The Impact of Supramolecular Forces on the Magnetic and Optical Properties of Bis(2-amino-6-bromopyridinium) Tetrachloridocuprate (C5H6BrN2)2[CuCl4]
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of (HABPy)2[CuCl4]
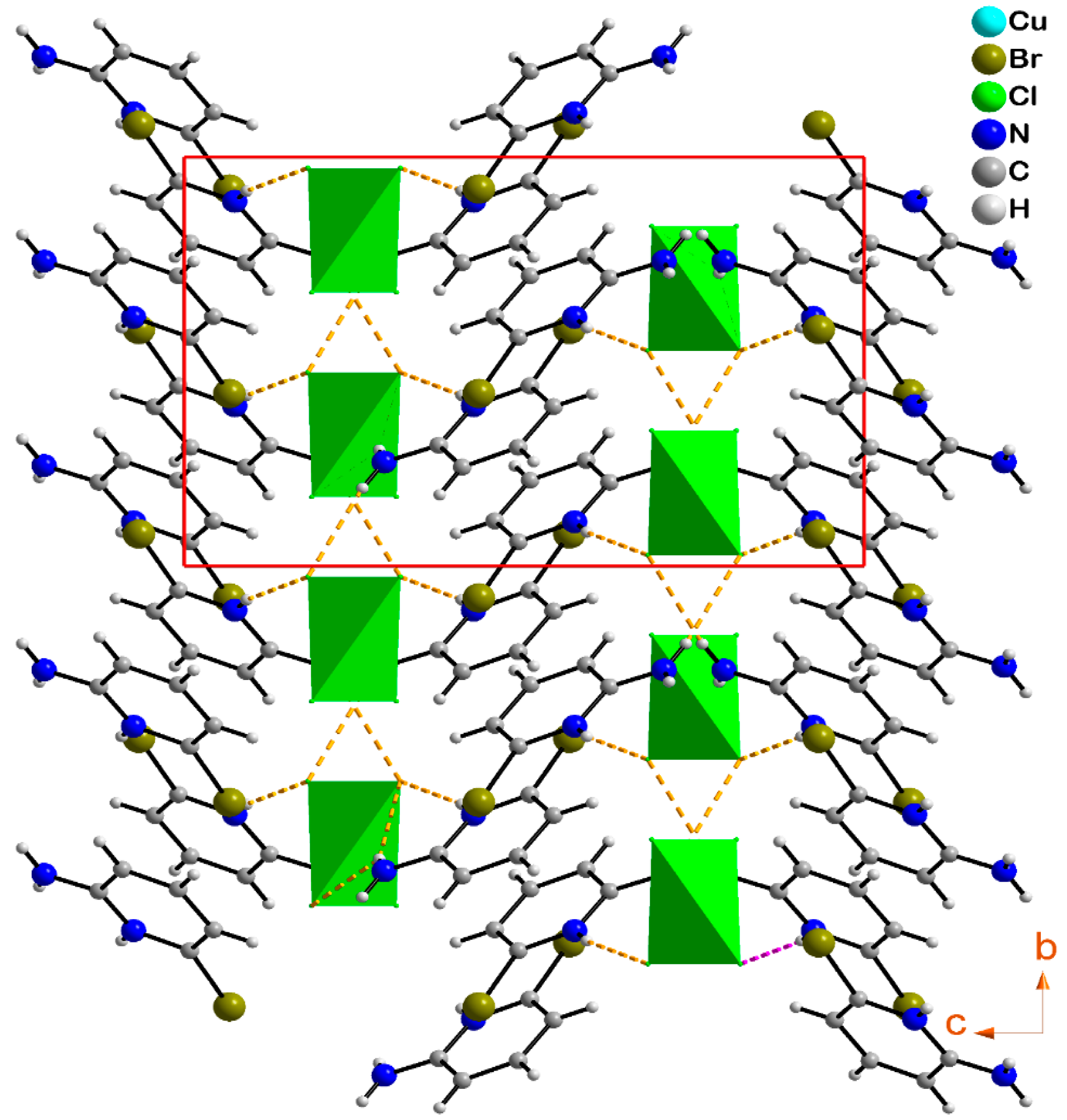
2.2. Structural Analysis
2.3. Hirshfeld Surface Analysis
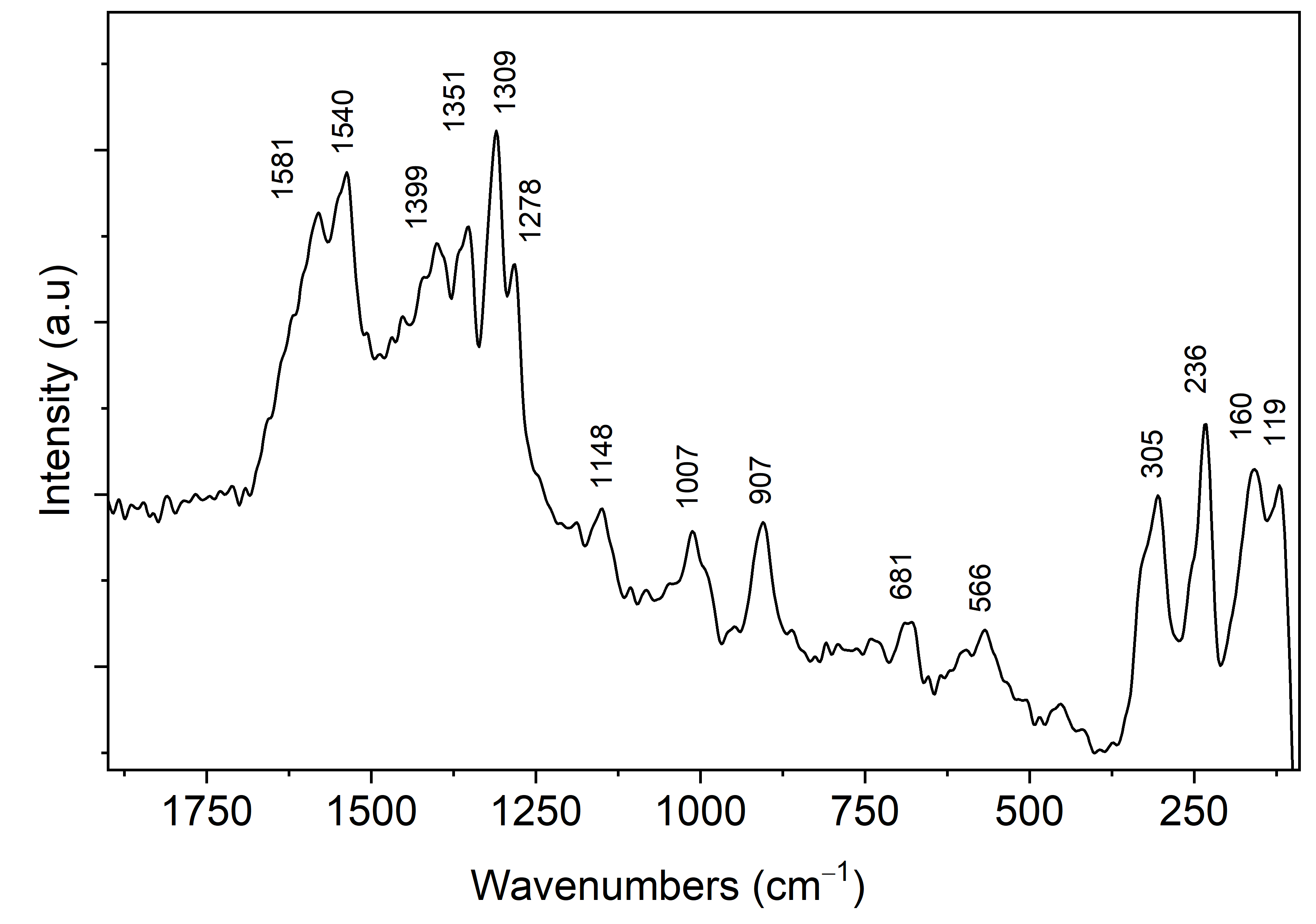
2.4. Fourier-Transform Infrared (FT-IR) and Raman Spectroscopy
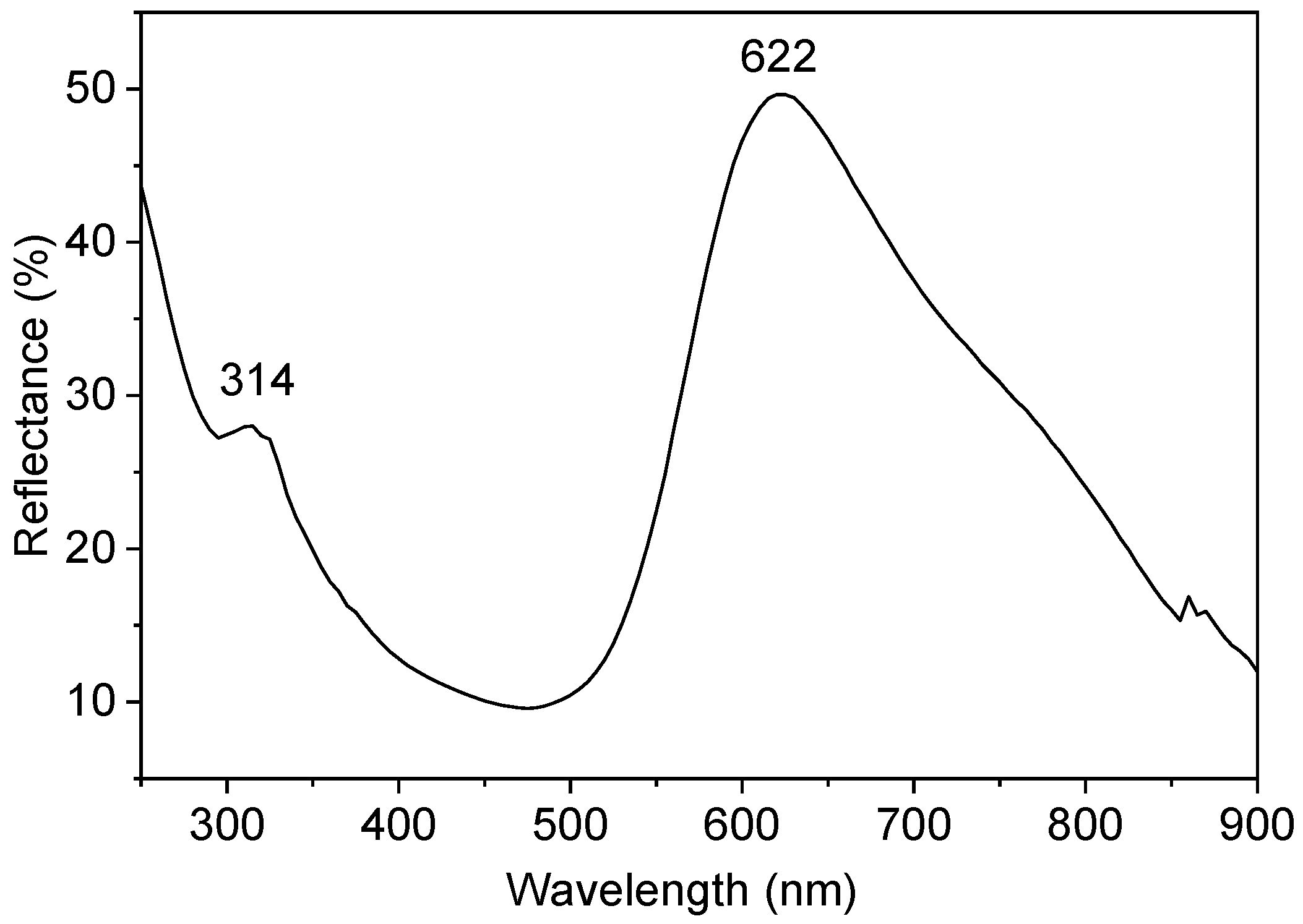
2.5. UV-Vis Diffuse Reflectance and Photoluminescence Spectroscopy
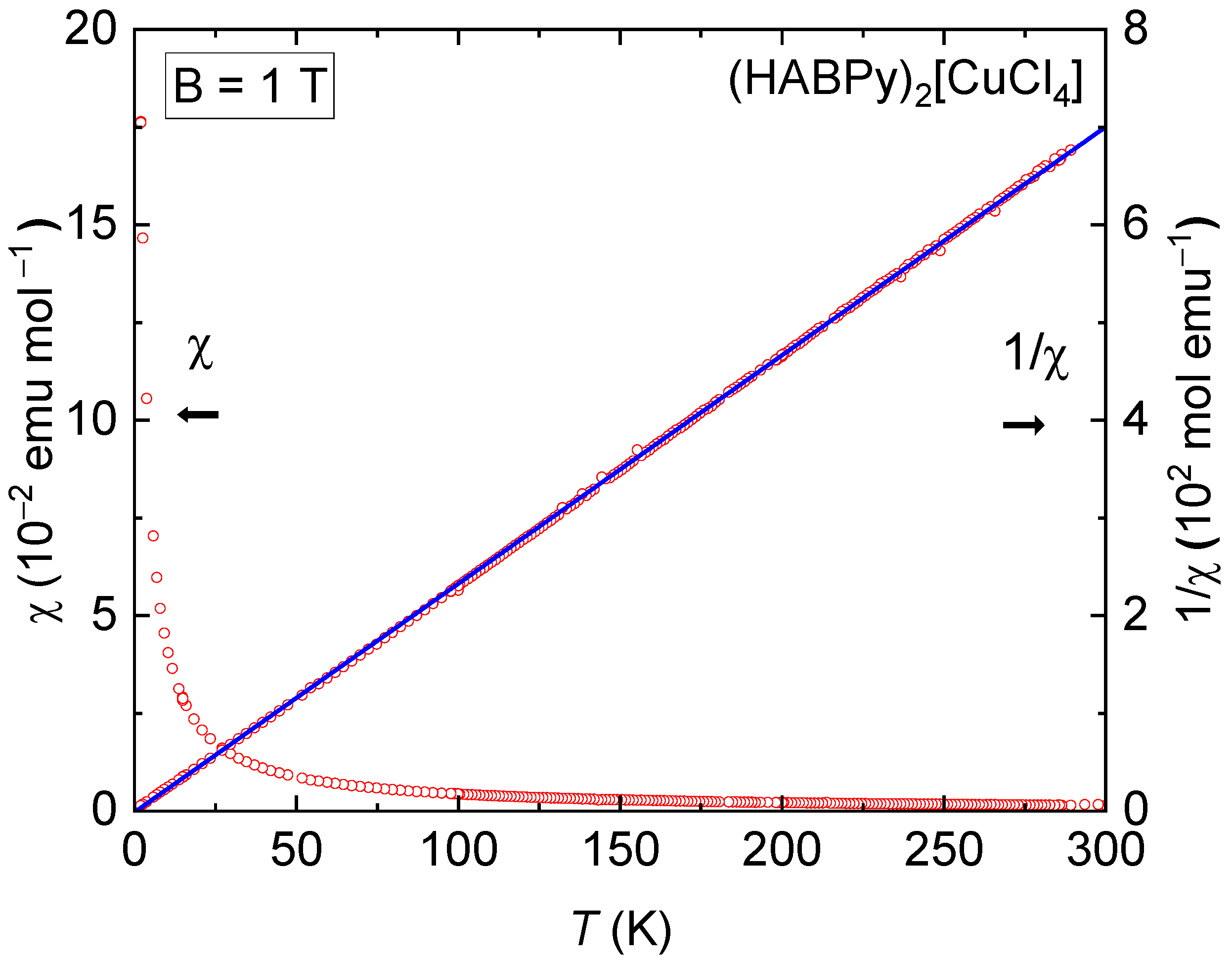
2.6. Magnetic Susceptibility
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of (HABPy)2[CuCl4]
3.4. Single-Crystal X-Ray Diffraction
3.5. Magnetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlachter, A.; Tanner, K.; Harvey, P.D. Copper halide-chalcogenoether and -chalcogenone networks: Chain and cluster motifs, polymer dimensionality and photophysical properties. Coord. Chem. Rev. 2021, 448, 214176. [Google Scholar] [CrossRef]
- Englert, U. Halide-bridged polymers of divalent metals with donor ligands—Structures and properties. Coord. Chem. Rev. 2010, 254, 537–554. [Google Scholar] [CrossRef]
- Zhou, K.; Qi, B.; Liu, Z.; Wang, X.; Sun, Y.; Zhang, L. Advanced Organic–Inorganic Hybrid Materials for Optoelectronic Applications. Adv. Funct. Mater. 2024, 34, 2411671. [Google Scholar] [CrossRef]
- Zheng, H.; Loh, K.P. Ferroics in Hybrid Organic–Inorganic Perovskites: Fundamentals, Design Strategies, and Implementation. Adv. Mater. 2024, 36, 2308051. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.-J.; Lee, S.; Lin, H.; Ma, B. Recent Advances in Luminescent Zero-Dimensional Organic Metal Halide Hybrids. Adv. Opt. Mater. 2021, 9, 2001766. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z. Recent progress of zero-dimensional luminescent metal halides. Chem. Soc. Rev. 2021, 50, 2626–2662. [Google Scholar] [CrossRef]
- Niu, T.; Xue, Q.; Yip, H.-L. Advances in Dion-Jacobson phase two-dimensional metal halide perovskite solar cells. Nanophotonics 2021, 10, 2069–2102. [Google Scholar] [CrossRef]
- Katan, C.; Mercier, N.; Even, J. Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Perovskite Semiconductors. Chem. Rev. 2019, 119, 3140–3192. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef]
- Theofylaktos, L.; Kosmatos, K.O.; Giannakaki, E.; Kourti, H.; Deligiannis, D.; Konstantakou, M.; Stergiopoulos, T. Perovskites with d-block metals for solar energy applications. Dalton Trans. 2019, 48, 9516–9537. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic−Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J. Prediction of Organic–Inorganic Hybrid Perovskite Band Gap by Multiple Machine Learning Algorithms. Molecules 2024, 29, 499. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, H.C.; Huang, Y.C.; Tan, C.S. Synthesis and Applications of Halide Perovskite Nanocrystals in Optoelectronics. Inorganics 2023, 11, 39. [Google Scholar] [CrossRef]
