Unraveling the Fe-Dependent Phase Evolution and Structure of Ni-Fe/γ-Al2O3 Catalysts: A Combined Experimental and Computational Study
Abstract
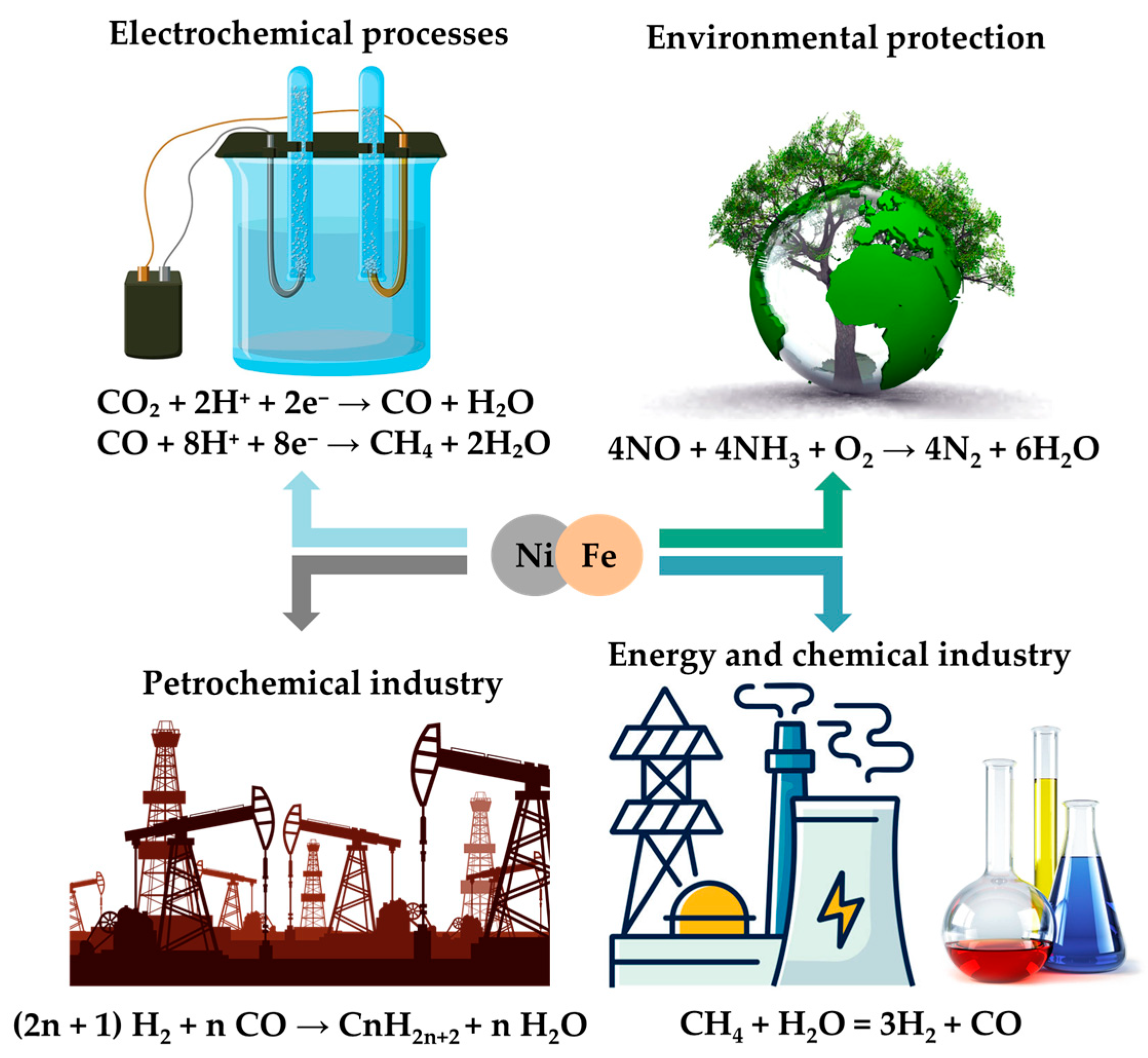
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Wang, Z.; Shen, Y.; Chen, L.; Wang, C. Achieving Sulfur Tolerance in CO2 Methanation over Ni-Based Catalysts: Protective Effects of Fe. Energy Fuels 2024, 38, 21287–21299. [Google Scholar] [CrossRef]
- Hantoko, D.; Khan, W.U.; Osman, A.I.; Nasr, M.; Rashwan, A.K.; Gambo, Y.; Shoaibi, A.A.; Chandrasekar, S. Carbon–neutral hydrogen production by catalytic methane decomposition: A review. Environ. Chem. Lett. 2024, 22, 1623–1663. [Google Scholar] [CrossRef]
- Alharthi, A.I. Nickel-iron catalyst for decomposition of methane to hydrogen and filamentous carbon: Effect of calcination and reaction temperatures. Alex. Eng. J. 2023, 67, 129–141. [Google Scholar] [CrossRef]
- Huynh, H.L.; Tucho, W.M.; Yu, X.; Yu, Z. Synthetic natural gas production from CO2 and renewable H2: Towards large-scale production of Ni–Fe alloy catalysts for commercialization. J. Clean. Prod. 2020, 264, 121720. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Fadlalla, M.I.; Babu, S.G.; Nyathi, T.M.; Weststrate, C.J.K.J.; Fischer, N.; Niemantsverdriet, J.W.H.; Claeys, M. Enhanced oxygenates formation in the Fischer–Tropsch synthesis over Co-and/or Ni-containing Fe alloys: Characterization and 2D gas chromatographic product analysis. ACS Catal. 2020, 10, 14661–14677. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Yang, Y.; Xiang, H.; Li, Y. Study on an iron–nickel bimetallic Fischer–Tropsch synthesis catalyst. Fuel Process. Technol. 2014, 118, 117–124. [Google Scholar] [CrossRef]
- Guo, F.; Jia, X.; Liang, S.; Zhou, N.; Chen, P.; Ruan, R. Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification. Bioresour. Technol. 2020, 298, 122263. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; K, L.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef]
- Putro, W.S.; Kojima, T.; Hara, T.; Ichikunia, N.; Shimazu, S. Selective hydrogenation of unsaturated carbonyls by Ni–Fe-based alloy catalysts. Catal. Sci. Technol. 2017, 7, 3637–3646. [Google Scholar] [CrossRef]
- Long, F.; Wu, S.; Chen, Y.; Cao, X.; Zhao, J.; Liu, P.; Jiang, J.; Zhang, X.; Xu, J. Hydrogenation of fatty acids to fatty alcohols over Ni3Fe nanoparticles anchored on TiO2 crystal catalyst: Metal support interaction and mechanism investigation. Chem. Eng. J. 2023, 464, 142773. [Google Scholar] [CrossRef]
- Nie, L.; de Souza, P.M.; Noronha, F.B.; An, W.; Sooknoi, T.; Resasco, D.E. Selective conversion of m-cresol to toluene over bimetallic Ni–Fe catalysts. J. Mol. Catal. A: Chem. 2014, 388, 47–55. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ariyama, K.; Furukawa, S.; Takayama, T.; Komatsu, T. Chemoselective hydrogenation of nitroarenes using Ni−Fe alloy catalysts at ambient pressure. ChemistrySelect 2021, 6, 5538–5544. [Google Scholar] [CrossRef]
- Petkar, D.R.; Kadu, B.S.; Chikate, R.C. Highly efficient and chemoselective transfer hydrogenation of nitroarenes at room temperature over magnetically separable Fe–Ni bimetallic nanoparticles. RSC Adv. 2014, 4, 8004–8010. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Yu, S.; Zhu, H.; Du, Y.; Zhang, Z.; You, C.; Jiang, X.; Jiang, J. Fe-promoted Ni catalyst with extremely high loading and oxygen vacancy for lipid deoxygenation into green diesel. Renew. Energy 2022, 197, 40–49. [Google Scholar] [CrossRef]
