. reported an anode material (Nb2Se9) for Li-ion batteries (LIBs). This electrode exhibited a reversible specific capacity of 542.2 mA h g−1 when it was tested at a current rate of 0.1 A g−1 over one hundred cycles. Then, after the increase of specific current up to 3.2 A g−1, further, it shows 272 mA h g−1 of capacity [87]. Tian et al. developed the Ge-CNF material specifically for use in high-performance energy storage devices. Nanotechnology and carbon coating modified this germanium/carbon nanofiber to enhance the LIBs’ capacity and charging speed. This material was very durable and had excellent electrochemical properties. It could hold the capacity of 281.6 mA h g−1 after 5000 cycles (charging/discharging) at the specific current of 5 A g−1 and 795.9 mA h g−1 after 100 cycles at 0.1 A g−1, with a coulombic efficiency of 99.76% [77].
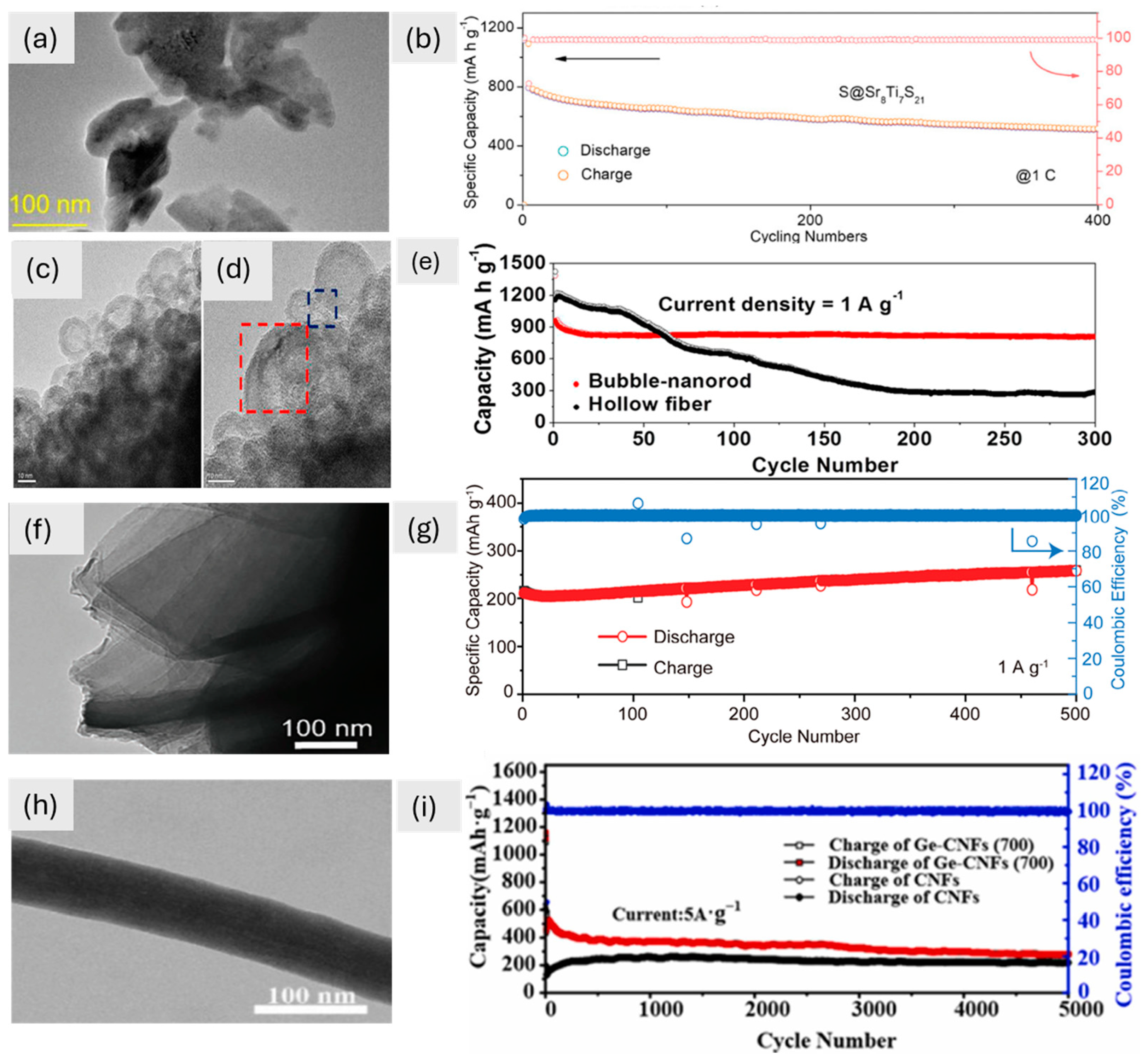
The examination of TEM images, alongside cyclic performance data, reveals a compelling narrative about the intricate relationship between the morphologies of 1D materials and their electrochemical behaviors (Figure 4). The TEM image shows that Sr8Ti7S21 perovskite has a flat structure (Figure 4a). The hexagonal Sr framework shows how well it is aligned, which improves lithium polysulphide binding and redox kinetics. This leads to a high capacity and great cycling stability, with only 0.08% decay over 400 cycles (Figure 4b). Fe2O3 nanostructures, on the other hand, have a 1D bubble-nanorod shape, with hollow nanospheres surrounded by carbon nanofibers, as seen in TEM images (Figure 4c,d). This one-of-a-kind structure, which is held together by a 3.0 nm shell and carbon matrix, keeps the volume from changing too much during cycling. This lets it keep 84% of its capacity after 300 cycles (Figure 4e). The Li1.2Ni2.5B2 has a 1D shape with Ni/B-based honeycomb channels and tunnel-type structures, which was confirmed by TEM (Figure 4f). The lattice spacing (0.42 nm) and even distribution of elements make it easy for Li⁺ to intercalate and diffuse. This leads to good stability over 500 cycles (Figure 4g). Finally, Ge-CNFs-(700) nanofibers have a flat shape with even widths and smooth surfaces, as seen in TEM images (Figure 4h). These features make them good for moving Li⁺ and electrons. This design, combined with a carbon coating, delivers ultra-high fast-charging performance, retaining 281.6 mA h g⁻1 after 5000 cycles (Figure 4i). These studies underscore how distinct 1D morphologies—ranging from hexagonal frameworks in Sr8Ti7S21 to hollow nanospheres in Fe2O3, honeycomb channels in Li1.2Ni2.5B2, and smooth nanofibers in Ge-CNFs—dictate their electrochemical behavior, emphasizing the importance of tailored structural design for optimizing energy storage performance.
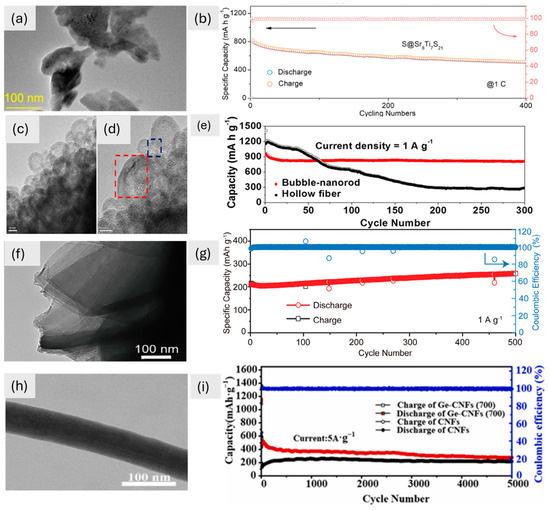
Figure 4.
(a) TEM image of Sr8Ti7S21 at 100 nm, (b) cycling performances of electrode S@Sr8Ti7S21 [16]. Copyright © 2024 Wiley. (c,d) Fe2O3/C composite TEM images at 10 nm, (e) cyclic performance of different materials at a current density of 1 A g−1 [81]. Copyright © 2015, American Chemical Society. (f) TEM image of Li1.2Ni2.5B2 at 100 nm, (g) cycling performance up to 500 cycles of Li1.2Ni2.5B2 [80]. Copyright © 2024 Wiley. (h) TEM image (100 nm) of Ge-CNFs-(700), (i) cycling performance up to 5000 cycles of CNFs and Ge-CNFs-(700) [77]. Copyright © 2024, Elsevier.
3.3. Two-Dimensional Inorganic Electrode Materials
The remarkable physical and chemical properties of two-dimensional inorganic materials, such as exfoliated graphene, together with their high surface-to-mass ratio, have sparked significant interest in the field of lithium-ion batteries. Two-dimensional transition metal carbides and nitrides, or MXenes, due to their remarkable structural flexibility and intricate surface chemistry, have garnered significant attention in the field of energy storage.
Among all the 2D materials, several investigations have indicated the potential uses of MXenes in LIBs [138,139,140]. Many new studies have put into evidence interesting qualities, including strong mechanical properties, variable bandgap energies, large electrochemically active surfaces, and great electrical conductivity. Multilayer MXenes may have a range of inorganic chemicals added to their structure to improve their effective dispersion in aqueous solutions. They are intriguing options for electrode material for LIBs because their layered structure makes it easier to store ions. In fact, Li+ can intercalate at low voltages (0.2 V to 0.6 V) with little diffusion barriers. It is necessary to assess the reversible potential of LIBs that include MXenes in light of the lowered diffusion barriers. Findings from theoretical studies indicate that MXenes with a smaller number of formula units have a higher reversible capacity and quicker diffusion rates than those with a larger number of formula units. On the other hand, the same relationship holds true in the inverse scenario [139].
