The Effect of the Glycine-to-Oxidant Ratio on the Characteristics and Catalytic Performance of VOx/MgO Catalysts for ODH of n-Octane
Abstract
1. Introduction
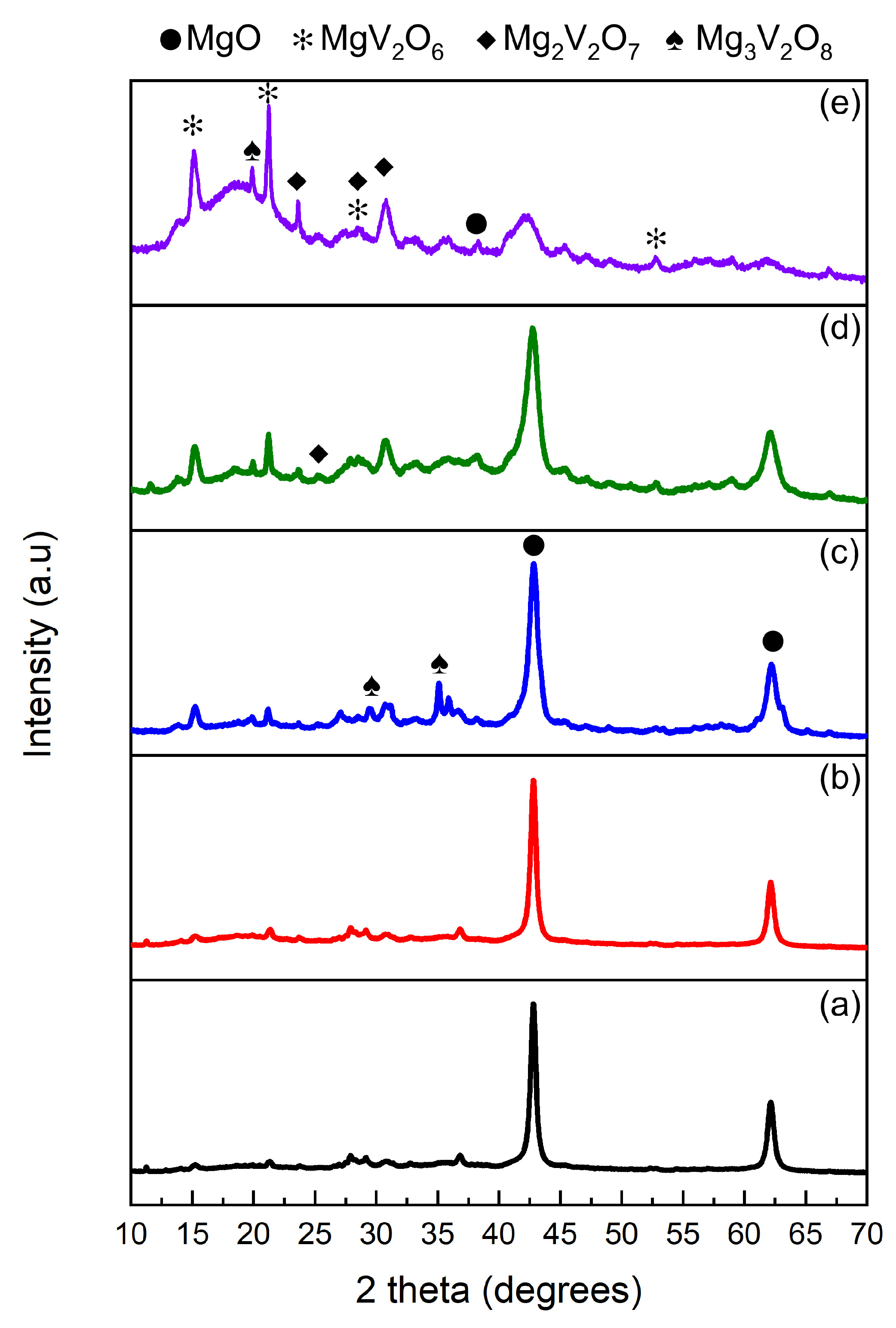
2. Results and Discussion
2.1. Thermodynamic Parameters
2.2. Elemental Composition
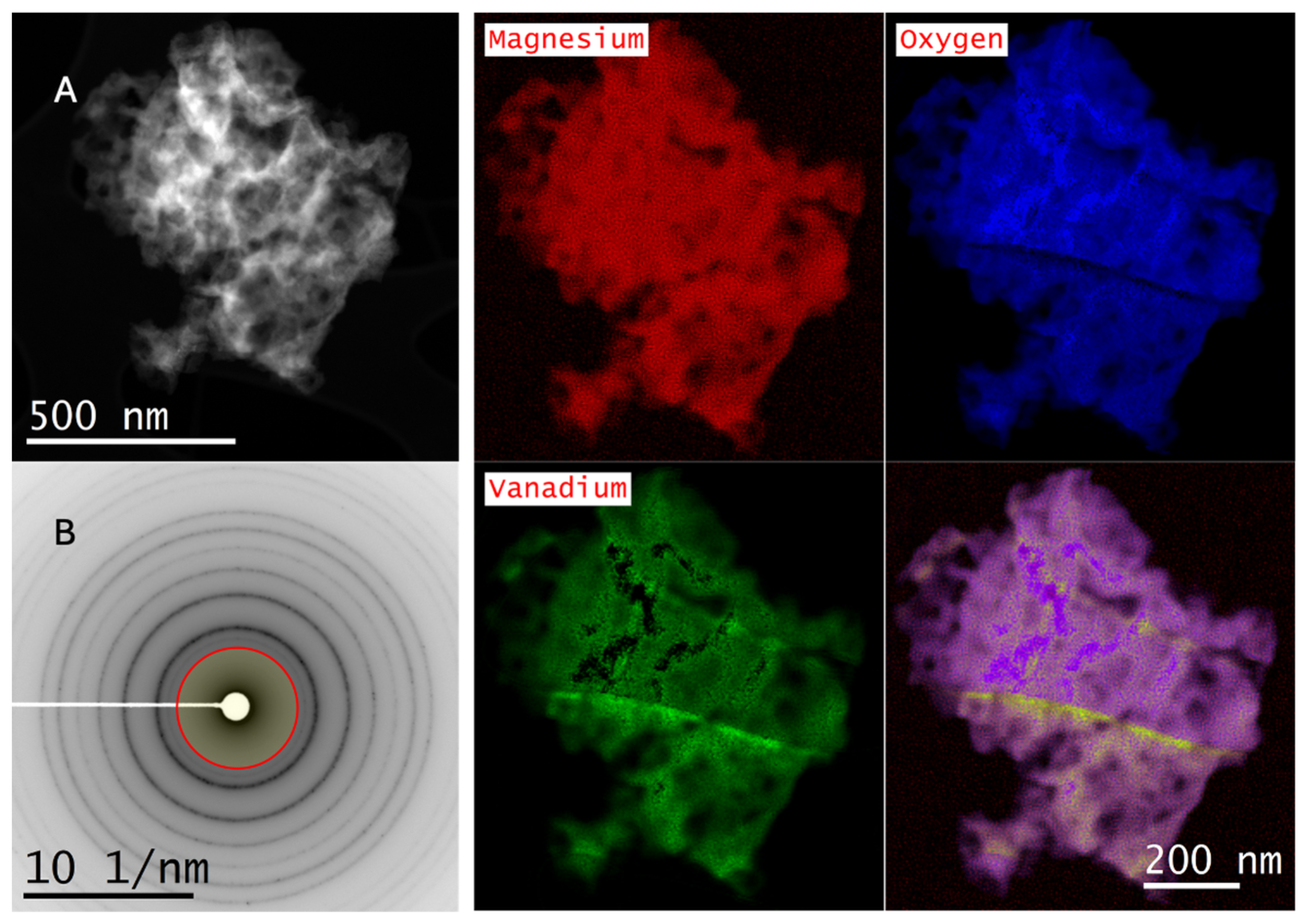
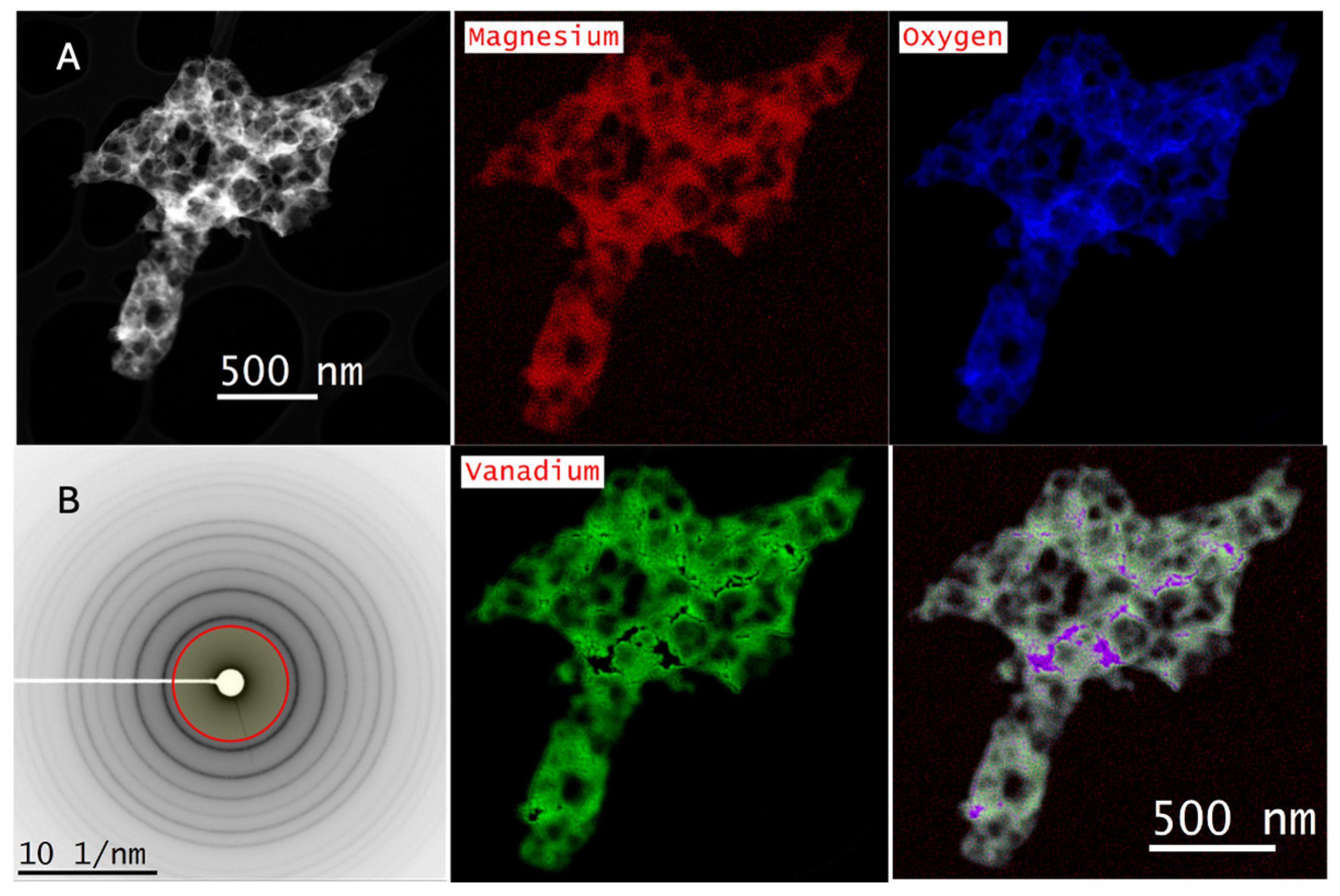
2.3. Scanning Transmission Electron Microscopy–Electron Energy Loss Spectroscopy
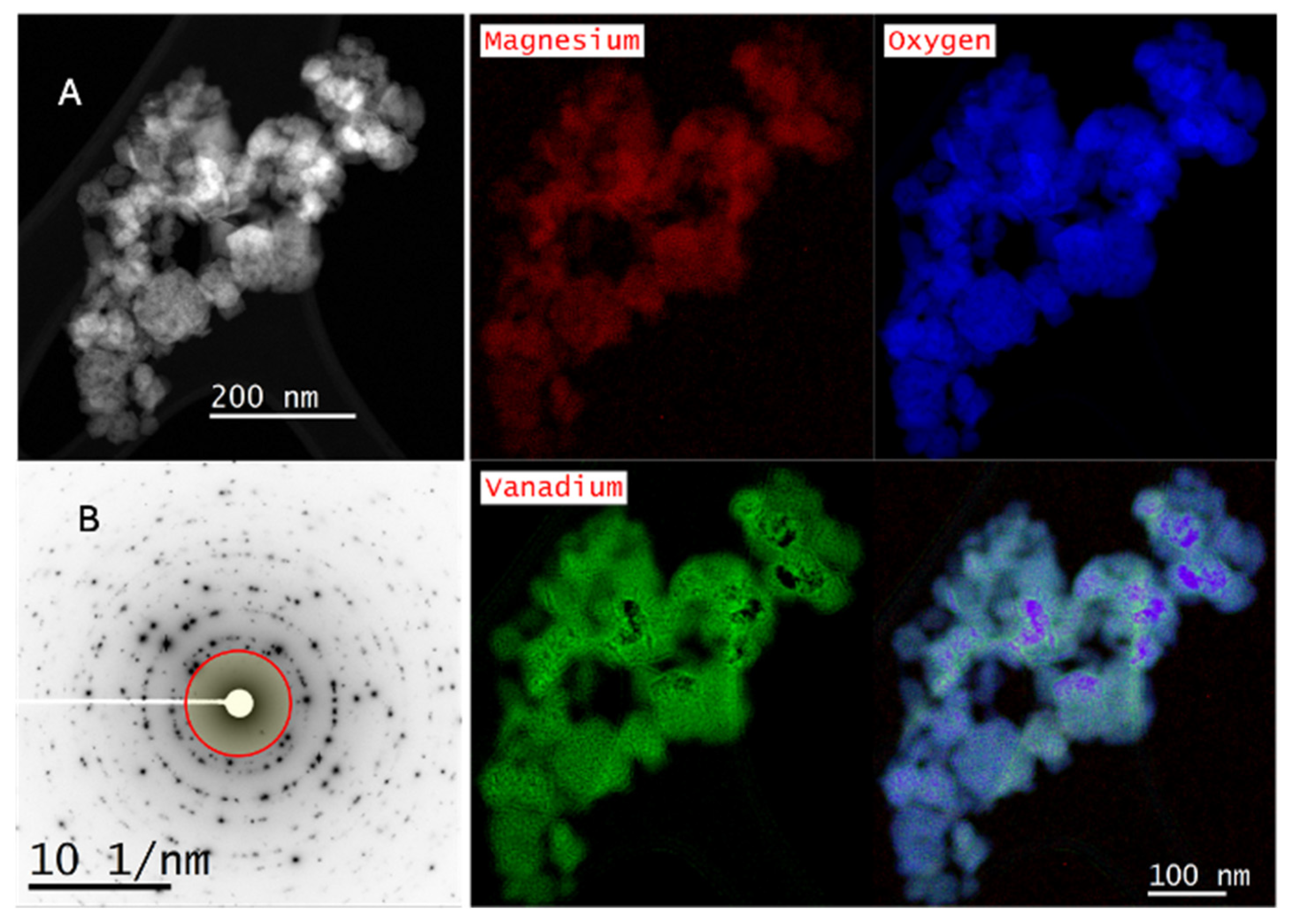
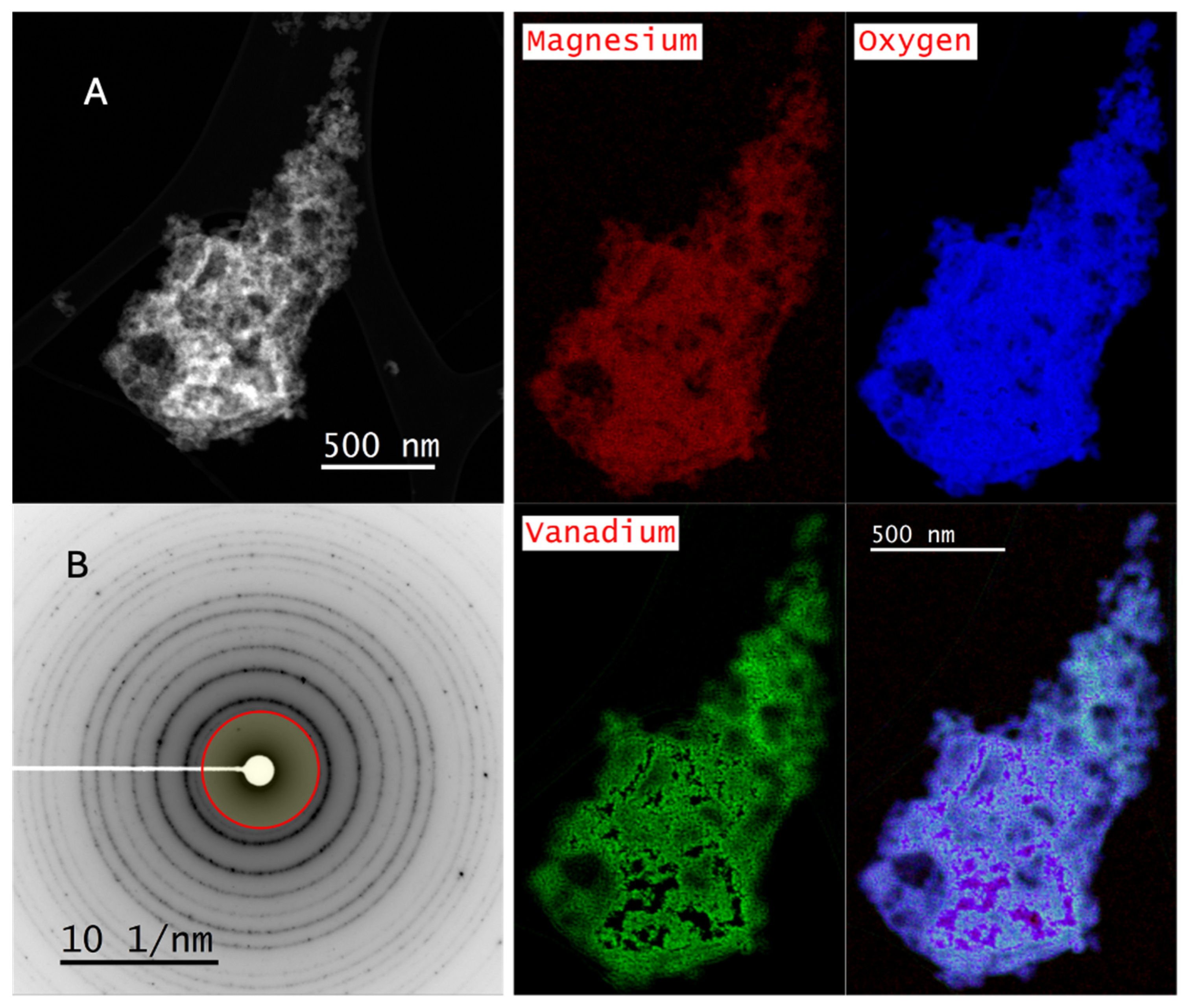
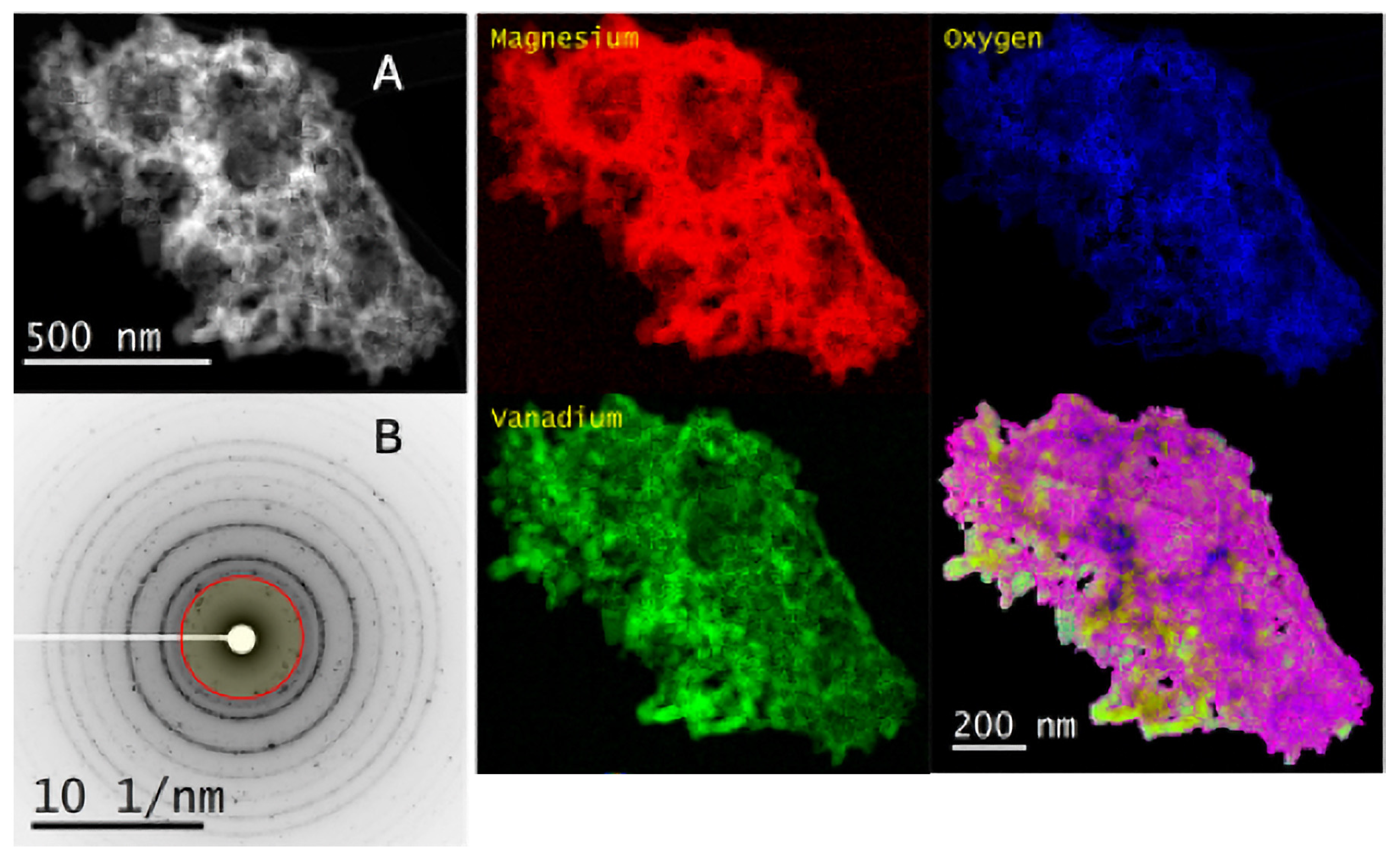
2.4. X-Ray Absorption Near-Edge Structure (XANES)
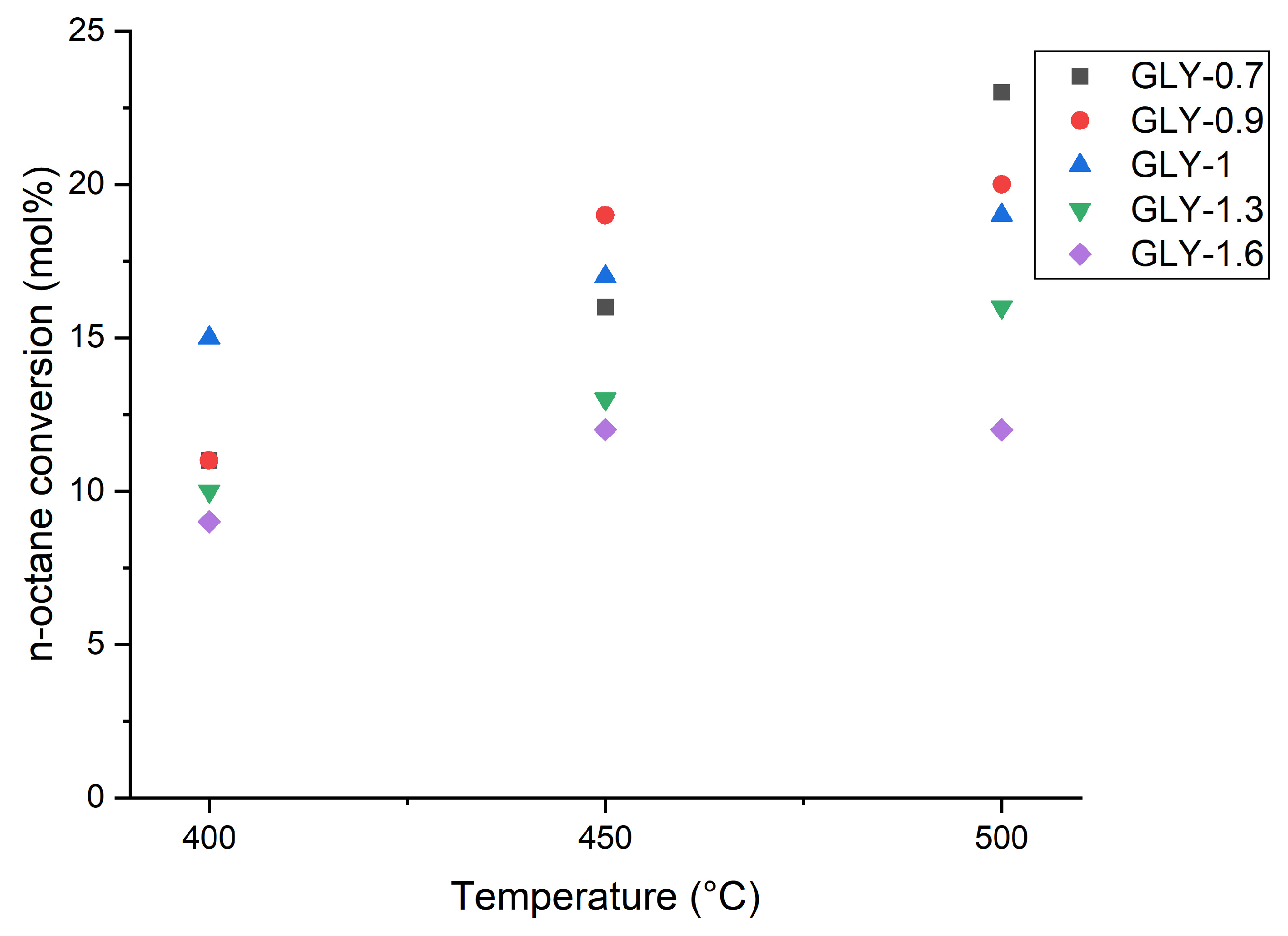
2.5. Catalytic Testing
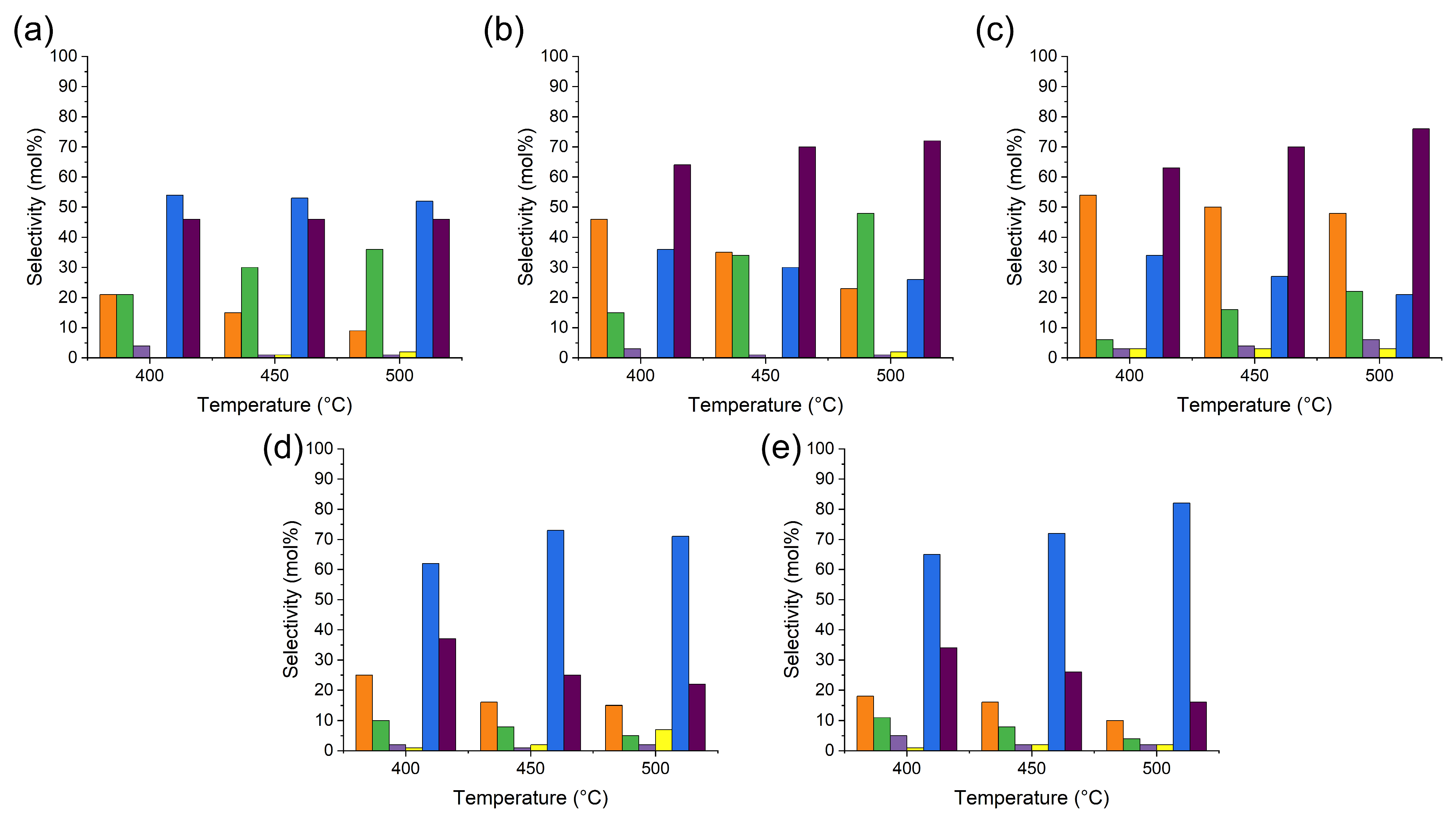
2.5.1. Product Selectivity at Iso-Conversion
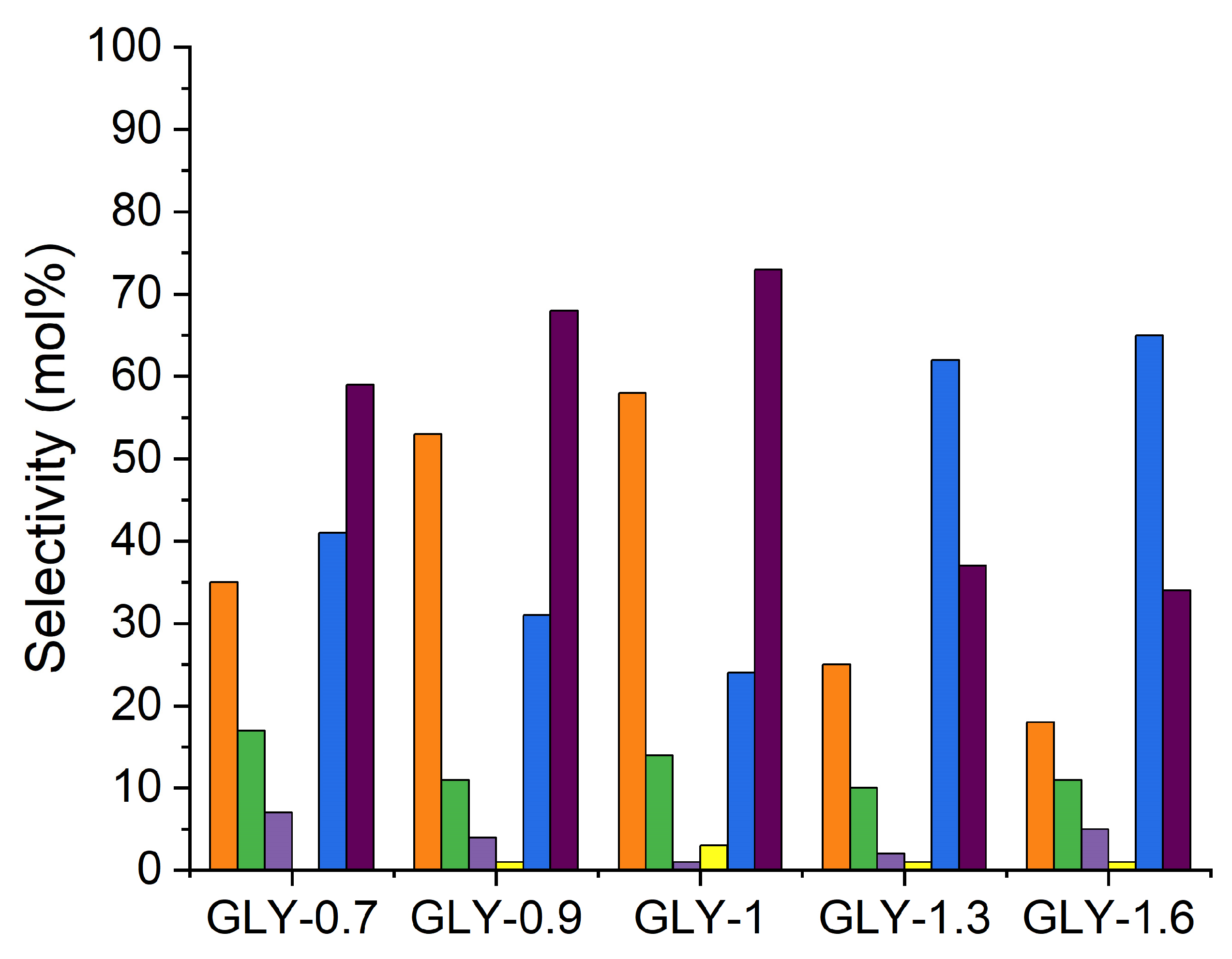
2.5.2. Effect of Temperature
2.6. Mechanistic Insights
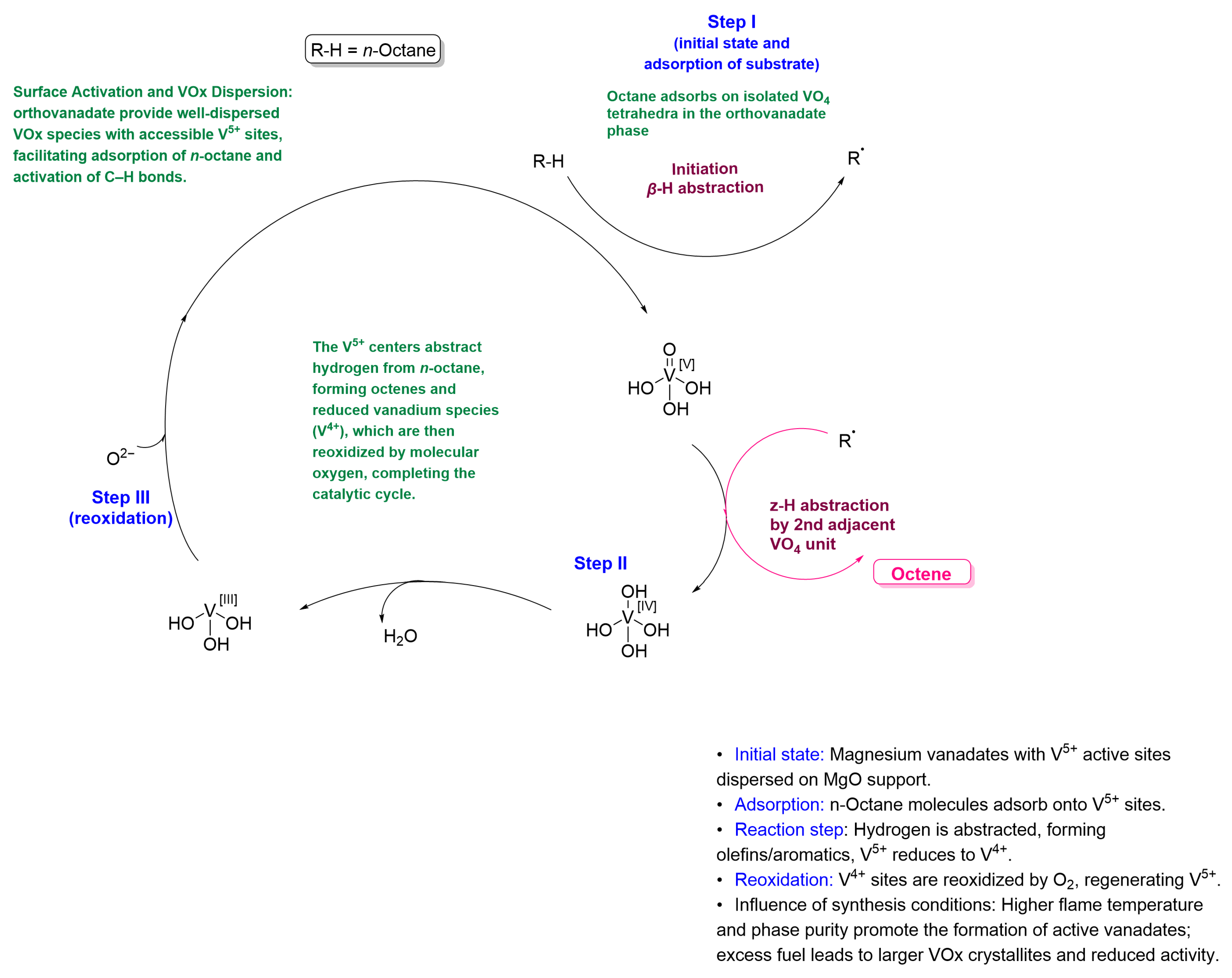
3. Experimental Section
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.2.1. X-Ray Studies
3.2.2. BET Surface Area Analysis
3.2.3. Scanning Electron Microscopy
3.2.4. Aberration-Corrected Transmission Electron Microscopy
3.3. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padayatchee, S.; Ibrahim, H.; Friedrich, H.B.; Olivier, E.J.; Ntola, P. Solution Combustion Synthesis for Various Applications: A Review of the Mixed-Fuel Approach. Fluids 2025, 10, 82. [Google Scholar] [CrossRef]
- Jadhav, L.D.; Patil, S.P.; Jamale, A.P.; Chavan, A.U. Solution Combustion Synthesis: Role of Oxidant to Fuel Ratio on Powder Properties. Mater. Sci. Forum 2013, 757, 85–98. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Costello, C.; Sherlock, K.P.; Lafarga, D.; Varma, A. Perovskite membranes by aqueous combustion synthesis: Synthesis and properties. Sep. Purif. Technol. 2001, 25, 117–126. [Google Scholar] [CrossRef]
