Abstract
The tumor microenvironment (TME) is characterized by hypoxia; acidic pH; oxidative stress; and immune suppression; all of which severely impair the efficacy of conventional cancer therapies. Recent advances in inorganic nanotechnology have led to the development of smart nanomaterials capable of modulating these abnormal features; thereby reprogramming the TME toward a more therapy-responsive state. Inorganic nanomaterials such as manganese dioxide; iron oxide; and cerium oxide can selectively alleviate hypoxia; buffer acidity; regulate redox balance; and even stimulate anti-tumor immunity through catalytic or structural mechanisms. These materials can further serve as carriers for stimuli-responsive drug delivery; enabling synergistic therapies that include chemodynamic; photothermal; and immunomodulatory treatments. This review summarizes recent developments in smart inorganic nanomaterials for TME modulation; discusses design considerations including biosafety and biodegradability; and evaluates the current translational status and future directions. Such strategies represent a promising leap toward precise and personalized cancer nanomedicine
1. Introduction
Cancer is not just a disease of rapidly dividing cells. It occurs within a complex and dynamic setting called the tumor microenvironment (TME) [1,2]. The TME includes not only tumor cells but also various non-malignant components. These include immune cells, fibroblasts, blood vessels, and extracellular matrix proteins [3,4]. The TME is not passive. It plays an active role in promoting tumor growth, invasion, and metastasis. It also protects tumors from therapeutic agents. Among its many characteristics, four stand out as key contributors to treatment resistance: hypoxia, acidity, oxidative stress, and immune suppression [5,6].
Hypoxia, or low oxygen levels, is common in solid tumors. Tumor cells often grow faster than their blood supply can support. This creates regions within the tumor that lack sufficient oxygen [7,8]. Hypoxia leads to the activation of specific genes that help cancer cells survive. It also reduces the effectiveness of therapies such as radiotherapy and some forms of chemotherapy. Acidic pH is another hallmark of the TME. Tumor cells often switch to a type of metabolism called aerobic glycolysis [4,9]. This produces large amounts of lactic acid. As a result, the tumor becomes acidic, especially in its extracellular environment. This acidic environment can degrade therapeutic drugs or prevent them from entering tumor cells effectively. High levels of reactive oxygen species (ROS) are also common in tumors [10,11]. While ROS can damage cells, cancer cells often tolerate and exploit them. High ROS can promote genetic mutations, drive cancer progression, and trigger resistance to treatment. Finally, the TME is often immunosuppressive. Tumor cells recruit immune cells like regulatory T cells (Tregs) or tumor-associated macrophages (TAMs) that suppress immune responses [12,13]. This makes it difficult for the immune system to recognize and destroy cancer cells. Immunosuppression in the TME is one of the major barriers to the success of immunotherapies.
Traditional cancer therapies include surgery, chemotherapy, radiotherapy, and immunotherapy. Each has made important contributions to cancer care. However, their effectiveness is often reduced by the hostile and abnormal conditions of the TME. Chemotherapy drugs rely on blood flow to reach the tumor [4,14]. But in many tumors, the blood vessels are irregular and leaky. This makes drug delivery inefficient. Even when the drugs reach the tumor, acidic pH and high glutathione (GSH) levels can deactivate them. Radiotherapy is also less effective in hypoxic environments [15,16]. Oxygen is required to generate free radicals that damage cancer cell DNA. Without oxygen, radiation becomes significantly less lethal to tumor cells. Immunotherapies have revolutionized treatment for many cancers [17,18]. But they often fail in “cold” tumors, which lack active immune cell infiltration. The immunosuppressive TME further limits the activation of T cells, even after checkpoint blockade therapies. These challenges reveal a major problem. Conventional therapies are designed for normal tissues, not for the unique biology of tumors. The abnormal features of the TME create a protective shield around cancer cells. Overcoming this shield requires new therapeutic strategies that can adapt to or modify the TME itself.
To address the challenges posed by the TME, researchers are turning to smart inorganic nanomaterials. These are tiny particles, often less than 100 nanometers in size, made from metals or metal oxides. They can be engineered to respond to the specific features of the TME. These materials are “smart” because they are stimuli-responsive. For example, some release their drug payload only in acidic conditions [19,20]. Others activate in the presence of high ROS or reducing agents like glutathione. This responsiveness allows them to deliver therapies directly to tumor sites while sparing healthy tissue. One key advantage of inorganic materials is their catalytic activity. Materials like manganese dioxide (MnO2) can react with tumor-derived hydrogen peroxide to produce oxygen [21,22]. This can relieve hypoxia and improve the effectiveness of radiotherapy or photodynamic therapy [23]. Iron oxide nanoparticles can trigger Fenton reactions in the tumor. These reactions convert hydrogen peroxide into hydroxyl radicals, which are toxic to cells. This forms the basis of chemodynamic therapy, a new approach that relies on the TME to generate therapeutic effects. Some inorganic nanoparticles also serve as imaging agents. Gold, cerium, and gadolinium-based nanoparticles enhance imaging techniques such as MRI, CT, and photoacoustic imaging. This allows for image-guided therapy, where clinicians can track treatment in real-time. In addition, inorganic nanomaterials can modulate the immune environment. For example, certain iron-based materials can reprogram tumor-associated macrophages from an immunosuppressive M2 type to an inflammatory M1 type. This can help “heat up” cold tumors and make them more responsive to immunotherapy. As research advances, these smart inorganic systems are becoming more multi-functional. They can carry drugs, generate oxygen, regulate redox balance, and support imaging—all in a single platform. This multi-modal approach is especially valuable in tackling the complexity of the TME.
This review focuses on the design, function, and application of smart inorganic nanomaterials for modulating the tumor microenvironment. We explore how these materials are used to target specific features of the TME—such as hypoxia, acidity, redox imbalance, and immune suppression. We provide a detailed overview of the components and characteristics of the TME. This sets the stage for understanding why it is such a critical barrier to effective treatment. We categorize the different types of inorganic nanomaterials used in cancer therapy. This includes metal oxides, noble metals, carbon-based materials, and more. We cover hypoxia relief, pH buffering, redox regulation, and immune reprogramming. We review how these strategies enable multi-modal therapies, including chemo, photo, radio, and immunotherapies. Finally, outlines current challenges and future perspectives. We discuss how nanotechnology, combined with machine learning and personalized medicine, might transform cancer therapy. Through this structured approach, we aim to provide a comprehensive and accessible overview of how smart inorganic nanomaterials are reshaping the future of cancer treatment by overcoming the long-standing barriers of the tumor microenvironment.
2. Overview of the Tumor Microenvironment (TME)
Cancer is not just a disease of rogue cells. It is deeply influenced by the biological environment in which it develops. This environment, known as the tumor microenvironment, plays a central role in cancer progression, treatment resistance, and immune evasion.
2.1. Key Components of the TME
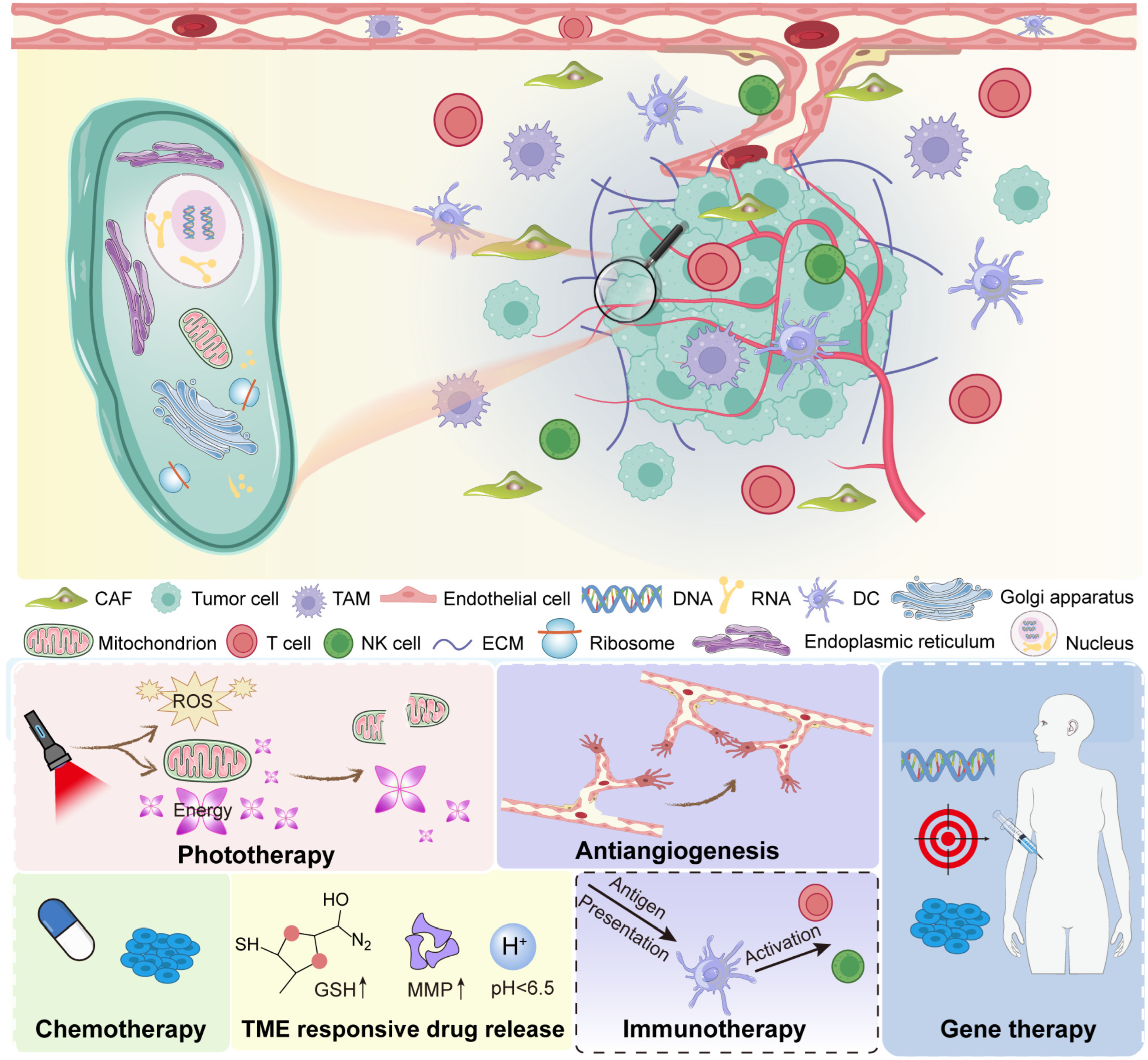
The tumor microenvironment is a complex and dynamic system (Figure 1). It contains both cancerous and non-cancerous elements. Together, they form an ecosystem that supports tumor survival. Cancer cells are the core of the tumor. They undergo uncontrolled growth due to genetic mutations and epigenetic changes [24,25]. However, they do not act alone. They communicate with surrounding cells and adapt to environmental stress. This ability to co-evolve with their surroundings is a major reason why cancer is hard to treat. Cancer cells release signals. These signals recruit and reprogram nearby cells. As a result, the tumor gains access to nutrients, avoids immune attack, and promotes blood vessel formation. These are classic examples of how cancer cells shape their environment. Stromal cells are non-cancerous cells found in the TME. These include fibroblasts, endothelial cells, and mesenchymal stem cells. One of the most important stromal cells in tumors is the cancer-associated fibroblast (CAF) [26,27]. CAFs differ from normal fibroblasts. They are activated by tumor signals and support cancer progression. CAFs secrete growth factors, remodel the extracellular matrix, and influence immune cell behavior [28]. Endothelial cells form the lining of blood vessels. In tumors, they help create abnormal vasculature. These blood vessels are often irregular, leaky, and poorly organized. This limits the delivery of oxygen and drugs into the tumor core.
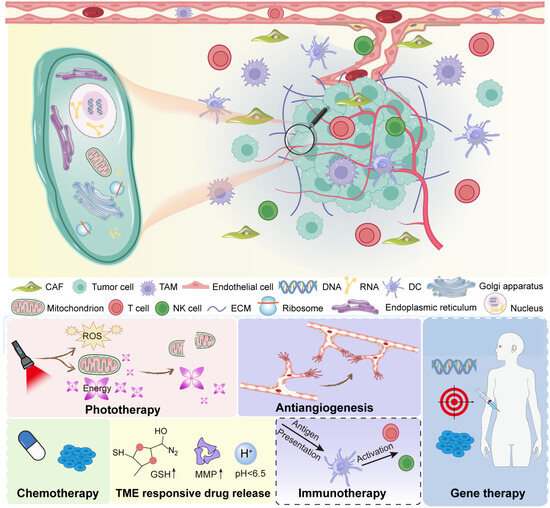
Figure 1.
Overview of the tumor microenvironment.
Immune cells are a major component of the TME. However, their role is complex. In some cases, immune cells fight cancer. Cytotoxic T cells and natural killer (NK) cells can recognize and destroy tumor cells [29,30]. But in many tumors, immune cells are reprogrammed to help the tumor. For example, TAMs often support tumor growth [31,32]. Instead of attacking cancer, they suppress other immune cells, promote angiogenesis, and enhance metastasis. Tregs also help the tumor by preventing effective immune responses. Myeloid-derived suppressor cells (MDSCs) further block immune activity. Together, these cells create an immunosuppressive environment. The ECM is a network of proteins and molecules surrounding the cells [33,34]. It provides structure and biochemical signals. In tumors, the ECM is often dense and disorganized. This stiff matrix can act as a barrier to drug penetration. It can also send signals that promote cancer cell survival and migration. Collagen, fibronectin, and hyaluronic acid are commonly overexpressed in the tumor ECM [35,36]. These molecules interact with integrins and other cell receptors to affect cell behavior. In short, the ECM is not just a scaffold. It is an active regulator of cell fate in the tumor setting.
