1. Introduction
The redox reaction of copper (Cu) complexes plays a key role in many biological systems [
1,
2,
3,
4]. Notably, blue Cu proteins such as plastocyanin and azurin function as biological electron carriers by virtue of the redox reactions between Cu
I and Cu
II at their active sites [
1,
2]. To achieve a rapid and reversible redox process between Cu
I and Cu
II, the coordination geometry must remain nearly unchanged throughout the redox event. However, the preferred coordination geometry of Cu
I and Cu
II complexes differs considerably owing to their distinct electronic configuration. Specifically, Cu
I adopts a tetrahedral coordination geometry due to its closed-shell d
10 configuration, whereas the d
9 configuration and characteristic Jahn–Teller distortion of Cu
II result in square-planar or distorted square-pyramidal complexes. Therefore, an appropriate ligand design is essential in order to control the redox properties and oxidation states of Cu complexes.
Cu complexes exhibit a rich redox chemistry, which can sometimes complicate the control of their oxidation states. For instance, several studies have reported the formation of Cu
I complexes from Cu
II precursors even in the absence of reducing agents [
5,
6,
7,
8,
9,
10]. Such a reduction, which is commonly termed spontaneous or complexation-induced reduction [
6,
7,
8,
9,
10], can be triggered by an intramolecular electron transfer from the ligand [
8,
10] or by the participation of solvents such as alcohols [
5]. The spontaneous or complexation-induced reduction of Cu
II complexes often occurs unintentionally, and limited efforts have so far been devoted to deliberately controlling this process [
5,
6,
7,
8,
9,
10]. Nevertheless, understanding and controlling the geometric preferences of both Cu
I and Cu
II may enable the deliberate induction of complexation-induced reduction.
Pyridine-based nitrogen ligands are widely used in Cu complexes due to their strong coordination ability with both Cu
I and Cu
II oxidation states [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. We envisioned that incorporating a biphenyl moiety into the pyridine-based ligand framework of Cu
II complexes could promote their complexation-induced reduction. The biphenyl unit consists of two aromatic rings with a dihedral angle of ~42° between them, which introduces a remarkable torsional distortion [
6]. Such a torsional distortion could facilitate the complexation-induced reduction of Cu
II by favoring the formation of the tetrahedral geometry, which is energetically preferred in Cu
I complexes. To test our hypothesis, we designed biphenyl-bridged pyridine-containing N
4 Schiff-base ligand
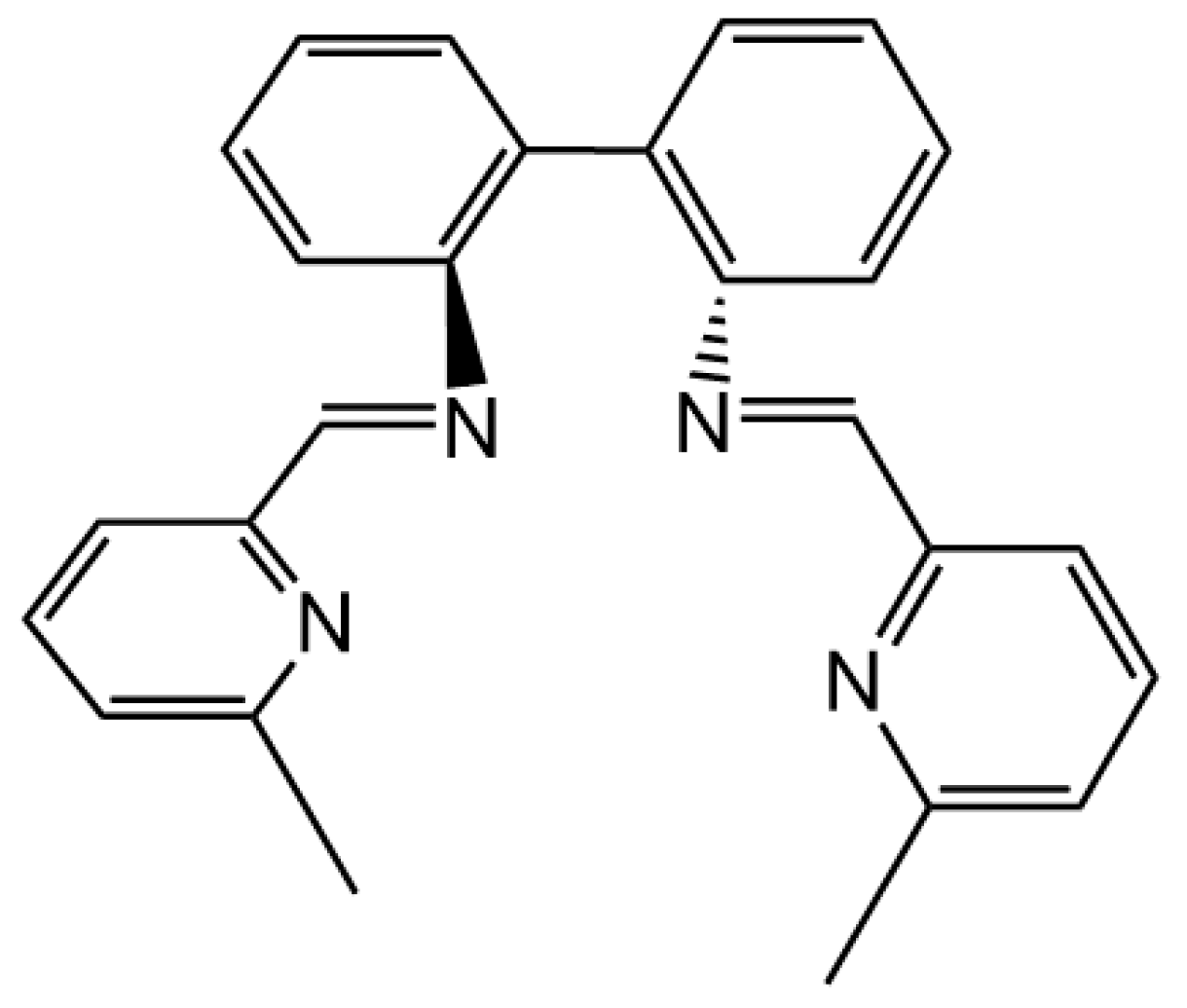
L (
Figure 1).
Herein, we report the design of ligand L and its reaction with a CuII precursor to yield a CuI complex via complexation-induced reduction. In addition, we discuss the effect of the biphenyl unit on the complexation-induced reduction of CuII on the basis of a comparison with other Cu complexes bearing pyridine-containing N4 Schiff-base ligands.