- Bulánek, R.; Kalužová, A.; Setnička, M.; Zukal, A.; Čičmanec, P.; Mayerová, J. Study of vanadium based mesoporous silicas for oxidative dehydrogenation of propane and n-butane. Catal. Today 2012, 179, 149–158. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, X.; Cai, R.; Ran, R.; Wang, H.; Shao, Z. Solution combustion synthesis of high-rate performance carbon-coated lithium iron phosphate from inexpensive iron (iii) raw material. J. Mater. Chem. 2012, 22, 2900–2907. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Imbert, F.E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl. Catal. A Gen. 2013, 452, 117–131. [Google Scholar] [CrossRef]
- Striker, T.; Ruud, J.A. Effect of Fuel Choice on the Aqueous Combustion Synthesis of Lanthanum Ferrite and Lanthanum Manganite. J. Am. Ceram. Soc. 2010, 93, 2622–2629. [Google Scholar] [CrossRef]
- Li, F.-t.; Ran, J.; Jaroniec, M.; Qiao, S.Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 2015, 7, 17590–17610. [Google Scholar] [CrossRef]
- Biamino, S.; Badini, C. Combustion synthesis of lanthanum chromite starting from water solutions: Investigation of process mechanism by DTA–TGA–MS. J. Eur. Ceram. Soc. 2004, 24, 3021–3034. [Google Scholar] [CrossRef]
- Toniolo, J.C.; Takimi, A.S.; Bergmann, C.P. Nanostructured cobalt oxides (Co3O4 and CoO) and metallic Co powders synthesized by the solution combustion method. Mater. Res. Bull. 2010, 45, 672–676. [Google Scholar] [CrossRef]
- Aali, H.; Azizi, N.; Baygi, N.J.; Kermani, F.; Mashreghi, M.; Youssefi, A.; Mollazadeh, S.; Khaki, J.V.; Nasiri, H. High antibacterial and photocatalytic activity of solution combustion synthesized Ni0.5Zn0.5Fe2O4 nanoparticles: Effect of fuel to oxidizer ratio and complex fuels. Ceram. Int. 2019, 45, 19127–19140. [Google Scholar] [CrossRef]
- Pederson, L.R.; Maupin, G.D.; Weber, W.J.; McReady, D.J.; Stephens, R.W. Combustion synthesis of YBa2Cu3O7−x: Glycine/metal nitrate method. Mater. Lett. 1991, 10, 437–443. [Google Scholar] [CrossRef]
