Fabrication of Cu-Doped Li4Ti5O12 Particles Embedded in Reduced Graphene Oxide Nanosheets for High-Rate Lithium-Ion Battery Anode
Abstract
1. Introduction
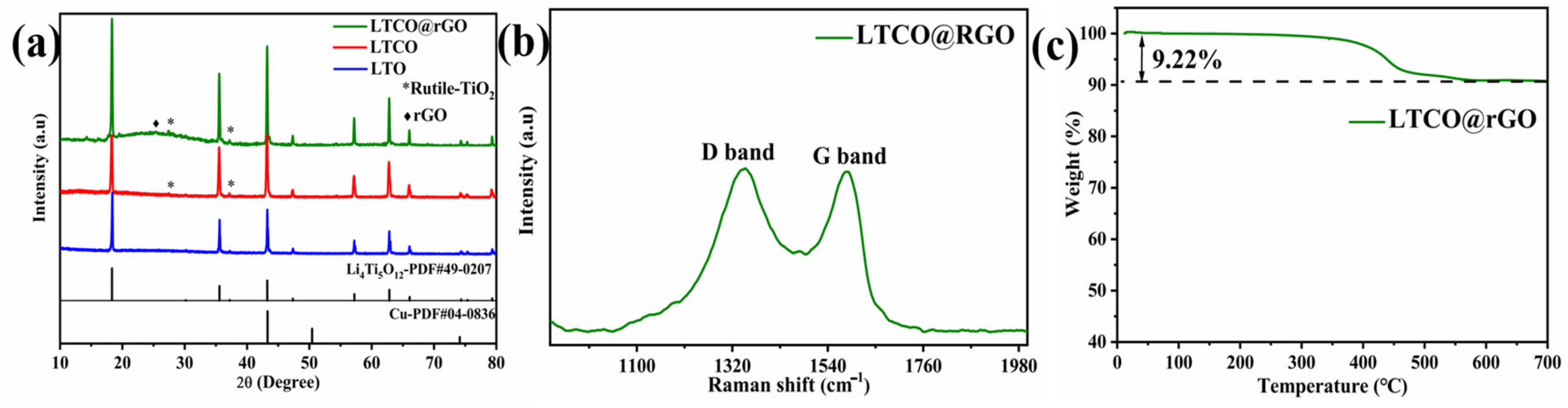
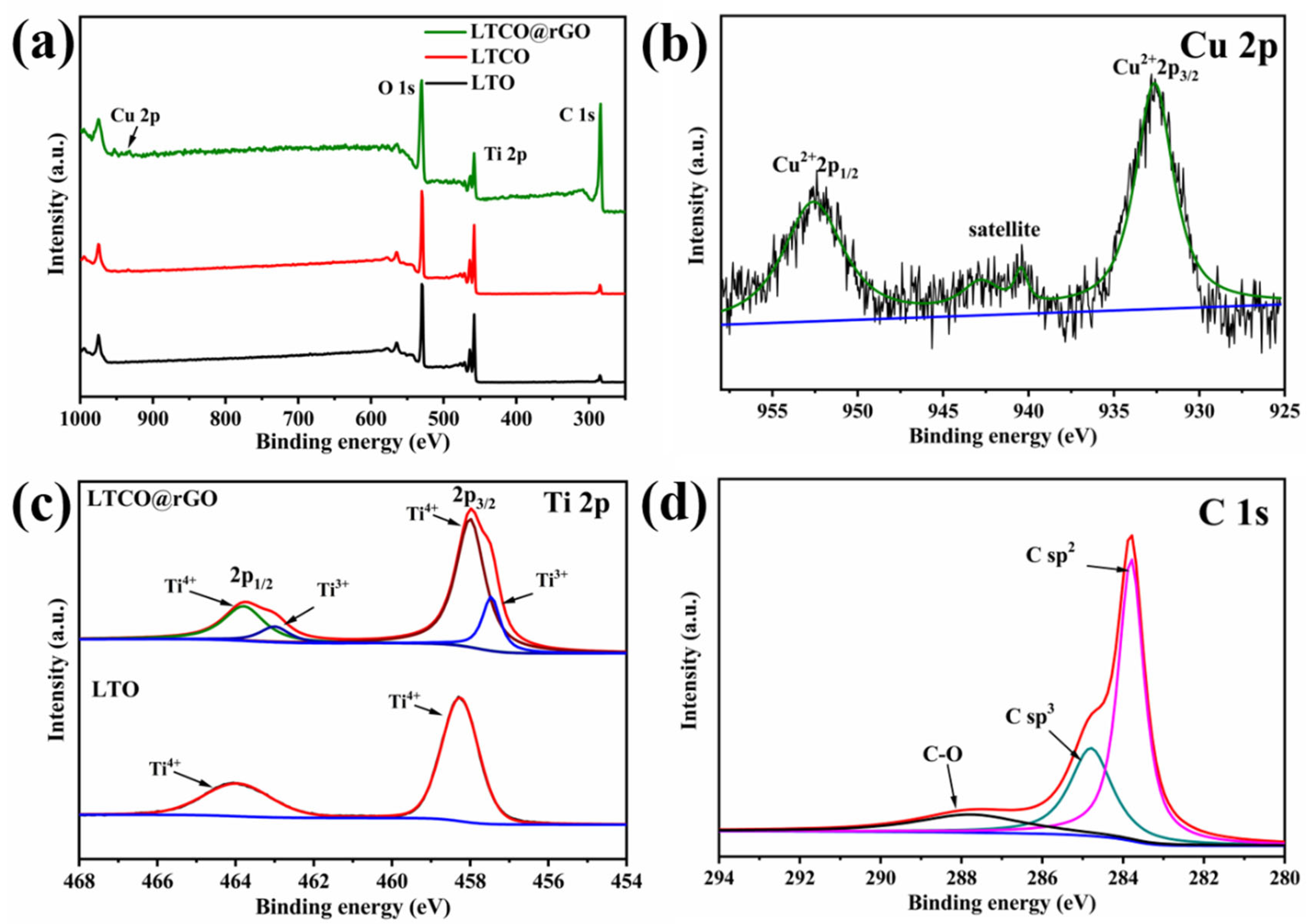
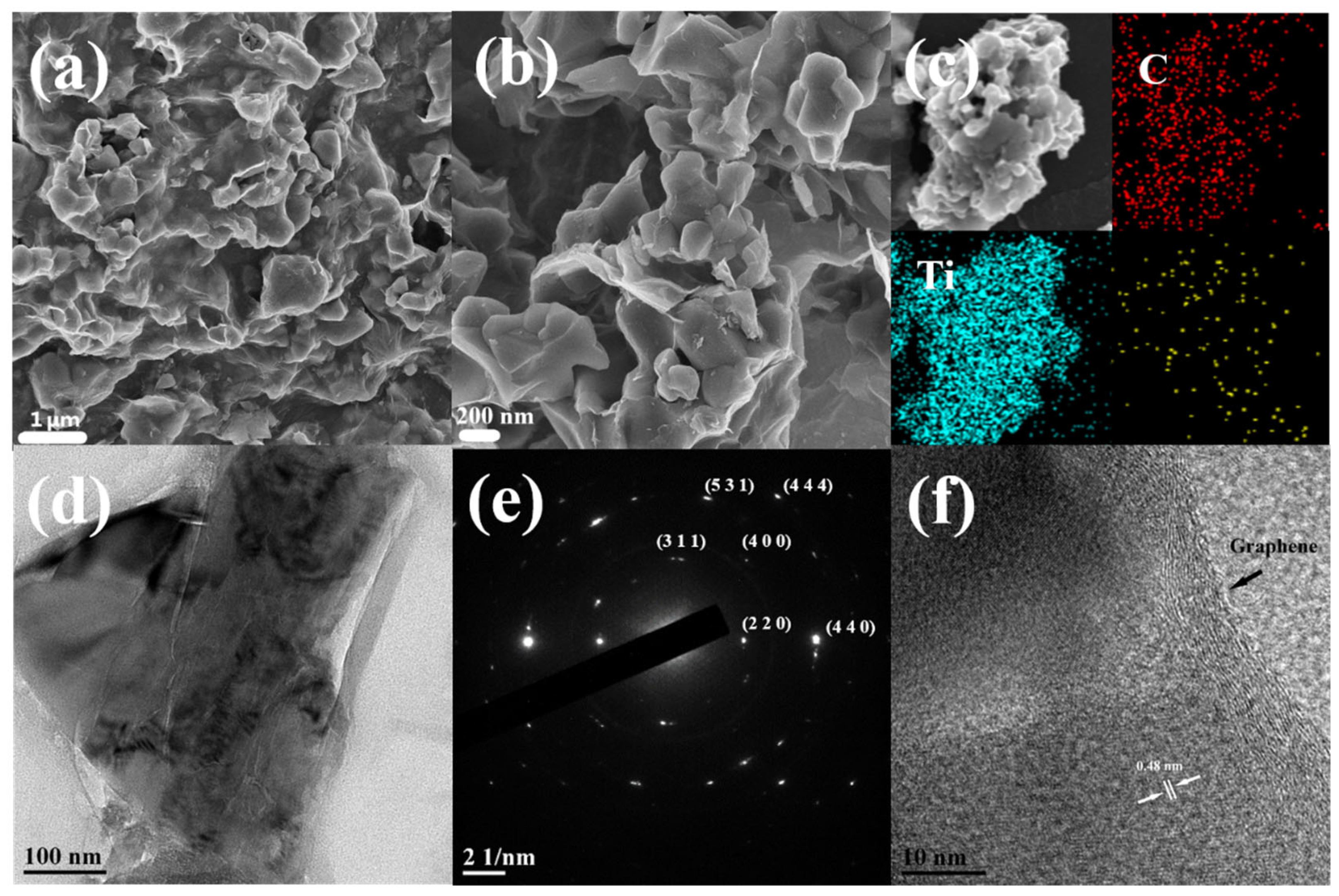
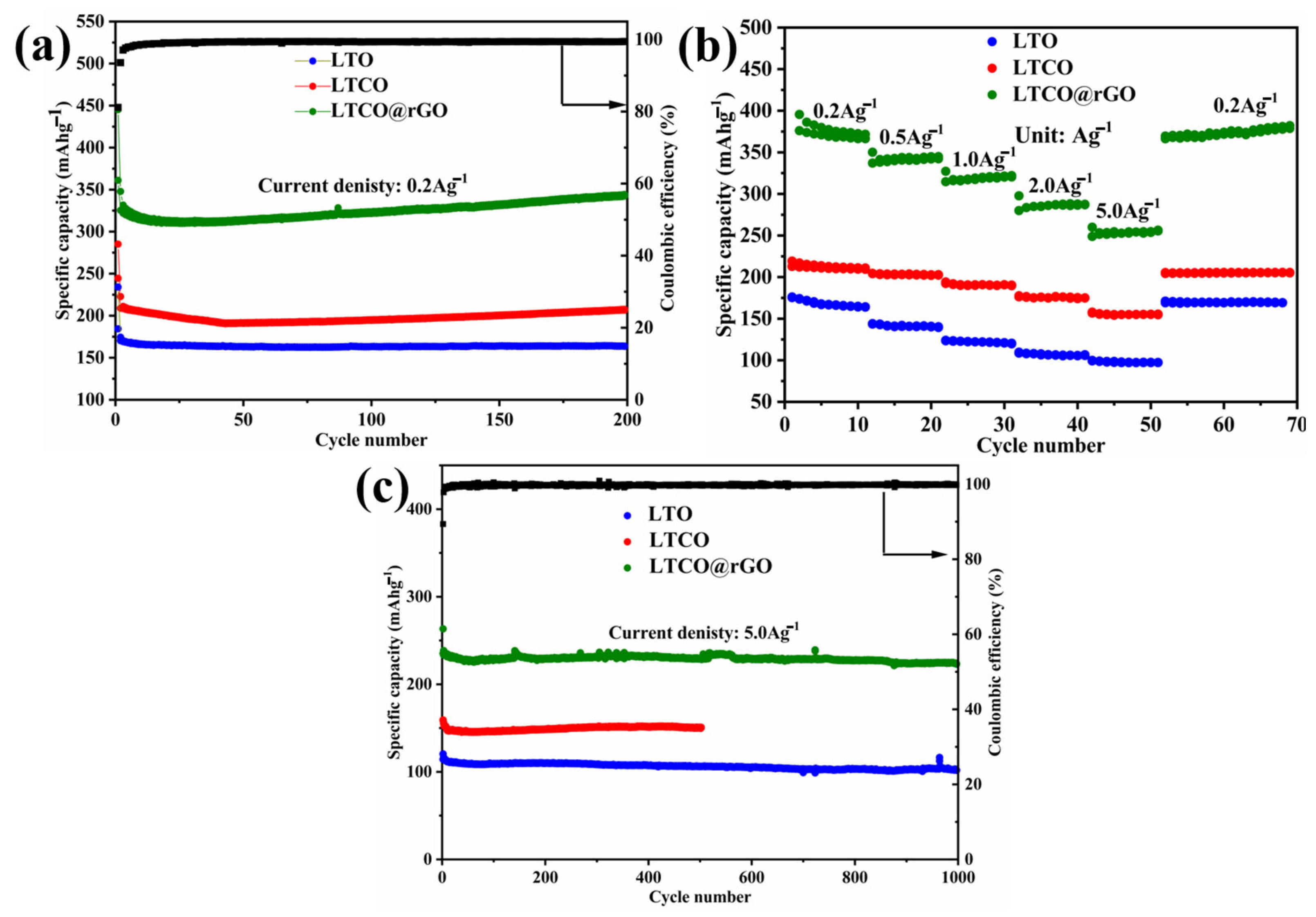
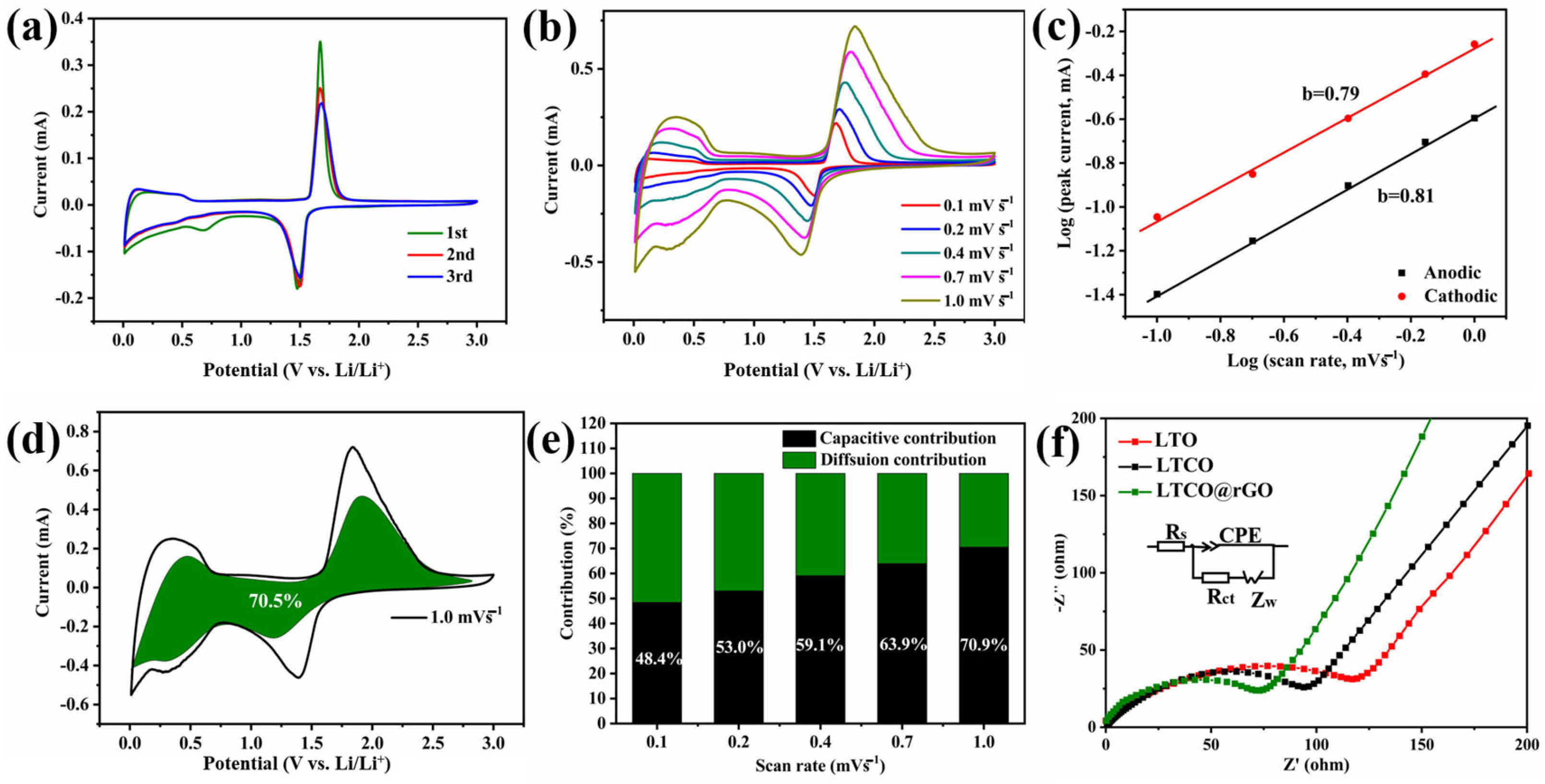
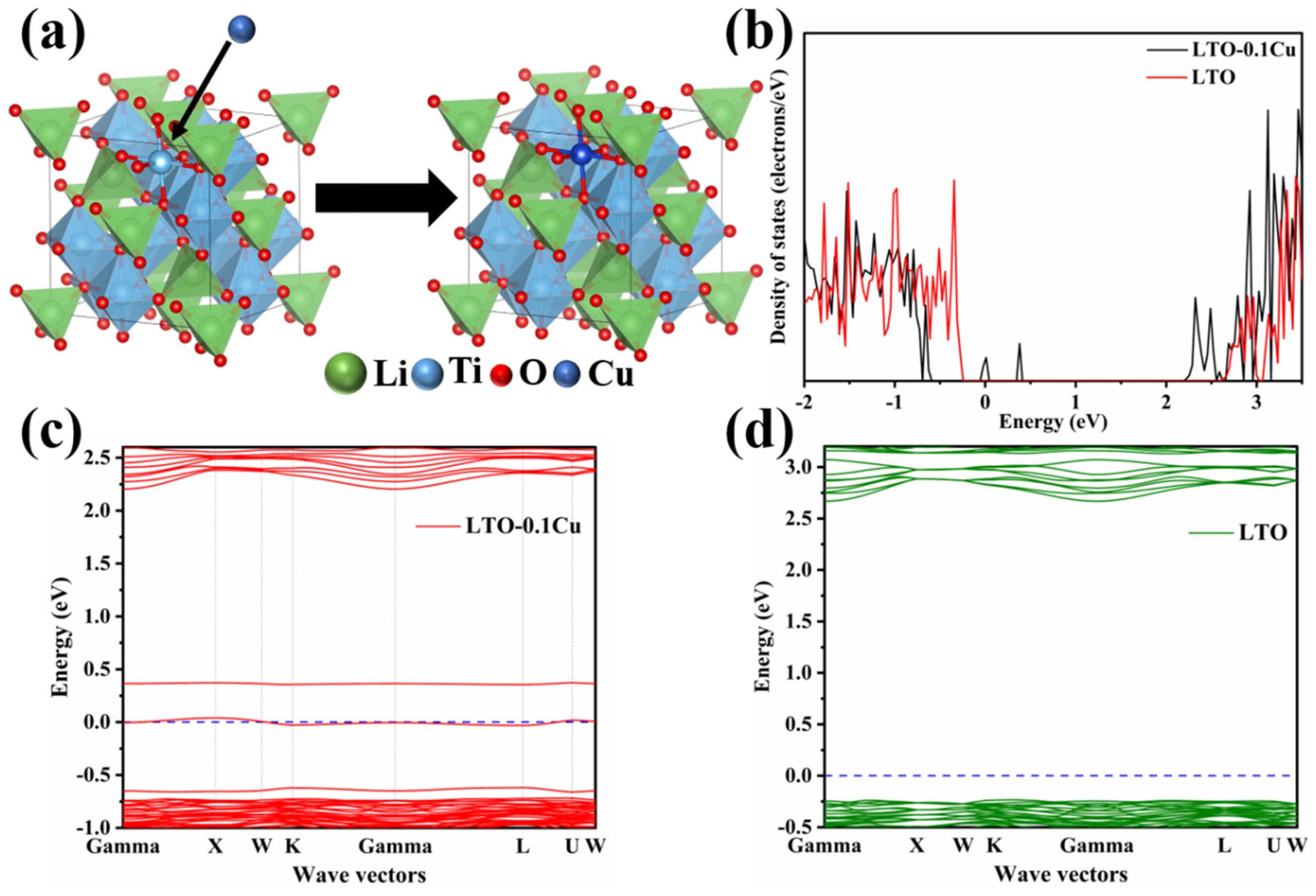
2. Results and Discussion
3. Experiment
3.1. Material Synthesis
3.2. Materials Characterization
3.3. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, M.; El-Hady, D.A.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Aravindan, V.; Lee, Y.S.; Madhavi, S. Research Progress on Negative Electrodes for Practical Li-Ion Batteries: Beyond Carbonaceous Anodes. Adv. Energy Mater. 2015, 5, 1402225. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Masse, R.C.; Cao, G. Novel Carbon-Encapsulated Porous SnO2 Anode for Lithium-Ion Batteries with Much Improved Cyclic Stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, G.; Choi, J. Binder-free SnO2–TiO2 composite anode with high durability for lithium-ion batteries. RSC Adv. 2019, 9, 6589–6595. [Google Scholar] [CrossRef]
- Yi, T.-F.; Xie, Y.; Zhu, Y.-R.; Zhu, R.-S.; Shen, H. Structural and thermodynamic stability of Li4Ti5O12 anode material for lithium-ion battery. J. Power Sources 2013, 222, 448–454. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li4Ti5O12—Based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R Rep. 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, H. Hydrothermal synthesis of Li4Ti5O12-TiO2 composites and Li4Ti5O12 and their applications in lithium-ion batteries. Ceram. Int. 2019, 45, 7419–7426. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; et al. Elucidating the Limit of Li Insertion into the Spinel Li4Ti5O12. ACS Mater. Lett. 2019, 1, 96–102. [Google Scholar] [CrossRef]
- Yan, B.; Li, M.S.; Li, X.F.; Bai, Z.M.; Yang, J.W.; Xiong, D.B.; Li, D.J. Novel understanding of carbothermal reduction enhancing electronic and ionic conductivity of Li4Ti5O12 anode. J. Mater. Chem. A 2015, 3, 11773–11781. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Lu, H.S.; Zhong, H.X.; Yan, X.D.; Ouyang, C.Y.; Zhang, L.Z. W6+ & Br- codoped Li4Ti5O12 anode with super rate performance for Li-ion batteries. J. Mater. Chem. A 2015, 3, 13706–13716. [Google Scholar]
- Yi, T.-F.; Wei, T.-T.; Li, Y.; He, Y.-B.; Wang, Z.-B. Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Mater. 2020, 26, 165–197. [Google Scholar] [CrossRef]
- Xue, X.; Yan, H.; Fu, Y. Preparation of pure and metal-doped Li4Ti5O12 composites and their lithium-storage performances for lithium-ion batteries. Solid State Ion. 2019, 335, 1–6. [Google Scholar] [CrossRef]
