Enhanced Phase Stability of Sm2(Fe, Al)17Cx
Abstract
1. Introduction
2. Results and Discussion
2.1. Formation Energy and Site Preference of Al in Sm2(Fe,Al)17 and Sm2(Fe,Al)17C3
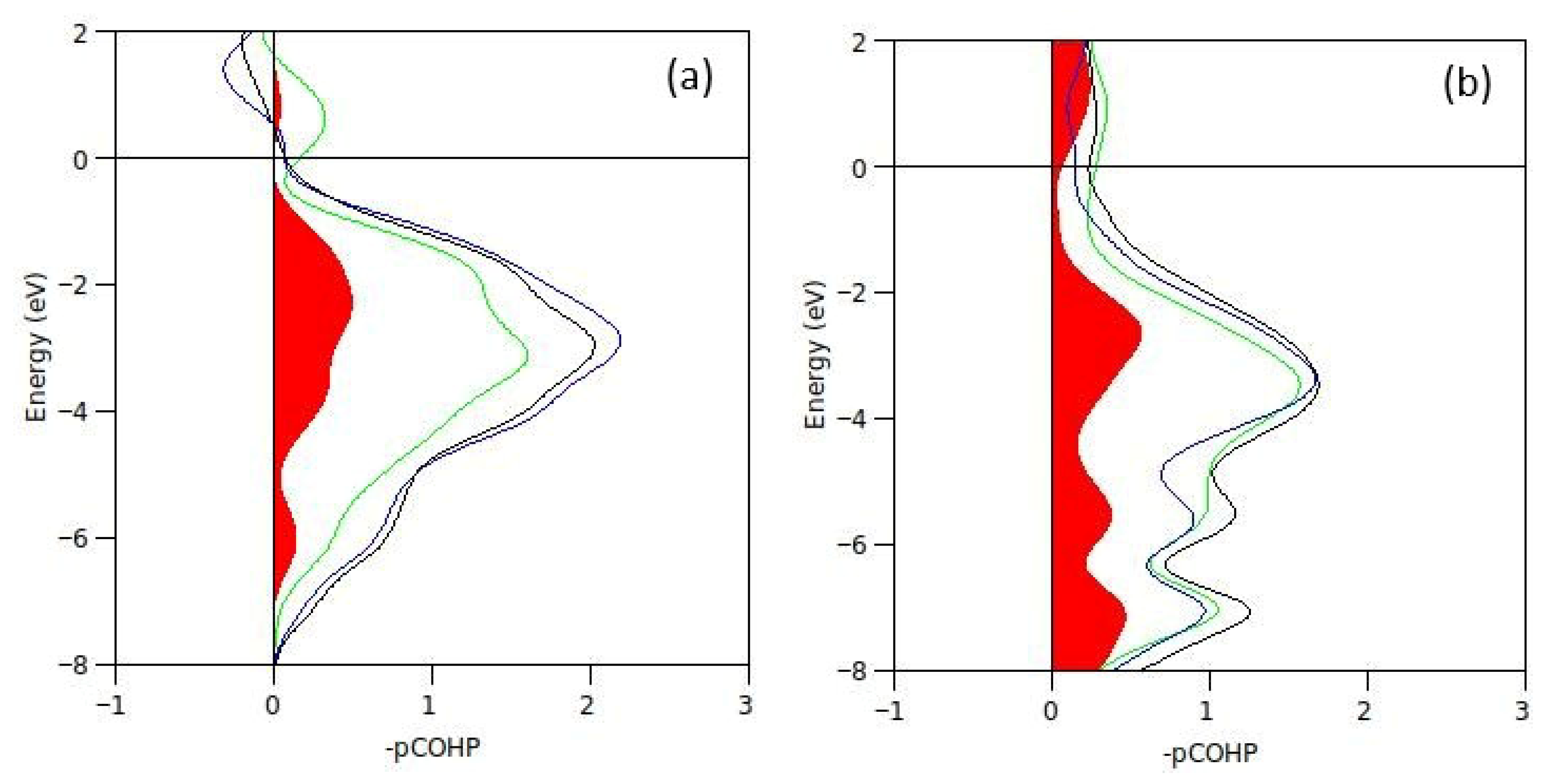
2.2. Chemical Bond in Sm2Fe17C3 and Sm2(Fe,Al)17C3
3. Computational Method and Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCallum, R.W.; Lewis, L.; Skomski, R.; Kramer, M.J.; Anderson, I.E. Practical aspects of modern and future permanent magnets. Annu. Rev. Mater. Res. 2014, 44, 451–477. [Google Scholar] [CrossRef]
- Sepehri-Amin, H.; Hirosawa, S.; Hono, K. Advances in Nd-Fe-B Based Permanent Magnets. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 269–372. [Google Scholar]
- Coey, J.M.D. Hard magnetic materials: A perspective. IEEE Trans. Magn. 2011, 47, 4671–4681. [Google Scholar] [CrossRef]
- Skoug, E.J.; Meyer, M.S.; Pinkerton, F.E.; Tessema, M.M.; Haddad, D.; Herbst, J.F. Crystal structure and magnetic properties of Ce2Fe14−xCoxB alloys. J. Alloys Compd. 2013, 574, 552–555. [Google Scholar] [CrossRef]
- Gabay, A.M.; Martín-Cid, A.; Barandiaran, J.M.; Salazar, D.; Hadjipanayis, G.C. Low-cost Ce1−xSmx(Fe, Co, Ti)12 alloys for permanent magnets. AIP Adv. 2016, 6, 056015. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Sun, H. Improved magnetic properties by treatment of iron-based rare earth intermetallic compounds in anmonia. J. Magn. Magn. Mater. 1990, 87, L251–L254. [Google Scholar] [CrossRef]
- Otani, Y.; Hurley, D.P.F.; Sun, H.; Coey, J.M.D. Magnetic properties of a new family of ternary rare-earth iron nitrides R2Fe17N3−δ (invited). J. Appl. Phys. 1991, 69, 5584–5589. [Google Scholar] [CrossRef]
- Matsuura, M.; Yamamoto, K.; Tezuka, N.; Sugimoto, S. Microstructural changes in high-coercivity Zn-bonded Sm-Fe-N magnets. J. Magn. Magn. Mater. 2020, 510, 166943. [Google Scholar] [CrossRef]
- Liu, X.B.; Gandha, K.; Wang, H.; Mungale, K.; Vaidya, U.K.; Nlebedim, I.C.; Paranthaman, M.P. Packing bimodal magnetic particles to fabricate highly dense anisotropic rare earth bonded permanent magnets. RSC Adv. 2023, 13, 17097–17101. [Google Scholar] [CrossRef]