2. Results and Discussion
Tetradentate ligand
L was synthesized via the condensation reaction between 6-methyl-2-pyridinecarboxaldehyde and 2,2′-diaminobiphenyl in ethanol. Interestingly, the reaction of
L with Cu
II(CF
3SO
3)
2 immediately furnished a black-green suspension and induced the spontaneous reduction of the Cu
II center to Cu
I. Recrystallization of the Cu
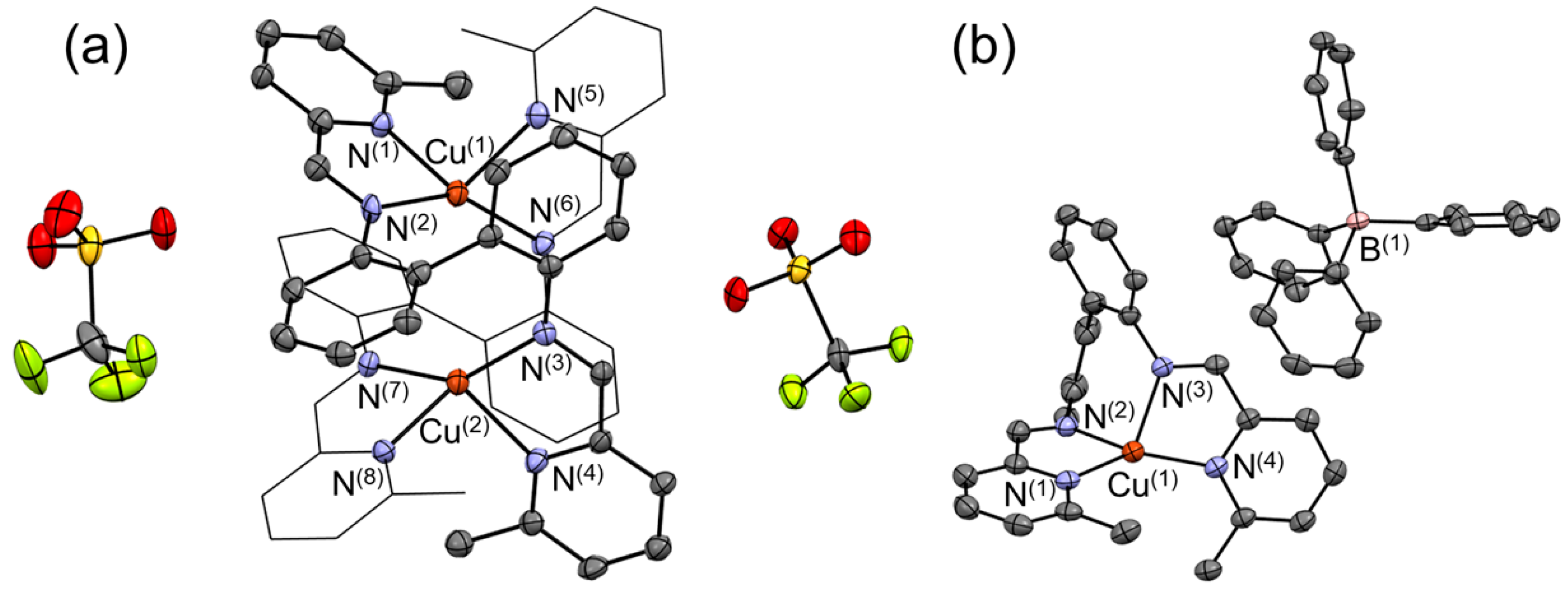
I complex using DMF and diethyl ether afforded deep-green crystals, which were subjected to a single-crystal X-ray diffraction (XRD) analysis. The resulting molecular structure is shown in
Figure 2a. The presence of two Cu centers and two CF
3SO
3‒ counter anions in the asymmetric unit indicates that the oxidation state of Cu is +I. The resulting molecular structure shows that the molecular formula of the obtained Cu
I complex is [Cu
I2(
L)
2](CF
3SO
3)
2. The bond distances between Cu and the pyridine N (N
py) are 2.097–2.114 Å, i.e., slightly shorter than those between Cu and the imine N (N
im) (2.055–2.074 Å). The N
im‒Cu‒N
py bond angles (80.9°–81.7°) are considerably smaller than the N
py‒Cu‒N
py (97.2°–97.7°) and N
im‒Cu‒N
im (144.2°–144.6°) bond angles. The dihedral angles between the N(1)–Cu(1)–N(2) and N(5)–Cu(1)–N(6) planes as well as between the N(3)–Cu(2)–N(4) and N(7)–Cu(2)–N(8) planes are 78.81° and 77.66°, respectively. Considering that the ideal square-planar and tetrahedral geometries are characterized by dihedral angles of 0° and 90°, respectively, the obtained values suggest that the coordination geometries of the Cu(1) and Cu(2) centers are slightly distorted from the ideal tetrahedral coordination geometry.
The reaction of
L with Cu
II(ClO
4)
2∙6H
2O also immediately furnished a dark-green suspension. The resulting solid was dissolved in acetonitrile, subjected to counter-anion exchange via the reaction with 10 equivalents of NaBPh
4, and recrystallized into dark brown crystals. The corresponding single-crystal XRD analysis provided the molecular structure shown in
Figure 2b. In contrast to [Cu
I2(
L)
2](CF
3SO
3)
2, the asymmetric unit contains only one Cu center and one BPh
4‒ anion, revealing that the molecular formula of the obtained Cu complex is [Cu(
L)]BPh
4. Considering the charge balance of [Cu(
L)]BPh
4, the oxidation state of Cu can also be assigned as +I. Therefore, the reaction between
L and a Cu
II source produced Cu
I complexes regardless of the counter anion. However, mononuclear or dinuclear Cu
I complexes were obtained depending on the counter anion.
The Cu‒N
py bond lengths in [Cu
I(
L)]BPh
4 are 1.985 and 2.027 Å, which are by 0.06–0.13 Å shorter than those of [Cu
I2(
L)
2](CF
3SO
3)
2 (2.097–2.114 Å). In contrast, the length of the Cu‒N
im bonds in [Cu
I(
L)]BPh
4 (2.053 and 2.100 Å) are comparable to those in [Cu
I2(
L)
2](CF
3SO
3)
2 (2.055–2.074 Å). The N
py(1)‒Cu‒N
py(4) bond angle (135.27°) is significantly larger than the N
py‒Cu‒N
im (81.26° and 81.38°) and N
im‒Cu‒N
im (88.93°) bond angles. The dihedral angle between the N(1)–Cu(1)–N(2) and N(3)–Cu(1)–N(4) planes (63.30°) is slightly smaller than the corresponding angles in [Cu
I2(
L)
2](CF
3SO
3)
2. These results indicate that [Cu
I(
L)]BPh
4 possesses a distorted tetrahedral coordination geometry. To discuss the differences in the structural geometries of [Cu
I(
L)]BPh
4 and [Cu
I2(
L)
2](CF
3SO
3)
2, we calculated their
τ4 values, as defined by Houser and co-workers [
21]. The
τ4 parameter is used to evaluate the geometry of four-coordinate transition metal complexes. It is defined as:
τ4 = [{360° – (
α +
β)}/141°], where
α and
β are the largest and second-largest bond angles around the metal center [
21]. The
τ4 values range from 1.00, corresponding to an ideal tetrahedral geometry, to 0, corresponding to a perfect square planar geometry [
21]. The estimated
τ4 values for the two Cu centers in [Cu
I2(
L)
2](CF
3SO
3)
2 are 0.83 and 0.84. In contrast,
τ4 of [Cu
I(
L)]BPh
4 is 0.96, indicating that [Cu
I(
L)]BPh
4 adopts a geometry closer to the ideal tetrahedral structure than [Cu
I2(
L)
2](CF
3SO
3)
2.
