Use of N-Heterocyclic Carbene Compounds (NHCs) Under Sustainable Conditions—An Update
Abstract
1. Introduction
2. Discussion
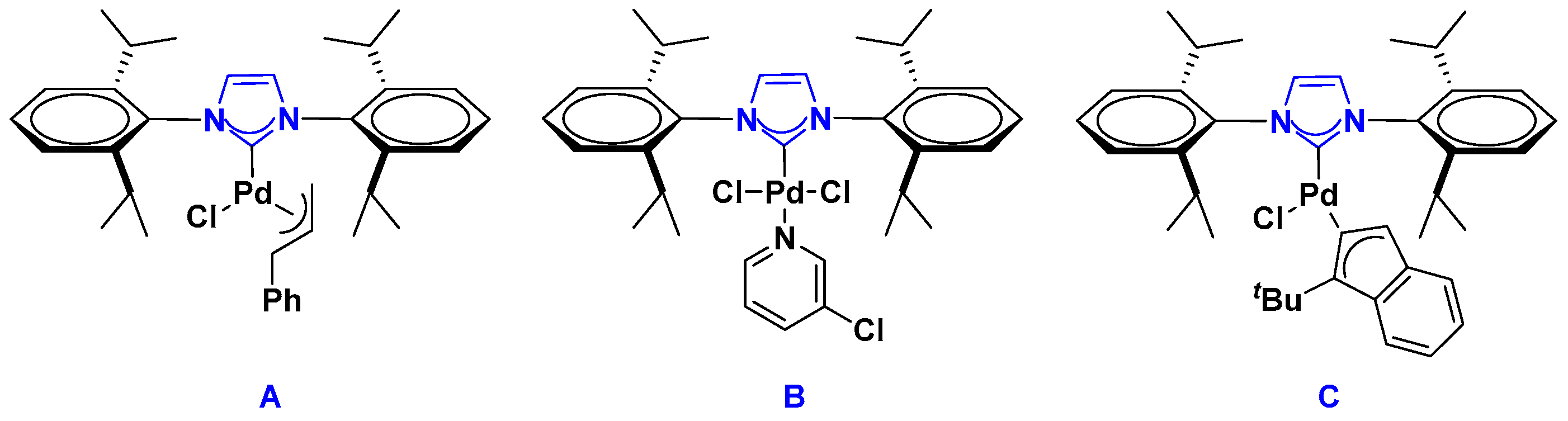
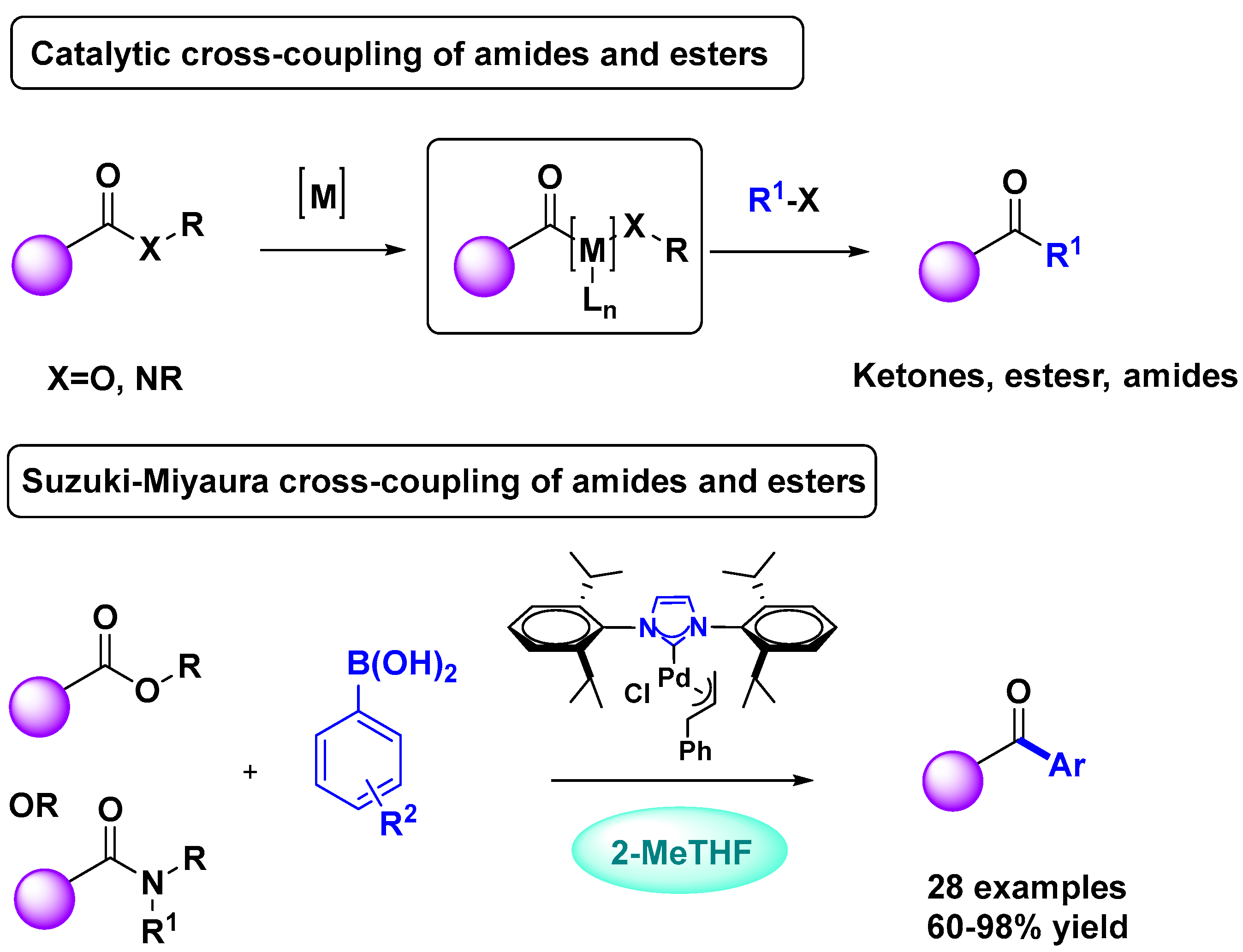
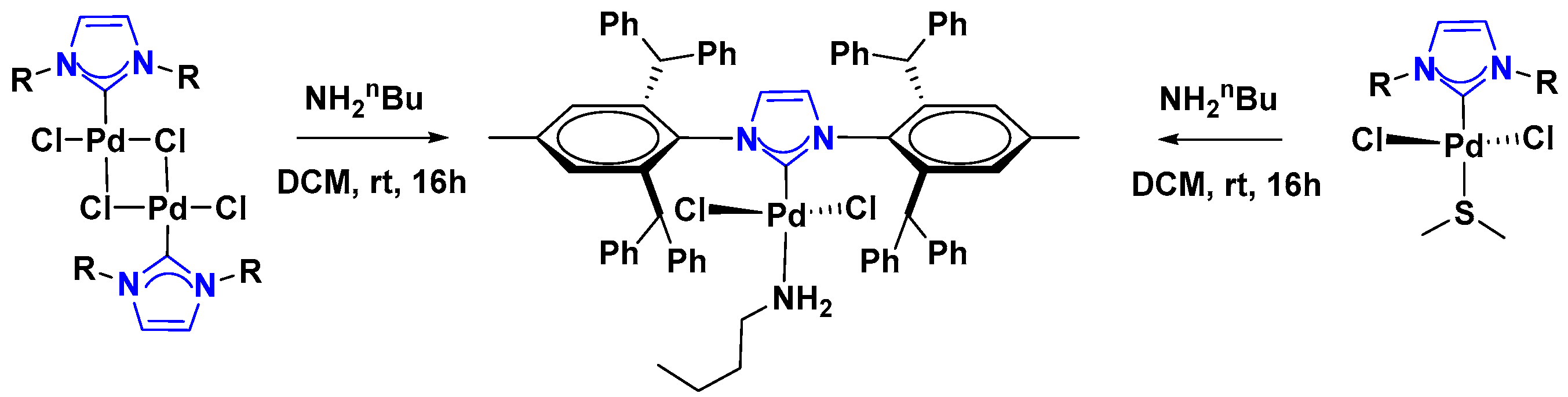
