Abstract
Treatment of N,N-dimethylhydrazinoalkenes with diethylzinc followed by exposure of the resulting ethylzinc amides to high vacuum drives a Schlenck redistribution metalloamination/cyclization to generate the corresponding bis(organozinc) intermediates in excellent conversions. Direct treatment of these with appropriate aryl or vinyl electrophiles in the presence of catalytic PdCl2 (DPEphos) provides the corresponding arylated or alkenylated pyrrolidines and piperidines with high efficiency.
1. Introduction
Nitrogen heterocycles are crucial drug scaffolds, accounting for approximately 75% of all small-molecule FDA-approved drugs available on the market [1]. They also serve as life’s building blocks, appearing in DNA, RNA, enzymes, and ribosomes [2]. Non-aromatic nitrogen heterocycles are among the most common drug moieties, with piperidine, piperazine, and pyrrolidine being, respectively, the most, third most, and fifth most frequently encountered azacycles [3]. The installation of stereocenters in saturated azacyclic sites poses a challenge that can be addressed by utilizing a hydroamination/cyclization or, more directly, by a metalloamination/cyclization-functionalization sequence [4,5]. Stahl reported the first substrate-based diastereoselective aminocyclization using Pd(TFA)2 as the catalyst (>20:1 dr). In these cases alkene containing products were obtained as a result of β-hydride elimination [6]. Waser subsequently described an efficient aminocyclization/alkynylation protocol mediated by a DPEphos•Pd(II) complex [7]. In sharp contrast to the Group 10 metals, examples of alkene amination processes mediated by Zn(II) are comparatively rare [8]. Our group first reported a diastereoselective synthesis of 2,5- and 3,5-substituted piperidines and pyrrolidines (up to >20:1 dr) using N,N-dimethylhydrazinoalkene substrates and diethylzinc in a cyclization/coupling tandem protocol via N-chelated organozinc intermediates [9]. This work was later optimized and expanded from allylation and acylation to propargylation and alkynylation [10,11,12,13]. In this contribution, we describe catalyst optimization for vinylation and arylation of organozinc metalloamination intermediates to obtain the corresponding C-SP2 functionalized products as well as the X-ray crystal structure for the essential spirobicyclic Zn(II) intermediate, for which only NMR evidence was previously available. For the arylation and vinylation, a Negishi-type cross-coupling presented the major advantage of using the zinc intermediate as is, without additional transmetallation. Negishi couplings generally require palladium(0) [13,14], palladium(II) [15,16], nickel(0) [17], or nickel(II) [18] catalysts to occur. But palladium catalysts tend to offer a higher functional-group tolerance [19].
2. Results and Discussion
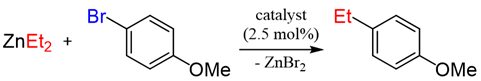
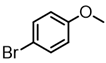
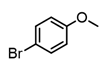
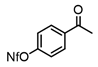
An initial catalyst evaluation was performed using diethylzinc as a model to investigate substitution vs. β-hydride elimination. Pd(II)-catalyzed coupling of diethylzinc with 4-bromoanisole using a total of ten catalysts was closely monitored by 1H NMR. The selection of 4-bromoanisole was based on its comparatively modest reactivity as a coupling partner. The use of bis(cyclooctadiene)Ni(0) lead to a mediocre consumption of diethylzinc, while alternative nickel(II) species were shown to be even less efficient, with the exception of NiCl2(DPPF), for which the selectivity towards cross-coupling was improved. Palladium catalysts typically showed higher reactivity than their nickel(II) counterparts. Accordingly, Pd2(dba)3 afforded limited conversion and selectivity. In addition, Pd(II) catalysts varied widely in selectivity, and these trends were ligand dependent. Phosphine-ligated catalysts were found to favor the desired Negishi pathway, particularly those with large bite angles (e.g., DPPF, DPEphos, and Xantphos) [20]. Of these, DPEphos [21] proved optimal. In contrast, Pd(II) complexes of monodentate phosphines, such as triphenylphosphine, were less efficient (Table 1).

Table 1.
Catalyst screening for Negishi cross-coupling using diethylzinc and 4-bromoanisole.
