Green Manufacturing of Rutile (TiO2) Welding Electrodes with Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials Used in the Rutile-Type Coating
- Slag formers: Feldspar (KAlSi3O2) or quartz (SiO2), rutile (TiO2) or ilmenite (FeTiO3), enhancing coating flux properties.
- Gas formers: Calcite (CaCO3) or dolomite (CaMg(CO3)2), and cellulose (C6H10O5), generating a protective gaseous atmosphere during welding.
- Deoxidizer and alloying agent: Low-carbon ferromanganese (FeMn, 75% Mn, 0.1–0.5% C), refining and improving weld metal properties.
- Binders: Sodium silicate (Na2(SiO3)nO) or potassium silicate (K2SiO3), ensuring robust adhesion of the coating to the core wire.
- Iron powder: 99% pure iron powder, enhancing coating efficiency and weld deposition.
2.2. Preparation of Blast Furnace Slags for Coating Integration
2.3. Production of Rutile-Type Coated Electrodes
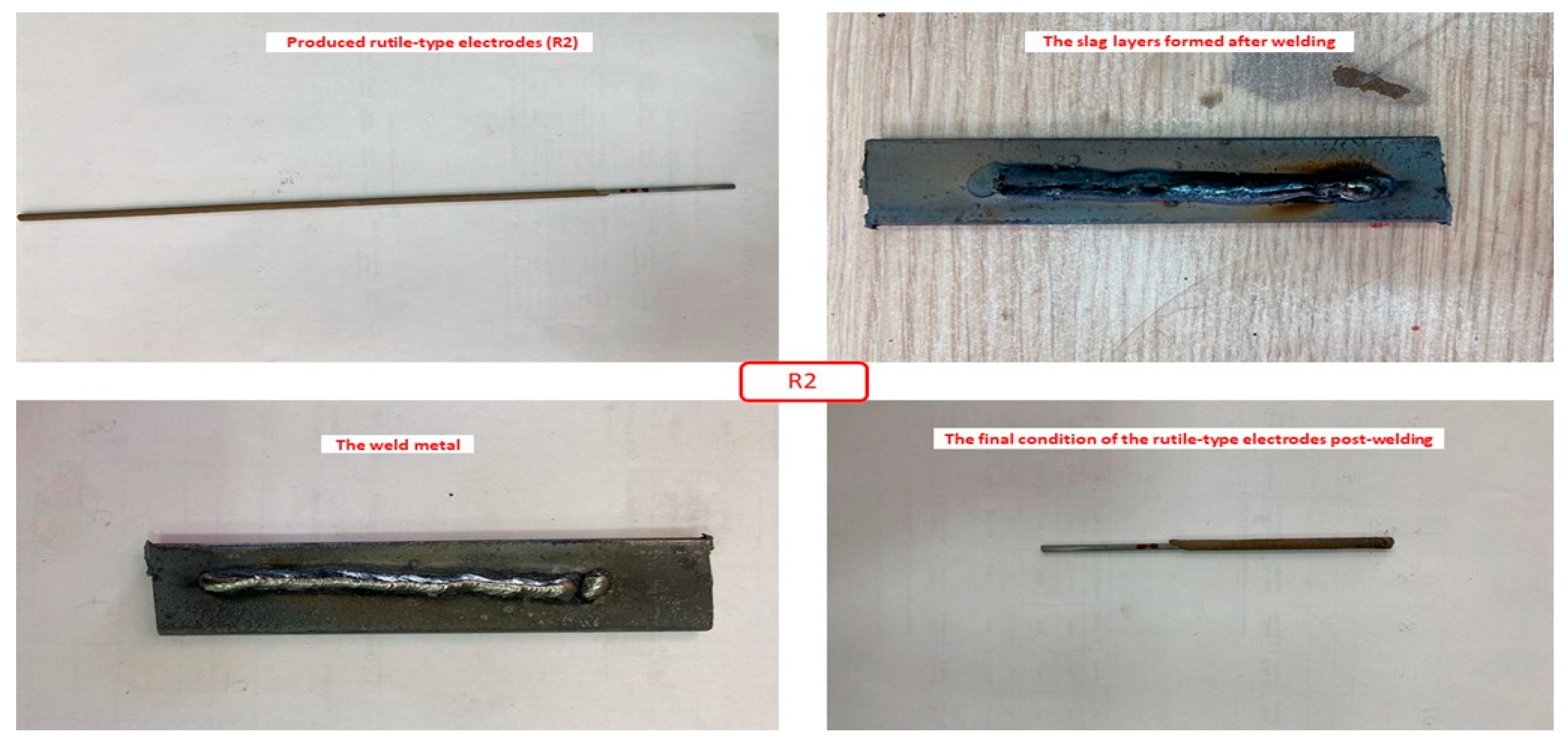