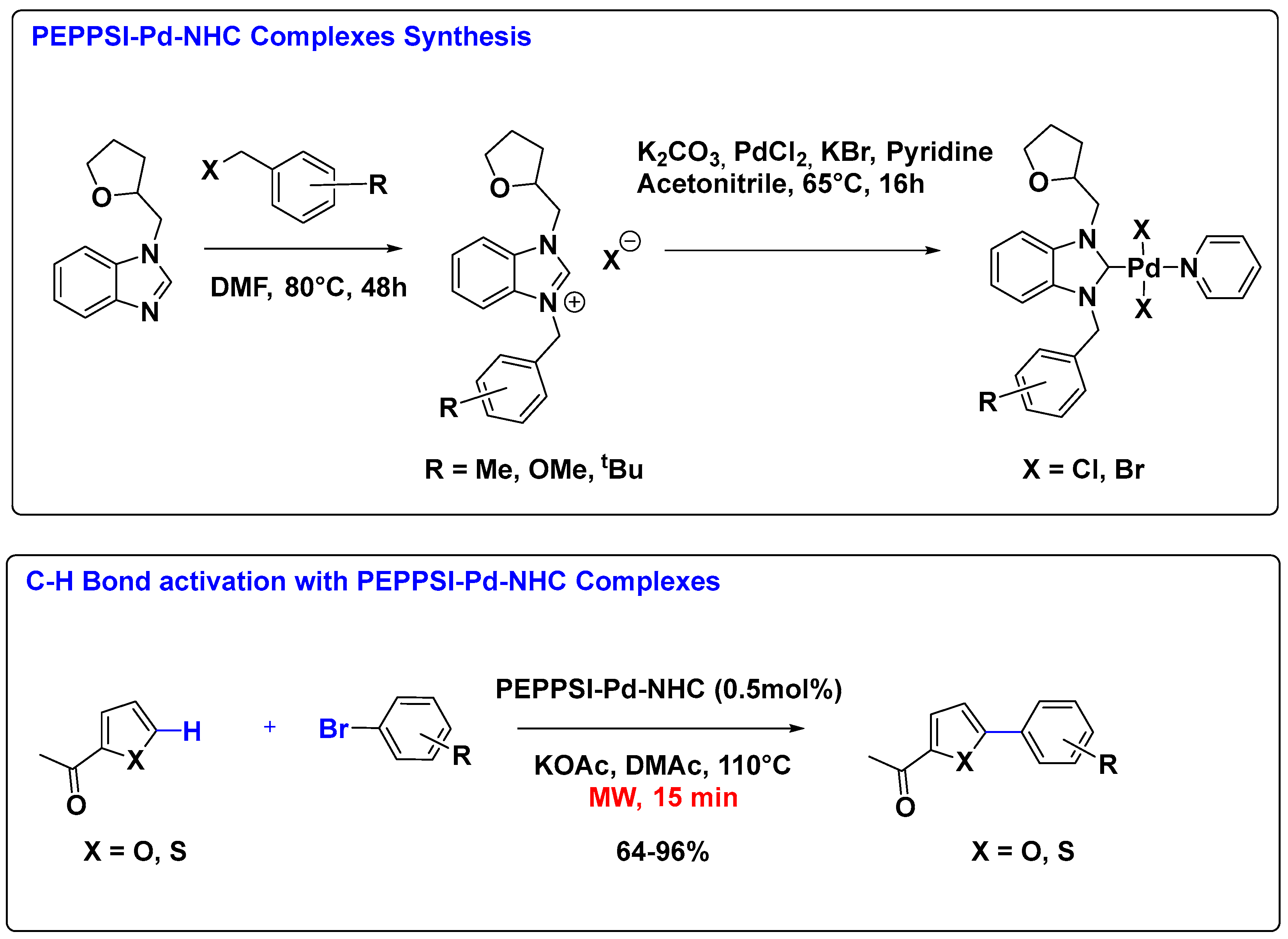
2.1. Palladium Catalysis
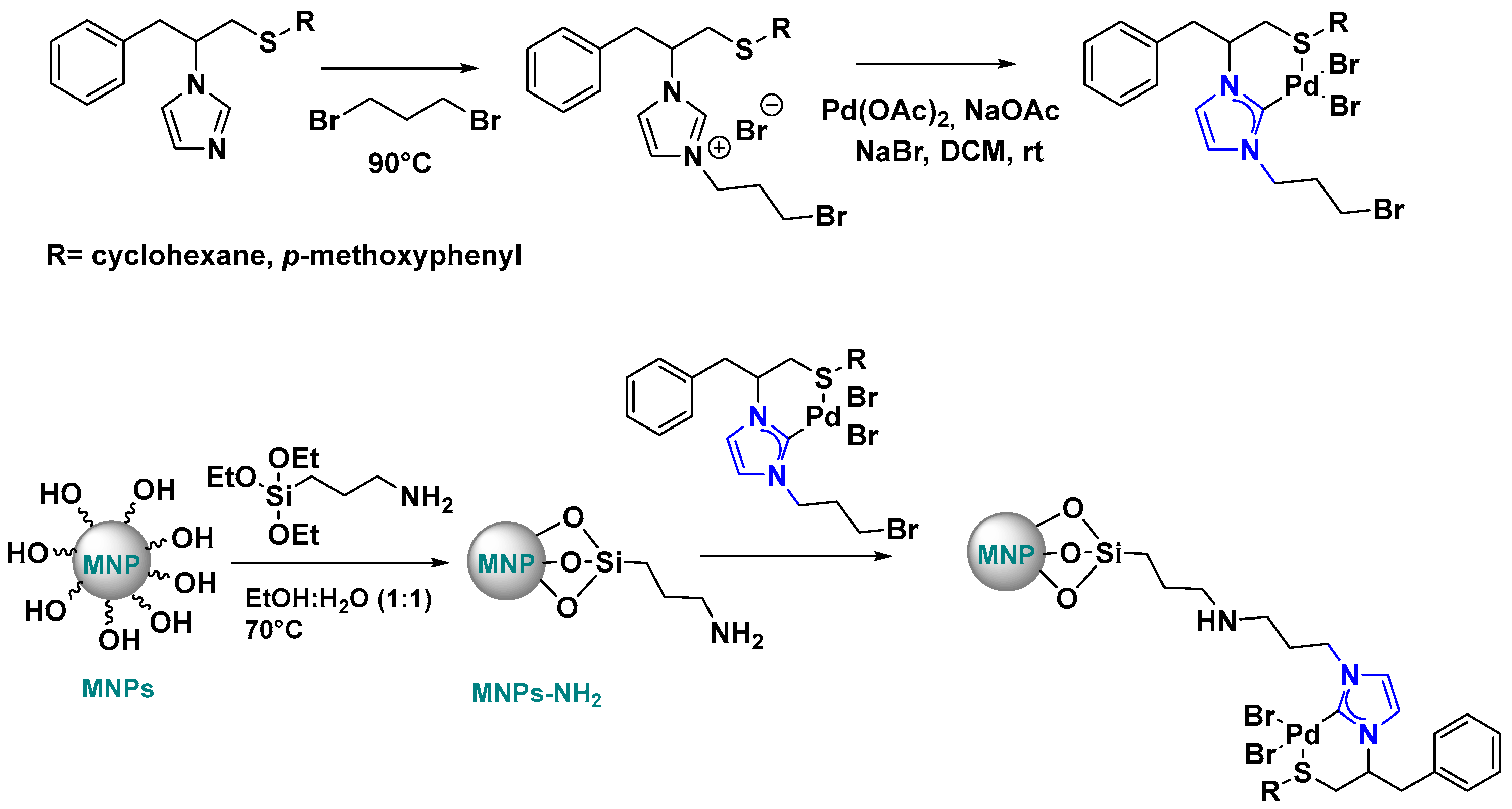
