A Theoretical Comparison on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2 for Adsorption and Sensing of Dissolved Gases (H2, C2H2, and C2H4) in Transformer Oil
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
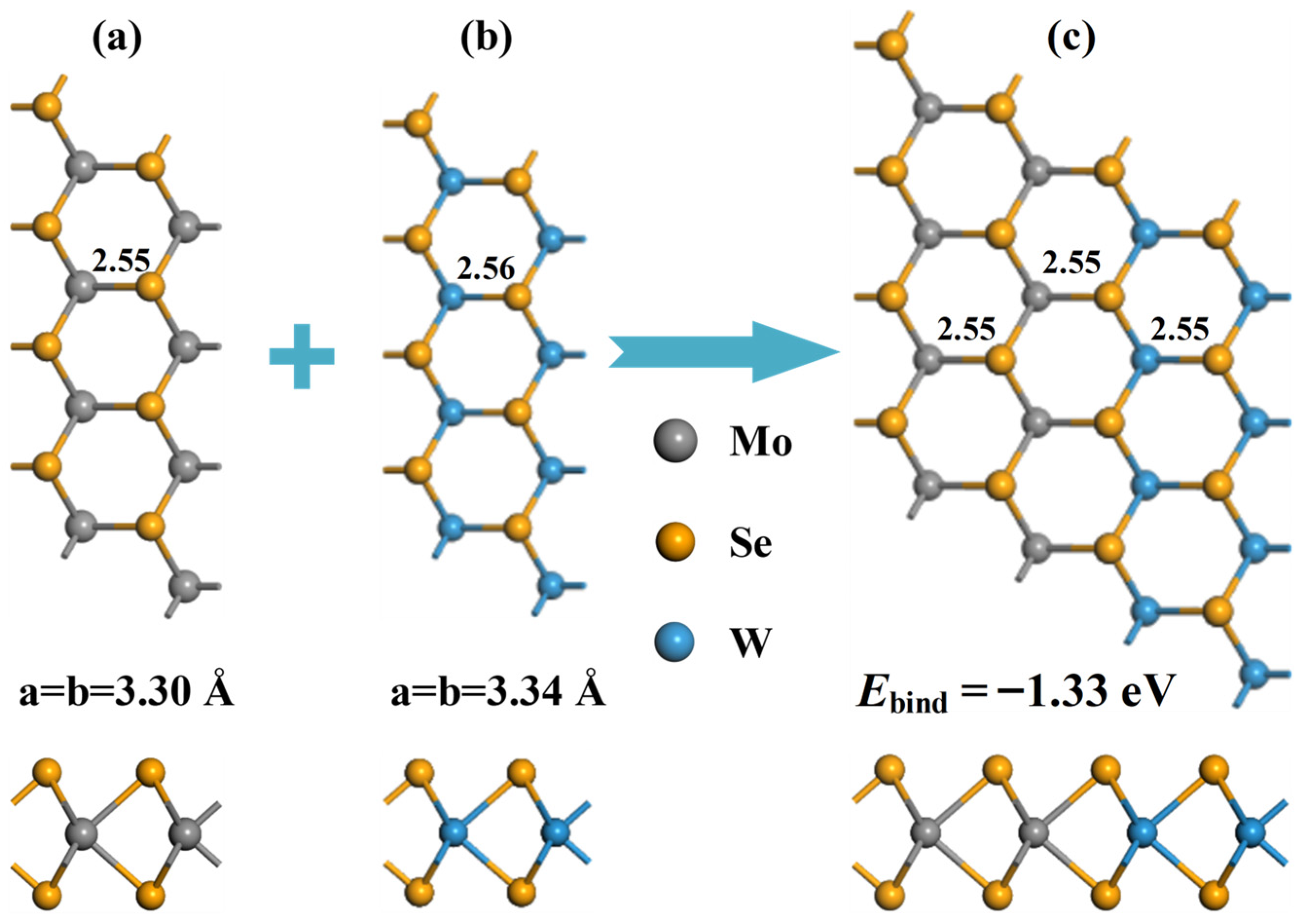
3.1. Analysis of Isolated and Pd-Doped MoSe2-WSe2 Configuration
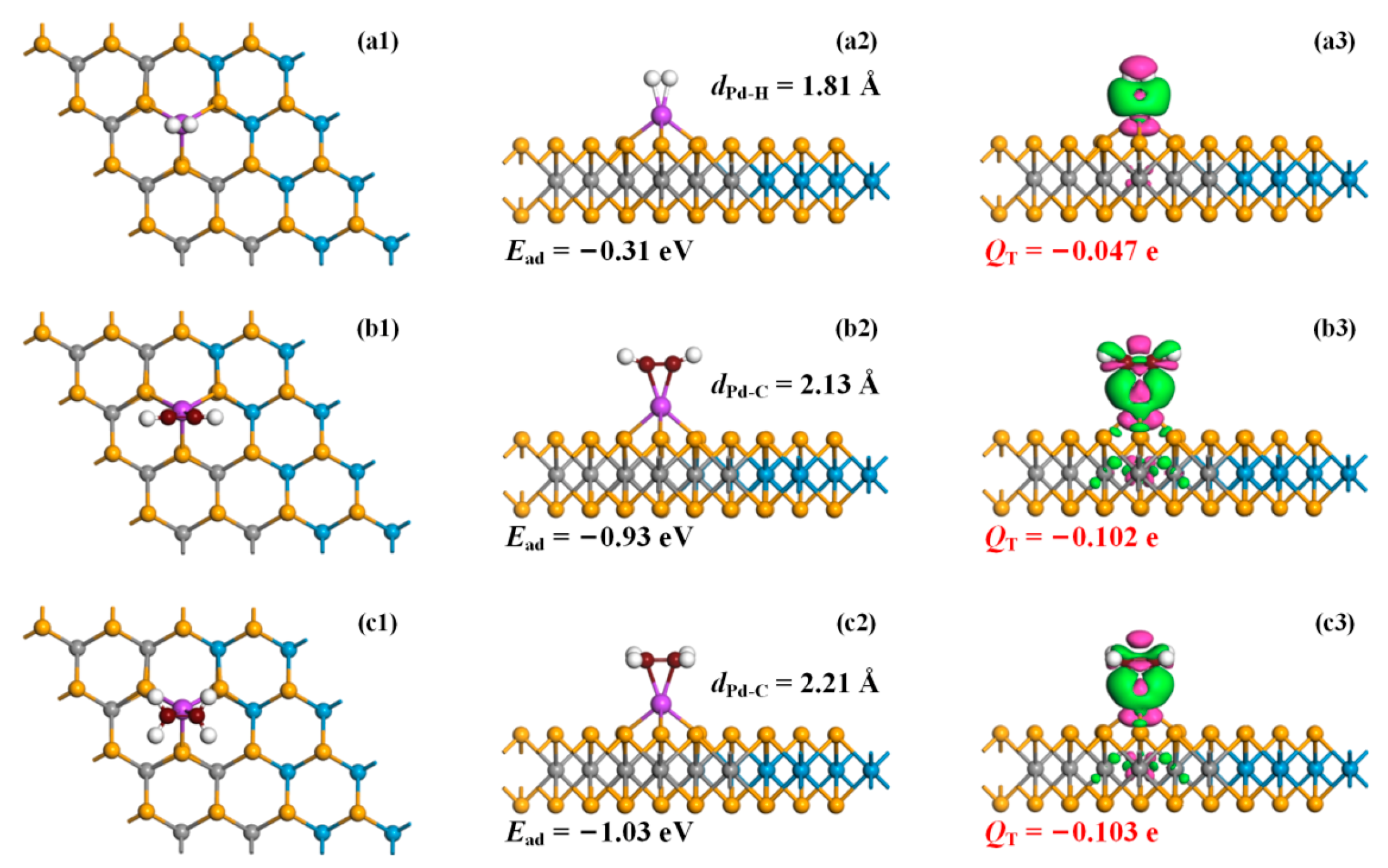
3.2. Gas Adsorptions on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2
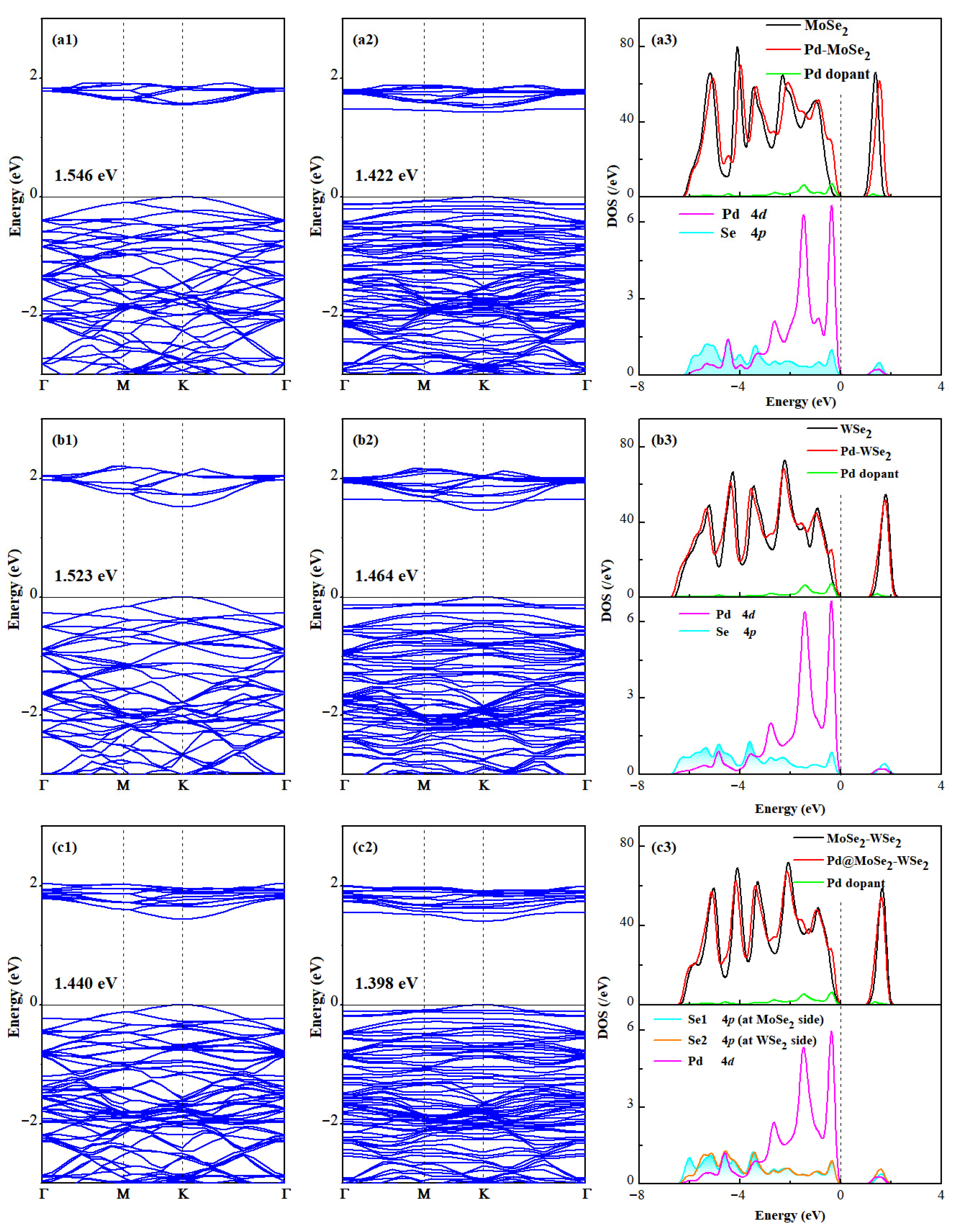
3.3. Analysis of Electronic Property in Gas Adsorption
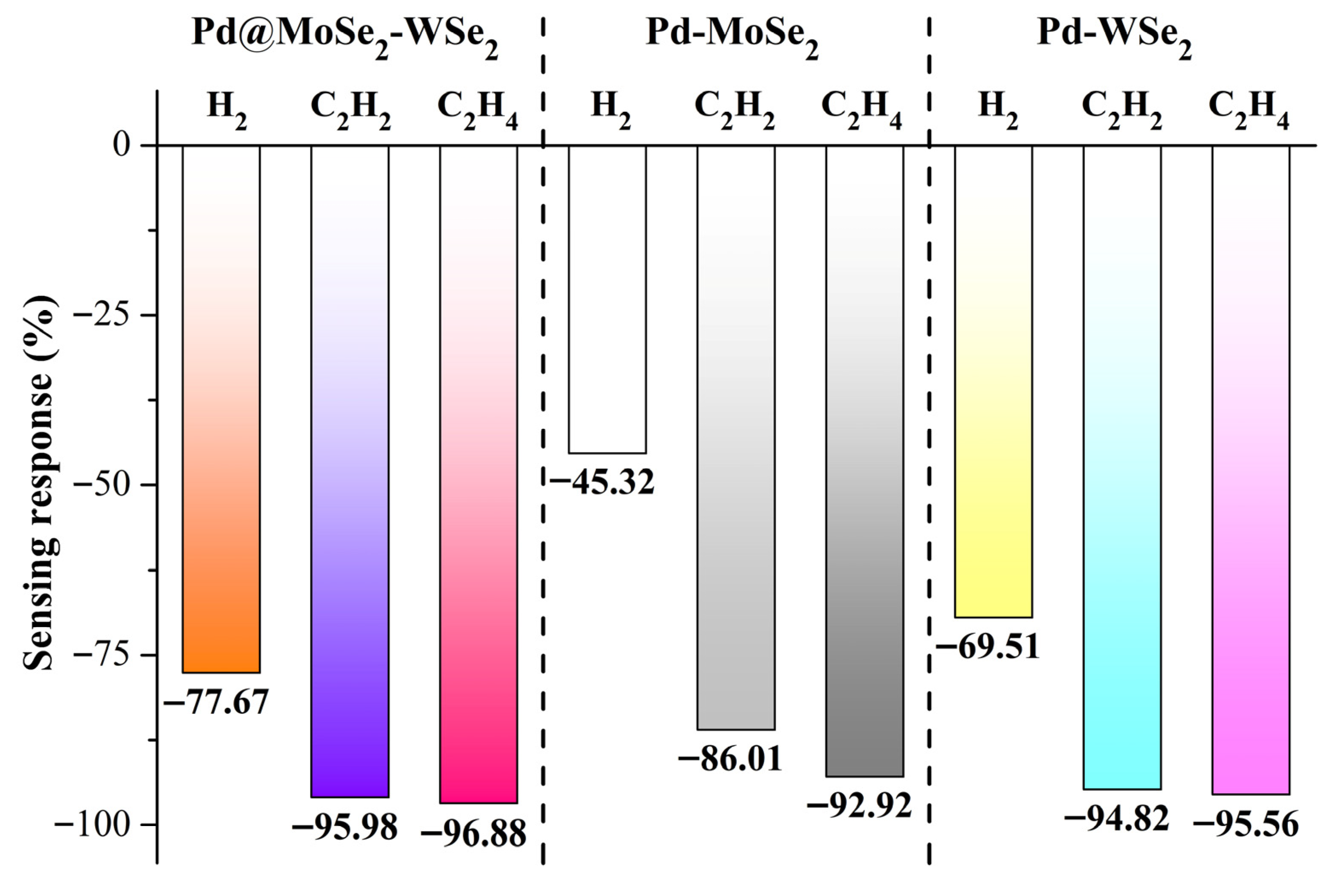
3.4. Gas Sensor Exploration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, G.; Tian, S.; Zhang, X. First-Principles Insight into Pd-Doped ZnO Monolayers as a Promising Scavenger for Dissolved Gas Analysis in Transformer Oil. ACS Omega 2020, 5, 17801–17807. [Google Scholar] [CrossRef]
- Baek, D.H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wei, L.; Chao, W.; Peng, W. The New Development Trend of Distribution Transformer. Appl. Mech. Mater. 2014, 672–674, 831–836. [Google Scholar] [CrossRef]
- Liao, R.; Zheng, H.; Grzybowski, S.; Yang, L.; Zhang, Y.; Liao, Y. An Integrated Decision-Making Model for Condition Assessment of Power Transformers Using Fuzzy Approach and Evidential Reasoning. IEEE Trans. Power Deliv. 2011, 26, 1111–1118. [Google Scholar] [CrossRef]
- Singh, S.; Bandyopadhyay, M.N. Dissolved gas analysis technique for incipient fault diagnosis in power transformers: A bibliographic survey. IEEE Electr. Insul. Mag. 2010, 26, 41–46. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Yang, F.; Jung, D.; Penner, R.M. Trace Detection of Dissolved Hydrogen Gas in Oil Using a Palladium Nanowire Array. Anal. Chem. 2011, 83, 9472–9477. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Cao, J.; Sheng, L.; Yin, L.; Xu, X. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sens. Actuators B Chem. 2014, 202, 232–239. [Google Scholar] [CrossRef]