- Elkhalifa, E.A.; Friedrich, H.B. Oxidative dehydrogenation and aromatization of n-octane over VMgO catalysts obtained by using different MgO precursors and different precursor treatments. J. Mol. Catal. A Chem. 2014, 392, 22–30. [Google Scholar] [CrossRef]
- Ntola, P.; Friedrich, H.B.; Singh, S.; Olivier, E.J.; Farahani, M.; Mahomed, A.S. Effect of the fuel on the surface VOx concentration, speciation and physico-chemical characteristics of solution combustion synthesised VOx/MgO catalysts for n-octane activation. Catal. Commun. 2023, 174, 106571. [Google Scholar] [CrossRef]
- Ntola, P.; Friedrich, H.B.; Mahomed, A.S.; Olivier, E.J.; Govender, A.; Singh, S. Exploring the role of fuel on the microstructure of VOx/MgO powders prepared using solution combustion synthesis. Mater. Chem. Phys. 2022, 278, 125602. [Google Scholar] [CrossRef]
- Lima, M.D.; Bonadimann, R.; de Andrade, M.J.; Toniolo, J.C.; Bergmann, C.P. Nanocrystalline Cr2O3 and amorphous CrO3 produced by solution combustion synthesis. J. Eur. Ceram. Soc. 2006, 26, 1213–1220. [Google Scholar] [CrossRef]
- Chick, L.A.; Pederson, L.R.; Maupin, G.D.; Bates, J.L.; Thomas, L.E.; Exarhos, G.J. Glycine-nitrate combustion synthesis of oxide ceramic powders. Mater. Lett. 1990, 10, 6–12. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Zhou, Q.; Mou, Y.; Ma, X.; Xue, L.; Yan, Y. Effect of fuel-to-oxidizer ratios on combustion mode and microstructure of Li2TiO3 nanoscale powders. J. Eur. Ceram. Soc. 2014, 34, 801–807. [Google Scholar] [CrossRef]
- Murugan, B.; Ramaswamy, A.V.; Srinivas, D.; Gopinath, C.S.; Ramaswamy, V. Effect of fuel and its concentration on the nature of Mn in Mn/CeO2 solid solutions prepared by solution combustion synthesis. Acta Mater. 2008, 56, 1461–1472. [Google Scholar] [CrossRef]
