Synthesis, Structural Characterization, Cytotoxicity, and Antibacterial Properties of Gold(III) Complexes with Hydrazones Derived from Vitamin B6
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Gold(III) Complexes
2.2. Cytotoxicity of Hydrazones 1a-l
2.3. Antibacterial Activity of Hydrazones and Their Gold(III) Complexes
3. Materials and Methods
3.1. Chemicals
3.2. Spectrometers
3.3. Cells
3.4. MTT Assay
3.5. Antibacterial Assay
3.6. Theory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LSQ | Least Squares Method |
| MW | Molecular Weight |
| MV | Molecular Volume |
| PSA | Polarizable Surface Area |
| INA | Institute of New Antibiotics |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MALDI TOF | matrix-assisted laser desorption/ionization time-of-flight |
| HMDSO | hexamethyldisiloxane |
References
- World Health Organization. Global Cancer Burden Growing, amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 15 October 2024).
- Teng, J.; Imani, S.; Zhou, A.; Zhao, Y.; Du, L.; Deng, S.; Li, J.; Wang, Q. Combatting Resistance: Understanding Multi-Drug Resistant Pathogens in Intensive Care Units. Biomed. Pharmacother. 2023, 167, 115564. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Munawar, K.S. Metal Complexes Incorporating Tridentate ONO Pyridyl Hydrazone Schiff Base Ligands: Crystal Structure, Characterization and Applications. Coord. Chem. Rev. 2024, 501, 215587. [Google Scholar] [CrossRef]
- Marzano, S.; Miglietta, G.; Morigi, R.; Marinello, J.; Arleo, A.; Procacci, M.; Locatelli, A.; Leoni, A.; Pagano, B.; Randazzo, A.; et al. Balancing Affinity, Selectivity, and Cytotoxicity of Hydrazone-Based G-Quadruplex Ligands for Activation of Interferon β Genes in Cancer Cells. J. Med. Chem. 2022, 65, 12055–12067. [Google Scholar] [CrossRef]
- Alam, A.; Khan, F.; Rehman, N.U.; Zainab; Elhenawy, A.A.; Islam, W.U.; Ali, M.; Aziz, S.; Al-Harrasi, A.; Ahmad, M. Flurbiprofen Clubbed Schiff’s Base Derivatives as Potent Anticancer Agents: In Vitro and In Silico Approach towards Breast Cancer. J. Mol. Struct. 2025, 1321, 139743. [Google Scholar] [CrossRef]
- Deepa, S.; Mathangi, N.; Mudavath, R.; Shekhar, I.; Aparna, A.V.; Sarala Devi, C. Heteroleptic Complexes of Hydrazone Scaffold of Picolinoyl N- Oxide and 2,4 Dihydroxy Phenyl Moieties; Evaluation of Antioxidant Activity, DNA and Protein Binding Properties and in Vitro Antiproliferation Studies. Inorganica Chim. Acta 2025, 574, 122391. [Google Scholar] [CrossRef]
- Firouzi, M.; Haghighijoo, Z.; Eskandari, M.; Mohabbati, M.; Miri, R.; Jamei, M.H.; Poustforoosh, A.; Nazari, S.; Firuzi, O.; Khoshneviszadeh, M.; et al. Synthesis and Cytotoxic Activity Evaluation of Novel Imidazopyridine Carbohydrazide Derivatives. BMC Chem. 2024, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, E.; Rezzola, S.; Filippi, E.; Turati, M.; Parrasia, S.; Bernardotto, S.; Stocco, M.; Szabò, I.; Mattarei, A.; Ronca, R.; et al. A Novel Mertansine Conjugate for Acid-Reversible Targeted Drug Delivery Validated through the Avidin-Nucleic-Acid-NanoASsembly Platform. Nanomed. Nanotechnol. Biol. Med. 2024, 62, 102784. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, O.G.; Morozova, T.D.; Kudyakova, Y.S.; Bazhin, D.N.; Kuratieva, N.V.; Klyushova, L.S.; Lavrov, A.N.; Lavrenova, L.G. Synthesis, Structure, and Properties of a Copper(II) Binuclear Complex Based on Trifluoromethyl Containing Bis(Pyrazolyl)Hydrazone. IJMS 2024, 25, 9414. [Google Scholar] [CrossRef]
- Kadi, I.; Şekerci, G.; Boulebd, H.; Zebbiche, Z.; Tekin, S.; Benarous, K.; Serseg, T.; Küçükbay, F.; Küçükbay, H.; Boumoud, T. Exploring the Anticancer Potential of New 3-cyanopyridine Derivatives Bearing N-acylhydrazone Motif: Synthesis, DFT Calculations, Cytotoxic Evaluation, Molecular Modeling, and Antioxidant Properties. J. Biochem. Mol. Toxicol. 2024, 38, e23819. [Google Scholar] [CrossRef]