For instance, despite having a low diffusion barrier of 0.42 eV, the material V4C3O2 exhibits a relatively low capacity of 148 mA h g−1 compared to other materials because of faster ion movement but may lead to lower capacity if the material cannot host many ions. On the other side, V2CO2 has a far higher diffusion barrier of 0.82 eV than other materials, even though it has a substantially higher capacity value of 276 mA h g−1. Also, the diffusion barrier of Ti3C2 is 0.07 eV, which is much lower than that of graphite, which is 0.3 eV. The previously mentioned benefits of MXenes are significant but we still need further study to fully understand their intercalated structures and the mechanisms associated with them [140]. Research on titanium carbide by M. Naguib et al. [141] revealed that Li+ intercalates inside the material’s layers. The study aimed to utilize titanium carbide as an anode material in lithium-ion batteries. Zhang et al. [142] reported highly conductive Ti3C2 MXene decorated with Sn4+ ions, emphasizing the material’s promising application in lithium-ion anodes. Researchers have investigated several MXene materials, including Ti2CTx, Nb2CTx, Ti3C2Tx, and V2CTx, for their potential use as LIBs. The letter Tx corresponds to the functional surface terminal groups in these materials. Because of its remarkable conductivity and primarily electrochemical behavior, MXene exhibits significant volumetric capacitances. The materials show substantial improvements in gravimetric performance as compared to activated carbon, demonstrating capacitances of around 210 farads per gram at a rate of 10 volts per second [143]. Lukatskaya et al. found that Ti3C2 in a microporous configuration reached a value of 300 Fg−1 capacitance at a scan rate of 101 mV s−1 [143]. This result was exceptional for electrochemical behavior, highlighting the significant potential of MXenes in energy storage applications.
The inorganic 2D materials, aside from transition metal carbides/nitrides (MXenes), that have been stated for use in high-performance LIBs can be categorized into several groups: graphene and the derivatives of graphene, such as graphene oxides, silicene, borophene, stanine, phosphorene, and germanene (called elemental analogs of graphene); 2D structures like hBN, transition metal dichalcogenides (TMDs), and transition metal oxides (TMOs); and newly emerging 2D materials.
Among the reported 2D materials, graphene and its derivatives have undergone the most extensive study. Pure graphene exhibits an exceptionally large surface area of 2630 m2 g−1, alongside remarkable charge carrier mobility, a thermal conductivity of 5000 W mK−1, and a Young’s modulus of 1 TPa [144,145]. Graphite, being the most commonly utilized electrode material for high-energy storage devices, has a theoretical capacity that falls short of meeting the growing demand; it also lacks sufficient rate capability. Derived from graphite, graphene exhibits a larger surface area and increased interlayer spacing compared to graphite, prompting extensive exploration of its potential as an electrode material. The ability of both sides of graphene to adsorb ions suggests that its capacity could potentially be twice that of graphite when utilized as an electrode material. The experimental studies on graphene anodes [146] and graphene cathodes [147] have demonstrated the feasibility of the concept of graphene electrodes.
Shu et al. [148] developed a porous graphene paper as an anode material for LIBs. This material demonstrates a discharge capacity of 400 mA h g−1, even at a high current density of 2000 mA g−1. The electrochemical characteristics of a material also depend upon the synthesis process and techniques used for the testing. Cohn et al. [149] created a nano-manufactured material specifically designed for LIBs. It was a hybrid graphene-single-walled CNT. By testing this material, they calculated the capacity of 2640 mA h g−1 at the rate of 0.186 A g−1. In another paper, Hassoun et al. [150] reported a capacity of 165 mA h g−1, which was basically calculated by a graphene-based battery with an energy density of 190 W h kg−1.
Researchers extensively examined graphene-based composites alongside other active materials that may achieve greater capacities. Layered transition metal oxides are very attractive options. They offer the potential for high capacity, exhibit excellent chemical stability, and are cost-effective. Ni et al. [91] developed a composite material of MoO2 nanosheets with a carbon matrix. MoO2 presents itself as a noteworthy electrode material, exhibiting moderate discharge and charge voltages. This composite, when utilized as the anode material in high-performance LIBs, has demonstrated a remarkable capacity of 1051 mA h g−1 over 100 cycles at a current of 0.5 A g−1. Nb2O5 nanosheets have been synthesized through experimental methods and evaluated for their performance as the anode in Li-ion batteries. They achieved a lithium capacity of 184 mA h g−1 and demonstrated commendable rate capability at intermediate discharge/charge voltages between 1.0 and 2.5 V [151].
Additionally, 2D transition metal dichalcogenides (TMDs) represent an expanding category of two-dimensional materials. Recently, there has been a big rise in the discovery and production of new 2D TMDs with a wide range of electric properties that can be used as LIB electrode materials.
Xiao et al. [152] developed a composite (MoS2/PEO), utilizing PEO to stabilize the disordered structure. The PEO/MoS2 composite demonstrated an impressive capacity of 1000 mA h g−1. Liu et al. [88] synthesized and reported the electrochemical characteristics of the graphene and MoS2 composite. At the specific current value of 100 mAg−1, the impressive capacity of 1351 mA h g−1 was shown by this material after 200 cycles (charging/discharging). Conversely, significant focus has been directed towards the fabrication of SnS2 and graphene composites, with reported significant capacities up to 1000 mA h g−1. SnS2 nanosheets represent a notable option for creating composites with graphene [153,154,155]. Researchers are finding ways and techniques not only to increase the LIBs performance but also the combinations of lithium with other metals, for example, Qu et al. [153] reported on the development of composite material for high-performance lithium/sodium batteries. This SnS2/rGO combined material shows a capacity of 544 mA h g−1 at a rate of 2A g−1. Alongside phosphorene, recent studies have also explored other two-dimensional (2D) monoelemental materials that have a similar lattice structure to graphene, including silicene, germanene, borophene, and stanine [156,157,158,159,160].
Bo et al. created a 2D carbon/Si composite using the ice template technique supported by ultrasonic atomization. They used this composite (NPCN/Si-2) as an electrode material to evaluate its electrochemical characteristics. This material showed a capacity value of 1977 mA h g−1 at the rate of 100 mA g−1 after 100 cycles (charging/discharging). The material even tested for 1000 charge-discharge cycles and it shows a stable capacity of 889 mA h g−1 [13]. Do et al. stated a unique synthesis method for improving the performance of Si-based anode material using the spray drying technique. The material demonstrates the capacity of 1224 mA h g−1 and an outstanding retention rate of 82% after the 100 cycles at 20 mA g−1 current density; this special combination produces high capacities and robust retention behaviors. They constructed a LIB consisting of silicon-based nanocarbon material as an anode. After 200 cycles, the lithium-ion battery full cell shows 65% capacity retention with an energy density of 350 Wh kg−1 [93].
Abdul et al. reported a B4C3 monolayer with a storage capacity of 2770 mA hg−1. They conducted theoretical studies using density functional theory to investigate the electrochemical viability of the material. Using the values of the diffusion coefficient and ionic conductivity, they explained the behavior of the material and predicted its potential for use as an anode in lithium-ion batteries [14]. Zaid et al. used a hydrothermal process to make titanium (TiO2) and C-TiO2 material, which improved the Li-battery’s electrical performance. As an anode material, the composite was a well-established and very effective carbon-doped TiO2 (C-TiO2). The findings demonstrated 400 and 500 mA hg−1 charge capabilities at 100 mAg−1 [161]. Siwei et al. synthesized the Cu3Si/Si nanowires that displayed an initial 89% coulombic efficiency and a specific capacity of 2630 mA h g−1, after 100 cycles of charging and discharging, resulting in a capacity of 1675 mA h g−1 at a current density of 200 mA g−1 [15].
The three studies (Figure 5) highlight the significance of 2D morphology in enhancing the electrochemical performance of Si-based materials, as revealed by TEM analysis. The first study demonstrates a 2D crumpled nanosheet morphology in NPCN/Si-2, where Si nanoparticles are uniformly embedded, creating a 3D interconnected network. This structure, confirmed by TEM (Figure 5a,b), ensures efficient Li-ion accessibility, reduces aggregation, and provides structural stability, resulting in high capacity and cycling stability (Figure 5c). In the second study, the 2D structure of the rGO and SWCNT layers creates an interconnected network (Figure 5d,e). This restores the sp2 carbon network, which makes the electrical conductivity and Li-ion transport better. This results in high capacity and retention (82%) after 100 cycles (Figure 5f). In the third study, Cu3Si/Si nanowires have a shape that is similar to that of a flat surface. This is because Cu3Si/Si nanoparticles are mixed in with Si nanowires, making a complex that can grow in size, as seen in the lattice fringes of TEM (Figure 5g). This morphology enhances cycling stability and supports high capacities (Figure 5l). In all cases, the inorganic materials (Si, Cu3Si) play a critical role in maintaining structural integrity and electrochemical performance while TEM analysis provides detailed insights into the morphological features that directly influence the material’s efficiency and stability (Figure 5).
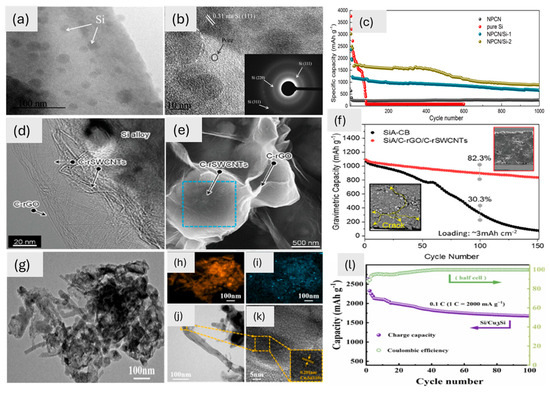
Figure 5.