- Ma, G.M.; Li, C.R.; Luo, Y.T.; Mu, R.D.; Wang, L. High sensitive and reliable fiber Bragg grating hydrogen sensor for fault detection of power transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]
- Benounis, M.; Aka-Ngnui, T.; Jaffrezic, N.; Dutasta, J.P. NIR and optical fiber sensor for gases detection produced by transformation oil degradation. Sens. Actuators A Phys. 2008, 141, 76–83. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Cheng, H.; Tang, J. Ladder-wise calculation method for z-coordinate of transformer PD source based on planar layout UHF antenna sensors. IEEJ Trans. Electr. Electron. Eng. 2019, 15, 340–345. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 2019, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.; Zhang, J.; Zhang, Y. Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Volt. 2019, 4, 242–258. [Google Scholar] [CrossRef]
- Sajjad, M.; Montes, E.; Singh, N.; Schwingenschlögl, U. Superior Gas Sensing Properties of Monolayer PtSe2. Adv. Mater. Interfaces 2017, 4, 1600911. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Ye, H.; Zhang, K.; Chen, X.; Zhang, G. Promoting sensitivity and selectivity of HCHO sensor based on strained InP3 monolayer: A DFT study. Appl. Surf. Sci. 2018, 459, 554–561. [Google Scholar] [CrossRef]
- Cui, H.; Ran, M.; Peng, X.; Zhang, G. First-principles design of noble metal (Rh and Pd) dispersed Janus WSTe monolayer for toxic gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112047. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65, 103974. [Google Scholar] [CrossRef]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Lin, Y.F.; Xu, Y.; Wang, S.T.; Li, S.L.; Yamamoto, M.; Aparecido-Ferreira, A.; Li, W.; Sun, H.; Shu, N.; Jian, W.B. Ambipolar MoTe2 Transistors and Their Applications in Logic Circuits. Adv. Mater. 2014, 26, 3263–3269. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Wu, E.; Xie, Y.; Yuan, B.; Zhang, H.; Hu, X.; Liu, J.; Zhang, D. Ultra-sensitive and Fully Reversible NO2 Gas Sensing based on p-type MoTe2 Under ultra-violet Illumination. ACS Sens. 2018, 3, 1719–1726. [Google Scholar]