The [Cu
I2(
L)
2](CF
3SO
3)
2 and [Cu
I(
L)]BPh
4 complexes are EPR silent (
Figure S1), indicating that they are diamagnetic species with a Cu oxidation state of +I. The
1H NMR spectra of [Cu
I2(
L)
2](CF
3SO
3)
2 and [Cu
I(
L)]BPh
4 in acetonitrile-
d3 are quite similar to each other (
Figures S2 and S3). All
1H NMR signals originating from the pyridine moieties in [Cu
I(
L)]BPh
4 are very slightly high-field shifted (~0.01 ppm) compared to the corresponding signals of [Cu
I2(
L)
2](CF
3SO
3)
2 (
Figure S3). Overall, the EPR and
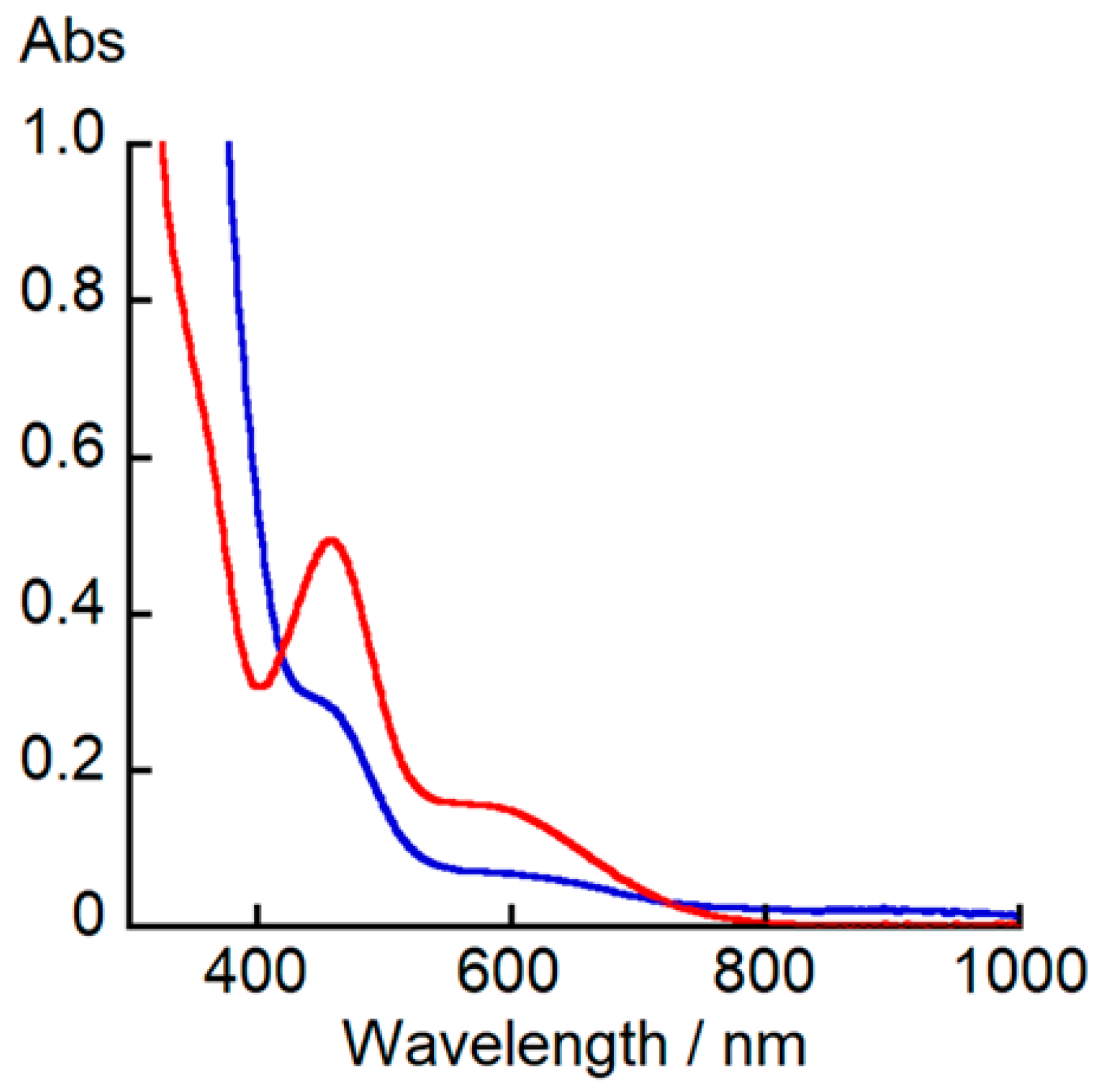
1H NMR spectroscopy results show no clear difference between mononuclear and dinuclear complexes. In contrast, the UV–vis–NIR spectrum of [Cu
I2(
L)
2](CF
3SO
3)
2 in acetonitrile clearly differs from that of [Cu
I(
L)]BPh
4. As shown in
Figure 3, the UV–vis–NIR spectrum of [Cu
I2(
L)
2](CF
3SO
3)
2 shows a shoulder band at 450 nm (
ε = 3000 M
−1∙cm
−1) and a low-intensity band at 590 nm (
ε = 700 M
−1∙cm
−1), while the corresponding spectrum of [Cu
I(
L)]BPh
4 exhibits an intense band at 450 nm (
ε = 5000 M
−1∙cm
−1) and a lower intensity band at 585 nm (
ε = 1500 M
−1∙cm
−1).
Considering that various Cu
II complexes have been synthesized by mixing a Cu
II source and organic Schiff-base ligands [
4,
22,
23,
24,
25,
26], the formation of the Cu
I complexes [Cu
I2(
L)
2](CF
3SO
3)
2 and [Cu
I(
L)]BPh
4 via the reaction between a Cu
II source and
L in the absence of additional reductants is unusual. Although the complexation-induced reduction of Cu
II has been previously reported, the reductant has not been unequivocally characterized in most cases [
6,
7,
9].