(a,b) High-resolution TEM images of NPCN/Si-2 with an inset of the SAED pattern and (c) electrochemical performance of pure Si, NPCN, and composites at the rate of 1 A g−1 [13]. Copyright © 2024 Elsevier. (d) TEM image (20 nm) of a SiA/NC, (e) SEM image (500 nm) of SiA/NC, (f) cyclic performance of materials with the inset showing cross-sectional images after 150 cycles of the materials, SiA and SiA/NC [93]. Copyright © 2023 Wiley. (g) TEM image (100 nm) of the produced nanowires, (h–k) Cu-Si 3:16 EDS map scanning, (l) cycle performance of Si/Cu3Si for 100 cycles at 0.2 A g−1 [15]. Copyright © 2024 Elsevier.
3.4. Three-Dimensional Inorganic Electrode Materials
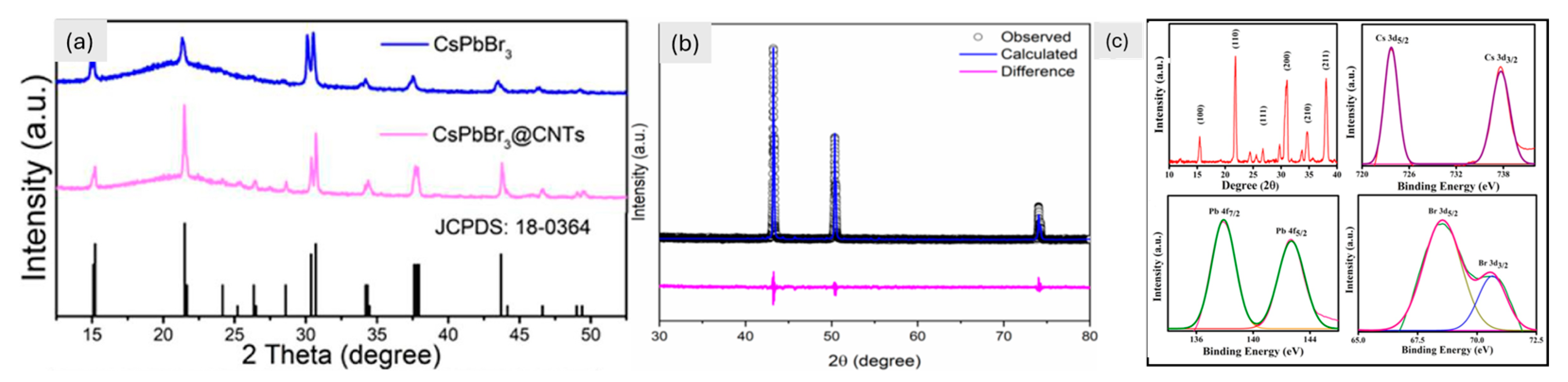
Additionally, 3D materials like SnO2, Fe2O3, TiO2, V2O5, and conducting polymers still face challenges because they have lower energy capacities than 1D and 2D materials [102,103,162,163]. A large-scale study is also being given to the electrochemical characteristics of 3D inorganic perovskite materials for LIBs. The three-dimensional shape of the highly linked crystal structure of the CsPbCl3 perovskite makes it easier for electrons to move and for Li-ions to diffuse. For lithium-ion batteries to operate well over an extended period, this 3D design enhances structural stability and ionic conductivity. Furthermore, the strong CsPbCl3 framework reduces volume expansion, improving cycle stability and capacity retention. CsPbCl3 material with a capacity of 612.3 mA h g⁻1 at a rate of 50 mA g⁻1 was reported by Pal et al. [106]. After 100 cycles, Paul et al. found that the CsPbBr3 material had a reduced capacity of 261 mA h g⁻1 at a current density of 60 mA g⁻1. When we look at CsPbBr3 with XPS, we can see that its chemical composition is stable (Figure 6c). This means that it will work well during cycling (charging/discharging) [100]. According to Kaisar et al., the CsPbl3 material can sustain performance for up to 100 cycles with a capacity of 235 mA h g⁻1 at 40 mA g⁻1. It was found that the crystal structures of the CsPbBr3 and CsPbI3 perovskites are orthorhombic by XRD. This is necessary for them to be stable in lithium-ion batteries from an electrochemical point of view. The Rietveld refinement of CsPbI3 shows that the unit cell volume changes during lithiation, affecting how much lithium can be stored (Figure 6b) [101]. According to Liu et al., the CsPbBr3@CNTs composite has an enhanced capacity of 470.2 mA h g⁻1 at 100 mA g⁻1 after 200 charge-discharge cycles. Improved conductivity and stability are shown by the CsPbBr3@CNTs composite’s ability to retain its structure (Figure 6a) [99]. The stability and lithium storage potential of 3D perovskites in energy storage applications are highlighted by these structural insights. Wu et al. found that the promising electrode material Cs2NaBiCl₆ has the highest capacity at 775 mA h g⁻1 when the current density is 75 mA g⁻1 for 25 cycles [105]. Another material, Cs2NaErCl₆, was described by Yang et al. in a different paper. It has a capacity of 120 mA hg⁻1 at a high current density of 300 mA g⁻1 after 500 cycles [98].
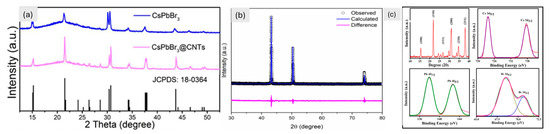
Figure 6.
(a) XRD of CsPbBr3 and composite [99], Copyright © 2021 Elsevier, (b) showing the XRD pattern of CsPbI3 after 100 charge-discharge cycles [101], Copyright © 2021 MDPI. (c) CsPbBr3 XRD pattern and XPS spectra of Cs/Pb/Br3 separately [100], Copyright © 2021, American Chemical Society.
At the moment, 3D-ordered porous (3DOP) electrode materials are being intensively recognized and investigated for their potential use in high-performance electrochemical energy storage systems. One of the advantages of 3DOP electrodes is that they have a periodic structure. This structure enables the connecting walls to reach into the unoccupied pores, which prevents the walls from being pulverized. These materials provide a high surface area along with interconnected pores, which enhance the efficiency of ion transport and electron pathways. The distinctive architecture boosts the charge and discharge rates as well as overall energy density. Furthermore, 3DOP materials demonstrate remarkable mechanical stability, accommodating volume fluctuations during cycling to enhance battery longevity [97].
Titanium dioxide (TiO2) has undergone a significant examination for use in high-performance LIBs. TiO2 is chemically stable, abundant in nature, very cost-effective, and environmentally friendly element. In most cases, it is used as an anode material for Li storage. It has been shown that the rutile TiO2 electrode has an early discharge capacity of 608 mA h g−1. This is a lot more than the theoretical capacity of TiO2, which is 168 mA h g−1 for Li0.5TiO2 and 336 mA h g−1 for LiTiO2. Furthermore, this 3DOP rutile TiO2 electrode demonstrates remarkable performance, reaching up to 5000 cycles without the use of any binders [97]. Transition cobalt oxides are also important materials for use in high-performance LIBs with impressive theoretical capacities. In the case of Co3O4, the theoretical value of 890 mA h g−1 capacity is reported. And in the case of CoO, the theoretical value of 716 mA h g−1 capacity is reported [164,165,166]. It is not possible to use CoO in LIBs as an anode material in experiments because it loses a lot of its capacity after long cycles, which is caused by big changes in volume. In addressing this issue, three-dimensional ordered porous carbon inverse opals have been employed to encapsulate CoO nanoparticles [104]. After 1000 cycles, the CoO/C electrode exhibits a notable value of 674 mA h g−1 capacity, which is 94% of the theoretical capacity. For active nanoparticles, the three-dimensional ordered porous carbon structure serves both a dimensional limitation and an interpenetrated, continuous conducting network.
On the other hand, iron oxides stand out among the numerous transition metal oxides as a widely examined anode material due to their numerous advantages. These include impressive theoretical capacities as well as their abundant availability and environmentally friendly properties. For example, Fe3O4 has a reported capacity value of 926 mA h g−1 whereas Fe2O3 has a reported capacity value of 1007 mA h g−1. In another publication, the capacities of 3DOP α-Fe2O3 are reported as 1883 (discharge) and 1139 mA h g−1 (charge), respectively [167].
Among all the many types of anode materials, investigations that compare Si anodes have shown that they have the greatest specific capacity of any of the other possibilities. As the need for energy storage solutions that work well and last a long time grows, studying bulk-silicon- and nano-silicon-based anodes in lithium-ion secondary cells looks like a good idea. That is the reason they have been studied on a large scale. It is thought that both bulk-silicon and nano-silicon (Si)-based anodes for lithium-ion secondary cells could be used to store energy in the future and they have a high theoretical capacity (about 4200 mA h g⁻1) [168]. One of the most notable drawbacks, however, is the large volume expansion of this material (about 400%) that takes place during the electrochemical processes with Li ions. This significantly restricts the practical use of these materials [169,170]. There are several Si anode composites with Ni (Si/Ni) that were manufactured using the method of electrodeposition in order to solve the pulverization problem [171,172].
Additional electrochemically active anode materials, including graphene [173], TiNb2O7 [174], MoS2 [175], and Ge [176,177], were also developed as 3DOP electrodes for Li-ion storage.