- Meng, W.-W.; Yan, B.-L.; Xu, Y.-J. Scalable synthesis of Ti3+ self-doped Li4Ti5O12 microparticles as an improved performance anode material for Li-ion batteries. J. Alloys Compd. 2019, 788, 21–29. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Lu, H.; Tang, D.; Ouyang, C.; Zhang, L. Ce 3+ -doped Li4Ti5O12 with CeO2 surface modification by a sol-gel method for high-performance lithium-ion batteries. Electrochim. Acta 2016, 189, 147–157. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; Hu, G.; Zhu, K.; Huang, H. Facile preparation of core-shell Si@Li4Ti5O12 nanocomposite as large-capacity lithium-ion battery anode. J. Energy Chem. 2020, 40, 89–98. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Yu, Y.; Hu, Y.; Chen, Y. Core-shell structured α-Fe2O3@Li4Ti5O12 composite as anode materials for high-performance lithium-ion batteries. J. Alloys Compd. 2020, 813, 152175. [Google Scholar] [CrossRef]
- Hong, H.-J.; Ban, G.; Lee, S.-M.; Park, I.-S.; Lee, Y.-J. Synthesis of 3D-structured Li4Ti5O12 from titanium(IV) oxysulfate (TiOSO4) solution as a highly sustainable anode material for lithium-ion batteries. J. Alloys Compd. 2020, 844, 156203. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, K.; Deng, X.; Ke, J.; Yang, B.; Dong, H.; Xiong, D.; He, M. Exfoliated Graphite Nanosheets Coating on Nano-grained SnO2/Li4Ti5O12 as a High-Performance Anode Material for Lithium-Ion Batteries. Langmuir 2020, 36, 14666–14675. [Google Scholar] [CrossRef] [PubMed]