To clarify why the Cu
I complex is formed by simply mixing Cu
II ions and
L, we investigated the redox behavior of [Cu(
L)]BPh
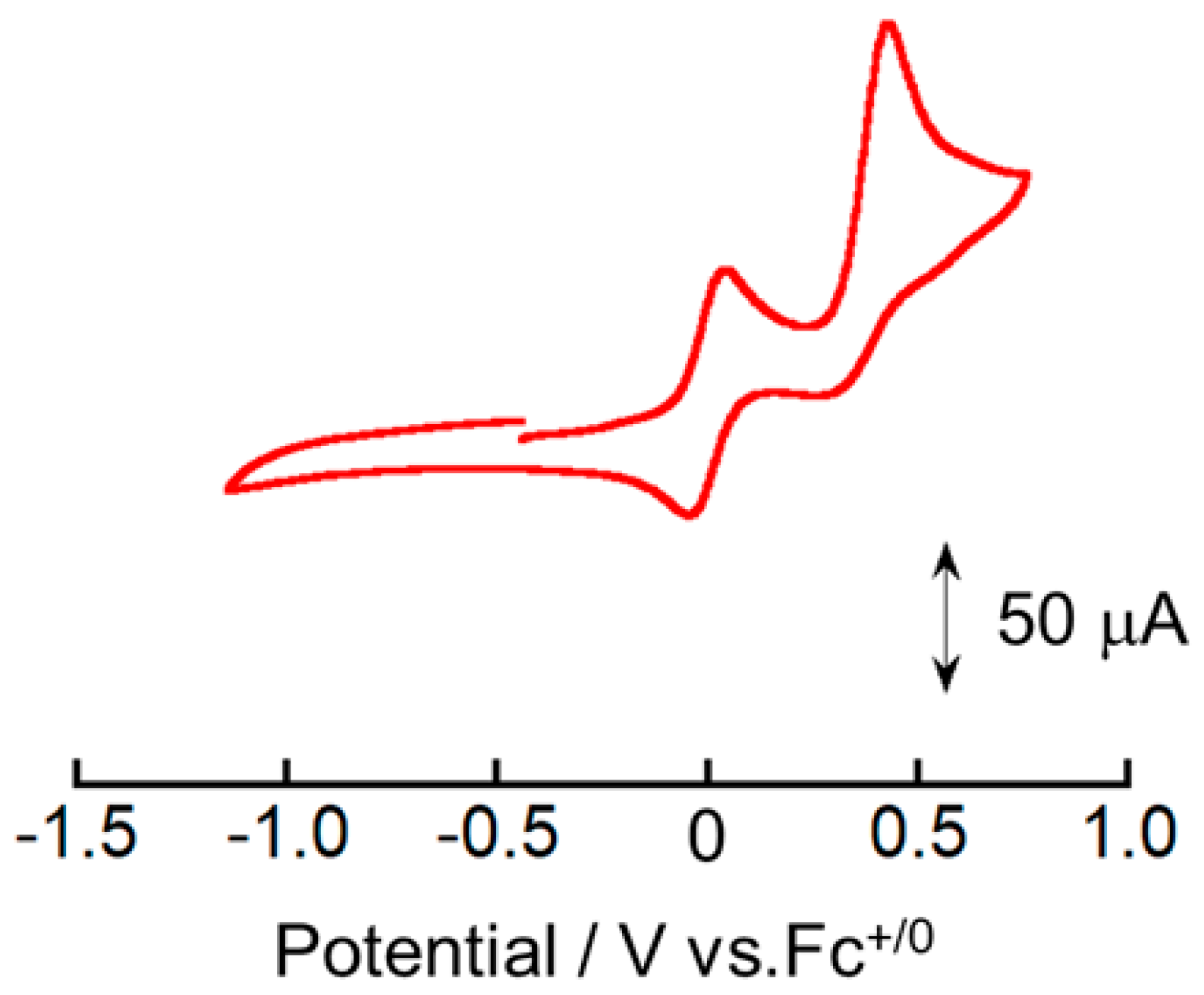
4. The cyclic voltammogram of [Cu
I(
L)]BPh
4 shows one reversible redox couple and one irreversible oxidation peak (
Figure 4). The irreversible oxidation peak at 0.42 V vs. Fc
+/0 is also observed in the cyclic voltammogram of NaBPh
4 in acetonitrile, but not in that of [Cu
I2(
L)
2](CF
3SO
3)
2 (
Figure S4). Therefore, it can be attributed to the oxidation of BPh
4‒ [
27]. The
E°’ value of the reversible redox event was estimated to be 0.05 V vs. Fc
+/0. To determine the electron stoichiometry (
n) of the reversible redox processes observed for [Cu
I(
L)]BPh
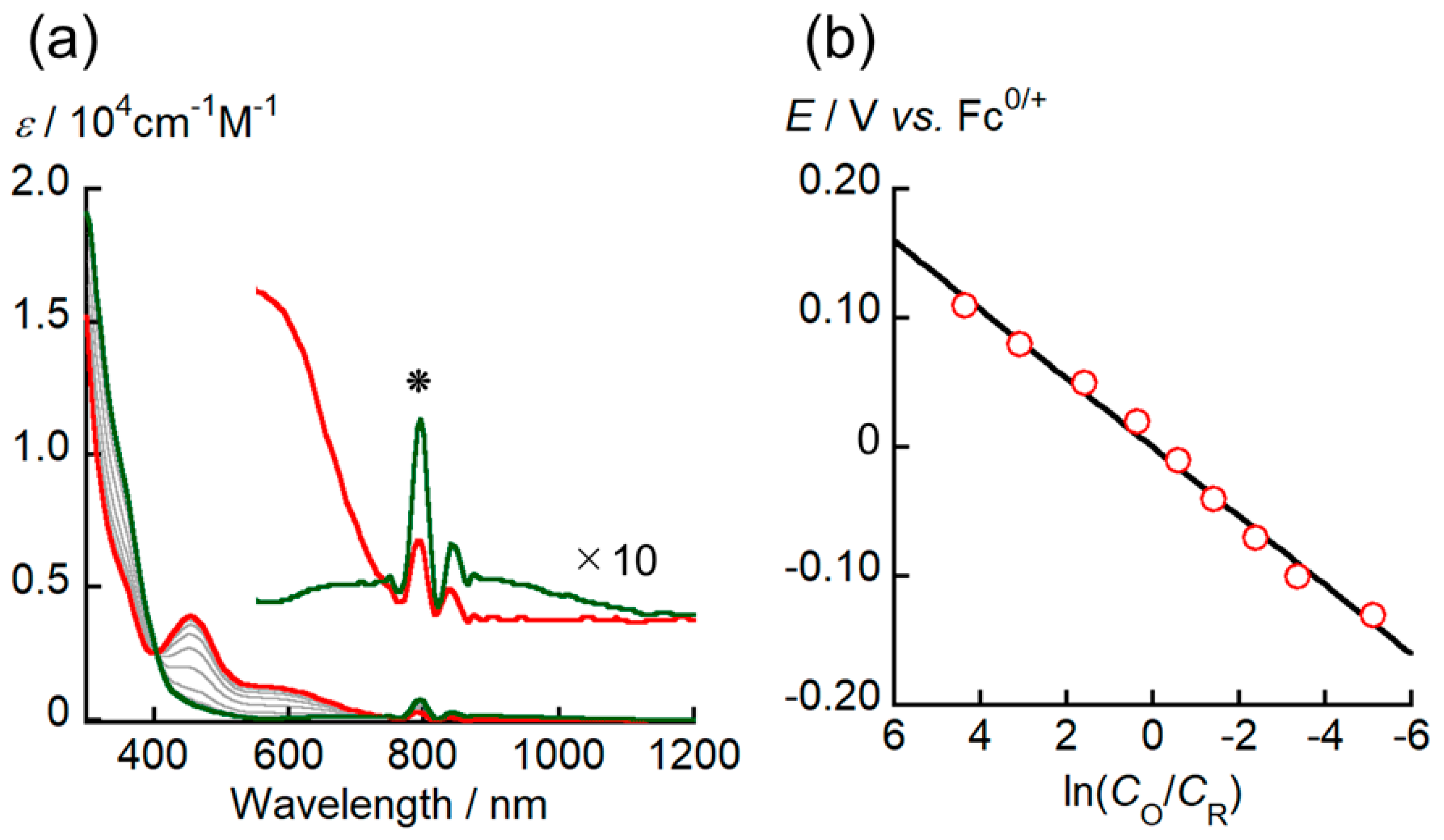
4, spectroelectrochemical measurements were conducted by recording the UV–vis–NIR spectra of [Cu
I(
L)]BPh
4 in acetonitrile at varying applied potentials between −0.13 and 0.11 V vs. Fc
+/0 (
Figure 5a).
As a general trend, the intensity of the absorption bands at ~453 and 530–720 nm decreases with decreasing applied potentials, while the intensity of the shoulder peak around 350 nm has increased, and new absorption band appear at ~800 nm. The presence of isosbestic points was clearly observed at ~402 and ~730 nm, indicating that a redox equilibrium of [Cu
I(
L)]BPh
4 solely occurred in this potential range. The spectral change of [Cu
I(
L)]BPh
4 converged at 0.11 V vs. Fc
+/0, implying that the oxidation of [Cu
I(
L)]BPh
4 was complete at this potential. The ratio between the concentrations of the oxidant (
CO) and the [Cu