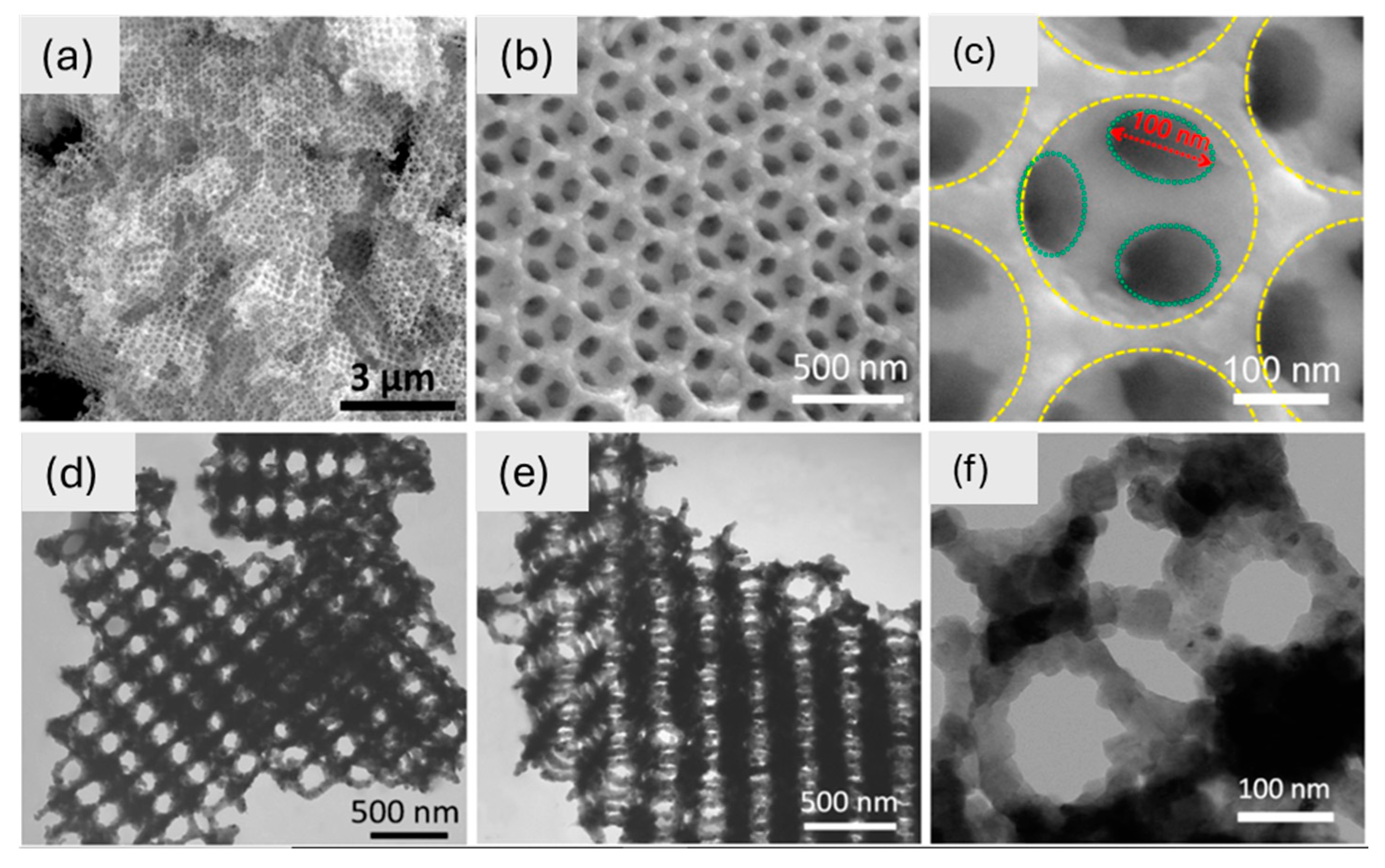
SEM and TEM confirm the 3D-ordered macroporous (3DOM) structure of TiNbO₇, which displays well-organized “honeycomb” pores with linked macropores (~100 nm diameter) and inorganic walls (~50 nm thick) (Figure 7). It makes it easier for lithium ions to move around and keeps the structure stable during cycling. This very porous three-dimensional framework is better at storing lithium ions. Because it is linked in three dimensions, this 3DOM-TiNbO₇ is a good material for the anode of high-performance lithium-ion batteries [174]. Also, tin dioxide is one of the examined anode materials for LIBs, with an irreversible conversion reaction alongside a reversible alloying reaction, (SnO2/Sn + Li2O) and (Sn/Li4.4Sn), respectively [178,179,180]. The investigation of commercial inorganic cathode nanomaterials for LIBs primarily centers on LiFePO4. The olivine-structured LiFePO4 is a highly favorable alternative to LiCoO2 due to its environmentally friendly nature. The primary drawback lies in its inadequate conductivity and rate capability [181]. To solve the problem of low conductivity, some conductive material can be used as a mixture with LiFePO4 electrodes. LiMnO2 has garnered significant attention thanks to its impressive theoretical capacity near 300 mA h g−1 along with good experimental capacities [182]. The LiMnO2 crystal has both a layered monoclinic structure and an orthorhombic structure. The zigzag arrangement of Li and Mn ions highlights the orthorhombic structure.
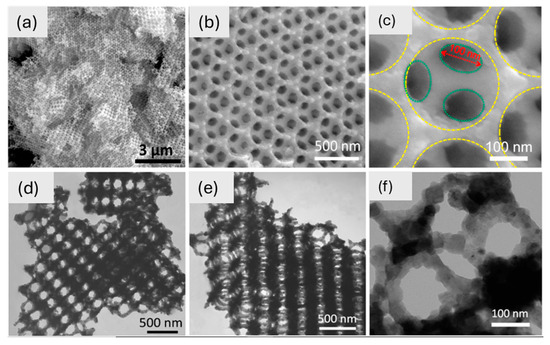
Figure 7.
(a–c) SEM images of TiNb2O7 at different magnifications and (d–f) TEM images of TiNb2O7 at different magnifications [174]. Copyright © 2017 Elsevier.
Although 3D materials have several benefits, poor cycle life; scaling issues; and expensive, complicated production restrict their use in high-performance LIBs. They are less appropriate for traditional usage since they often increase power density while decreasing volumetric energy density. Efficiency and longevity may be impacted by side reactions and unequal electrolyte penetration brought on by large surface areas. It is challenging to compete with cost-effective, optimized materials like graphite. Three-dimensional materials still have challenges with cost, performance, and scalability, despite continuous research aimed at enhancing their capabilities.
4. Conclusions and Future Outlook
Extensive efforts have been invested to develop novel and efficient electrode materials for high-performance energy storage devices based on inorganic oxides. However, the devices made up of these electrodes are characterized by lower energy and power density [183,184]. This study reveals that the electrode material properties like surface areas, charge storage sites, chemical reactions, unique bonding, and interactions with the electrolyte play a crucial role in defining the electrochemical performances of electrodes used in lithium-ion batteries.
LIBs can utilize zero-dimensional nanoparticles, such as quantum dots, and three-dimensional structures like graphene multilayers; however, these nanostructures are less prevalent compared to one- and two-dimensional alternatives [185,186,187,188,189,190]. One-dimensional nanoparticles, such as nanowires and nanotubes, show high conductivity and ability to improve charge transfer. Graphene and other layered materials exhibit exceptional electrical conductivity and a large surface area, both of which are essential for effective energy storage. Each type of nanostructure addresses specific performance metrics for LIBs. The most promising silicon-based architectures for high energy density are 0D and 1D structures. Additionally, 1D CNTs and 2D graphene provide a better rate performance for rapid charge/discharge. MXenes and 3D hierarchical structures are particularly noteworthy for long-term cycle stability.
This study allows us to highlight that optimizing the morphology of nanomaterials significantly enhances the electrochemical performance of LIB electrodes. To obtain high energy density, fast charge/discharge rates, and long-term stability, future research should focus on scalable ways to make hybrid structures that combine 0D, 1D, 2D, and 3D materials. A hybrid method that combines many nanostructures (for example, 3D frameworks with built-in 0D or 2D materials) could improve battery performance. This would bridge the gap between lab-based innovation and commercial scalability. Furthermore, investigating innovative surface alterations and electrolyte interactions may enhance economic viability even further.