2.2. Major Abnormalities in the Tumor Microenvironment
The TME is not like normal tissue. It is hostile, stressful, and highly altered. These changes affect how the tumor behaves and how it responds to therapy. Here, we discuss the four most prominent abnormalities: hypoxia, acidity, oxidative stress, and glutathione (GSH) overexpression. Hypoxia refers to low oxygen levels. It is a defining feature of many solid tumors [37,38]. As tumors grow, they often outpace their blood supply. This results in regions that receive little to no oxygen. Cells in these regions adapt by activating hypoxia-inducible factors (HIFs). These are transcription factors that help cells survive under low oxygen. HIFs trigger the expression of genes that promote angiogenesis, anaerobic metabolism, and drug resistance [39,40]. They also increase the expression of immune checkpoint molecules, which helps the tumor evade immune detection. Hypoxia makes tumors more aggressive. It also reduces the effectiveness of radiotherapy, which relies on oxygen to generate free radicals that kill cancer cells.
Tumors are acidic. This is mainly due to the Warburg effect, where cancer cells rely on glycolysis even in the presence of oxygen [41,42]. This produces lactic acid, which is then secreted into the extracellular space. The result is a low extracellular pH, often around 6.5 or lower. Normal tissues typically have a pH of 7.4. Acidic pH has multiple effects. It impairs drug uptake, especially for weakly basic drugs. It also alters enzyme activity and immune cell function. Moreover, acidic conditions can promote the activation of certain proteases that help cancer cells invade surrounding tissues. Importantly, acidity is not uniform. Some regions of the tumor are more acidic than others. This creates a patchy landscape that complicates treatment.
Cancer cells often have high levels of ROS. These are chemically reactive molecules that include superoxide, hydrogen peroxide, and hydroxyl radicals [43,44]. ROS are produced during metabolism and signaling. In tumors, their levels are elevated due to mitochondrial dysfunction, rapid growth, and inflammation. High ROS levels can damage DNA, proteins, and lipids. This drives mutations and genomic instability. But cancer cells often adapt. They develop antioxidant defenses to tolerate the oxidative stress [45,46]. ROS also influence signaling pathways. They activate NF-κB, HIF-1, and MAPK pathways, all of which promote survival, proliferation, and invasion. ROS can also affect the immune system [47,48]. Moderate ROS levels can suppress T cell activation and contribute to immune escape.
To counter high ROS, cancer cells increase their levels of antioxidants. The most important of these is glutathione (GSH). GSH is a tripeptide that directly neutralizes ROS. It is present at high concentrations in many tumors, often several times higher than in normal tissue [49,50]. High GSH helps cancer cells resist oxidative damage. It also contributes to drug resistance. Many chemotherapy agents work by generating ROS. When GSH levels are high, these drugs become less effective. GSH also plays a role in detoxification. It conjugates with drugs and toxins to make them easier to eliminate. This further reduces treatment effectiveness. In summary, elevated GSH levels give cancer cells a survival advantage in the harsh tumor environment.
2.3. The Tumor Microenvironment as a Therapeutic Target
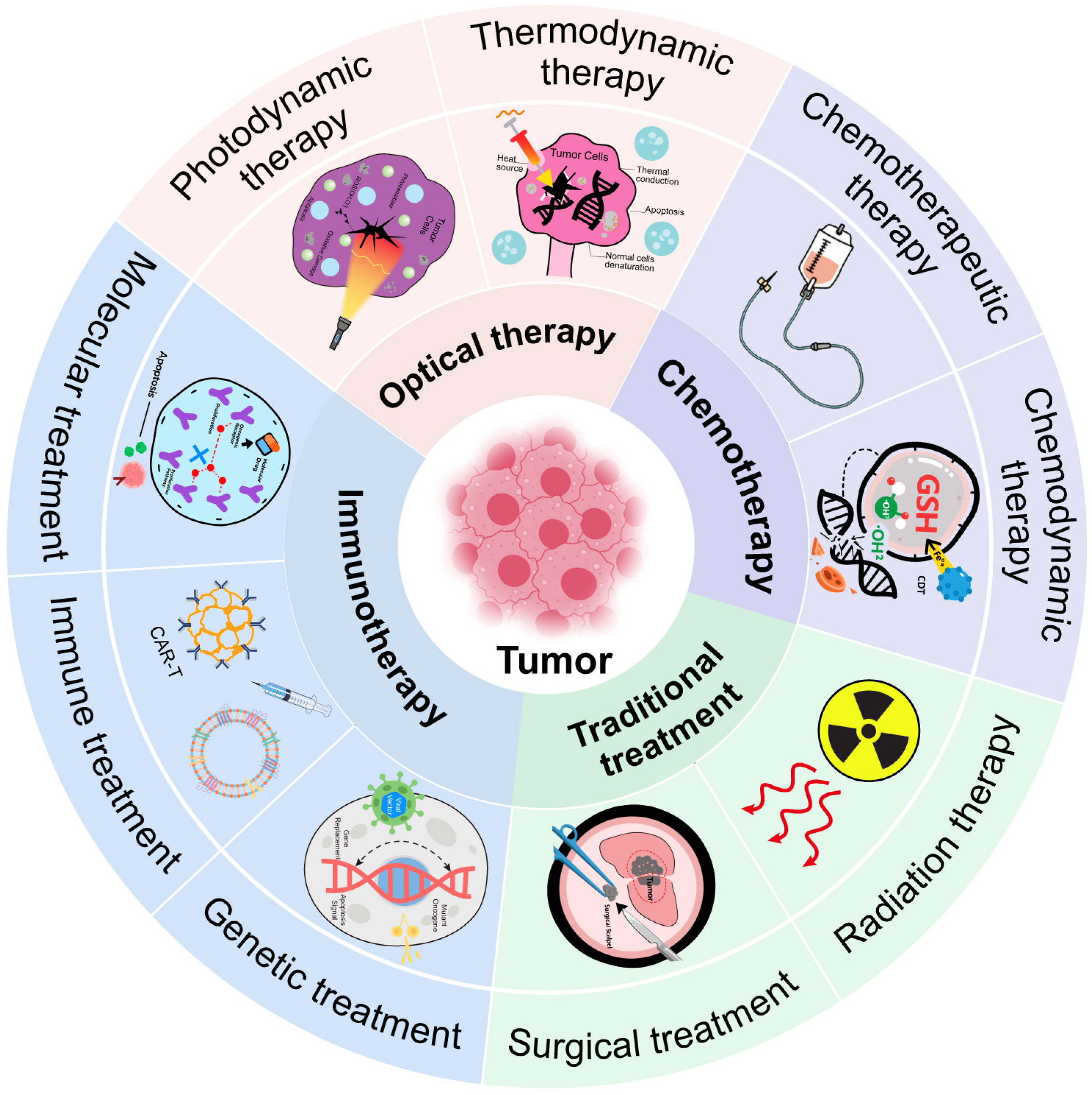
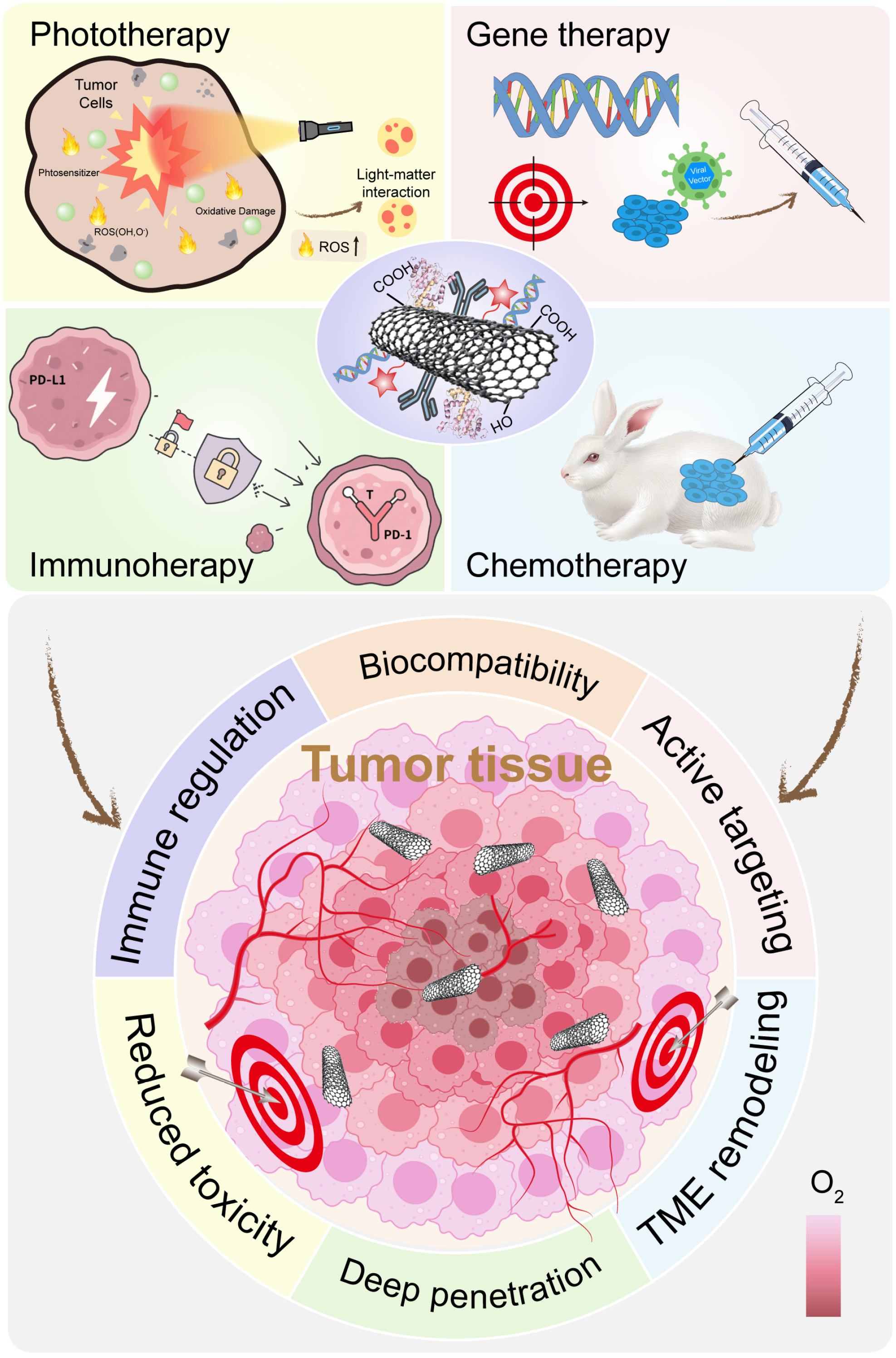
The unique features of the TME present both challenges and opportunities. While they help the tumor resist treatment, they also offer targets for therapy (Figure 2). One approach is to deliver oxygen or generate it in situ. Materials like manganese dioxide (MnO2) can react with tumor hydrogen peroxide to produce oxygen [51,52]. This relieves hypoxia and improves the efficacy of radiotherapy or photodynamic therapy. Another approach is to exploit hypoxia. Certain prodrugs are activated only in low-oxygen conditions. These selectively kill hypoxic tumor cells while sparing normal tissue. Acidic pH can be used as a trigger for drug release. pH-sensitive materials can release their payloads only in acidic conditions. This improves selectivity and reduces side effects. Buffering the tumor pH is another strategy. Compounds like bicarbonate can neutralize acidity, although clinical translation remains difficult.
Figure 2.
Summary of tumor therapy.
Chemodynamic therapy (CDT) uses Fenton or Fenton-like reactions to convert tumor hydrogen peroxide into toxic hydroxyl radicals [53,54]. Iron, copper, or manganese-based materials can catalyze these reactions. This increases ROS and kills tumor cells from within. To enhance CDT, researchers are designing nanoparticles that also deplete GSH. Reducing GSH makes tumor cells more vulnerable to ROS. This dual approach maximizes oxidative damage. The TME’s immunosuppressive nature can also be reversed. Certain nanoparticles can repolarize tumor-associated macrophages from an M2 (suppressive) state to an M1 (pro-inflammatory) state. Others can deliver antigens or adjuvants to dendritic cells to improve immune activation. Nanomaterials are also being used to deliver checkpoint inhibitors more precisely.
3. Classes of Inorganic Nanomaterials Used in TME Modulation
Inorganic nanomaterials have emerged as powerful tools in the fight against cancer. They offer unique physical and chemical properties that can be harnessed to interact with the TME. These materials can respond to specific features such as acidity, hypoxia, and oxidative stress.