- Wang, X.; Ding, C.; Long, H.; Wu, Y.; Zhao, H.; Jiang, F.; Chang, R.; Xue, S.; Shen, M.; Yang, X. Catalytic reduction of nitrogen monoxide using iron–nickel oxygen carriers derived from electroplating sludge: Novel method for the collaborative emission decrease of polluting gases. Sci. Total Environ. 2024, 927, 172315. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Valenzuela, M.A. Ni-based catalysts used in heterogeneous catalytic ozonation for organic pollutant degradation: A minireview. Environ. Sci. Pollut. Res. 2022, 29, 84056–84075. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Mokhtar, A.; Hachemaoui, M.; Beldjilali, M.; Meliani, M.F.; Zahmani, H.H.; Hacini, S.; Boukoussa, B. Fe and Ni nanoparticles-loaded zeolites as effective catalysts for catalytic reduction of organic pollutants. Microporous Mesoporous Mater. 2021, 310, 110597. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhi, M.; Hong, Z. Development trends on nickel-based electrocatalysts for direct hydrazine fuel cells. ChemCatChem 2021, 13, 81–110. [Google Scholar] [CrossRef]
- Zeng, Z.; Gan, L.Y.; Bin Yang, H.; Su, X.; Gao, J.; Liu, W.; Matsumoto, H.; Gong, J.; Zhang, J.; Cai, W.; et al. Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution. Nat. Commun. 2021, 12, 4088. [Google Scholar] [CrossRef]
- Yan, P.; Peng, H.; Zhang, X.; Rabiee, H.; Ahmed, M.; Weng, Y.; Rozhkovskaya, A.; Vogrin, J.; Konarova, M.; Zhu, Z. Unlocking the role of Ni-Fe species in CO2 methanation. Fuel 2024, 374, 132373. [Google Scholar] [CrossRef]
- Medina, O.E.; Amell, A.A.; López, D.; Santamaría, A. Comprehensive review of nickel-based catalysts advancements for CO2 methanation. Renew. Sustain. Energy Rev. 2025, 207, 114926. [Google Scholar] [CrossRef]
- Görlin, M.; Ferreira de Araújo, J.; Schmies, H.; Bernsmeier, D.; Dresp, S.; Gliech, M.; Jusys, Z.; Chernev, P.; Kraehnert, R.; Dau, H.; et al. Tracking catalyst redox states and reaction dynamics in Ni–Fe oxyhydroxide oxygen evolution reaction electrocatalysts: The role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 2017, 139, 2070–2082. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Yi, C.; Yang, H.; Hou, X.; Liao, X.; Chen, C.; Yu, D.; Zhou, X. An efficient NiFe binary alloy anode catalyst for direct borohydride fuel cells. Chem. Eng. J. 2023, 472, 145097. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Hu, E.; Hong, R.; Li, T.; Yuan, X.; Cheng, X.B.; Cai, N.; Xiao, R.; Zhang, H. Nickel-iron nanoparticles encapsulated in carbon nanotubes prepared from waste plastics for low-temperature solid oxide fuel cells. iScience 2022, 25, 104855. [Google Scholar] [CrossRef]
- Van Loon, J.; Kubarev, A.V.; Roeffaers, M.B.J. Correlating catalyst structure and activity at the nanoscale. ChemNanoMat 2018, 4, 6–14. [Google Scholar] [CrossRef]
- Venezia, A.M.; La Parola, V.; Liotta, L.F. Structural and surface properties of heterogeneous catalysts: Nature of the oxide carrier and supported particle size effects. Catal. Today 2017, 285, 114–124. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R.; Valle, M. Crystal structure prediction using evolutionary approach. In Modern Methods of Crystal Structure Prediction; Oganov, A.R., Ed.; Wiley-VCH, Weinheim: Berlin, Germany, 2010; pp. 147–180. [Google Scholar] [CrossRef]
