Effect of CaO/SiO2 and MgO/Al2O3 on the Metallurgical Properties of Low Boron-Bearing High-Alumina Slag
Abstract
1. Introduction
2. Results and Discussion
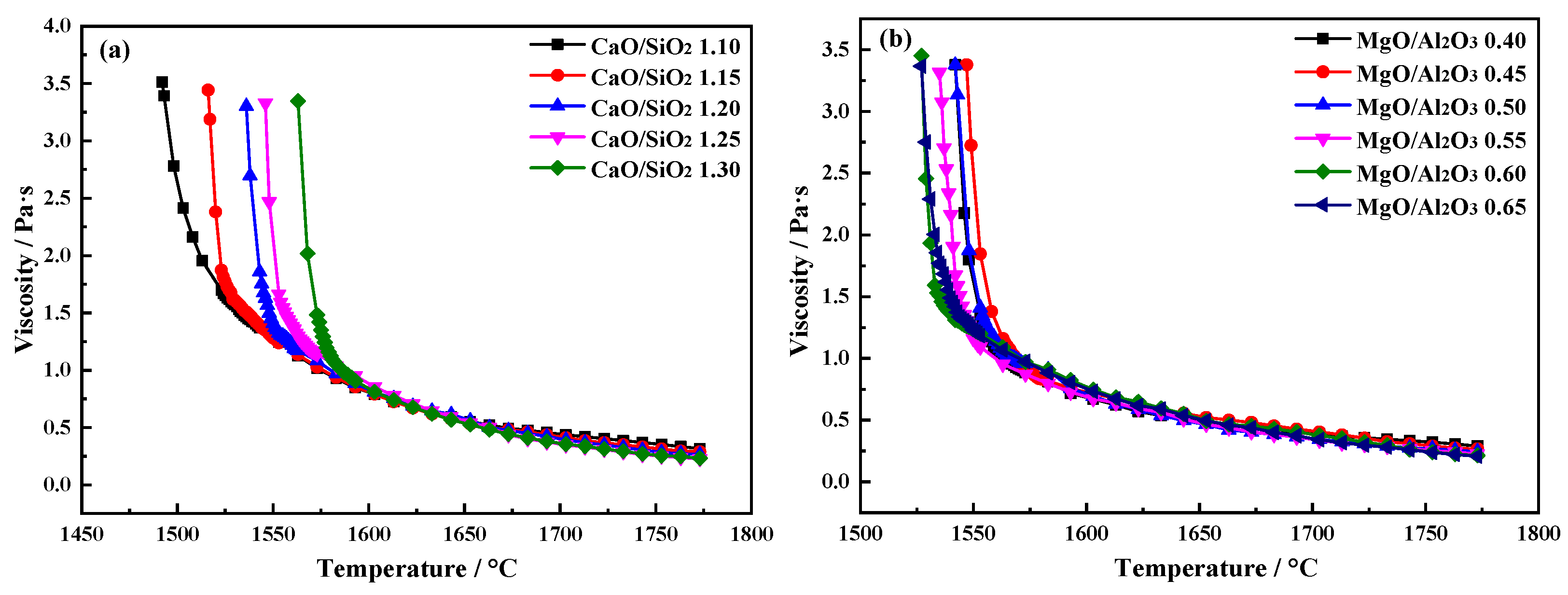
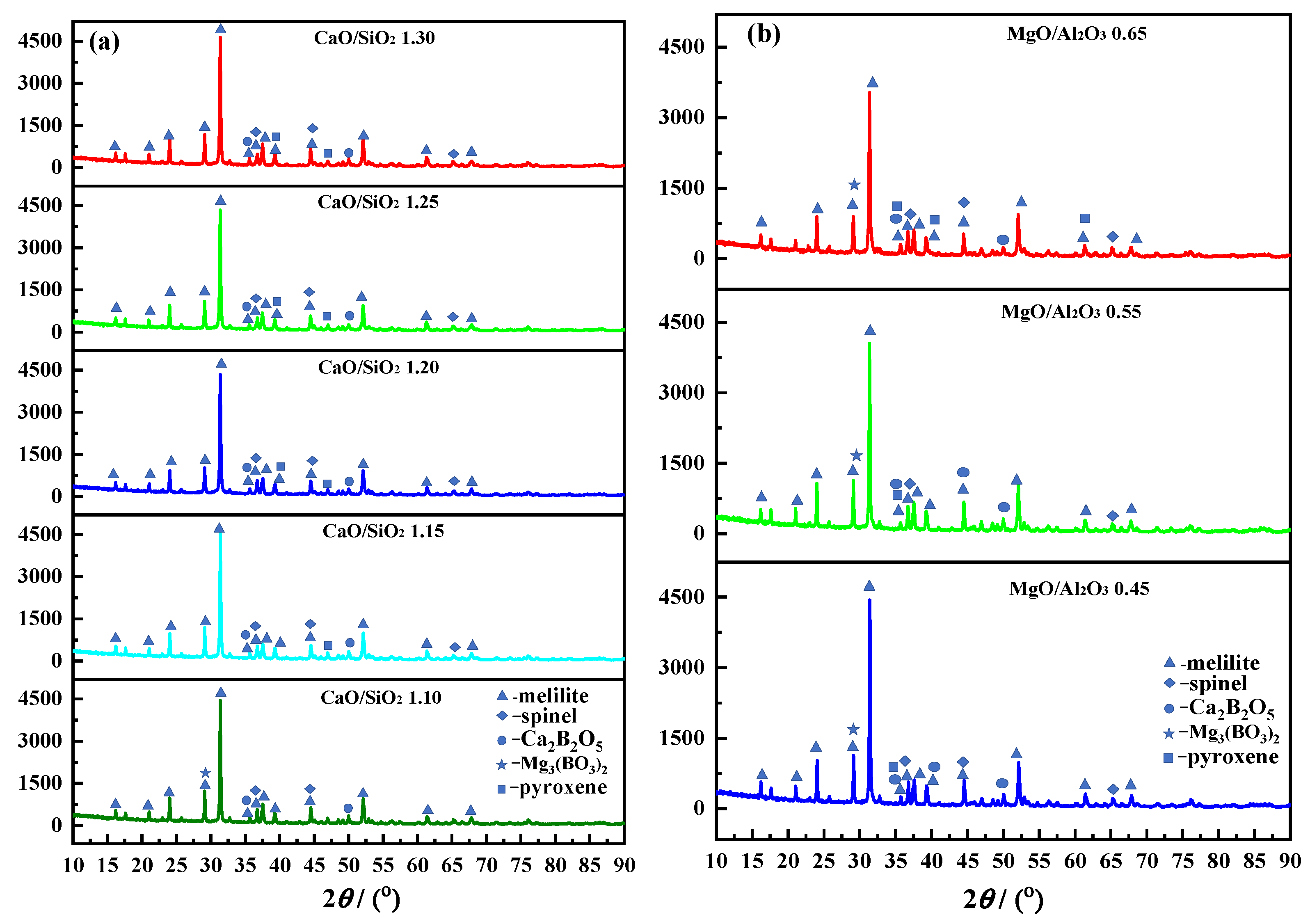
2.1. Effects of CaO/SiO2 and MgO/Al2O3 on the Viscous Behaviors of the Slag
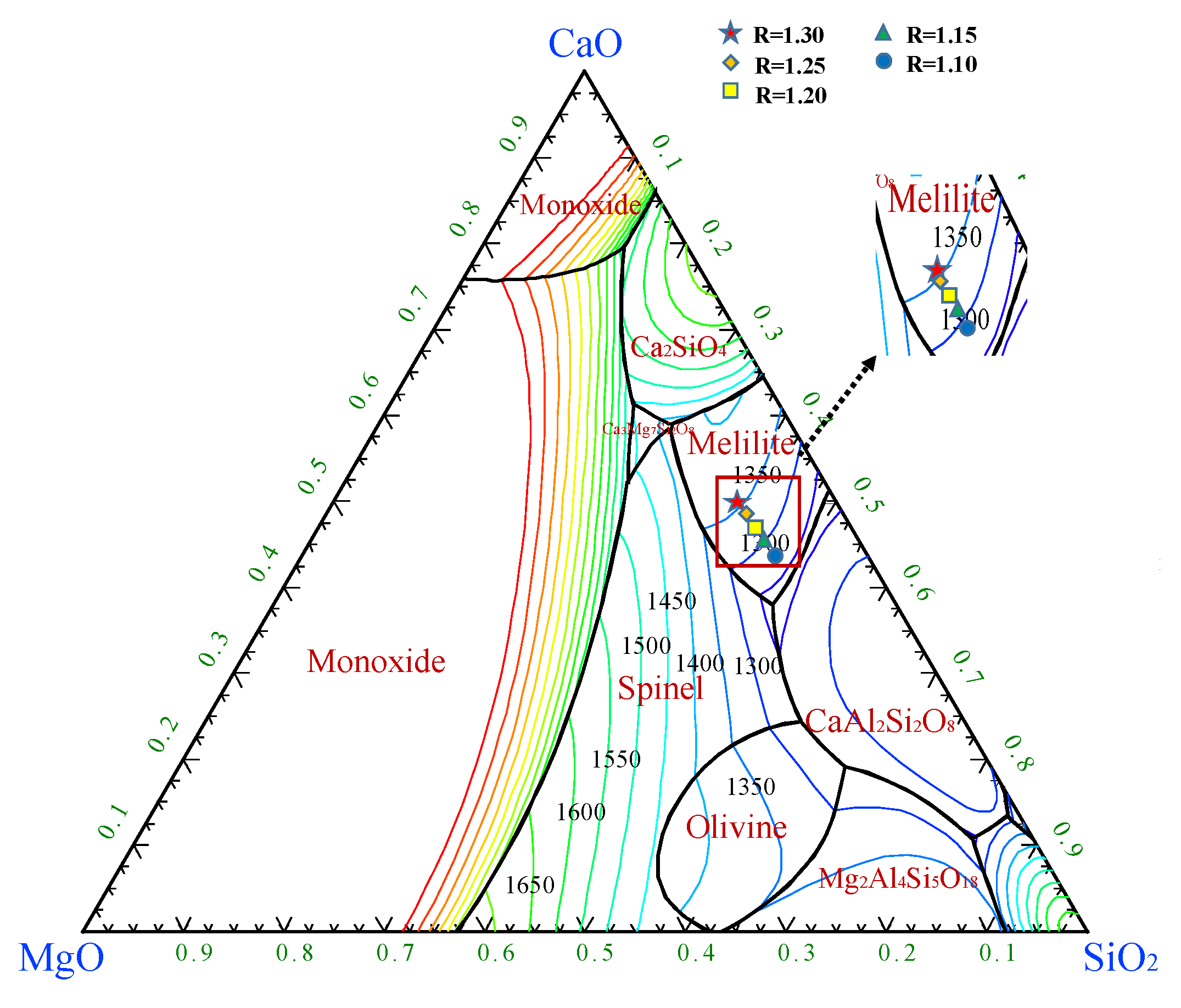