- Salunkhe, A.; Khot, V.; Phadatare, M.R.; Pawar, S. Combustion synthesis of cobalt ferrite nanoparticles—Influence of fuel to oxidizer ratio. J. Alloys Compd. 2012, 514, 91–96. [Google Scholar] [CrossRef]
- Poth, J.; Haberkorn, R.; Beck, H.P. Combustion-synthesis of SrTiO3 Part I. synthesis and properties of the ignition products. J. Eur. Ceram. Soc. 2000, 20, 707–713. [Google Scholar] [CrossRef]
- Masokano, D.S.; Ntola, P.; Mahomed, A.S.; Bala, M.D.; Friedrich, H.B. Influence of support properties on the activity of 2Cr-Fe/MgO-MO2 catalysts (M= Ce, Zr, CeZr and Si) for the dehydrogenation of n-octane with CO2. J. CO2 Util. 2024, 86, 102909. [Google Scholar] [CrossRef]
- Pradhan, S.; Bartley, J.K.; Bethell, D.; Carley, A.F.; Conte, M.; Golunski, S.; House, M.P.; Jenkins, R.L.; Lloyd, R.; Hutchings, G.J. Non-lattice surface oxygen species implicated in the catalytic partial oxidation of decane to oxygenated aromatics. Nat. Chem. 2012, 4, 134–139. [Google Scholar] [CrossRef]
- Asinger, F. Paraffins: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ntola, P.; Shozi, M. Gas-Phase Oxidative Dehydrogenation of n-Octane over Metal Oxide Catalysts: A Review. Catalysts 2024, 14, 100. [Google Scholar] [CrossRef]
- Kharton, V.V.; Figueiredo, F.M.; Kovalevsky, A.V.; Viskup, A.P.; Naumovich, E.N.; Yaremchenko, A.A.; Bashmakov, I.A.; Marques, F.M.B. Processing, microstructure and properties of LaCoO3−δ ceramics. J. Eur. Ceram. Soc. 2001, 21, 2301–2309. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Wu, T.-Y. Combustion synthesis of nanocrystalline ZnO powders using zinc nitrate and glycine as reactants—Influence of reactant composition. J. Mater. Sci. 2004, 39, 6111–6115. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Mohanty, T.; Prakash, D.; Tyagi, A.; Sinha, P. Glycine-nitrate solution combustion synthesis of lithium zirconate: Effect of fuel-to oxidant ratio on phase, microstructure and sintering. J. Eur. Ceram. Soc. 2020, 40, 136–144. [Google Scholar] [CrossRef]