3.1. Metal Oxides
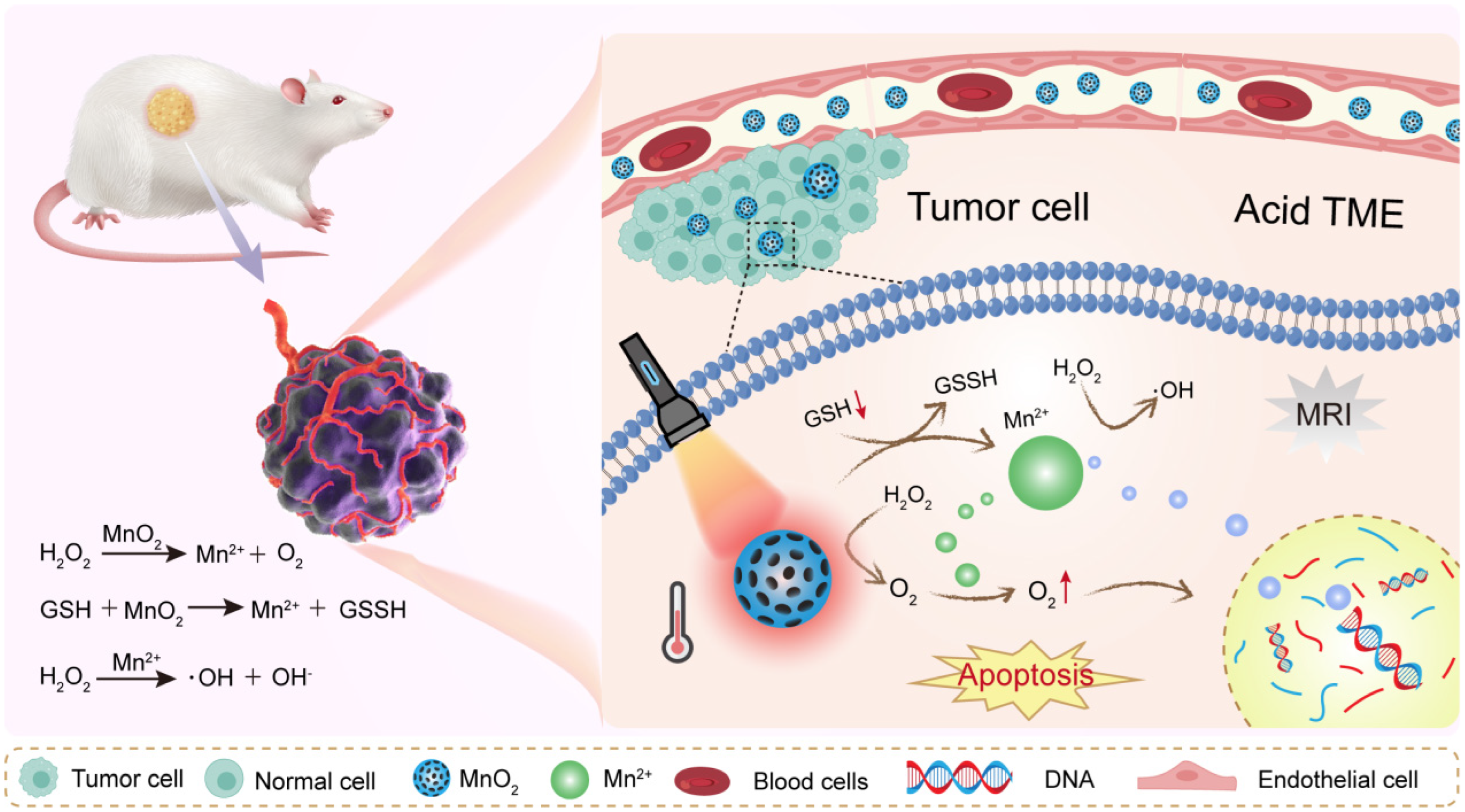
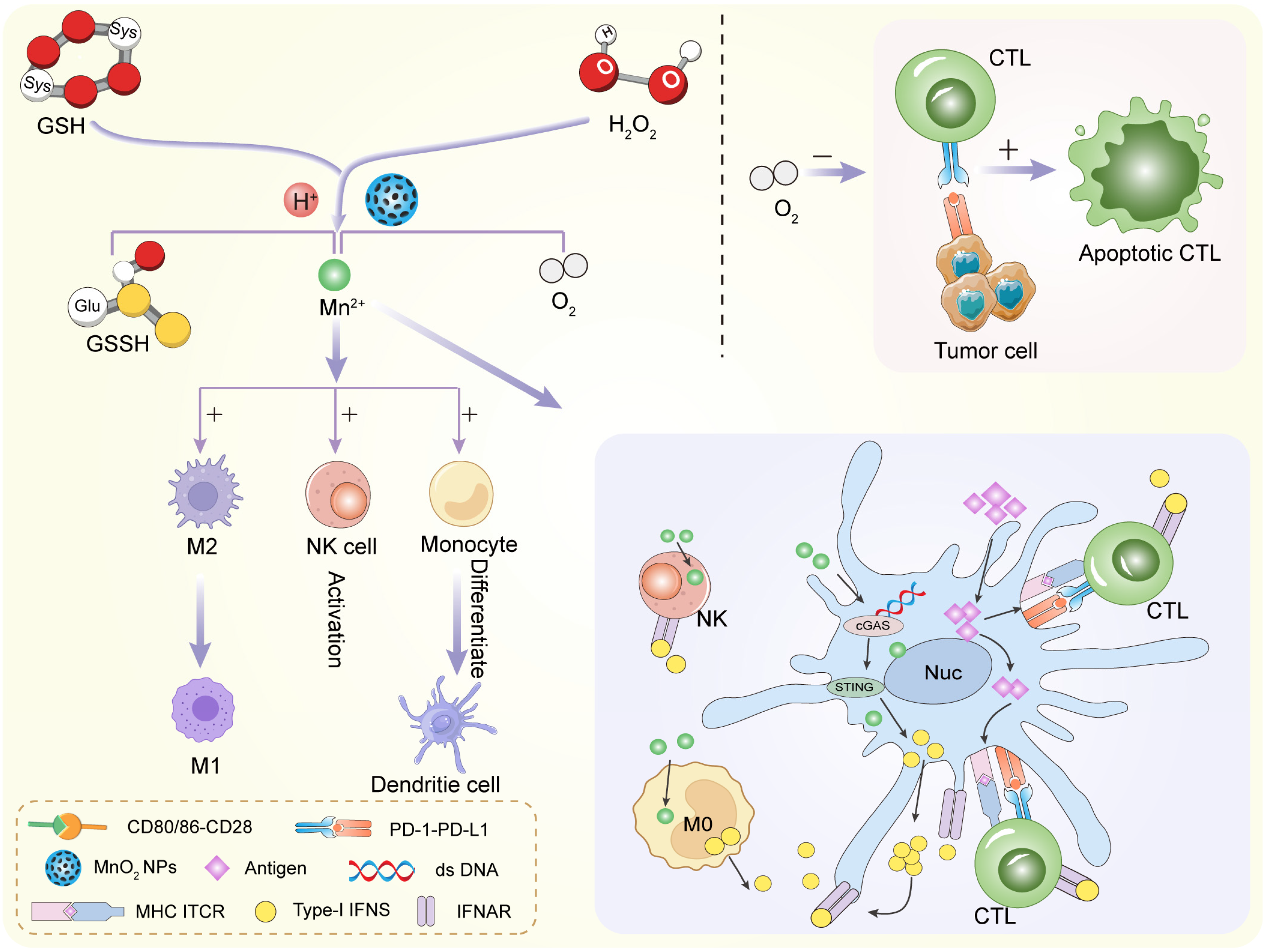
Metal oxides are among the most widely studied materials for TME modulation. They are chemically versatile and often exhibit catalytic or redox activity. Their ability to react with molecules like hydrogen peroxide or glutathione makes them especially useful in hostile tumor environments. Manganese dioxide is a standout in TME-focused research. It reacts efficiently with hydrogen peroxide (H2O2), which is abundant in tumors. This reaction produces oxygen, which helps relieve hypoxia. In doing so, MnO2 can enhance the performance of radiotherapy and photodynamic therapy [55,56] (Figure 3). MnO2 also degrades in acidic conditions. This is useful because many tumors have a low extracellular pH. When MnO2 breaks down, it releases Mn2+ ions. These ions can serve as contrast agents in magnetic resonance imaging (MRI), enabling real-time monitoring [57,58]. Moreover, MnO2 can reduce levels of glutathione (GSH). This weakens the tumor’s antioxidant defenses and enhances oxidative damage. That is why MnO2 is often used in chemodynamic therapy (CDT) strategies. In summary, MnO2 is multifunctional. It relieves hypoxia, lowers GSH, and enhances both imaging and therapy. These features make it a central material in smart cancer nanomedicine.
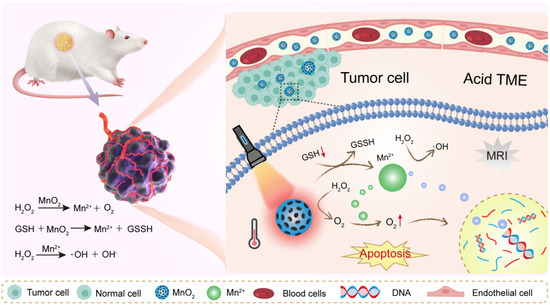
Figure 3.
MnO2 NPs remodel tumor microenvironment by reacting with H2O2 to release oxygen and Mn2+.
Iron oxide nanoparticles are already approved for clinical use in imaging. They also show promise in cancer therapy. Their value lies in their ability to catalyze the Fenton reaction. In this reaction, ferrous ions (Fe2+) convert hydrogen peroxide into hydroxyl radicals. These radicals are extremely reactive and toxic to cells [59,60]. By generating reactive oxygen species (ROS) within tumors, iron oxide promotes chemodynamic therapy. This is especially effective in tumors with high H2O2 levels [61]. Iron oxide nanoparticles can also modulate immune responses. Some studies suggest they help repolarize tumor-associated macrophages. This can turn the tumor from an immunosuppressive to an immunostimulatory environment. Additionally, Fe3O4 can serve as a delivery vehicle for drugs [62,63]. It can be guided to tumors using external magnetic fields. This improves targeting and reduces off-target toxicity. In short, iron oxide is a flexible and clinically relevant material. It works both as a therapeutic agent and an imaging enhancer.
Cerium oxide is known for its redox-switching behavior. It can shift between Ce3+ and Ce4+ oxidation states. This makes it useful for regulating oxidative stress in tumors [64,65]. Interestingly, CeO2 shows dual activity. In normal cells, it scavenges excess ROS and protects tissues. In tumor cells, where the redox balance is already disrupted, it may behave differently. Some studies suggest it can actually increase ROS under certain conditions. CeO2 nanoparticles also influence inflammation [66,67]. They can inhibit pro-inflammatory cytokines and reduce tumor-promoting inflammation. This immune-regulatory effect is still being explored. Its antioxidant and pro-oxidant duality give cerium oxide a unique role. It is promising in cases where fine-tuned redox control is needed.
3.2. Noble Metals
Noble metal nanoparticles have distinct surface chemistry and photothermal properties. They are chemically stable and easy to modify. Among these, gold and platinum are the most commonly used in TME-targeted therapies. Gold nanoparticles (AuNPs) are highly popular in cancer nanomedicine. They are biocompatible and easily functionalized. Their surfaces can be modified with drugs, peptides, or targeting ligands [67,68]. One of their key strengths is their optical property. They absorb near-infrared light and convert it into heat. This makes them excellent agents for photothermal therapy (PTT). In tumors, this localized heating can disrupt cancer cells without damaging surrounding tissues. In addition to photothermal effects, AuNPs can act as redox modulators. Their surfaces can be engineered to interact with glutathione or deliver redox-active compounds. This helps disturb the tumor’s antioxidant defense. Gold nanoparticles are also valuable in imaging. They enhance contrast in computed tomography (CT) and other imaging modalities. This supports image-guided treatment strategies [69,70]. AuNPs are safe, versatile, and multifunctional. Their ease of use makes them ideal platforms for combined diagnostic and therapeutic applications.
Platinum-based drugs like cisplatin have long been used in chemotherapy. However, platinum nanoparticles offer a new level of control. PtNPs can catalyze ROS production in tumors. Like iron-based materials, they can participate in redox reactions that generate toxic radicals [71,72]. These nanoparticles can amplify oxidative stress and kill tumor cells from within. PtNPs can also degrade in acidic environments. This makes them suitable for pH-sensitive drug delivery. Some formulations combine PtNPs with chemotherapeutic agents to create synergistic systems [73,74]. Moreover, platinum has high X-ray absorption. This enhances its role in radiotherapy. PtNPs can increase the radiation dose absorbed by the tumor while sparing healthy tissues. While toxicity remains a concern, new surface coatings and targeting methods are improving their safety profiles.