In these studies, major side products were identified as ethylene (singlet, 5.5 ppm) and ethane (singlet, 0.0 ppm), which most likely resulted from β-hydride elimination followed by reductive elimination. The formation of ethylene would imply a more rapid β-hydride elimination than reductive elimination. This contrasts with the literature examples, which state that the small atomic radius of nickel reduces its ability to undergo β-hydride elimination compared to palladium [22]. Recent work has also shown the propensity for nickel catalysts to create clusters with zinc and undergo alternative pathways through one-electron transfer mechanisms [23,24].
Among all the catalysts that were examined, PdCl2(DPEphos) furnished the highest conversion to the cross-coupled product, second only to PdCl2(Xantphos), in accordance with Waser’s findings [7,25]. Experiments were subsequently conducted using the optimized cross-coupling conditions on the bis(amido)zinc intermediates 4 and 5 (Table 2) instead of diethylzinc. Intermediates 4 and 5 were obtained from their respective N,N-dimethylhydrazinoalkenes 1 and 2, followed by removal of the volatile components in vacuo, subsequent addition of THF, PdCl2(DPEphos), and an appropriate electrophile. The conformationally biased 2,2-dimethyl-bearing hydrazinoalkene 1 underwent facile conversion to the intermediate 5, while 1-methyl-bearing substrate 2 gave a slightly lower conversion (c.a., 95%, Scheme 1). The halide electrophiles were chosen to probe reaction scope and limitations. These included aryl, vinyl, pyridyl and indolyl halides, and spanned the range from poorly to highly activated electrophiles as well as varied steric hinderance levels.

Table 2.
Cross-coupling reactions between a range of electrophiles and organozinc intermediates 4–6.
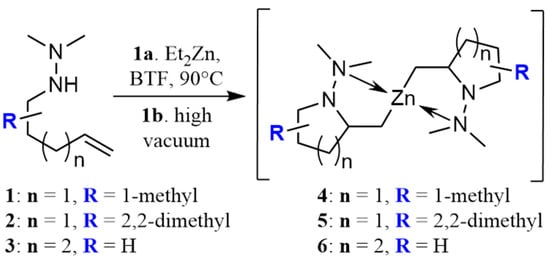
Scheme 1.
Synthesis of organozinc intermediates 4–6 using diethylzinc and hydrazinoalkenes 1–3.
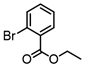
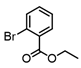
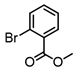
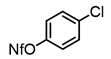
2-Bromopropene, (E)-1-bromo-2-phenylethene, and methyl α-bromoacrylate were selected to probe the ability of vinylic bromides to serve as cross-coupling participants. (E)-β-bromostyrene provided an example of lower hindrance compared to 2-bromopropene, while methyl α-bromoacrylate differs from the latter with higher electrophilicity. In addition, the alleneic halide 1-Bromo-1,2-propadiene was examined. Diverse aryl halides were evaluated, where trends in reactivity were explored by comparing 4-bromoanisole, 2-bromo benzoate esters, 2-bromotoluene, 2-bromo-5-chlorotoluene and 2-bromomesitylene, as were 3-bromo- and 7-bromo-1-tosyl-1H-indole, in cross-coupling reactions with organizinc intermediate 4. 2-Bromo- and 2-chloropyridine were also examined as pyridine-based substrates possessing activated 2-positions. 2-Bromo and 2-iodothiophene were also tested using organozinc intermediates 4 and 5. (E)-β-Bromostyrene-derived products were isolated in excellent yields, whereas 2-bromopropene proved an inefficient coupling partner. Similarly, 1-bromo-1,2-propadiene and methyl α-bromoacrylate were comparatively inefficient as reactants.
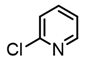
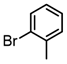
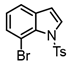
It is also of interest that 4-bromoanisole and the more electrophilic 2-bromobenzoate esters gave similar yields. As expected, bromine was the only targeted halide in 2-bromo-5-chlorotoluene reactions and was similar to 2-bromotoluene in speed of consumption and yield. The more hindered 2-bromomesitylene proved unreactive toward all three organozinc intermediates. 7-Bromo-1-tosyl-1H-indole led to the desired product in its reaction with organozinc 4. 2-Chloropyridine and 2-bromopyridine provided full conversions and in good yields. Both 2-bromo and 2-iodothiophene were not suitable under these conditions presumably due to high zinc–sulfur affinity [26].