- Toniolo, J.; Lima, M.; Takimi, A.; Bergmann, C. Synthesis of alumina powders by the glycine–nitrate combustion process. Mater. Res. Bull. 2005, 40, 561–571. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: Generalized one-pot synthesis, band structures and visible-light photocatalytic properties. J. Mater. Chem. 2012, 22, 22840–22843. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Kumar, V.; Bhalerao, G.; Choudhary, R.; Phase, D. Surface and spectral studies of Eu3+ doped α-Al2O3 synthesized via solution combustion synthesis. Adv. Powder Technol. 2015, 26, 1263–1268. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Aliotta, C.; Barbucci, A.; Viviani, M.; Clematis, D.; Carpanese, M.P.; Presto, S. Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts. Catalysts 2020, 10, 1465. [Google Scholar] [CrossRef]
- Li, P.; Zhou, W.; Wang, X.; Zhang, Y.; Umezawa, N.; Abe, H.; Ye, J.; Wang, D. Effects of cation concentration on photocatalytic performance over magnesium vanadates. APL Mater. 2015, 3, 104405. [Google Scholar] [CrossRef]
- Song, J.-D.; Song, T.-Y.; Zhang, T.-T.; Wang, Y.; Luo, M.-F.; Lu, J.-Q. High performance V2O5/MgF2 catalysts for gas-phase dehydrofluorination of 1, 1, 1, 3, 3-pentafluoropropane: Support-induced evolution of new active sites. J. Catal. 2018, 364, 271–281. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, M.A.R. Synthesis, characterization and physical properties of high quality MgV2O6 crystals by solid-state reaction and ab-initio methods. J. Alloys Compd. 2019, 797, 630–639. [Google Scholar] [CrossRef]
- Brown, G.E.; Calas, G.; Waychunas, G.; Petiau, J.; Hawthorne, F. Spectroscopic Methods in Mineralogy and Geology; Mineralogical Society of America: Washington, DC, USA, 1988. [Google Scholar]