- Tapera, M.; Doğan, E.; Şahin, K.; Gözkamane, G.A.; Kekeçmuhammed, H.; Sandal, S.; Gurkan, A.C.; Bora, R.E.; Anber, A.; Durdagi, S.; et al. Imidazole-Based Hydrazones as Potent Anti-Colon Cancer Agents: Design, Synthesis, Biological Evaluation and Computational Studies. J. Mol. Struct. 2024, 1318, 139240. [Google Scholar] [CrossRef]
- Elgohary, M.K.; Elkotamy, M.S.; Al-Warhi, T.; Eldehna, W.M.; Abdel-Aziz, H.A. Development of New LSM-83177 Analogues as Anti-Tumor Agents against Colorectal Cancer Targeting P53-MDM2 Interaction. Bioorganic Chem. 2024, 153, 107766. [Google Scholar] [CrossRef] [PubMed]
- Basu Baul, T.S.; Hlychho, B.; Das Pramanik, S.; Lyčka, A.; Roy, P.; Mahmoud, A.G.; Guedes Da Silva, M.F.C. Organotin(IV) Complexes Derived from 2,6-Diacetylpyridine Bis(2-Hydroxybenzoylhydrazone) as Prospective Anti-Proliferative Agents: Synthesis, Characterization, Structures and in Vitro Anticancer Activity. J. Inorg. Biochem. 2024, 261, 112693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Oh, J.M.; Prabhakaran, P.; Awasti, A.; Kim, H.; Mathew, B. Isatin-Tethered Halogen-Containing Acylhydrazone Derivatives as Monoamine Oxidase Inhibitor with Neuroprotective Effect. Sci. Rep. 2024, 14, 1264. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Ertorun, İ.; Akalın Çiftçi, G.; Özdemir, A. Design, Synthesis and Biological Evaluation of a New Series of Imidazothiazole-Hydrazone Hybrids as Dual EGFR and Akt Inhibitors for NSCLC Therapy. Eur. J. Med. Chem. 2024, 276, 116698. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, T.H.; Truong, H.N.; Dang, T.M.; Nguyen, M.T.T.; Nguyen, N.T.; Dang, P.H. Synthesis, Cytotoxicity, and Quantitative Structure–Activity Relationship Studies of Alkyl Triphenylphosphonium Pinostrobin Derivatives. ChemistrySelect 2024, 9, e202402190. [Google Scholar] [CrossRef]
- Smirnova, A.; Tretyakova, E.; Kazakova, O. New Cytotoxic α-Aminoacylamide and Bis -1,5-Disubstituted Tetrazole Adducts From Amino-Diterpene Molecules by Ugi Reaction. Chem. Biol. Drug Des. 2024, 104, e14632. [Google Scholar] [CrossRef]
- Çınarlı, M.; Ataol, Ç.Y.; Zeyrek, C.T.; Öğütcü, H.; Açık, L.; Batı, H. Design, Synthesis, Characterization, Theoretical Calculations, Molecular Docking Studies, and Biological Evaluation of New Fe(II) and Cu(II) Complexes of 2-Acetylpyridine Derivative Sulfonyl Hydrazone Schiff Base. J. Mol. Struct. 2025, 1321, 140112. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, Y.; Xu, Z.; Zhao, D.; Li, D.; Yang, H.; Zhu, S.; Xu, H.; Peng, S.; Miao, Z.; et al. Design, Synthesis and Evaluation of Novel LpxC Inhibitors Containing a Hydrazone Moiety as Gram-Negative Antibacterial Agents. Eur. J. Med. Chem. 2024, 279, 116892. [Google Scholar] [CrossRef]
- Kamat, V.; Venuprasad, K.D.; Shadakshari, A.J.; Bhat, R.S.; D’souza, A.; Chapi, S.; Kumar, A.; Kuthe, P.V.; Sankaranarayanan, M.; Venugopala, K.N. Synthesis, Anti-Inflammatory, Antibacterial, and Antioxidant Evaluation of Novel Pyrazole-Linked Hydrazone Derivatives. J. Mol. Struct. 2024, 1312, 138634. [Google Scholar] [CrossRef]
- Zang, Z.-L.; Wang, Y.-X.; Battini, N.; Gao, W.-W.; Zhou, C.-H. Synthesis and Antibacterial Medicinal Evaluation of Carbothioamido Hydrazonyl Thiazolylquinolone with Multitargeting Antimicrobial Potential to Combat Increasingly Global Resistance. Eur. J. Med. Chem. 2024, 275, 116626. [Google Scholar] [CrossRef] [PubMed]
- Siutkina, A.I.; Chashchina, S.V.; Makhmudov, R.R.; Novikova, V.V.; Igidov, N.M.; Chernov, I.N. Synthesis, Analgesic and Antimicrobial Activity of N-Hetarylamides of 2-(2-(Diarylmethylene)Hydrazono)-5,5-Dimethyl-4-Oxohexanoic Acid. Izv. Vyss. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnologiya 2022, 65, 74–82. [Google Scholar] [CrossRef]