Author Contributions
Conceptualization, M.H.K. and V.T.; methodology M.H.K. and V.T.: investigation, M.H.K. resources, P.L. and V.T.; data curation, M.H.K.; writing—original draft preparation, M.H.K.; writing—review and editing, M.H.K., P.L. and V.T.; supervision, V.T.; funding acquisition, P.L. and V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The development of this work is framed in the Environmentally Friendly Aviation for All Classes of Aircraft (EFACA) project. This project is funded by the European Union Horizon Europe research and innovation programme (HORIZON-CL5-2021-D5-01-05) under grant agreement no. 101056866.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
MHK acknowledges the support by the Dottorato di Ricerca Nazionale in Photovoltaics funded by Italian University Ministry.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Evolution of strategies for modern rechargeable batteries. ACC Chem. Res. 2013, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices System power ratings, module size. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Chodankar, N.R.; Dubal, D.P.; Kwon, Y.; Kim, D.H. Direct growth of FeCo₂O₄ nanowire arrays on flexible stainless steel mesh for high-performance asymmetric supercapacitor. NPG Asia Mater. 2017, 9, e419. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Chang, Z.; Zhu, Y.; Fu, L.; Liu, X.; Wu, Y. Electrode materials with tailored facets for electrochemical energy storage. Nanoscale Horiz. 2016, 1, 272–289. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Goodman, M.D.; Zhang, H.G.; Epstein, E.S.; Huang, B.; Pan, Z.; Kim, J.; Choi, J.H.; Huang, X. Mechanically and chemically robust sandwich-structured C@Si@C nanotube array Li-ion battery anodes. ACS Nano 2015, 9, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, C.; Xu, Y.; Liang, L.; Zhou, M.; Jiang, J.; Singh, S.; Zhao, H.; Schober, A.; Lei, Y. Article type: Communication A selectively permeable membrane for enhancing cyclability of organic sodium-ion batteries. Adv. Mater. 2016, 28, 9182–9187. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/ niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries; Nature Publishing Group: London, UK, 2016. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. LigO2 and LigS Batteries with High Energy Storage; Nature Publishing Group: London, UK, 2012. [Google Scholar] [CrossRef]
- Liang, B.; Tan, W.; Chen, M.; Yi, M.; Hu, J.; Zeng, K.; Wang, Y.; Li, Y.; Yang, G. Facile synthesis of two-dimensional carbon/Si composite assembled by ultrasonic atomization-assisted-ice template technology as electrode for lithium-ion battery. J. Alloys Compd. 2024, 976, 173030. [Google Scholar] [CrossRef]
- Majid, A.; Najam, U.; Ahmad, S.; Alkhedher, M. On the prospects of using B4C3 as a potential electrode material for lithium-ion batteries. Mater. Sci. Semicond. Process 2024, 176, 108320. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, J.; Nayaka, G.P.; Dong, P.; Zhang, Y.; Xing, Y.; Zhang, X.; Du, N.; Zhou, Z. Efficient electrochemical synthesis of Cu3Si/Si hybrids as negative electrode material for lithium-ion battery. J. Alloys Compd. 2024, 998, 174996. [Google Scholar] [CrossRef]
- Yang, D.; Han, Y.; Li, M.; Li, C.; Bi, W.; Gong, Q.; Zhang, J.; Zhang, J.; Zhou, Y.; Gao, H.; et al. Highly Conductive Quasi-1D Hexagonal Chalcogenide Perovskite Sr8 Ti7 S21 with Efficient Polysulfide Regulation in Lithium-Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2401577. [Google Scholar] [CrossRef]
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage; Wiley-VCH Verlag: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Zhang, S.; Wang, J.; Huang, Q.; Yu, N.; Zhu, Y.; Fu, L.; Wang, F.; Chen, Y.; et al. Three-Dimensional Ordered Porous Electrode Materials for Electrochemical Energy Storage; Nature Publishing Group: London, UK, 2019. [Google Scholar] [CrossRef]
- Cui, G.; Gu, L.; Thomas, A.; Fu, L.; van Aken, P.A.; Antonietti, M.; Maier, J. A Carbon/Titanium Vanadium Nitride Composite for Lithium Storage. ChemPhysChem 2010, 11, 3219–3223. [Google Scholar] [CrossRef]
- Lee, K.T.; Lytle, J.C.; Ergang, N.S.; Oh, S.M.; Stein, A. Synthesis and rate performance of monolithic macroporous carbon electrodes for lithium-ion secondary batteries. Adv. Funct. Mater. 2005, 15, 547–556. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.-G.; Dai, S. Soft-templated mesoporous carbon-carbon nanotube composites for high performance lithium-ion batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Lv, W.; Yu, W.; Wu, S.; Hou, P.; Yang, Q.; Meng, Q.; Liu, C.; Cheng, H.-M. Vertically aligned carbon nanotubes grown on graphene paper as electrodes in lithium-ion batteries and dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High Rate Capabilities Fe3O4-Based Cu Nano-Architectured Electrodes for Lithium-Ion Battery Applications; Nature Publishing Group: London, UK, 2006. [Google Scholar] [CrossRef]
- Kim, W.T.; Jeong, Y.U.; Lee, Y.J.; Kim, Y.J.; Song, J.H. Synthesis and lithium intercalation properties of Li3VO4 as a new anode material for secondary lithium batteries. J. Power Sources 2013, 244, 557–560. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Zhang, X.; Cui, G. Nanostructured transition metal nitrides for energy storage and fuel cells. Coord. Chem. Rev. 2013, 257, 1946–1956. [Google Scholar] [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Diez Perez, I.; Maret, W.; Chen, F.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. In AIChE Annual Meeting, Conference Proceedings; American Institute of Chemical Engineers: New York, NY, USA, 2019. [Google Scholar]
- Lee, J.T.; Zhao, Y.; Thieme, S.; Kim, H.; Oschatz, M.; Borchardt, L.; Magasinski, A.; Cho, W.-I.; Kaskel, S.; Yushin, G. Sulfur-infiltrated micro-and mesoporous silicon carbide-derived carbon cathode for high-performance lithium sulfur batteries. Adv. Mater. 2013, 25, 4573–4579. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Seng, K.H.; Park, M.H.; Guo, Z.P.; Liu, H.K.; Cho, J. Catalytic role of ge in highly reversible GeO2/Ge/C nanocomposite anode material for lithium batteries. Nano Lett. 2013, 13, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Luo, S.; Wang, K.; Wang, J.; Jiang, K.; Li, Q.; Fan, S. Binder-free LiCoO2/carbon nanotube cathodes for high-performance lithium ion batteries. Adv. Mater. 2012, 24, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, F.; Liu, F.; Xiang, Q.; Feng, X.; Liu, L.; Qiu, G. Facile hydrothermal synthesis and electrochemical properties of orthorhombic LiMnO2 cathode materials for rechargeable lithium batteries. RSC Adv. 2014, 4, 13693–13703. [Google Scholar] [CrossRef]
- Kobayashi, G.; Yamada, A.; Nishimura, S.-I.; Kanno, R.; Kobayashi, Y.; Seki, S.; Ohno, Y.; Miyashiro, H. Shift of redox potential and kinetics in Lix(MnyFe1-y)PO4. J. Power Sources 2009, 189, 397–401. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Min, D.H. Durable large-area thin films of graphene/carbon nanotube double layers as a transparent electrode. Langmuir 2009, 25, 11302–11306. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zeng, Y.; Zhang, C.; Lu, X.; Zeng, C.; Yao, C.; Yang, Y.; Tong, Y. Titanium dioxide@polypyrrole core-shell nanowires for all solid-state flexible supercapacitors. Nanoscale 2013, 5, 10806–10810. [Google Scholar] [CrossRef]
- Yu, M.; Zhai, T.; Lu, X.; Chen, X.; Xie, S.; Li, W.; Liang, C.; Zhao, W.; Zhang, L.; Tong, Y. Manganese dioxide nanorod arrays on carbon fabric for flexible solid-state supercapacitors. J. Power Sources 2013, 239, 64–71. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.H.; Gong, H. A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/Carbon nanotube electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, X.-H.; Xiao, X.; Zhai, T.; Dai, J.; Zhang, F.; Hu, B.; Wang, X.; Gong, L.; Chen, J.; et al. Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure. ACS Nano 2012, 6, 656–661. [Google Scholar] [CrossRef]
- Lu, X.; Zhai, T.; Zhang, X.; Shen, Y.; Yuan, L.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.; Zhou, J.; et al. WO3-x@Au@MnO2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv. Mater. 2012, 24, 938–944. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Wang, W.; Fang, P.; Lu, X.; Tong, Y. Recent Advances in Metal Nitrides as High-Performance Electrode Materials for Energy Storage Devices. J. Mater. Chem. A 2015, 3, 1364–1387. [Google Scholar] [CrossRef]
- Li, Y.; Levine, A.M.; Zhang, J.; Lee, R.J.; Naskar, A.K.; Dai, S.; Paranthaman, M.P. Carbon/tin oxide composite electrodes for improved lithium-ion batteries. J. Appl. Electrochem. 2018, 48, 811–817. [Google Scholar] [CrossRef]
- Yi, H.; Huang, Y.; Sha, Z.; Zhu, X.; Xia, Q.; Xia, H. Facile synthesis of Mo2N quantum dots embedded N-doped carbon nanosheets composite as advanced anode materials for lithium-ion batteries. Mater. Lett. 2020, 276, 128205. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Liu, X.; Zheng, X.; Zhao, Y.; Zhang, D. Facile synthesis of Fe24N10/porous carbon as a novel high-performance anode material for lithium-ion batteries. Mater. Lett. 2021, 300, 130196. [Google Scholar] [CrossRef]
