Hollow-Structured Carbon-Coated CoxNiySe2 Assembled with Ultrasmall Nanoparticles for Enhanced Sodium-Ion Battery Performance
Abstract
1. Introduction
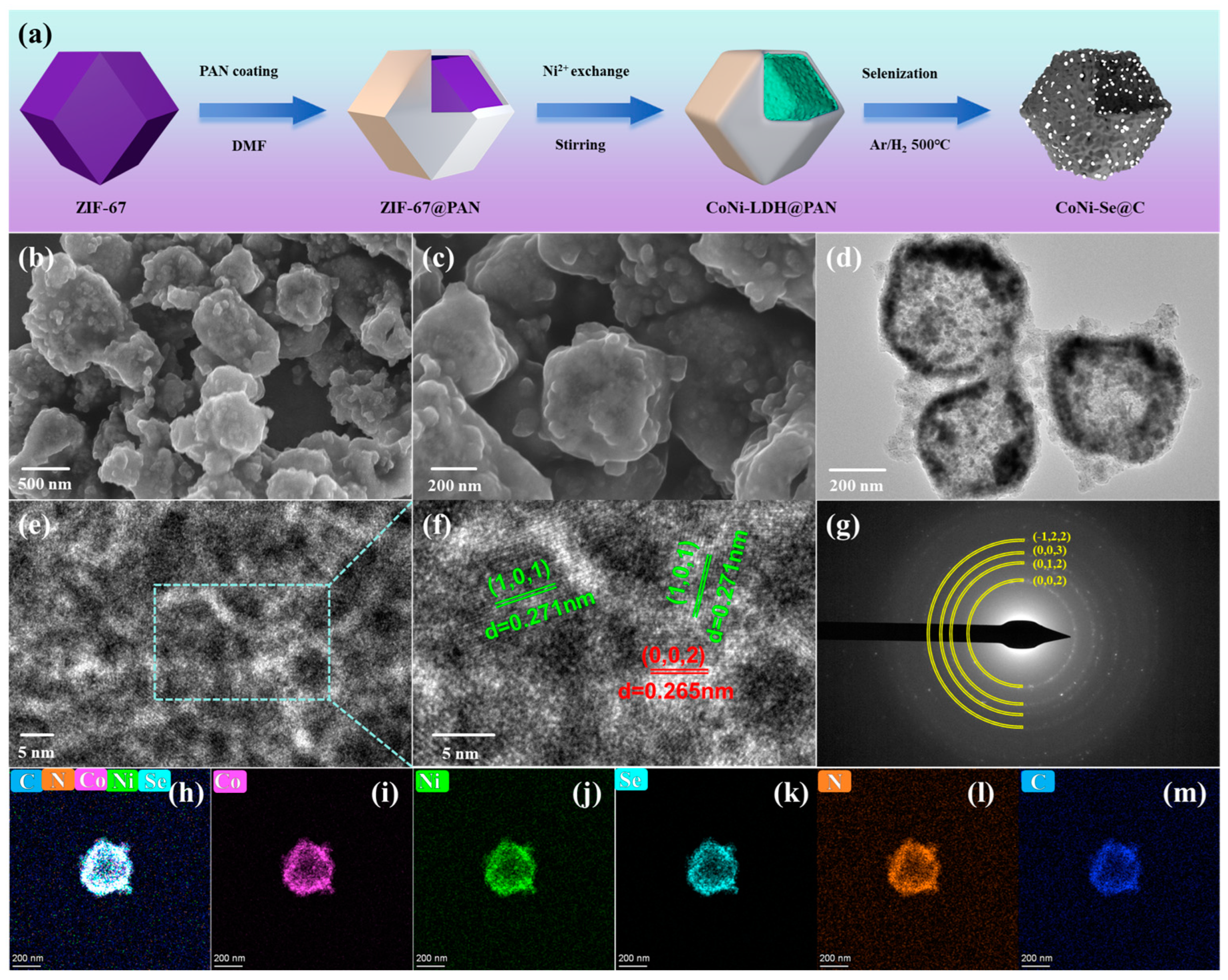
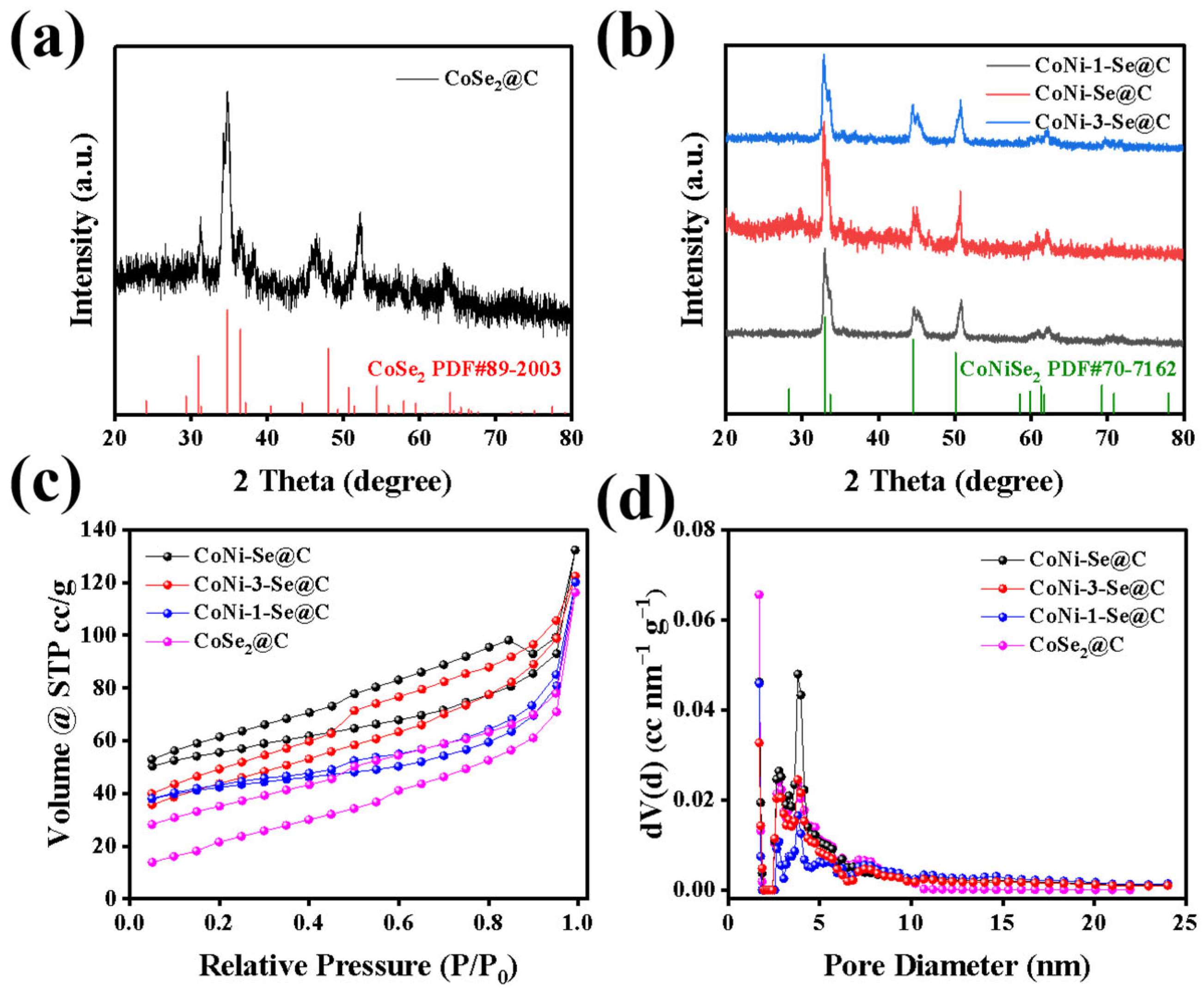
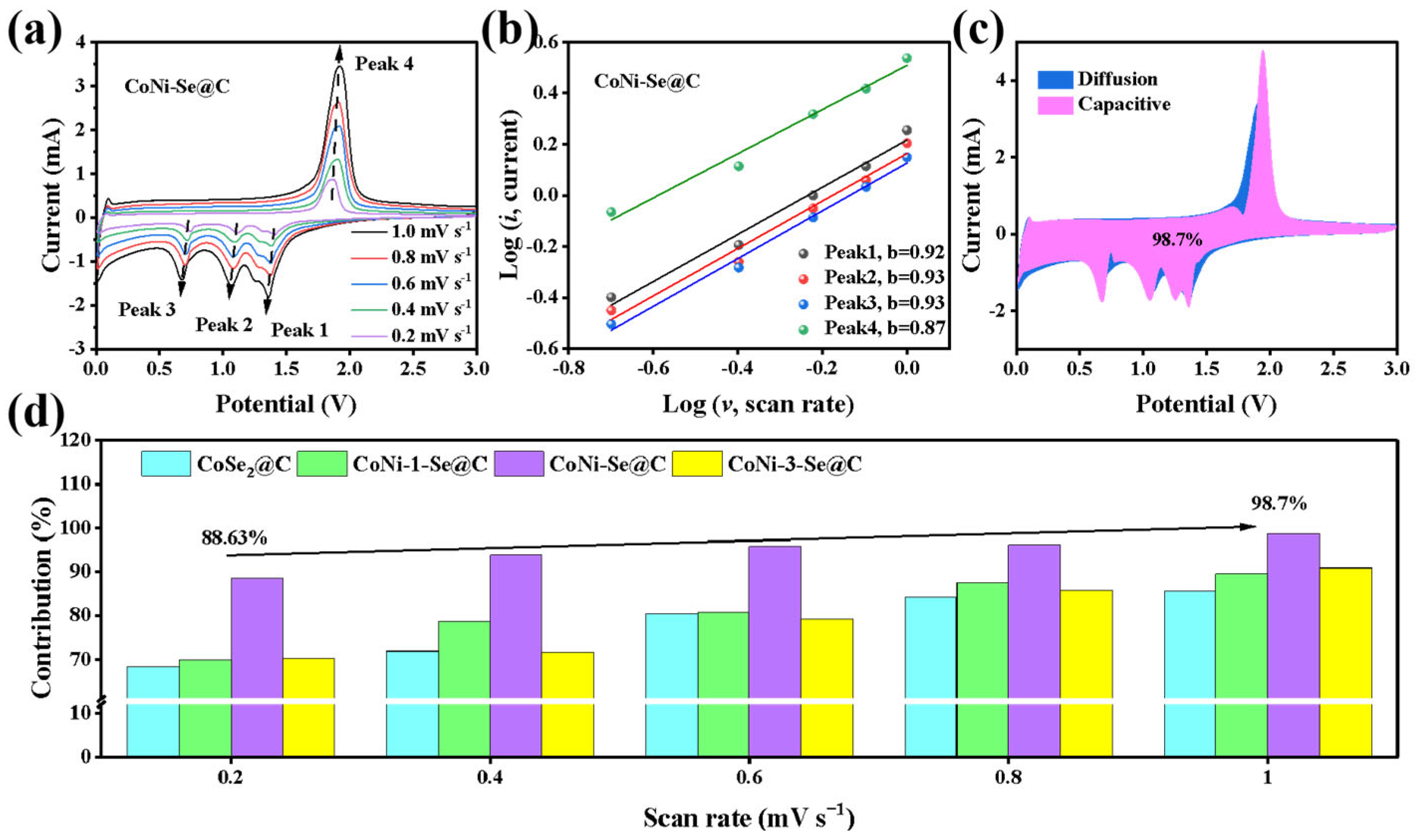
2. Results
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of ZIF-67
3.3. Synthesis of ZIF-67@PAN
3.4. Synthesis of CoNi-1-LDH@PAN, CoNi-LDH@PAN and CoNi-3-LDH@PAN
3.5. Synthesis of CoNi-Se@C, CoNi-1-Se@C, CoNi-3-Se@C and CoSe2@C
3.6. Materials Characterization
3.7. Coin Cell Assembly
3.8. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Men, S.; Zheng, H.; Ma, D.; Huang, X.; Kang, X. Unraveling the stabilization mechanism of solid electrolyte interface on ZnSe by rGO in sodium ion battery. J. Energy Chem. 2021, 54, 124–130. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.; Men, S.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. CoSe2 Nanoparticles Encapsulated by N-Doped Carbon Framework Intertwined with Carbon Nanotubes: High-Performance Dual-Role Anode Materials for Both Li- and Na-Ion Batteries. Adv. Sci. 2018, 5, 1800763. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, Q.; Liao, Z.; Chen, Y.; Liu, Y.; Liu, Q.; Xiong, X. Steric Hindrance Engineering to Modulate the Closed Pores Formation of Polymer-Derived Hard Carbon for High-Performance Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202409906. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Zhao, S.; Che, H.; Chen, S.; Tao, H.; Liao, J.; Liao, X.-Z.; Ma, Z.-F. Research Progress on the Solid Electrolyte of Solid-State Sodium-Ion Batteries. Electrochem. Energy Rev. 2024, 7, 3. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Tang, F.; Wu, M.; Yang, W.; Xu, C.; Rui, X. Controllable fabrication of vanadium selenium nanosheets for a high-performance Na-ion battery anode. Chem. Commun. 2023, 59, 11365–11368. [Google Scholar] [CrossRef]