2.4. Welding Tests of Rutile-Type Coated Electrodes Containing BFS
2.5. Microstructure, XRD, and Chemical Composition Analyses
2.6. Hardness Measurements
2.7. Tensile and Impact Tests
2.8. Environmental and Economic Impact Assessment
3. Results and Discussion
3.1. Visual Inspection Results of the Produced Rutile-Type Coated Electrodes
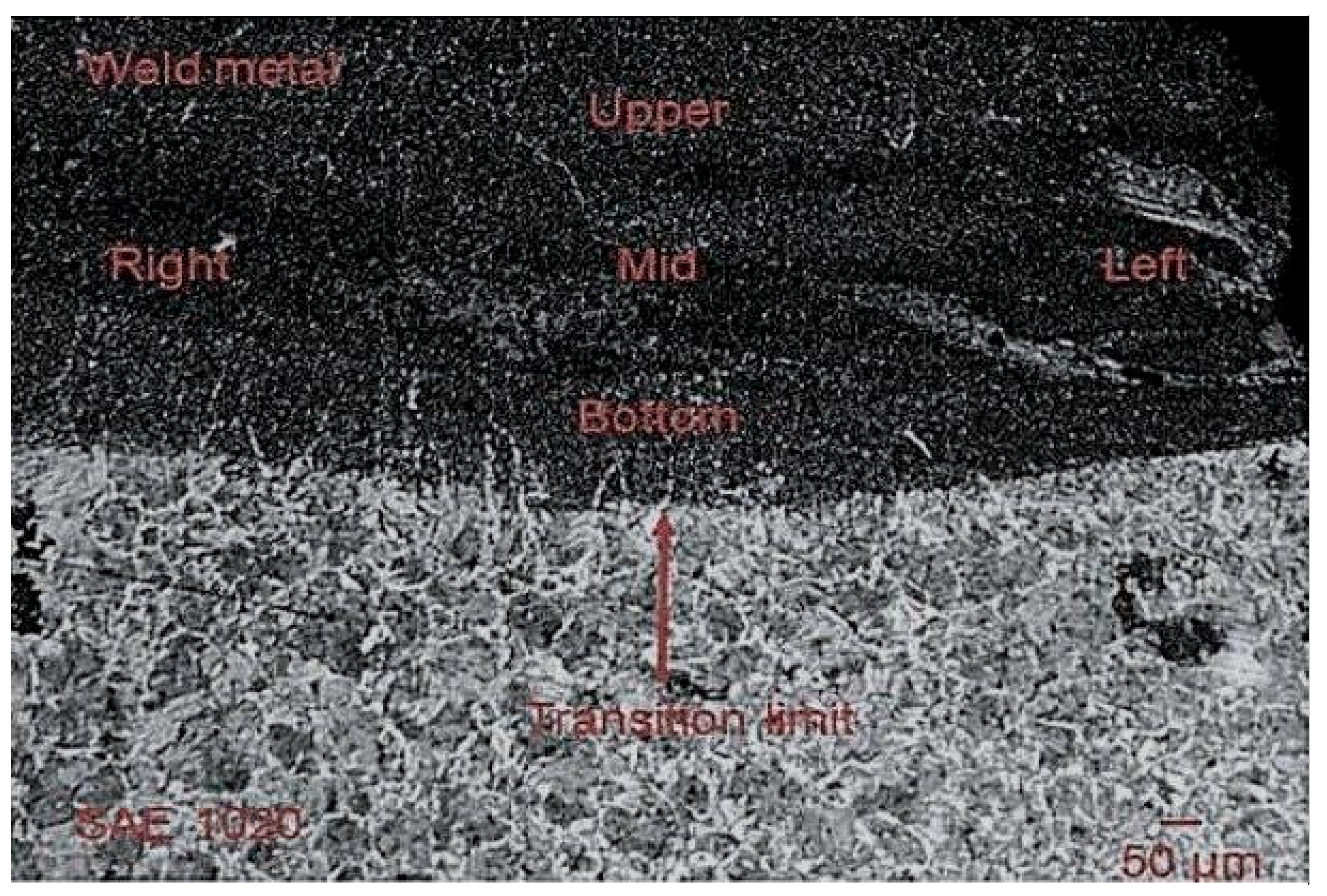
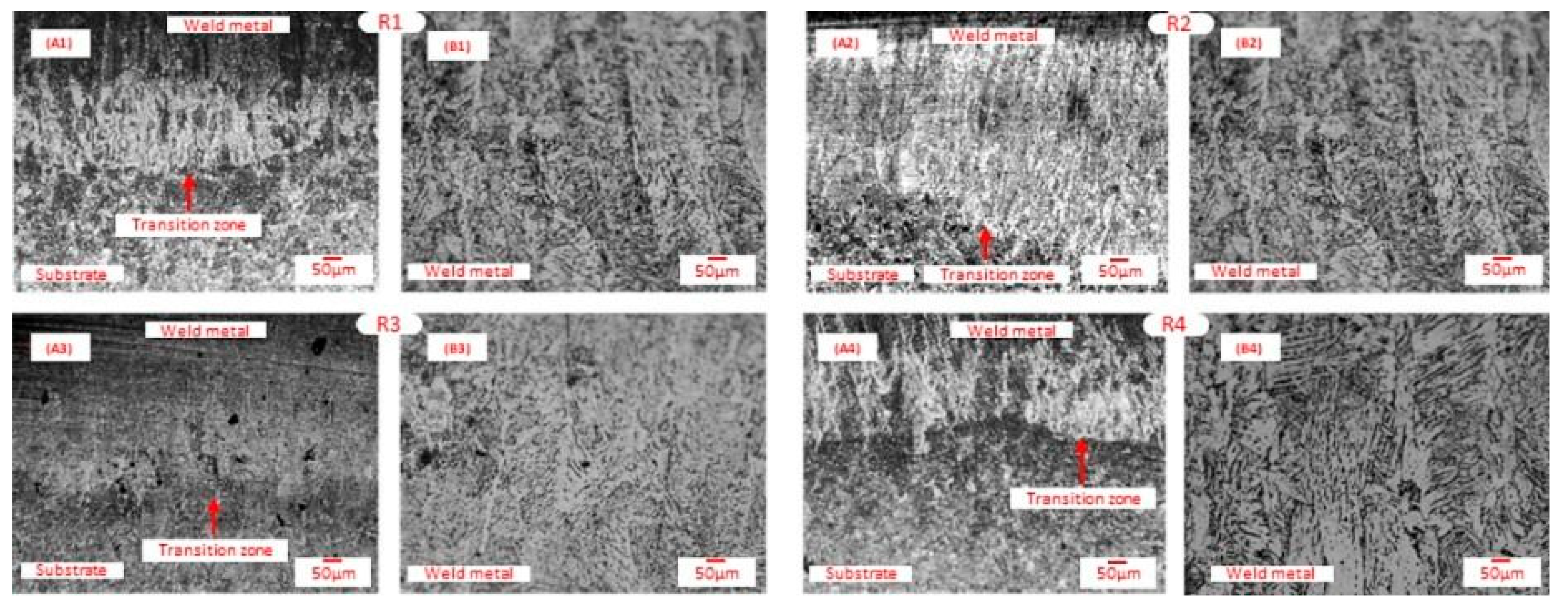
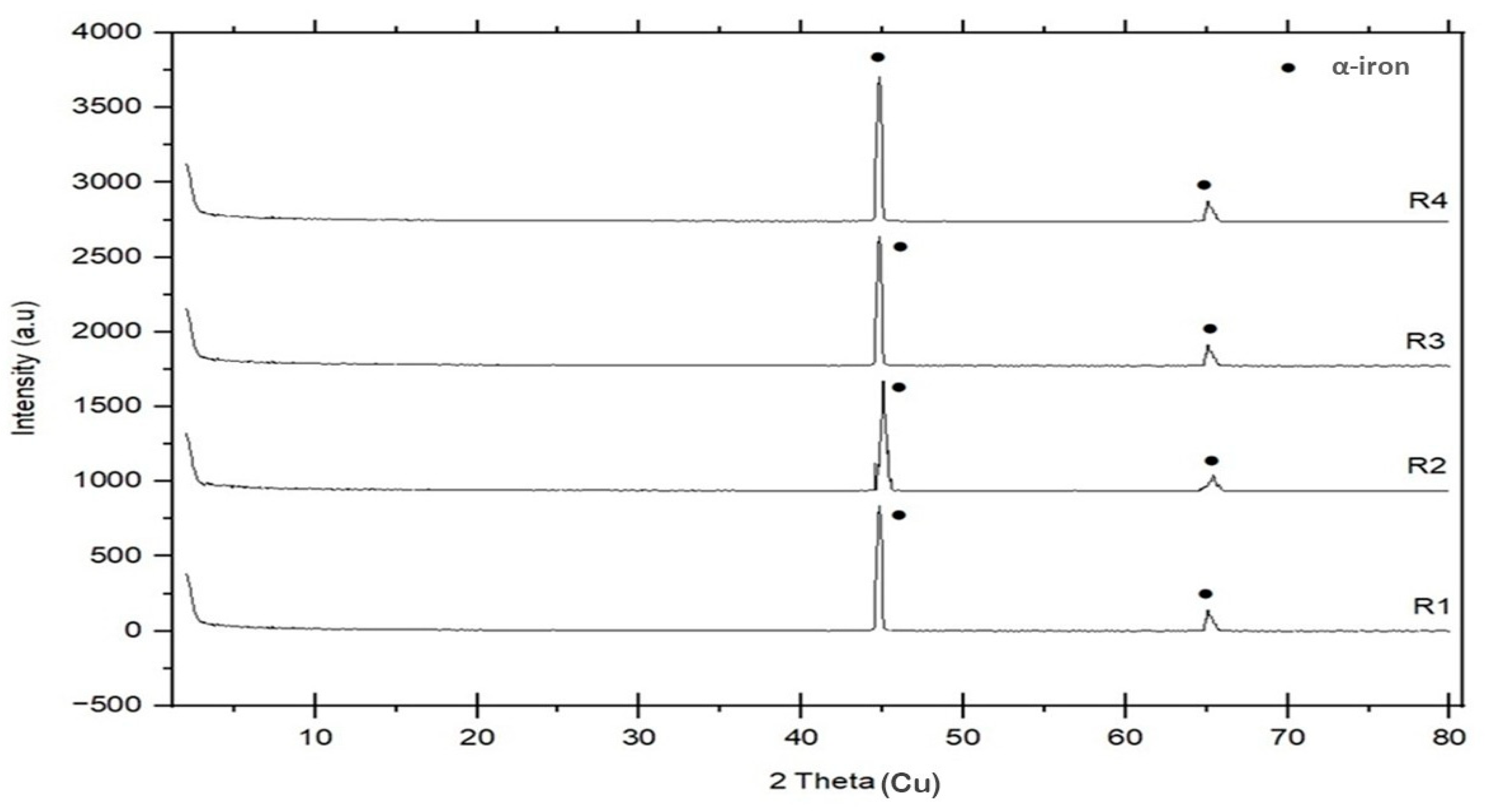