- Long, B.; Balogun, M.-S.; Luo, L.; Luo, Y.; Qiu, W.; Song, S.; Zhang, L.; Tong, Y. Encapsulated Vanadium-Based Hybrids in Amorphous N-Doped Carbon Matrix as Anode Materials for Lithium-Ion Batteries. Small 2017, 13, 1702081. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, L.; Jia, D.; Yang, Y.; Liu, X.; Sun, M.; Zhao, Z.; Qiu, J. Ni@Ni3 N Embedded on Three-Dimensional Carbon Nanosheets for High-Performance Lithium/Sodium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 48536–48545. [Google Scholar] [CrossRef]
- Ma, C.; Jia, X.; Liu, X.; Wang, J.; Qiao, W.; Yu, J.; Ling, L. Ultrafine NbN nanoparticle decorated nitrogen-doped carbon nanosheets with efficient polysulfide catalytic conversion for superior Li–S batteries. J. Power Sources 2022, 520, 230764. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.L.; Deng, Y.P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges; Wiley-VCH Verlag: Weinheim, Germany, 2020. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, Y.; Ma, Y.; Li, C.; Fu, L.; Zeng, W.; Wang, X.; Li, X.; Wang, M.; Guo, B. Organic active materials in rechargeable batteries: Recent advances and prospects. Energy Storage Mater. 2023, 63, 103046. [Google Scholar] [CrossRef]
- Jiang, B.; Su, Y.; Liu, R.; Sun, Z.; Wu, D. Calcium Based All-Organic Dual-Ion Batteries with Stable Low Temperature Operability. Small 2022, 18, 2200049. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lu, X.; Peng, L.; Xu, K.; Peng, X.; Huang, J.; Yu, G.; Xie, Y. Two-dimensional vanadyl phosphate ultrathin nanosheets for high energy density and flexible pseudocapacitors. Nat. Commun. 2013, 4, 2431. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, Z.; Tao, Z.; Chen, J. Inorganic &organic materials for rechargeable Li batteries with multi-electron reaction. Sci. China Mater. 2014, 57, 42–58. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode material for lithium ion batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ. Sci. 2011, 4, 3223–3242. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Malode, S.J.; Shetti, N.P.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Energy Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Nanophase ZnCo2O4 as a high performance anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2855–2861. [Google Scholar] [CrossRef]
- Hu, Y.S.; Kienle, L.; Guo, Y.G.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 18, 1421–1426. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, S.; Cho, J. Highly Reversible Li-Ion Intercalating MoP2 Nanoparticle Cluster Anode for Lithium Rechargeable Batteries. J. Electrochem. Soc. 2009, 156, A89. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Ezema, F.I.; Lokhande, C.D.; Lokhande, A.C. Chemically Deposited Metal Chalcogenide-Based Carbon Composites for Versatile Applications; Springer International Publishing: Heidelberg, Germany, 2023; ISBN 978-3-03-123400-2. [Google Scholar] [CrossRef]
- Yu, W.-J.; He, W.; Wang, C.; Liu, F.; Zhu, L.; Tian, Q.; Tong, H.; Guo, X. Confinement of TiO2 quantum dots in graphene nanoribbons for high-performance lithium and sodium ion batteries. J. Alloys Compd. 2022, 898, 162856. [Google Scholar] [CrossRef]
- Yin, X.; Zhi, C.; Sun, W.; Lv, L.-P.; Wang, Y. Multilayer NiO@Co3O4 @graphene quantum dots hollow spheres for high-performance lithium-ion batteries and supercapacitors. J. Mater. Chem. A Mater. 2019, 7, 7800–7814. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Liu, J.; Geng, D.; Zhang, Y.; Norouzi Banis, M.; Li, Y.; Yang, J.; Li, R.; Sun, X.; et al. Tin oxide with controlled morphology and crystallinity by atomic layer deposition onto graphene nanosheets for enhanced lithium storage. Adv. Funct. Mater. 2012, 22, 1647–1654. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Z.; Feng, X.; Zhang, Y.; Cao, S.; Zhang, J. Engineering the micro/nano structure of Ca3Co4O9 anode material for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2024, 35, 17. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Park, H.; Lee, S.; Jeong, H.-D. Synthesis and Electrochemical Performance of π-Conjugated Molecule Bridged Silicon Quantum Dot Cluster as Anode Material for Lithium-Ion Batteries. ACS Omega 2020, 5, 8629–8637. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Wang, J.; Yang, J.; Geng, D.; Li, R.; Cai, M.; Sham, T.-K.; Sun, X. Defect-rich crystalline SnO2 immobilized on graphene nanosheets with enhanced cycle performance for li ion batteries. J. Phys. Chem. C 2012, 116, 22149–22156. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Zhang, H.; Zeng, W.; Yan, F.; Li, C.C.; Duan, H. High-performance and ultra-stable lithium-ion batteries based on MOF-derived ZnO@ZnO quantum dots/C core-shell nanorod arrays on a carbon cloth anode. Adv. Mater. 2015, 27, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.-W.; Park, Y.-U.; Gwon, H.; Seo, D.-H.; Kim, Y.; Kang, K. SnO2/graphene composite with high lithium storage capability for lithium rechargeable batteries. Nano Res. 2010, 3, 813–821. [Google Scholar] [CrossRef]
- Rana, M.; Boaretto, N.; Mikhalchan, A.; Santos, M.V.; Marcilla, R.; Vilatela, J.J. Composite Fabrics of Conformal MoS2Grown on CNT Fibers: Tough Battery Anodes without Metals or Binders. ACS Appl. Energy Mater. 2021, 4, 5668–5676. [Google Scholar] [CrossRef]
- Paek, S.M.; Yoo, E.J.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, T.-T.; Li, X.-J.; Wang, K.; Wang, L.-Y.; Liang, J.-F.; Song, Y.-X.; Li, P.-Y.; Zhang, Y.-G.; Zhang, Y.-H.; et al. Ultra-high rapid-charging performance of 1D germanium anode materials for lithium-ion batteries. J. Alloys Compd. 2024, 976, 173287. [Google Scholar] [CrossRef]
- Pinilla, S.; Park, S.-H.; Fontanez, K.; Márquez, F.; Nicolosi, V.; Morant, C. 0D-1D Hybrid Silicon Nanocomposite as Lithium-Ion Batteries Anodes. Nanomaterials 2020, 10, 515. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xu, S.; Zhang, C.; Hou, L.; Yuan, C. Scalable Synthesis of One-Dimensional Mesoporous ZnMnO3 Nanorods with Ultra-Stable and High Rate Capability for Efficient Lithium Storage. Chem. A Eur. J. 2019, 25, 16683–16691. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zong, K.; Ghani, U.; Saad, A.; Liu, D.; Deng, Y.; Raza, W.; Li, Y.; Hussain, A.; Ye, P.; et al. Ternary Lithium Nickel Boride with 1D Rapid-Ion-Diffusion Channels as an Anode for Use in Lithium-Ion Batteries. Small 2024, 20, 2309918. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and Synthesis of Bubble-Nanorod-Structured Fe2O3 –Carbon Nanofibers as Advanced Anode Material for Li-Ion Batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, S.; Zhang, Y.; Cao, M. High-performance lithium storage of Co3O4 achieved by constructing porous nanotube structure. Electrochim. Acta 2015, 182, 507–515. [Google Scholar] [CrossRef]
- Cao, F.-F.; Deng, J.-W.; Xin, S.; Ji, H.-X.; Schmidt, O.G.; Wan, L.-J.; Guo, Y.-G. Cu-Si Nanocable Arrays as High-Rate Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2011, 23, 4415–4420. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Kim, C.; Jung, J.-W.; Yoon, K.R.; Kim, I.-D. Porous SnO2-CuO nanotubes for highly reversible lithium storage. J. Power Sources 2018, 373, 11–19. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, F.; Xia, Y.; Zheng, G. Zn4 Sb3 Nanotubes as Lithium Ion Battery Anodes with High Capacity and Cycling Stability. Adv. Energy Mater. 2013, 3, 286–289. [Google Scholar] [CrossRef]
- Choi, W.; Oh, S.; Hwang, S.; Chae, S.; Park, H.; Lee, W.; Woo, C.; Dong, X.; Choi, K.H.; Ahn, J.; et al. One-dimensional van der Waals transition metal chalcogenide as an anode material for advanced lithium-ion batteries. J. Mater. Chem. A Mater. 2024, 12, 7122–7131. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Jiao, L.; Chen, J. A graphene-like MoS2 /graphene nanocomposite as a highperformance anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 13109–13115. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable Silicon–Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Zhang, C.; Liu, F.; Zhu, J.; Hou, Y. Hybrid of Co3 Sn2 @Co Nanoparticles and Nitrogen-Doped Graphene as a Lithium Ion Battery Anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Li, L.; Mai, L. Ultrathin MoO2 nanosheets for superior lithium storage. Nano Energy 2015, 11, 129–135. [Google Scholar] [CrossRef]
- Sun, F.; Huang, K.; Qi, X.; Gao, T.; Liu, Y.; Zou, X.; Wei, X.; Zhong, J. A rationally designed composite of alternating strata of Si nanoparticles and graphene: A high-performance lithium-ion battery anode. Nanoscale 2013, 5, 8586. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Cho, J.Y.; Kim, J.H.; Ryoo, G.; Yoon, J.; Jo, A.; Lee, M.H.; Park, J.H.; Yoo, J.-K.; Lee, D.Y.; et al. Dispersant-Free Colloidal and Interfacial Engineering of Si-Nanocarbon Hybrid Anode Materials for High-Performance Li-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2311353. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Wang, B.; Zhang, Y.; Ning, J.; Xiao, Z.; Zhang, X.; Chang, Y.; Zhi, L. High-Performance Silicon Battery Anodes Enabled by Engineering Graphene Assemblies. Nano Lett. 2015, 15, 6222–6228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Qu, B.; Lu, B.; Xu, Z. Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays. Nano Energy 2014, 3, 88–94. [Google Scholar] [CrossRef]