- Yamaguchi, W.; Hosokawa, A.; Takagi, K. Anomalous Reduction in Magnetization of Sm2Fe17N3Anisotropic Sintered Magnets During Pressure Current Sintering. IEEE Trans. Magn. 2023, 59, 1–6. [Google Scholar]
- Zhang, D.T.; Yue, M.; Zhang, J.X. Study on bulk Sm2Fe17Nx sintered magnets prepared by spark plasma sintering. Powder Metall. 2007, 50, 215–222. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, S.; Huang, H.; Li, R.; Cai, W.; Chen, H.; Li, J.; Qiao, L.; Ying, Y.; Li, W.; et al. Magnetic Properties and Microstructure of Ce-Cu-Al Low Melting Alloy Bonding Sm2Fe17N3 Magnet Fabricated by the Hot-Pressing Method. Magnetochemistry 2022, 8, 149. [Google Scholar] [CrossRef]
- Takagi, K.; Soda, R.; Jinno, M.; Yamaguchi, W. Possibility of high-performance Sm2Fe17N3 sintered magnets by low-oxygen powder metallurgy process. J Magn. Magn. Mater. 2020, 506, 166811. [Google Scholar] [CrossRef]
- Kuchi, R.; Schlagel, D.; Cozzini, T.S.; Zaikina, J.V.; Hlova, I.Z. Exploiting mechanochemical activation and molten-salt-assisted reduction-diffusion approach in bottom-up synthesis of Sm2Fe17N3. J. Alloys Compd. 2024, 980, 173532. [Google Scholar] [CrossRef]
- Liao, L.X.; Chen, X.; Altounian, Z.; Ryan, D.H. Structure and magnetic properties of R2Fe17Cx (x∼2.5). Appl. Phys. Lett. 1992, 60, 129–131. [Google Scholar] [CrossRef]
- Buschow, K.H.J. New developments in hard magnetic materials. Rep. Prog. Phys. 1991, 54, 1123–1213. [Google Scholar] [CrossRef]
- Haije, W.G.; Jacobs, T.H.; Buschow, K.H.J. Magnetic structure of ternary rare earth carbides of the type R2Fe17C. J. Less-Common Met. 1990, 163, 353–359. [Google Scholar] [CrossRef]
- Shen, B.-G.; Gong, H.-Y.; Cheng, Z.-H.; Kong, L.-S.; Wang, F.-W.; Liang, B.; Zhang, J.-X.; Guo, H.-Q.; Zhao, J.-G. Effects of Ga substitution on the hard magnetic properties of the Sm2Fe17C1.5 compounds. J. Magn. Magn. Mater. 1996, 153, 332–336. [Google Scholar] [CrossRef]