- Oganov, A.R.; Kruglov, I.; Zhang, J.; Mahdi Davari Esfahani, M. Computational materials discovery using evolutionary algorithms. In Computational Materials Discovery; Oganov, A.R., Saleh, G., Kvashnin, A.G., Eds.; Special Collection: 2018 eBook collection; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 15–65. [Google Scholar] [CrossRef]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How Evolutionary Crystal Structure Prediction Works–and Why. Acc. Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.F. Synthesis, catalysis, surface chemistry and structure of bimetallic nanocatalysts. Chem. Soc. Rev. 2012, 41, 7977–7979. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Jiang, H.L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Campbell, C.T. The energetics of supported metal nanoparticles: Relationships to sintering rates and catalytic activity. Acc. Chem. Res. 2013, 46, 1712–1719. [Google Scholar] [CrossRef]
- Gu, C.; Chen, L.; Liu, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Wang, C.; Ma, L. One-pot conversion of biomass-derived levulinic acid to furanic biofuel 2-methyltetrahydrofuran over bimetallic NiCo/γ-Al2O3 catalysts. Mol. Catal. 2022, 524, 112317. [Google Scholar] [CrossRef]
- Kouachi, K.; Lafaye, G.; Pronier, S.; Bennini, L.; Menad, S. Mo/γ-Al2O3 catalysts for the Biginelli reaction. Effect of Mo loading. J. Mol. Catal. A Chem. 2014, 395, 210–216. [Google Scholar] [CrossRef]
- Pandey, A.; Bjurström, A.; Birdsong, B.K.; Arvidsson, R.; Dezfoli, P.R.; Tjus, K.; Andrée, S.; Sädbom, S.; Björk, A.; Olsson, R.T. Carbon fibres as electrodes for the recovery of nickel from industrial wastewater. RSC Appl. Interfaces 2025, 2, 1031–1040. [Google Scholar] [CrossRef]
- Judanova, L.I.; Logvinenko, V.A.; Judanov, N.F. Homogeneous Nanoparticles of Nickel Coated with Shell and Metod of Their Production. R.U. Patent RU2466098C1, 29 March 2011. [Google Scholar]
- Asaldoust, F.; Mabhouti, K.; Jafari, A.; Taleb-Abbasi, M. Structural, magnetic, and optical characteristics of undoped and chromium, iron, cobalt, copper, and zinc doped nickel oxide nanopowders. Sci. Rep. 2025, 15, 1088. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kim, Y.S.; Pawar, S.M.; Kim, J.H.; Im, H.; Kim, H. Chemically grown, porous, nickel oxide thin-film for electrochemical supercapacitors. J. Power Sources 2011, 196, 2393–2397. [Google Scholar] [CrossRef]
- Zeifert, B.H.; Salmones, J.; Hernández, J.A.; Reynoso, R.; Nava, N.; Reguera, E.; Cabañas-Moreno, J.G.; Aguilar-Ríos, G. X-ray diffraction and Mössbauer characterization of Raney Fe-Ni catalysts. J. Radioanal. Nucl. Chem. 2000, 245, 637–639. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Sepúlveda-Escribano, A.; Rodriguez-Ramos, I. Carbon supported bimetallic catalysts containing iron: I. Preparation and characterization. Appl. Catal. A Gen. 1992, 81, 81–100. [Google Scholar] [CrossRef]
- Boretskaya, A.; Il’yasov, I.D.; Lamberov, A.; Popov, A. Identification of amorphous and crystalline phases in alumina entity and their contribution to the properties of the palladium catalyst. Appl. Surf. Sci. 2019, 496, 143635. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Phase Diagram Creation. Available online: https://oqmd.org/analysis/phase_diagram/ (accessed on 11 June 2025).
- Automatic-Flow for Materials Discovery. Available online: https://www.aflowlib.org/aflow-chull/ (accessed on 11 June 2025).
- Silman, G.I. Compilative Fe–Ni phase diagram with author’s correction. Met. Sci. Heat. Treat. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Crystallography Open Database. Available online: http://www.crystallography.net/cod/result.php (accessed on 11 June 2025).