- Fei, L.; Lin, Q.; Yuan, B.; Chen, G.; Xie, P.; Li, Y.; Xu, Y.; Deng, S.; Smirnov, S.; Luo, H. Reduced Graphene Oxide Wrapped FeS Nanocomposite for Lithium-Ion Battery Anode with Improved Performance. ACS Appl. Mater. Interfaces 2013, 5, 5330–5335. [Google Scholar] [CrossRef] [PubMed]
- McNulty, D.; Carroll, E.; O’Dwyer, C. Rutile TiO2 Inverse Opal Anodes for Li-Ion Batteries with Long Cycle Life, High-Rate Capability, and High Structural Stability. Adv. Energy Mater. 2017, 7, 1602291. [Google Scholar] [CrossRef]
- Yang, S.; Liang, Q.; Wu, H.; Pi, J.; Wang, Z.; Luo, Y.; Liu, Y.; Long, Z.; Zhou, D.; Wen, Y.; et al. Lead-Free Double Perovskite Cs2 NaErCl6: Li + as High-Stability Anodes for Li-Ion Batteries. J. Phys. Chem. Lett. 2022, 13, 4981–4987. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, K.; Tan, L.; Qi, S.; Liu, G.; Chen, J.; Lou, Y. All-inorganic halide perovskite CsPbBr3@CNTs composite enabling superior lithium storage performance with pseudocapacitive contribution. Electrochim. Acta 2021, 367, 137352. [Google Scholar] [CrossRef]
- Paul, T.; Maiti, S.; Chatterjee, B.K.; Bairi, P.; Das, B.K.; Thakur, S.; Chattopadhyay, K.K. Electrochemical Performance of 3D Network CsPbBr 3 Perovskite Anodes for Li-Ion Batteries: Experimental Venture with Theoretical Expedition. J. Phys. Chem. C 2021, 125, 16892–16902. [Google Scholar] [CrossRef]
- Kaisar, N.; Paul, T.; Chi, P.-W.; Su, Y.-H.; Singh, A.; Chu, C.-W.; Wu, M.-K.; Wu, P.M. Electrochemical Performance of Orthorhombic CsPbI3 Perovskite in Li-Ion Batteries. Materials 2021, 14, 5718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Pan, L.; Yu, G. 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ. Sci. 2013, 6, 2856–2870. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Lu, Z.; Yu, H.; Yan, Q.; Hng, H.H. Carbon inverse opal entrapped with electrode active nanoparticles as high-performance anode for lithium-ion batteries. Sci. Rep. 2013, 3, 2317. [Google Scholar] [CrossRef]
- Wu, H.; Pi, J.; Liu, Q.; Liang, Q.; Qiu, J.; Guo, J.; Long, Z.; Zhou, D.; Wang, Q. All-Inorganic Lead Free Double Perovskite Li-Battery Anode Material Hosting High Li + Ion Concentrations. J. Phys. Chem. Lett. 2021, 12, 4125–4129. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Ghosh, A. Three-Dimensional Perovskite Anode for Quasi-Solid-State-Ion and Dual-Ion Batteries: Mechanism of Conversion Process in Perovskite. Phys. Rev. Appl. 2020, 14, 064010. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.; Yang, W.; Yu, Z.; Sun, X.; Zhang, Y.; Yang, X.; Kimura, H.; Hou, C.; Guo, Z.; et al. Recent Advances in Transition Metal Oxides with Different Dimensions as Electrodes for High-Performance Supercapacitors; Springer Science and Business Media B.V.: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mo, L.-E.; Zhang, Y.; Chen, S.; Zhang, X.; Hu, L. NiCo2S4 quantum dots with high redox reactivity for hybrid supercapacitors. Chem. Eng. J. 2020, 388, 124109. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Hosono, E.; Wang, K.; Zhou, H. The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew. Chem. Int. Ed. 2008, 47, 7461–7465. [Google Scholar] [CrossRef]
- Fang, T.; Duh, J.-G.; Sheen, S.-R. Improving the Electrochemical Performance of LiCoO2 Cathode by Nanocrystalline ZnO Coating. J. Electrochem. Soc. 2005, 152, A1701. [Google Scholar] [CrossRef]
- Fu, L.J.; Liu, H.; Li, C.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Surface Modifications of Electrode Materials for Lithium Ion Batteries; Elsevier Masson SAS: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Wang, B.; Luo, B.; Li, X.; Zhi, L. Graphene-Inorganic Composites as Electrode Materials for Lithium-Ion Batteries. In Chemical Synthesis and Applications of Graphene and Carbon Materials; Wiley: Hoboken, NJ, USA, 2016; pp. 217–249. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Ivanovici, S.; Müllen, K. Fabrication of Graphene-Encapsulated Oxide Nanoparticles: Towards High-Performance Anode Materials for Lithium Storage. Angew. Chem. 2010, 122, 8586–8589. [Google Scholar] [CrossRef]
- Yang, S.; Cui, G.; Pang, S.; Cao, Q.; Kolb, U.; Feng, X.; Maier, J.; Mllen, K. Fabrication of cobalt and cobalt oxide/graphene composites: Towards high-performance anode materials for lithium ion batteries. ChemSusChem 2010, 3, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, D.H.; Kim, S.W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Xia, F.; Hu, X.; Sun, Y.; Luo, W.; Huang, Y. Layer-by-layer assembled MoO2-graphene thin film as a high-capacity and binder-free anode for lithium-ion batteries. Nanoscale 2012, 4, 4707–4711. [Google Scholar] [CrossRef]
- Mai, Y.J.; Tu, J.P.; Gu, C.D.; Wang, X.L. Graphene anchored with nickel nanoparticles as a high-performance anode material for lithium ion batteries. J. Power Sources 2012, 209, 1–6. [Google Scholar] [CrossRef]
- Tao, H.C.; Fan, L.Z.; Mei, Y.; Qu, X. Self-supporting Si/Reduced Graphene Oxide nanocomposite films as anode for lithium ion batteries. Electrochem. Commun. 2011, 13, 1332–1335. [Google Scholar] [CrossRef]
- Cheng, J.; Du, J. Facile synthesis of germanium-graphene nanocomposites and their application as anode materials for lithium ion batteries. CrystEngComm 2012, 14, 397–400. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cho, S.I.; Lee, S.B. Poly(3,4-ethylenedioxythiophene) nanotubes as electrode materials for a high-powered supercapacitor. Nanotechnology 2008, 19, 215710. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Kim, D.-W.; Yoo, P.J.; Chiang, C.-Y.; Meethong, N.; Hammond, P.T.; Chiang, Y.-M.; Belcher, A.M. Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science 2006, 312, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Wang, G.; Kang, Y.; Wexler, D.; Dou, S.; Liu, H. Preparation and Electrochemical Properties of SnO2 Nanowires for Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2007, 46, 750–753. [Google Scholar] [CrossRef]
- Sides, C.R.; Croce, F.; Young, V.Y.; Martin, C.R.; Scrosati, B. A High-Rate, Nanocomposite LiFePO4∕Carbon Cathode. Electrochem. Solid-State Lett. 2005, 8, A484. [Google Scholar] [CrossRef]
- Cho, S.I.; Lee, S.B. Fast Electrochemistry of Conductive Polymer Nanotubes: Synthesis, Mechanism, and Application. ACC Chem. Res. 2008, 41, 699–707. [Google Scholar] [CrossRef]
- Kim, D.K.; Muralidharan, P.; Lee, H.-W.; Ruffo, R.; Yang, Y.; Chan, C.K.; Peng, H.; Huggins, R.A.; Cui, Y. Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes. Nano Lett. 2008, 8, 3948–3952. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Khoo, E.; Sumboja, A.; Lee, P.S. Facile Coating of Manganese Oxide on Tin Oxide Nanowires with High-Performance Capacitive Behavior. ACS Nano 2010, 4, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, L.; Zhang, X. Preparation and electrochemistry of one-dimensional nanostructured MnO2/PPy composite for electrochemical capacitor. Appl. Surf. Sci. 2010, 256, 4339–4343. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Guo, B.; Chen, J. Template-Synthesized LiCoO2, LiMn2O4, and LiNi0.8Co0.2O2 Nanotubes as the Cathode Materials of Lithium Ion Batteries. J. Phys. Chem. B 2005, 109, 14017–14024. [Google Scholar] [CrossRef]
- Wei, Q.; An, Q.; Chen, D.; Mai, L.; Chen, S.; Zhao, Y.; Hercule, K.M.; Xu, L.; Minhas-Khan, A.; Zhang, Q. One-Pot Synthesized Bicontinuous Hierarchical Li3V2(PO4)3 /C Mesoporous Nanowires for High-Rate and Ultralong-Life Lithium-ion Batteries. Nano Lett. 2014, 14, 1042–1048. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, Y.; Gu, L.; Weichert, K.; Maier, J. Electrospinning of Highly Electroactive Carbon-Coated Single-Crystalline LiFePO 4 Nanowires. Angew. Chem. Int. Ed. 2011, 50, 6278–6282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liang, Y.; Cui, L.-F.; Sanchez Casalongue, H.; Li, Y.; Hong, G.; Cui, Y.; Dai, H. LiMn1-xFexPO4 Nanorods Grown on Graphene Sheets for Ultra-High Rate Performance Lithium Ion Batteries. arXiv 2011, arXiv:1107.0111. [Google Scholar] [CrossRef]
- Trinh, D.V.; Nguyen, M.T.T.; Dang, H.T.M.; Dang, D.T.; Le, H.T.T.; Le, H.T.N.; Tran, H.V.; Huynh, C.D. Hydrothermally synthesized nanostructured LiMnxFe1−xPO4(x = 0 – 0.3) cathode materials with enhanced properties for lithium-ion batteries. Sci. Rep. 2021, 11, 12280. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.Q.; Tu, J.P.; Mai, Y.J.; Cheng, L.J.; Wang, X.L.; Gu, C.D. Enhanced electrochemical performances of multi-walled carbon nanotubes modified Li3V2(PO4)3/C cathode material for lithium-ion batteries. J. Alloys Compd. 2011, 509, 7181–7185. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Cui, L.-F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-Amorphous Core−Shell Silicon Nanowires for High Capacity and High Current Battery Electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Eames, C.; Islam, M.S. Ion Intercalation into Two-Dimensional Transition-Metal Carbides: Global Screening for New High-Capacity Battery Materials. J. Am. Chem. Soc. 2014, 136, 16270–16276. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Ultra-High-Rate Pseudocapacitive Energy Storage in Two-Dimensional Transition Metal Carbides. In MXenes; Jenny Stanford Publishing: New York, NY, USA, 2023; pp. 723–743. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1565–1596. [Google Scholar] [CrossRef]