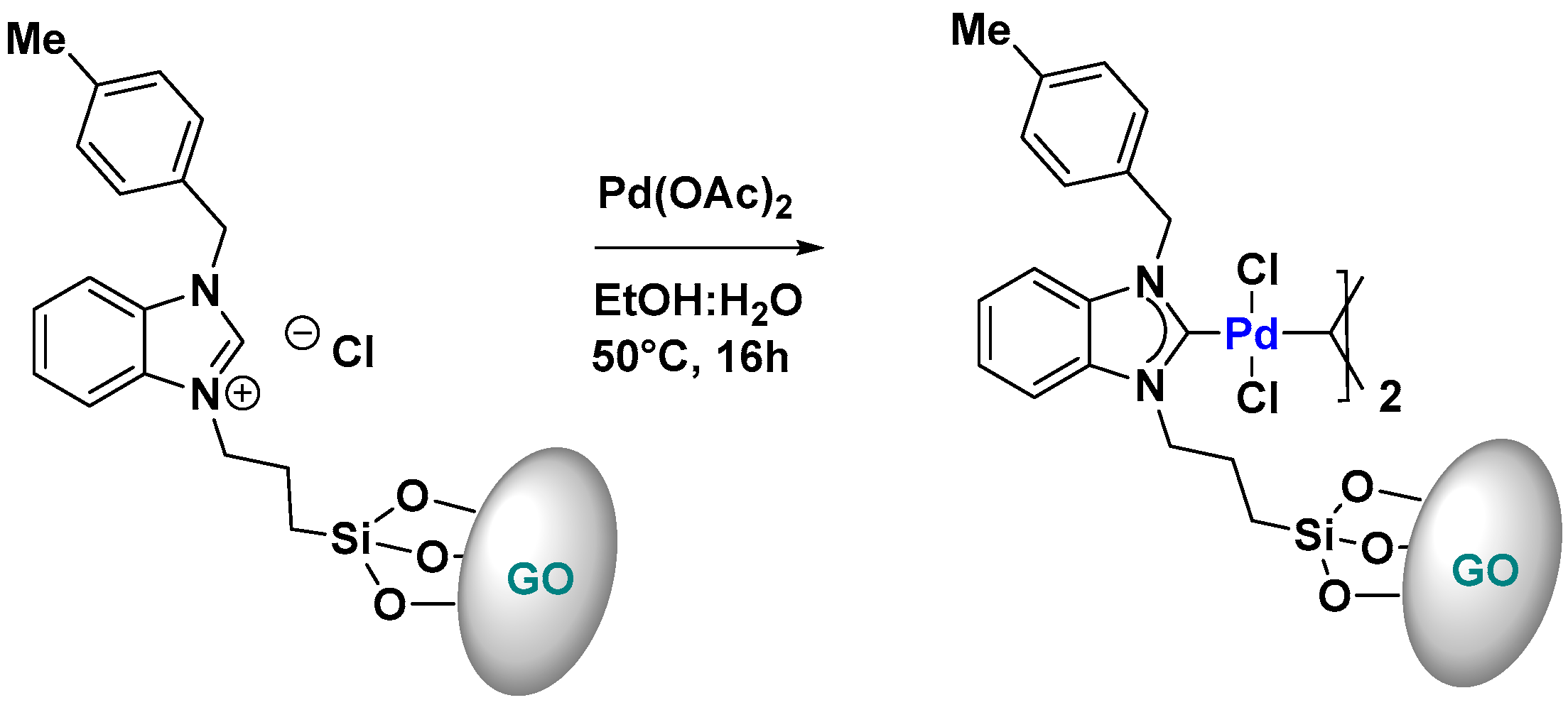
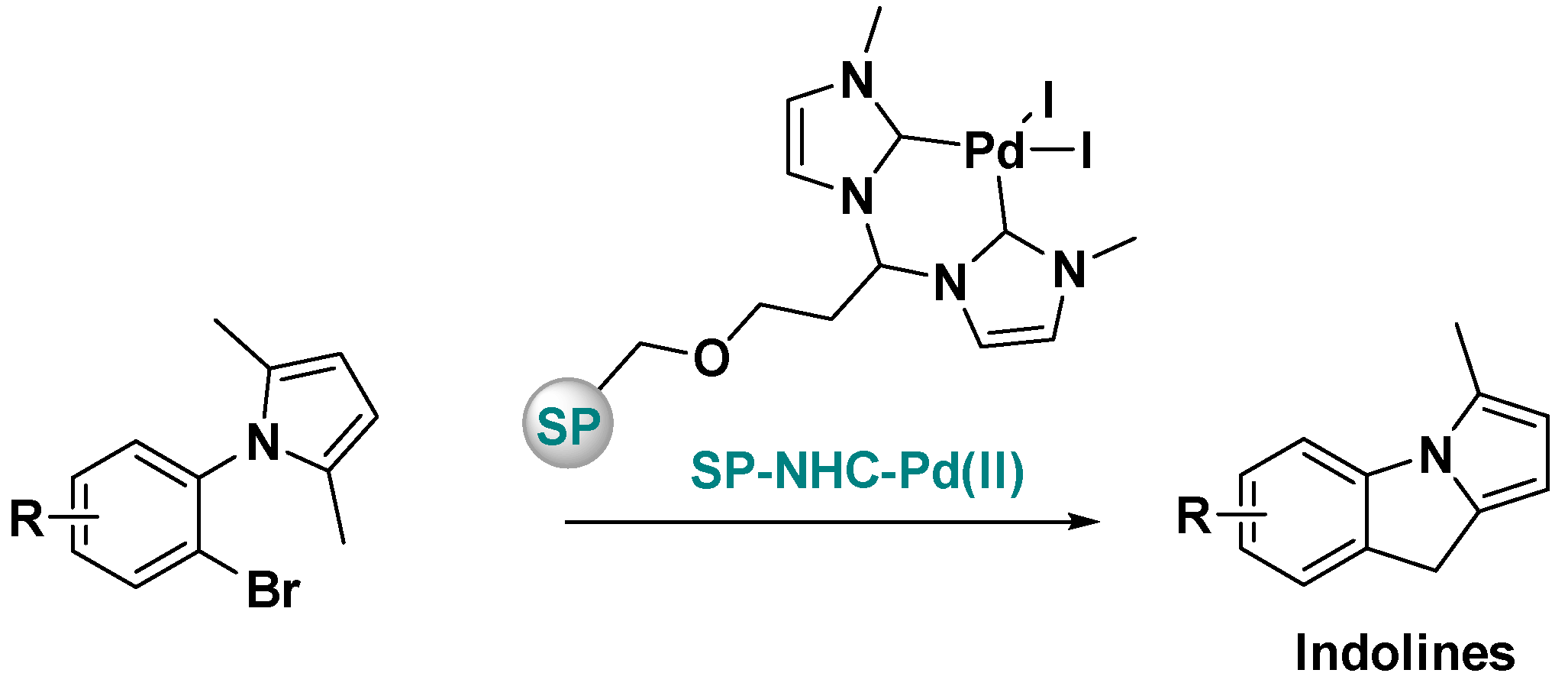
Supported Pd-NHC Catalysts
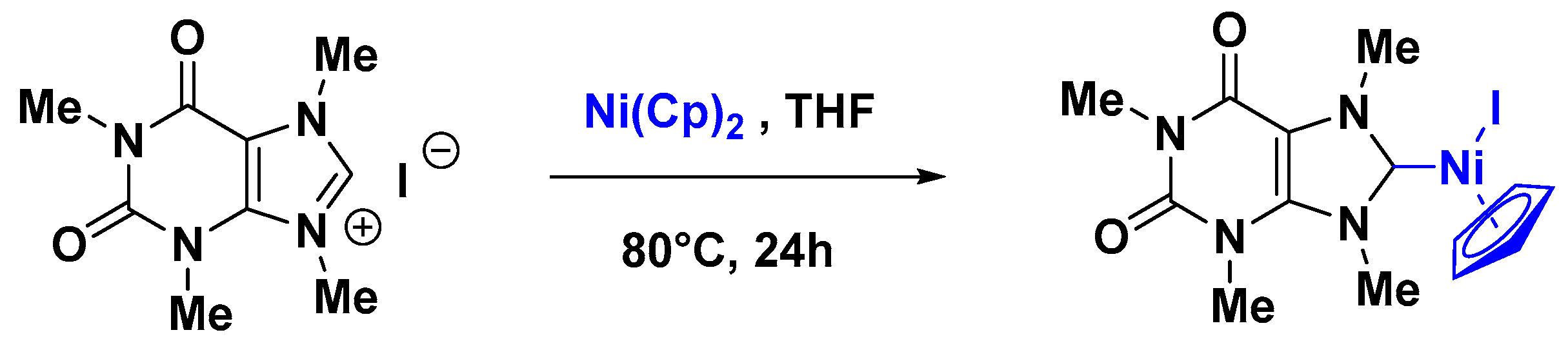