- Coccia, C.; Moroni, M.; Malavasi, L. Chiral Metal Halide Perovskites: Focus on Lead-Free Materials and Structure-Property Correlations. Molecules 2023, 28, 6166. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, F.; Jung, E.; Öz, S.; Choi, H.; Fischer, T.; Mathur, S. Chemical Processing of Mixed-Cation Hybrid Perovskites: Stabilizing Effects of Configurational Entropy. In Perovskite Solar Cells: Materials, Processes, and Devices, 1st ed.; Ahmad, S., Kazim, S., Grätzel, M., Eds.; Chapter 1; WILEY-VCH GmbH: Weinheim, Germany, 2022; pp. 1–31. ISBN 978-3-527-34715-5. [Google Scholar]
- Chatterjee, S.; Pal, A.J. Influence of metal substitution on hybrid halide perovskites: Towards lead-free perovskite solar cells. J. Mater. Chem. A 2018, 6, 3793–3823. [Google Scholar] [CrossRef]
- Cui, F.; García-López, V.; Wang, Z.; Luo, Z.; He, D.; Feng, X.; Dong, R.; Wang, X. Two-Dimensional Organic−Inorganic van der Waals Hybrids. Chem. Rev. 2025, 125, 445–520. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B. Zero-dimensional hydrazine iodobismuthate as a lead-free perovskite-like light absorber in a self-powered photodetector. J. Alloys Compd. 2022, 893, 162347. [Google Scholar] [CrossRef]
- Zhou, J.; Yun, X.Y.; Fang, C.; Xu, D.H.; Li, X.; Cui, Y.Y.; Zhang, Z.C. Ternary zero-dimensional hybrid metal halides with dual emission. Mater. Today Chem. 2022, 23, 100757. [Google Scholar] [CrossRef]
- Yu, T.; Mao, X.; Xu, X.; Wang, Z.; Zhang, R. Ultrafast dynamics of a highly-emissive zero-dimensional organic tin bromide perovskite. Chem. Phys. Lett. 2021, 769, 138411. [Google Scholar] [CrossRef]
- Vijayakanth, T.; Liptrot, D.J.; Gazit, E.; Boomishankar, R.; Bowen, C.R. Recent Advances in Organic and Organic–Inorganic Hybrid Materials for Piezoelectric Mechanical Energy Harvesting. Adv. Funct. Mater. 2022, 32, 2109492. [Google Scholar] [CrossRef]
- Novikov, A.S. Non-Covalent Interactions in Organic, Organometallic, and Inorganic Supramolecular Systems Relevant for Medicine, Materials Science, and Catalysis. Crystals 2022, 12, 246. [Google Scholar] [CrossRef]
- Speetzen, E.D.; Nwachukwu, C.I.; Bowling, N.P.; Bosch, E. Complementary, Cooperative Ditopic Halogen Bonding and Electron Donor-Acceptor π-π Complexation in the Formation of Cocrystals. Molecules 2022, 27, 1527. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, S.; Meng, Y.; Yip, S.P.; Ho, J.C. Aromatic spacer engineering for 2D halide perovskites and their application in solar cells. Mater. Today Electron. 2024, 8, 100100. [Google Scholar] [CrossRef]
