3. Materials and Methods
All chemicals were purchased from Apollo Scientific (Manchester, UK) or Fluorochem (Hadfield, UK) commercial sources and used without further purification unless otherwise stated.
L5 was prepared by the literature method [
25]. NMR spectra were recorded on Bruker Fourier 300, DPX 400, and Avance 500 or 600 MHz NMR spectrometers (Billerica, MA, USA).
1H and
13C{
1H} NMR chemical shifts were referenced relative to the residual solvent resonances in the deuterated solvent. Mass spectra (
Supplementary Materials) were recorded on a Waters LCT premier XE spectrometer (Temecula, CA, USA). UV/Vis spectra were obtained on a Cary 60 spectrophotometer and recorded over the range of 800 to 250 nm, with a 600 nm min
−1 scan rate using a 1 cm path length quartz cuvette. Emission spectra were collected using a Cary Eclipse spectrophotometer from 700 to 450 nm, with an excitation wavelength of 410 nm and a 600 nm min
−1 scan rate.
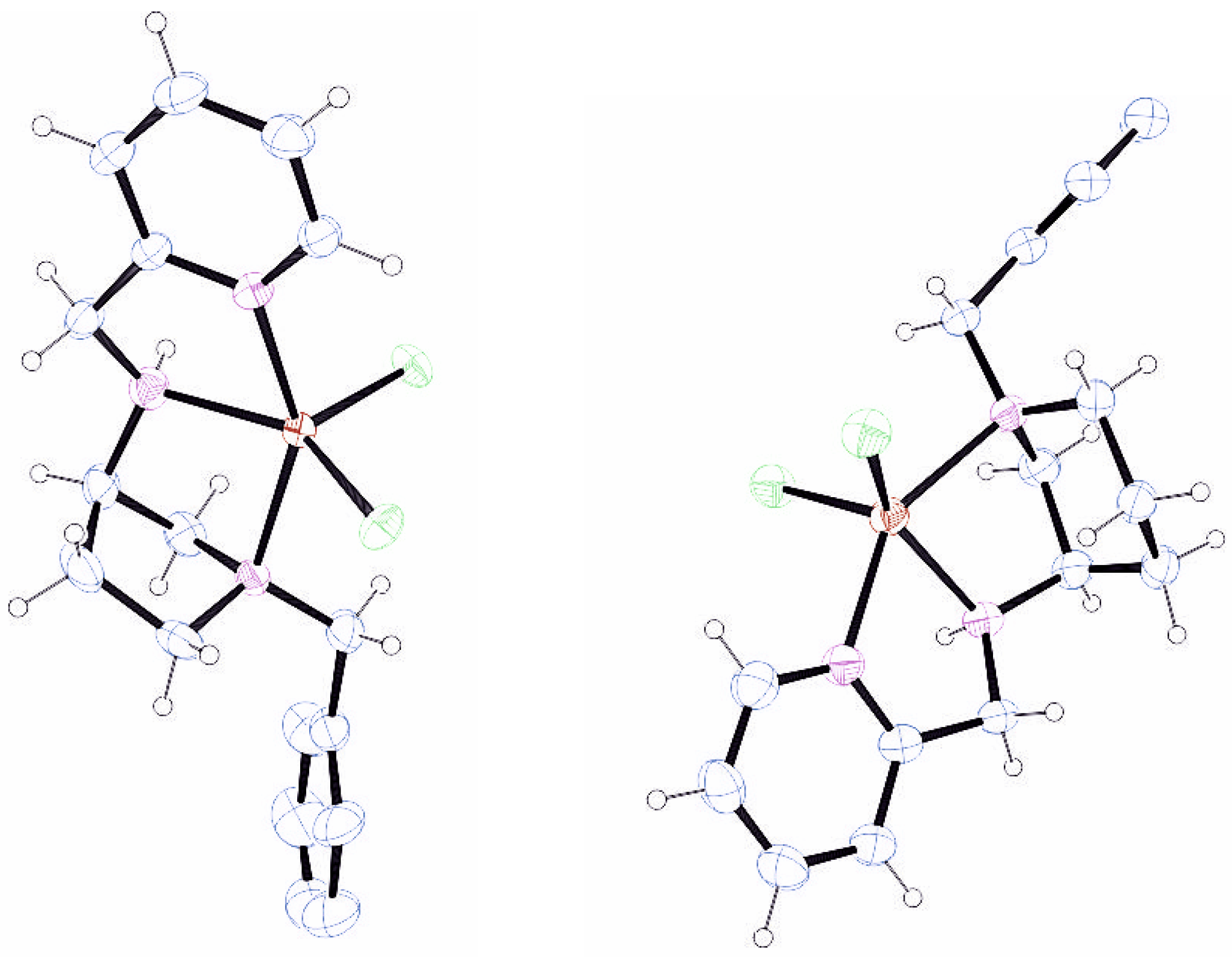
An Agilent SuperNova Dual Atlas (Santa Clara, CA, USA) diffractometer was used to record the single-crystal X-ray diffraction data using either Cu or Mo radiation. Data were recorded at room temperature or at 200 K with an Oxford Cryosystems cooling apparatus used for temperature regulation. The data were processed using CrysAlisPro 1.171.43.90 [
26] and the crystal structures were solved using SHELXT [
27] and refined using SHELXL [
28]. Non-hydrogen atoms were generally refined with anisotropic displacement parameters. In the final cycles of refinement, ideal hydrogen atom geometry was applied, and a riding model was used with displacement parameters set to either 1.2 or 1.5 times the U
eq values for the atoms to which the hydrogen atoms are bonded. CCDC 2500951-2500958 contain the supplementary crystallographic data for this paper. These data can be obtained free-of-charge via
https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 June 2024) or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033; e-mail:
deposit@ccdc.cam.ac.uk. A table of pertinent details of the data collection and refinement is included in the
Supplementary Materials along with relevant spectroscopic and analytical data in
Figures S1–S113 and Tables S1–S7.