- Zhang, Y.; Yang, R.; Li, H.; Zeng, Z. Boosting Electrocatalytic Reduction of CO2 to HCOOH on Ni Single Atom Anchored WTe2 Monolayer. Small 2022, 18, 2203759. [Google Scholar] [CrossRef]
- Xu, L.; Gui, Y.; Li, W.; Li, Q.; Chen, X. Gas-sensing properties of Ptn-doped WSe2 to SF6 decomposition products. J. Ind. Eng. Chem. 2021, 97, 452–459. [Google Scholar] [CrossRef]
- Cui, H.; Wu, H.; He, D.; Ma, S. Noble metal (Pd, Pt)-functionalized WSe2 monolayer for adsorbing and sensing thermal runaway gases in LIBs: A first-principles investigation. Environ. Res. 2025, 269, 120847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, H.; Chang, J.; Wang, B.; Kuang, A.; Chen, H. ZnO/MoX 2 (X= S, Se) composites used for visible light photocatalysis. RSC Adv. 2018, 8, 10828–10835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, S.; Wang, X.; Huang, J.; Pan, W.; Zhang, J.; Meteku, B.E.; Zeng, J. UV illumination-enhanced ultrasensitive ammonia gas sensor based on (001) TiO2/MXene heterostructure for food spoilage detection. J. Hazard. Mater. 2022, 423, 127160. [Google Scholar] [CrossRef]
- Ikram, M.; Liu, L.; Liu, Y.; Ma, L.; Lv, H.; Ullah, M.; He, L.; Wu, H.; Wang, R.; Shi, K. Fabrication and characterization of a high-surface area MoS2@ WS2 heterojunction for the ultra-sensitive NO2 detection at room temperature. J. Mater. Chem. A 2019, 7, 14602–14612. [Google Scholar] [CrossRef]