2.1.1. Effects of CaO/SiO2 on the Viscous Behaviors of the Slag
2.1.2. Effects of MgO/Al2O3 on the Viscous Behaviors of the Slag
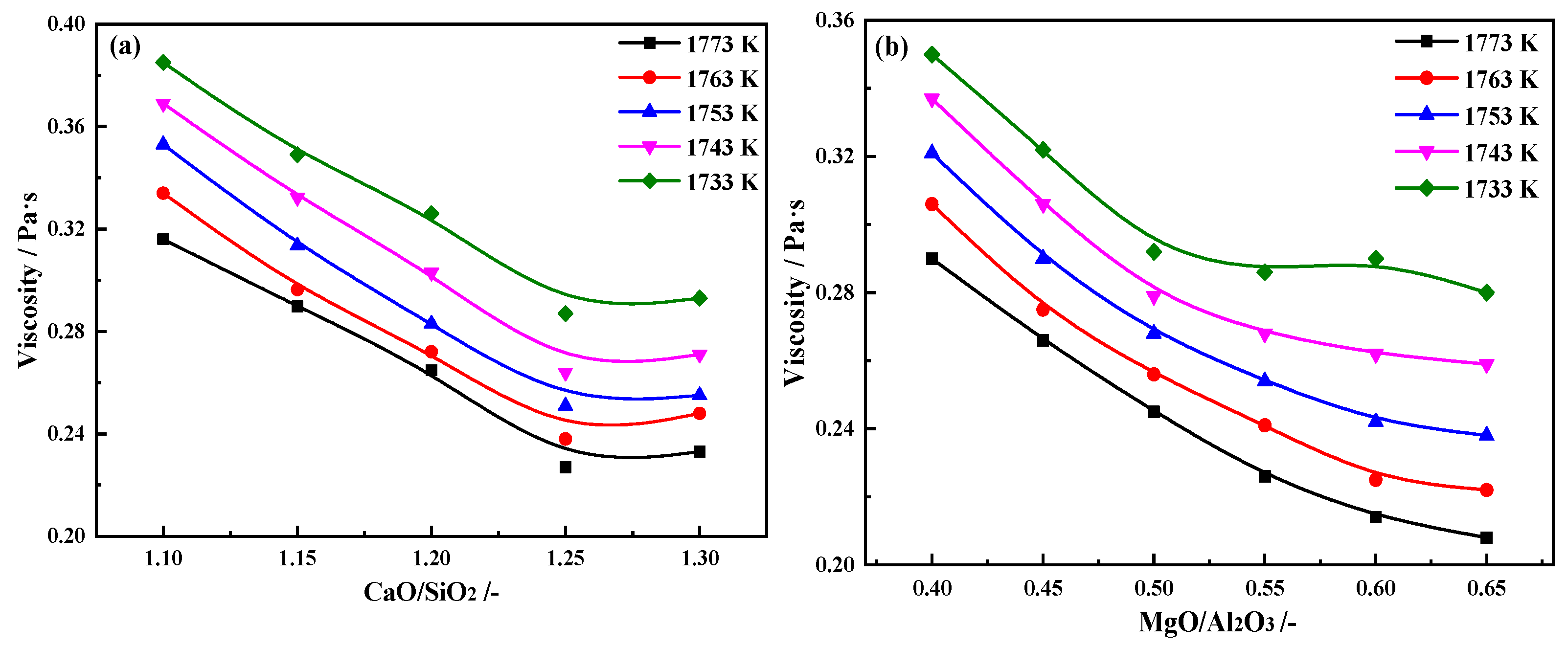
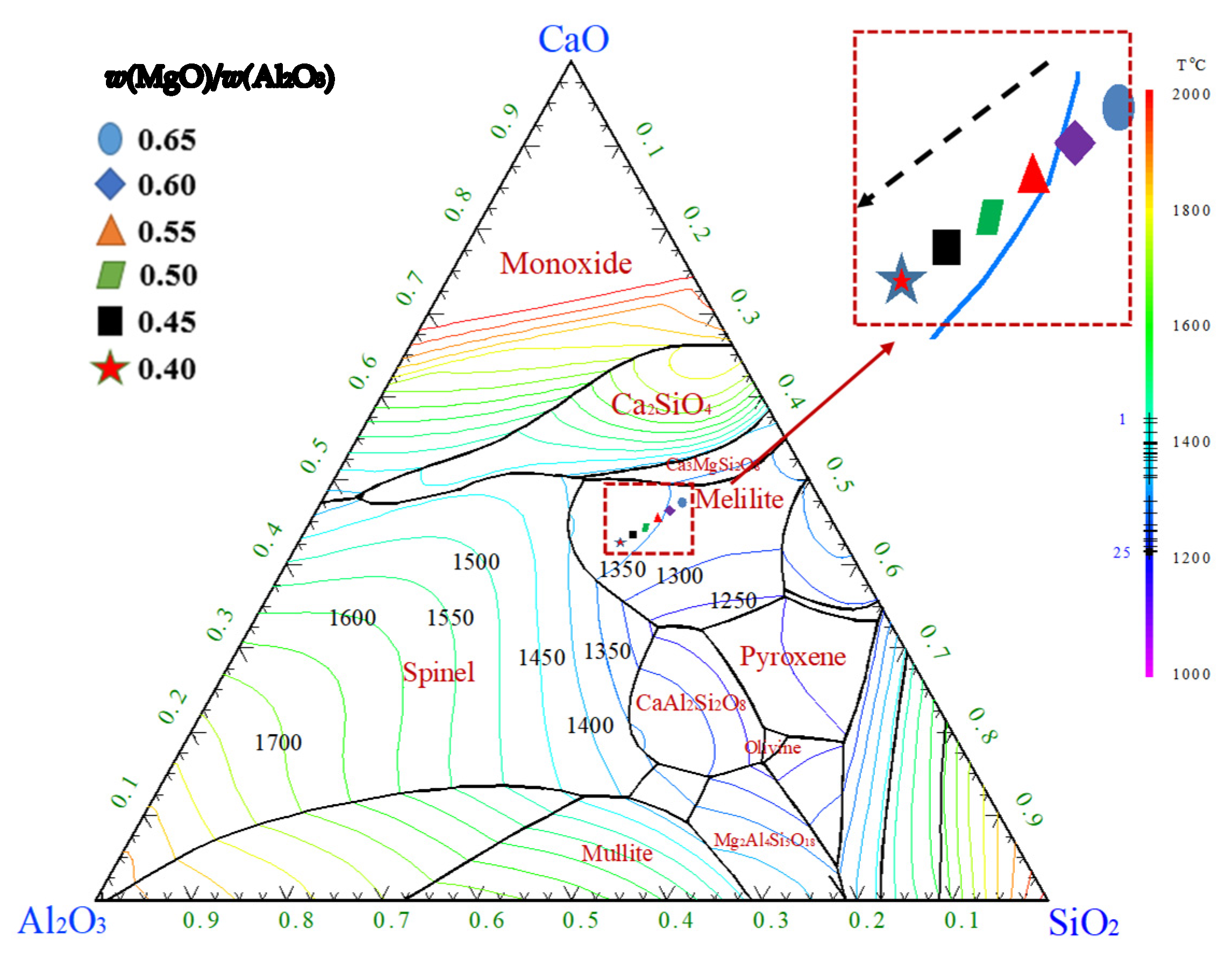
2.2. Effects of CaO/SiO2 and MgO/Al2O3 on the Break Point Temperature of Slag
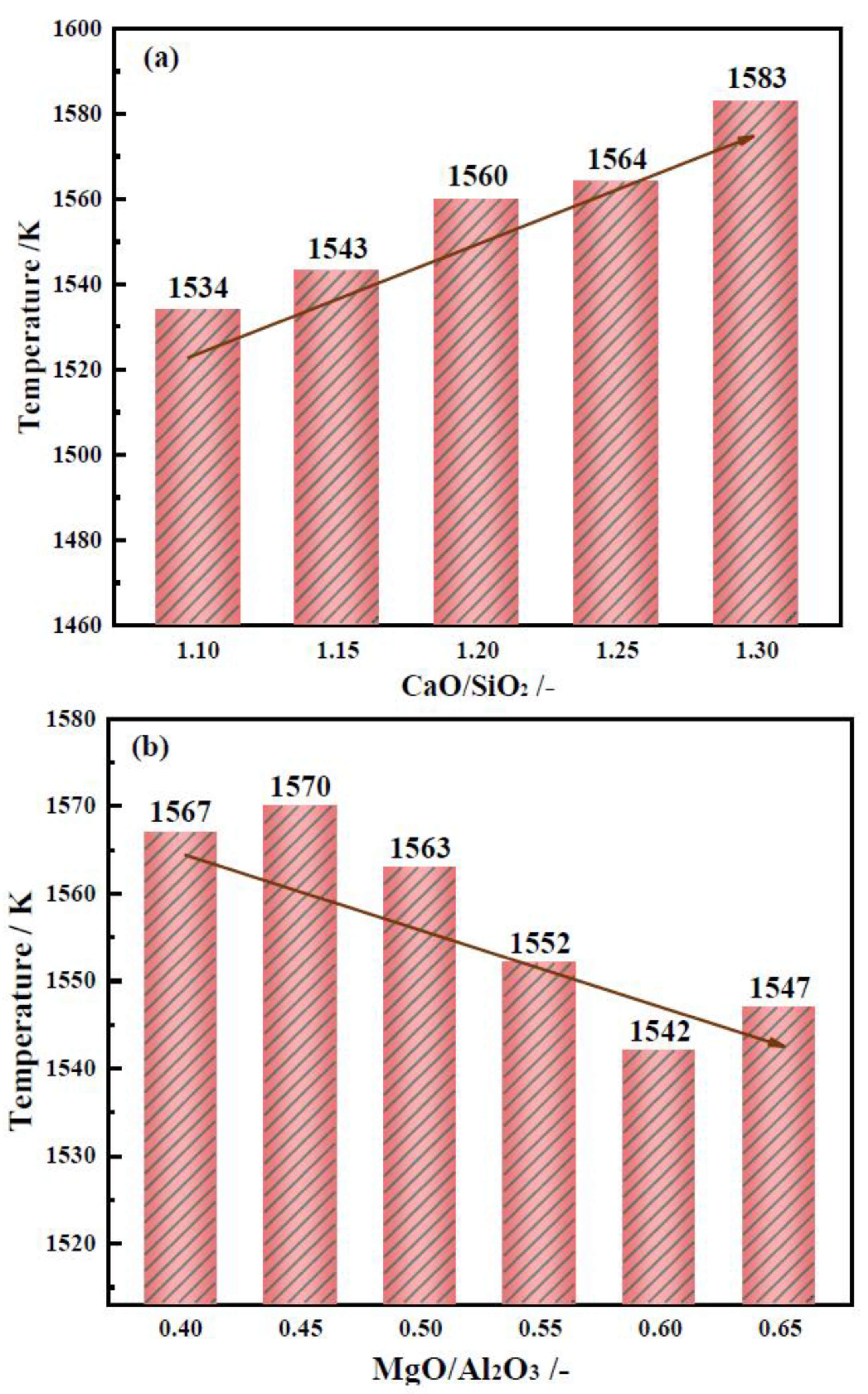