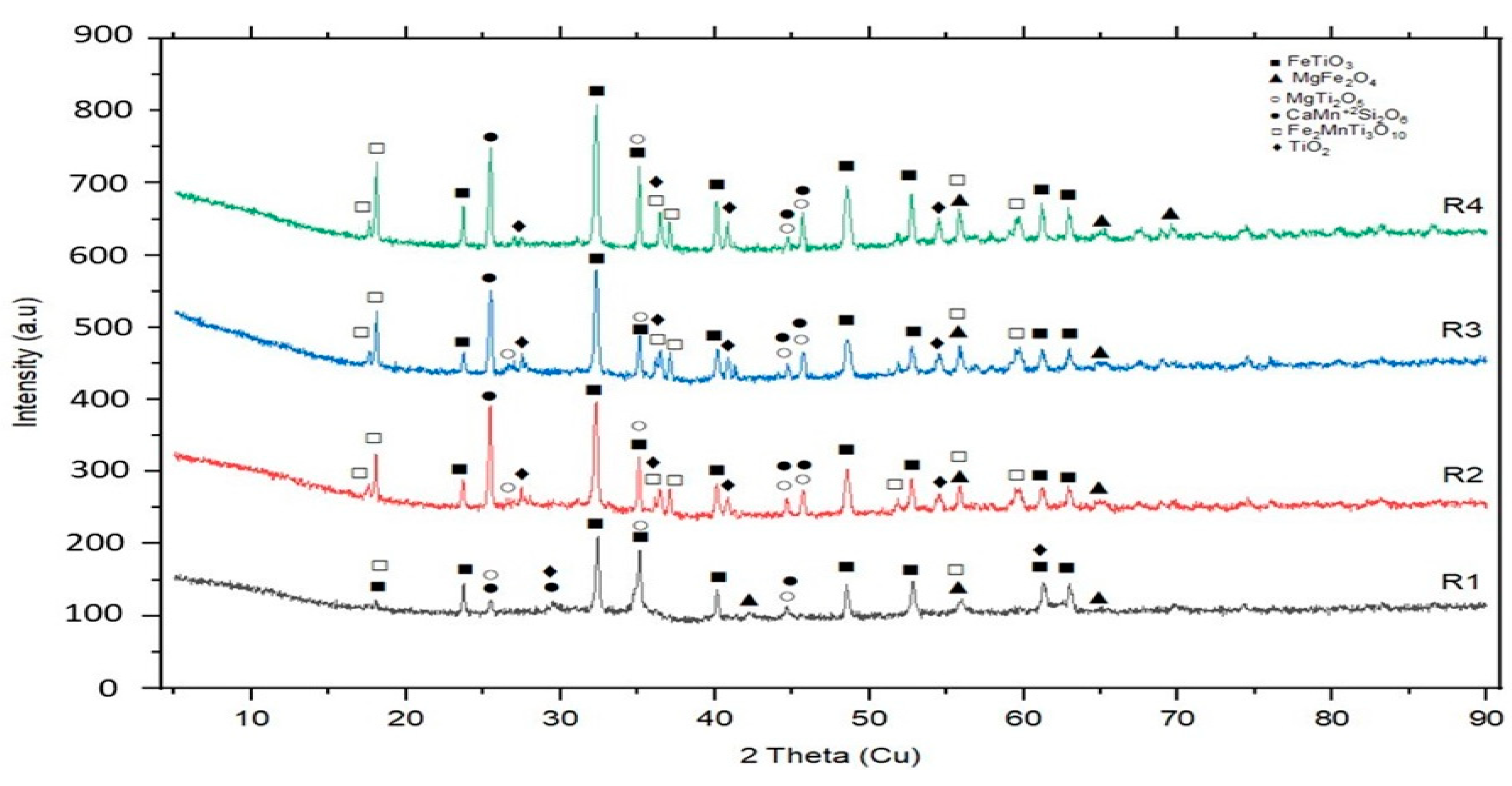
3.2. Microstructure, XRD, and Chemical Composition Analysis Results
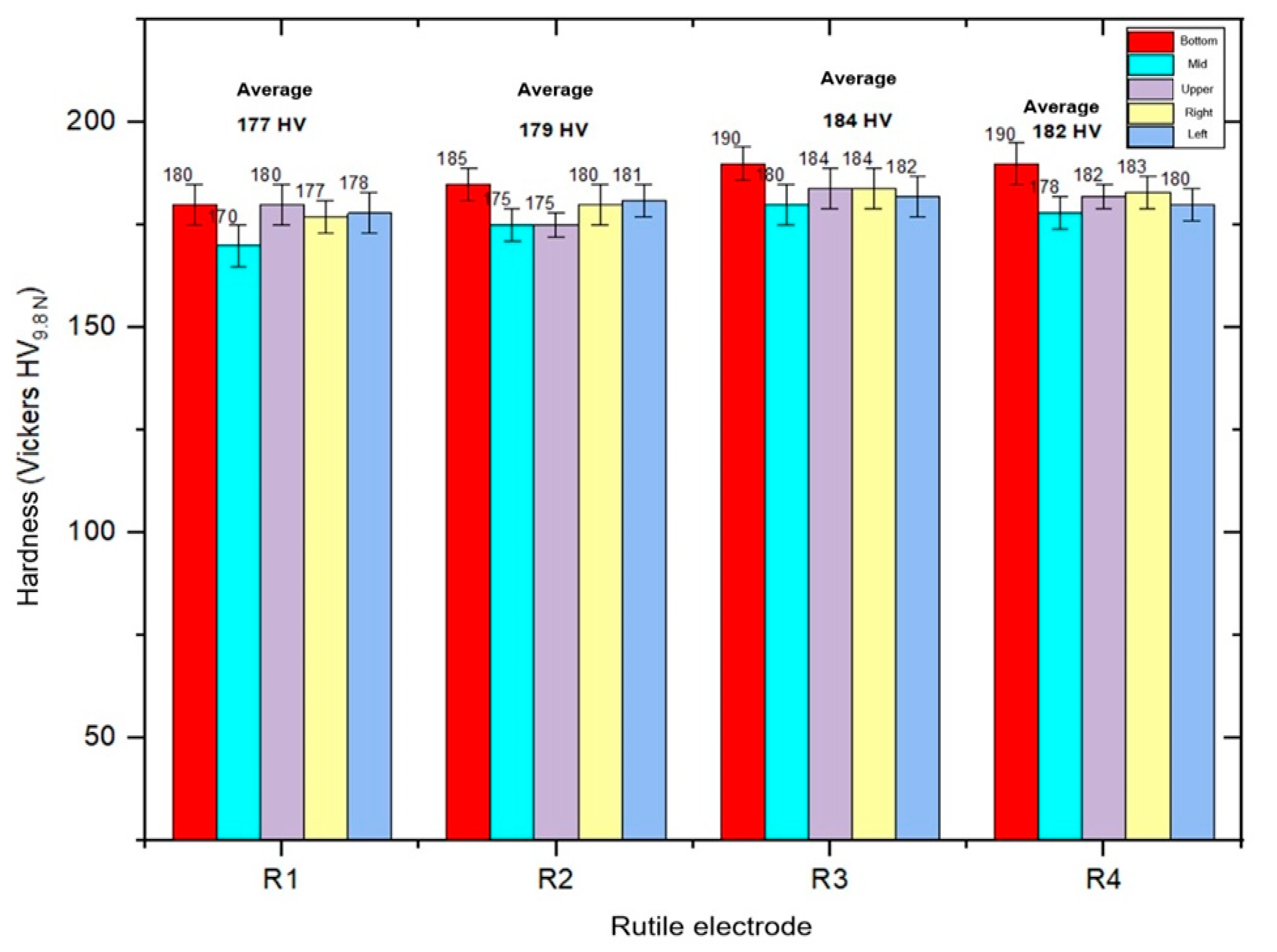
3.3. Hardness Measurement Results
3.4. Tensile and Impact Test Results
3.5. Environmental and Economic Impact Results
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey. Iron and steel slag. Mineral Commodity Summaries. 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-iron-steel-slag.pdf (accessed on 6 October 2025).