- Shen, B.-G.; Liang, B.; Wang, F.-W.; Cheng, Z.-H.; Gong, H.-Y.; Zhang, S.-Y.; Zhang, J.-X. Magnetic properties of Sm2Fe17−xSix and Sm2Fe17−xSixC compounds. J. Appl. Phys. 1995, 77, 2637–2640. [Google Scholar] [CrossRef]
- Cheng, Z.-H.; Shen, B.-G.; Zhang, J.-X.; Wang, F.-W.; Gong, H.-Y.; Zhan, W.-S.; Zhao, J.-G. Structure and magnetic anisotropy of Sm2Fe17−xAlxC (x = 2–8) compounds prepared by arc melting. J. Appl. Phys. 1994, 76, 6734–6736. [Google Scholar] [CrossRef]
- Teresiak, A.; Kubis, M.; Mattern, N.; Müller, K.-H.; Wolf, B. Crystal structure of Sm2Fe17−yMy compounds with M = Al, Si, Ga. J. Alloys Compd. 2001, 319, 168–173. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Cheng, Z.-H.; Shen, B.-G. High coercivity of melt–spun Sm2Fe15Al2C1.5 compound. J. Appl. Phys. 1996, 79, 5528–5529. [Google Scholar] [CrossRef]
- Altounian, Z.; Liu, X.B.; Girl, E. Formation, structure and hard magnetic properties of Sm2Fe17−xCoxCy compounds. J. Phys. Condens. Matter 2003, 15, 3315–3322. [Google Scholar] [CrossRef]
- Girt, E.; Altounian, Z. Origin of Fe substitutions in Nd2Fe17−δXδ. Phys. Rev. B 1998, 57, 5711–5714. [Google Scholar] [CrossRef]
- Miedema, A.R. Energy effects and charge transfer in metal physics; modelling in real space. Phys. B Condens. Matter 1992, 182, 1–17. [Google Scholar] [CrossRef]
- Sabirianov, R.F.; Jaswal, S.S. Electronic structure and magnetism in Sm2Fe17−xAx (A = Al, Ga, Si). J. Appl. Phys. 1996, 79, 5942–5944. [Google Scholar] [CrossRef]
- Steinbeck, L.; Richter, M.; Nitzsche, U.; Eschrig, H. Ab initio calculation of electronic structure, crystal field, and intrinsic magnetic properties of Sm2Fe17, Sm2Fe17N3, Sm2Fe17C3 and Sm2Co17. Phys. Rev. B 1996, 53, 7111–7127. [Google Scholar] [CrossRef]
- Pandey, T.; Du, M.-H.; Parker, D.S. Tuning the Magnetic Properties and Structural Stabilities of the 2-17-3 Magnets Sm2Fe17X3 (X=C, N) by Substituting La or Ce for Sm. Phys. Rev. Appl. 2018, 9, 034002. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef]
- Jepsen, O.; Andersen, O.K. The STUTTGART TB-LMTO program. Cell 1998. Available online: https://www2.fkf.mpg.de/andersen/LMTODOC/LMTODOC.html (accessed on 12 October 2000).