2.2.1. Effects of CaO/SiO2 on the Break Point Temperature of Slag
2.2.2. Effects of MgO/Al2O3 on the Break Point Temperature of Slag
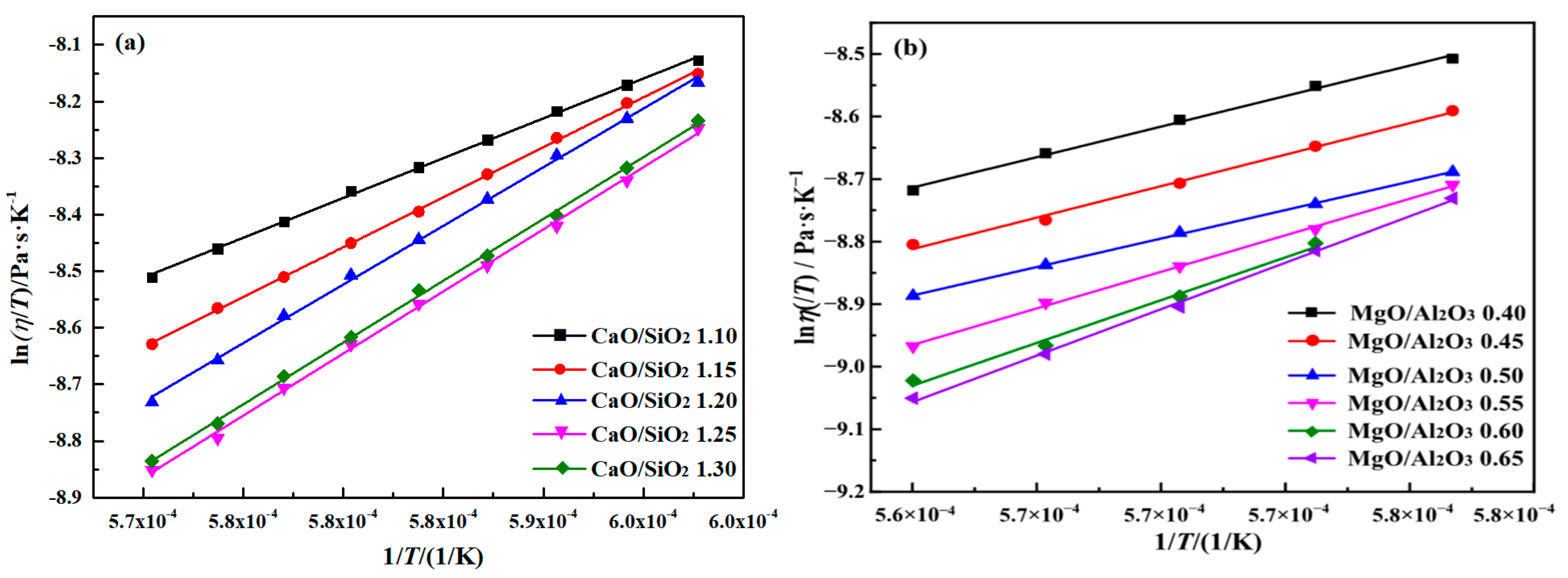
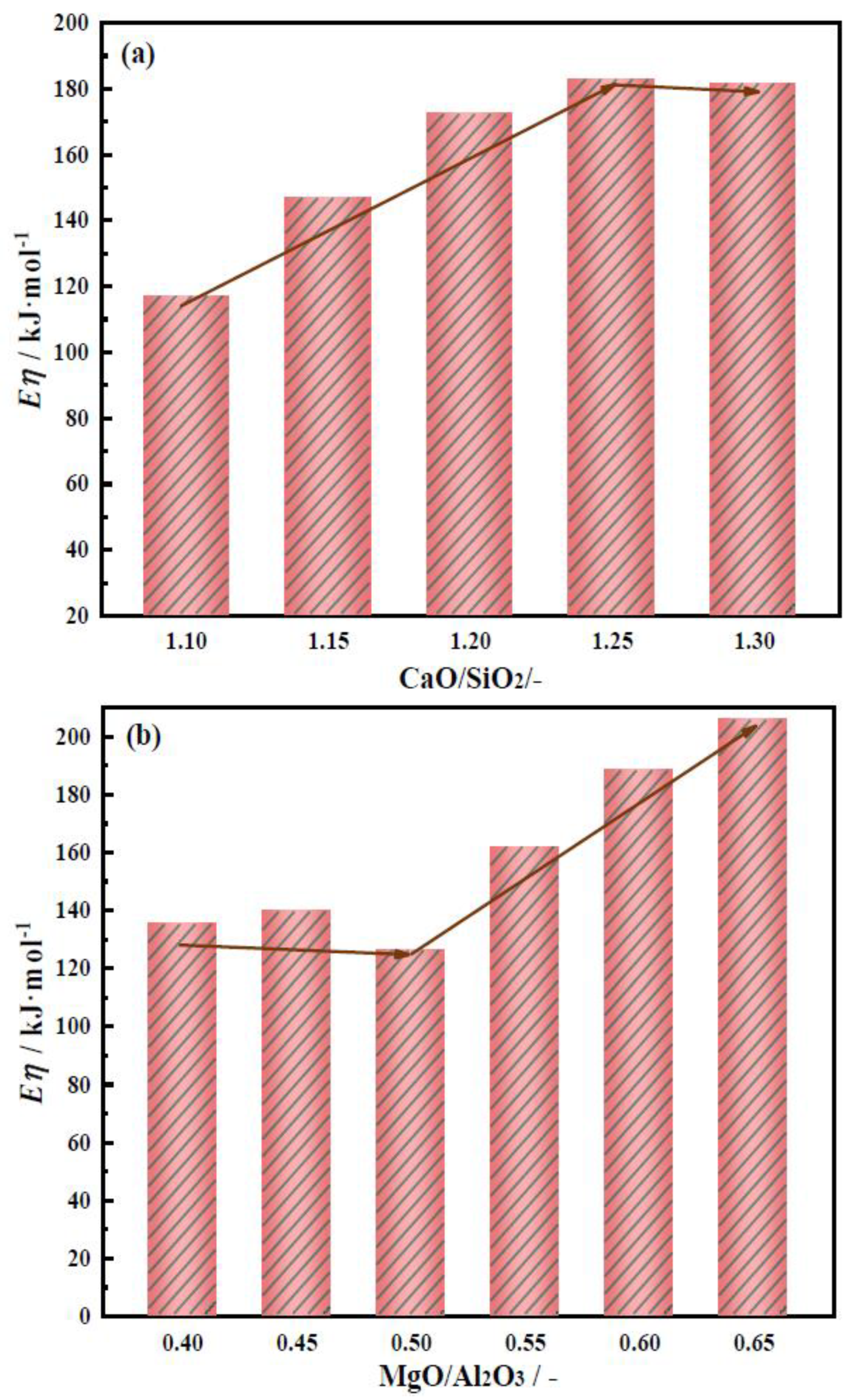
2.3. Effects of CaO/SiO2 and MgO/Al2O3 on Activation Energy of Slag Viscous Flow