3.3. Carbon-Based Nanomaterials
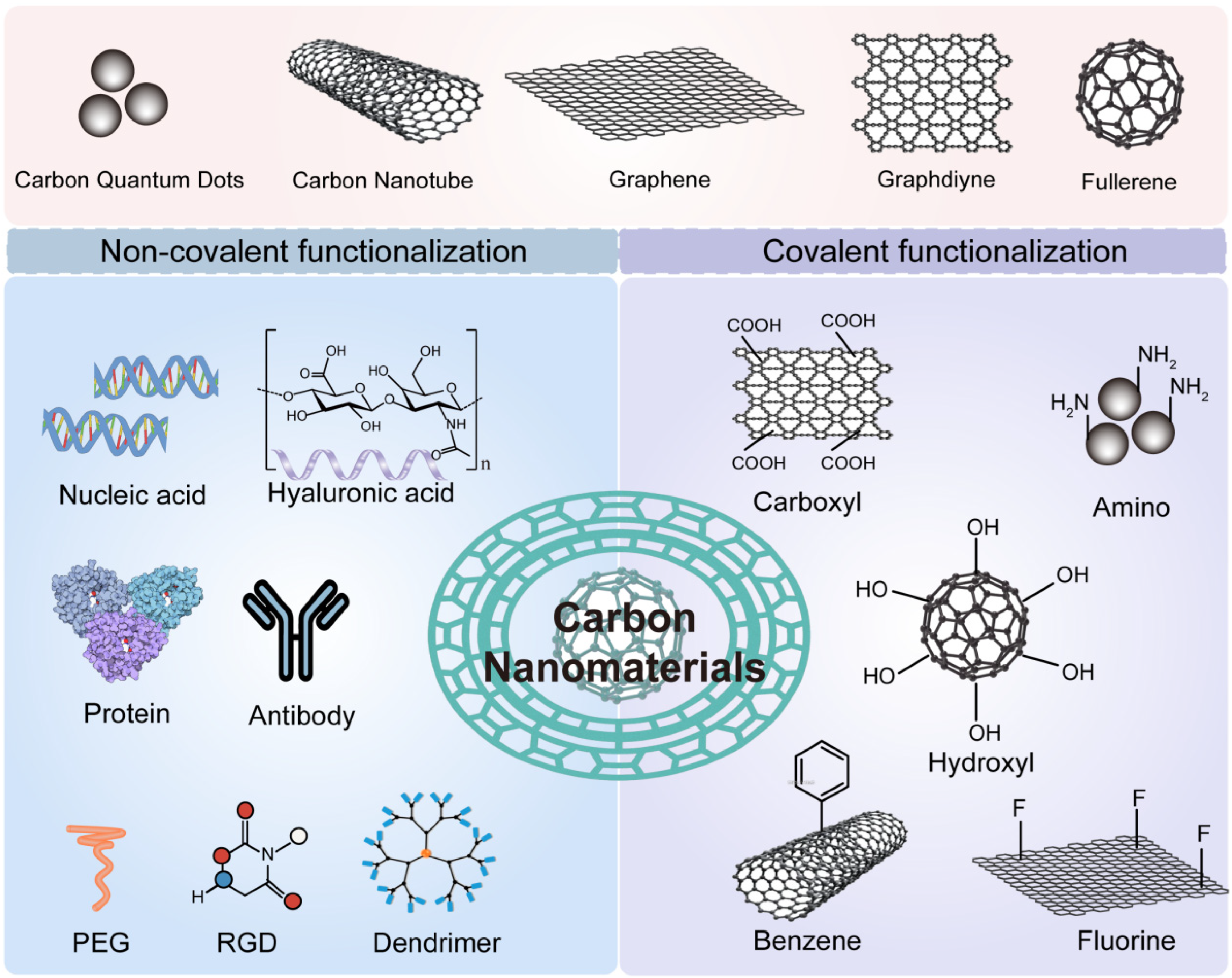
Carbon-based materials have emerged as a versatile category in cancer therapy. They are lightweight, biocompatible, and rich in surface functionalities (Figure 4). Their ability to modulate ROS and serve as photothermal agents is especially valuable in the TME context. Graphene oxide (GO) is a single-atomic-layer material with oxygen-containing groups. It offers a large surface area and good dispersibility in water [75,76]. GO can absorb near-infrared light and generate heat. This makes it a powerful tool for photothermal therapy. It can also carry drugs and release them in response to TME stimuli such as pH or GSH. Additionally, GO has redox-modulating properties. It can interact with ROS and contribute to oxidative stress in tumor cells. Functionalization improves its performance. Modified GO with polyethylene glycol (PEG) or targeting ligands shows improved circulation and tumor accumulation [77,78]. Its 2D structure also allows for easy loading of imaging agents. This opens opportunities for multimodal theranostics.
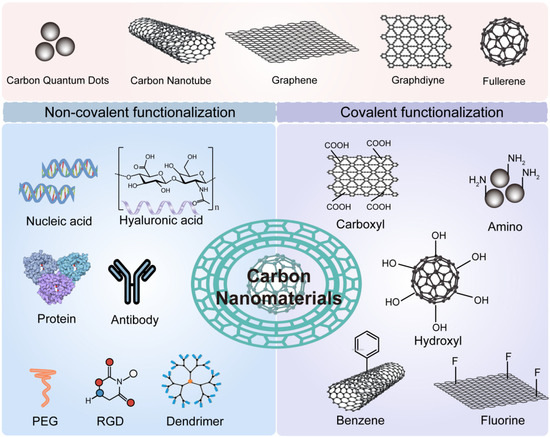
Figure 4.
Schematic of Carbon Nanomaterial Functionalization. Carbon nanomaterials can be functionalized via covalent or non-covalent approaches using diverse chemical groups and biomolecules. This modification enhances their anticancer therapeutic efficacy.
Carbon dots (CDs) are small, fluorescent carbon-based nanoparticles. They are highly tunable and photostable. Their small size allows for deep tumor penetration. CDs can scavenge ROS or generate ROS, depending on their surface chemistry. This makes them adaptable to different tumor environments. They are also excellent imaging agents. Many CDs exhibit intrinsic fluorescence, which is useful for optical tracking [79,80]. Some carbon dots have also been developed to carry therapeutic cargo. Their tunable surface allows for stimuli-sensitive drug release. Overall, carbon-based materials offer a flexible platform for TME modulation. They combine diagnostic and therapeutic capabilities in compact designs.
3.4. Other Inorganic Materials
Beyond metal oxides, noble metals, and carbon forms, several other inorganic materials are gaining attention. These include calcium carbonate, silica, black phosphorus, and metal sulfides. Calcium carbonate is a naturally occurring, biocompatible material. It dissolves in acidic environments, making it ideal for pH-responsive drug delivery [81,82]. In the acidic TME, CaCO3 particles degrade and release calcium ions. These ions can disrupt calcium signaling in cancer cells and trigger cell death. CaCO3 also helps buffer the acidic TME. This makes it a useful adjunct to therapies that are pH-sensitive. The simplicity and safety of CaCO3 make it appealing for clinical translation. Mesoporous silica nanoparticles (MSNs) are well-studied in nanomedicine. They have a tunable pore structure, large surface area, and good biocompatibility [83]. MSNs can load various drugs and release them in response to TME triggers. Acid-sensitive gatekeepers or enzyme-cleavable coatings allow precise control over release kinetics [84]. Silica is also used to coat other nanomaterials, improving their stability and safety. While inert on their own, MSNs gain functionality through surface modifications. This adaptability allows for customization based on tumor needs.
Black phosphorus is an emerging 2D material with excellent biodegradability. It breaks down into phosphate, a harmless by-product. BP absorbs near-infrared light and efficiently converts it to heat [85,86]. This makes it a potent photothermal agent. It also contributes to ROS production under light exposure, supporting photodynamic therapy. Its soft, layered structure allows it to carry drugs or nucleic acids. However, its instability in aqueous environments remains a technical challenge. Metal sulfides such as copper sulfide (CuS) and bismuth sulfide (Bi2S3) are gaining interest. These materials are photoactive and generate heat or ROS under light or ultrasound stimulation. CuS, for instance, is a strong absorber of NIR light. It has been used in photothermal and photoacoustic imaging. Bismuth-based sulfides offer high X-ray attenuation. This makes them excellent for CT imaging and radiosensitization. Metal sulfides can also modulate redox balance. Some can deplete glutathione or generate ROS in situ. These features support TME-targeted therapies.
4. Strategies for Tumor Microenvironment Modulation Using Inorganic Nanomaterials
The tumor microenvironment is an abnormal and hostile landscape. It is shaped by low oxygen levels, acidic pH, oxidative stress, and immune evasion. These features help cancer cells survive and resist therapy. However, these same features can be used as targets. Smart inorganic nanomaterials are designed to exploit these characteristics. They can respond to local cues and activate therapeutic actions only within the tumor.
4.1. Alleviating Hypoxia
Hypoxia means low oxygen. It is one of the most common features in solid tumors. Poor blood supply and rapid tumor growth both contribute to it. Hypoxia makes tumors more aggressive and resistant to treatments like radiotherapy and some chemotherapies. Radiation therapy depends on oxygen to generate free radicals. When oxygen is low, the effect is weakened. Hypoxia also supports immune evasion and promotes metastasis. It is a serious barrier to effective cancer treatment.
To overcome hypoxia, researchers have turned to oxygen-generating or oxygen-carrying nanoparticles. These materials can increase oxygen levels directly at the tumor site [87,88]. One of the most studied materials isMnO2. MnO2 reacts with hydrogen peroxide (H2O2), which is naturally overproduced in tumors (Figure 5). The reaction produces oxygen: 2 H2O2 + MnO2 → O2 + 2 H2O + Mn2+. This simple reaction helps supply oxygen locally. The produced oxygen enhances the effects of radiotherapy or photodynamic therapy. At the same time, MnO2 breaks down in the acidic tumor environment. This improves its biodegradability and biocompatibility.
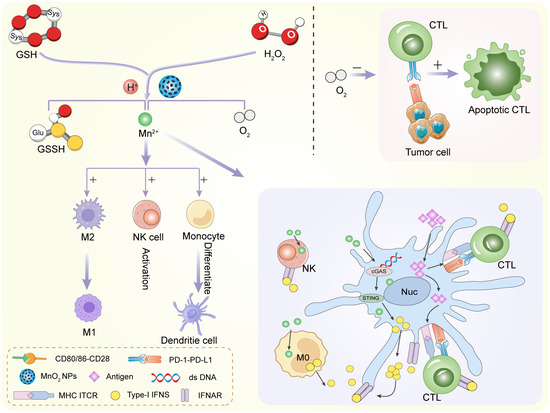
Figure 5.
MnO2 NPs regulate innate and adaptive immune cells against tumor and infections.
Other strategies involve perfluorocarbon (PFC)-loaded nanoparticles. PFCs can physically carry and release oxygen [89,90]. These particles are often combined with inorganic carriers like iron oxide or silica for imaging and targeting. After injection, they accumulate in tumors and gradually release oxygen. Some nanoparticles mimic the enzyme catalase, which naturally decomposes hydrogen peroxide into water and oxygen. Materials like platinum, cerium oxide, and iron oxide can perform this reaction. They act as nanozymes—catalytic nanomaterials that behave like enzymes. These materials do not just deliver oxygen. They also relieve oxidative stress and improve cell function. This is helpful for activating immune responses that are otherwise shut down by hypoxia. In short, alleviating hypoxia is a promising way to enhance the success of many therapies. Inorganic nanomaterials offer smart and effective ways to generate oxygen inside tumors.
4.2. pH Modulation
Tumors are acidic. This acidity results from altered metabolism. Cancer cells rely heavily on glycolysis, even in the presence of oxygen. This leads to lactic acid accumulation. Proton pumps and poor blood flow make the situation worse.
The extracellular pH in tumors can fall below 6.5, compared to 7.4 in normal tissues. This low pH affects drug stability, uptake, and cell signaling. It also helps tumors invade nearby tissues. Inorganic nanomaterials can respond to pH. Some materials degrade or change structure in acidic conditions [91,92]. This feature is used for controlled drug release. For example, calcium carbonate dissolves in low pH. In the acidic tumor, CaCO3 breaks down into calcium ions and carbon dioxide. This neutralizes local acidity and also promotes cell stress.
Another widely used material is ZIF-8, a zinc-based metal–organic framework. ZIF-8 is stable in blood but dissolves in acidic conditions. The main products are Zn2+ and 2-methylimidazole, both of which are involved in antitumor effects. This allows it to release drugs specifically inside the tumor. These pH-responsive behaviors improve drug precision. They help minimize damage to normal tissues. Some materials do more than just degrade. They can actively buffer the acidity. Magnesium oxide, CaCO3, and other proton-scavenging materials absorb protons and raise the pH. This can normalize the extracellular environment. A more neutral pH improves the function of T cells and natural killer (NK) cells. It also enhances drug uptake and reduces tumor invasiveness. By altering pH, nanomaterials not only make drugs more effective but also improve immune activation. This dual effect makes pH modulation a powerful therapeutic strategy.
4.3. Redox Balance Regulation
Tumors have an abnormal redox environment. They contain high levels of ROS like hydrogen peroxide. At the same time, they also overexpress antioxidants, mainly GSH. ROS promote mutations and tumor growth. But cancer cells must control ROS to avoid death [93,94]. They do this by boosting GSH levels. High GSH allows cancer cells to survive under oxidative stress. Unfortunately, it also helps them resist chemotherapy and radiation. Smart nanomaterials are being developed to deplete GSH inside tumors [95,96]. This disrupts the redox balance and sensitizes cells to oxidative damage. Materials like copper, manganese, and iron-based nanoparticles can react with GSH. In doing so, they consume GSH and reduce the tumor’s ability to handle ROS. Some nanoplatforms are coated with disulfide bonds. These bonds are cleaved by GSH, triggering drug release while depleting GSH at the same time. Lowering GSH increases the effect of chemodynamic therapy and photodynamic therapy. It also helps convert an immunosuppressive tumor into an immunogenic one.