- Chaurand, P.; Rose, J.; Briois, V.; Salome, M.; Proux, O.; Nassif, V.; Olivi, L.; Susini, J.; Hazemann, J.-L.; Bottero, J.-Y. New methodological approach for the vanadium K-edge X-ray absorption near-edge structure interpretation: Application to the speciation of vanadium in oxide phases from steel slag. J. Phys. Chem. B 2007, 111, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.-F.; Zhang, Y. The effect of pore size or iron particle size on the formation of light olefins in Fischer–Tropsch synthesis. RSC Adv. 2015, 5, 29002–29007. [Google Scholar] [CrossRef]
- Concepción, P.; Nieto, J.L.; Pérez-Pariente, J. Oxidative dehydrogenation of propane on VAPO-5, V2O5/ALPO4-5 and V2O5/MgO catalysts. Nature of selective sites. J. Mol. Catal. A Chem. 1995, 97, 173–182. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Cao, Y.; Zhu, K.-K.; Yan, S.-R.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Highly efficient VO x/SBA-15 mesoporous catalysts for oxidative dehydrogenation of propane. Chem. Commun. 2002, 2832–2833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Cao, Y.; Yan, S.-R.; Dai, W.-L.; Fan, K.-N. Highly effective oxidative dehydrogenation of propane over vanadia supported on mesoporous SBA-15 silica. Catal. Lett. 2003, 88, 61–67. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Novitskaya, E.; Kelly, J.P.; Bhaduri, S.; Graeve, O.A. A review of solution combustion synthesis: An analysis of parameters controlling powder characteristics. Int. Mater. Rev. 2021, 66, 188–214. [Google Scholar] [CrossRef]
- Blackburn, D.W. Catalysis of Organic Reactions; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Slyemi, S.; Blanchard, J.; Barama, S.; Barama, A.; Messaoudi, H.; Casale, S.; Calers, C.; Ihdene, Z. Effect of the preparation method and of the vanadium content on the physicochemical and surface properties of vanadium–magnesium-based catalysts for the selective oxidation of n-butane. Comptes Rendus Chim. 2017, 20, 1062–1071. [Google Scholar] [CrossRef]