2.3.1. Effects of CaO/SiO2 on Activation Energy of Slag Viscous Flow
2.3.2. Effects of MgO/Al2O3 on Activation Energy of Slag Viscous Flow
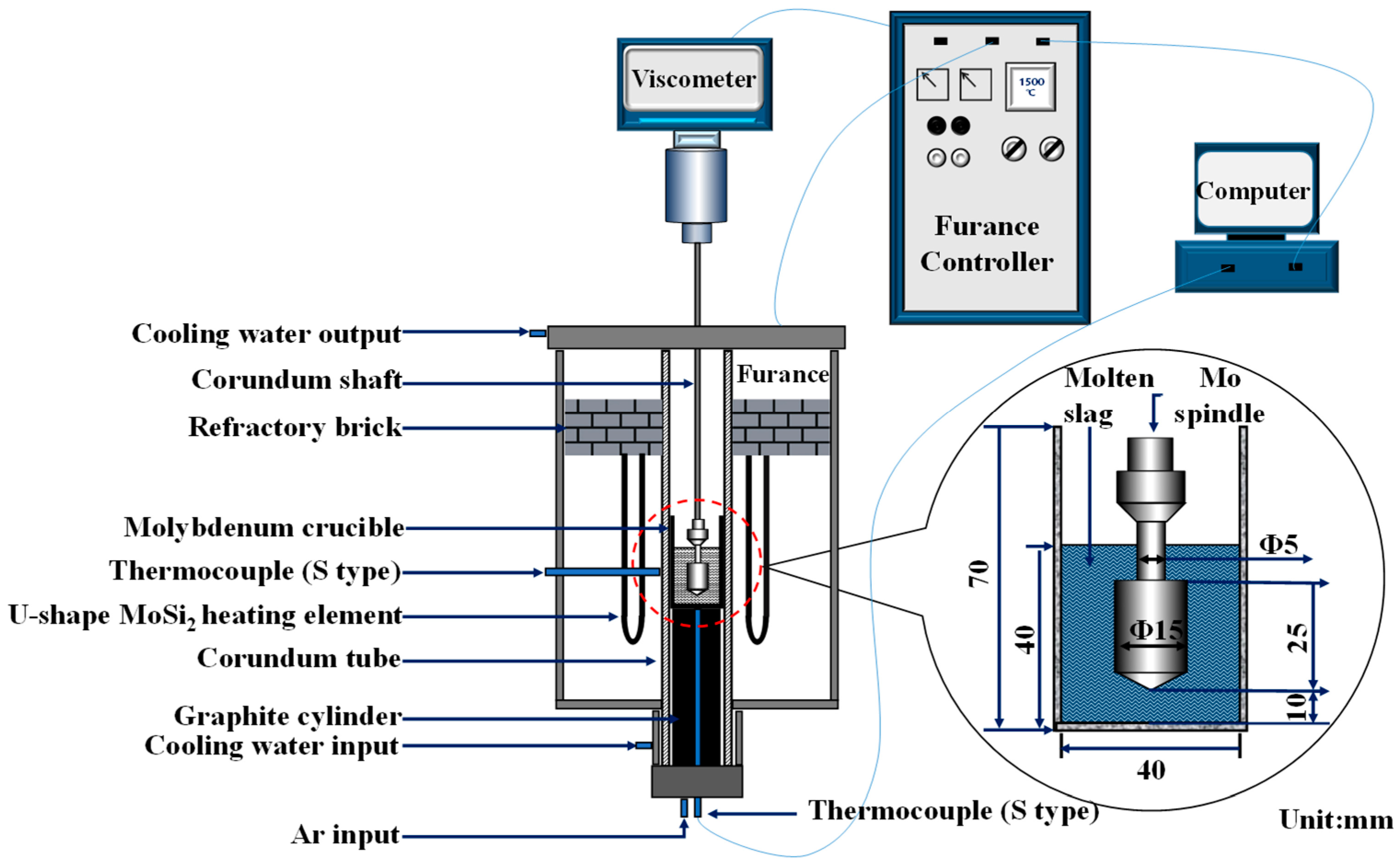
3. Experimental
3.1. Raw Material
3.2. Experimental Procedure
4. Conclusions
- (1)
- With CaO/SiO2 increasing from 1.10 to 1.30, viscosity first decreased significantly and then slowed down. When CaO/SiO2 is 1.25, η1773K is 0.227 Pa·s. TBr shows an increasing trend, increasing from 1534 K to 1583 K. Eη increases from 117.01 to 182.86 kJ·mol−1, and the thermal stability of the slag deteriorates first and then improves. At this point, the slag system has a better performance.
- (2)
- With MgO/Al2O3 increasing from 0.40 to 0.65, viscosity first decreased significantly and then slowed down. When MgO/Al2O3 is 0.55, η1773K is 0.226 Pa·s. TBr decreases from 1570 K to 1542 K. The Eη increases from 126.20 to 205.86 kJ·mol−1, and the thermal stability of the slag first improves and then deteriorates. At this point, the slag system has a better performance.