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Dovesi, R.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.; D’Arco, P.; Noël, Y.; Rérat, M.; Carbonnière, P.; et al. The CRYSTAL code, 1976–2020 and beyond, a long story. J. Chem. Phys. 2020, 152, 204111. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Ertural, C.; Hempelmann, J.; Dronskowski, R. Crystal Orbital Bond Index: Covalent Bond Orders in Solids. J. Phys. Chem. C 2021, 125, 7959–7970. [Google Scholar] [CrossRef]
- Villars, P. Sm2Fe17C (Sm2Fe17C3) Crystal Structure: Datasheet from ‘PAULING FILE Multinaries Edition–2022’ in SpringerMaterials; Springer: Berlin/Heidelberg, Germany, 2022; Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0554563 (accessed on 16 March 2024).
- Villars, P. Sm2Fe17 Crystal Structure: Datasheet from ‘PAULING FILE Multinaries Edition–2022’ in SpringerMaterials; Springer: Berlin/Heidelberg, Germany, 2022; Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0251341 (accessed on 16 March 2024).
- Koch, E.; Fischem, W. DIDO95 and VOID95–programs for the calculation of Dirichlet domains and coordination polyhedra. Z. Krist. 1996, 211, 251–253. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Corso, A.D. Pseudopotentials periodic table: From H to Pu. Comput. Mater. Sci. 2014, 95, 337–350. [Google Scholar] [CrossRef]
- Richter, M. Band structure theory of magnetism in 3d-4f compounds. J. Phys. D Appl. Phys. 1998, 31, 1017–1048. [Google Scholar] [CrossRef]