- Reuter, M.A.; Xiao, Y.; Boin, U. Recycling and environmental issues of metallurgical slags and salt fluxes. In Proceedings of the VII International Conference on Molten Slags, Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; pp. 349–356. [Google Scholar]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Oge, M.; Ozkan, D.; Celik, M.D.; Sabankaya, G.; Gok, M.S. An overview of utilization of blast furnace and steelmaking slag in various applications. Mater. Today Proc. 2019, 11, 516–525. [Google Scholar] [CrossRef]
- Buddhdev, B.G.; Timani, K.L. Critical review for utilization of blast furnace slag in geotechnical applications. In Problematic Soils and Geoenvironmental Concerns; Gali, M.L., Rao, P.R., Eds.; Springer: Singapore, 2021; pp. 103–114. [Google Scholar] [CrossRef]
- Zakira, U.; Zheng, K.; Xie, N.; Jin, F. Development of high-strength geopolymers from red mud and blast furnace slag. J. Clean. Prod. 2023, 383, 135439. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, B. Development of unsintered construction materials from red mud wastes produced in the sintering alumina process. Constr. Build. Mater. 2008, 22, 2299–2307. [Google Scholar] [CrossRef]
- Cechin, L.; Ceron, C.; Oliveira, M.; Oliveira, A.; Souza, R.; Hotza, D. Ceramic composites from iron ore and blast furnace slag. Ceram. Int. 2021, 47, 10785–10791. [Google Scholar] [CrossRef]
- Cheah, C.B.; Tan, E.L.; Ramli, M. Recent advances in slag-based binder and chemical activators derived from industrial by-products: A review. Constr. Build. Mater. 2021, 272, 121657. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hou, G.; Yan, P. Preparation of sustainable and green cement-based composite binders with high-volume steel slag powder and ultrafine blast furnace slag powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Boura, P.; Lučaník, A. Application of a clay-slag geopolymer matrix for repairing damaged concrete: Laboratory and industrial-scale experiments. Mater. Test. 2017, 59, 929–937. [Google Scholar] [CrossRef]
- Ministry of Environment and Urbanization; Turkish Steel Producers Association. Iron and Steel Slag Report; Turkish Steel Producers Association: Ankara, Turkey, 2015; Available online: https://celik.org.tr/en/ (accessed on 6 October 2025).
- Gursel, A.; Kurt, A. Radiation emission during SMAW applications on SS304 and A36 steels. Mater. Test. 2014, 56, 826–830. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal. COM(2019) 640 final. Brussels, Belgium: European Commission, 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52019DC0640 (accessed on 6 October 2025).
- Balos, S.; Sidjanin, L.; Dramicanin, M.; Labus, D. Rutile electrodes enhanced with TiO2 nanoparticles. Adv. Mater. Res. 2016, 1138, 69–74. [Google Scholar] [CrossRef]
- ESAB. Welding Solutions. 2025. Available online: https://esab.com (accessed on 6 October 2025).
- Gedik Kaynak. Welding Products and Services. 2025. Available online: https://gedik.com.tr/en/welding (accessed on 6 October 2025).
- Kobelco Welding. Welding Technologies. 2025. Available online: https://www.kobelcowelding.nl/ (accessed on 6 October 2025).
- Lincoln Electric. Welding Equipment and Consumables. 2025. Available online: https://www.lincolnelectric.com (accessed on 6 October 2025).
- Magmaweld. Welding Products and Solutions. 2025. Available online: https://www.magmaweld.com/ (accessed on 6 October 2025).