- Klein, A.; Krakor, S.; Payen, L.; Walleriu, C. Azo- and Hydrazo-Dipyridines in Halidometallate Structures. Inorg. Chem. Res. 2024, 7, 65–75. [Google Scholar] [CrossRef]
- Selivanov, N.I.; Rozhkova, Y.A.; Kevorkyants, R.; Emeline, A.V.; Bahnemann, D.W. The effect of organic cations on the electronic, optical and luminescence properties of 1D piperidinium, pyridinium, and 3-hydroxypyridinium lead trihalides. Dalton Trans. 2020, 49, 4390–4403. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, W.T.M.; Herckens, R.; Van Hecke, K.; Ruttens, B.; D’Haen, J.; Lutsen, L.; Vanderzande, D. Low-Dimensional Hybrid Perovskites Containing an Organic Cation with an Extended Conjugated System: Tuning the Excitonic Absorption Features. Chem. Nanomater. Energy Biol. More 2019, 5, 323–327. [Google Scholar] [CrossRef]
- García-Espejo, G.; Rodríguez-Padrón, D.; Pérez-Morales, M.; Luque, R.; de Miguel, G.; Camacho, L. Mechanochemical synthesis of one-dimensional (1D) hybrid perovskites incorporating polycyclic aromatic spacers: Highly fluorescent cation-based materials. J. Mater. Chem. C 2018, 6, 7677–7682. [Google Scholar] [CrossRef]
- Ju, M.-G.; Dai, J.; Ma, L.; Zhou, Y.; Zeng, X.C. Zero-Dimensional Organic-Inorganic Perovskite Variant: Transition between Molecular and Solid Crystal. J. Am. Chem. Soc. 2018, 140, 10456–10463. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, H.; Hu, Z.; Fu, Q.; Tao, H.; Weng, J.; Xiong, L.; Cheng, Z. Magnetic hybrid organic–inorganic perovskite (CH3NH3)2XCl4 (X = Mn, Cu, Co) crystals. CrystEngComm 2021, 23, 5208–5213. [Google Scholar] [CrossRef]
- Kusumoto, S.; Nagasawa, S.; Suzuki, R.; Tachibana, M.; Tsuji, T.; Zenno, H.; Nakashima, Y.; Hayami, S.; Kim, Y.; Koide, Y. Ferromagnetic and plastically deformable organic–inorganic hybrid crystal: (C7H9NH3)2CuCl4, Chem. Commun. 2025, 61, 10303–10306. [Google Scholar] [CrossRef]
- Baker, P.J.; Lancaster, T.; Franke, I.; Hayes, W.; Blundell, S.J.; Pratt, F.L.; Jain, P.; Wang, Z.-M.; Kurmoo, M. Muon spin relaxation investigation of magnetic ordering in the hybrid organic-inorganic perovskites [(CH3)2NH2]M(HCOO)3 (M = Ni, Co, Mn, Cu). Phys. Rev. B 2010, 82, 012407. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Puigjaner, C.; Garcia, J.; Fernandez, A.I.; Barreneche, C. Copper- and manganese-based layered hybrid organic–inorganic compounds with polymorphic transitions as energy storage materials. J. Mater. Chem. A 2024, 12, 18544–18553. [Google Scholar] [CrossRef]