- Althagafy, H.S.; El-Aziz, M.K.A.; Ibrahim, I.M.; Abd-alhameed, E.K.; Hassanein, E.H.M. Pharmacological Updates of Nifuroxazide: Promising Preclinical Effects and the Underlying Molecular Mechanisms. Eur. J. Pharmacol. 2023, 951, 175776. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Kole, K.; Yadav, T.; Bhavar, A.; Waghmare, P.; Bhokare, R.; Manjappa, A.; Jha, N.K.; Chellappan, D.K.; Shinde, S.; et al. Drug Repurposing: An Emerging Strategy in Alleviating Skin Cancer. Eur. J. Pharmacol. 2022, 926, 175031. [Google Scholar] [CrossRef]
- Sah, P.P.T.; Peoples, S.A. Isonicotinyl Hydrazones as Antitubercular Agents and Derivatives for Identification of Aldehydes and Ketones. J. Am. Pharm. Assoc. (Sci. Ed.) 1954, 43, 513–524. [Google Scholar] [CrossRef]
- Palmer, L.D.; Skaar, E.P. Transition Metals and Virulence in Bacteria. Annu. Rev. Genet. 2016, 50, 67–91. [Google Scholar] [CrossRef]
- Sada, P.K.; Bar, A.; Jassal, A.K.; Kumar, P.; Srikrishna, S.; Singh, A.K.; Kumar, S.; Singh, L.; Rai, A. A Novel Rhodamine Probe Acting as Chemosensor for Selective Recognition of Cu2+ and Hg2+ Ions: An Experimental and First Principle Studies. J. Fluoresc. 2024, 34, 2035–2055. [Google Scholar] [CrossRef]
- Anitha, O.; Ghorai, S.; Thiruppathiraja, T.; Amir, H.; Murugan, A.; Natarajan, R.; Lakshmipathi, S.; Viswanathan, C.; Jothi, M.; Murugesapandian, B. Pyridine Appended Pyrimidine Bis Hydrazone: Zn2+/ATP Detection, Bioimaging and Functional Properties of Its Dinuclear Zn(II) Complex. Talanta 2024, 273, 125900. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Huang, Z.; Ma, Q.; Liang, Y.; Xue, Z.; Feng, L.; Zhao, L. A Pyridine Modified Naphthol Hydrazone Schiff Base Chemosensor for Al3+ via Intramolecular Charge Transfer Process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122961. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Si, G.; Xu, G.; Zhou, S.; Xue, X. A New Turn on Coumarin-Based Fluorescence Probe for Cr3+ Detection in Aqueous Solution. J. Inorg. Biochem. 2023, 247, 112302. [Google Scholar] [CrossRef]
- Dewangan, S.; Mishra, A.; Halder, B.; Mishra, A.; Dhiman, R.; Chatterjee, S. Unsymmetrically Bi-Functionalized 1,1′-Ferrocenyl Bi-Hydrazone and Hydrazone-Cyanovinyl Molecules as Fluorescent “on–off” Sensor: Synthesis, Cytotoxicity and Cancer Cell Imaging Behavior. Inorganica Chim. Acta 2023, 552, 121511. [Google Scholar] [CrossRef]
- Turhan, O.; Yaman, M.; Dikmen, G.; Nural, Y.; Sarıboyacı, A.E.; Tasa, B.A.; Soykan, M.N.; Seferoğlu, Z. Novel Fluorescent Sensors Based on Coumarin-Hydrazide-Hydrazone Hybrid for the Detection of CN−, Co2+ and Ni2+ Ions: DFT and Bioimaging in Living Cells. J. Mol. Liq. 2023, 392, 123440. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhou, Z. Dual-Site Lysosome-Targeted Fluorescent Sensor for Fast Distinguishing Visualization of HClO and ONOO– in Living Cells and Zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 312, 124064. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-F.; Wang, B.-B.; Wu, W.-N.; Bi, W.-Y.; Xu, Z.-H.; Fan, Y.-C.; Bian, L.-Y.; Wang, Y. A Pyrazine-Containing Hydrazone Derivative for Sequential Detection of Al3+ and F−. J. Mol. Struct. 2022, 1251, 132073. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Tang, H.; Meier, H.; Cao, D. An Efficient Probe for Sensing Different Concentration Ranges of Glutathione Based on AIE-Active Schiff Base Nanoaggregates with Distinct Reaction Mechanism. Sens. Actuators B Chem. 2018, 273, 1085–1090. [Google Scholar] [CrossRef]