- Men, S.; Lin, J.; Zhou, Y.; Kang, X. N-doped porous carbon wrapped FeSe2 nanoframework prepared by spray drying: A potential large-scale production technique for high-performance anode materials of sodium ion batteries. J. Power Sources 2021, 485, 229310. [Google Scholar] [CrossRef]
- Weng, J.; Zou, D.; Yuan, W.; Zhou, P.; Ding, M.; Zhou, J.; Cong, H.; Cheng, F. Bimetallic selenide heterostructure with directional built-in electric-field confined in N-doped carbon nanofibers for superior sodium storage with ultralong lifespan. J. Energy Chem. 2024, 91, 407–416. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Xu, S.; Xu, C.; Rui, X. Advanced Vanadium Oxides for Sodium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2306055. [Google Scholar] [CrossRef]
- Ju, X.; Hou, X.; Liu, Z.; Du, L.; Zhang, L.; Xie, T.; Paillard, E.; Wang, T.; Winter, M.; Li, J. Revealing the Effect of High Ni Content in Li-Rich Cathode Materials: Mitigating Voltage Decay or Increasing Intrinsic Reactivity. Small 2023, 19, 2207328. [Google Scholar] [CrossRef]
- Gao, H.; Ning, S.; Zhou, Y.; Men, S.; Kang, X. Polyacrylonitrile-induced formation of core-shell carbon nanocages: Enhanced redox kinetics towards polysulfides by confined catalysis in Li-S batteries. Chem. Eng. J. 2021, 408, 127323. [Google Scholar] [CrossRef]
- He, P.; Yu, X.-Y.; Lou, X.W. Carbon-Incorporated Nickel–Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Z.; Zhang, Y.; Zhang, Y.; Li, J.; Wang, H.; Jiang, Y.; Xing, G.; Liu, H.; Tang, Y. Revitalizing sodium-ion batteries via controllable microstructures and advanced electrolytes for hard carbon. eScience 2024, 4, 100181. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zou, L.; Xu, Q. A Hydrangea-Like Superstructure of Open Carbon Cages with Hierarchical Porosity and Highly Active Metal Sites. Adv. Mater. 2019, 31, 1904689. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, W.; Xiao, Y.; Shi, Z.; Cao, X.; Tang, Y.; Gao, Q. CoNiSe2 heteronanorods decorated with layered-double-hydroxides for efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 242, 132–139. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, T.; Zi, B.; Zhao, J.; Li, D.; Chen, M.; Sun, H.; Zhang, J.; Zhang, Y.; Liu, Q. Dual Supports by Cation Vacancies and Surface Optimization for CoNiSe2-Based Hybrid Supercapacitors with High Energy Density. ACS Energy Lett. 2023, 8, 3420–3429. [Google Scholar] [CrossRef]
- Shi, X.; Wang, H.; Ji, S.; Linkov, V.; Liu, F.; Wang, R. CoNiSe2 nanorods directly grown on Ni foam as advanced cathodes for asymmetric supercapacitors. Chem. Eng. J. 2019, 364, 320–327. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, M.; Wang, L.; Huang, Q.; Su, Z.; Xu, P.; Zou, R.; Wang, X.; Zeng, C.; Ba, K. MOFs-Derived Flower-Like Hierarchically Porous Zn-Mn-Se/C Composite for Extraordinary Rate Performance and Durable Anode of Sodium-Ion and Potassium-Ion Batteries. Small 2022, 18, 2203964. [Google Scholar] [CrossRef]
- Feng, J.; Luo, S.-H.; Yan, S.-X.; Zhan, Y.; Wang, Q.; Zhang, Y.-H.; Liu, X.; Chang, L.-J. Rational Design of Yolk–Shell Zn—Co—Se@N-Doped Dual Carbon Architectures as Long-Life and High-Rate Anodes for Half/Full Na-Ion Batteries. Small 2021, 17, 2101887. [Google Scholar] [CrossRef]
- Ma, S.; Yan, W.; Dong, Y.; Su, Y.; Ma, L.; Li, Y.; Fang, Y.; Wang, B.; Wu, S.; Liu, C.; et al. Recent advances in carbon-based anodes for high-performance sodium-ion batteries: Mechanism, modification and characterizations. Mater. Today 2024, 75, 334–358. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wang, J.-L.; Yan, H.-Y.; Zhao, J.-T.; Wang, D.-D.; Luo, S.-H.; Wang, Q.; Liu, X. Short rod-like NiCoSe2 binary-metal selenide nanomaterials of carbon-coated as high-performance anode for sodium-ion batteries. Ionics 2023, 29, 3505–3515. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, Y.; Wen, J.; Si, W.; Gao, H.; Wang, G.; Kang, X. Pyrrole derivatives as interlayer modifier of Li-S batteries: Modulation of electrochemical performance by molecular perturbation. J. Energy Chem. 2022, 75, 164–172. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, L.; Zhang, M.; Wu, F.; Huang, Q.; Su, Z.; Xu, P.; Liao, M.; Hu, Y.; Lin, X. Controllable MOF-Derived Hierarchical Hollow CoNiSe2 with Enhanced Mechanics and Kinetics for Extraordinary Rate Performance and Durable Anode of Sodium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 7129–7137. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, P.; Zhang, X.; Zhu, B.; Liu, T.; Yu, J. Facile synthesis of NiCoSe2@carbon anode for high-performance sodium-ion batteries. J. Colloid Interf. Sci. 2024, 662, 1075–1085. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, W.; Wang, C.; Zhao, S.; Hao, Z.; Huang, X.; Miao, K.; Kang, X. MOF-Derived Fe2CoSe4@NC and Fe2NiSe4@NC Composite Anode Materials towards High-Performance Na-Ion Storage. Inorganics 2024, 12, 165. [Google Scholar] [CrossRef]
- Ou, X.; Li, J.; Zheng, F.H.; Wu, P.; Pan, Q.C.; Xiong, X.H.; Yang, C.H.; Liu, M.L. X-ray diffraction characterization of NiSe as a promising anode material for sodium ion batteries. J. Power Sources 2017, 343, 483–491. [Google Scholar] [CrossRef]
- Santoni, F.; De Angelis, A.; Moschitta, A.; Carbone, P.; Galeotti, M.; Cinà, L.; Giammanco, C.; Di Carlo, A. A guide to equivalent circuit fitting for impedance analysis and battery state estimation. J. Energy Storage 2024, 82, 110389. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhões, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Kemp, N.T. A Tutorial on Electrochemical Impedance Spectroscopy and Nanogap Electrodes for Biosensing Applications. IEEE Sens. J. 2021, 21, 22232–22245. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Huang, X.; Tang, T.; Hou, Y. Hierarchically Porous Fe2CoSe4 Binary-Metal Selenide for Extraordinary Rate Performance and Durable Anode of Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1802745. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Reddy, A.E.; Kim, H.-J. Wearable superhigh energy density supercapacitors using a hierarchical ternary metal selenide composite of CoNiSe2 microspheres decorated with CoFe2Se4 nanorods. J. Mater. Chem. A 2018, 6, 7439–7448. [Google Scholar] [CrossRef]
- Vivekanantha, M.; Sundhar Arul Saravanan, R.; Kumar Nayak, P.; Prakash, R.; Kamala Bharathi, K. Synergistic-effect of high Ni content and Na dopant towards developing a highly stable Li-Rich cathode in Li-ion batteries. Chem. Eng. J. 2022, 444, 136503. [Google Scholar] [CrossRef]
- Piontek, S.; Andronescu, C.; Zaichenko, A.; Konkena, B.; Junge Puring, K.; Marler, B.; Antoni, H.; Sinev, I.; Muhler, M.; Mollenhauer, D.; et al. Influence of the Fe:Ni Ratio and Reaction Temperature on the Efficiency of (FexNi1–x)9S8 Electrocatalysts Applied in the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 987–996. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Yin, Z.; Tong, H.; Jiang, H.; Ding, Z.; Zhou, L. Bimetallic heterojunction of CuSe/ZnSe@Nitrogen-doped carbon with modified band structures for fast sodium-ion storage. Chem. Eng. J. 2022, 446, 137366. [Google Scholar] [CrossRef]
- Wang, D.; Chao, Y.; Guo, K.; Wang, Z.; Yang, M.; Zhu, J.; Cui, X.; Xu, Q. Engineering Metal Electron Spin Polarization to Regulate p-Band Center of Se for Enhanced Sodium-Ion Storage. Adv. Funct. Mater. 2024, 34, 2405642. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, N.; Fernandes, R.; Kadrekar, R.; Dashora, A.; Yadav, A.K.; Bhattacharyya, D.; Jha, S.N.; Miotello, A.; Kothari, D.C. Co–Ni–B nanocatalyst for efficient hydrogen evolution reaction in wide pH range. Appl. Catal. B Environ. 