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Surface-engineered Li4Ti5O12 nanoparticles by TiO2 coating for superior rate capability and electrochemical stability at elevated temperature. Appl. Surf. Sci. 2019, 480, 817–821. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Wang, D.; Xiao, L.; Guo, X.; Zhang, Y.; Wang, Z. Carbon-coated Li4Ti5O12–TiO2 microspheres as anode materials for lithium ion batteries. Surf. Eng. 2017, 33, 559–566. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.V.; Santhanagopalan, D. Interface-engineered Li4Ti5O12-TiO2 dual-phase nanoparticles and CNT additive for supercapacitor-like high-power Li-ion battery applications. Nanotechnology 2018, 29, 095402. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; An, C.; Zhang, H.; Wang, Y.; Jiao, L.; Yuan, H. Copper-doped dual phase Li4Ti5O12-TiO2 nanosheets as high-rate and long cycle life anodes for high-power lithium-ion batteries. ChemSusChem 2015, 8, 114–122. [Google Scholar] [CrossRef]
- Sun, J.; Teng, D.; Liu, Y.; Chi, C.; Yu, Y.; Lan, J.-L.; Yang, X. Enhanced lithium storage capability of a dual-phase Li4Ti5O12–TiO2–carbon nanofiber anode with interfacial pseudocapacitive effect. RSC Adv. 2014, 4, 48632–48638. [Google Scholar] [CrossRef]