- Mitelea, I.; Uţu, I.D.; Karancsi, O.; Urlan, S.D.; Crăciunescu, C.M. Investigation of the microstructure of dissimilar welds in duplex stainless steel and low alloyed steel. Mater. Test. 2019, 61, 120–124. [Google Scholar] [CrossRef]
- Çevik, B. Effect of welding processes on mechanical and microstructural properties of S275 structural steel joints. Mater. Test. 2018, 60, 863–868. [Google Scholar] [CrossRef]
- Ozdemir, U.; Sozeri, M.; Findik, T.; Kilicli, V. Effect of buttering on the wear behavior of the SMA welded hardfacing layer in a low-carbon steel. Mater. Test. 2023, 65, 494–504. [Google Scholar] [CrossRef]
- Uzunali, U.Y.; Cuvalci, H. Effect of welding parameters on cruciform weld joints made of armor steel. Mater. Test. 2024, 66, 364–379. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Ozgurluk, Y.; Gulec, A.; Ozkan, D.; Binal, G. Effect of coating degradation on the hot corrosion behavior of yttria-stabilized zirconia (YSZ) and blast furnace slag (BFS) coatings. Surf. Coat. Technol. 2023, 473, 130000. [Google Scholar] [CrossRef]
- Karaoglanli, A.C. Structure and durability evaluation of blast furnace slag coatings and thermal barrier coatings (TBCs) under high temperature conditions. Surf. Coat. Technol. 2022, 452, 129087. [Google Scholar] [CrossRef]
- Keßler, S.; Ziehensack, E.; Gehlen, C. Performance test to evaluate the corrosion resistance of stainless steel in concrete. Mater. Test. 2019, 61, 459–466. [Google Scholar] [CrossRef]
- Eroglu, M. Boride coatings on steel using shielded metal arc welding electrode: Microstructure and hardness. Surf. Coat. Technol. 2009, 203, 2229–2235. [Google Scholar] [CrossRef]
- Vaz, C.T.; Bracarense, A.Q.; Felizardo, I.; Pessoa, E.M. Impermeable low hydrogen covered electrodes: Weld metal, slag, and fumes evaluation. J. Mater. Res. Technol. 2012, 1, 64–70. [Google Scholar] [CrossRef]
- Nagentrau, M.; Tobi, A.L.M.; Sambu, M.; Jamian, Z. The influence of welding condition on the microstructure of WC hardfacing coating on carbon steel substrate. Int. J. Refract. Met. Hard Mater. 2019, 82, 43–57. [Google Scholar] [CrossRef]
- Kocaman, E.; Kilinc, B.; Sen, S.; Sen, U. In-situ TiB2 and Fe2Ti intermetallic assisted hard coatings by Fe-Ti-B based hardfacing electrodes. J. Alloys Compd. 2022, 900, 163478. [Google Scholar] [CrossRef]
- Trinh, N.Q.; Le, D.K.; Tashiro, S.; Bui, H.V.; Tanaka, T. Optimization of metal transfer in rutile flux-cored arc welding through controlled CO2 concentration in argon–CO2 shielding gas. J. Manuf. Process. 2024, 124, 590–603. [Google Scholar] [CrossRef]
- Le, D.K.; Tashiro, S.; Trinh, N.Q.; Tanaka, T.; Bui, H.V. Elucidation of alkali element’s role in optimizing metal transfer behavior in rutile-type flux-cored arc welding. J. Manuf. Process. 2025, 139, 105–125. [Google Scholar] [CrossRef]
- Pradeep, A.V. Effect of blast furnace slag on mechanical properties of glass fiber polymer composites. Procedia Mater. Sci. 2015, 10, 230–237. [Google Scholar] [CrossRef]
- Binici, H.; Aksogan, O. The use of ground blast furnace slag, chrome slag and corn stem ash mixture as a coating against corrosion. Constr. Build. Mater. 2011, 25, 4197–4201. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Sharma, P.; Thakur, P.; Singh, R.K. Application of Biobased Substances in the Synthesis of Nanostructured Magnetic Core-Shell Materials. Inorganics 2023, 11, 406. [Google Scholar] [CrossRef]
- Al-Attar, M.; Al-Khafaji, A.; Al-Zubaidi, A.; Al-Attar, A. Waste-to-Reuse Foam Glasses Produced from Soda-Lime-Silicate Glass, Cathode Ray Tube Glass, and Aluminium Dross. Inorganics 2022, 10, 1. [Google Scholar] [CrossRef]
- Kovářík, T.; Šoukal, F.; Všianský, D.; Vávrová, K. The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum. Inorganics 2023, 11, 298. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung (DIN). DIN 17145; Weldable Normalized Fine-Grain Structural Steels–Technical Delivery Conditions. Beuth Verlag: Berlin, Germany, 1983.
- Moore, J.J. Chemical Metallurgy; Butterworths: London, UK, 1981. [Google Scholar]
- Cankut, S. Extractive Metallurgy; Istanbul Technical University Publications: Istanbul, Turkey, 1972. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Gaskell, D.R. Introduction to Metallurgical Thermodynamics, 2nd ed.; Hemisphere: New York, NY, USA, 1981. [Google Scholar]
- Oğuz, B. Arc Welding; Oerlikon-Magmaweld Publication: Istanbul, Turkey, 1989. [Google Scholar]
- Anık, S.; Tülbentçi, K.; Kaluç, E. Electric Arc Welding with the Covered Electrode; Gedik Education Foundation Publications: Istanbul, Turkey, 1991. [Google Scholar]
- Eryürek, B. Covered Electrode Selection for Steels; Askaynak-Lincoln Electric Publications: Istanbul, Turkey, 2007. [Google Scholar]
- Mikhailitsyn, S.; Sheksheev, M.; Platov, S.; Emelyushin, A.; Naumov, S. Investigation of the viscosity of liquid welding slags and melts of electrode coatings. Izv. Vysshikh Uchebnykh Zavedenii, Chernaya Metall. 2018, 61, 280–287. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Liu, X.; Zhou, Y.; Shi, Y. Influence of welding method on residual stress and metallography of a mild steel welded butt-joint plate. J. Constr. Steel Res. 2022, 199, 107640. [Google Scholar] [CrossRef]
- ISO 9018:2015; Destructive Tests on Welds in Metallic Materials—Tensile Test on Cruciform and Lapped Joints. ISO: Geneva, Switzerland, 2015.
- ISO 148-1:2016; Metallic Materials—Charpy Pendulum Impact Test—Part 1: Test Method. ISO: Geneva, Switzerland, 2016.
- ASTM E23:2023; Standard Test Methods for Notched Bar Impact Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2023.
- ISO 2560:2020; Welding Consumables—Covered Electrodes for Manual Metal Arc Welding of Non-Alloyed and Fine-Grained Steels—Classification. ISO: Geneva, Switzerland, 2020.