- Guo, X.; Gao, W.; Cheng, Z.-Z.; Huang, Y.-Y.; Yao, Z.-Y.; Li, Q.-Z.; Qiao, X.; Xie, C.-Z.; Xu, J.-Y. Highly Selective Fluorescent Detection Platform Based on Isoquinoline Schiff Base Ligand Monitors Glutathione in Biological Systems. J. Photochem. Photobiol. A Chem. 2022, 428, 113864. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, X.; Zheng, S.; Guo, H.; Yang, F. An Effect Fluorescence Sensor for Arginine Based on Bis-Cyanodistyrene Schiff-Base. J. Mol. Struct. 2024, 1305, 137798. [Google Scholar] [CrossRef]
- Gnocchini, E.; Pilesi, E.; Schiano, L.; Vernì, F. Vitamin B6 Deficiency Promotes Loss of Heterozygosity (LOH) at the Drosophila Warts (Wts) Locus. IJMS 2022, 23, 6087. [Google Scholar] [CrossRef]
- Barile, A.; Graziani, C.; Antonelli, L.; Parroni, A.; Fiorillo, A.; Di Salvo, M.L.; Ilari, A.; Giorgi, A.; Rosignoli, S.; Paiardini, A.; et al. Identification of the Pyridoxal 5′-phosphate Allosteric Site in Human Pyridox(Am)Ine 5′-phosphate Oxidase. Protein Sci. 2024, 33, e4900. [Google Scholar] [CrossRef]
- Pilesi, E.; Angioli, C.; Graziani, C.; Parroni, A.; Contestabile, R.; Tramonti, A.; Vernì, F. A Gene-nutrient Interaction between Vitamin B6 and Serine Hydroxymethyltransferase (SHMT) Affects Genome Integrity in Drosophila. J. Cell. Physiol. 2023, 238, 1558–1566. [Google Scholar] [CrossRef]
- Moosa, N.Y.; Azeem, S.A.; Lodge, J.K.; Cheung, W.; Ahmed, S.U. Vitamin B6 Pathway Maintains Glioblastoma Cell Survival in 3D Spheroid Cultures. IJMS 2024, 25, 10428. [Google Scholar] [CrossRef]
- Graziani, C.; Barile, A.; Antonelli, L.; Fiorillo, A.; Ilari, A.; Vetica, F.; Di Salvo, M.L.; Paiardini, A.; Tramonti, A.; Contestabile, R. The Z Isomer of Pyridoxilidenerhodanine 5′-phosphate Is an Efficient Inhibitor of Human Pyridoxine 5′-phosphate Oxidase, a Crucial Enzyme in Vitamin B6 Salvage Pathway and a Potential Chemotherapeutic Target. FEBS J. 2024, 291, 4984–5001. [Google Scholar] [CrossRef]
- Gálvez-Navas, J.M.; Molina-Montes, E.; Rodríguez-Barranco, M.; Ramírez-Tortosa, M.; Gil, Á.; Sánchez, M.-J. Molecular Mechanisms Linking Genes and Vitamins of the Complex B Related to One-Carbon Metabolism in Breast Cancer: An In Silico Functional Database Study. IJMS 2024, 25, 8175. [Google Scholar] [CrossRef] [PubMed]
- Pilesi, E.; Tesoriere, G.; Ferriero, A.; Mascolo, E.; Liguori, F.; Argirò, L.; Angioli, C.; Tramonti, A.; Contestabile, R.; Volontè, C.; et al. Vitamin B6 Deficiency Cooperates with Oncogenic Ras to Induce Malignant Tumors in Drosophila. Cell Death Dis. 2024, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, D.; Shukla, S.K.; Hu, T.; Thakur, R.; Fu, X.; King, R.J.; Kollala, S.S.; Attri, K.S.; Murthy, D.; et al. Vitamin B6 Competition in the Tumor Microenvironment Hampers Antitumor Functions of NK Cells. Cancer Discov. 2024, 14, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Borova, J.; Neuwirt, J.; Fuchs, O. Mobilization of Iron from Reticulocytes: Identification of Pyridoxal Isonicotinoyl Hydrazone as a New Iron Chelating Agent. FEBS Lett. 1979, 97, 317–321. [Google Scholar] [CrossRef]
- Ponka, P.; Richardson, D.R.; Edward, J.T.; Chubb, F.L. Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class. Relationship of the Lipophilicity of the Apochelator to Its Ability to Mobilise Iron from Reticulocytes in Vitro. Can. J. Physiol. Pharmacol. 1994, 72, 659–666. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Nagy, E.; Ponka, P.; Schulman, H.M. The Iron Chelator Pyridoxal Isonicotinoyl Hydrazone (PIH) Protects Plasmid pUC-18 DNA against OH-Mediated Strand Breaks. Free Radic. Biol. Med. 1998, 25, 875–880. [Google Scholar] [CrossRef]
- Szuber, N.; Buss, J.L.; Soe-Lin, S.; Felfly, H.; Trudel, M.; Ponka, P. Alternative Treatment Paradigm for Thalassemia Using Iron Chelators. Exp. Hematol. 2008, 36, 773–785. [Google Scholar] [CrossRef]