Another strategy is to generate ROS inside the tumor. Many metal-based nanoparticles can catalyze Fenton or Fenton-like reactions. For example, iron-based materials convert H2O2 into hydroxyl radicals, which are highly toxic. This is the core of CDT [97,98]. It kills cancer cells without requiring external triggers like light or heat. Copper and manganese-based materials also show Fenton-like activity. They broaden the types of reactions possible in the tumor environment. When ROS levels are pushed too high, cancer cells undergo oxidative stress-induced death. This offers a non-traditional way to kill tumors by exploiting their own metabolism.
4.4. TME-Triggered Drug Release
One of the major limitations of chemotherapy is poor selectivity. Many anticancer drugs harm both healthy and cancerous cells. This causes severe side effects. Smart drug delivery systems aim to change that. They use TME-responsive triggers to release drugs only where needed. These triggers include acidic pH, high GSH, and elevated H2O2 levels. By linking drug release to these local conditions, therapies become more targeted and safer. As discussed earlier, acidic conditions are ideal for triggering drug release. Materials like ZIF-8, CaCO3, and black phosphorus degrade in low pH. These systems can carry chemotherapeutics or photosensitizers. Once they reach the acidic tumor, they break apart and release their payload.
This reduces systemic toxicity and improves drug accumulation at the tumor site. Many tumors have high levels of GSH. Nanoparticles can be designed with disulfide bonds or redox-cleavable linkers. These break down in reductive environments, releasing drugs specifically inside cancer cells. This method is especially useful for delivering genetic material, peptides, or immunotherapy agents. Some systems respond to high ROS levels. These include boronate- or thioketal-linked carriers. ROS cleave these chemical bonds, which open the structure and release the drug. ROS-triggered systems can be combined with CDT to create a feedback loop. As CDT increases ROS levels, it accelerates drug release, boosting therapeutic efficiency. In short, TME-triggered drug release enhances precision. It ensures that drugs are released where they are needed most.
4.5. Immune Modulation
The immune system should be able to destroy cancer cells. However, many tumors avoid immune detection. They build a microenvironment that suppresses immune responses. They recruit regulatory T cells, secrete inhibitory cytokines, and express immune checkpoint molecules. The result is a “cold” tumor that is invisible to the immune system. Immune checkpoint inhibitors have helped. But their success is limited in cold tumors. New approaches are needed to stimulate anti-tumor immunity.
Some therapies can cause immunogenic cell death (ICD) [99,100]. This is a type of cell death that releases signals to alert the immune system. Inorganic nanoparticles like iron oxide, black phosphorus, or manganese dioxide can induce ICD. They do this by generating ROS or delivering drugs that cause endoplasmic reticulum stress. ICD helps turn cold tumors into hot tumors, which are more responsive to immunotherapy. Nanoparticles that trigger ICD may be combined with checkpoint inhibitors for better outcomes.
TAMs are often of the M2 type, which supports tumor growth. Reprogramming them to the M1 type can enhance inflammation and immune activation. Some nanomaterials, like iron oxide or manganese-based particles, help polarize TAMs to the M1 phenotype. They act through ROS generation or delivery of immunomodulatory agents. This shift in macrophage behavior improves T cell recruitment and tumor clearance. Ferroptosis is a non-apoptotic form of cell death driven by iron and lipid peroxidation. It leads to massive oxidative stress and cell rupture. Nanoparticles that deliver iron or inhibit antioxidant enzymes can trigger ferroptosis in tumors. This not only kills cancer cells but also releases danger signals that activate immune cells. Ferroptosis is being studied as a method to both kill tumors and stimulate anti-tumor immunity.
5. Multimodal Therapies Enabled by Tumor Microenvironment (TME) Modulation
Modifying the tumor microenvironment opens new paths for treating cancer. In its natural state, the TME works against therapy. It blocks drug delivery, weakens immune cells, and protects cancer cells from stress. By changing the TME, treatment becomes more effective. Inorganic nanomaterials play a big role in this transformation. They enable therapies to work better by responding to local tumor conditions. They also make it possible to combine different therapies into a single treatment strategy.
5.1. Chemo/Chemodynamic Therapy (CDT)
Chemotherapy remains a standard cancer treatment. It uses drugs that kill fast-growing cells. But in many cases, the TME reduces drug effectiveness. Poor blood flow limits drug entry. High glutathione levels neutralize drug-induced oxidative stress. Acidic pH can degrade drugs before they reach their target. TME modulation helps solve these problems. By delivering drugs in a smart way—such as through pH-sensitive nanocarriers—chemotherapy becomes more precise. Some nanomaterials also deplete glutathione, which makes cancer cells more sensitive to chemotherapy. For example, calcium carbonate or ZIF-8-based systems can release drugs only in acidic environments. This reduces side effects and boosts drug concentration at the tumor site [100,101].
CDT is a newer approach. It uses the TME’s own chemistry to kill tumor cells. CDT is based on the Fenton reaction. In this process, transition metals like iron or copper react with hydrogen peroxide to produce hydroxyl radicals. These radicals cause oxidative damage and cell death. Tumors often contain high levels of hydrogen peroxide and low pH. These are perfect conditions for the Fenton reaction. Nanoparticles made from Fe3O4, CuO, or MnO2 can catalyze this reaction within the tumor. CDT is unique because it does not need external activation like light or heat. It uses the tumor’s own biochemistry [102]. This makes it useful for deep-seated tumors or patients who cannot tolerate other therapies. CDT is also highly selective. Normal tissues have lower H2O2 and higher pH, so the reaction does not happen there [103]. This improves safety. Combining CDT with chemotherapy creates a dual attack. One weakens the tumor’s antioxidant system, while the other delivers direct cytotoxic agents.
5.2. Photothermal and Photodynamic Therapy
Photothermal therapy uses light to kill cancer cells. Nanoparticles absorb near-infrared (NIR) light and convert it into heat. This heat kills tumor cells by damaging their membranes and proteins. Gold nanoparticles, carbon nanotubes, and black phosphorus are common photothermal agents. These materials accumulate in the tumor and respond to external light. The TME affects PTT. Hypoxia, acidity, and poor blood supply can limit the effect. But nanomaterials that modulate the TME can overcome this [104,105]. For example, MnO2 can generate oxygen. This improves local blood flow and oxygenation. The result is better light absorption and stronger thermal effects. Some nanocarriers are also designed to change shape or surface charge after TME exposure. This improves retention and penetration.
PTT is non-invasive and can be precisely controlled. It works well with imaging, making image-guided therapy possible. Photodynamic therapy is similar to PTT, but instead of heat, it uses light to produce ROS. A photosensitizer molecule absorbs light and transfers energy to nearby oxygen. This produces singlet oxygen, a highly reactive form that kills cells. PDT depends on oxygen. This is a problem in hypoxic tumors [106,107] (Figure 6). TME modulation can help. Oxygen-generating nanoparticles like MnO2 or catalase-mimicking materials supply O2 where needed. This allows the photosensitizer to work even in low-oxygen conditions. Additionally, pH-sensitive systems can release the photosensitizer only in the acidic tumor. This reduces off-target effects. When combined, PTT and PDT offer synergistic effects. Heat from PTT can increase blood flow, helping with oxygen delivery for PDT. Meanwhile, ROS from PDT can stress tumor cells, making them more sensitive to heat. Many nanoplatforms now carry both photothermal and photodynamic agents. They respond to a single light source, delivering a powerful one-two punch to tumors.
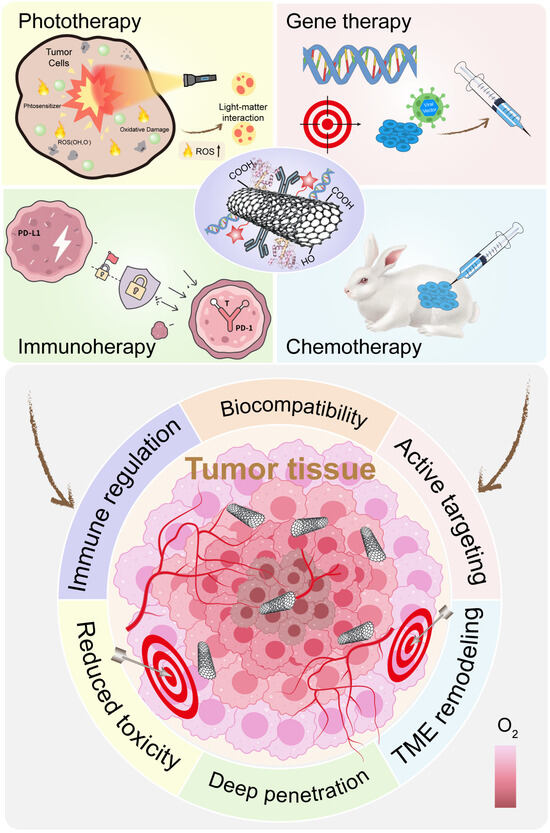
Figure 6.
Advantages and Applications of Functionalized CNTs in Cancer Therapy. Functionalized carbon nanotubes (CNTs) are widely applied in cancer treatments, including chemotherapy, phototherapy, immunotherapy, and gene therapy. Surface modification enhances their biocompatibility, tumor penetration, and active targeting, while also reducing inherent toxicity.
5.3. Radiotherapy Enhancement
Radiotherapy is one of the oldest cancer treatments. It works by using ionizing radiation to damage DNA. The goal is to cause lethal mutations in cancer cells. However, the success of radiotherapy depends on oxygen. Radiation creates free radicals from water molecules. These radicals damage DNA. But in hypoxic tumors, this process is less efficient. The DNA damage is not enough to kill the cells. Also, tumors often repair radiation damage quickly. High antioxidant levels like GSH contribute to this resistance. Nanomaterials can enhance radiotherapy in several ways. One method is oxygen generation. As discussed earlier, MnO2, catalase mimics, and PFC-based carriers improve oxygen levels in tumors. This restores radiosensitivity.
Another approach is using high-Z (atomic number) materials like gold, bismuth, or hafnium. These materials absorb radiation more effectively than soft tissue. When exposed to X-rays, they produce a burst of secondary electrons. This increases DNA damage in nearby cells. Some nanocarriers are also designed to deliver radiosensitizers—agents that make cancer cells more vulnerable to radiation. These may include platinum compounds or ROS-generating agents. Other systems release drugs after radiation triggers them. This turns radiation into both a treatment and a drug-release signal. By integrating nanotechnology, radiotherapy becomes more targeted and effective. Lower doses may be used, reducing damage to healthy tissue.
5.4. Synergistic Immunotherapy
Immunotherapy activates the body’s own immune system to fight cancer. Immune checkpoint inhibitors, like anti-PD-1 and anti-CTLA-4, have shown success in melanoma and lung cancer. But many tumors remain unresponsive. These “cold tumors” lack active immune cells. They are surrounded by suppressive signals and immune-silent stromal cells. The TME blocks T cell entry, reduces antigen presentation, and increases regulatory cell activity. TME modulation can make immunotherapy more effective. Nanomaterials can reverse immunosuppression and trigger immune recognition. One key strategy is inducing immunogenic cell death (ICD) [108,109]. This form of death alerts the immune system. Nanoparticles that produce ROS, trigger ferroptosis, or disrupt cell membranes can cause ICD. This turns dying tumor cells into a signal that activates dendritic cells and T cells. Another method is macrophage repolarization. Tumors often have M2-type macrophages, which promote growth and suppress inflammation. Certain nanomaterials—like iron oxide or manganese-based systems—can shift these to the M1 type. M1 macrophages release pro-inflammatory cytokines and help recruit killer T cells.
Smart nanoparticles can deliver checkpoint inhibitors directly into tumors. This avoids systemic exposure and reduces side effects. Combining photothermal or photodynamic therapy with immunotherapy is also promising. Heat or ROS from these therapies not only kill tumor cells but also expose tumor antigens. This boosts the immune response when combined with anti-PD-1 or anti-CTLA-4 drugs. Chemodynamic therapy also supports immune activation. The ROS generated from CDT causes cell damage that is recognized by the immune system. When GSH is depleted, the immune-suppressive environment weakens. These multimodal combinations are now in clinical trials. They offer a more complete way to treat tumors that resist single-mode treatments.
6. Design Considerations for Smart TME-Modulating Nanoplatforms
Designing nanomaterials to modulate the TME is a complex task. The tumor is a harsh and unpredictable space. It is acidic, oxygen-deprived, full of reactive molecules, and often shielded from the immune system. For a nanoparticle to work well, it must be engineered with care.
6.1. Surface Functionalization and Targeting
The surface of a nanoparticle is the first thing it presents to its biological surroundings. It determines how the material interacts with cells, proteins, and the immune system. A poorly designed surface can lead to quick clearance or toxicity. A well-designed one can lead to precise targeting and enhanced therapeutic effects. One common method of surface functionalization is PEGylation. This means coating the nanoparticle with polyethylene glycol (PEG). PEG helps the nanoparticle avoid detection by the immune system [110,111]. It reduces opsonization, a process where blood proteins tag foreign objects for clearance. PEG also improves the stability and circulation time of nanoparticles in the bloodstream. However, PEG is not perfect. Some patients develop anti-PEG antibodies. This can lead to rapid clearance after repeated doses. Newer materials such as zwitterionic polymers or biomimetic coatings are being explored as alternatives.