- Topic, E.; Rubcic, M. Structural Insights into Layered Tetrahalocuprates(II) Based on Small Unsaturated and Cyclic Primary Ammonium Cations. Materials 2023, 16, 2236. [Google Scholar] [CrossRef]
- Topić, E.; Senjug, P.; Barisic, D.; Loncaric, I.; Pajic, D.; Rubcic, M. Structure-Related Evolution of Magnetic Order in Anisidinium Tetrachlorocuprates(II). Cryst. Growth Des. 2023, 23, 4262–4272. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.-F.; Li, X.-Y.; Zhang, Y.; Shao, X.; Yang, D.; Zhang, H.-L. Two-Dimensional Perovskite Chiral Ferromagnets. Chem. Mater. 2020, 32, 8914–8920. [Google Scholar] [CrossRef]
- Ferreira, C.F.; Pérez-Cordero, E.E.; Abboud, K.A.; Talham, D.R. Reversible Medium-Dependent Solid–Solid Phase Transformations in Two-Dimensional Hybrid Perovskites. Chem. Mater. 2016, 28, 5522–5529. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Chen, H.Z.; Shi, M.M.; Wu, G.; Zhou, R.J.; Yang, Z.S.; Wang, M.; Tang, B.Z. Preparation and characterization of organic–inorganic hybrid perovskite (C4H9NH3)2CuCl4. Mater. Sci. Eng. B 2005, 117, 313–316. [Google Scholar] [CrossRef]
- Czugler, M.; Kotai, L.; Sreedhar, B.; Rockenbauer, A.; Gacs, I.; Holly, S. The Effect of HCl on the Copper(II) Chloride/Pyridine/Water System: Synthesis, Properties and Crystal Structure of [(pyH)2CuCl4] and [(pyH)2Cu3Cl8(H2O)2]n. Eur. J. Inorg. Chem. 2002, 2002, 3298–3304. [Google Scholar] [CrossRef]
- Bourwina, M.; Msalmi, R.; Walha, S.; Turnbull, M.M.; Roisnel, T.; Guesmi, A.; Houas, A.; Hamadi, N.B.; Naïli, H. Crystal Chemistry, Optic and Magnetic Characterizations of a New Copper Based Material Templated by Hexahydrodiazepine. ACS Omega 2023, 8, 15075–15082. [Google Scholar] [CrossRef]
- Rogalewicz, B.; Szczesio, M.; Poleszak, E.; Kowalczyk, J.; Szewczyk, B.; Camargo, B.C.; Szczytko, J.; Witkowski, M.; Fruzinski, A.; Raducka, A.; et al. Influence of Incorporation of Different dn-Electron Metal Cations into Biologically Active System on Its Biological and Physicochemical Properties. Int. J. Mol. Sci. 2021, 22, 12909. [Google Scholar] [CrossRef]
- Li, Y.-T.; Huang, L.; Li, X.-R.; Hu, B.-Y.; Li, X.-L.; Jiang, Y.; Yu, J.-Y.; Ni, C.-L. Preparation, Crystal Structure, Optical Properties, Hirshfeld Surface Analysis and Antibacterial Activity of 4-Nitrobenzyl-4-aminopyridinium Tetrachlorocuprate(II). J. Chem. Crystallogr. 2024, 54, 291–304. [Google Scholar] [CrossRef]
- Bedford, C.P.; Landee, C.P.; Wikaira, J.L.; Turnbull, M.M. Copper(II) halide salts of 5-bromo-2-aminopyridine and 3,5-dibromo-2-aminopyridine: Syntheses, structures and magnetic behavior. J. Coord. Chem. 2023, 76, 1353–1369. [Google Scholar] [CrossRef]