DFT calculations were performed using the ORCA quantum chemistry software package (version 6.0.0) [
29]. The relevant structures were subjected to geometry optimisation and confirmed as stationary points via vibrational frequency calculations. The conductor-like polarisable continuum model (CPCM) was used to model solvation [
30]. The geometry optimisation and vibrational frequency calculations were performed at the PBE-D3BJ level of theory with def2-SVP basis, followed by singe-point energy calculations at the PBE0-D3BJ/def2-TZVP level of theory to obtain accurate electronic energies [
31,
32,
33,
34]. Calculations were performed using the facility operated by Advanced Research Computing at Cardiff (ARCCA) on behalf of Supercomputing Wales (SCW). The approach is common practice as detailed in a recent publication [
35].
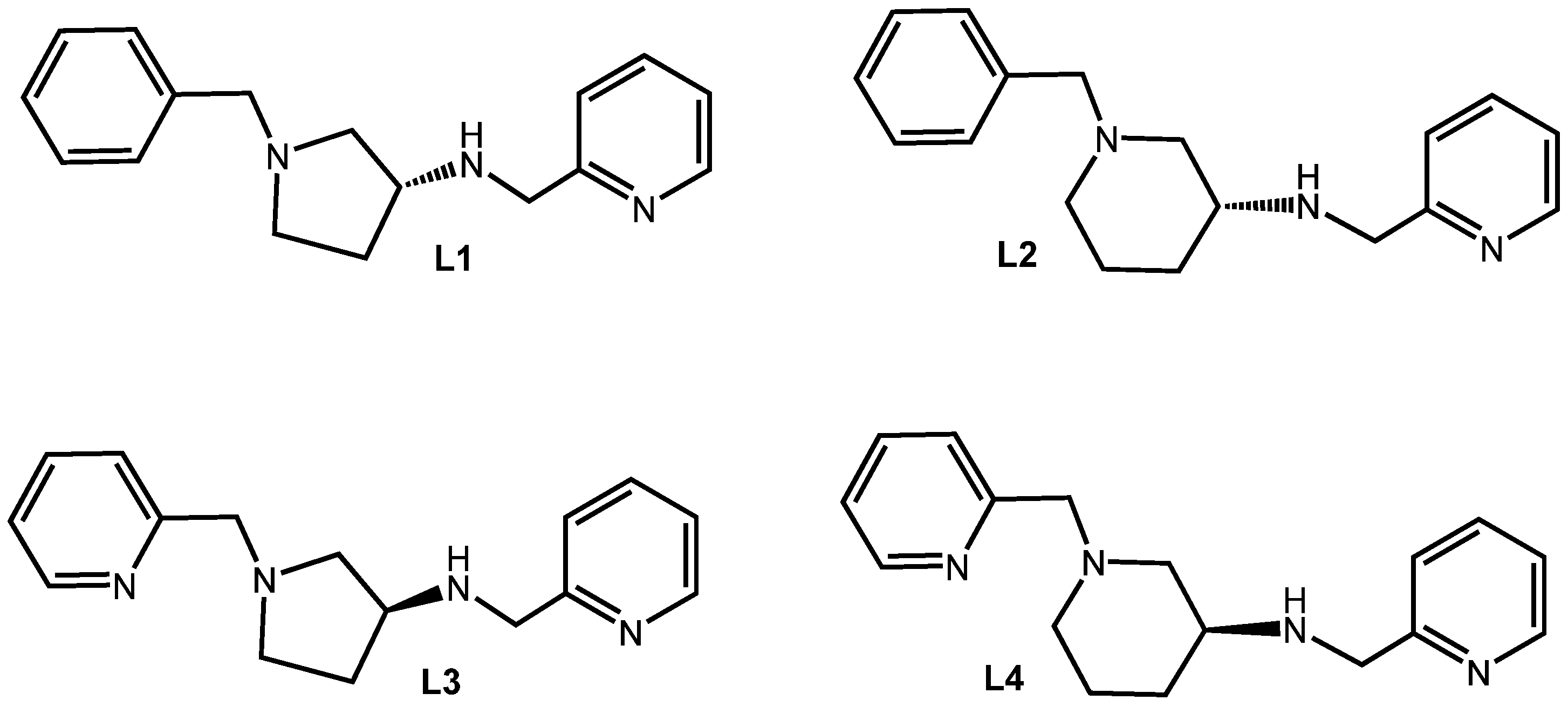
3.1. Synthesis of (R)-1-Benzyl-N-(Pyridin-2-Ylmethyl)Pyrrolidin-3-Amine, L1
A 1:1 mixture of (R)-1-benzyl-3-aminopyrrolidine (0.50 g, 2.84 mmol) and 2-pyridinecarboxaldehyde (0.3 g, 2.84 mmol) were heated at 50 °C for 4 h in MeOH (20 mL), allowed to cool to RT, and the solvent was removed in vacuo. The residue was dissolved in MeOH (25 mL) and excess solid NaBH4 (0.4 g) added portion-wise over the course of 3 hrs. After removing the MeOH, the residue was treated with water and made basic with solid NaOH (care! Heat). The amine product was extracted into CH2Cl2 (2 × 40 mL), the organic phase was dried over MgSO4, filtered, and all volatiles were removed in vacuo to yield the product as a clear yellow oil. Yield = 508 mg (67%). 1H (CDCl3, 400 MHz): δH 8.45 (d, J 4.9 Hz, 1H), 7.56 (td, J 7.6, 1.7 Hz, 1H), 7.27–7.13 (m, 6H), 7.06 (dd, J 7.6, 4.9 Hz, 1H), 3.79 (d, J 14.2 Hz, 1H), 3.74 (d, J 13.2 Hz, 1H), 3.53 (s, 2H), 3.29 (m, 1H), 2.73 (dd, J 9.4, 6.7 Hz, 1H), 2.52 (m, 2H), 2.32 (dd, J 9.4, 5.1 Hz, 1H), 2.06 (m, 1H), 1.58 (m, 1H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 159.6 (C), 149.2 (CH), 148.9 (CH), 139.0 (C), 136.5 (CH), 128.9 (CH), 128.2 (CH), 127.0 (CH), 122.4 (CH), 122.0 (CH) 60.8 (CH2), 60.6 (CH2), 58.0 (CH), 53.7 (CH2) 53.4 (CH2), 32.1 (CH2) ppm. HRMS (ES): m/z 268.1814 (calc. 268.1814) [L]+, 100%.