Overall, yields obtained for pyrrolidines (e.g., 7a–8e) were acceptable, with higher yields being obtained for 2-chloropyridine and (E)-β-bromostyrene compared to typical aryl bromides or 7-bromo-1-tosyl-1H-indole. When closely followed by 1H NMR, the cross-coupling reaction can be accompanied by retro-metalloamination/cyclization of the organozinc intermediates in the cases of organozincs 4 and 6, which results in back-conversion to the starting N,N-dimethylhydrazinoalkene. This was found to be electrophile-dependent in nature, and the use of two equivalents of the electrophilic component mitigated this side reaction.
Lower conversion of the pro-6-membered zinc amide corresponding to 3 to organozinc 6 also impeded the cross-coupling step. Accordingly, more reactive nonaflate electrophiles [27] were needed for successful cross-couplings to occur in respectable yields. A series of aryl nonaflates of varying reactivity were examined as shown in Table 2. Piperidine derivatives were thus obtained in yields as high as 72% through the use of two equivalents of nonaflate.
A more detailed study on the generation of the organozinc intermediates for use in cross-coupling was needed to clarify the mechanism of metalloamination/cyclization. The mechanism was initially investigated by 1H NMR using the 2,2-dimethyl bearing substrate 2 since the Thorpe–Ingold effect favors complete conversion to organozinc 5. Instantaneous coordination of diethylzinc to the hydrazine’s N-H nitrogen was observed through the deshielding of this hydrogen (Δδ = 0.49 ppm, C6D6). Subsequent heating at 90 °C for 16 h resulted in full conversion to the ethylzinc bearing species 11 as observed by 1H NMR (Scheme 1). Removal of the volatile components under high vacuum caused a shift in the Schlenk equilibrium [28], which favored the formation of the d18 dialkylzinc dimer 5 that crystallized as a solid. This was identified by 1H NMR and X-ray crystallography (Figure 1). Addition of triethylamine after cyclization to the ethyl alkylzinc intermediate 11 favored the evaporation of the more volatile amine-chelated diethylzinc under high vacuum, quickly promoting the zinc dimer complex 5 (Scheme 2) [29]. This crystal appears to be the first identified hydrazine-organozinc dimer. The closest structure previously reported contains an amine-alkyl zinc dimer and presents a similar metal center, as both have a seesaw-like geometry [30]. The obtained angles around the metal center were 160.34° (C-Zn-C) and 112.39° (N-Zn-N); thus, a respective relative difference of 11% and 6% was found compared to seesaw. Additionally, the intermediate 5 withstands long exposures to the air, eventually reacting with water, and not oxygen, leading to ring opening, giving the starting hydrazinoalkene.
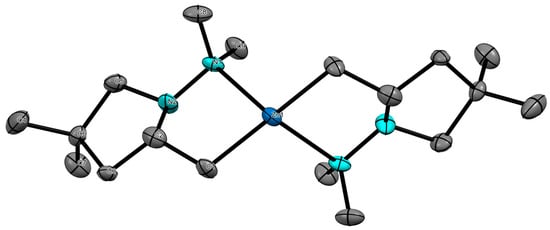
Figure 1.
Thermal ellipsoid plot of the zinc dimer intermediate 5, with thermal ellipsoids drawn at 50% probability. All hydrogen atoms are omitted for clarity. Carbon atoms are shown in grey, nitrogen atoms are shown in teal, and the zinc atom is shown in dark blue for clarity.