Another important strategy is active targeting. This involves attaching ligands—such as peptides, antibodies, aptamers, or sugars—to the surface. These ligands recognize specific receptors overexpressed on tumor cells or tumor-associated cells. For example, the folate receptor is overexpressed in several cancers [112,113]. Nanoparticles with folic acid ligands can bind selectively to those tumor cells. Similarly, RGD peptides target integrins, which are common on angiogenic blood vessels in tumors. Targeting does not guarantee entry into every tumor cell, but it helps improve the local accumulation of therapeutic agents. Combining passive targeting (via the enhanced permeability and retention effect) with active targeting improves therapeutic outcomes. More recently, cell membrane-coated nanoparticles have gained attention. These use membranes from red blood cells, platelets, or cancer cells themselves. This gives the nanoparticles a “self” identity, helping them evade the immune system. Cancer cell membranes, for instance, can provide homotypic targeting. This means the particles are more likely to bind to other cells from the same tumor type. These biomimetic systems blend the natural and synthetic worlds for better performance.
6.2. Biodegradability and Biosafety
Biodegradability is essential. After a nanoparticle completes its job, it should break down into safe, easily excretable components. If it does not, it can accumulate in organs and cause long-term toxicity. This is a major concern in clinical translation. Many early nanomaterials showed promise in the lab but failed in trials due to poor clearance and toxicity. Some inorganic materials naturally degrade under TME conditions. Calcium carbonate, for example, dissolves in acidic environments. It breaks down into calcium and carbon dioxide—both safe and easily processed by the body. Black phosphorus is another biodegradable material. It degrades into phosphate, a common and safe ion found in cells. Its degradation is accelerated in water or in the acidic tumor environment.
Iron oxide nanoparticles are metabolized through natural iron pathways in the liver and spleen. This makes them safer than some synthetic metal-based systems. Designers must also consider the speed of degradation. Too fast, and the material may lose its effect. Too slow, and it may linger in tissues. An ideal nanoparticle should remain stable in blood but degrade in the tumor. To achieve this, some materials are coated with pH-sensitive shells. These protect the core during circulation but dissolve when exposed to acidic tumor pH. Researchers must strike a balance. The material needs to be robust enough to reach the tumor, but soft enough to break down afterward.
6.3. Stimuli-Responsiveness (pH, Redox, Enzyme, Light)
Stimuli-responsive systems are at the heart of smart nanomedicine. They allow the nanoparticle to stay dormant during circulation and only activate at the tumor site. This improves specificity and reduces side effects. The TME offers several triggers. These include low pH, high GSH, elevated hydrogen peroxide (H2O2), overexpressed enzymes, and even external stimuli like light or ultrasound.
Tumor environments are more acidic than normal tissues. This difference is exploited by pH-sensitive systems. Materials like ZIF-8 or CaCO3 degrade in acidic pH. They release their contents only when they reach the tumor. This makes pH-responsiveness one of the most widely used strategies. Glutathione levels are much higher inside tumor cells than in the bloodstream. Nanoparticles designed with disulfide bonds or redox-cleavable linkers respond to this difference. Once inside the cell, high GSH levels break the disulfide bonds. This leads to the release of drugs or other agents. Redox-responsive systems are especially useful for intracellular delivery.
ROS are elevated in tumors. Some systems use boronate esters or thioketal linkers, which are sensitive to ROS. When triggered, these systems degrade and release their contents. This is useful for combined therapies. For instance, a photodynamic agent may raise ROS levels, which then triggers drug release from nearby nanoparticles. Tumors often overexpress enzymes like matrix metalloproteinases (MMPs) [114,115]. These enzymes break down the extracellular matrix. Enzyme-sensitive coatings can be cleaved by MMPs, triggering release. This adds another layer of selectivity. Light, ultrasound, and magnetic fields can also trigger nanoparticle activation. For example, gold nanoshells respond to near-infrared light for photothermal therapy. External triggers give researchers precise control. They allow activation only in the tumor area, minimizing systemic toxicity. The use of combined stimuli—such as pH and light, or ROS and enzyme—adds further sophistication. These multi-responsive systems are gaining popularity in advanced cancer therapy.
6.4. Size, Charge, and Biodistribution
Even the best-designed nanomaterial can fail if it does not reach the tumor. Size, shape, and charge determine how a nanoparticle moves through the body, how it accumulates in tumors, and how it is cleared. Size is a critical factor. Nanoparticles must be large enough to avoid kidney filtration but small enough to pass through leaky tumor blood vessels. The ideal size for passive targeting is often between 30 and 150 nm. This range allows particles to accumulate in tumors via the enhanced permeability and retention (EPR) effect. Smaller particles (below 10 nm) may be rapidly cleared by the kidneys. Larger particles (above 200 nm) may get trapped in the liver or spleen. Researchers must fine-tune the size for each application. Drug loading, circulation time, and tumor penetration all depend on it.
Charge affects how particles interact with cell membranes and proteins. Positively charged nanoparticles are taken up more easily by cells. However, they are also more likely to bind serum proteins and get cleared quickly. They may also trigger unwanted immune responses. Neutral or slightly negative particles tend to circulate longer. They are less likely to activate immune clearance. PEGylation can help mask the charge and improve behavior in the bloodstream. Charge must also be compatible with targeting strategies. For example, cationic surfaces are good for nucleic acid delivery but not always ideal for systemic circulation. The goal is to maximize tumor accumulation while minimizing exposure to healthy organs. This depends on a combination of size, charge, surface chemistry, and shape. Rod-shaped or disk-like particles may behave differently from spherical ones. Some shapes may penetrate tumors better or avoid clearance longer. Advanced imaging tools like PET, CT, or fluorescence imaging are used to track biodistribution. These tools help refine nanoparticle design over time.
7. Challenges and Future Perspectives
Smart inorganic nanomaterials have shown strong potential in modulating the tumor microenvironment. They offer new tools for cancer diagnosis and therapy. They respond to local stimuli. They improve the precision of treatments. They even help overcome drug resistance. But despite these advances, challenges remain. Several barriers still limit the full clinical translation of TME-modulating nanoplatforms. The path forward requires new ideas, better designs, and more personalized strategies.
7.1. Tumor Heterogeneity in TME Responsiveness
One of the biggest challenges is tumor heterogeneity. This means that tumors differ not just between patients but also within the same tumor mass. Some regions may be hypoxic. Others may have normal oxygen levels. Some areas are acidic. Others are not. This makes it difficult to design one-size-fits-all nanomaterials. A system that works well in one tumor may fail in another. Even in the same patient, different metastatic sites may respond differently to treatment. Nanomaterials often rely on the presence of specific triggers—like low pH, high glutathione, or excess hydrogen peroxide. But these triggers are not consistent. Their levels vary widely across tumor types and stages. This variation affects the efficiency of TME-responsive systems. A nanoparticle designed to release drugs in acidic conditions may not activate in a tumor with near-neutral pH. Similarly, chemodynamic therapy may fail if the tumor lacks sufficient hydrogen peroxide. To address this, future systems must include real-time feedback. Nanomaterials that can both diagnose and treat are needed. These “theranostic” platforms will help determine when and where the therapy should be activated. Advanced imaging or biosensing components could provide this feedback. This will allow doctors to adjust treatment based on the actual TME profile of each patient or tumor site.
7.2. Immune Evasion and Nanoparticle–Immune System Interaction
Nanoparticles are foreign bodies. The immune system is trained to detect and remove anything that does not belong. This includes smart nanocarriers. When injected, nanoparticles may be quickly coated by plasma proteins. This process is called opsonization. It marks them for removal by macrophages. As a result, fewer particles reach the tumor. Some nanoparticles may also activate immune cells unintentionally. This leads to inflammation or cytokine release. In some cases, it may cause serious side effects. Even when particles are designed for immune modulation, the response can be unpredictable. For example, a nanoparticle meant to polarize macrophages to the M1 state may instead get trapped by other immune cells, reducing its therapeutic effect.
To overcome this, surface modification is key. Coating nanoparticles with PEG can reduce recognition. However, repeated use may lead to anti-PEG antibodies. Alternative coatings—such as zwitterionic polymers or cell membrane cloaking—are being explored. Another approach is to use immune-informed design. Nanomaterials should not only avoid immune clearance but also cooperate with immune cells. For instance, biomimetic nanoparticles that use cancer cell membranes can enhance immune compatibility and improve homing to tumors. Future research must aim to balance stealth and immune engagement. The goal is to evade unwanted immune responses while enabling therapeutic immune activation.
7.3. Integration of AI for Nanoparticle Design
Designing effective nanomaterials involves many variables. Material type, size, charge, shape, drug load, and release profile all matter. Changing one feature affects many others. Testing every combination in the lab is time-consuming and expensive. This is where AI can help. AI tools, especially machine learning algorithms, can analyze large datasets. They can find patterns in how nanoparticles behave in different conditions. They can predict outcomes based on design features. For example, by predicting protein corona formation through machine learning, researchers can precisely control the surface properties of nanoparticles, significantly improve their tumor targeting efficiency, and provide a new data-driven approach to personalized nanodrug design. AI can also optimize formulation development. It can speed up screening for new materials. It can suggest new combinations that researchers might not consider. To support this, large and consistent datasets are needed. Many labs collect data differently. Without standardization, AI models may be inaccurate. Creating open-access nanoparticle databases will be essential. These should include detailed information on particle design, in vivo behavior, and therapeutic outcomes. With more data, AI will become an even more powerful tool in nanomedicine.
7.4. Potential Nanoparticle Toxicity
Inorganic nanomaterials are widely used in cancer treatment due to their unique optical, magnetic, and catalytic properties, but their potential toxicity seriously restricts clinical translation. First, soluble nanoparticles release metal ions, leading to oxidative stress and apoptosis directly induce membrane damage, mitochondrial dysfunction and DNA breakage. Second, long-term risks, such as the fibrous structure of carbon nanotubes may cause cancer and metal oxide nanoparticles induce genotoxicity. At present, clinical translation is facing challenges such as lack of standardized toxicity tests, insufficient long-term data, and species differences, and it is necessary to establish an international toxicology framework, develop degradable materials, and integrate organoids and AI to simulate human metabolic responses, and finally promote safe and efficient inorganic nanomaterials from the laboratory to clinical practice through precise modification, intelligent design and AI-assisted prediction.
8. Conclusions
Over the past decade, smart inorganic nanomaterials have transformed the field of cancer research. They have opened new doors in how we think about treating tumors. They do more than carry drugs. They interact with the tumor microenvironment. They respond to it. They change it. This review has explored the various ways these nanomaterials can be used. It has also discussed the strategies behind their design and the barriers that still remain. As the field continues to grow, it is important to reflect on what we have achieved—and where we are heading.
One of the most important contributions of inorganic nanomaterials is their ability to modulate the tumor microenvironment. Traditional cancer treatments often fail because the TME is hostile. It is acidic, oxygen-deprived, and full of antioxidants like glutathione. These conditions protect cancer cells and reduce therapy effectiveness. Smart inorganic nanomaterials are designed to work with these conditions, not against them. They use TME features as triggers for activation. This allows therapies to become more targeted and safer. For example, MnO2 and similar materials generate oxygen from hydrogen peroxide [116,117]. This helps relieve hypoxia and boosts radiotherapy and photodynamic therapy. Iron and copper-based materials catalyze Fenton-like reactions, generating toxic hydroxyl radicals. This forms the basis of CDT, a treatment that uses the tumor’s own biochemistry against it.
Nanoparticles also help manage pH and redox balance. Materials like calcium carbonate or ZIF-8 break down in acidic environments. Others release drugs in response to high levels of glutathione or ROS. These features allow for stimuli-responsive drug delivery, improving precision and reducing harm to healthy tissues. Another key advance is the development of immune-modulating nanoplatforms. Certain inorganic materials reprogram immune cells, turning “cold” tumors into “hot” ones. Some induce ICD. Others promote macrophage polarization or trigger ferroptosis, a unique form of cell death that stimulates immune activity [107,118]. Together, these features make smart nanomaterials versatile tools. They support multimodal therapies that combine chemotherapy, phototherapy, radiotherapy, and immunotherapy. Instead of using one weapon, doctors can now use several—all guided by the same platform. The design principles behind these platforms are also advancing. Nanoparticles are now tailored for specific size, shape, and surface chemistry. They are made biodegradable and safe. They include surface ligands for active targeting. Many are even multi-responsive, reacting to more than one TME feature at once. We also see progress in integrating diagnosis and therapy into the same system. These theranostic platforms allow real-time tracking of treatment. They help doctors adjust strategies as needed. This is a big step toward personalized, adaptive medicine.
Looking ahead, the role of smart inorganic nanomaterials in precision oncology will become even more central. They align perfectly with the goals of personalized medicine. They can adapt to each patient’s tumor environment. They can deliver treatment based on real-time biological cues. However, future progress will require addressing key challenges. Tumor heterogeneity remains a major barrier. Not all tumors respond the same way. Some may not have enough hydrogen peroxide for CDT. Others may not be acidic enough for pH-triggered release. Future nanoplatforms must include feedback systems to assess the local TME before acting.
The immune system is another complex factor. Nanoparticles must avoid immune clearance but still interact with immune cells when needed. This balance is difficult but essential. Better biomimetic designs and immune-compatible coatings will be important moving forward.