- Shimada, H.; Akazawa, T.; Ikenaga, N.-o.; Suzuki, T. Dehydrogenation of isobutane to isobutene with iron-loaded activated carbon catalyst. Appl. Catal. A Gen. 1998, 168, 243–250. [Google Scholar] [CrossRef]
- Elkhalifa, E.A.; Friedrich, H.B. Oxidative dehydrogenation of n-octane using vanadium-magnesium oxide catalysts with different vanadium loadings. Appl. Catal. A Gen. 2010, 373, 122–131. [Google Scholar] [CrossRef]
- Elkhalifa, E.A.; Friedrich, H.B. Magnesium oxide as a catalyst for the dehydrogenation of n-octane. Arab. J. Chem. 2018, 11, 1154–1159. [Google Scholar] [CrossRef]
- Damoyi, N.E.; Friedrich, H.B.; Kruger, G.H.; Willock, D. A DFT mechanistic study of the ODH of n-hexane over isolated H3VO4. Mol. Catal. 2018, 452, 83–92. [Google Scholar] [CrossRef]
- Montalvo-Castro, H.; Loaiza-Orduz, Á.; Meyer, R.J.; Plaisance, C.; Hibbitts, D. Electronic and geometric features controlling the reactivity of Mg-vanadate and V2O5 surfaces toward the initial C–H activation of C1–C3 alkanes–A DFT+ U study. J. Catal. 2025, 442, 115800. [Google Scholar]
- Damoyi, N.E.; Friedrich, H.B.; Kruger, G.H.; Willock, D.J. A DFT study of the catalytic ODH of n-hexane over a cluster model of vanadium oxide. Mol. Catal. 2023, 541, 113078. [Google Scholar] [CrossRef]
- Egerton, R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A chemical data resource on the internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]


| Catalyst Name | Adiabatic Flame Temperature | Qs | Ds | Dsmod | Time Factor |
|---|---|---|---|---|---|
| GLY-1.6 | 2246 | 472 | 4.5 | 2.2 | 3.6 |
| GLY-1.3 | 2065 | 492 | 4.6 | 1.9 | 4.0 |
| GLY-1 | 1962 | 503 | 4.7 | 1.7 | 4.4 |
| GLY-0.9 | 1670 | 533 | 4.8 | 1.3 | 5.5 |
| GLY-0.7 | 1476 | 556 | 4.8 | 1.1 | 6.7 |
| Catalyst Name | Fuel-to-Oxidant Molar Ratio | V2O5 wt% (XRF) |
|---|---|---|
| GLY-1.6 | 1.6:1 | 13 |
| GLY-1.3 | 1.3:1 | 16 |
| GLY-1 | 1:1 | 14 |
| GLY-0.9 | 0.9:1 | 15.3 |
| GLY-0.7 | 0.7:1 | 14.1 |