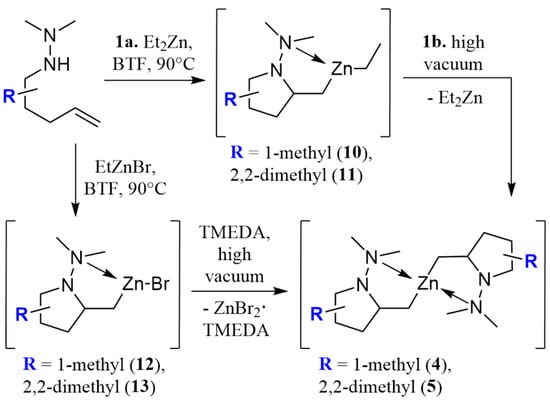
Scheme 2.
Synthesis and structure of intermediates 4–5 and 10–13.
Ethylzinc bromide was also evaluated for N-metallation, leading to the formation of ethane and the generation of the intermediate alkylzinc bromides (Scheme 2, 12 and 13). Organozinc halides benefit from lower reactivities than the corresponding dialkyls [31], and therefore benefit from higher functional group tolerances [32]. In the case of organozinc bromide 12 (d16 complex) in benzotrifluoride, the addition of TMEDA lead to the precipitation of ZnBr2(TMEDA) thereby driving transmetallation to the zinc dimer 5 (d18 complex, Scheme 2) [33,34]. This experimental protocol was repeated with the 1-methyl substituted N,N-dimethylhydrazine 1 and its structural isomer 2 to generate organozincs 4 and 6. Although both were formed as oils at room temperature [35], these intermediates were identified by 1H and 13C NMR and proved to be very effective cross-coupling partners.
3. Materials and Methods
The synthesis and characterization of all reported products are presented in sections S-I through S-IV of the electronic Supplementary Information File.
Oven- or flame-dried glassware was employed under nitrogen unless otherwise noted. All materials were used as received from commercial sources. Syntheses of hydrazines adapted from previously reported procedures [10]. Thin-layer chromatography (TLC) employed 0.25 mm glass silica gel plates with UV indicator and visualized with UV light (254 nm) or potassium permanganate staining. Nuclear magnetic resonance (NMR) data was obtained from a Bruker AVANCE III HD NMR spectrometer equipped with an Ascend 500 (500 MHz) magnet from Bruker Corporation, Billerica, United States. High-resolution mass spectra (HRMS) were obtained from a Bruker MicroTOF with a Dart 100—SVP 100 ion source from Bruker Daltonics GmbH & Co. KG, Bremen, Germany, or an Agilent 7890A/5975C TAD series GC/MSD from Agilent Technologies, Santa Clara, United States. The X-ray diffraction data were measured at 100 K on a Bruker D8 Venture Duo X-ray diffractometer from Bruker AXS GmbH, Karlsruhe, Germany, equipped with a CMOS IμS 3.0 Mo source from Incoatec GmbH, Geesthacht, Germany, and a PHOTON detector from Bruker AXS GmbH, Karlsruhe, Germany.
4. Representative Experimental Procedures
1.0 mmol scale: Compound 7c was prepared using the following procedure: In a nitrogen-filled glovebox, a 10 mL Schlenk flask equipped with a magnetic stirring bar was charged with diethylzinc (0.128 mL, 1.2 eq.), benzotrifluoride (4 mL) and 2-(hex-5-en-2-yl)-1,1-dimethylhydrazine (0.185 mL, 1.0 mmol). The reactant mixture was removed from the glovebox and placed in a 90 °C oil bath for 8 h. The reaction mixture was returned to room temperature and the solvent was removed under vacuum. The flask was returned to the glovebox and was charged with THF (4 mL), 2-bromo-5-chlorotoluene (0.160 mL, 1.2 mmol), and PdCl2 (DPEPhos) (35.8 mg, 5 mol%). After 16 h of stirring, the resulting mixture was dispersed into diethyl ether (10 mL) and washed with water (2 × 10 mL). The combined aqueous phases were then back-extracted with diethyl ether (3 × 10 mL); then, the combined organics were washed with brine (20 mL), dried over Na2SO4, and concentrated in vacuo. The crude product was further purified via flash column chromatography (hexanes to 1:9 ethyl acetate/hexanes gradient) to afford 0.209 g (78%) of compound 7c as a clear oil.