- Barber, C.; Dobkin, D.; Huhdanpaa, H. The quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef]
- Stevanović, V.; Lany, S.; Zhang, X.; Zunger, A. Correcting density functional theory for accurate predictions of compound enthalpies of formation: Fitted elemental-phase reference energies. Phys. Rev. B 2012, 85, 115104. [Google Scholar] [CrossRef]
- Sandu, M.P.; Sidelnikov, V.S.; Geraskin, A.A.; Chernyavskii, A.V.; Kurzina, I.A. Influence of the method of preparation of the Pd-Bi/Al2O3 catalyst on catalytic properties in the reaction of liquid-phase oxidation of glucose into gluconic acid. Catalysts 2020, 10, 271. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef]
- Abzaev, Y.A.; Guda, S.A.; Guda, A.A.; Zelenkov, A.A.; Kolesnikov, V.I. Structural Phase State of High-Entropy NbTiHfVZr Alloy. Phys. Met. Metallogr. 2023, 124, 807–815. [Google Scholar] [CrossRef]
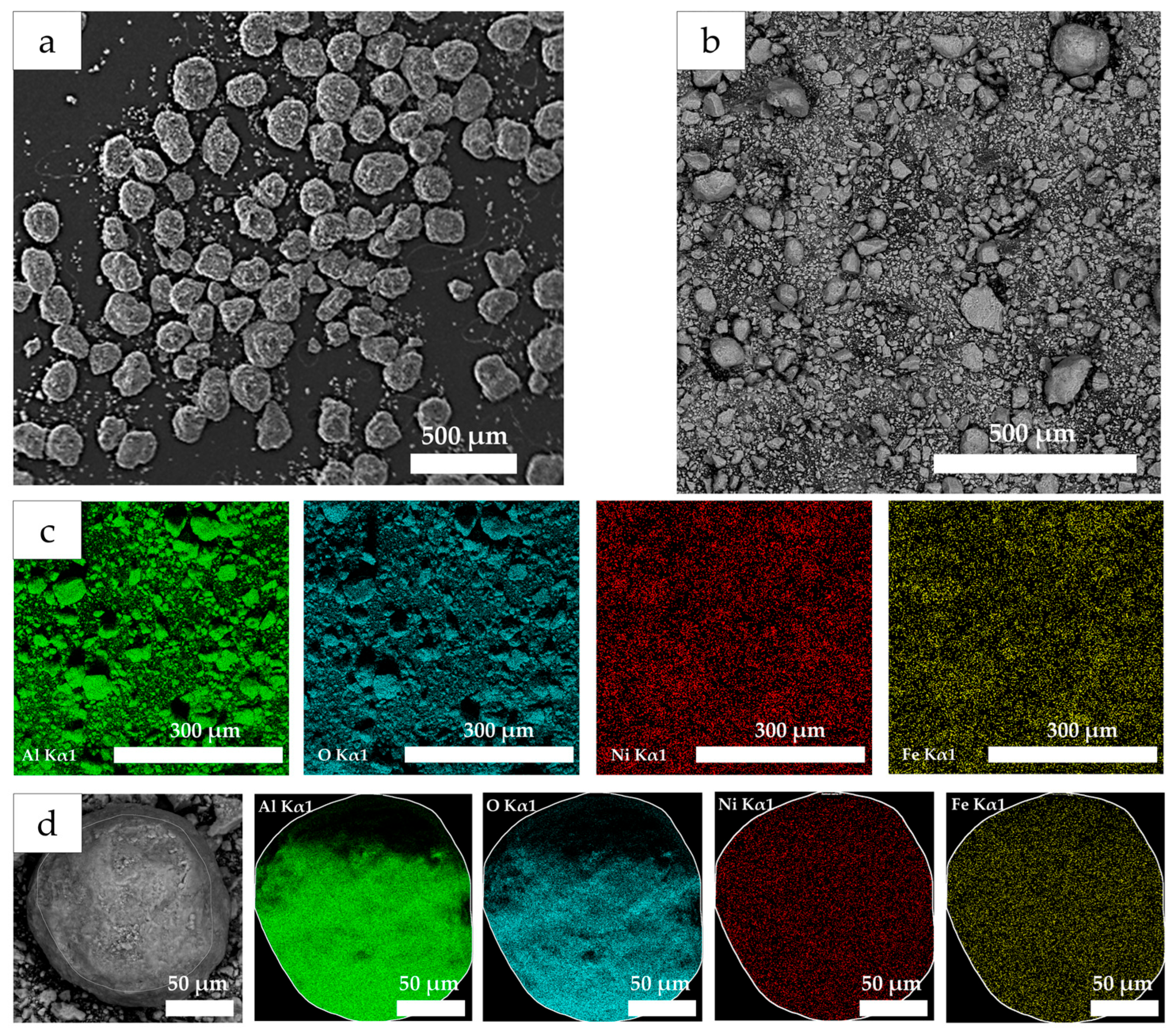
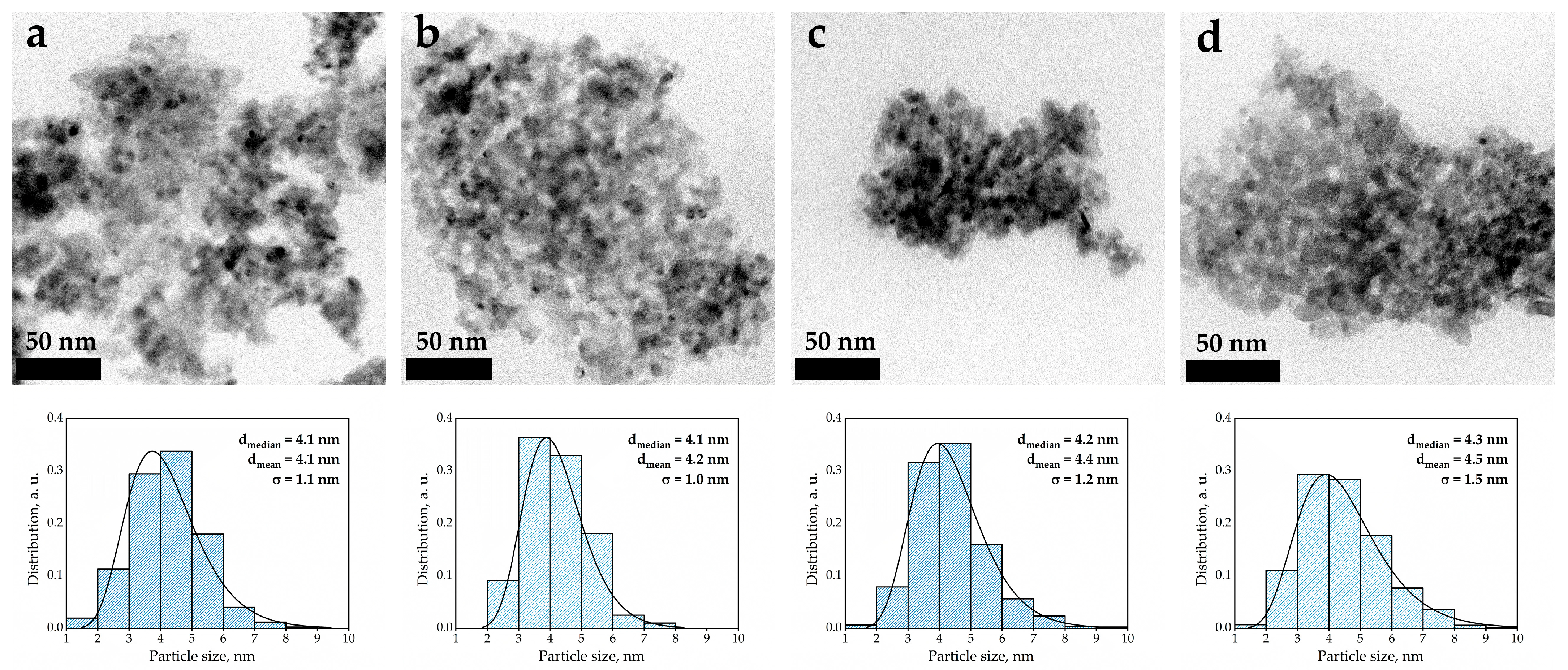
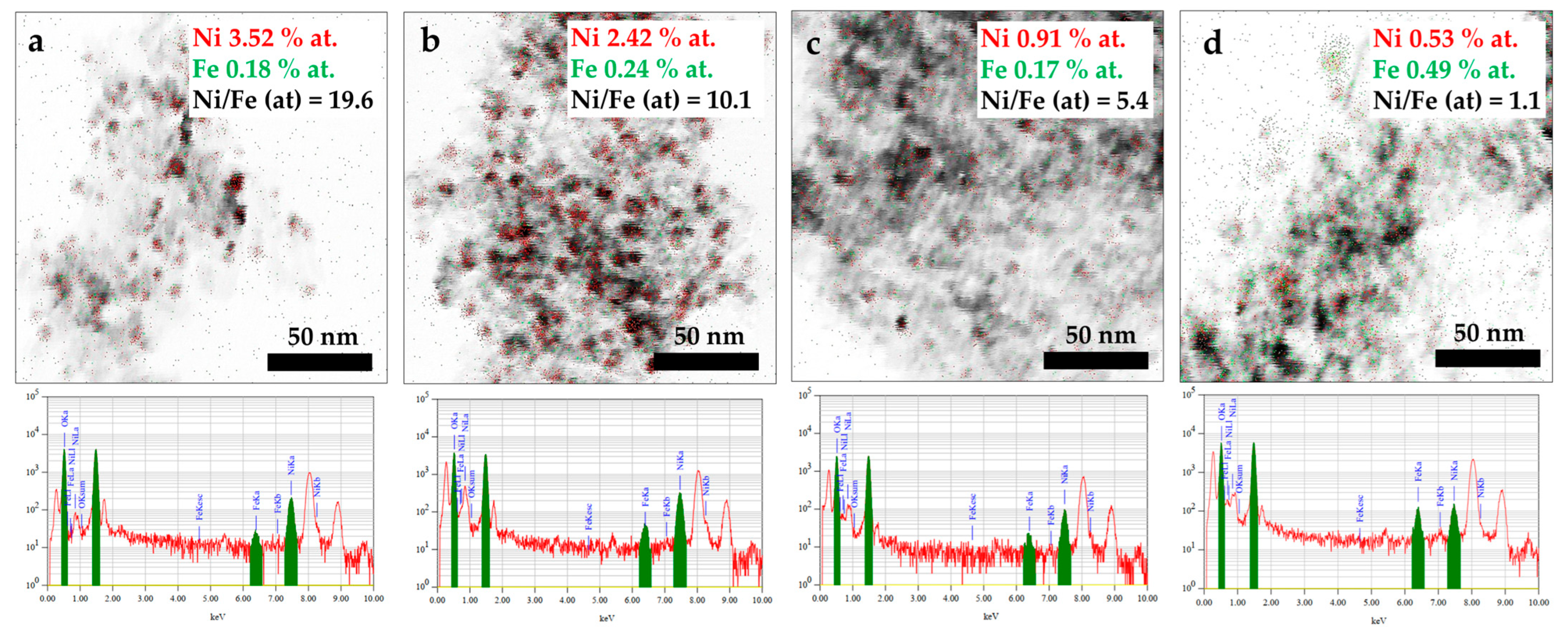


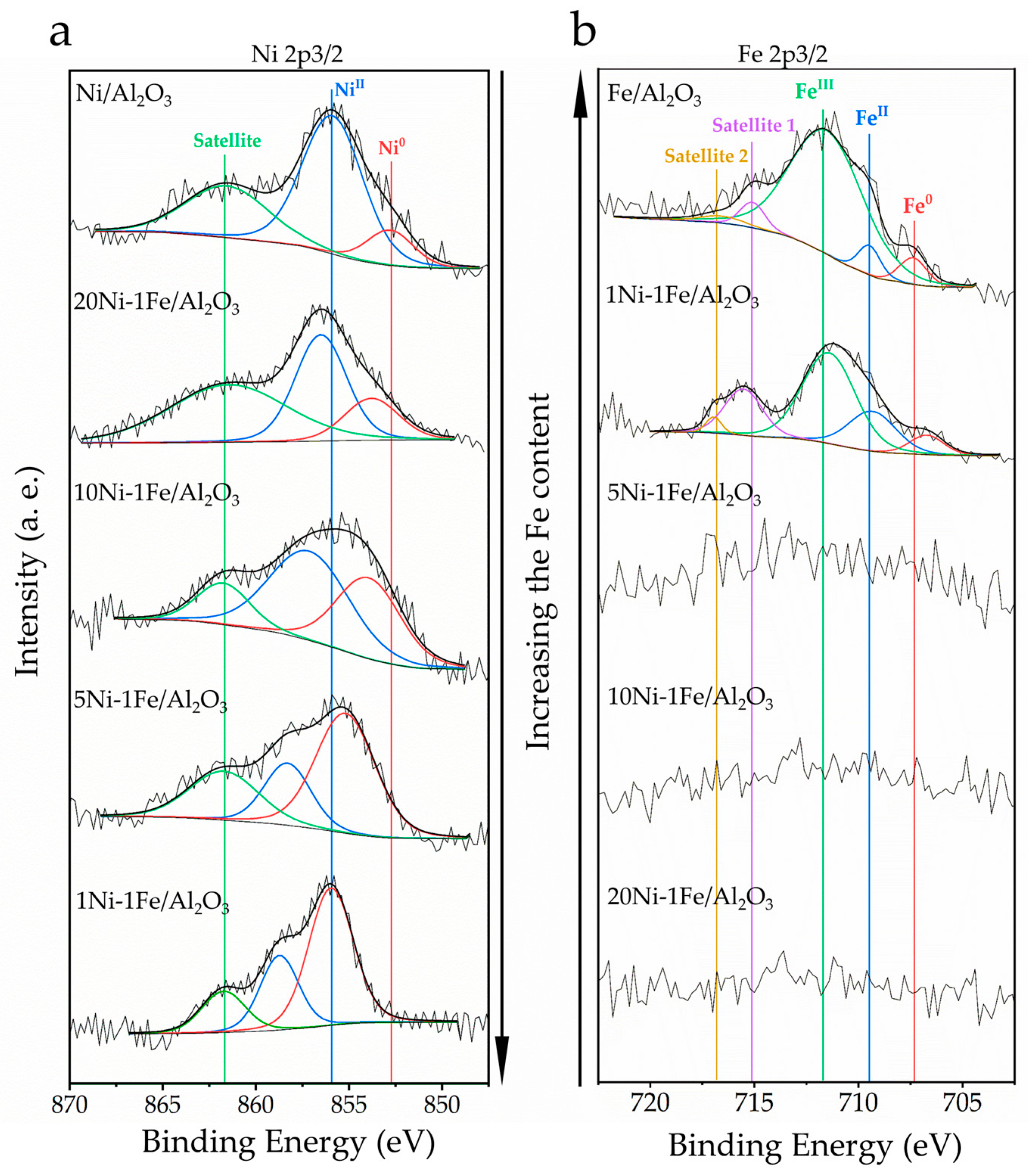

| Sample | Metal Content (wt.%) | Total Metal Content (Ni + Fe) (wt.%) | Ni/Fe (Atomic Ratio) | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | |
|---|---|---|---|---|---|---|---|
| Ni | Fe | ||||||
| γ-Al2O3 | - | - | - | - | 138 | 0.30 | 6.0 |
| 1Ni-1Fe/Al2O3 | 2.29 | 2.13 | 4.42 | 1.0 | 132 | 0.28 | 6.3 |
| 5Ni-1Fe/Al2O3 | 4.43 | 0.71 | 5.14 | 5.4 | 136 | 0.28 | 6.0 |
| 10Ni-1Fe/Al2O3 | 4.99 | 0.08 | 5.07 | 9.9 | 138 | 0.29 | 6.0 |
| 20Ni-1Fe/Al2O3 | 5.29 | 0.03 | 5.32 | 18.6 | 138 | 0.29 | 6.0 |