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Falsafi, M.; Fornasiero, R. Explorative multiple-case research on the scrap-based steel slag value chain: Opportunities for circular economy. Sustainability 2022, 14, 2284. [Google Scholar] [CrossRef]
- Cabrera-Luna, K.; Burciaga-Diaz, O.; Santana-Carrillo, J.L.; Escalante-Garcia, J.I. Environmental performance of sustainable supersulfated cements based on blast furnace slag: A life cycle study. Environ. Res. 2025, 279, 121876. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, G.; Bier, T.; Waida, S.; Dous, A.; Heinemann, S.; Herr, P.; Charitos, A. Treatment of energy from waste plant fly-ash for blast furnace slag substitution as a supplementary cementitious material. J. Clean. Prod. 2025, 490, 144693. [Google Scholar] [CrossRef]
- EREF; Waste Today. Average Cost to Landfill Municipal Solid Waste in the United States in 2022 and 2023, by Region (in U.S. Dollars Per Ton). Statista. 2024. Available online: https://www.statista.com/statistics/692063/cost-to-landfill-municipal-solid-waste-by-us-region/ (accessed on 18 June 2025).
- Ceylan, I.; Gokdemir, H.; Cengiz, T.; Cicek, B. Development of CaO-rich blast furnace slag containing fluorine mica-based glass ceramic coatings. Ceram. Int. 2021, 47, 29988–29994. [Google Scholar] [CrossRef]
- Easterling, K. Introduction to the Physical Metallurgy of Welding, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1992. [Google Scholar]
- Pardo, N.; Moya, J.A. Prospective scenarios on energy efficiency and CO2 emissions in the European iron & steel industry. Energy 2013, 54, 113–128. [Google Scholar] [CrossRef]

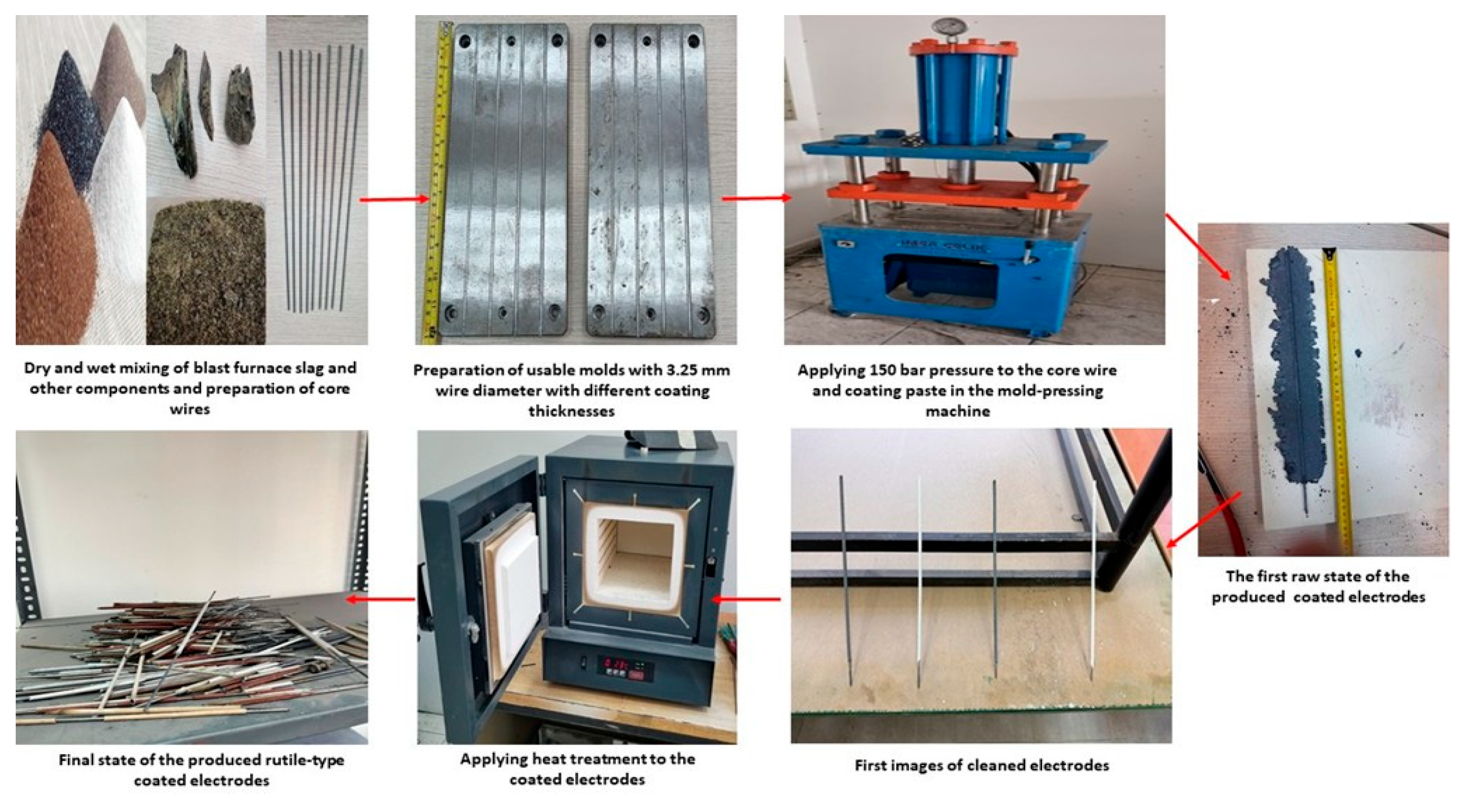



| Material | SiO2 | CaO | Al2O3 | MgO | TiO2 | Na2O + K2O | Fe2O3 | MnO | S2− | PO43− | Rest |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 39 (±0.5) | 37.5 (±0.4) | 12.4 (±0.2) | 5 (±0.1) | 1.6 (±0.2) | 1.1 (±0.05) | 0.9 (±0.1) | 0.4 (±0.5) | 0.06 (±0.01) | 0.05 (±0.01) | Others |
| Chemical Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | C | Si | Cu | Mn | P | S | Ni | Cr | Fe |
| Core wire | 0.08 | 0.022 | 0.089 | 0.544 | 0.009 | 0.011 | 0.046 | 0.041 | Bal. |
| SAE 1020 | 0.20 | 0.001 | - | 0.400 | 0.026 | 0.022 | - | 0.064 | Bal. |
| Flux Ratios (wt%) | |||||
|---|---|---|---|---|---|
| Electrode Code (Rutile) | BFS | Rutile (TiO2) | Other Flux Components | Binder (K2SiO3) | Water |
| R1 | 50 | 35–50 | 0–15 | 20–25 | 1–2 |
| R2 | 45 | 35–50 | 0–15 | 20–25 | 1–2 |
| R3 | 40 | 35–50 | 0–15 | 20–25 | 1–2 |
| R4 | 35 | 35–50 | 0–15 | 20–25 | 1–2 |
| Parameters | Properties |
|---|---|
| Electrode Type | Rutile (Thick coating) |
| Electrode outer diameter (mm) | 5.17 mm |
| Electrode Core Wire Diameter (mm) | 3.25 mm |
| Electrode Core Wire weight (g) | 23 g |
| Electrode coating thickness (mm) | 1.92 mm |
| Electrode coating weight (g) | 12 g |
| Coating/Core wire (%) | 59 |
| Total electrode length (cm) | 35 cm |
| Welding Machine (type) | Shielded metal arc welding-SMAW |
| Current (A) | 70–100 |