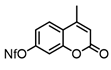
0.1 mmol scale: Compound 9c was prepared using the following procedure: In a nitrogen-filled glove box, a J. Young tube equipped with a C6D6 insert was charged with benzotrifluoride (0.4 mL), 2-(hex-5-en-1-yl)-1,1-dimethylhydrazine (0.1 mmol), and diethylzinc (12.3 μL, 1.2 eq.). The reactant mixture heated at 60 °C for 16 h, and then tetramethylethylenediamine (30.0 μL, 2.0 eq.) was added. Completion of the reaction was monitored by the disappearance of the alkene peaks by 1H NMR. Upon completion, volatiles were removed under high vacuum and the tube was charged with THF (0.3 mL), 4-methyl-7-nonafluorobutylsulfonyloxy coumarin (2.0 eq.), and DPEphos-PdCl2 (3.58 mg, 5 mol%). The reactant mixture was allowed to stand at room temperature overnight. The resulting mixture was dispersed into diethyl ether (1 mL) and washed with water (2 mL). The combined aqueous phases were then back-extracted with diethyl ether (3 × 1 mL); then, the combined organics were washed with brine (2 mL), dried over Na2SO4, and concentrated in vacuo. The crude product was further purified via flash column chromatography (1:19 to 1:1 ethyl acetate/hexanes gradient) to afford 17.5 mg (58%) of compound 9c as a clear oil.
5. Conclusions
Treatment of N,N-dimethylhydrazinoalkenes with diethylzinc followed by exposure of the resulting ethylzinc amide to high vacuum drives a Schlenck redistribution metalloamination/cyclization to generate the corresponding bis(organozinc) intermediates in excellent conversions. The metalloamination event is noteworthy in that it represents one of the very few migratory insertion reactions involving the participation of a zinc(II) intermediate and an alkene. Interception of the bis(organozinc)s containing 5-membered azacycles 4 and 5 with appropriate aryl or vinyl bromides in the presence of catalytic PdCl2(DPEphos) provides the corresponding arylated or alkenylated pyrrolidines 7a–h and 8a–e with high efficiency. In the case of the bis(organozinc) 6, leading to 6-membered azacycles, more reactive nonaflates should be used to mitigate retro-metalloamination/cyclization, thereby furnishing the corresponding piperidines 9a–e in acceptable yields.
Supplementary Materials
Supporting information for this article is available online at https://www.mdpi.com/article/10.3390/inorganics13100328/s1, Figure S1: Thermal ellipsoid plot of the zinc dimer intermediate 5, with thermal ellipsoids drawn at 50% probability. All hydrogen atoms are omitted for clarity. References [36,37] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, T.L. and J.L.; methodology, J.L.; validation, T.L. and J.L.; formal analysis, J.L. and C.F.; investigation, J.L. and C.F.; resources, T.L.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, J.L., T.L. and C.F.; supervision, T.L.; project administration, T.L.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Public Health Service of the U.S. Department of Health and Human Services grant number [R01GM116949]. Support for MSU’s NMR Center and the 400 MHz NMR spectrometer used in this research has been provided by the NSF (NSF-MRI: CHE-2018388) and MSU’s office of the Vice President for Research and Economic Development. Funding for the Proteomics, Metabolomics and Mass Spectrometry Facility used in this publication was made possible in part by the MJ Murdock Charitable Trust, the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P20GM103474 and S10OD28650, and the MSU Office of Research, Economic Development and Graduate Education.
Data Availability Statement
The crystallographic data for the zinc dimer intermediate 5 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2286606 and can be accessed via www.ccdc.cam.ac.uk/structures, (accessed on 29 August 2025).
Acknowledgments
We want to thank Isaac Sanchez for checking selected experimental procedures and acknowledge the assistance provided by Brian Tripet (NMR facility, Montana State University) and Navamoney Arulsamy (X-Ray Inorganic Facility, University of Wyoming).
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships influencing the work reported in this paper.