| Ni/Al2O3 | 4.65 | - | 4.65 | - | 137 | 0.28 | 6.0 |
| Fe/Al2O3 | - | 4.80 | 4.80 | - | 123 | 0.25 | 6.3 |
| Sample | Measured d-Spacing (Å) | Intensity | Phase Assignment | Reference d-Spacing (Å) | Δd (%) | Description | ICDD Reference (PDF#) |
|---|---|---|---|---|---|---|---|
| 20Ni-1Fe/Al2O3 | 2.44 | High | γ-Al2O3 (311) | 2.40 | −1.6% | Support background | 00-010-0425 |
| 2.03 | High | Ni (111) | 2.03 | 0% | Main phase | 00-004-0850 | |
| 1.43 | High | α-Fe (200) | 1.43 | 0% | Local Fe clusters | 00-006-0696 | |
| 1.17 | Weak | α-Fe (211) | 1.17 | 0% | Local Fe clusters | 00-006-0696 | |
| 1.01 | Very weak | Ni (222) | 1.04 | +2.9% | High-order reflection | 00-004-0850 | |
| 10Ni-1Fe/Al2O3 | 2.46 | High | γ-Al2O3 (311) | 2.40 | −2.4% | Support background | 00-010-0425 |
| 2.03 | High | Ni (111) | 2.03 | 0% | Main phase | 00-004-0850 | |
| 1.80 | Spotty | FeNi (200) | 1.79 | −0.5% | Fe dissolution in Ni/FeNi formation | 00-047-1405 | |
| 1.43 | High | α-Fe (200) | 1.43 | 0% | Main phase | 00-006-0696 | |
| 1.28 | Spotty | FeNi (220) | 1.27 | −0.8% | Confirms FeNi | 00-047-1405 | |
| 1.17 | Very weak | α-Fe (211) | 1.17 | 0% | Confirms Fe | 00-006-0696 | |
| 5Ni-1Fe/Al2O3 | 2.43 | High | γ-Al2O3 (311) | 2.40 | −1.2% | Support background | 00-010-0425 |
| 2.07 | High | FeNi (111) | 2.03/2.07 | −2.0%/0% | Main phase | 00-047-1405 | |
| 1.42 | High | α-Fe (200) | 1.43 | +0.7% | Main phase | 00-006-0696 | |
| 1.29 | Weak | FeNi (220) | 1.27 | −1.6% | Confirms FeNi | 00-047-1405 | |
| 1.17 | Spotty | α-Fe (211) | 1.17 | 0% | Confirms Fe | 00-006-0696 | |