| Welding Angle (°) | 45 |
| Pole (±) | (+) |
| Substrate | SAE 1020 |
| Substrate Dimensions (mm) | 20 × 100 × 3 mm |
| Pre-heat for substrate | No |
| Pre-heat for electrodes | No |
| (a) Performance Criteria of Rutile-Type Covered Electrodes | |||||||||
| Rating | Arc Stability | Burning | Spatter | Slag Uniformity | Slag Removal | Seam Appearance | Odor and Smoke | Seam or Slag Porosity | |
| Point | |||||||||
| 1–2 | Very Bad | Very difficult | Excess | Very poor | Very difficult | Matt | Excess | Very high | |
| 3–4 | Bad | Difficult | Increased | Poor | Difficult | Low gloss | Less | High | |
| 5–6 | Good | Medium | Moderate | Average | Easy | Medium | Medium | Medium | |
| 7–8 | Very good | Easy | Minimal | Good | Very easy | Glossy | Minimal | Low | |
| 9–10 | Excellent | Very easy | None | Very good | Spontane | High gloss | None | None | |
| (b) Scores received by therutile-type coveredelectrodes | |||||||||
| Electrode numbers | Arc stability | Burning | Spatter | Slag uniformity | Slag removal | Seam appearance | Odor and smoke | Seam porosity | |
| R1 | Point | 8 | 9 | 7 | 7 | 9 | 7 | 8 | 7 |
| R2 | 10 | 10 | 8 | 10 | 10 | 10 | 8 | 8 | |
| R3 | 10 | 10 | 8 | 10 | 10 | 10 | 8 | 10 | |
| R4 | 10 | 10 | 8 | 10 | 10 | 7 | 8 | 10 | |
| Chemical Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrode | C | Mn | Si | S | Cr | P | O | N | Fe |
| R1 | 0.12 | 1.06 | 0.25 | 0.026 | 0.05 | 0.030 | 1.18 | 0.04 | Bal. |
| R2 | 0.13 | 1.08 | 0.31 | 0.027 | 0.05 | 0.028 | 1.06 | 0.04 | Bal. |
| R3 | 0.11 | 1.17 | 0.40 | 0.024 | 0.05 | 0.027 | 0.93 | 0.04 | Bal. |
| R4 | 0.11 | 1.19 | 0.47 | 0.023 | 0.04 | 0.027 | 0.90 | 0.04 | Bal. |
| Electrode Numbers | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation A5 (%) | Izod V-Notch Impact Strength (Joule) | |
|---|---|---|---|---|---|
| 20 °C | 0 °C | ||||
| R1 | 470 | 560 | 24 | 79 | 48 |
| R2 | 474 | 562 | 25 | 83 | 53 |
| R3 | 477 | 570 | 26 | 86 | 58 |
| R4 | 482 | 573 | 26 | 86 | 59 |
| Commercial E6013 | 400–500 | 500–560 | 23–28 | 70–100 | 50–60 |
| Minimum Yield Strength (MPa) | Tensile Strength Range (MPa) | Minimum Elongation A5 (%) |
|---|---|---|
| 355 | 440–570 | 22 |
| 380 | 470–600 | 20 |
| 420 | 500–640 | 20 |
| 460 | 530–680 | 20 |
| 500 | 560–720 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaptanoglu, M. Green Manufacturing of Rutile (TiO2) Welding Electrodes with Blast Furnace Slag. Inorganics 2025, 13, 361. https://doi.org/10.3390/inorganics13110361
Kaptanoglu M. Green Manufacturing of Rutile (TiO2) Welding Electrodes with Blast Furnace Slag. Inorganics. 2025; 13(11):361. https://doi.org/10.3390/inorganics13110361
Chicago/Turabian StyleKaptanoglu, Mustafa. 2025. "Green Manufacturing of Rutile (TiO2) Welding Electrodes with Blast Furnace Slag" Inorganics 13, no. 11: 361. https://doi.org/10.3390/inorganics13110361
APA StyleKaptanoglu, M. (2025). Green Manufacturing of Rutile (TiO2) Welding Electrodes with Blast Furnace Slag. Inorganics, 13(11), 361. https://doi.org/10.3390/inorganics13110361