2.2. Nickel Catalysis
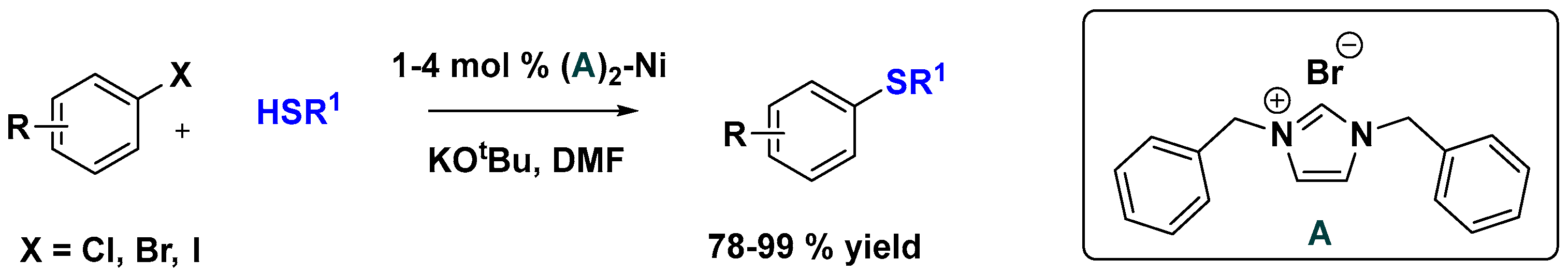
Supported Ni-NHC Catalysts