- Richardson, D.R.; Milnes, K. The Potential of Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class as Effective Antiproliferative Agents II: The Mechanism of Action of Ligands Derived From Salicylaldehyde Benzoyl Hydrazone and 2-Hydroxy-1-Naphthylaldehyde Benzoyl Hydrazone. Blood 1997, 89, 3025–3038. [Google Scholar] [CrossRef]
- Gao, J.; Richardson, D.R. The Potential of Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class as Effective Antiproliferative Agents, IV: The Mechanisms Involved in Inhibiting Cell-Cycle Progression. Blood 2001, 98, 842–850. [Google Scholar] [CrossRef]
- Karwt, R.; Bondar, O.V.; Pugachev, M.V.; Mohammad, T.; Kadyrova, A.S.; Pavelyev, R.S.; Alrhmoun, S.; Gnezdilov, O.I.; Shtyrlin, Y.G. Anticancer Potential of Pyridoxine-Based Doxorubicin Derivatives: An In Vitro Study. Life 2024, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Ponka, P.; Hardy, P.; Hanna, N.; Varma, D.R.; Lachapelle, P.; Chemtob, S. Prevention of Postasphyxia Electroretinal Dysfunction with a Pyridoxal Hydrazone. Free Radic. Biol. Med. 1997, 22, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Schulman, H.M.; Wilczynska, A. Ferric Pyridoxal Isonicotinol Hydrazone Can Provide Iron for Heme Synthesis in Reticulocytes. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1982, 718, 151–156. [Google Scholar] [CrossRef]
- Huang, A.; Ponka, P. A Study of the Mechanism of Action of Pyridoxal Isonicotinoyl Hydrazone at the Cellular Level Using Reticulocytes Loaded with Non-Heme 59Fe. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1983, 757, 306–315. [Google Scholar] [CrossRef]
- Buss, J.L.; Neuzil, J.; Gellert, N.; Weber, C.; Ponka, P. Pyridoxal Isonicotinoyl Hydrazone Analogs Induce Apoptosis in Hematopoietic Cells Due to Their Iron-Chelating Properties. Biochem. Pharmacol. 2003, 65, 161–172. [Google Scholar] [CrossRef]
- Buss, J.L.; Neuzil, J.; Ponka, P. The Role of Oxidative Stress in the Toxicity of Pyridoxal Isonicotinoyl Hydrazone (PIH) Analogues. Biochem. Soc. Trans. 2002, 30, 755–758. [Google Scholar] [CrossRef]
- Casas, J.S.; Couce, M.D.; Sordo, J. Coordination Chemistry of Vitamin B6 and Derivatives: A Structural Overview. Coord. Chem. Rev. 2012, 256, 3036–3062. [Google Scholar] [CrossRef]
- Sahoo, S.K. Chromo-Fluorogenic Sensing Using Vitamin B6 Cofactors and Their Derivatives: A Review. New J. Chem. 2021, 45, 8874–8897. [Google Scholar] [CrossRef]
- Richardson, D.R.; Wis Vitolo, L.M.; Hefter, G.T.; May, P.M.; Clare, B.W.; Webb, J.; Wilairat, P. Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class Part I. Ionisation Characteristics of the Ligands and Their Relevance to Biological Properties. Inorganica Chim. Acta 1990, 170, 165–170. [Google Scholar] [CrossRef]
- Wis Vitolo, L.M.; Hefter, G.T.; Clare, B.W.; Webb, J. Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class Part II. Formation Constants with Iron(III) and Iron(II). Inorganica Chim. Acta 1990, 170, 171–176. [Google Scholar] [CrossRef]
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) Complexes for Antitumor Applications: An Overview. Chem. A Eur. J 2018, 24, 11840–11851. [Google Scholar] [CrossRef]
- Gurba, A.; Taciak, P.; Sacharczuk, M.; Młynarczuk-Biały, I.; Bujalska-Zadrożny, M.; Fichna, J. Gold (III) Derivatives in Colon Cancer Treatment. IJMS 2022, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Ratia, C.; Sueiro, S.; Soengas, R.G.; Iglesias, M.J.; López-Ortiz, F.; Soto, S.M. Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance. Antibiotics 2022, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Kuranova, N.N.; Pimenov, O.A.; Zavalishin, M.N.; Gamov, G.A. Complexes of Gold(III) with Hydrazones Derived from Pyridoxal: Stability, Structure, and Nature of UV-Vis Spectra. IJMS 2024, 25, 5046. [Google Scholar] [CrossRef] [PubMed]