- (3)
- For comprehensive considerations, when CaO/SiO2 is 1.25 and MgO/Al2O3 is 0.55, the η1773K, TBr, and Eη are at a reasonable value. The low boron-bearing high-alumina slag system has the best metallurgical performance at this value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.Y.; Wang, S.Z.; Zhao, J.; Wu, Z.; Geng, Y.; Ma, Y.; Xue, R. Influence of B2O3 on the thermophysical properties of molten blast furnace slag. J. Non-Cryst. Solids 2023, 615, 122429. [Google Scholar] [CrossRef]
- Bi, Z.; Li, K.; Jiang, C.; Zhang, J.; Ma, S.; Sun, M.; Wang, Z.; Li, H. Effects of B2O3 on the structure and properties of blast furnace slag by molecular dynamics simulation. J. Non-Cryst. Solids 2021, 551, 120412. [Google Scholar] [CrossRef]
- Talapaneni, T.; Chaturvedi, V. Proposing a suitable slag composition by estimating the fusion behavior, viscosity and desulphurization ability for blast furnaces running with high alumina. Mater. Today Proc. 2022, 67, 558–565. [Google Scholar] [CrossRef]
- Li, T.; Zhao, C.; Sun, C.; Song, S.; Wang, Q. Roles of MgO and Al2O3 in viscous and structural behavior of blast furnace primary slag with C/S = 1.4. Metall. Mater. Trans. B 2020, 51, 2724–2734. [Google Scholar] [CrossRef]
- Liang, H.L.; Chu, M.S.; Feng, C.; Tang, J.; Liu, Z.; Wang, W. Optimisation study and affecting mechanism of CaO/SiO2 and MgO on viscous behaviours of titanium-bearing blast furnace slag. Ironmak. Steelmak. 2018, 47, 106–117. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Hong, C.; Ma, X.J.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt%Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Huang, X.H.; Liao, J.L.; Zheng, K.; Hu, H.H.; Wang, F.M.; Zhang, Z.T. Effect of B2O3 addition on viscosity of mould slag containing low silica content. Ironmak. Steelmak. 2014, 41, 67–74. [Google Scholar] [CrossRef]
- Sukenaga, S.; Higo, T.; Shibata, H.; Saito, N. Effect of CaO/SiO2 ratio on surface tension of CaO-SiO2-Al2O3-MgO Melts. ISIJ Int. 2015, 55, 1299–1304. [Google Scholar] [CrossRef]
- Saito, N.; Hori, N.; Nakashima, K.; Mori, K. Viscosity of blast furnace type slags. Metall. Mater. Trans. B 2003, 34, 509–516. [Google Scholar] [CrossRef]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Liang, W.; Sun, M. Molecular dynamics simulation on the effect of MgO/Al2O3 ratio on structure and properties of blast furnace slag under different basicity conditions. Metall. Mater. Trans. B 2018, 50, 367–375. [Google Scholar] [CrossRef]
- Qiu, G.X.; Miao, D.J.; Wei, X.L.; Bai, C.; Li, X.M. Effect of MgO/Al2O3 and CaO/SiO2 on the metallurgical properties of CaO-SiO2-Al2O3-MgO-TiO2 slag. J. Non-Cryst. Solids 2022, 585, 121545. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; Zhang, J.; Qin, Y.; Bai, C. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5%TiO2. Can. Metall. Quart. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Jiao, K.; Liu, Z. Influence of basicity and MgO/Al2O3 ratio on the viscosity of blast furnace slags containing chloride. Metall. Res. Technol. 2017, 114, 395. [Google Scholar] [CrossRef]
- Das, K.; Agrawal, A.; Reddy, A.S.; Ramna, R.V. FactSage studies to identify the optimum slag regime for blast furnace operation. Trans. Indian Inst. Met. 2021, 74, 419–428. [Google Scholar] [CrossRef]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Liang, W.; Sun, M.; Wang, Z. The effect of CaO(MgO) on the structure and properties of aluminosilicate system by molecular dynamics simulation. J. Mol. Liq. 2018, 268, 762–769. [Google Scholar] [CrossRef]
- Jin, H.B.; Yang, S.Y.; Liu, H.; Chen, Y.; He, S.; Wang, Q. Effect of CaO partial substituted by BaO on structure of aluminotitanate melts by molecular dynamics simulation. J. Non-Cryst. Solids 2023, 605, 122160. [Google Scholar] [CrossRef]
- Liu, W.G.; Zuo, H.B. Effect of MnO and CaO substitution for BaO on the viscosity and structure of CaO-SiO2-MgO-Al2O3-BaO-MnO slag. J. Non-Cryst. Solids 2021, 567, 120940. [Google Scholar] [CrossRef]
- Bi, Z.S.; Li, K.J.; Jiang, C.H.; Zhang, J.L.; Ma, S.F. Effects of amphoteric oxide (Al2O3 and B2O3) on the structure and properties of SiO2-CaO melts by molecular dynamics simulation. J. Non-Cryst. Solids 2021, 559, 120687. [Google Scholar] [CrossRef]