I(
L)]BPh
4 reductant ([Cu
I(
L)]BPh
4,
CR) at each potential was calculated using the absorbance change.
Figure 5b shows the relationship between ln(
CO/
CR) and the applied potential (
E vs. Fc
+/0), which should theoretically follow the Nernst equation (Equation (1)):
where
R,
T, and
F are the gas constant (8.314 J·mol
−1·K
−1), the absolute temperature (295 K), and the Faraday constant (96,485 C·mol
−1), respectively. The
n and
E°’ values for the redox reaction of [Cu
I(
L)]BPh
4 were determined to be 0.98(4) and 0.00(1) V vs. Fc
+/0, respectively, by least-squares fitting eq 1 to the plot of
E as a function of ln(
CO/
CR) (
Figure 5b). This result suggests that the redox reactions of [Cu
I(
L)]BPh
4 are single-electron processes. In addition, the
E°’ value is consistent within an acceptable margin of error with the redox potential determined via CV.
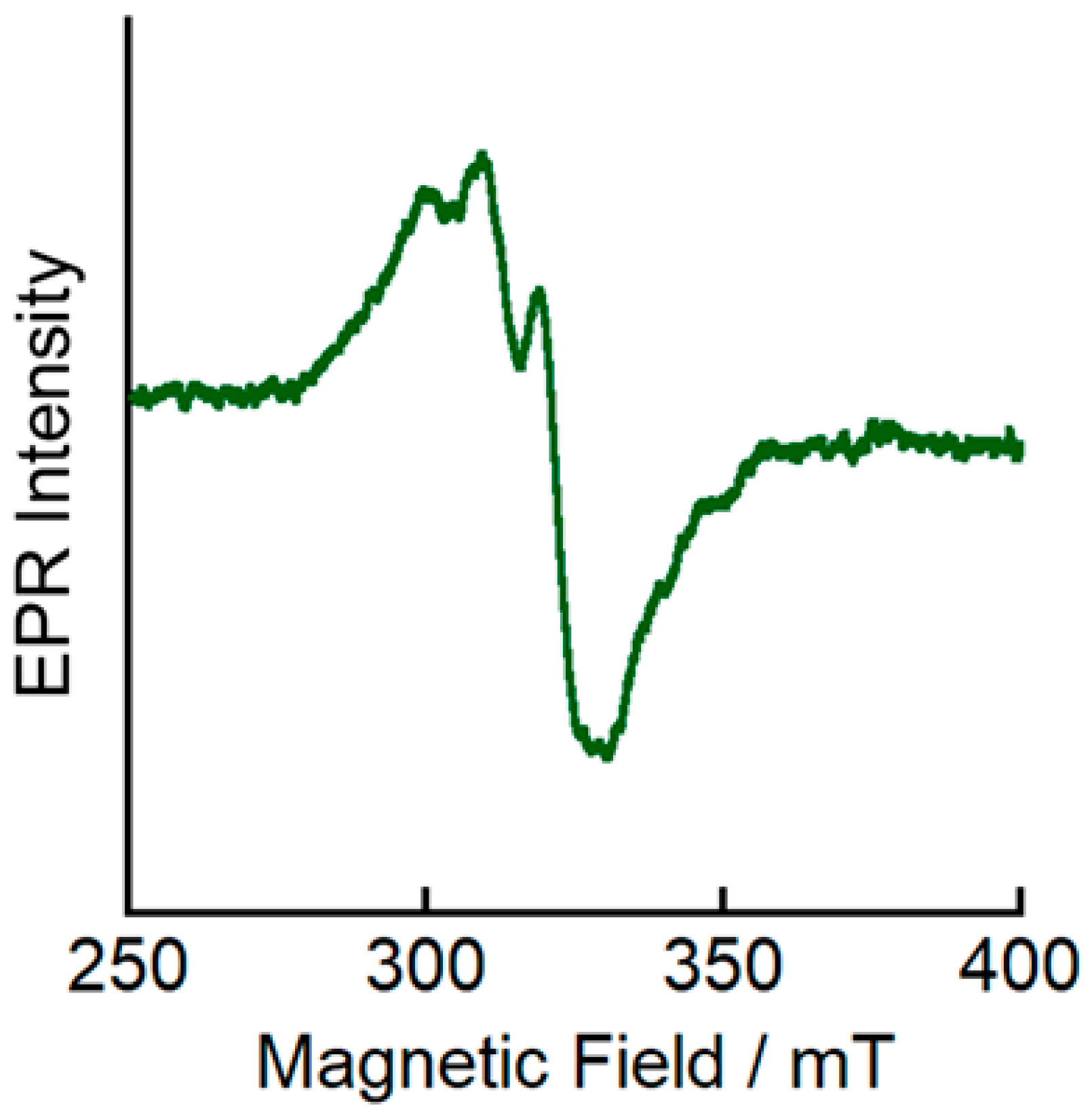
To elucidate the electronic structure of the electrochemically generated one-electron oxidized complex [Cu(
L)]
2+, we measured its EPR spectrum at 77 K. Axial signals typical for mononuclear Cu
II systems (
g1 = 2.25 and
g2 = 2.10) were observed (
Figure 6), suggesting that the one-electron oxidation of [Cu
I(
L)]
+ could provide a Cu
II complex. However, the EPR spectral features of [Cu(
L)]
2+ are more similar to those observed for distorted square-planar Cu
II complexes [
24] than to those of tetrahedral Cu
II complexes [
28,
29]. These results suggest that the oxidation from [Cu
I(
L)]
+ to [Cu(
L)]
2+ is accompanied by a structural change toward a more planar coordination geometry.