2016, 192, 126–133. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Wang, Z.; Mao, T.; Hong, Z.; Han, J.; Peng, D.-L.; Yue, G. Van der Waals Forces between S and P Ions at the CoP-C@MoS2/C Heterointerface with Enhanced Lithium/Sodium Storage. Adv. Funct. Mater. 2023, 33, 2302830. [Google Scholar] [CrossRef]
- Xu, J.; Xie, L.; Niu, Y.; Chen, H.; Zhang, Y.; Jiang, Y.; Han, Q.; Qiu, X.; Miao, Y.; Zhu, L.; et al. Nitrogen-doped carbon decorated 3D NiCoSe2 micro-flowers as high-performance anode materials for lithium-ion batteries. Phys. Chem. Chem. Phys. 2023, 25, 11530–11544. [Google Scholar] [CrossRef]
- Gao, L.; Cao, M.; Zhang, C.; Li, J.; Zhu, X.; Guo, X.; Toktarbay, Z. Zinc selenide/cobalt selenide in nitrogen-doped carbon frameworks as anode materials for high-performance sodium-ion hybrid capacitors. Adv. Compos. Hybrid Mater. 2024, 7, 144. [Google Scholar] [CrossRef]
- Xu, L.-J.; Wang, X.-J.; Tang, G.-Y.; Zhu, B.-C.; Yu, J.-G.; Zhang, L.-Y.; Liu, T. NiSe nanoparticles anchored on hollow carbon nanofibers with enhanced rate capability and prolonged cycling durability for sodium-ion batteries. Rare Met. 2025, 44, 185–194. [Google Scholar] [CrossRef]
- Wang, L.; Huang, F.; Song, X.; Li, J.; Zhu, G.; Jin, Z.; Dai, Z. Rational Design of Quasi-1D Multicore–Shell MnSe@N-Doped Carbon Nanorods as High-Performance Anode Material for Sodium-Ion Batteries. Nano Lett. 2024, 24, 11349–11357. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Li, T.; Qiao, S.; Yang, Y.-C.; Liu, Z.; Yang, J.; Tuan, H.-Y.; Cao, G.; Huang, W. Boosting Manganese Selenide Anode for Superior Sodium-Ion Storage via Triggering α → β Phase Transition. ACS Nano 2024, 18, 3801–3813. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, F.; Tang, Y.; Liu, R.; Pang, J.; Cheng, G.; Hu, M.; Ding, J. Hollow porous Co0.85Se/MoSe2@MXene heterostructured anode for sodium-ion hybrid capacitors. Chem. Eng. J. 2024, 500, 157001. [Google Scholar] [CrossRef]