- Lei, J.; Yang, W.; Sheng, G.Y.; Zhang, C.; Wu, T.; Wang, H.C. Effects of BaO Content and CaO/Al2O3 ratio on the properties and structure of aluminate slag. Metall. Mater. Trans. B 2022, 53, 2239–2247. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, Y.; Wu, T.; Bao, G.D.; Lei, J.; Wang, H.C. Effect of B2O3 on the structure and properties of aluminate slag. Metall. Res. Technol. 2022, 119, 507. [Google Scholar] [CrossRef]
- Bo, W.; Jian, X.; Zhang, H.L.; Sun, S.; Feng, Z. Influence of MgO on calcium aluminate slag system with lower calcium. Adv. Mater. Res. 2014, 3226, 941–944. [Google Scholar] [CrossRef]
- Qin, J.H.; Liu, W.G.; Wu, H.J.; Wang, J.; Xue, Q.; Zhao, H.; Zuo, H. A comprehensive investigation on the viscosity and structure of CaO-SiO2-Al2O3-MgO-BaO slag with different Al2O3/SiO2 ratios. J. Mol. Liq. 2022, 365, 120060. [Google Scholar] [CrossRef]
- Wang, W.; Dai, S.; Zhou, L.; Zhang, J.; Tian, W.; Xu, J. Viscosity and structure of MgO-SiO2-based slag melt with varying B2O3 content. Ceram. Int. 2020, 46, 3631–3636. [Google Scholar] [CrossRef]
- Chen, Z.W.; Meng, Z.; Liu, L.L.; Wang, H.; Sun, Y.; Wang, X. Structural and viscous insight into impact of MoO3 on molten slags. Metall. Mater. Trans. B 2021, 52, 3730–3743. [Google Scholar] [CrossRef]
- Liu Hao Qin, Y.L.; Yang, Y.H.; Zhang, Q.; Deng, N. Influence of Al2O3 content on the melting and fluidity of blast furnace type slag with low TiO2 content. J. Chem. 2018, 2018, 9502304. [Google Scholar] [CrossRef]
- Zhang, W.; He, F.; Xiao, Y.; Xie, M.; Xie, J.; Li, F.; Yang, H.; Li, Z. Structure, crystallization mechanism, and properties of glass ceramics from molten blast furnace slag with different B2O3/Al2O3. Mater. Chem. Phys. 2020, 243, 122664. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, B.; Liu, Z.; Yang, H.; Zou, M. Effects of Fe/SiO2 ratio and MgO content on the viscous behaviors of the SiO2-FeO-MgO-12 wt pct Fe2O3-8wt pct CaO-3 wt pct Al2O3 slag system. Metall. Mater. Trans. B 2022, 53, 902–915. [Google Scholar] [CrossRef]
- Kong, W.G.; Liu, J.H.; Yu, Y.W.; Hou, X.M.; He, Z.J. Effect of w(MgO)/w(Al2O3) ratio and basicity on microstructure and metallurgical properties of blast furnace slag. J. Iron Steel Res. Int. 2021, 28, 1223–1232. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, H.; Wang, J.; Pang, Z.; Pei, G.; Yan, Z.; Mao, H.; Qiu, G.; Lv, X. Influence of TiO2 on viscosity, phase composition and structure of chromium-containing high-titanium blast furnace slag. J. Mater. Res. Technol. 2021, 12, 1615–1622. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Qin, J.; Li, F.; Liu, Z.G. Effects of MgO and TiO2 on the viscous behaviors and phase compositions of titanium-bearing slag. Int. J. Min. Met. Mater. 2016, 23, 868–880. [Google Scholar] [CrossRef]


| CaO | SiO2 | MgO | Al2O3 | B2O3 |
|---|---|---|---|---|
| 38.01 | 30.37 | 7.98 | 17.00 | 0.47 |
| No. | CaO | SiO2 | MgO | Al2O3 | B2O3 | CaO/SiO2 | MgO/Al2O3 |
|---|---|---|---|---|---|---|---|
| 1 | 35.03 | 31.85 | 7.98 | 17.00 | 0.47 | 1.10 | 0.47 |
| 2 | 35.77 | 31.11 | 7.98 | 17.00 | 0.47 | 1.15 | 0.47 |
| 3 | 36.48 | 30.40 | 7.98 | 17.00 | 0.47 | 1.20 | 0.47 |
| 4 | 37.15 | 29.72 | 7.98 | 17.00 | 0.47 | 1.25 | 0.47 |
| 5 | 37.80 | 29.08 | 7.98 | 17.00 | 047 | 1.30 | 0.47 |
| 6 | 37.81 | 30.25 | 6.80 | 17.00 | 0.47 | 1.25 | 0.40 |
| 7 | 37.74 | 29.87 | 7.65 | 17.00 | 0.47 | 1.25 | 0.45 |
| 8 | 36.87 | 29.49 | 8.50 | 17.00 | 0.47 | 1.25 | 0.50 |
| 9 | 36.39 | 29.11 | 9.35 | 17.00 | 0.47 | 1.25 | 0.55 |
| 10 | 35.92 | 28.74 | 10.20 | 17.00 | 0.47 | 1.25 | 0.60 |
| 11 | 35.45 | 28.36 | 11.05 | 17.00 | 0.47 | 1.25 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Z.; Wu, C.; Liu, Z. Effect of CaO/SiO2 and MgO/Al2O3 on the Metallurgical Properties of Low Boron-Bearing High-Alumina Slag. Inorganics 2025, 13, 346. https://doi.org/10.3390/inorganics13110346
Sun Y, Zhang Z, Wu C, Liu Z. Effect of CaO/SiO2 and MgO/Al2O3 on the Metallurgical Properties of Low Boron-Bearing High-Alumina Slag. Inorganics. 2025; 13(11):346. https://doi.org/10.3390/inorganics13110346
Chicago/Turabian StyleSun, Ye, Zuoliang Zhang, Chunlei Wu, and Zhenggen Liu. 2025. "Effect of CaO/SiO2 and MgO/Al2O3 on the Metallurgical Properties of Low Boron-Bearing High-Alumina Slag" Inorganics 13, no. 11: 346. https://doi.org/10.3390/inorganics13110346
APA StyleSun, Y., Zhang, Z., Wu, C., & Liu, Z. (2025). Effect of CaO/SiO2 and MgO/Al2O3 on the Metallurgical Properties of Low Boron-Bearing High-Alumina Slag. Inorganics, 13(11), 346. https://doi.org/10.3390/inorganics13110346