3.2. Synthesis of (R)-1-Benzyl-N-(Pyridin-2-Ylmethyl)Piperidin-3-Amine, L2
Prepared as for L1 using (R)-1-benzyl-3-aminopiperidine. Yield = 498 mg (71%). 1H (CDCl3, 400 MHz): δH 8.46 (d, J 4.8 Hz, 1H), 7.54 (td, J 7.6, 1.6 Hz, 1H), 7.25–7.14 (m, 6H), 7.06 (dd, J 6.9, 5.5 Hz, 1H), 3.85 (d, J 14.0 Hz, 1H), 3.81 (d, J 13.2 Hz, 1H), 3.44 (s, 2H), 2.82 (d, J 10.0 Hz, 1H), 2.69 (m, 1H), 2.57 (m, 1H), 2.01 (t, J 10.0 Hz, 1H), 1.92 (t, J 9.0 Hz, 1H), 1.82 (m, 1H), 1.63 (m, 1H), 1.49 (m, 1H), 1.17 (m, 1H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 159.9 (C), 149.2 (CH), 138.3 (C), 136.4 (CH), 129.2 (CH), 128.2 (CH), 127.0 (CH), 122.3 (CH), 121.9 (CH) 63.3 (CH2), 59.4 (CH2), 54.1 (CH), 53.8 (CH2) 52.4 (CH2), 31.0 (CH2), 23.7 (CH2) ppm. HRMS (ES): m/z 268.1814 (calc. 268.1814) [L]+, 100%.
3.3. Synthesis of (S)-N,1-Bis(Pyridin-2-Ylmethyl)Pyrrolidin-3-Amine, L3
A 1:1 mixture of 3S-Bocaminopyrrolidine (0.5 g, 2.7 mmol) and 2-pyridinecarboxaldehyde (0.29 g, 2.7 mmol) was heated at 50 °C for 4 h in MeOH (20 mL), allowed to cool to RT, and the solvent was removed in vacuo. The residue was dissolved in MeOH (25 mL) and solid NaBH4 added portion-wise over the course of 3 hrs. After removing the MeOH, the residue was treated with water whereupon an oil separated out (the pH was around neutral). The protected amine was extracted into Et2O (2 × 50 mL) and the combined organic extracts were washed with water, dried over MgSO4, filtered and taken to dryness in vacuo to yield a colourless oil. The Boc-protected amine was dissolved in 3 M HCl in MeOH and the mixture was heated at 50 °C for 8 hrs. After stirring overnight at RT, the volatiles were removed and the residue was dissolved in water (30 mL) and basified to pH > 14. Extraction into CH2Cl2 (2 × 40 mL), drying over MgSO4, filtration and subsequent removal of volatiles gave (S)-1-(pyridin-2-ylmethyl)pyrrolidin-3-amine as a clear yellow oil. Yield = 0.45 g (88%). The oil was used directly for the preparation of (S)-N,1-bis(pyridin-2-ylmethyl)pyrrolidin-3-amine (L3) by adding to a solution of 2-pyridinecarboxaldehyde (1.1 equiv.) in MeOH (30 mL). After stirring for 2 hrs, the solvent was removed and fresh MeOH was added (30 mL) whereupon an excess of NaBH4 (0.5 g) was added portion-wise over a period of 2 hrs. After leaving to stir overnight, the volatiles were removed, the residue dissolved in water (40 mL), which was made strongly basic by the addition of solid NaOH (care heat!) with ice cooling. The mixture was extracted into CH2Cl2 (2 × 40 mL) and the organic phase was dried over MgSO4, filtered, and taken to dryness in vacuo to give a dark viscous oil, which was heated in vacuo using a short path distillation apparatus to remove a small amount of 2-pyridylmethanol. The undistilled brown oil was the (S)-N,1-bis(pyridin-2-ylmethyl)pyrrolidin-3-amine (L3) product. Yield = 0.57 g (79%). 1H (CDCl3, 400 MHz): δH 8.47 (m, 2H), 7.56 (m, 2H), 7.32 (d, J 7.9 Hz, 1H), 7.22 (d, J 7.8 Hz, 1H), 7.08 (m, 2H), 3.81 (d, J 14.0 Hz, 1H), 3.77 (d, J 14.0 Hz, 1H), 3.73 (d, J 13.6 Hz, 1H), 3.68 (d, J 13.6 Hz, 1H), 3.33 (m, 1H), 2.80 (dd, J 9.3, 6.7 Hz, 1H), 2.64 (m, 1H), 2.57 (m, 1H), 2.42 (dd, J 9.3, 5.2 Hz, 1H), 2.10 (m, 1H), 1.62 (m, 1H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 159.6 (C), 159.1 (C), 149.3 (CH), 149.2 (CH), 136.5 (CH), 136.4 (CH), 123.0 (CH), 122.4 (CH), 122.0 (CH), 121.9 (CH), 62.1 (CH2), 60.9 (CH2), 57.1 (CH), 53.8 (CH2) 53.3 (CH2), 32.1 (CH2) ppm. HRMS (ES): m/z 269.1776 (calc. 269.1766) [L]+, 100%.