- Kalita, P.; Sutradhar, M.K.; Mondal, B. Hybrid MoSe2/WSe2 Nanomaterials: Enhancing Humidity Tolerant Gas Response. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Creazzo, F. Engineering of MoSe2 and WSe2 Monolayers and Heterostructures by DFT-Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2025, 17, 39676–39693. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, F. Detection of Dissolved Gas in Transformer Oil Using Pt-Doped WTe 2 Based Sensor: A First Principles Study. In Proceedings of the 2023 IEEE 6th International Electrical and Energy Conference (CIEEC), Hefei, China, 12–14 May 2023; pp. 733–736. [Google Scholar]
- Ding, H.; Qin, Z.; Sun, X.; Wang, B.; Wang, F. Pd-Adsorbing CrS2 monolayer as sensing material for detecting CO, C2H2, and C2H4 in dissolved gases: A first-principles study. Chem. Phys. Lett. 2024, 857, 141700. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Zhao, Q.; Zhang, G. Pd-doped PtSe2 monolayer with strain-modulated effect for sensing SF6 decomposed species: A first-principles study. J. Mater. Res. Technol. 2022, 18, 629–636. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Xiong, H.; Li, Y.; Zhang, D. A first-principles study of the SF6 decomposed products adsorbed over defective WS2 monolayer as promising gas sensing device. IEEE Trans. Device Mater. Reliab. 2019, 19, 473–483. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Di Stasio, R.A., Jr.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 094106. [Google Scholar] [CrossRef]
- Yang, A.; Pan, J.; Wang, D.; Lan, T.; Liu, Z.; Yuan, H.; Chu, J.; Wang, X.; Rong, M. Tunable SO2-sensing performance of arsenene induced by Stone-Wales defects and external electric field. Appl. Surf. Sci. 2020, 523, 146403. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Cui, H.; Hu, J.; Jiang, X.; Zhang, X. A first-principles study of SOF2 and SO2F2 adsorption onto PdSe2-based monolayers: Favorable sensitivity and selectivity by doping single Cu or Rh atom. Environ. Res. 2025, 269, 120843. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zeng, Z.; Liu, L.; Jia, Y. Theoretical screening of the transition metal heteronuclear dimer anchored graphdiyne for electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 54, 501–509. [Google Scholar] [CrossRef]