Next, to test the validity of our hypothesis, we performed density-functional-theory (DFT) and time-dependent DFT (TD-DFT) calculations on [Cu
I(
L)]
+ and [Cu(
L)]
2+. The corresponding DFT-optimized structures are shown in
Figure S5, and selected structural parameters are summarized in
Table S1.
The bond lengths between the Cu center and the imino N in [Cu(
L)]
2+, i.e., Cu(1)–N(2) and Cu(1)–N(3) (1.994 Å), are ~0.13 Å shorter than those of [Cu
I(
L)]
+ (Cu(1)–N(2) = 2.116 Å; Cu(1)–N(3) = 2.122 Å). This suggests that the increase in the positive charge of Cu through the oxidation from [Cu
I(
L)]
+ to [Cu(
L)]
2+ strengthens these coordination bonds. To discuss the structural change upon oxidation from [Cu
I(
L)]
+ to [Cu(
L)]
2+, we focused on the dihedral angles between the N(1)–Cu(1)–N(2) and N(3)–Cu(1)–N(4) planes in both complexes. The dihedral angle of DFT-optimized [Cu
I(
L)]
+ (64.36°) (
Figure S6) is in good agreement with the XRD value (63.30°). In contrast, the dihedral angle of DFT-optimized [Cu(
L)]
2+ exhibits a considerably smaller value (56.04°). These results indicate that oxidation is accompanied by a structural change toward a more planar coordination geometry. The spin-density plot of [Cu(
L)]
2+ clearly shows that the unpaired electron is mainly distributed on the Cu center and the coordinated N atoms (
Figure 7). In addition, the total spin-density value of Cu and N (0.98) indicates that Cu has a 3d
9 electron configuration, i.e., the most common state of Cu
II complexes.
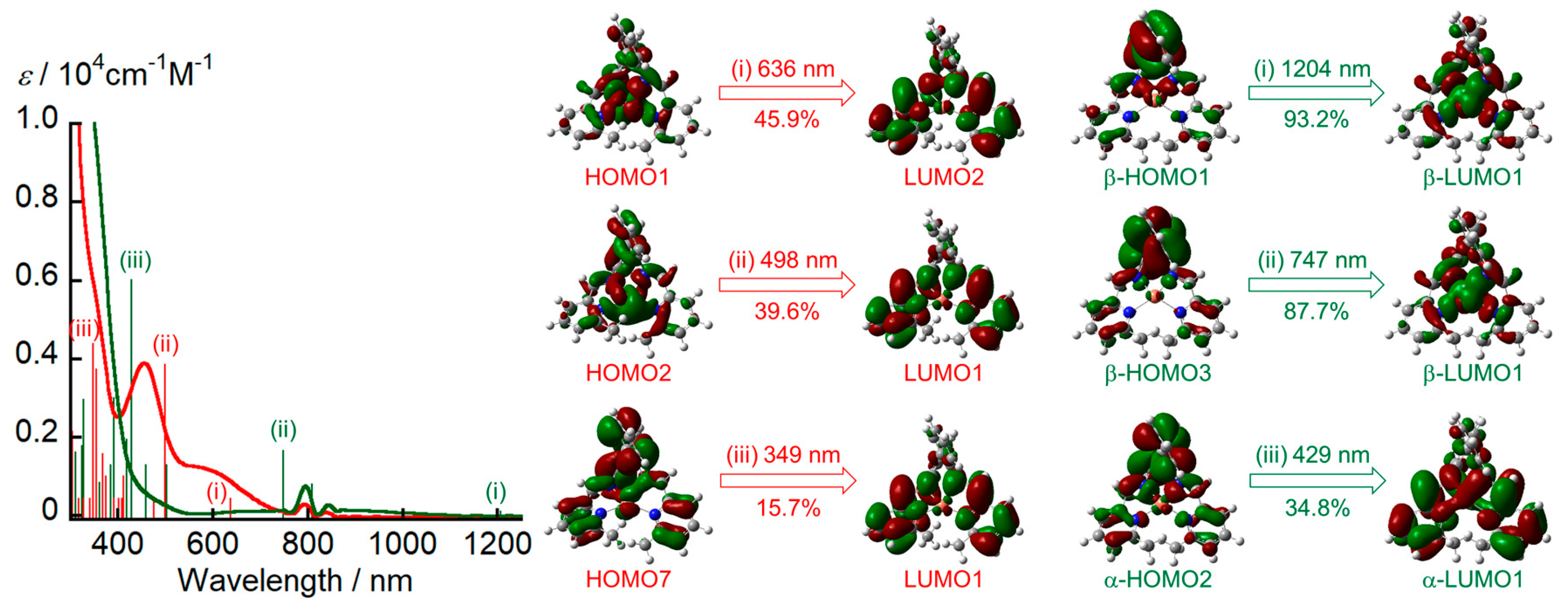
Using the DFT-optimized structure, we estimated the electronic absorption spectra of [Cu
I(
L)]
+ and [Cu(
L)]
2+ by TD-DFT calculations (
Figure 8). The TD-DFT calculations reproduced the experimental spectral features well but underestimated the transition energies. Several intense bands of [Cu
I(
L)]
+ between 415 and 770 nm are attributable to the charge transfer from Cu to the ligand (MLCT). These MLCT bands significantly decreased upon the one-electron oxidation of [Cu
I(
L)]
+, while a new absorption band attributed to the charge transfer from
L to Cu (LMCT) appeared at ~830 cm
−1. Consequently, [Cu(
L)]
2+ can be categorized as a Cu
II complex ([Cu
II(
L)]
2+).