- Marzari, N.; Vanderbilt, D.; De Vita, A.; Payne, M.C. Thermal Contraction and Disordering of the Al(110) Surface. Phys. Rev. Lett. 1999, 82, 3296–3299. [Google Scholar] [CrossRef]
- Liu, X.B.; Nlebedim, I.C. Phase stability and coercivity in La2Fe14B magnet. AIP Adv. 2023, 13, 025211. [Google Scholar] [CrossRef]
- Tolman, R.C. The Principles of Statistical Mechanics; Dover Pub. Inc.: New York, NY, USA, 1979. [Google Scholar]
- Liu, X.; Nlebedim, I.C. Site Occupancy Preference and Magnetic Properties in Nd2(Fe,Co)14B. Crystals 2024, 14, 370. [Google Scholar] [CrossRef]
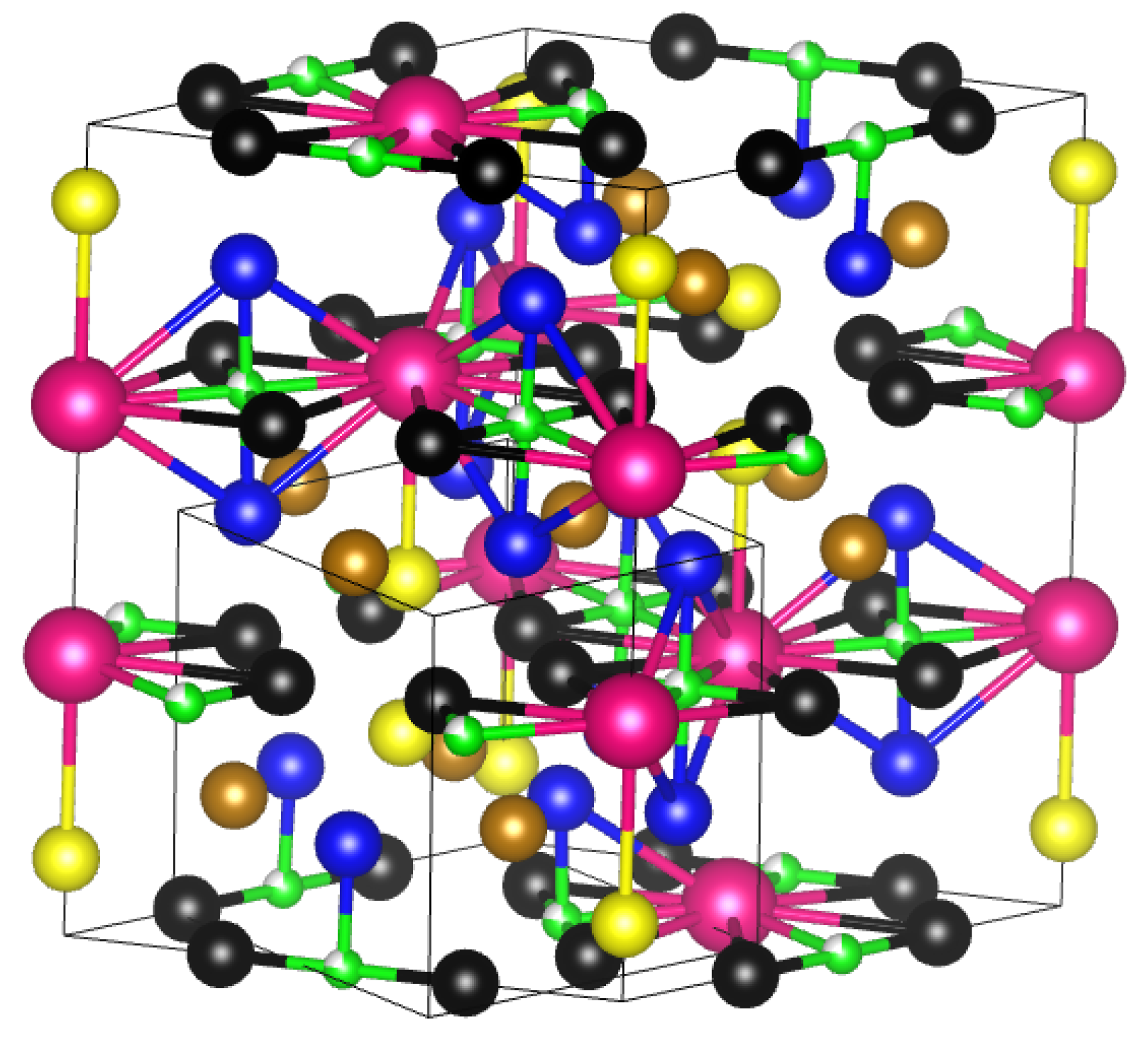

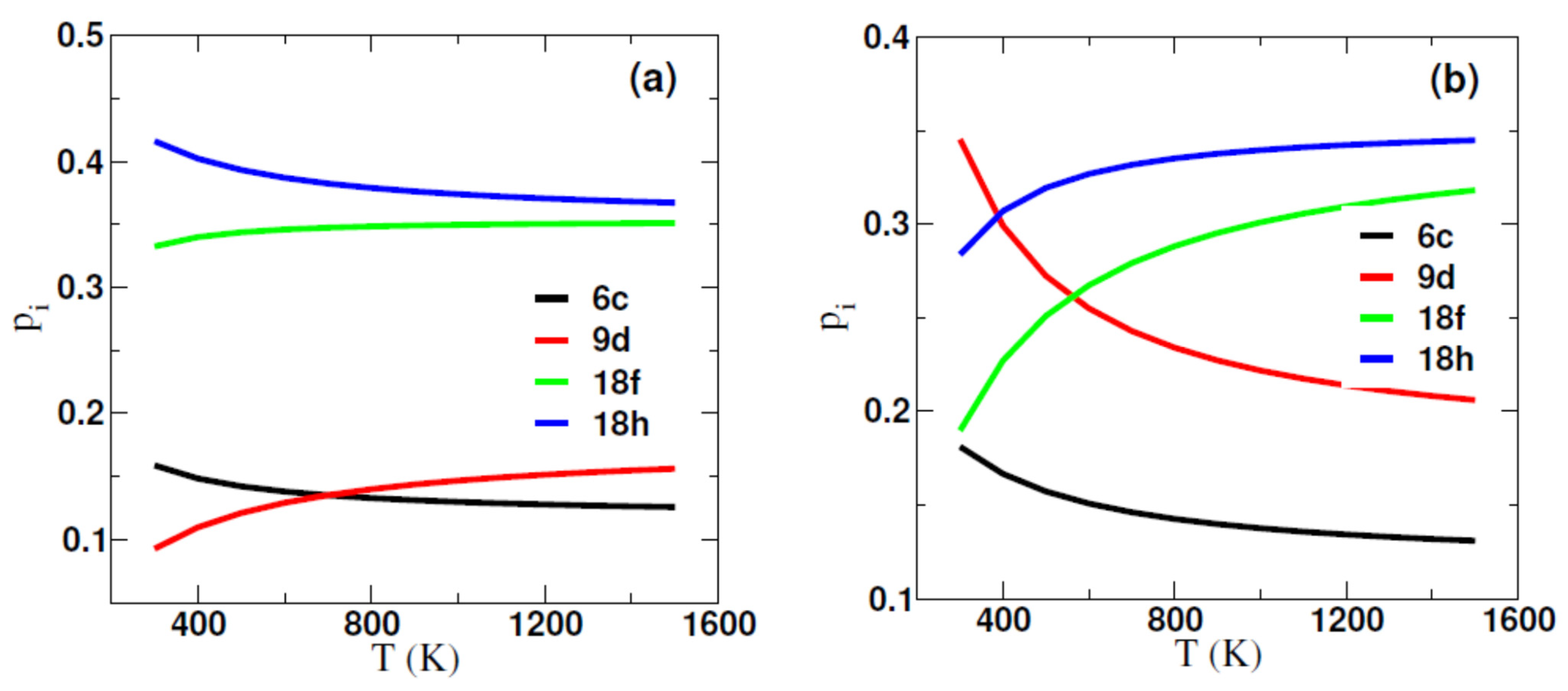
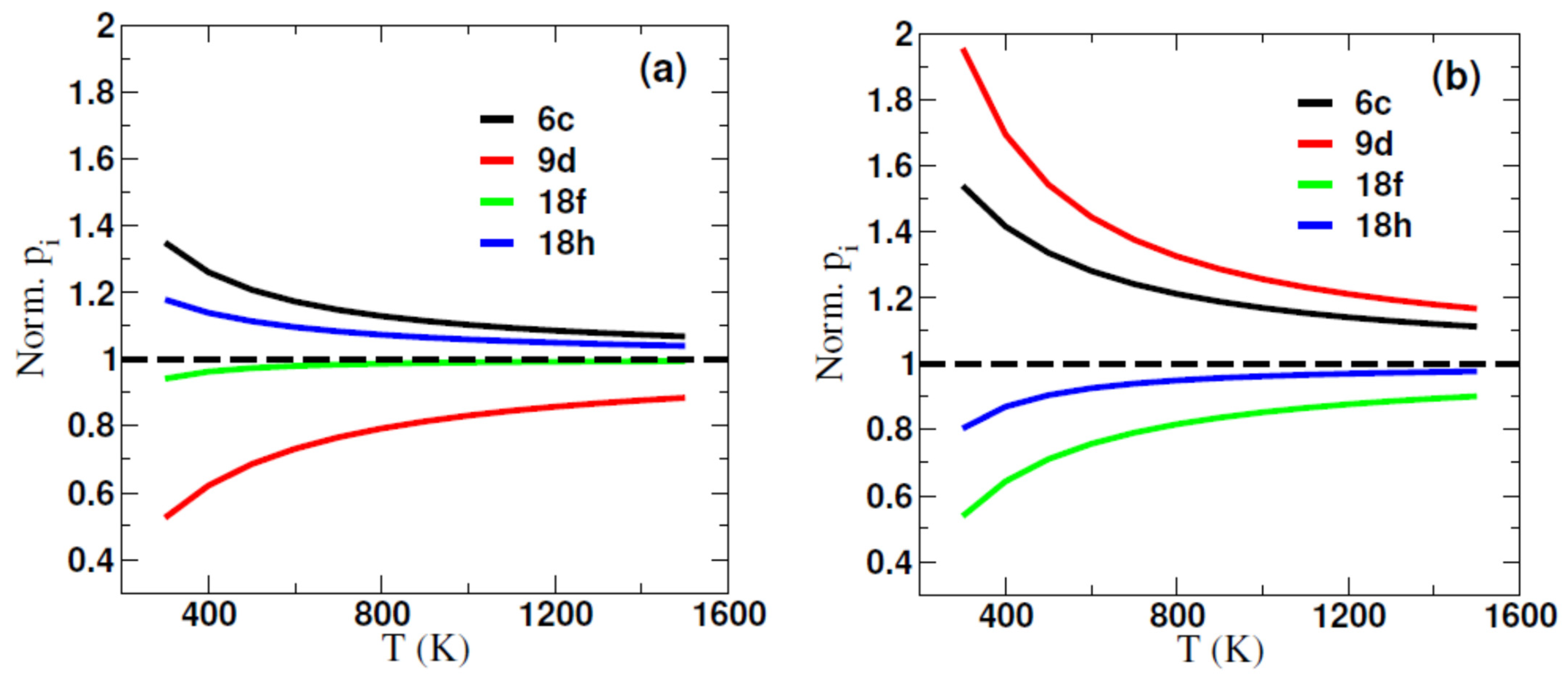