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Walsh, C.T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075–3081. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among US FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Chemler, S.R. The enantioselective intramolecular aminative functionalization of unactivated alkenes, dienes, allenes and alkynes for the synthesis of chiral nitrogen heterocycles. Org. Biomol. Chem. 2009, 7, 3009–3019. [Google Scholar] [CrossRef]
- Nanda, S.K.; Mallik, R. Transition Metal-Catalyzed Carboamination of Alkenes and Allenes: Recent Progress. Asian J. Org. Chem. 2022, 11, 41–65. [Google Scholar] [CrossRef]
- Redford, J.E.; McDonald, R.I.; Rigsby, M.L.; Wiensch, J.D.; Stahl, S.S. Stereoselective Synthesis of cis-2,5-Disubstituted Pyrrolidines via Wacker-Type Aerobic Oxidative Cyclization of Alkenes with tert-Butanesulfinamide Nucleophiles. Org. Lett. 2012, 14, 1242–1245. [Google Scholar] [CrossRef]
- Nicolai, S.; Waser, J. Pd(0)-Catalyzed Oxy- and Aminoalkynylation of Olefins for the Synthesis of Tetrahydrofurans and Pyrrolidines. Org. Lett. 2011, 13, 6324–6327. [Google Scholar] [CrossRef]
- Sunsdahl, B.; Smith, A.R.; Livinghouse, T. Intramolecular Metalloamination of N,N-Dimethylhydrazinoalkenes: A Versatile Method to Access Functionalized Piperidines and Pyrrolidines. Angew. Chem.-Int. Ed. 2014, 53, 14352–14356. [Google Scholar] [CrossRef]
- Mickelsen, K.; Zabawa, S.; Livinghouse, T. Diethylzinc-Mediated Metalloamination-Alkylation of N,N-Dimethylhydrazinoalkenes. Catalysis of C-Zn Alkylation Using Simple Cu(I) Salts. Synlett 2018, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Frabitore, C.; Lepeule, J.; Towey, B.; Livinghouse, T.; Robinson, W.C. Efficient Reductions of Dimethylhydrazones using Preformed Primary Amine Boranes. Synth. Commun. 2022, 52, 185–189. [Google Scholar] [CrossRef]
- Frabitore, C.; Lepeule, J.; Livinghouse, T. Copper(I)-Catalyzed Cross-Coupling of 1-Bromoalkynes with N-Heterocyclic Organozinc Reagents. Molecules 2022, 27, 4561. [Google Scholar] [CrossRef]
- Frabitore, C.; Livinghouse, T. On the Copper(I)-Catalyzed Cross-Coupling of 1-Bromoalkynes with N-Heterocyclic Organozinc Reagents: Substrate Scope and Catalyst Evaluation. Synth.-Stuttg. 2023, 55, 2370–2376. [Google Scholar] [CrossRef]
- Zhou, J.R.; Fu, G.C. Palladium-catalyzed Negishi cross-coupling reactions of unactivated alkyl iodides, bromides, chlorides, and tosylates. J. Am. Chem. Soc. 2003, 125, 12527–12530. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, K.; Serizawa, H.; Ishii, K.; Mikami, K. Palladium-Catalyzed Negishi Cross-Coupling Reaction of Aryl Halides with (Difluoromethyl)zinc Reagent. Org. Lett. 2016, 18, 3690–3693. [Google Scholar] [CrossRef]
- Han, C.; Buchwald, S.L. Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2009, 131, 7532–7533. [Google Scholar] [CrossRef]
- Yang, Y.; Oldenhuis, N.J.; Buchwald, S.L. Mild and General Conditions for Negishi Cross-Coupling Enabled by the Use of Palladacycle Precatalysts. Angew. Chem.-Int. Ed. 2013, 52, 615–619. [Google Scholar] [CrossRef]
- Gong, H.G.; Gagne, M.R. Diastereoselective Ni-catalyzed Negishi cross-coupling approach to saturated, fully oxygenated C-alkyl and C-aryl glycosides. J. Am. Chem. Soc. 2008, 130, 12177–12183. [Google Scholar] [CrossRef]
- Schley, N.D.; Fu, G.C. Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc. 2014, 136, 16588–16593. [Google Scholar] [CrossRef]