- Wu, H.; Fang, J.; Yuan, S.; Liu, Y.; Zeng, J.; Jiang, T. Adsorption and gas sensing properties of Cun and Pdn (n = 1–3) clusters modified MoSe2 for lithium battery thermal runaway gases. Appl. Surf. Sci. 2024, 648, 158963. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Jiang, J.; Zeng, W.; Zhou, Q. DFT study of Cun (n = 1–3) cluster-modified WSe2 monolayers for the detection of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2). Surf. Interfaces 2024, 52, 104904. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular single-bond covalent radii for elements 1-118. Chemistry 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, D.; Wang, D.; Deng, J.; Kong, D.; Zhang, H. Performance prediction of 2D vertically stacked MoS2-WS2 heterostructures base on first-principles theory and Pearson correlation coefficient. Appl. Surf. Sci. 2022, 596, 153498. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Z.; Yun, M.; Liu, D.; Pan, Z.; Yang, D. The adsorption characteristics of MoS2–CuO heterojunction to polar gas molecules (CO, NO and NO2): A first–principle study. Appl. Surf. Sci. 2023, 610, 155564. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J. Highly Selective NH3 Sensor Based on MoS2/WS2 Heterojunction. Nanomaterials 2023, 13, 1835. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lu, G.; Liu, X.; Fan, S.; Hu, Y.; Pinna, N.; Zhang, J. Mixed-dimensional heterojunction by 3D CdS nanowire arrays bridged with 2D WSe2 for ultrafast photoelectric gas sensor. Matter 2024, 8, 101914. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, X.; Wu, D.; Chen, L.; Su, Y.; Cui, H.; Wu, F. Single Rh atom decorated pristine and S-defected PdS2 monolayer for sensing thermal runaway gases in a lithium-ion battery: A first-principles study. Surf. Interfaces 2023, 37, 102735. [Google Scholar] [CrossRef]