3.4. Synthesis of (S)-N,1-Bis(Pyridin-2-Ylmethyl)Piperidin-3-Amine, L4
This was prepared in a similar manner to that described for L3 and obtained as a clear yellow oil without the need for short path distillation. Yield = 0.70 g (92%). 1H (CDCl3, 400 MHz): δH 8.47 (m, 2H), 7.55 (m, 2H), 7.33 (d, J 7.8 Hz, 1H), 7.21 (d, J 7.8 Hz, 1H), 7.08 (m, 2H), 3.84 (s, 2H), 3.59 (s, 2H), 2.87 (d, J 10.1 Hz, 1H), 2.72 (m, 1H), 2.63 (m, 1H), 2.08 (t, J 10.5 Hz, 1H), 1.98 (t, J 9.6 Hz, 1H), 1.87 (m, 2H), 1.65 (m, 1H), 1.53 (m, 1H), 1.18 (m, 1H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 160.0 (C), 158.9 (C), 149.3 (CH), 149.2 (CH), 136.5 (CH), 136.3 (CH), 123.2 (CH), 122.3 (CH), 121.9 (CH), 121.9 (CH), 64.9 (CH2), 59.8 (CH2), 54.1 (CH2), 54.0 (CH), 52.5 (CH2) 31.0 (CH2), 23.8 (CH2) ppm. HRMS (ES): m/z 269.1776 (calc. 269.1766) [L]+, 100%.
3.5. Synthesis of [Zn(L1)Cl2]
A solution of ligand L1 (100 mg, 3.75 × 10−4 mol) in EtOH (10 mL) was added to a solution of ZnCl2 (51 mg, 3.75 × 10−4 mol) in EtOH (10 mL) to give an immediate precipitate that was filtered and air-dried. Yield = 120 mg (79%). 1H (d6-dmso, 400 MHz): δH 8.50 (br, 1H), 7.95 (t, J 7.7 Hz, 1H), 7.51 (d, J 8.0 Hz, 2H), 7.47 (br, 1H), 7.43–7.24 (m, 5H), 3.97 (br, 3H), 3.74 (d, J 10.8 Hz, 1H), 3.42 (br, 1H), 2.92 (br, 2H), 2.29 (m br, 2H), 1.91 (br, 1H) 1.68 (br, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 156.7 (C), 147.7 (CH), 139.2 (C), 130.6 (CH), 128.5 (CH), 127.7 (CH), 124.0 (CH), 123.3 (CH), 60.8 (CH2), 58.7 (CH2), 57.4 (CH), 55.9 (CH2) 49.5 (CH2), 28.8 (CH2) ppm. HRMS (ES): m/z 366.0719 (calc. 366.0715) [L]+, 95%.
3.6. Synthesis of [Zn(L2)Cl2]
As above. Yield = 120 mg (76%). 1H (d6-dmso, 400 MHz): δH 8.64 (d, J 4.7 Hz, 1H), 8.05 (t, J 7.7 Hz, 1H), 7.58 (m, 2H), 7.42–7.32 (m, 5H), 4.70 (br, 1H), 4.08 (br, 2H), 3.91 (br, 2H), 3.19 (br, 1H), 2.83 (br, 2H), 2.44 (br, 1H), 2.16 (br, 1H), 1.82 (br, 2H), 1.52 (br, 1H), 1.44 (m, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 155.4 (C), 146.4 (CH), 138.7 (C), 130.1 (CH), 127.5 (CH), 127.5 (CH), 127.0 (CH), 123.2 (CH), 122.3 (CH), 60.5 (CH2), 55.3 (CH2), 50.7 (CH), 50.2 (CH2) 47.3 (CH2), 25.2 (CH2), 19.4 (CH2) ppm. HRMS (ES): m/z 380.0873 (calc. 380.0872) [L]+, 20%.
3.7. Synthesis of [Zn(L3)Cl2]
The tetramine L3 (300 mg, 1.12 mmol) was dissolved in EtOH (25 mL) and added to a solution of 1 equivalent of ZnCl2 in EtOH (15 mL). The homogeneous solution was stirred overnight before concentrating in vacuo and precipitation of a cream solid on addition of diethyl ether. Attempts to filter were frustrated by the hygroscopic nature of the solid, so it was extracted into CH2Cl2 and taken to dryness at the pump to give a pale yellow solid. Yield = 362 mg (80%). 1H (CDCl3, 400 MHz): δH 8.84 (d, J 5.0 Hz, 1H), 8.52 (d, J 5.0 Hz, 1H), 7.77 (td, J 7.8, 1.2 Hz, 1H), 7.62 (td, J 7.9, 1.2 Hz, 1H), 7.36 (m, 1H), 7.28 (d, J 7.7 Hz, 1H), 7.21 (d, J 7.8 Hz, 1H), 7.16 (dd, 7.5, 4.9 Hz, 1H), 4.43 (d, J 15.3 Hz, 1H), 4.29 (dd, J 15.5, 5.6 Hz, 1H), 4.02 (dd, J 15.6, 7.8 Hz, 1H), 3.68 (d, J 15.3 Hz, 1H), 3.46 (m, 1H), 3.38 (d, J 9.5 Hz, 1H), 3.31 (dd, J 9.2, 3.9 Hz, 1H), 2.55 (dd, J 9.7, 2.3 Hz, 1H), 2.29 (m, 1H), 2.11 (m, 2H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 159.6 (C), 159.1 (C), 149.3 (CH), 149.2 (CH), 139.2 (CH), 137.0 (CH), 124.4 (CH), 123.3 (CH), 122.7 (CH), 122.6 (CH), 62.1 (CH2), 60.9 (CH2), 57.1 (CH), 53.8 (CH2) 53.3 (CH2), 32.1 (CH2) ppm. HRMS (ES): m/z 367.0675 (calc. 367.0668) [M − Cl]+, 50%.