- Althobaiti, M.G.; Hermi, S.; Alotaibi, A.A.; Alotaibi, K.M.; Hassan, H.A.; Mi, J.-X.; Ben Nasr, C.; Mrad, M.H. A New Cu(II) Metal Complex Template with 4-tert-Butyl-Pyridinium Organic Cation: Synthesis, Structure, Hirshfeld Surface, Characterizations and Antibacterial Activity. Crystals 2022, 12, 254. [Google Scholar] [CrossRef]
- Gatfaoui, S.; Issaoui, N.; Noureddine, O.; Roisnel, T.; Marouani, H. Self assembly of a novel Cu(II) complex, (C6H9N2)2[CuCl4]: Experimental, computational, and molecular docking survey. J. Iran. Chem. Soc. 2021, 18, 2331–2343. [Google Scholar] [CrossRef]
- Mehmood, T.; Bhosale, R.S.; Reddy, J.P. Bis(2-methylpyridinium) tetrachloridocuprate(II): Synthesis, structure and Hirshfeld surface analysis. Acta Crystallogr. E Res. Commun. 2021, 77, 726–729. [Google Scholar] [CrossRef]
- Monroe, J.C.; Landee, C.P.; Rademeyer, M.; Turnbull, M.M. Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium. Inorganics 2020, 8, 18. [Google Scholar] [CrossRef]
- Ben Hmida, W.; Jellali, A.; Abid, H.; Hamdi, B.; Naili, H.; Zouari, R. Synthesis, crystal structure, vibrational studies, optical properties and DFT calculation of a new luminescent material based Cu(II). J. Mol. Struct. 2019, 1184, 604–614. [Google Scholar] [CrossRef]
- Azouzi, K.; Hamdi, B.; Zouari, R.; Ben Salah, A. Crystal structure, Hirshfeld surface analysis, vibrational properties, and electrical and dielectric studies of the bis(4-benzylpyridinium) tetrachlorocuprate (II). Ionics 2016, 22, 1669–1680. [Google Scholar] [CrossRef]
- Ben Nasr, M.; Lefebvre, F.; Ben Nasr, C. Synthesis, Crystal Structure and Infrared Characterization of Bis(4-dimethylamino-pyridinium) Tetrachlorocuprate. Am. J. Anal. Chem. 2015, 6, 446–456. [Google Scholar] [CrossRef]
- Wikaira, J.L.; Landee, C.P.; Ludy, S.J.; Turnbull, M.M. Tetrahalocuprate salts of substituted 4-aminopyridines: Synthesis, structure and magnetic properties. Polyhedron 2013, 52, 770–780. [Google Scholar] [CrossRef]
- Abdalrahman, M.A.; Awwadi, F.F.; Jameson, G.B.; Landee, C.P.; Saunders, C.G.; Turnbull, M.M.; Wikaira, J.L. Effects of halogen and hydrogen bonding on defect disorder: The ladder that wasn’t there. CrystEngComm. 2013, 15, 4309–4320. [Google Scholar] [CrossRef]
- Herringer, S.N.; Turnbull, M.M.; Landee, C.P.; Wikaira, J.L. Synthesis, structure, and magnetic properties of bis(3-amino-2-chloropyridinium)tetrahalocuprate(II) [halide = Cl or Br]. J. Coord. Chem. 2009, 62, 863–875. [Google Scholar] [CrossRef]
- Al-Far, R.H.; Ali, B.F. Bis(2-amino-4-methyl pyridinium) tetrachloridocuprate(II). Acta Crystallogr. E Crystal. Commun. 2009, 65, m73. [Google Scholar] [CrossRef]