- Gao, J.; Gao, K.; Miao, L.; Ding, J.; Chen, H.; Fu, H.; Peng, J. First principle investigation on gas sensing properties of MoS2/ZnO heterojunction. Microchem. J. 2024, 206, 111414. [Google Scholar] [CrossRef]
- Murphy, L.R.; Meek, T.L.; Allred, A.L.; Allen, L.C. Evaluation and test of pauling’s electronegativity scale. J. Phys. Chem. A 2000, 104, 5867–5871. [Google Scholar] [CrossRef]
- Xingyi, T.; Qiang, L.; Hua-Hua, D.F. The device performance limit of in-plane monolayer VTe2/WTe2 heterojunction-based field-effect transistors. Nanoscale 2023, 15, 19726–19734. [Google Scholar]
- Li, F.; Chen, F.; Cui, H.; Jiang, X. Pristine and Ni-doped WTe2 monolayer for adsorption and sensing of C2H2 and C2H4 in oil-immersed transformers: A DFT study. Comput. Theor. Chem. 2023, 1226, 114187. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, D.; Chen, Q.; Wang, Z.; Wang, D.; Yang, Z.; Xu, W.; Wang, L.; Zhu, L.; An, F. Heterostructure construction of SnS2 Debye nanowires modified with ZnO nanorods for chemiresistive H2S detection in sulfur hexafluoride decomposition products. Sens. Actuators B Chem. 2023, 390, 133952. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, W.; Zhang, T.; Yuan, H.; Bi, M.; Zhou, X. Adsorption and gas-sensing performances of C2H2, C2H4, CO, H2 in transformer oil on Pt-doped MoTe2 monolayer: A DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 146, 115568. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wei, Z.; Wang, Q.; Liang, Z.; Yuan, T. Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory. Chemosensors 2022, 10, 307. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Mi, H.; Wang, J.; Zeng, W. Gas-sensing mechanism of Cr doped SnP3 monolayer to SF6 partial discharge decomposition components. Appl. Surf. Sci. 2021, 546, 149084. [Google Scholar] [CrossRef]