AI will also play a role. AI can help predict which nanoparticle designs will work best for specific tumors. It can accelerate screening and optimization. It can even help design personalized formulations on demand. The future will also bring modular platforms. These will allow doctors to mix and match drug types, targeting ligands, and surface coatings. One core nanoparticle could be customized for different patients or cancer types. This modularity will reduce cost, improve speed, and enhance treatment outcomes. To truly bring smart nanomaterials into everyday clinical practice, we also need regulatory progress. New evaluation frameworks must be developed for these complex systems. Safety testing must include both short- and long-term effects. Manufacturing must be standardized and scalable. Despite these hurdles, the potential benefits are too great to ignore. Smart inorganic nanomaterials can improve how we deliver drugs. They can enhance how tumors respond to treatment. They can guide therapy in real-time. And most importantly, they can help doctors tailor treatments to individual patients.
Cancer is a complex and evolving disease. It adapts quickly and resists simple solutions. To beat it, we need tools that are just as smart and adaptable. Inorganic nanomaterials are one of those tools. They bring intelligence to therapy. They bring precision to treatment. And they bring hope for better outcomes. As we move forward, collaboration between scientists, clinicians, and engineers will be key. Together, we can refine these materials and bring them from the lab to the clinic. Smart inorganic nanomaterials are not just a research trend. They are part of the future of cancer care.
Funding
This research was funded by Young Elite Scientists Sponsorship Program by BATSA (BYESS2023244), and Beijing Institute of Technology Research Fund Program for Young Scholars.
Acknowledgments
The authors also want to thank the Analysis & Testing Center at the Beijing Institute of Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, C.; Shao, F.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Chen, S.; Liao, C.; Hu, H.; Liao, J.; Chen, Z.; Li, S.; Zeng, X.; Peng, B.; Shen, S.; Li, D.; et al. Hypoxia-driven tumor stromal remodeling and immunosuppressive microenvironment in scirrhous HCC. Hepatology 2024, 79, 780–797. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.; Yang, X. Hypoxia-Driven Changes in Tumor Microenvironment: Insights into Exosome-Mediated Cell Interactions. Int. J. Nanomed. 2024, 19, 8211–8236. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Malla, R.; Kumari, S.; Ganji, S.P.; Srilatha, M.; Nellipudi, H.R.; Nagaraju, G.P. Reactive oxygen species of tumor microenvironment: Harnessing for immunogenic cell death. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189154. [Google Scholar] [CrossRef]
- Malla, R.; Surepalli, N.; Farran, B.; Malhotra, S.V.; Nagaraju, G.P. Reactive oxygen species (ROS): Critical roles in breast tumor microenvironment. Crit. Rev. Oncol. 2021, 160, 103285. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Rivadeneira, D.B.; Lontos, K.; Dean, V.G.; Gunn, W.G.; Watson, M.J.; Yao, T.; Wilfahrt, D.; Hinck, C.; Wieteska, L.; et al. An oncolytic virus–delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment. J. Exp. Med. 2023, 220, e20230053. [Google Scholar] [CrossRef] [PubMed]
- O’COnnell, B.C.; Hubbard, C.; Zizlsperger, N.; Fitzgerald, D.; Kutok, J.L.; Varner, J.; Ilaria, R.; Cobleigh, M.A.; Juric, D.; Tkaczuk, K.H.R.; et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J. ImmunoTher. Cancer 2024, 12, e009160. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A Game Changer in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Ozpiskin, O.M.; Zhang, L.; Li, J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9, 1215–1231. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Khosravi, G.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Song, X.; Hao, C.; Li, Y.; Li, Y.; Dong, H.; Wei, Q.; Wei, M.; Li, H.; Zhao, L. Chiral inorganic nanomaterials in the tumor microenvironment: A new chapter in cancer therapy. Pharmacol. Res. 2024, 208, 107386. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, 2101526. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, D.; Zhao, Y.; Wang, X.; Yao, S.; Huang, W.; Yang, Y.; Dong, X.; Zhang, L.; Yang, J. Tumor microenvironment-responsive manganese-based nano-modulator activate the cGAS-STING pathway to enhance innate immune system response. J. Nanobiotechnol. 2024, 22, 535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Barkley, D.; Moncada, R.; Pour, M.; Liberman, D.A.; Dryg, I.; Werba, G.; Wang, W.; Baron, M.; Rao, A.; Xia, B.; et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 2022, 54, 1192–1201. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Gabai, Y.; Assouline, B.; Ben-Porath, I. Senescent stromal cells: Roles in the tumor microenvironment. Trends Cancer 2023, 9, 28–41. [Google Scholar] [CrossRef]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.K. Imitating Hypoxia and Tumor Microenvironment with Immune Evasion by Employing Three Dimensional In vitro Cellular Models: Impressive Tool in Drug Discovery. Recent Pat. Anti-Cancer Drug Discov. 2022, 17, 80–91. [Google Scholar] [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef]
- Dekker, Y.; Le Dévédec, S.E.; Danen, E.H.J.; Liu, Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes 2022, 13, 1585. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Dharmaratne, N.U.; Kaplan, A.R.; Glazer, P.M. Targeting the Hypoxic and Acidic Tumor Microenvironment with pH-Sensitive Peptides. Cells 2021, 10, 541. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Med. Cell Longev. 2016, 2016, 1580967. [Google Scholar] [CrossRef]
- Jafari, M.; Sriram, V.; Premnauth, G.; Merino, E.; Lee, J.-Y. Modified peroxamide-based reactive oxygen species (ROS)-responsive doxorubicin prodrugs. Bioorganic Chem. 2022, 127, 105990. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Zong, L.; Chen, X.; Chen, K.; Jiang, Z.; Nan, L.; Li, X.; Li, W.; Shan, T.; et al. Reactive Oxygen Species and Targeted Therapy for Pancreatic Cancer. Oxidative Med. Cell Longev. 2016, 2016, 1616781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Wu, Q.; Chen, Y.; Deng, Y.; Yang, Z.; Zhang, L.; Liu, B. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019, 22, 101116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Lin, Z.; Jiang, C.; Chen, X.; Wang, K.; Liu, L.; Shao, L.; Pan, J.; Li, J.; et al. Methylglyoxal from gut microbes boosts radiosensitivity and radioimmunotherapy in rectal cancer by triggering endoplasmic reticulum stress and cGAS-STING activation. J. ImmunoTher. Cancer 2023, 11, e007840. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, G.; Zhang, Z.; Yong, T.; Yang, X.; Gan, L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics 2021, 11, 1937–1952. [Google Scholar] [CrossRef]
- Wen, X.; Wang, C.; Bi, S.; Xu, Y.; Wu, Z.; Huang, H.; Liu, Z.; Zeng, S. Tumor Microenvironment Cascade Activated Biodegradable Nano-Enzymes for Glutathione-Depletion and Ultrasound-Enhanced Chemodynamic Therapy. Small 2024, 20, e2405457. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Zhang, D. Manganese Dioxide-Based Nanomaterials for Medical Applications. ACS Biomater. Sci. Eng. 2024, 10, 2680–2702. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, Y.; Ma, Y.; Chen, D.; Zhang, T.; Fan, S.; Lin, W.; Huang, Y.; Lu, H.; Xu, J.-F.; et al. Immunomodulatory activity of manganese dioxide nanoparticles: Promising for novel vaccines and immunotherapeutics. Front. Immunol. 2023, 14, 1128840. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, Y.; Wu, F. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, 2103868. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Zhang, Z.; He, D.; Zhu, J.; Chen, Y.; Zhang, Y. Remodeling of tumor microenvironment for enhanced tumor chemodynamic/photothermal/chemo-therapy. J. Nanobiotechnol. 2022, 20, 388. [Google Scholar]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, W.; Xiao, X.; Liu, S.; Liu, L.; Zhang, L.; Li, L.; Li, Z.; Li, Z.; Xu, M.; et al. Reprogramming exosomes for immunity-remodeled photodynamic therapy against non-small cell lung cancer. Bioact. Mater. 2024, 39, 206–223. [Google Scholar] [CrossRef]
- Liang, L.; Jia, M.; Zhao, M.; Deng, Y.; Tang, J.; He, X.; Liu, Y.; Yan, K.; Yu, X.; Yang, H.; et al. Progress of Nanomaterials Based on Manganese Dioxide in the Field of Tumor Diagnosis and Therapy. Int. J. Nanomed. 2024, 19, 8883–8900. [Google Scholar] [CrossRef]
- Yue, Z.; Zhao, Q.; Wang, S.; Yao, S.; Wan, X.; Hu, Q.; Wen, K.; Zhao, Y.; Li, L. Manganese Dioxide Coated Piezoelectric Nanosonosensitizer for Cancer Therapy with Tumor Microenvironment Remodeling and Multienzyme-Like Catalysis. Small Methods 2024, 8, e2400018. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, Y.; Zheng, R.; Xu, K.; Yan, J.; Song, P.; Wang, Y.; Rauf, A.; Pan, Y.; Zhang, H. Immunomodulation of Tumor Microenvironment by Arginine-Loaded Iron Oxide Nanoparticles for Gaseous Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 19825–19835. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, D.; Ding, M.; Yu, N.; Liu, J.; Li, J.; Lin, L. Tumor-targeting biomimetic sonosensitizer-conjugated iron oxide nanocatalysts for combinational chemodynamic–sonodynamic therapy of colorectal cancer. J. Mater. Chem. B 2022, 10, 4595–4604. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Wu, Y.; Li, C.; Xu, Z.; Zhang, N.; Wang, X.; Zhao, Y.; Zu, T.; He, Q.; et al. Biomimetic Iron-Based Nanoparticles Remodel Immunosuppressive Tumor Microenvironment for Metabolic Immunotherapy. Int. J. Nanomed. 2024, 19, 9333–9349. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xia, Q.; Shang, J.; He, Y.; Li, Z.; Chen, Y.; Gao, F.; Yu, X.; Yuan, Z.; et al. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J. Nanobiotechnol. 2024, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Magnetic nanomaterials with near-infrared pH-activatable fluorescence via iron-catalyzed AGET ATRP for tumor acidic microenvironment imaging. J. Mater. Chem. B 2015, 3, 2786–2800. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, S.; Zheng, H.; Yang, S.; Zhou, L.; Liu, K.; Zhang, Q.; Zhang, H. Cu-doped cerium oxide-based nanomedicine for tumor microenvironment-stimulative chemo-chemodynamic therapy with minimal side effects. Colloids Surf. B Biointerfaces 2021, 205, 111878. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cheng, H.; Dai, Y.; Su, Z.; Wang, C.; Lei, L.; Lin, D.; Li, X.; Chen, H.; Fan, K.; et al. In Vivo Regenerable Cerium Oxide Nanozyme-Loaded pH/H2O2-Responsive Nanovesicle for Tumor-Targeted Photothermal and Photodynamic Therapies. ACS Appl. Mater. Interfaces 2021, 13, 233–244. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, N.; Wu, Q.; Chen, Z.; Wang, Y.; Fu, C.; Huang, Z.; Meng, X.; Qiao, B. Cerium oxide nanozymes enhance microwave immunotherapy by reshaping the tumor microenvironment. Nanoscale 2025, 17, 14614–14623. [Google Scholar] [CrossRef]