| Sample | Amount of Gases Formed (mol) a | Crystallite Size (nm) b | Surface Area (m2/g) |
|---|---|---|---|
| GLY-0.7 | 2.68 | 21(40) | 15 |
| GLY-0.9 | 2.83 | 30 (32) | 19 |
| GLY-1 | 3.61 | 23 (18) | 20 |
| GLY-1.3 | 3.4 | 29 (25) | 34 |
| GLY-1.6 | 3.75 | 34 (29) | 26 |
| Catalyst Name | Fuel-to-Oxidant Molar Ratio | Description |
|---|---|---|
| GLY-1.6 | 1.6:1 | glycine richest |
| GLY-1.3 | 1.3:1 | glycine rich |
| GLY-1 | 1:1 | stoichiometric |
| GLY-0.9 | 0.9:1 | glycine lean |
| GLY-0.7 | 0.7:1 | glycine leanest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntola, P.; Singh, S.; Mahomed, A.S.; Olivier, E.J.; Shozi, M.; Russell, A.; Celorrio, V.; Friedrich, H.B. The Effect of the Glycine-to-Oxidant Ratio on the Characteristics and Catalytic Performance of VOx/MgO Catalysts for ODH of n-Octane. Inorganics 2025, 13, 389. https://doi.org/10.3390/inorganics13120389
Ntola P, Singh S, Mahomed AS, Olivier EJ, Shozi M, Russell A, Celorrio V, Friedrich HB. The Effect of the Glycine-to-Oxidant Ratio on the Characteristics and Catalytic Performance of VOx/MgO Catalysts for ODH of n-Octane. Inorganics. 2025; 13(12):389. https://doi.org/10.3390/inorganics13120389
Chicago/Turabian StyleNtola, Pinkie, Sooboo Singh, Abdul S. Mahomed, Ezra J. Olivier, Mzamo Shozi, Andrea Russell, Veronica Celorrio, and Holger B. Friedrich. 2025. "The Effect of the Glycine-to-Oxidant Ratio on the Characteristics and Catalytic Performance of VOx/MgO Catalysts for ODH of n-Octane" Inorganics 13, no. 12: 389. https://doi.org/10.3390/inorganics13120389
APA StyleNtola, P., Singh, S., Mahomed, A. S., Olivier, E. J., Shozi, M., Russell, A., Celorrio, V., & Friedrich, H. B. (2025). The Effect of the Glycine-to-Oxidant Ratio on the Characteristics and Catalytic Performance of VOx/MgO Catalysts for ODH of n-Octane. Inorganics, 13(12), 389. https://doi.org/10.3390/inorganics13120389