- Ren, M.; Zang, H.; Cao, S.; Liu, W.; Li, M.; Yao, J.; Cai, F.; Cui, J.; Wang, Y. Fe3Se4 decorating carbon nanotubes with superior sodium storage performance for sodium-ion batteries. J. Energy Storage 2024, 81, 110486. [Google Scholar] [CrossRef]
- Xu, C.; Yang, J.; Chen, K.; Ma, G.; Wang, Y.; Li, Z.; Zhou, Z.; Wu, Z.; Che, S.; Ding, C.; et al. CoSe2-Modified multidimensional porous carbon frameworks as high-Performance anode for fast-Charging sodium-Ion batteries. Chem. Eng. J. 2024, 497, 154875. [Google Scholar] [CrossRef]
- Buğday, N.; Wang, H.; Hong, N.; Zhang, B.; Deng, W.; Zou, G.; Hou, H.; Yaşar, S.; Ji, X. Fabrication of a Stable and Highly Effective Anode Material for Li-Ion/Na-Ion Batteries Utilizing ZIF-12. Small 2024, 20, 2403736. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Si, W.; Kang, X. Hollow-Structured Carbon-Coated CoxNiySe2 Assembled with Ultrasmall Nanoparticles for Enhanced Sodium-Ion Battery Performance. Inorganics 2025, 13, 96. https://doi.org/10.3390/inorganics13030096
Wang C, Si W, Kang X. Hollow-Structured Carbon-Coated CoxNiySe2 Assembled with Ultrasmall Nanoparticles for Enhanced Sodium-Ion Battery Performance. Inorganics. 2025; 13(3):96. https://doi.org/10.3390/inorganics13030096
Chicago/Turabian StyleWang, Chao, Weijie Si, and Xiongwu Kang. 2025. "Hollow-Structured Carbon-Coated CoxNiySe2 Assembled with Ultrasmall Nanoparticles for Enhanced Sodium-Ion Battery Performance" Inorganics 13, no. 3: 96. https://doi.org/10.3390/inorganics13030096
APA StyleWang, C., Si, W., & Kang, X. (2025). Hollow-Structured Carbon-Coated CoxNiySe2 Assembled with Ultrasmall Nanoparticles for Enhanced Sodium-Ion Battery Performance. Inorganics, 13(3), 96. https://doi.org/10.3390/inorganics13030096