3.8. Synthesis of [Zn(L3)Br]Br]
Prepared as for the chlorido complex. The cream precipitate obtained on concentration of the reaction mixture was filtered and air-dried. Yield = 387 mg (70%). 1H (d6-dmso, 400 MHz): δH 8.78 (d, J 5.0 Hz, 1H), 8.26 (d, J 5.0 Hz, 1H), 8.19 (td, J 7.7, 1.6 Hz, 1H), 8.15 (td, J 7.7, 1.4 Hz, 1H), 7.71 (m, 3H), 7.60 (t, J 6.2 Hz, 1H), 5.17 (dd, J 10.0, 6.3 Hz, 1H), 4.36–4.05 (m, 4H), 3.62 (d, J 6.0 Hz, 1H), 3.42 (m, 1H), 3.03 (d, J 10.3 Hz, 1H), 2.78 (m, 1H), 2.41 (d, J 10.6 Hz, 1H), 2.28 (m, 1H), 1.75 (m, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 157.7 (C), 155.5 (C), 148.4 (CH), 148.2 (CH), 141.4 (CH), 125.3 (CH), 125.2 (CH), 125.0 (CH), 124.8 (CH), 56.0 (CH), 56.0 (CH2), 53.3 (CH2), 50.0 (CH2) 49.8 (CH2), 29.5 (CH2) ppm. HRMS (ES): m/z 413.0128 (calc. 413.0142) [M − Br]+, 20%.
3.9. Synthesis of [Zn(L3)I]I]
Prepared as for the chlorido complex and isolated in several crops upon slow evaporation of the reaction mixture. Each crop was isolated by filtration and air-dried. Combined yield = 586 mg (89%). 1H (d6-dmso, 400 MHz): δH 8.65 (d, J 5.1 Hz, 1H), 8.32 (s br, 1H), 8.18 (q, J 7.7 Hz, 1H), 7.72 (d, J 7.8 Hz, 1H), 7.68 (d, J 7.9 Hz, 1H), 7.64 (t, J 6.6 Hz, 1H), 5.16 (t, J 6.6 Hz, 1H), 4.40–4.07 (m, 4H), 3.62 (d, J 5.0 Hz, 1H), 3.42 (m, 2H), 2.92 (d, J 10.2 Hz, 1H), 2.69 (m, 1H), 2.44 (d, J 10.1 Hz, 1H), 2.27 (m br, 1H), 1.74 (m br, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 157.4 (C), 155.3 (C), 149.1 (CH), 148.7 (CH), 139.9 (CH), 139.6 (CH), 124.7 (CH), 124.1 (CH), 123.8 (CH), 58.0 (CH), 57.7 (CH2), 54.9 (CH2), 50.9 (CH2) 49.0 (CH2), 27.4 (CH2) ppm. HRMS (ES): m/z 459.0029 (calc. 459.0024) [M − I]+, 40%.
3.10. Synthesis of [Cd(L3)Cl2]
Prepared as for [Zn(L3)Cl2]. Cream solid isolated in several crops and recrystallised from EtOH. Combined yield = 440 mg (87%). 1H (CD2Cl2, 400 MHz): δH 9.12 (s br, 1H), 9.06 (d, J 4.2 Hz, 1H), 7.80 (m, 2H), 7.36 (t, J 6.6 Hz, 1H), 7.32 (t, J 6.8 Hz, 1H), 7.27 (d, J 7.8 Hz, 1H), 7.21 (d, J 7.8 Hz, 2H), 4.55 (d, J 12.4 Hz, 1H), 4.42 (dd, J 17.2, 7.9 Hz, 1H), 3.92 (m, 1H), 3.68 (m, 2H), 3.46 (m, 1H), 3.33 (td, J 9.1, 3.7 Hz, 1H), 3.12 (d, J 7.3 Hz, 1H), 2.90 (d, J 9.8 Hz, 1H), 2.47 (m, 1H), 2.20 (m, 2H), 1.81 (m br, 1H), 1.43 (m br, 1H) ppm. 13C{1H} (CD2Cl2, 100 MHz): δC 156.7 (C), 154.8 (C), 149.8 (CH), 149.7 (CH), 138.4 (CH), 138.3 (CH), 123.4 (CH), 123.3 (CH), 123.1 (CH), 122.3 (CH), 58.7 (CH2), 57.8 (CH), 55.6 (CH2), 50.7 (CH2) 48.8 (CH2), 26.5 (CH2) ppm. HRMS (ES): m/z 417.0411 (calc. 417.0410) [M − Cl]+, 10%.
3.11. Synthesis of [Cd(L3)Br2]]
Prepared as for the chlorido complex. The cream precipitate obtained on concentration of the reaction mixture was filtered and air-dried. Yield = 587 mg (97%). 1H (d6-dmso, 300 MHz): δH 9.01 (d, J 4.6 Hz, 2H), 7.99 (dd, J 7.7, 1.8 Hz, 1H), 7.94 (dd, J 7.7, 1.8 Hz, 1H), 7.57–7.44 (m, 4H), 4.52 (d, J 6.2 Hz, 1H), 4.39 (d, J 15.1 Hz, 1H), 4.18 (dd, J 17.5, 7.4 Hz, 1H), 3.93 (d, J 17.4 Hz, 1H), 3.69 (d, J 15.1 Hz, 1H), 3.51 (m br, 1H), 3.15 (m, 1H), 2.72 (d, J 9.5 Hz, 1H), 2.41 (m, 1H), 2.18 (dd, J 9.5, 2.5 Hz, 1H), 1.77 (m, 1H), 1.23 (m, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 157.7 (C), 155.5 (C), 149.2 (CH), 148.9 (CH), 139.3 (CH), 139.2 (CH), 124.3 (CH), 123.7 (CH), 123.3 (CH), 58.2 (CH2), 57.8 (CH), 54.9 (CH2), 50.6 (CH2) 48.6 (CH2), 26.6 (CH2) ppm. HRMS (ES): m/z 460.9897 (calc. 460.9905) [M − Br]+, 3%.