| 1Ni-1Fe/Al2O3 | 2.42 | High | γ-Al2O3 (311) | 2.40 | −0.8% | Support background | 00-010-0425 |
| 2.07 | High | FeNi (111) | 2.07 | 0% | Main phase | 00-047-1405 | |
| 1.43 | High | α-Fe (200) | 1.43 | 0% | Main phase | 00-006-0696 | |
| 1.18 | Weak | α-Fe (211) | 1.17 | −0.8% | Confirms Fe | 00-006-0696 | |
| 1.03 | Weak | FeNi (222) | 1.04 | +1.0% | Confirms FeNi | 00-047-1405 |
| Sample | Detected Phases | Description |
|---|---|---|
| 20Ni-1Fe/Al2O3 | γ-Al2O3 | Support |
| Ni | Main phase | |
| α-Fe | Local clusters | |
| 10Ni-1Fe/Al2O3 | γ-Al2O3 | Support |
| Ni | Main phase | |
| FeNi intermetallic/Fe-Ni solid solution | Initial formation | |
| α-Fe | Main phase | |
| 5Ni-1Fe/Al2O3 | γ-Al2O3 | Support |
| FeNi intermetallic | Confirmed phase | |
| α-Fe | Main phase | |
| 1Ni-1Fe/Al2O3 | γ-Al2O3 | Support |
| FeNi intermetallic | Main phase | |
| α-Fe | Main phase |
| Sample | Binding Energy, eV | Contribution of State, % |
|---|---|---|
| Ni/Al2O3 | Ni0 852.8 | 16.5 |
| NiII 855.9 | 83.5 | |
| Fe/Al2O3 | Fe0 707.4 | 7.0 |
| FeII 709.5 | 6.4 | |
| FeIII 711.5 | 86.6 | |
| 1Ni-1Fe/Al2O3 | Ni0 855.7 | 68.2 |
| NiII 858.7 | 31.8 | |
| Fe0 706.7 | 9.0 | |
| FeII 709.2 | 25.6 | |
| FeIII 711.2 | 65.3 | |
| 5Ni-1Fe/Al2O3 | Ni0 855.2 | 71.1 |
| NiII 858.3 | 28.9 | |
| 10Ni-1Fe/Al2O3 | Ni0 854.0 | 42.2 |
| NiII 857.1 | 57.8 | |
| 20Ni-1Fe/Al2O3 | Ni0 853.8 | 29.5 |
| NiII 856.5 | 70.5 |
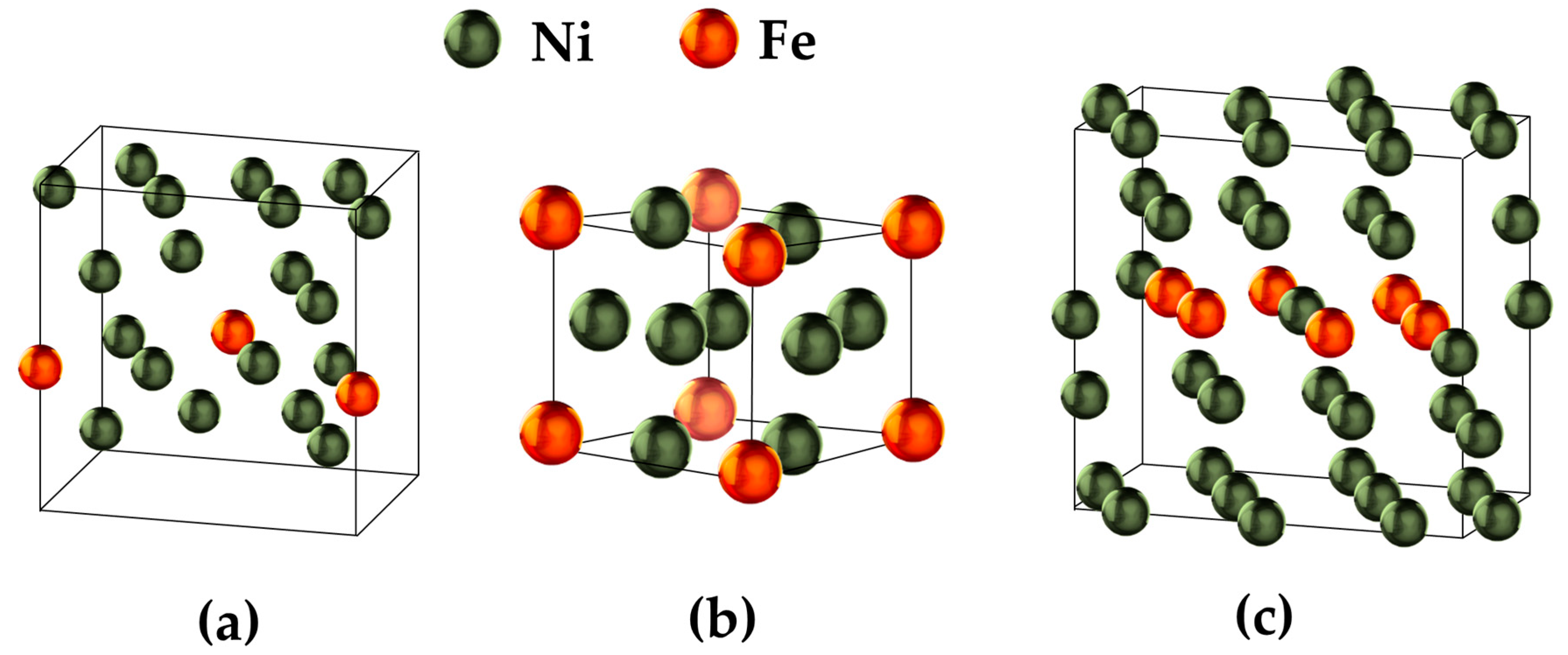
| Structure | Composition | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Space Group |
|---|---|---|---|---|---|---|---|---|