- Patanjali, P.; Kumar, R.; Sourabh; Kumar, A.; Chaudhary, P.; Singh, R. Reviewing Gold(III) Complexes as Effective Biological Operators. Main Group Chem. 2018, 17, 35–52. [Google Scholar] [CrossRef]
- Wróblewska, A.; Sadowski, M.; Jasiński, R. Selectivity and Molecular Mechanism of the Au(III)-Catalyzed [3+2] Cycloaddition Reaction between (Z)-C,N-Diphenylnitrone and Nitroethene in the Light of the Molecular Electron Density Theory Computational Study. Chem Heterocycl Comp 2024, 60, 639–645. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Maltseva, M.A.; Osokin, V.S.; Aleksandriiskii, V.V.; Petrova, U.A.; Knyazeva, A.A.; Eroshin, A.V.; Zhabanov, Y.A.; Gamov, G.A. Synthesis and Characterization of a Vitamin B6-Tetrazole Hydrazone as a Fluorescence Probe for Selective Detection of Cd2+ and Ga3+ Ions. Opt. Mater. 2025, 158, 116493. [Google Scholar] [CrossRef]
- Gamov, G.A.; Kiselev, A.N.; Aleksandriiskii, V.V.; Sharnin, V.A. Influence of Regioisomerism on Stability, Formation Kinetics and Ascorbate Oxidation Preventive Properties of Schiff Bases Derived from Pyridinecarboxylic Acids Hydrazides and Pyridoxal 5′-Phosphate. J. Mol. Liq. 2017, 242, 1148–1155. [Google Scholar] [CrossRef]
- Gamov, G.A.; Khodov, I.A.; Belov, K.V.; Zavalishin, M.N.; Kiselev, A.N.; Usacheva, T.R.; Sharnin, V.A. Spatial Structure, Thermodynamics and Kinetics of Formation of Hydrazones Derived from Pyridoxal 5′-Phosphate and 2-Furoic, Thiophene-2-Carboxylic Hydrazides in Solution. J. Mol. Liq. 2019, 283, 825–833. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Gamov, G.A.; Pogonin, A.E.; Isagulieva, A.K.; Shibaeva, A.V.; Klimovich, M.A.; Morozov, V.N. A New Fluorescent Vitamin B6-Based Probe for Selective and Sensitive Detection Ga3+ Ions in the Environment and Living Cells. Dye. Pigment. 2023, 219, 111621. [Google Scholar] [CrossRef]
- Kuranova, N.N.; Petrova, D.V.; Zavalishin, M.N.; Kiselev, A.N.; Gamov, G.A. Prediction of Protonation Constants of Hydrazones and Schiff Bases Derived from Pyridoxal 5′-Phosphate, Pyridoxal, 3-Hydroxyisonicotinaldehyde and Salicylic Aldehyde. J. Mol. Liq. 2023, 390, 123049. [Google Scholar] [CrossRef]
- Molinspiration Calculation of Molecular Properties and Bioactivity Score. Available online: https://www.molinspiration.com/cgi/properties (accessed on 17 October 2024).
- Zavalishin, M.N.; Gamov, G.A.; Nikitin, G.A.; Pimenov, O.A.; Aleksandriiskii, V.V.; Isagulieva, A.K.; Shibaeva, A.V. A Simple Vitamin B6-Based Fluorescent Chemosensor for Selective and Sensitive Al3+ Recognition in Water: A Spectral and DFT Study. Microchem. J. 2024, 197, 109791. [Google Scholar] [CrossRef]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing Powerhouse in Colon Cancer Cells by Hydrazide–Hydrazone-Based Small Molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef]
- Srovnalová, A.; Kaplánek, R.; Kejík, Z.; Hajduch, J.; Gurská, S.; Martásek, P.; Hajdúch, M.; Džubák, P.; Jakubek, M. Synthesis and Evaluation of Cyclobut-3-Ene-1,2-Dione-3-Hydrazones with Benzothiazole Moiety as Novel Anticancer Agents Inducing Nonapoptotic Oncosis-like Cell Death. Biomed. Pharmacother. 2025, 190, 118404. [Google Scholar] [CrossRef]
- Negru, G.; Kamus, L.; Bîcu, E.; Shova, S.; Sendid, B.; Dubar, F.; Ghinet, A. Attempts to Access a Series of Pyrazoles Lead to New Hydrazones with Antifungal Potential against Candida Species Including Azole-Resistant Strains. Molecules 2021, 26, 5861. [Google Scholar] [CrossRef]
- Başaran, E.; Tür, G.; Akkoc, S.; Taskin-Tok, T. Design, Synthesis, and In Silico and In Vitro Cytotoxic Activities of Novel Isoniazid–Hydrazone Analogues Linked to Fluorinated Sulfonate Esters. ACS Omega 2024, 9, 17551–17562. [Google Scholar] [CrossRef]
- Liu, R.; Cui, J.; Ding, T.; Liu, Y.; Liang, H. Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules 2022, 27, 8393. [Google Scholar] [CrossRef]