- Liang, K.; Huang, X.; Hong, X.; Liao, Y.; Ren, Y.; Wang, H. Sulfur and nitrogen-doped Li4Ti5O12/rGO as an anode material for advanced sodium-ion batteries. J. Alloys Compd. 2021, 857, 158190. [Google Scholar] [CrossRef]
- Uceda, M.; Chiu, H.-C.; Gauvin, R.; Zaghib, K.; Demopoulos, G.P. Electrophoretically co-deposited Li4Ti5O12/reduced graphene oxide nanolayered composites for high-performance battery application. Energy Storage Mater. 2020, 26, 560–569. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Zhao, X. In-situ constructing of mesoporous Li4Ti5O12@rGO hybrid spheres as anode materials for lithium-ion batteries. Ionics 2020, 26, 2791–2801. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, H.; Hu, G. A flexible mesoporous Li4Ti5O12-rGO nanocomposite film as free-standing anode for high rate lithium ion batteries. J. Power Sources 2018, 375, 59–67. [Google Scholar] [CrossRef]
- Ge, H.; Hao, T.; Osgood, H.; Zhang, B.; Chen, L.; Cui, L.; Song, X.M.; Ogoke, O.; Wu, G. Advanced Mesoporous Spinel Li4Ti5O12/rGO Composites with Increased Surface Lithium Storage Capability for High-Power Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 9162–9169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cao, X.; Zhao, D.; Zhu, L.; Xie, L.; Li, J.; Miao, Y. Enhancing Lithium Storage Performances of the Li4Ti5O12 Anode by Introducing the CuV2O6 Phase. ACS Appl. Mater. Interfaces 2020, 12, 39170–39180. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, T.; Bai, Y.J. Capacity degradation of Li4Ti5O12 during long-term cycling in terms of composition and structure. Dalton Trans. 2020, 49, 10003–10010. [Google Scholar] [CrossRef]