| S-1 | Ni20Fe2 | 7.372 | 5.493 | 6.502 | 114.93 | 78.99 | 77.16 | P1, Triclinic |
| S-2 | Ni11Fe10 | 6.111 | 6.914 | 6.132 | 100.02 | 94.01 | 103.62 | P1, Triclinic |
| S-3 | Ni5Fe11 | 6.938 | 6.938 | 3.455 | 90.00 | 90.00 | 90.00 | P4/mmm, Tetragonal |
| S-4 | Ni5Fe11 | 15.922 | 2.464 | 8.516 | 95.35 | 90.00 | 90.00 | 8, CmMonoclinic |
| Structure | Composition | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Space Group |
|---|---|---|---|---|---|---|---|---|
| S-5 | Ni9Fe | 4.163 | 7.330 | 7.987 | 90.00 | 99.80 | 90.00 | 8, Cm Monoclinic |
| S-6 | Ni5Fe | 4.277 | 4.277 | 4.058 | 90.00 | 90.00 | 120.00 | 189, P-62m, Hexagonal |
| S-7 | Ni10Fe2 | 4.283 | 7.420 | 8.184 | 90.00 | 80.13 | 90.00 | 12 C2/m, Monoclinic |
| Material ID | Formula | Functional | Enthalpy Formation (eV/Atom) | Temperature (K) |
|---|---|---|---|---|
| mp-13 | Fe | GGA | 0 | 0 |
| mp-2213 | FeNi | GGA | −0.076 | |
| mp-1418 | FeNi3 | GGA | −0.097 | |
| mp-23 | Ni | GGA | 0 | |
| mp-13 | Fe | GGA | 0 | 300 |
| mp-2213 | FeNi | GGA | −0.079 | |
| mp-1418 | FeNi3 | GGA | −0.096 | |
| mp-23 | Ni | GGA | 0 | |
| mp-13 | Fe | GGA | 0 | 700 |
| mp-2213 | FeNi | GGA | −0.079 | |
| mp-1418 | FeNi3 | GGA | −0.094 | |
| mp-23 | Ni | GGA | 0 | |
| mp-13 | Fe | GGA | 0 | 900 |
| mp-2213 | FeNi | GGA | −0.025 | |
| mp-1418 | FeNi3 | GGA | −0.045 | |
| mp-23 | Ni | GGA | 0 | |
| mp-13 | Fe | GGA | 0 | 1500 |
| mp-2213 | FeNi | GGA | +0.008 | |
| mp-1418 | FeNi3 | GGA | −0.014 | |
| mp-23 | Ni | GGA | 0 | |
| mp-13 | Fe | GGA | 0 | 1700 |
| mp-2213 | FeNi | GGA | +0.03 | |
| mp-1418 | FeNi3 | GGA | +0.009 | |