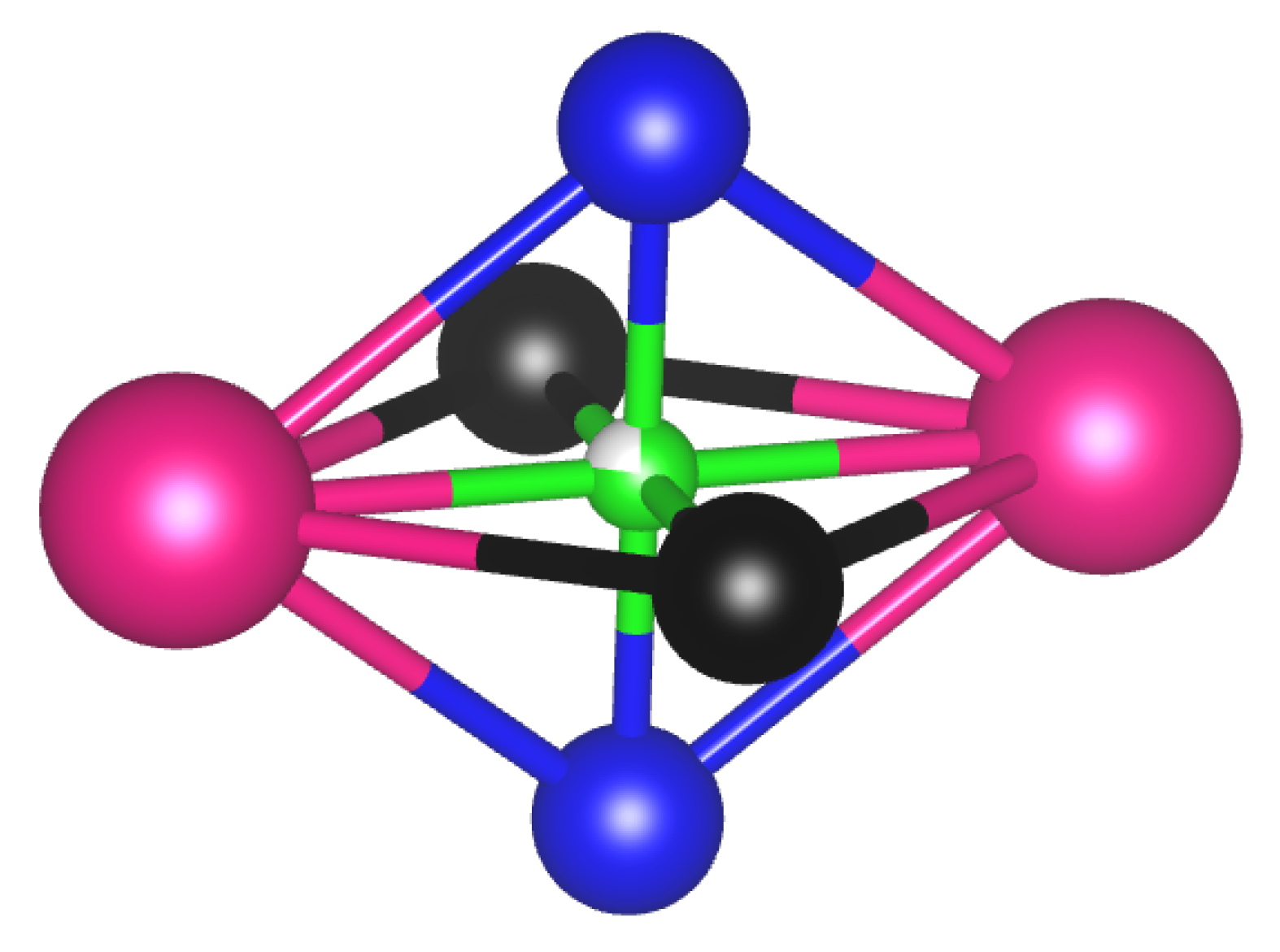


| Al@6c | Al@9d | Al@18f | Al@18h | |
|---|---|---|---|---|
| Sm2Fe16Al | −0.0996 | −0.0752 | −0.0903 | −0.0961 |
| Sm2Fe16AlC3 | −0.1904 | −0.1966 | −0.1632 | −0.1736 |
| Site | Sm2Fe17 | Sm2Fe17C3 | ||
|---|---|---|---|---|
| NN | WSV | NN | WSV | |
| Sm (6c) | 6Fe3, 1Fe1, 9Fe4, 3Fe2 | 31.7 | 3C, 3Fe4, 1Fe1, 6Fe3, 6Fe4, 3Fe2 | 32.8 |
| Fe1 (6c) | 1Fe1, 1Sm, 3Fe2, 3Fe4, 6Fe3 | 12.2 | 1Fe1, 1Sm, 3Fe4, 3Fe2, 6Fe3 | 12.8 |
| Fe2 (9d) | 4Fe3, 4Fe4, 2Fe1, 2Sm | 11.1 | 4Fe3, 4Fe4, 2Fe1, 2Sm | 11.9 |
| Fe3 (18f) | 2Fe2, 2Sm, 2Fe3, 4Fe4, 2Fe1 | 11.5 | 1C, 2Fe2, 2Fe3, 2Fe4, 2Sm, 2Fe4, 2Fe1 | 11.9 |
| Fe4 (18h) | 2Fe2, 2Fe4, 3Sm, 2Fe3, 1Fe1, 2Fe3 | 11.7 | 1C, 2Fe2, 2Fe3, 3Sm, 2Fe4, 1Fe1, 2Fe3 | 12.5 |
| C (9e) | 4Fe3, 4Fe4, 2Sm | 2.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Nlebedim, I.C. Enhanced Phase Stability of Sm2(Fe, Al)17Cx. Inorganics 2025, 13, 358. https://doi.org/10.3390/inorganics13110358
Liu X, Nlebedim IC. Enhanced Phase Stability of Sm2(Fe, Al)17Cx. Inorganics. 2025; 13(11):358. https://doi.org/10.3390/inorganics13110358
Chicago/Turabian StyleLiu, Xubo, and Ikenna C. Nlebedim. 2025. "Enhanced Phase Stability of Sm2(Fe, Al)17Cx" Inorganics 13, no. 11: 358. https://doi.org/10.3390/inorganics13110358
APA StyleLiu, X., & Nlebedim, I. C. (2025). Enhanced Phase Stability of Sm2(Fe, Al)17Cx. Inorganics, 13(11), 358. https://doi.org/10.3390/inorganics13110358