- Ho, C.-K.; Li, C.-Y.V.; Chan, K.-Y.; Yung, H.; Tay, Y.-Y. Interfacing TiO2(B) Nanofibers with Li4Ti5O12 Towards Highly Reversible and Durable TiO2-based Anode for Li−Ion Batteries. Energy Technol. 2019, 7, 107–112. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, I.L.; Jiang, Y.-R.; Lin, J.-Y. Synthesis of spinel lithium titanate anodes incorporated with rutile titania nanocrystallites by spray drying followed by calcination. Solid State Ion. 2011, 201, 60–67. [Google Scholar] [CrossRef]
- Qian, D.; Gu, Y.; Guo, S.; Liu, H.; Chen, Y.; Wang, J.; Ma, G.; Wu, C. Effect of rich R-TiO2 on the rate and cycle properties of Li4Ti5O12 as anode for lithium ion batteries. J. Energy Chem. 2019, 32, 182–188. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, K.; Ke, J.; Dong, H.; Huang, X.; Bai, C.; Xiong, D.; He, M. Exfoliated graphite nanosheets wrapping on MoO2–SnO2 nanoparticles as a high performance anode material for lithium ion batteries. J. Power Sources 2020, 467, 228357. [Google Scholar] [CrossRef]
- Liang, Q.; Cao, N.; Song, Z.; Gao, X.; Hou, L.; Guo, T.; Qin, X. Co-doped Li4Ti5O12 nanosheets with enhanced rate performance for lithium-ion batteries. Electrochim. Acta 2017, 251, 407–414. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Xu, H.; Chen, J. Fabrication of continuous conductive network for Li4Ti5O12 anode by Cu-doping and graphene wrapping to boost lithium storage. J. Alloys Compd. 2019, 780, 1–7. [Google Scholar] [CrossRef]
- Xing, L.-L.; Huang, K.-J.; Cao, S.-X.; Pang, H. Chestnut shell-like Li4Ti5O12 hollow spheres for high-performance aqueous asymmetric supercapacitors. Chem. Eng. J. 2018, 332, 253–259. [Google Scholar] [CrossRef]
- Kahrizi, M.; Kashani, H.; Ghaffarinejad, A. Improving the Cyclability and Rate Capability of Li4Ti5O12 Anode Material by Cu2+ and F− co-Doping. ChemistrySelect 2023, 8, e202204198. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Wang, J.; Yang, Q. Electrochemical Characteristics of Cu-Doping Li4Ti5O12 as Anode for Lithium-Ion Batteries. ECS Meet. Abstr. 2010, MA2010-03, 282. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Li, W.-R.; Zhu, M.-H.; Xiong, D.-P.; He, M. Synthesis of Cu-doped Li4Ti5O12 anode materials with a porous structure for advanced electrochemical energy storage: Lithium-ion batteries. Solid State Ion. 2021, 364, 115614. [Google Scholar] [CrossRef]
- Feng, Y.; Bai, C.; Wu, K.; Dong, H.; Ke, J.; Huang, X.; Xiong, D.; He, M. Fluorine-doped porous SnO2@C nanosheets as a high performance anode material for lithium ion batteries. J. Alloys Compd. 2020, 843, 156085. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Wang, H.; Xie, S.; Yao, T.; Wang, J.; She, Y.; Shi, J.-W.; Shan, G.; Zhang, Q.; Han, X.; Leung, M.K.H. Casting amorphorized SnO2/MoO3 hybrid into foam-like carbon nanoflakes towards high-performance pseudocapacitive lithium storage. J. Colloid Interface Sci. 2019, 547, 299–308. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Zhu, M.; He, M.; Feng, Z.; Zhang, B. Fabrication of Cu-Doped Li4Ti5O12 Particles Embedded in Reduced Graphene Oxide Nanosheets for High-Rate Lithium-Ion Battery Anode. Inorganics 2025, 13, 394. https://doi.org/10.3390/inorganics13120394
Deng X, Zhu M, He M, Feng Z, Zhang B. Fabrication of Cu-Doped Li4Ti5O12 Particles Embedded in Reduced Graphene Oxide Nanosheets for High-Rate Lithium-Ion Battery Anode. Inorganics. 2025; 13(12):394. https://doi.org/10.3390/inorganics13120394
Chicago/Turabian StyleDeng, Xiaoqian, Menghan Zhu, Miao He, Zuyong Feng, and Beibei Zhang. 2025. "Fabrication of Cu-Doped Li4Ti5O12 Particles Embedded in Reduced Graphene Oxide Nanosheets for High-Rate Lithium-Ion Battery Anode" Inorganics 13, no. 12: 394. https://doi.org/10.3390/inorganics13120394
APA StyleDeng, X., Zhu, M., He, M., Feng, Z., & Zhang, B. (2025). Fabrication of Cu-Doped Li4Ti5O12 Particles Embedded in Reduced Graphene Oxide Nanosheets for High-Rate Lithium-Ion Battery Anode. Inorganics, 13(12), 394. https://doi.org/10.3390/inorganics13120394