- Haddad, S.F.; Al-Far, R.H. Crystal Structure of Three Isomorphous Compounds of 2,5-Dibromopyridine with Tetrahalometalate(II) Ions. J. Chem. Crystallogr. 2008, 38, 663–669. [Google Scholar] [CrossRef]
- Haddad, S.; Willett, R.D. Polymorphism in Bis(4-dimethylamino-pyridinium)tetrachlorocuprate(II). Inorg. Chem. 2001, 40, 2457–2460. [Google Scholar] [CrossRef]
- Woodward, F.M.; Landee, C.P.; Giantsidis, J.; Turnbull, M.M.; Richardson, C. Structure and magnetic properties of (5BAP)2CuBr4: Magneto-structural correlations of layered S = 1/2 Heisenberg antiferromagnets. Inorg. Chim. Acta 2001, 324, 324–330. [Google Scholar] [CrossRef]
- Riley, M.J.; Boutchard, C.; Krausz, E.R.; Hitchman, M.A. Observation of a magnetic dipole origin in the spectrum of the square planar CuCl42– ion. Chem. Phys. Lett. 1996, 254, 403–409. [Google Scholar] [CrossRef]
- Halvorson, K.E.; Patterson, C.; Willett, R.D. Structures of Bis(4-aminopyridinium) Tetrachlorocuprate(II) Monohydrate, [C5H7N2]2[CuCl4].H2O, and Bis(2-amino-3-hydroxypyridinium) Tetrachlorocuprate(II), [C5H7N2O]2[CuCl4]: Correlation of CuCl42– Geometry with Hydrogen Bonding and Electronic Structure. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1990, 46, 508–519. [Google Scholar] [CrossRef]
- Garci, F.; Chebbi, H.; Rouzbeh, N.; Rochels, L.; Disch, S.; Haseloer, A.; Sebastian, S.S.; Ruschewitz, U.; Anthony, E.T.; Klein, A.; et al. Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives. Molecules 2023, 28, 731. [Google Scholar] [CrossRef]
- Hoffmann, C.; Kolks, N.; Smets, D.; Haseloer, A.; Gröner, B.; Urusova, E.A.; Endepols, H.; Neumaier, F.; Ruschewitz, U.; Klein, A.; et al. Next Generation Copper Mediators for the Efficient Production of 18F-Labeled Aromatics. Chem. Eur. J. 2023, 29, e202202965. [Google Scholar] [CrossRef]
- Wackerbarth, I.; Widhyadnyani, N.N.A.T.; Schmitz, S.; Stirnat, K.; Butsch, K.; Pantenburg, I.; Meyer, G.; Klein, A. CuII Complexes and Coordination Polymers with Pyridine or Pyrazine Amides and Amino Benzamides—Structures and EPR Patterns. Inorganics 2020, 8, 65. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Di Nicola, C.; Pettinari, C.; Skelton, B.W.; Somers, N.; White, A.H. Mechanochemical synthesis in copper(II) halide/pyridine systems: Single crystal X-ray diffraction and IR spectroscopic studies. Dalton Trans. 2011, 40, 5102–5115. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Sertucha, J.; Lezama, L.; Rojo, T.; Román, P. Synthesis, characterisation and crystal structure of 2-aminopyridinium (2-amino-5-bromopyridine)tribromocuprate(II) and bis(2-aminopyridinium) tetrabromocuprate(II). J. Chem. Soc. Dalton Trans. 1997, 1997, 847–854. [Google Scholar] [CrossRef]