Isolating the oxidized complex [CuII(L)]2+ would provide valuable information to clarify the complexation-induced reduction of CuII to CuI. However, the electrochemically generated [CuII(L)]2+ gradually decomposed once the electrolysis was stopped. Unfortunately, attempts to synthesize and isolate [CuII(L)]2+ via chemical oxidation of [CuI(L)]+ proved unsuccessful.
Regardless, we were also interested in identifying the reducing agent responsible for the reduction of Cu
II. In some of the previous studies on the complexation-induced reduction of Cu
II to Cu
I [
5,
6,
7,
8,
9,
10], ethanol, which was used as the reaction solvent, was identified as the reducing agent [
5,
8,
10]. Initially, we hypothesized that this could also be the case in the present study. However, [Cu
I(
L)]BPh
4 was successfully synthesized in the absence of ethanol (for details, see the
Supporting Information) both under ambient conditions and a N
2 atmosphere. These results suggest that neither the solvent nor the atmosphere gas is involved in the reduction of Cu
II.
The [Cu
I(
L)]BPh
4 complex was synthesized in a yield of approximately 45%, which was consistently reproduced across multiple experiments. Given that the yield remained relatively low despite the reproducibility, we hypothesized that ligand
L or its precursors (6-methyl-2-pyridinecarboxaldehyde and 2,2′-diaminobiphenyl) might be involved in the complexation-induced reduction of Cu
II during complex formation. To examine this hypothesis, the complex was synthesized using a slight excess of either 6-methyl-2-pyridinecarboxaldehyde or 2,2′-diaminobiphenyl relative to the stoichiometric ratio. However, the yield of the complex remained in the range of 45–48% (for details, see the
Supporting Information) without significant improvement. In contrast, when two equivalents of
L were reacted with the Cu
II source, the yield of [Cu
I(
L)]BPh
4 increased to 70% (for details, see the
Supporting Information). This result suggests that uncoordinated
L may participate in the reduction of Cu
II to Cu
I. To gain a better understanding of this reduction process, we made extensive efforts to identify the byproducts. However, the formation of multiple decomposition products hindered their identification.
Previously, Fabbrizzi and co-workers have synthesized various Cu
II complexes with pyridine-containing N
4 Schiff-base ligands (
Figure S7) by mixing a Cu
II source with the corresponding ligands [
22]. Specifically, they employed Schiff-base ligands derived from two 6-methyl-2-carboxypyridine moieties bridged by ethylenediamine or 1,2-cyclohexanediamine [
22]. These ligand frameworks possess relatively high planarity. In fact, the dihedral angles between the N(1)–Cu(1)–N(2) and N(3)–Cu(1)–N(4) planes in the Cu
II complex (~22°) [
22] is significantly smaller than that of [Cu
II(
L)]
2+ (56.04°). At present, we hypothesize that such a difference in dihedral angle may play a key role in the reduction of Cu
II to Cu
I. The torsion of
L induced by the biphenyl moiety may favor the formation of the tetrahedral geometry preferred by Cu
I complexes over the coordination to Cu
II. Therefore, approaching a four-coordinated tetrahedral coordination geometry around the Cu center would facilitate the reduction of Cu
II to Cu
I during complexation. According to Shimazaki and co-workers, modification of the coordination geometry in Cu–phenolate complexes from a trigonal-bipyramidal to a tetrahedral four-coordinate structure induces a change in the electronic structure from a Cu
II–(phenolate) species to a Cu
I–(phenoxyl radical) species [
4,
30]. This distortion of the coordination geometry around the Cu center facilitates the intramolecular electron transfer from the coordinating phenolate to the Cu
II ion, resulting in the formation of the Cu
I–(phenoxyl radical) species. The fate of the oxidation state of the metal center in a Cu complex is sometimes governed by its coordination structure.
3. Materials and Methods
Materials.
All chemicals used in this work were of reagent grade and used as received, unless otherwise specified.
Caution! Although there were no incidents in our laboratory, transition-metal perchlorates may explode violently. They should be prepared only in small quantities and handled with the utmost care.
Synthesis of CuI complexes.
[Cu2(L)2](CF3SO3)2. A solution of 6-methyl-2-pyridinecarboxaldehyde (52.6 mg, 0.434 mmol, TCI) and 2,2′-diaminobiphenyl (40.0 mg, 0.217 mmol, TCI) in ethanol (0.5 mL) was heated at 60 °C for 1 h, then slowly treated with CuII(CF3SO3)2 (80.0 mg, 0.221 mmol, TCI) in ethanol (0.5 mL). The resulting mixture was stirred for 1 h. The green precipitate formed was collected by filtration, dissolved in a mixture of DMF (200 µL) and ethanol (800 µL), and recrystallized into deep-green crystals via vapor diffusion with diethyl ether. Yield: 35 mg (13%). 1H NMR (399.78 MHz, acetonitrile-d3, δ /ppm vs. TMS): 2.61 (s, 12H, –CH3), 7.17 (d, 4H, aryl, JH–H = 7.6 Hz), 7.32–7.46 (m, 12H, aryl) 7.72 (dd, 8H, py, JH–H = 18.6 Hz, JH–H = 7.6 Hz), 8.02 (t, 4H, py, JH–H = 8.0 Hz), 8.56 (s, 4H, –CH=N–).
[Cu(L)]BPh4. A solution of 6-methyl-2-pyridinecarboxaldehyde (26.0 mg, 0.215 mmol, TCI) and 2,2′-diaminobiphenyl (20.0 mg, 0.109 mmol, TCI) in ethanol (1 mL) was heated at 60 °C for 1 h, then slowly treated with CuII(ClO4)2∙6H2O (40.6 mg, 0.110 mmol, TCI) in ethanol (1 mL). The mixture was stirred for 1 h. The resulting precipitate was collected by filtration and dissolved in acetonitrile. NaBPh4 (0.35 g, 1.02 mmol, TCI) was added to this solution, and the mixture was stirred for several minutes. The mixture was then concentrated under reduced pressure, and ethanol (1 mL) was added to the residue to yield a dark brown solid, which was collected by filtration, dissolved in a mixture of DMF (200 µL) and ethanol (800 µL), and recrystallized into dark brown crystals via vapor diffusion with diethyl ether. Yield: 31 mg (37%). 1H NMR (399.78 MHz, acetonitrile-d3, δ /ppm vs. TMS): 2.60 (s, 6H, –CH3), 6.84 (t, 4H, –B–Ph4, JH–H = 7.2 Hz), 6.99 (t, 8H, –B–Ph4, JH–H = 7.2 Hz), 7.17 (d, 2H, aryl, JH–H = 8.0 Hz), 7.27 (m, 8H, –B–Ph4), 7.31–7.46 (m, 6H, aryl), 7.71 (dd, 4H, py, JH–H = 18.0 Hz, JH–H = 8.0 Hz), 8.02 (t, 2H, py, JH–H = 7.6 Hz), 8.55 (s, 2H, –CH=N–). Elemental analysis (%) Calcd. for [Cu(L)]BPh4 (C50H42BN4Cu): C, 77.66; H, 5.47; N, 7.25. Found: C, 76.94; H, 5.43; N, 7.21.