- Reza Khoshnobish, S.; Ahmed, T.; Arefin, T.; Akter Piya, A.; Ud Daula Shamim, S. Assessing the sensing performance of Janus transition metal dichalcogenides (ScSSe, TiSSe and ZrSSe) for oxygen-containing toxic gas molecules such as CO, NO, NO2 and SO2. Appl. Surf. Sci. 2025, 679, 161301. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Li, P.; Pang, M.; Luo, Y. Fabrication of Pd-decorated MoSe 2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Xiong, H.; Deng, G.; Gan, L. Theoretical investigations of adsorption and sensing properties of M2Pc (M= Cr, Mo) monolayers towards volatile organic compounds. Colloids Surf. A Physicochem. Eng. Asp. 2025, 717, 136750. [Google Scholar] [CrossRef]
- He, W.; Xu, Y.; Wu, Z.; Hou, Z.; Qin, W.; Liu, X.; Shi, J.; Long, Y. Adsorption of Nitrogen-Containing Toxic Gases on Transition Metal (Pt, Ag, Au)-Modified Janus ZrSSe Monolayers for Sensing Applications: A DFT Study. ACS Appl. Nano Mater. 2025, 8, 3163–3174. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Zhang, C.; Xie, S.; Wu, S.; Cui, H. Adsorptions of C5F10O decomposed compounds on the Cu-decorated NiS2 monolayer: A first-principles theory. Mol. Phys. 2023, 121, e2163715. [Google Scholar] [CrossRef]
- Verlag, S. Semiconductor Physical Electronics. Semicond. Phys. Electron. 2006, 28, 363–364. [Google Scholar]
- Liu, L.; Li, Y.; Jiang, X.; Zhang, Z.; Li, T.; Ma, L.; Niu, S.; Chen, Z.; Xiao, S.; Dan, M.; et al. Ir-doped MoSe2: A promising candidate for C4F7N decomposed species detection and scavenging. Surf. Interfaces 2024, 51, 104634. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, S.; Bin, Y.; Jiang, X.; Cui, H. Ni-decorated WS2-WSe2 heterostructure as a novel sensing candidate upon C2H2 and C2H4 in oil-filled transformers: A first-principles investigation. Mol. Phys. 2025, e2492391. [Google Scholar] [CrossRef]