- Tafere, D.A.; Gebrezgiabher, M.; Elemo, F.; Sani, T.; Atisme, T.B.; Ashebr, T.G.; Ahmed, I.N. Hydrazones, Hydrazones-Based Coinage Metal Complexes, and Their Biological Applications. RSC Adv. 2025, 15, 6191–6207. [Google Scholar] [CrossRef]
- Yeo, C.I.; Goh, C.H.P.; Tiekink, E.R.T.; Chew, J. Antibiotics: A “GOLDen” Promise? Coord. Chem. Rev. 2024, 500, 215429. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Kiselev, A.N.; Pechnikova, N.L.; Shagalov, E.V.; Nikitin, G.A.; Gamov, G.A. Benzotiazole-Based Colorimetric Chemosensor for the Effective Detection of Hazardous Cyanide Ions. ChemistrySelect 2023, 8, e202301302. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N. La3+, Ce3+, Eu3+, and Gd3+ Complex Formation with Hydrazones Derived from Pyridoxal 5’-Phosphate in a Neutral Tris–HCl Buffer. Russ. J. Inorg. Chem. 2021, 66, 1561–1568. [Google Scholar] [CrossRef]
- Khodov, I.A.; Belov, K.V.; Pogonin, A.E.; Savenkova, M.A.; Gamov, G.A. Spatial Structure and Conformations of Hydrazones Derived from Pyridoxal 5′-Phosphate and 2-, 3-Pyridinecarbohydrazide in the Light of NMR Study and Quantum Chemical Calculations. J. Mol. Liq. 2021, 342, 117372. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Gamov, G.A.; Pimenov, O.A.; Pogonin, A.E.; Aleksandriiskii, V.V.; Usoltsev, S.D.; Marfin, Y.S. Pyridoxal 5′-Phosphate 2-Methyl-3-Furoylhydrazone as a Selective Sensor for Zn2+ Ions in Water and Drug Samples. J. Photochem. Photobiol. A Chem. 2022, 432, 114112. [Google Scholar] [CrossRef]
- Liu, M.; Mao, X.; Ye, C.; Huang, H.; Nicholson, J.K.; Lindon, J.C. Improved WATERGATE Pulse Sequences for Solvent Suppression in NMR Spectroscopy. J. Magn. Reson. 1998, 132, 125–129. [Google Scholar] [CrossRef]

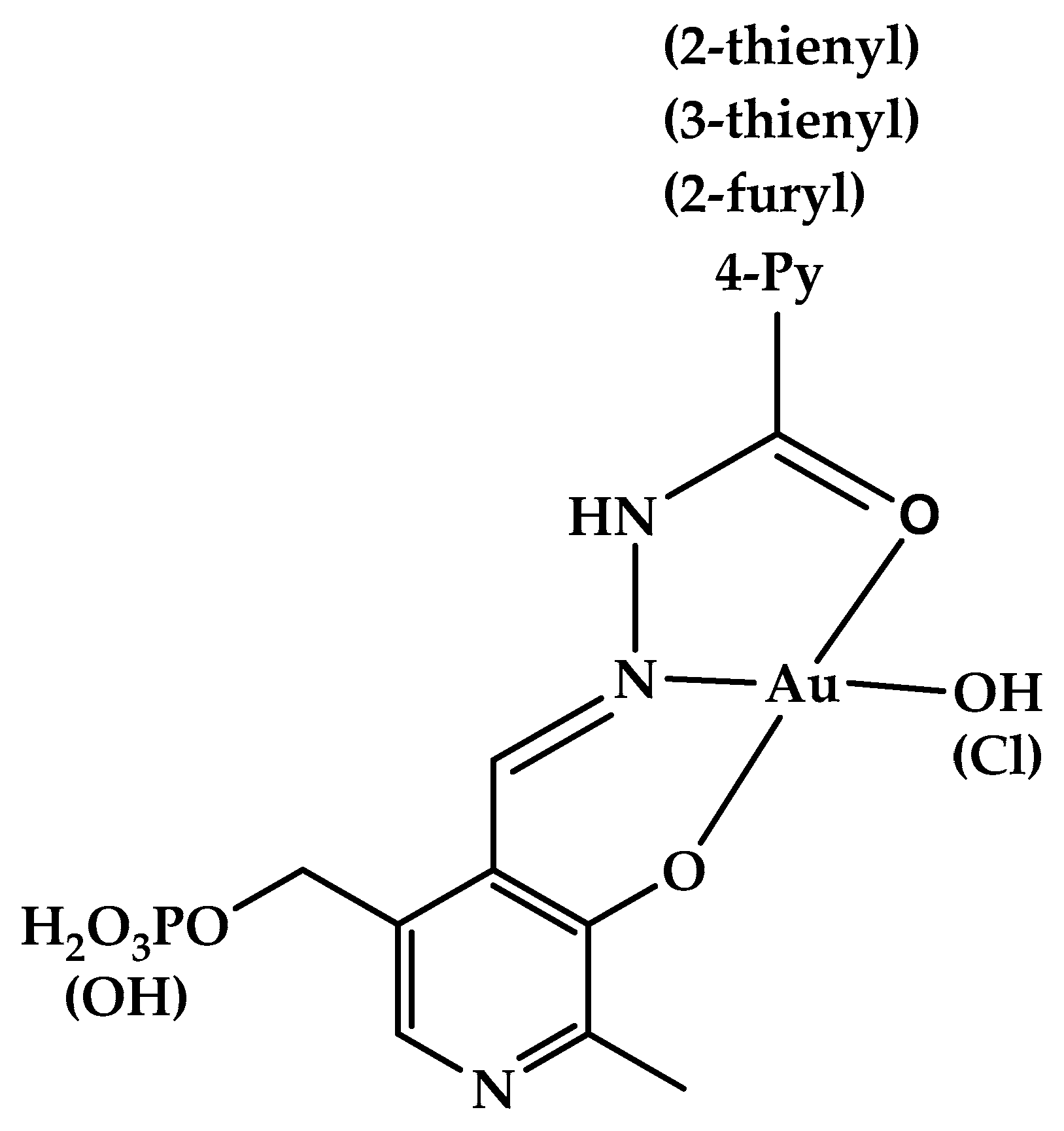
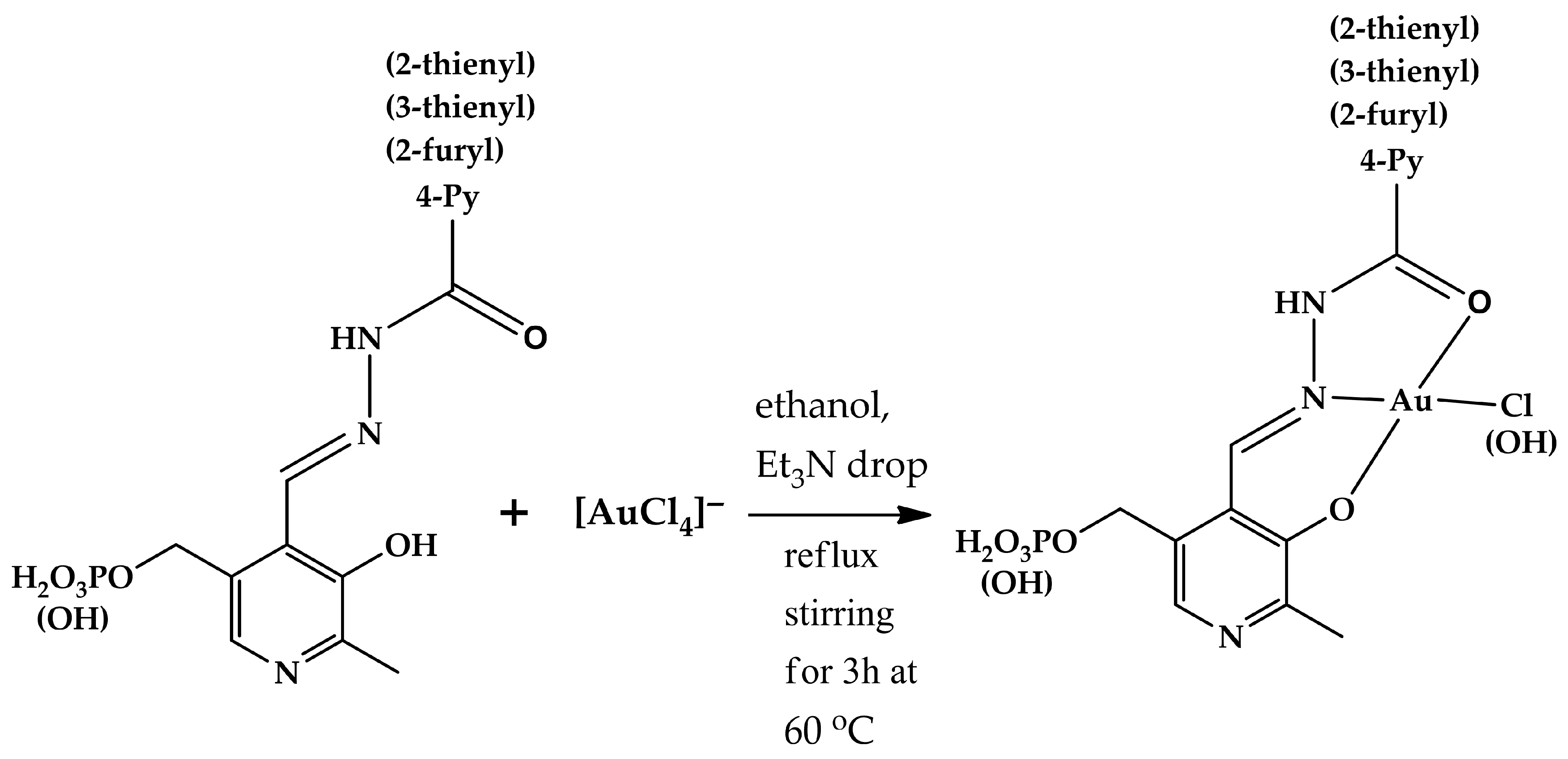
| Nucleus | 1a | 3a | Nucleus | 1g | 3g | 1h | 3h | 1j | 3j |
| –NH– | 12.97s | 13.23s | –NH– | 12.76s | 13.05s | 12.82s | 13.03s | 12.60s | 13.01s |
| –OH | 12.28s | 12.88s | –OH | 12.30s | 13.05s | 12.33s | 13.03s | 12.40s | 13.01s |
| H6 | 8.89s | 8.91s | H6 | 8.87s | 8.89s | 8.83s | 8.83s | 8.81s | 8.83s |
| H11, 13 | 8.84d | 8.91s | H11 | 8.03s | 8.08s | 7.99d | 8.02d | 8.41d | 8.02dd |
| H7 | 8.03s | 8.26s | H7 | 8.01s | 8.26s | 8.02s | 8.25s | 8.01s | 8.24s |
| H10, 14 | 7.89d | 7.96d | H13 | 7.41d | 7.46d | 7.97d | 8.02d | 7.64d | 7.31dd |
| H12 | 6.76d | 6.80d | 7.28t | 7.32t | 7.73dd | 8.01d | |||
| H5′ | 5.06d | 5.20d | H5' | 5.05d | 5.19d | 5.06d | 5.20d | 5.05d | 5.19d |
| H2′ | 2.46s | 2.61s | H2′ | 2.44s | 2.61s | 2.45s | 2.60s | 2.45s | 2.59s |
| 31P | −1.52s | −1.41s | 31P | −1.51s | −1.43s | −1.53s | −1.42s | −1.53s | −1.43s |
| Nucleus | 2a | 4a | Nucleus | 2g | 4g | 2h | 4h | 2j | 4j |
| –NH– | 12.88s | 13.04s | –NH– | 12.97s | 13.17s | 12.97s | 13.20s | 13.06s | 13.31s |
| –OH | 13.30s | 13.46s | –OH | 13.07s | 13.14s | 13.15s | 13.20s | 12.94s | 13.02s |
| H6 | 9.07s | 9.07s | H6 | 8.99s | 8.98s | 8.99s | 8.97s | 8.98s | 8.97s |
| H11, 13 | 8.88d | 8.96d | H7 | 8.22s | 8.28s | 8.22s | 8.29s | 8.21s | 8.29s |
| H7 | 8.22s | 8.31s | H11 | 8.07s | 8.08s | 8.09d | 8.08d | 8.50s | 8.50s |
| H10, 14 | 7.97d | 8.09d | H13 | 7.50d | 7.50d | 8.02d | 8.03d | 7.75dd | 7.76dd |
| H12 | 6.79d | 6.80d | 7.31t | 7.32t | 7.68dd | 7.68dd | |||
| H5′ | 4.77s | 4.82s | H5′ | 4.76s | 4.79s | 4.77s | 4.81s | 4.76s | 4.80s |
| H2′ | 2.60s | 2.65s | H2′ | 2.60s | 2.64s | 2.60s | 2.65s | 2.60s | 2.65s |
| Cell Line | 1a | 1b | 1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | 1k | 1l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEK293T | >50 | 16 ± 3 | 16 ± 3 | 4 ± 2 | 32 ± 2 | 13 ± 4 | 21 ± 2 | 18 ± 2 | 27 ± 2 | 41 ± 4 | 10 ± 3 | 10 ± 7 |