- Ahmadi, R.A.; Hasanvand, F.; Bruno, G.; Rudbari, H.A.; Amani, S. Synthesis, Spectroscopy, and Magnetic Characterization of Copper(II) and Cobalt(II) Complexes with 2-Amino-5-bromopyridine as Ligand. Russ. J. Coord. Chem. 2013, 39, 867–871. [Google Scholar] [CrossRef]
- Atanasov, M.; Ganyushin, D.; Sivalingam, K.; Neese, F. A Modern First-Principles View on Ligand Field Theory Through the Eyes of Correlated Multireference Wavefunctions. Struct. Bond. 2012, 143, 149–220. [Google Scholar] [CrossRef]
- Ferguson, J. Electronic Absorption Spectrum and Structure of CuCl42−. J. Chem. Phys. 1964, 40, 3406–3410. [Google Scholar] [CrossRef]
- Winter, A.; Thiel, K.; Zabel, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 2: Tetrachloridocuprates(II). New J. Chem. 2014, 38, 1019–1030. [Google Scholar] [CrossRef]
- Farra, R.; Thiel, K.; Winter, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 1: Tetrabromidocuprates(II). New J. Chem. 2011, 35, 2793–2803. [Google Scholar] [CrossRef]
- Karoui, K.; Ben Bechir, M.; Bulou, A.; Guidara, K.; Ben Rhaiem, A. [N(CH3)3H]2CuCl4: Ab initio calculations and characterization of phase transitions by Raman spectroscopy. J. Mol. Struct. 2016, 1114, 161–170. [Google Scholar] [CrossRef]
- Hitchman, M.A.; Cassidy, P.J. Polarized Crystal Spectrum of Bis(methylphenethylammonium) Tetrachlorocuprate(II): Analysis of the Energies, Vibrational Fine Structure, and Temperature Dependence of the “d-d” Transitions of the Planar CuCl42– Ion. Inorg. Chem. 1979, 18, 1745–1754. [Google Scholar] [CrossRef]
- Kapustyanyk, V.B.; Korchak, Y.M. Thermochromic phase transition in [NH2(C2H5)3]2CuCl4 crystals. J. Appl. Spectrosc. 2000, 67, 1045–1049. [Google Scholar] [CrossRef]
- Shymkiv, R.M.; Sveleba, S.A.; Karpa, I.V.; Katerynchuk, I.N.; Kunyo, I.M.; Phitsych, E.I. Electronic Spectra and Phase Transitions in Thin [N(CH3)4]2CuCl4 Microcrystals. J. Appl. Spectr. 2012, 78, 823–828. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Loy, C.; Zeller, M.; Rosokha, S.V. Halogen Bonding in the Complexes of Brominated Electrophiles with Chloride Anions: From a Weak Supramolecular Interaction to a Covalent Br–Cl Bond. Crystals 2020, 10, 1075. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 2000, 3885–3896. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Technol. Phys. 1931, 12, 593–601, See Also English Translation by Westin, S. (An Article on Optics of Paint Layers). Available online: http://www.graphics.cornell.edu/~westin/pubs/kubelka.pdf (accessed on 8 August 2025).