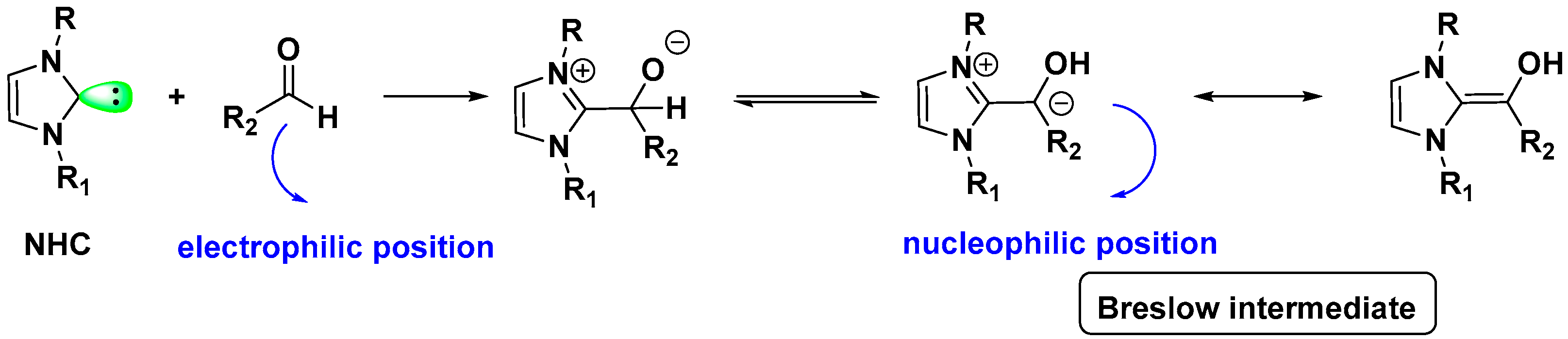
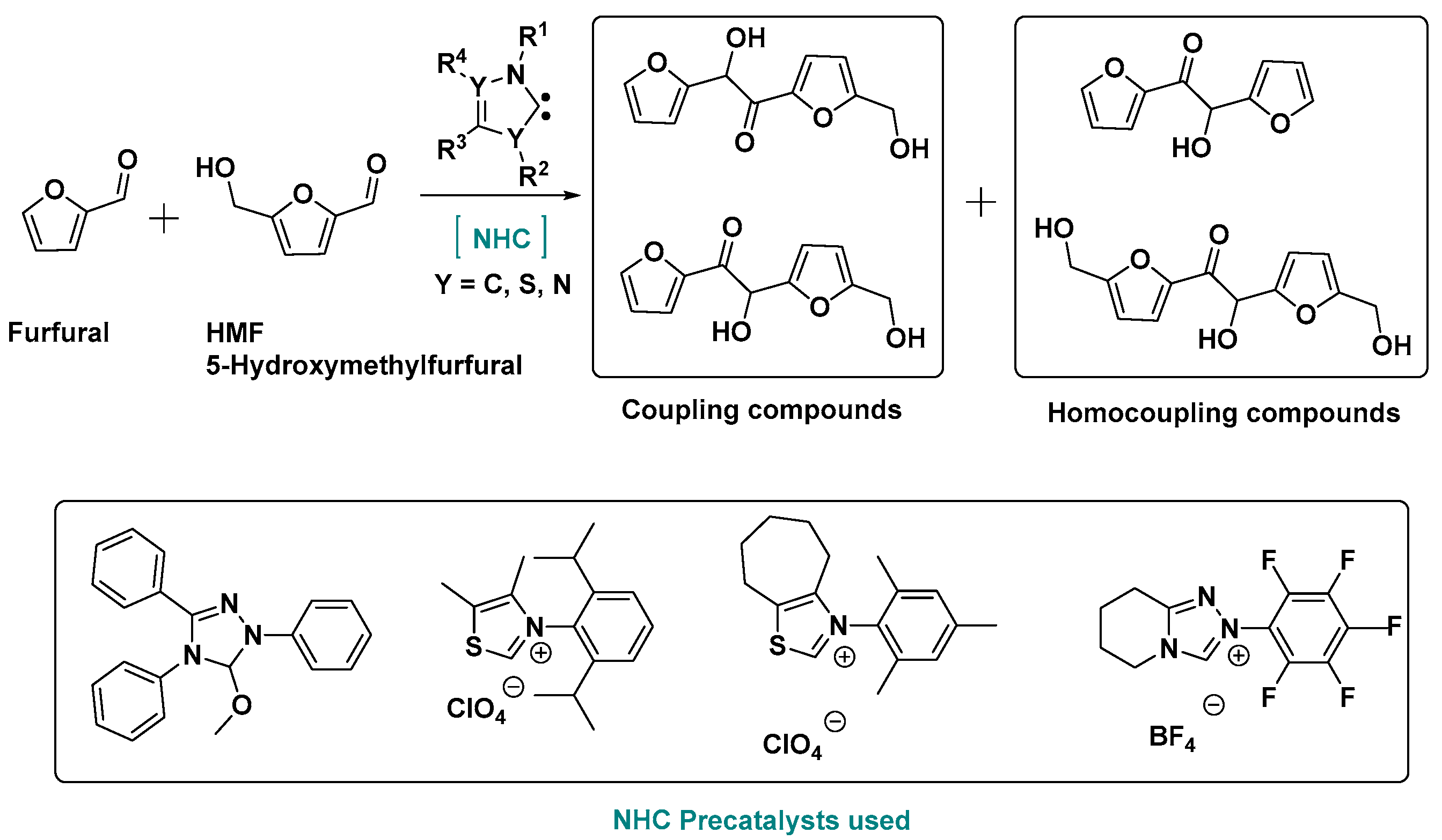
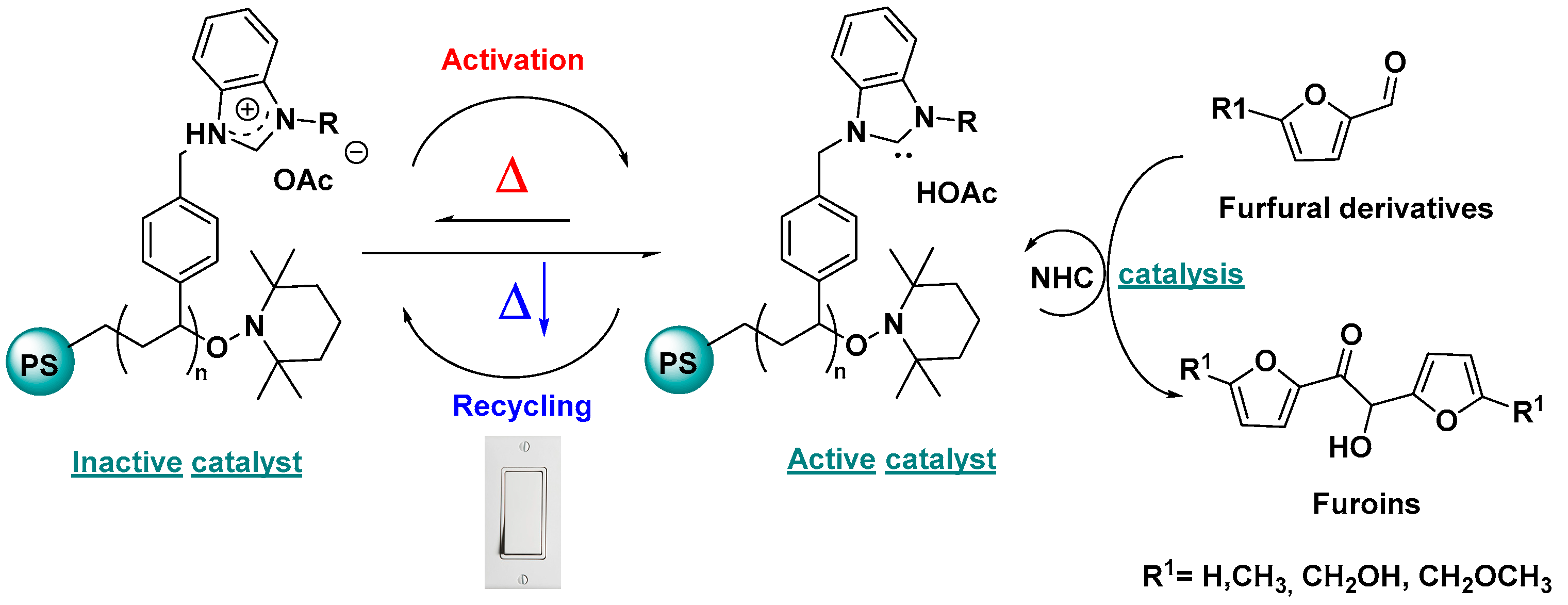