| HCT116 | >50 | 17 ± 2 | 16 ± 2 | 6 ± 3 | 27 ± 5 | 22 ± 3 | 12 ± 2 | 12 ± 2 | 26 ± 6 | 42 ± 11 | 7 ± 3 | 9 ± 7 |
| Compound | Concentration, µg mL−1 | Microorganism | pH | ||||
|---|---|---|---|---|---|---|---|
| S. aureus INA 00985 | B. subtilis ATCC 6633 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 14053 | |||
| [HAuCl4] | 10 | 50 | 40 | 40 | 42 | 30 | 1 |
| 1 | 35 | 24 | 24 | 25 | 17 | ||
| 3a | 5 | no effect | no effect | no effect | no effect | no effect | 8 |
| 1 | no effect | no effect | no effect | no effect | no effect | ||
| 3g | 5 | 20 | 15 | 15 | 18 | 13 | 8 |
| 1 | 12 | 11 | 13 | 13 | no effect | ||
| 3h | 5 | 12 | no effect | 11 | 10 | no effect | 6 |
| 1 | no effect | no effect | no effect | no effect | no effect | 8 | |
| 3j | 5 | 25 | 20 | 20 | 24 | 18 | 6 |
| 1 | 15 | 13 | 15 | 18 | 11 | 8 | |
| 4a | 5 | no effect | no effect | no effect | no effect | no effect | 7 |
| 1 | no effect | no effect | no effect | no effect | no effect | 8 | |
| 4g | 5 | 17 | 13 | 16 | 15 | no effect | 8 |
| 1 | 11 | no effect | 12 | 12 | no effect | 8 | |
| 4h | 5 | 11 | no effect | 11 | 11 | no effect | 7 |
| 1 | no effect | no effect | no effect | no effect | no effect | 8 | |
| 4j | 5 | 12 | no effect | 10 | no effect | no effect | 7 |
| 1 | no effect | no effect | no effect | no effect | no effect | 8 | |
| Mixtures of [HAuCl4] and hydrazones solutions in 0.025 M Tris-HCl buffer | |||||||
| [HAuCl4] + 1a | 10 | 22 | 16 | 23 | 24 | 14 | |
| 1 | 22 | 14 | 20 | 22 | no effect | 7 | |
| [HAuCl4] + 1g | 10 | 24 | 15 | 23 | 25 | 14 | |
| 1 | 22 | 13 | 18 | 22 | no effect | 7 | |
| [HAuCl4] + 1h | 10 | 28 | 20 | 25 | 25 | 15 | |
| 1 | 22 | 12 | 16 | 25 | no effect | 7 | |
| [HAuCl4] + 1i | 10 | 17 | no effect | 17 | 16 | no effect | |
| 1 | 22 | 14 | 18 | 24 | no effect | 7 | |
| [HAuCl4] + 1j | 10 | 25 | 20 | 24 | 25 | 15 | |
| 1 | 22 | 15 | 20 | 25 | no effect | 7 | |
| [HAuCl4] + 2a | 10 | 18 | no effect | 15 | 15 | no effect | |
| 1 | 20 | 12 | 20 | 25 | no effect | 7 | |
| [HAuCl4] + 2g | 10 | 27 | 17 | 23 | 25 | 15 | |
| 1 | 22 | 12 | 18 | 24 | no effect | 7 | |
| [HAuCl4] + 2h | 10 | 15 | no effect | 16 | 18 | no effect | |
| 1 | 22 | 12 | 15 | 25 | no effect | 7 | |
| [HAuCl4] + 2i | 10 | 25 | 20 | 25 | 25 | 15 | |
| 1 | 22 | 13 | 20 | 25 | no effect | 7 | |
| [HAuCl4] + 2j | 10 | 18 | no effect | 15 | 15 | no effect | |
| 1 | 22 | 13 | 18 | 25 | no effect | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, D.V.; Isagulieva, A.K.; Sineva, O.N.; Sadykova, V.S.; Zavalishin, M.N.; Gamov, G.A. Synthesis, Structural Characterization, Cytotoxicity, and Antibacterial Properties of Gold(III) Complexes with Hydrazones Derived from Vitamin B6. Inorganics 2025, 13, 335. https://doi.org/10.3390/inorganics13100335
Petrova DV, Isagulieva AK, Sineva ON, Sadykova VS, Zavalishin MN, Gamov GA. Synthesis, Structural Characterization, Cytotoxicity, and Antibacterial Properties of Gold(III) Complexes with Hydrazones Derived from Vitamin B6. Inorganics. 2025; 13(10):335. https://doi.org/10.3390/inorganics13100335
Chicago/Turabian StylePetrova, Daria V., Aleksandra K. Isagulieva, Olga N. Sineva, Vera S. Sadykova, Maksim N. Zavalishin, and George A. Gamov. 2025. "Synthesis, Structural Characterization, Cytotoxicity, and Antibacterial Properties of Gold(III) Complexes with Hydrazones Derived from Vitamin B6" Inorganics 13, no. 10: 335. https://doi.org/10.3390/inorganics13100335
APA StylePetrova, D. V., Isagulieva, A. K., Sineva, O. N., Sadykova, V. S., Zavalishin, M. N., & Gamov, G. A. (2025). Synthesis, Structural Characterization, Cytotoxicity, and Antibacterial Properties of Gold(III) Complexes with Hydrazones Derived from Vitamin B6. Inorganics, 13(10), 335. https://doi.org/10.3390/inorganics13100335