Physical measurements.
The 1H NMR spectra were recorded on a JEOL ECX-400 NMR spectrometer (JEOL Ltd., Tokyo, Japan) (1H: 399.78 MHz). Chemical shifts are referenced to TMS (δ = 0 ppm). Electron paramagnetic resonance (EPR) spectra were obtained using a JEOL JES-X320 X-band spectrometer (JEOL Ltd., Tokyo, Japan) equipped with a standard low-temperature apparatus. The spectra for the solid samples were recorded at 77 K by using quartz tubes with a 5-mm inner diameter. Microwave frequency was standardized against a Mn(II) marker.CV measurements were performed on the CuI complexes (1.00 mM) dissolved in acetonitrile containing 0.1 M tetra-n-butylammonium hexafluorophosphate perchlorate (TBAPF6) at 295 K under a dry Ar atmosphere using an Autolab NOVA 2 electrochemical analyzer (Metrohm Japan Ltd., Tokyo, Japan). A three-electrode system consisting of a GC disk working electrode, a Pt wire counter electrode, and a Ag0/+ reference electrode (0.1 M TBAP + 1 mM AgNO3/CH3CN) was employed. The ferrocene/ferrocenium ion redox couple (Fc0/+) was used as the external standard redox system. All samples were prepared under an inert Ar atmosphere. Dissolved O2 in the sample solutions was removed by purging with Ar gas for at least 10 min prior to starting the experiments.
UV–vis–NIR spectroelectrochemical measurements of Cu
I complexes in acetonitrile were performed with a HITACHI UH4150 spectrophotometer (Hitachi High-Tech Corporation, Tokyo, Japan) equipped with an optically transparent thin layer electrode (OTTLE) cell at 295 K [
31,
32]. The optical path length was calibrated spectrophotometrically (1.0 × 10
−2 cm) [
31,
32]. The three-electrode system was the same as that in the aforementioned electrochemical experiments except that the working electrode was a Pt gauze (80 mesh). The potential applied on OTTLE was controlled using Autolab NOVA 2(Metrohm Japan Ltd., Tokyo, Japan). The absorption spectrum at each potential step was recorded after equilibration of the electrochemical reaction at the applied potential on the working electrode, which was completed within 3 min. The sample solution in the OTTLE cell was prepared in a similar manner to that for the electrochemical measurements. EPR spectroelectrochemical measurements of Cu
I complex in acetonitrile were performed by using an EPR tube equipped with a gold electrode and a helix coil electrode ( KSL-HE01, Kyoto Spin Lab Co., Ltd., Kyoto, Japan). The applied potential was set to 0.2 V vs. Fc
+/0 using Autolab NOVA 2.
Crystallographic analysis..
XRD data for [Cu
2(
L)
2](CF
3SO
3)
2 and [Cu(
L)]BPh
4 were collected on a Rigaku XtaLab Synergy-R-DW-TT diffractometer (Rigaku Holdings Corporation, Tokyo, Japan) equipped with a hybrid pixel array detector and graphite-monochromated Cu
Kα (
λ = 1.54184 Å) radiation at 100 K. The sample was mounted on a MiTeGen Dual Thickness MicroMount (MiTeGen, LLC, Ithaca, NY, USA) and placed in a temperature-controlled N
2 gas flow. Intensity data were collected by taking oscillation photographs. Reflection data were corrected for both Lorentz and polarization effects. The structures were solved using direct methods and refined anisotropically using the SHELX (2019/3) program suite [
33] for non-hydrogen atoms via full-matrix least-squares calculations. Each refinement was continued until all shifts were smaller than one-third of the standard deviation of the parameters involved. Hydrogen atoms were placed at calculated positions. All calculations were performed using the crystallographic software package
Olex2-1.5
[
34].
Crystallographic data for [Cu2(L)2](CF3SO3)2: Fw = 1206.17, 0.08 × 0.05 × 0.03 mm3, monoclinic, Pc, a = 11.8459(3) Å, b = 19.8379(4) Å, c = 11.8919(3) Å, β = 117.494(3)°, V = 2478.95(12) Å3, Z = 2, T = 100 K, Dcalcd = 1.616 g cm−3, μ(Cu Kα) = 2.578 mm−1, GOF = 1.032, R1(I > 2σ) = 0.0452, wR2(all) = 0.1157.
Crystallographic data for [Cu(L)]BPh4: Fw = 773.22, 0.11 × 0.05 × 0.02 mm3, triclinic, P‒1, a = 11.4008(3) Å, b = 11.5395(4) Å, c = 15.8744(6) Å, α = 92.725(3)°, β = 108.344(3)°, γ = 99.419(3)°, V = 1944.61(12) Å3, Z = 2, T = 100 K, Dcalcd = 1.321 g cm−3, μ(Cu Kα) = 1.103 mm−1, GOF = 1.022, R1(I > 2σ) = 0.0508, wR2(all) = 0.1424.
Theoretical calculations.
DFT calculations on the molecular and electronic structures of [Cu
I(
L)]
+ and [Cu(
L)]
2+ were performed using the Gaussian 16 program suite (Revision C.02) [
35]. Atomic coordinates for geometry optimization of [Cu
I(
L)]
+ were taken from those determined by the single-crystal XRD analysis. Geometry optimization of [Cu(
L)]
2+ was performed using the XRD determined structure of [Cu
I(
L)]
+ as the initial geometry, while the net electric charge of +2 and spin multiplicity of doublet spin state were considered. B3LYP [
36] was employed as the functional and a conductor-like polarized continuum model (dielectric constant: 35.688) was included in all calculations because this method is known to give reasonable accuracy [
37]. The 6-311++G(d,p) basis sets were used. Vibrational-frequency calculations were performed at the same level of theory to confirm that no imaginary frequency was present. Single-point calculations for energetic analysis were performed on the optimized geometry using the same condition. The spin-density plots and molecular orbitals were visualized using GaussView 6.1 [
38]. TD-DFT calculations were performed on the optimized geometry using the same conditions. Theoretical electronic transition spectra were recorded for low-lying excited states using 50 roots to generate absorption spectra for >300 nm.