3.12. Synthesis of [Cd(L3)I2]]
Prepared as above. White solid. Yield = 647 mg (91%). 1H (d6-dmso, 300 MHz): δH 8.92 (d, J 4.5 Hz, 1H), 8.81 (d, J 4.7 Hz, 1H), 8.03 (td, J 7.8, 1.6 Hz, 1H), 7.99 (td, J 7.6, 1.8 Hz, 1H), 7.61 (m, 1H), 7.53 (m, 2H), 4.58 (m br, 1H), 4.27 (d, J 15.4 Hz, 1H), 4.16 (dd, J 17.3, 7.0 Hz, 1H), 4.03 (d, J 17.6 Hz, 1H), 3.84 (d, J 15.8 Hz, 1H), 3.55 (m, 1H), 3.08 (m, 1H), 2.76 (d, J 9.8 Hz, 1H), 2.43 (m, 1H), 2.25 (d, J 10.2 Hz, 1H), 1.91 (m br, 1H), 1.38 (m br, 1H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 157.4 (C), 155.3 (C), 149.1 (CH), 148.7 (CH), 139.9 (CH), 139.6 (CH), 124.7 (CH), 124.1 (CH), 123.8 (CH), 58.0 (CH2), 57.7 (CH), 54.9 (CH2), 50.9 (CH2) 48.9 (CH2), 27.4 (CH2) ppm. HRMS (ES): m/z 508.9771 (calc. 508.9766) [M − I]+, 2%.
3.13. Synthesis of [Zn(L4)Cl2]
This was prepared in a similar manner to that described for L3 and ZnCl2. The compound did not precipitate from EtOH, so all volatiles were removed in vacuo, the residue was dissolved in CH2Cl2 (10 mL), filtered, and the solvent was removed under dynamic vacuum to give a pale yellow solid. Yield = 390 mg (83%). 1H (CD2Cl2, 400 MHz): δH 8.55 (d, J 4.8 Hz, 1H), 8.29 (d, J 5.0 Hz, 1H), 7.95 (t, J 7.7 Hz, 1H), 7.88 (t, J 7.7 Hz, 1H), 7.50 (d, J 4.7 Hz, 1H), 7.48 (d, J 4.6 Hz, 1H), 7.42 (m, 2H), 4.54 (t, J 7.5 Hz, 1H),4.40 (dd, J 15.7, 7.4 Hz, 1H), 4.06 (d, J 15.6 Hz, 1H), 3.96 (d, J 15.6 Hz, 1H), 3.92 (dd, J 15.2, 8.0 Hz, 1H), 2.98 (m, 2H), 2.58 (m, 2H), 2.37 (m, 1H), 2.20 (d, J 14.1 Hz, 1H), 1.62 (m, 2H) ppm. 13C{1H} (CD2Cl2, 100 MHz): δC 157.1 (C), 154.0 (C), 148.0 (CH), 147.8 (CH), 140.4 (CH), 139.0 (CH), 124.6 (CH), 124.6 (CH), 124.1 (CH), 123.7 (CH), 59.7 (CH2), 55.7 (CH2), 53.8 (CH2), 51.7 (CH), 51.5 (CH2) 27.3 (CH2), 20.2 (CH2) ppm. HRMS (ES): m/z 381.0829 (calc. 381.0824) [L]+, 40%.
3.14. Synthesis of [Zn(L4)Br2]
This was prepared in a similar manner to that described for L4 and ZnCl2. Yield = 518 mg (91%). 1H (CD2Cl2, 400 MHz): δH 8.55 (d, J 5.0 Hz, 1H), 7.95 (m, 2H), 7.77 (d, J 5.1 Hz, 1H), 7.51 (m, 3H), 7.34 (m, 1H), 5.17 (dd, J 10.1, 6.4 Hz, 1H), 4.51 (dd, J 15.3, 6.1 Hz, 1H), 4.05 (d, J 15.8 Hz, 1H), 3.92 (d, J 15.7 Hz, 1H), 3.65 (dd, J 15.4, 10.6 Hz, 1H), 3.23 (m, 1H), 3.15 (d, J 11.5 Hz, 1H), 2.67 (m, 2H), 2.46 (d, J 12.3 Hz, 1H), 2.37 (m, 2H), 1.61 (m, 2H) ppm. 13C{1H} (CD2Cl2, 100 MHz): δC 156.7 (C), 154.3 (C), 147.3 (CH), 147.3 (CH), 141.2 (CH), 140.7 (CH), 125.2 (CH), 125.0 (CH), 124.8 (CH), 124.7 (CH), 59.2 (CH2), 54.7 (CH2), 54.4 (CH2), 52.0 (CH), 51.3 (CH2) 27.3 (CH2), 20.2 (CH2) ppm. HRMS (ES): m/z 427.0302 (calc. 427.0299) [L]+, 45%.
3.15. Synthesis of [Zn(L4)I]I
This was prepared in a similar manner to that described for L4 and ZnCl2. Several crops were obtained upon slow evaporation of the EtOH. Combined yield = 573 mg (85%). 1H (d6-dmso, 400 MHz): δH 8.78 (d, J 4.9 Hz, 1H), 8.21 (td, J 7.7, 1.5 Hz, 1H), 8.15 (td, J 7.7, 1.5 Hz, 1H), 7.79 (m, 2H), 7.74 (d, J 8.0 Hz, 1H), 7.69 (d, J 7.8 Hz, 1H), 7.55 (m, 1H), 5.33 (dd, J 11.1, 5.3 Hz, 1H), 4.26 (dd, J 15.4, 5.4 Hz, 1H), 4.06 (m, 3H), 3.22 (m, 1H), 3.13 (d, J 10.9 Hz, 1H), 2.87 (d, J 11.9 Hz, 1H), 2.70 (td, J 12.2, 3.0 Hz, 1H), 2.30 (m, 1H), 1.93 (d, J 13.3 Hz, 1H), 1.68 (m, 2H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 157.0 (C), 154.7 (C), 147.9 (CH), 147.5 (CH), 141.7 (CH), 141.6 (CH), 125.5 (CH), 125.5 (CH), 125.4 (CH), 124.7 (CH), 58.5 (CH2), 54.1 (CH2), 53.9 (CH2), 51.5 (CH), 50.8 (CH2) 27.2 (CH2), 20.3 (CH2) ppm. HRMS (ES): m/z 473.0187 (calc. 473.0181) [L]+, 15%.