- Li, Z.; Rao, Y.; Wang, Z.; Zhang, T.; Wu, G.; Sun, L.; Ren, Y.; Tao, L. Universal Synthesis of Core–Shell-Structured Ordered Mesoporous Transition Metal Dichalcogenides/Metal Oxides Heterostructures with Active Edge Sites. Small Struct. 2025, 6, 2400376. [Google Scholar] [CrossRef]
- Wang, K.; Fu, Y.; Kong, D.; Wang, S.; Li, L. First-principles insight into adsorption behavior of a Pd-doped PtTe2 monolayer for CO and C2H2 and the effect of an applied electric field. J. Phys. Chem. Solids 2023, 177, 111289. [Google Scholar] [CrossRef]




| Gas Species | Bandgaps of Various Systems (eV) | ||
|---|---|---|---|
| Pd@MoSe2-WSe2 | Pd-MoSe2 | Pd-WSe2 | |
| Isolated | 1.398 | 1.422 | 1.464 |
| H2 | 1.321 | 1.391 | 1.403 |
| C2H2 | 1.233 | 1.321 | 1.312 |
| C2H4 | 1.220 | 1.286 | 1.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Ma, S.; Cui, H. A Theoretical Comparison on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2 for Adsorption and Sensing of Dissolved Gases (H2, C2H2, and C2H4) in Transformer Oil. Inorganics 2025, 13, 360. https://doi.org/10.3390/inorganics13110360
Guo X, Ma S, Cui H. A Theoretical Comparison on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2 for Adsorption and Sensing of Dissolved Gases (H2, C2H2, and C2H4) in Transformer Oil. Inorganics. 2025; 13(11):360. https://doi.org/10.3390/inorganics13110360
Chicago/Turabian StyleGuo, Xinyu, Shouxiao Ma, and Hao Cui. 2025. "A Theoretical Comparison on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2 for Adsorption and Sensing of Dissolved Gases (H2, C2H2, and C2H4) in Transformer Oil" Inorganics 13, no. 11: 360. https://doi.org/10.3390/inorganics13110360
APA StyleGuo, X., Ma, S., & Cui, H. (2025). A Theoretical Comparison on Pd-Doped MoSe2, WSe2, and MoSe2-WSe2 for Adsorption and Sensing of Dissolved Gases (H2, C2H2, and C2H4) in Transformer Oil. Inorganics, 13(11), 360. https://doi.org/10.3390/inorganics13110360