| mp-23 | Ni | GGA | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulevich, S.A.; Shcherbakova-Sandu, M.P.; Meshcheryakov, E.P.; Abzaev, Y.A.; Guda, S.A.; Kumar, R.; Sonwane, A.K.; Samal, S.; Kushwaha, A.K.; Kurzina, I.A. Unraveling the Fe-Dependent Phase Evolution and Structure of Ni-Fe/γ-Al2O3 Catalysts: A Combined Experimental and Computational Study. Inorganics 2025, 13, 349. https://doi.org/10.3390/inorganics13110349
Gulevich SA, Shcherbakova-Sandu MP, Meshcheryakov EP, Abzaev YA, Guda SA, Kumar R, Sonwane AK, Samal S, Kushwaha AK, Kurzina IA. Unraveling the Fe-Dependent Phase Evolution and Structure of Ni-Fe/γ-Al2O3 Catalysts: A Combined Experimental and Computational Study. Inorganics. 2025; 13(11):349. https://doi.org/10.3390/inorganics13110349
Chicago/Turabian StyleGulevich, Semyon A., Mariya P. Shcherbakova-Sandu, Eugene P. Meshcheryakov, Yurij A. Abzaev, Sergey A. Guda, Ritunesh Kumar, Akshay K. Sonwane, Sonali Samal, Ajay K. Kushwaha, and Irina A. Kurzina. 2025. "Unraveling the Fe-Dependent Phase Evolution and Structure of Ni-Fe/γ-Al2O3 Catalysts: A Combined Experimental and Computational Study" Inorganics 13, no. 11: 349. https://doi.org/10.3390/inorganics13110349
APA StyleGulevich, S. A., Shcherbakova-Sandu, M. P., Meshcheryakov, E. P., Abzaev, Y. A., Guda, S. A., Kumar, R., Sonwane, A. K., Samal, S., Kushwaha, A. K., & Kurzina, I. A. (2025). Unraveling the Fe-Dependent Phase Evolution and Structure of Ni-Fe/γ-Al2O3 Catalysts: A Combined Experimental and Computational Study. Inorganics, 13(11), 349. https://doi.org/10.3390/inorganics13110349