3.16. Synthesis of [Cd(L4)Cl2]
This was prepared in a similar manner to that described for L4 and ZnCl2. The compound did not precipitate from EtOH, so all volatiles were removed in vacuo to give a white solid. Yield = 517 mg (99%). 1H (CD2Cl2, 400 MHz): δH 9.26 (s br, 1H), 9.02 (s br, 1H), 7.77 (m, 2H), 7.33 (m, 1H), 7.27 (m, 3H), 4.34 (dd, J 16.4, 8.2 Hz, 1H), 4.18 (s br, 1H), 3.88 (d, J 16.1 Hz, 1H), 3.57 (s br, 1H), 3.30 (s br, 1H), 3.15 (m br, 2H), 2.63 (s br, 1H), 2.29 (m br, 2H), 1.50 (m br, 2H), 1.16 (s br, 1H) ppm. 13C{1H} (CD2Cl2, 100 MHz): δC 155.8 (C), 154.5 (C), 150.3 (CH), 148.8 (CH), 138.7 (CH), 138.3 (CH), 124.0 (CH), 123.5 (CH), 123.4 (CH), 122.7 (CH), 60.4 (CH2), 57.0 (CH2), 54.1 (CH2), 51.7 (CH), 48.2 (CH2), 24.0 (CH2), 20.2 (CH2) ppm. HRMS (ES): m/z 431.0569 (calc. 431.0567) [L]+, 10%.
3.17. Synthesis of [Cd(L4)Br2]
This was prepared in a similar manner to that described for L4 and CdCl2. The compound precipitated from EtOH as a cream solid which was filtered and air-dried. Yield = 274 mg (44%). 1H (CD2Cl2, 400 MHz): δH 9.42 (s br, 1H), 9.13 (s br, 1H), 7.80 (m, 2H), 7.39 (m, 2H), 7.29 (m, 2H), 4.49 (br, 1H), 4.30 (d br, 1H), 3.893 (m br, 1H), 3.52 (br, 1H), 3.34 (br, 1H), 3.22 (br, 1H), 2.97 (br, 1H), 2.68 (br, 1H), 2.31 (m br, 2H), 1.74–1.28 (m br, 3H) ppm. 13C{1H} (CD2Cl2, 100 MHz): δC 155.3 (C), 154.2 (C), 150.3 (CH), 148.6 (CH), 138.8 (CH), 138.3 (CH), 124.2 (CH), 123.4 (CH), 123.4 (CH), 122.7 (CH), 60.1 (CH2), 57.0 (CH2), 54.0 (CH2), 51.7 (CH), 47.9 (CH2), 23.9 (CH2), 20.2 (CH2) ppm. HRMS (ES): m/z 475.0061 (calc. 475.0061) [L]+, 5%.
3.18. Synthesis of [Cd(L4)I2]
This was prepared in a similar manner to that described for L4 and CdCl2. The compound precipitated from EtOH as a pale-yellow solid which was filtered and air-dried. Yield = 472 mg (65%). 1H (d6-dmso, 400 MHz): δH 9.17 (br, 1H), 9.02 (br, 1H), 8.00 (m, 2H), 7.54 (m, 4H), 4.65 (br, 1H), 4.07 (m, 3H), 3.62 (br, 1H), 3.16 (m, 1H), 3.02 (d, J 9.5 Hz, 1H), 2.59 (br, 1H), 2.35 (m br, 2H), 1.48 (br, 2H), 1.19 (br, 2H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 156.4 (C), 154.6 (C), 149.6 (CH), 147.6 (CH), 139.9 (CH), 139.6 (CH), 125.2 (CH), 124.0 (CH), 124.0 (CH), 124.0 (CH), 59.0 (CH2), 55.8 (CH2), 53.6 (CH2), 52.0 (CH), 47.5 (CH2), 23.5 (CH2), 20.7 (CH2) ppm. HRMS (ES): m/z 522.9928 (calc. 522.9923) [L]+, 3%.