- Duisenberg, A.J.M. Indexing in Single-Crystal Diffractometry with an Obstinate List of Reflections. J. Appl. Crystallogr. 1992, 25, 92–96. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]



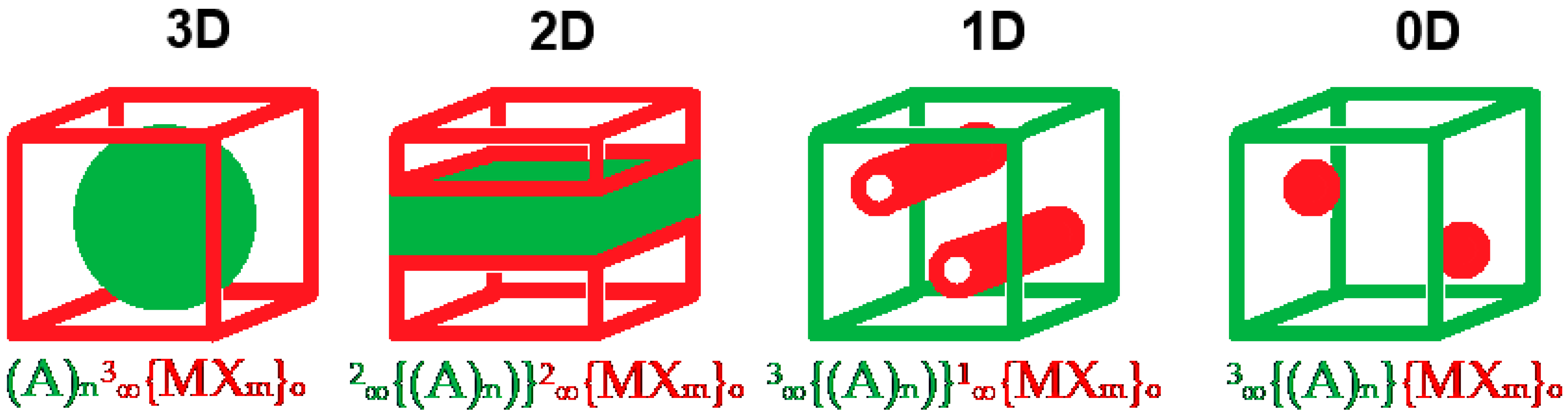
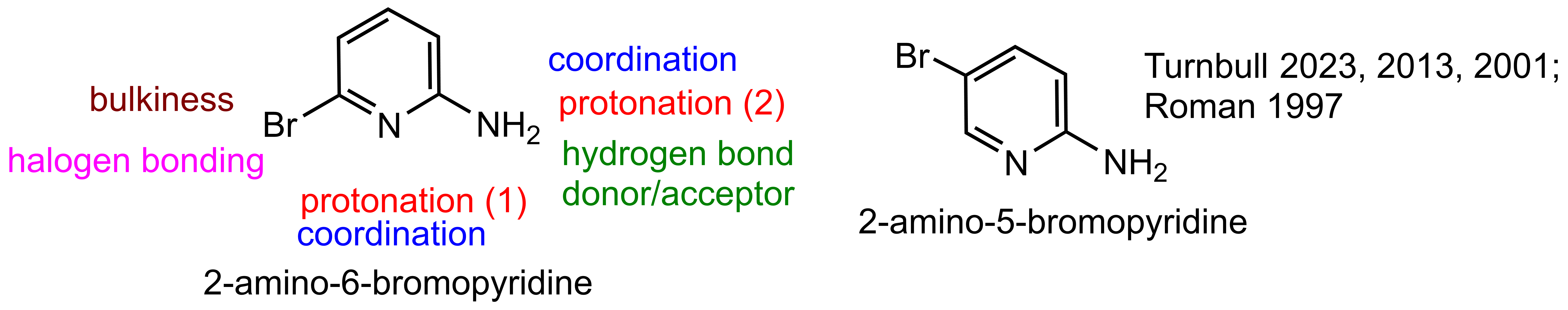
| A+ Cation | Formulae | τ4 Values | Reference |
|---|---|---|---|
| 2-amino-6-bromopyridinium | (C5H6BrN2)2[CuCl4] | 0.69 | this work |
| 2-amino-5-bromopyridinium | (C5H6BrN2)2[CuCl4] | 0.57 | [43] |
| 3-amino-2-chloropyridinium | (C5H6ClN4)2[CuCl4] | 0.58 | [54] |
| 4-tert-butyl-pyridinium | (C9H14N)2[CuCl4] | 0.58, 0.55 a | [44] |
| 2-amino-5-methylpyridinium | (C6H9N2)2[CuCl4] | 0.57 | [45] |
| 1,4′-bipyridine-1,1’-diium | (C10H10N2)[CuCl4] | 0.53 | [47] |
| 2-amino-4-methylpyridinium | (C6H9N2)2[CuCl4] | 0.48 | [53] |
| 2,2,6,6-tetramethylpiperidinium-1-yl)oxidanyl | (C9H19NO)2[CuCl4] | 0.70 | [48] |
| 4-amino-2-methylpyridinium | (C6H9N2)2[CuCl4] | 0.44 | [51] |
| 4-amino-2-chloropyridinium | (C5H6ClN2)2[CuCl4] | 0.62 | [51] |
| 2-methyl-pyridinium | (C6H8N)2[CuCl4] | 0.67 | [46] |
| 4-dimethylamino-pyridinium | (C7H11N2)2 [CuCl4] b | 0.42/0.86 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbali, L.; Stojadinovic, V.K.; Klein, A.; Chebbi, H. The Impact of Supramolecular Forces on the Magnetic and Optical Properties of Bis(2-amino-6-bromopyridinium) Tetrachloridocuprate (C5H6BrN2)2[CuCl4]. Inorganics 2025, 13, 339. https://doi.org/10.3390/inorganics13100339
Ghorbali L, Stojadinovic VK, Klein A, Chebbi H. The Impact of Supramolecular Forces on the Magnetic and Optical Properties of Bis(2-amino-6-bromopyridinium) Tetrachloridocuprate (C5H6BrN2)2[CuCl4]. Inorganics. 2025; 13(10):339. https://doi.org/10.3390/inorganics13100339
Chicago/Turabian StyleGhorbali, Lokmen, Vladimir Kjartan Stojadinovic, Axel Klein, and Hammouda Chebbi. 2025. "The Impact of Supramolecular Forces on the Magnetic and Optical Properties of Bis(2-amino-6-bromopyridinium) Tetrachloridocuprate (C5H6BrN2)2[CuCl4]" Inorganics 13, no. 10: 339. https://doi.org/10.3390/inorganics13100339
APA StyleGhorbali, L., Stojadinovic, V. K., Klein, A., & Chebbi, H. (2025). The Impact of Supramolecular Forces on the Magnetic and Optical Properties of Bis(2-amino-6-bromopyridinium) Tetrachloridocuprate (C5H6BrN2)2[CuCl4]. Inorganics, 13(10), 339. https://doi.org/10.3390/inorganics13100339