- Wei, D.; Haque, S.; Andrew, P.; Kivioja, J.; Ryhänen, T.; Pesquera, A.; Centeno, A.; Alonso, B.; Chuvilin, A.; Zurutuza, A.; et al. Ultrathin rechargeable all-solid-state batteries based on monolayer graphene. J. Mater. Chem. A Mater. 2013, 1, 3177. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.-Y.; Hong, J.; Kang, K. All-graphene-battery: Bridging the gap between supercapacitors and lithium ion batteries. Sci. Rep. 2014, 4, 5278. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Wang, C.; Wang, M.; Zhao, C.; Wallace, G.G. Graphene cryogel papers with enhanced mechanical strength for high performance lithium battery anodes. J. Mater. Chem. A 2014, 2, 1325–1331. [Google Scholar] [CrossRef]
- Cohn, A.P.; Oakes, L.; Carter, R.; Chatterjee, S.; Westover, A.S.; Share, K.; Pint, C.L. Assessing the improved performance of freestanding, flexible graphene and carbon nanotube hybrid foams for lithium ion battery anodes. Nanoscale 2014, 6, 4669–4675. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An Advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Zhang, Y. Fabrication of Nb2O5 Nanosheets for High-rate Lithium Ion Storage Applications. Sci. Rep. 2015, 5, 8326. [Google Scholar] [CrossRef]
- Xiao, J.; Choi, D.; Cosimbescu, L.; Koech, P.; Liu, J.; Lemmon, J.P. Exfoliated MoS2 Nanocomposite as an Anode Material for Lithium Ion Batteries. Chem. Mater. 2010, 22, 4522–4524. [Google Scholar] [CrossRef]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-Reduced Graphene Oxide Composite—A High-Capacity, High-Rate, and Long-Cycle Life Sodium-Ion Battery Anode Material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, P.; Huang, L.; Xie, J.; Zhang, S.; Cao, G.; Zhao, X. Few-Layered SnS2 on Few-Layered Reduced Graphene Oxide as Na-Ion Battery Anode with Ultralong Cycle Life and Superior Rate Capability. Adv. Funct. Mater. 2015, 25, 481–489. [Google Scholar] [CrossRef]
- Prikhodchenko, P.V.; Yu, D.Y.W.; Batabyal, S.K.; Uvarov, V.; Gun, J.; Sladkevich, S.; Mikhaylov, A.A.; Medvedev, A.G.; Lev, O. Nanocrystalline tin disulfide coating of reduced graphene oxide produced by the peroxostannate deposition route for sodium ion battery anodes. J. Mater. Chem. A Mater. 2014, 2, 8431. [Google Scholar] [CrossRef]
- Zhu, F.-F.; Chen, W.-J.; Xu, Y.; Gao, C.-L.; Guan, D.-D.; Liu, C.-H.; Qian, D.; Zhang, S.-C.; Jia, J.-F. Epitaxial growth of two-dimensional stanene. Nat. Mater. 2015, 14, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Zhang, G.; Zhang, Y.-W. Ultrafast and Directional Diffusion of Lithium in Phosphorene for High-Performance Lithium-Ion Battery. Nano Lett. 2015, 15, 1691–1697. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Tritsaris, G.A.; Kaxiras, E.; Meng, S.; Wang, E. Adsorption and Diffusion of Lithium on Layered Silicon for Li-Ion Storage. Nano Lett. 2013, 13, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Lu, Z.; Wu, M.C.; Ciucci, F.; Zhao, T.S. Borophene: A promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 2016, 23, 97–104. [Google Scholar] [CrossRef]
- Mahmoud, Z.H.; Ajaj, Y.; Ghadir, G.K.; Al-Tmimi, H.M.; Jasim, H.H.; Al-Salih, M.; Alubiady, M.H.S.; Al-Ani, A.M.; Jumaa, S.S.; Azat, S.; et al. Carbon-doped titanium dioxide (TiO2) as Li-ion battery electrode: Synthesis, characterization, and performance. Results Chem. 2024, 7, 101422. [Google Scholar] [CrossRef]
- Stein, A.; Wilson, B.E.; Rudisill, S.G. Design and functionality of colloidal–crystal–templated materials—Chemical applications of inverse opals. Chem. Soc. Rev. 2013, 42, 2763–2803. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Wang, X.; Chen, D.; Chen, X.; Li, D.; Shen, G. Three-Dimensional Structural Engineering for Energy-Storage Devices: From Microscope to Macroscope. ChemElectroChem 2014, 1, 975–1002. [Google Scholar] [CrossRef]
- Peng, C.; Chen, B.; Qin, Y.; Yang, S.; Li, C.; Zuo, Y.; Liu, S.; Yang, J. Facile ultrasonic synthesis of coo quantum dot/graphene nanosheet composites with high lithium storage capacity. ACS Nano 2012, 6, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, X.-L.; Guo, Y.-G.; Zhong, Y.; Cao, X.; Ma, Y.; Yao, J. Synthesis and lithium storage properties of Co3O4 nanosheet-assembled multishelled hollow spheres. Adv. Funct. Mater. 2010, 20, 1680–1686. [Google Scholar] [CrossRef]
- Wang, J.; Yang, N.; Tang, H.; Dong, Z.; Jin, Q.; Yang, M.; Kisailus, D.; Zhao, H.; Tang, Z.; Wang, D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew. Chem. Int. Ed. 2013, 52, 6417–6420. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, J.; Yang, Y.; Li, J.; Tan, X. Synthesis, characterization and electrochemical properties of three-dimensionally ordered macroporous α-Fe2O3. Mater. Sci. Eng. B 2012, 177, 1612–1617. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fu, L.; Gao, J.; Yang, L.; Wu, Y.; Wu, H. Core-shell Si/C nanocomposite as anode material for lithium ion batteries. Pure Appl. Chem. 2006, 78, 1889–1896. [Google Scholar] [CrossRef]
- Jin, Y.; Munakata, H.; Okada, N.; Kanamura, K. Design and evaluation of a three dimensionally ordered macroporous structure within a highly patterned cylindrical Sn-Ni electrode for advanced lithium ion batteries. J. Nanomater. 2013, 2013, 937019. [Google Scholar] [CrossRef]
- Kim, D.; Suk, J.; Kim, D.W.; Kang, Y.; Im, S.H.; Yang, Y.; Park, O.O. Electrochemically grown three-dimensional porous Si@Ni inverse opal structure for higherperformance Li ion battery anode. J. Mater. Chem. A 2014, 2, 6396–6401. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Wang, H.G.; Wu, Z.; Zhang, X.B. In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 2013, 7, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Cheng, X.; Zhao, Y.; Lushington, A.; Gao, J.; Li, Q.; Zuo, P.; Wang, B.; Gao, Y.; Ma, Y.; et al. Superior performance of ordered macroporous TiNb2O7 anodes for lithium ion batteries: Understanding from the structural and pseudocapacitive insights on achieving high rate capability. Nano Energy 2017, 34, 15–25. [Google Scholar] [CrossRef]
- Deng, Z.; Jiang, H.; Hu, Y.; Liu, Y.; Zhang, L.; Liu, H.; Li, C. 3D Ordered Macroporous MoS2@C Nanostructure for Flexible Li-Ion Batteries. Adv. Mater. 2017, 29, 1603020. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, J.; Hao, J.; Su, B.L.; Li, Y. 3D ordered macroporous germanium fabricated by electrodeposition from an ionic liquid and its lithium storage properties. J. Mater. Chem. A Mater. 2013, 1, 15076–15081. [Google Scholar] [CrossRef]
- Song, T.; Jeon, Y.; Samal, M.; Han, H.; Park, H.; Ha, J.; Yi, D.K.; Choi, J.-M.; Chang, H.; Choi, Y.-M.; et al. A Ge inverse opal with porous walls as an anode for lithium ion batteries. Energy Environ. Sci. 2012, 5, 9028–9033. [Google Scholar] [CrossRef]
- Choi, H.; Takahashi, D.; Kono, K.; Kim, E. Evidence of supersolidity in rotating solid helium. Science 2010, 330, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, G.; Huang, Y.; Wang, B.; Yao, B.; Wu, Y. Preparation of nanowire arrays of amorphous carbon nanotube-coated single crystal SnO2. Chem. Mater. 2008, 20, 2612–2614. [Google Scholar] [CrossRef]
- Zhao, N.H.; Yang, L.C.; Zhang, P.; Wang, G.J.; Wang, B.; Yao, B.D.; Wu, Y.P. Polycrystalline SnO2 nanowires coated with amorphous carbon nanotube as anode material for lithium ion batteries. Mater. Lett. 2010, 64, 972–975. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, H.P.; Fu, L.J.; Wu, Y.P.; Wu, H.Q. Kinetic study on LiFePO4/C nanocomposites synthesized by solid state technique. J. Power Sources 2006, 159, 717–720. [Google Scholar] [CrossRef]
- Kong, F.; Longo, R.C.; Park, M.-S.; Yoon, J.; Yeon, D.-H.; Park, J.-H.; Wang, W.; KC, S.; Doo, S.-G.; Cho, K. Ab initio study of doping effects on LiMnO2 and Li2MnO3 cathode materials for Li-ion batteries. J. Mater. Chem. A 2015, 3, 8489–8500. [Google Scholar] [CrossRef]
- Shouji, E.; Buttry, D.A. New Organic-Inorganic Nanocomposite Materials for Energy Storage Applications. Langmuir 1999, 15, 669–673. [Google Scholar] [CrossRef]
- Zhuang, G.V.; Yang, H.; Blizanac, B.; Ross, P.N., Jr. A Study of Electrochemical Reduction of Ethylene and Propylene Carbonate Electrolytes on Graphite Using ATR-FTIR Spectroscopy. Electrochem. Solid-State Lett. 2005, 8, A441. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Z.; Li, J. A hybrid supercapacitor fabricated with a carbon nanotube cathode and a TiO2-B nanowire anode. Adv. Funct. Mater. 2006, 16, 2141–2146. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Yadav, S.; Daniel, S. Green Synthesis of Zero-Dimensional Carbon Nanostructures in Energy Storage Applications—A Review; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829. [Google Scholar] [CrossRef]
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Rhodes, C.P.; McEvoy, T.M.; Bour, M.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).