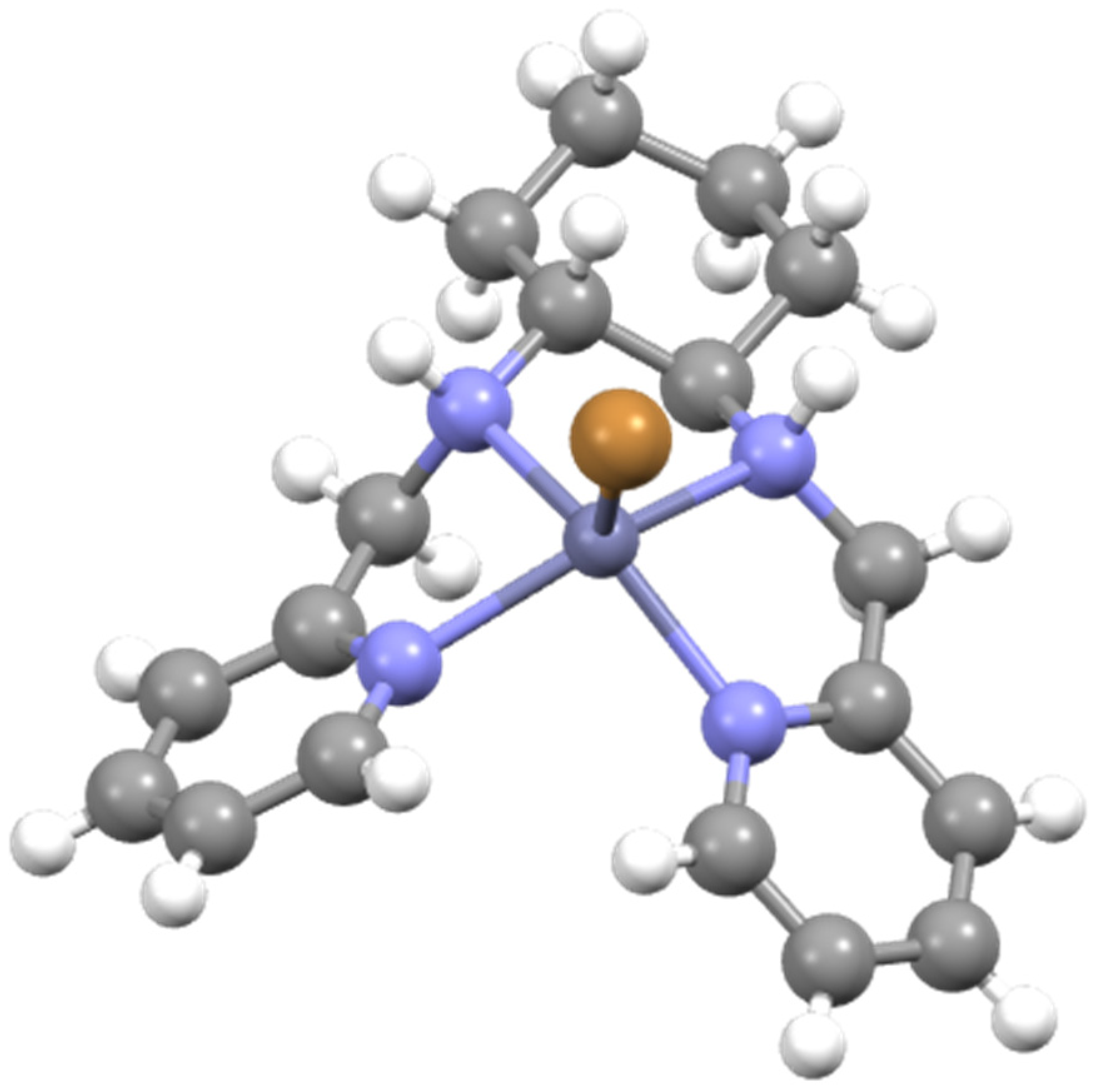
3.19. Synthesis of [Zn(L5)Cl2]
This was prepared in a similar manner to that described for L3 and ZnCl2 using L5 (0.30 g, 1.01 mmol). The compound did not precipitate from EtOH, so all volatiles were removed in vacuo, the residue was dissolved in CH2Cl2 (10 mL), filtered, and the solvent was removed under dynamic vacuum to give a hygroscopic cream solid. Yield = 411 mg (94%). 1H (CDCl3, 400 MHz): δH 8.91 (br, 2H), 7.85 (td, J 7.7, 1.6 Hz, 2H), 7.41 (t, J 6.6 Hz, 2H), 7.36 (d, J 7.8 Hz, 2H), 4.66 (d, J 12.6 Hz, 2H),4.05 (dd, J 13.3, 2.5 Hz, 2H), 3.29 (br, 2H), 2.31 (m, 2H), 1.79 (m, 2H), 1.25 (m, 4H) ppm. 13C{1H} (CDCl3, 100 MHz): δC 155.5 (C), 148.5 (CH), 139.4 (CH), 124.1 (CH), 123.8 (CH), 60.3 (CH), 48.6 (CH2), 31.0 (CH2) 24.7 (CH2) ppm. HRMS (ES): m/z 395.0981 (calc. 395.0983) [L]+, 90%.
3.20. Synthesis of [Zn(L5)Br]Br
This was prepared in a similar manner to that described for L3 and ZnCl2. The compound did not precipitate from EtOH immediately but formed lovely colourless crystals on standing. Filtered and air-dried. Yield = 417 mg (79%). 1H (d6-dmso, 400 MHz, major isomer): δH 8.90 (d, J 4.8 Hz, 2H), 8.05 (td, J 7.7, 1.6 Hz, 2H), 7.58 (m, 4H), 4.30 (dd, J 15.9, 4.3 Hz, 2H), 4.06 (d, J 16.6 Hz, 2H), 3.98 (m, 2H), 2.16 (d, J 9.6 Hz, 2H), 1.66 (m, 4H), 1.02 (m, 4H) ppm. HRMS (ES): m/z 441.0453 (calc. 441.0455) [L]+, 10%.
3.21. Synthesis of [Zn(L5)I]I
This was prepared in a similar manner to that described for L3 and ZnCl2. The compound did not precipitate from EtOH immediately but formed lovely pale-yellow crystals on standing. Filtered and air-dried. Yield = 361 mg (58%). 1H (d6-dmso, 400 MHz): δH 8.72 (dd, J 5.4, 1.2 Hz, 2H), 8.13 (td, J 7.7, 1.6 Hz, 2H), 7.65 (m, 4H), 4.19 (m, 4H), 2.21 (br, 2H), 1.75 (br, 2H), 1.65 (br, 2H), 1.06 (m, 4H) ppm. 13C{1H} (d6-dmso, 100 MHz): δC 156.1 (C), 148.5 (CH), 140.5 (CH), 125.1 (CH), 124.6 (CH), 59.6 (CH), 47.7 (CH2), 29.6 (CH2) 24.8 (CH2) ppm. HRMS (ES): m/z 487.0350 (calc. 487.0337) [L]+, 50%.