- Cooper, A.K.; Burton, P.M.; Nelson, D.J. Nickel versus Palladium in Cross-Coupling Catalysis: On the Role of Substrate Coordination to Zerovalent Metal Complexes. Synth.-Stuttg. 2020, 52, 565–573. [Google Scholar] [CrossRef]
- Johns, A.M.; Utsunomiya, M.; Incarvito, C.D.; Hartwig, J.F. A highly active palladium catalyst for intermolecular hydroamination. Factors that control reactivity and additions of functionalized anilines to dienes and vinylarenes. J. Am. Chem. Soc. 2006, 128, 1828–1839. [Google Scholar] [CrossRef]
- Birkholz, M.N.; Freixa, Z.; van Leeuwen, P. Bite angle effects of diphosphines in C-C and C-X bond forming cross coupling reactions. Chem. Soc. Rev. 2009, 38, 1099–1118. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 2017, 1, 0025. [Google Scholar] [CrossRef]
- Day, C.S.; Somerville, R.J.; Martin, R. Deciphering the dichotomy exerted by Zn(ii) in the catalytic sp(2) C-O bond functionalization of aryl esters at the molecular level. Nat. Catal. 2021, 4, 124–133. [Google Scholar] [CrossRef]
- Nicolai, S.; Sedigh-Zadeh, R.; Waser, J. Pd(0)-Catalyzed Alkene Oxy- and Aminoalkynylation with Aliphatic Bromoacetylenes. J. Org. Chem. 2013, 78, 3783–3801. [Google Scholar] [CrossRef]
- Maret, W. Zinc and sulfur: A critical biological partnership. Biochemistry 2004, 43, 3301–3309. [Google Scholar] [CrossRef]
- Espino, G.; Kurbangalieva, A.; Brown, J.M. Aryl bromide/triflate selectivities reveal mechanistic divergence in palladium-catalysed couplings; the Suzuki-Miyaura anomaly. Chem. Commun. 2007, 17, 1742–1744. [Google Scholar] [CrossRef]
- Gauvin, R.M.; Buch, F.; Delevoye, L.; Harder, S. Well-Defined Silica-Supported Calcium Reagents: Control of Schlenk Equilibrium by Grafting. Chem.-A Eur. J. 2009, 15, 4382–4393. [Google Scholar] [CrossRef]
- Smith, L.M.; Coward, K.M.; Jones, A.C.; Bickley, J.F.; Steiner, A.; Petroni, S.; Roberts, J.S. Purification of dialkylzinc precursors using tertiary amine ligands. J. Electron. Mater. 2001, 30, 1433–1437. [Google Scholar] [CrossRef]
- Dekker, J.; Boersma, J.; Fernholt, L.; Haaland, A.; Spek, A.L. Molecular Structure of Bis(3-(Dimethylamino)propyl)zinc, Zn (CH2)3N(CH3)2)2, by X-ray and Gas Electron-Diffraction and Bis(3-Mercapto)zinc, Zn (CH2)3SCH3)2, by Gas Electron-Diffraction. Organometallics 1987, 6, 1202–1206. [Google Scholar] [CrossRef]
- Eckert, P.; Organ, M.G. The Role of LiBr and ZnBr2 on the Cross-Coupling of Aryl Bromides with Bu2Zn or BuZnBr. Chem.-A Eur. J. 2019, 25, 15751–15754. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.J.; Shannon, J.; Stephens, J.C.; Woodward, S. Demonstration of promoted zinc schlenk equilibria, their equilibrium values and derived reactivity. Chem.-A Eur. J. 2007, 13, 2462–2472. [Google Scholar] [CrossRef]
- Dessy, R.E.; Coe, G.R. Structure of Organozinc Reagents. J. Org. Chem. 1963, 28, 3592–3593. [Google Scholar] [CrossRef]
- Abraham, M.H.; Rolfe, P.H. Organometallic Compounds 4. Constitution of Ethylzinc Halides. J. Organomet. Chem. 1967, 7, 35–43. [Google Scholar] [CrossRef]
- Yalkowsky, S.H. Carnelley’s Rule and the Prediction of Melting Point. J. Pharm. Sci. 2014, 103, 2629–2634. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. CStructural Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- APEX4, Software Suite V2022. 1-1; Bruker AXS Inc.: Madison, WI, USA, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).