- Yan, S.; Gao, Z.; Ding, J.; Chen, S.; Wang, Z.; Jin, W.; Qu, B.; Zhang, Y.; Yang, L.; Guo, D.; et al. Nanocomposites based on nanoceria regulate the immune microenvironment for the treatment of polycystic ovary syndrome. J. Nanobiotechnol. 2023, 21, 412. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Zhang, C.; Niu, X.; Sun, M.; Yan, Z.; Wang, W.; Yuan, Z. Tumor acid microenvironment-activated self-targeting & splitting gold nanoassembly for tumor chemo-radiotherapy. Bioact. Mater. 2021, 7, 377–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Hu, B.; Zhong, S.; Yan, K.; Wu, Y.; Li, S.; Yang, Y.; Xu, Z.; Lu, Y.; et al. MDSC-targeting gold nanoparticles enhance PD-1 tumor immunotherapy by inhibiting NLRP3 inflammasomes. Biomaterials 2024, 307, 122533. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Z.; Tong, Z.; Zhang, S.; Min, J.; Xu, Q.; Zhou, L.; Mao, Z.; Xia, H.; Wang, W. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics 2020, 10, 5195–5208. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, L.; Chen, B.; Luo, X.; Xu, P.; Cai, J.; Hu, Y. Platinum prodrug nanoparticles inhibiting tumor recurrence and metastasis by concurrent chemoradiotherapy. J. Nanobiotechnol. 2022, 20, 129. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Lu, Y.; Chen, X.; Zhang, Y.; Zhou, W.; Guo, Q.; Li, C.; Zhang, Y.; Zhang, Y.; et al. Tumor Microenvironment-Triggered Aggregated Magnetic Nanoparticles for Reinforced Image-Guided Immunogenic Chemotherapy. Adv. Sci. 2019, 6, 1802134. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Zhang, Y.; Wang, Y.; Sun, L.; Jiang, J.; Jiao, L.; Li, R.; Zhang, Y.; Zhang, M.; et al. A versatile nanoplatform carrying cascade Pt nanozymes remodeling tumor microenvironment for amplified sonodynamic/chemo therapy of thyroid cancer. Biomaterials 2025, 313, 122778. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, W.; Zhu, M.; Zhao, L.; Gao, L.; Liang, H.; Zhang, Z.; Yang, F. Human Serum Albumin-Platinum(II) Agent Nanoparticles Inhibit Tumor Growth Through Multimodal Action Against the Tumor Microenvironment. Mol. Pharm. 2023, 21, 346–357. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Q.; Ye, J.; Yao, S.; Li, Q.; Fan, Z.; Ge, S.; Wang, Y.; Xu, D.; Zhou, J.; et al. Tumor microenvironment modulation innovates combinative cancer therapy via a versatile graphene oxide nanosystem. Biomater. Sci. 2025, 13, 3123–3148. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, L.; Zhao, Y.; Yi, X.; Zhu, L.; Ge, F.; Mou, X.; Chen, L.; Sun, L.; Yang, K. Nano-graphene oxide-manganese dioxide nanocomposites for overcoming tumor hypoxia and enhancing cancer radioisotope therapy. Nanoscale 2018, 10, 5114–5123. [Google Scholar] [CrossRef]
- Du, P.; Yan, J.; Long, S.; Xiong, H.; Wen, N.; Cai, S.; Wang, Y.; Peng, D.; Liu, Z.; Liu, Y. Tumor microenvironment and NIR laser dual-responsive release of berberine 9-O-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2020, 8, 4046–4055. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, M.-M.; Luo, Q.-W.; Zhang, Y.-C.; Liu, T.-T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.-W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Bao, Y.; Li, G.; Li, S.; Zhang, H.; Wu, X.; Yan, R.; Wang, Z.; Guo, C.; Jin, Y. Multifunctional Tumor-Targeting Carbon Dots for Tumor Microenvironment Activated Ferroptosis and Immunotherapy in Cancer Treatment. ACS Appl. Mater. Interfaces 2023, 15, 56834–56845. [Google Scholar] [CrossRef]
- Guo, L.; Ding, J.; Zhou, W. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm. Sin. B 2023, 13, 5074–5090. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Z.; Deng, L.; Yang, Y.; Zhu, Y.; Sun, X.; Hao, Y.; He, Y.; Fu, J. Hydrogen Therapy Reverses Cancer-Associated Fibroblasts Phenotypes and Remodels Stromal Microenvironment to Stimulate Systematic Anti-Tumor Immunity. Adv. Sci. 2024, 11, e2401269. [Google Scholar] [CrossRef]
- Gharehbaba, A.M.; Soltanmohammadi, F.; Eskandani, M.; Adibkia, K. From barriers to breakthroughs: Mesoporous silica nanoparticles in targeting the tumor microenvironment. Int. J. Pharm. 2025, 682, 125979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Du, H.; Chen, X.; Hu, N.; Yu, T.; Hou, M.; Yu, X. An enzyme-responsive hydrogel functionalized with mesoporous silica nanoparticles for co-delivery of cisplatin and shRNA to overcome chemotherapy resistance in non-small cell lung cancer. RSC Adv. 2025, 15, 23966–23977. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dong, H.; Gao, L.; Liu, M.; Wang, C. Mechanism of selenium-doped black phosphorus nanosheets wrapped with biomimetic tumor cell membrane for prostate cancer immunotherapy. Mater. Sci. Eng. C 2025, 176, 214339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mu, M.; Wei, Y.; Yan, B.; Liu, H.; Guo, K.; Zhang, M.; Dai, X.; Sun, X.; Leong, D.T. Novel ultrathin ferrous sulfide nanosheets: Towards replacing black phosphorus in anticancer nanotheranostics. Bioact. Mater. 2025, 43, 564–578. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Akbari, A.; Jassim, A.Y.; Hussein, U.A.-R.; Salavati-Niasari, M. Recent advances in targeting cancer stem cells by using nanomaterials. Int. J. Pharm. 2025, 673, 125381. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, K.; Saini, P.; Sethi, M.; Meena, P.; Dandia, A.; Weigand, W.; Parewa, V. Elucidating the Synergistic Promotional Mechanism of Water and Oxygen in the Aerobic C–C Homocoupling Reaction Catalyzed by Visible-Light-Derived Core–Shell Pd@A-CQDs Nanostructures. ACS Appl. Mater. Interfaces 2024, 16, 46200–46215. [Google Scholar] [CrossRef]
- Mali, A.; Nayak, N.U.; van Doesburg, J.; Fokkink, R.; van Riessen, K.; de Kruijf, R.; Srinivas, M. Polymeric (Poly(lactic-co-glycolic acid)) Particles Entrapping Perfluorocarbons Are Stable for a Minimum of Six Years. ACS Omega 2025, 10, 6768–6779. [Google Scholar] [CrossRef]
- Krafft, M.; Riess, J. Corrigendum to Therapeutic oxygen delivery by perfluorocarbon-based colloids. Adv. Colloid Interface Sci. 2021, 295, 102505. [Google Scholar] [CrossRef]
- Srinivasan, M.K.; Aruchamy, M.; Namasivayam, N.; Muthupandian, S. Protective effect of pH-sensitive carvacrol zinc oxide quantum dots on Nrf-2, MAPK and NF-κB pathways and protein-bound carbohydrates in DMBA-induced mammary carcinogenesis. Pathol. Res. Pract. 2025, 272, 156117. [Google Scholar] [CrossRef]
- Aydın, B.; Bozoğlu, S.; Karatepe, N.; Güner, F.S. Folic acid-conjugated magnetic carbon nanotube nanocarriers for targeted delivery of mitoxantrone. Nanotechnology 2025, 36, 305701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, Z.; Gu, Y.; Bing, L.; Xie, Y.; Shen, Z.; Chen, W. Powerful Tribocatalytic Degradation of Methyl Orange Solutions with Concentrations as High as 100 mg/L by BaTiO3 Nanoparticles. Nanomaterials 2025, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Chen, X.; Yan, B.; Li, S.; Deng, H.; Lu, H. Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process. Nanomaterials 2025, 15, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, A.; Kornaś, A.; Gil, J.; Sękara, A.; Húska, D.; Rudolphi-Szydło, E.; Sieprawska, A.; Gawrońska, K.; Kulak, M.; Fotopoulos, V.; et al. Exogenously applied putrescine and chitosan–putrescine nanocomposite alleviate the negative effects of chilling stress on iceberg lettuce seedlings. Sci. Rep. 2025, 15, 26963. [Google Scholar] [CrossRef]
- Madbouly, N.A.; Ali, D.M.; Farid, A.A. Nanoparticles from grape seed extract inhibit inflammatory cytokines and ameliorate CCl4-induced hepatotoxicity. BMC Complement. Med. Ther. 2025, 25, 276. [Google Scholar] [CrossRef]
- Chauhan, A.; Saini, A.; Sharma, D. The evolution of integrated magnetic hyperthermia and chemodynamic therapy for combating cancer: A comprehensive viewpoint. Nanoscale Adv. 2025, 7, 4820–4836. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chen, H.; Chen, Y.; Ni, Y.; Ji, J.; Wang, K.; Jin, Q. Self-assembled copper-amino acid nanoleaves for targeted treatment of deep-seated bacterial infections via chemodynamic therapy and cuproptosis-like death. Biomaterials 2025, 325, 123566. [Google Scholar] [CrossRef]
- Kim, E.; Jang, D.; Kim, M.; Heo, J.; Shin, J.; Chung, J.-Y.; Choi, M.; Yang, W.-H.; Lee, H.; Cha, J.-H. High-throughput screening system for immunogenic cell death inducers using artificial intelligence-based real-time image analysis. Comput. Biol. Med. 2025, 196, 110727. [Google Scholar] [CrossRef]
- Isaeva, A.S.; Yeriomenko, A.D.T.; Idota, E.; Volodina, S.I.; Porozova, N.O.; Bezsonov, E.E.; Malogolovkin, A.S. Shaping viral immunotherapy towards cancer-targeted immunological cell death. Front. Oncol. 2025, 15, 1540397. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Chang, Y.; Tang, L.; Ma, G.; Liu, X.; Gao, F.; Ma, X.; Guo, Y. Tumor microenvironment responsive smart nanoplatform for synergistic tumor therapy through co-enhancement of GSH depletion and hypoxia relief. J. Inorg. Biochem. 2025, 272, 113005. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Tong, W.; Peng, B.; Gao, W.; Wang, Y.; Wang, H.; Liu, J.; Liu, Y.; Sun, B.; Ma, J.; et al. Mesoporous Nanoplatform That Efficiently Delivers DOX as an H2O2 Generator to Trigger Mutual Amplification of Cuproptosis and Chemodynamic Therapy. Adv. Healthc. Mater. 2025, 14, e2500933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, H.; Lei, L.; Scherman, D.; Liu, Y. Tumor microenvironment-responsive MIL-53(Fe)@MnO2- induced glutathione depletion and sustained hydroxyl radical generation for enhanced chemodynamic cancer therapy. J. Colloid Interface Sci. 2025, 700, 138342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Hou, Y.; Yunlong, W.; Feng, X. Immunomodulatory effects of photothermal therapy in breast cancer: Advances and challenges. Front. Immunol. 2025, 16, 1544693. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, W.; Shi, X.; Lv, L.; Fang, Z.; Li, J. A TME-activated photothermal agent with photodegradability for accurate breast tumor photothermal therapy. RSC Adv. 2025, 15, 25062–25066. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, T.; Hu, L.; Vankann, V.; Bohn, T.; Bopp, T.; Kuan, S.L.; Weil, T. Autonomous Activation of a Gated Chemiluminescent Photosensitizer Enables Targeted Photodynamic Therapy in Tumor Cells. J. Am. Chem. Soc. 2025, 147, 27822–27834. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, L.; Pan, Z.; Wang, X.; Lin, L.; Fang, Y.; Huang, Y.; Feng, X.; Chen, X. Oxygen-boosted nanodrug for amplified ferroptosis-photodynamic immunotherapy together with PD-1 checkpoint blockade against triple-negative breast cancer. Colloids Surf. B Biointerfaces 2025, 255, 114963. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Hu, X.; Quan, R.; Lu, D.; Li, Z.; Yu, C.; Li, X.; Ma, S.; Li, X.; et al. Cascade reaction–driven biomimetic scintillant/metal–organic frameworks for X-ray triggered combinational therapy against glioma. Mater. Today Bio 2025, 33, 102069. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Liu, L.; Wu, Y.; Li, W.; Liang, J.; Shen, H.; Fang, S.; Huang, X.; Chu, Z.; et al. Gelatin Methacryloyl Xerogel Puncture Implants Loaded with Cu0.5Mn2.5O4 Nanoparticles Synergizes Cuproptosis and STING Activation for Enhanced Breast Cancer Immunotherapy. ACS Nano 2025, 19, 27902–27918. [Google Scholar] [CrossRef]
- Graván, P.; Peña-Martín, J.; de Andrés, J.L.; Pedrosa, M.; Villegas-Montoya, M.; Galisteo-González, F.; Marchal, J.A.; Sánchez-Moreno, P. Exploring the Impact of Nanoparticle Stealth Coatings in Cancer Models: From PEGylation to Cell Membrane-Coating Nanotechnology. ACS Appl. Mater. Interfaces 2024, 16, 2058–2074. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, W.; Wang, P.; Zhang, Y.; Meng, J.; Zhang, L. Tumor Microenvironment-Responsive Shell/Core Composite Nanoparticles for Enhanced Stability and Antitumor Efficiency Based on a pH-Triggered Charge-Reversal Mechanism. Pharmaceutics 2021, 13, 895. [Google Scholar] [CrossRef]
- Liu, B.; Shen, J.; Wu, Y.; Wong, C.F.; Hei, T.Y.; Li, J.W.-T.; Hummel, S.N.; Jin, G.; Petrucci, N.R.; Wang, D.M.; et al. Novel strategy for tumor immunotherapy using FITC-folate bispecific adapter bridged CAR immune cell cocktails. Bioact. Mater. 2025, 52, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Dong, J.; Xie, F.; Feng, X.; Wang, J.; Xu, X.; Tang, B.; Sun, C.; Wang, Y.; Zhong, W.; et al. Polyvalent folate receptor-targeting chimeras for degradation of membrane proteins. Nat. Chem. Biol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, M.; Mubeen, F.; Sundas, A.; Naz, R.; Hu, X.; Chen, X. CDKN2A and matrix metalloproteinases: Key regulators of cellular senescence in squamous cell carcinoma. Am. J. Transl. Res. 2025, 17, 4573–4589. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Wei, W. Research advances of matrix metalloproteinases family in uveal melanoma. Eur. J. Med. Res. 2025, 30, 609. [Google Scholar] [CrossRef]
- Sun, M.; Ni, C.; Li, A.; Liu, J.; Guo, H.; Xu, F.; Li, K.; Cao, X.; Shi, X.; Guo, R. A biomimetic nanoplatform mediates hypoxia-adenosine axis disruption and PD-L1 knockout for enhanced MRI-guided chemodynamic-immunotherapy. Acta Biomater. 2025, 201, 618–632. [Google Scholar] [CrossRef]
- Tang, Z.; Luo, W.; Xu, M.; Liu, Y.; Yu, Q.; Wang, L. A TME-responsive oxygen-self-supplying hybridized polymersome for synergistic triple-modal therapy and precision theranostics in hypoxic tumors. J. Mater. Chem. B 2025, 13, 8136–8148. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Cui, H.; Geng, H.; Ruan, L.; Zhao, Y.; Zhou, C.; Dai, W.; Chen, J.; Yu, J.; et al. EGCG and DOX dual-drug-loaded enzyme-responsive nanovesicles boost mitochondrial-mediated ICD for improved immunotherapy. Front. Pharmacol. 2025, 16, 1624109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).