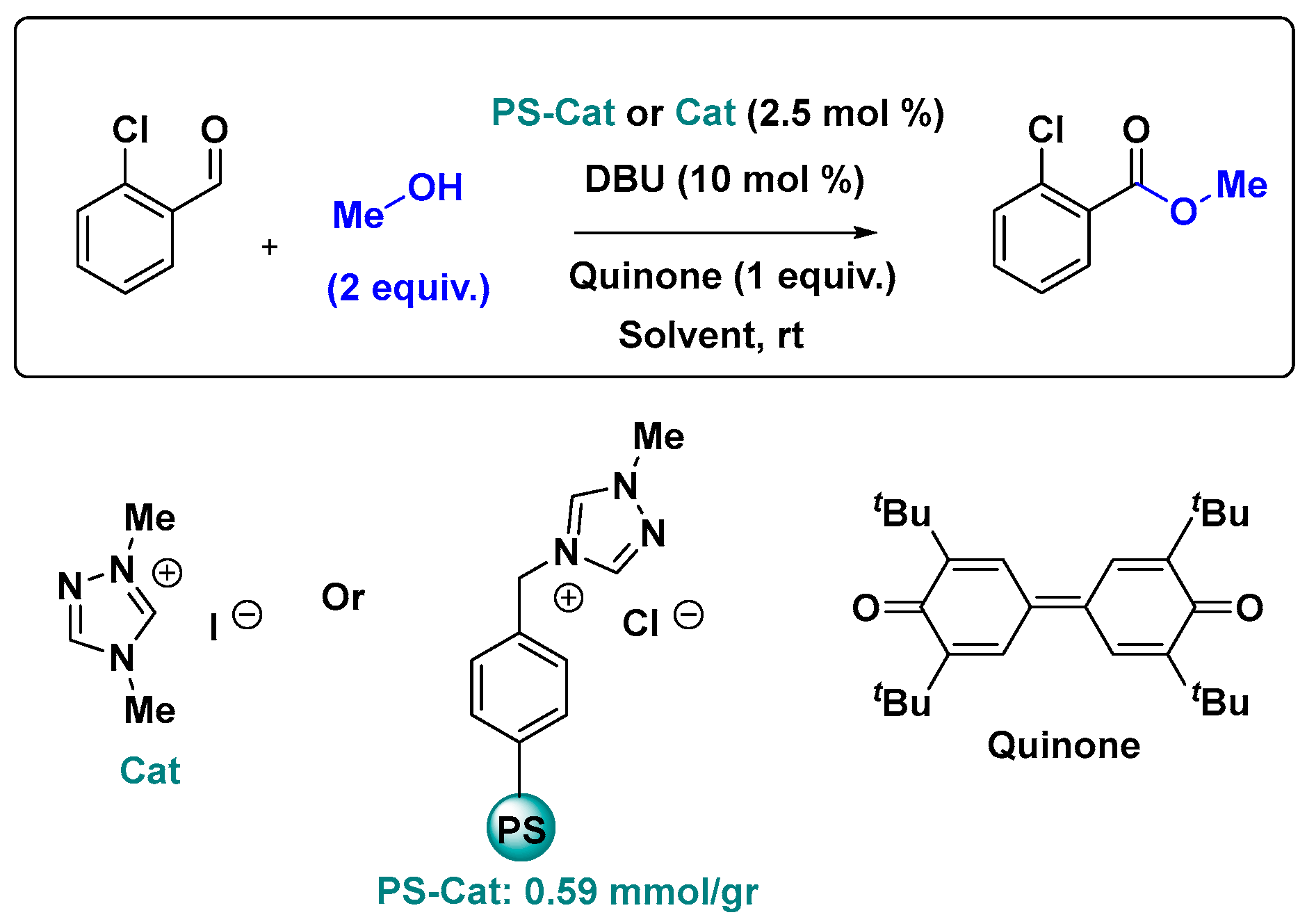
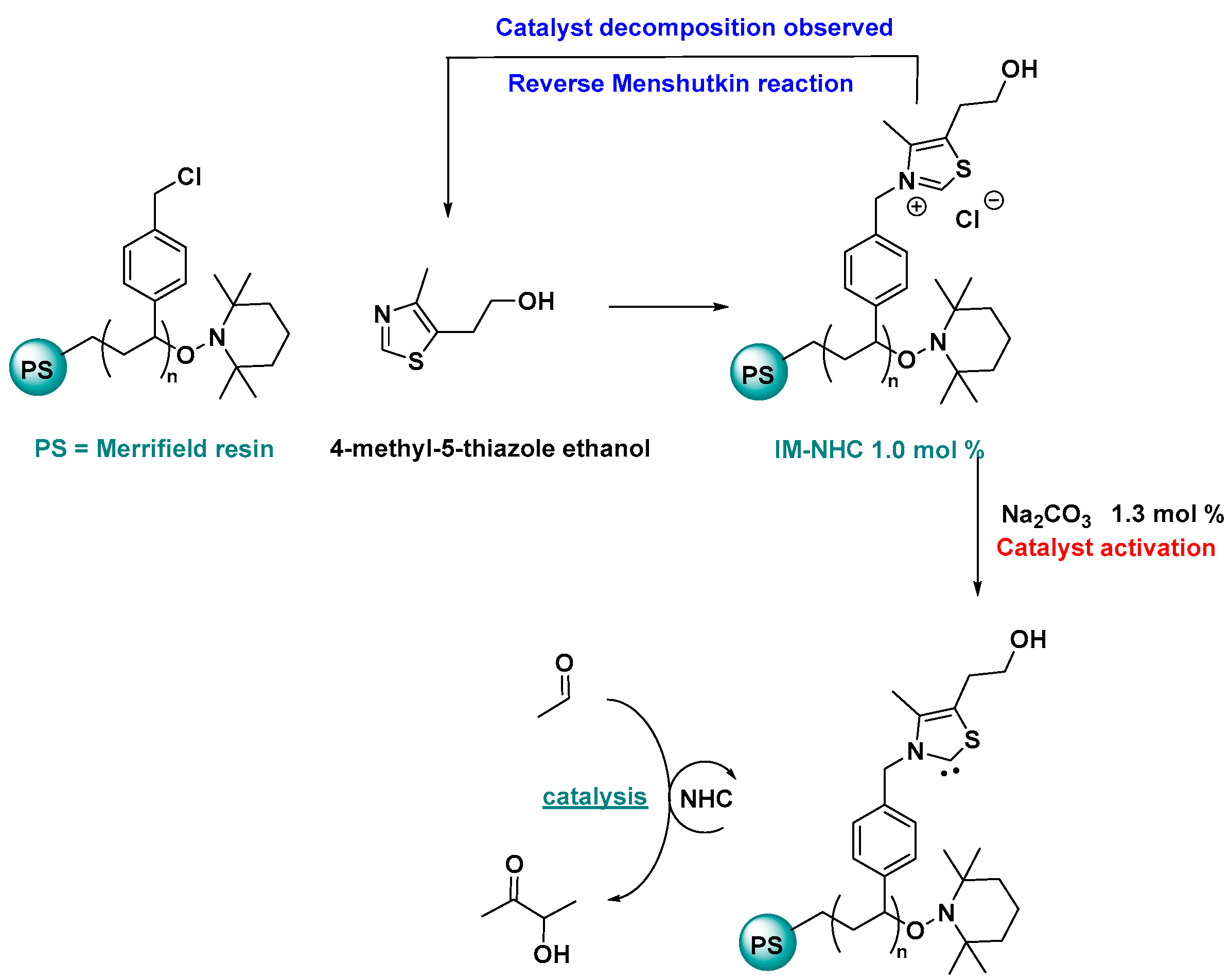
2.3. NHC Catalysis Without Metal
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHCs | N-heterocyclic carbenes |
| RTIL | Room-temperature ionic liquid |
| NPs | Nanoparticles |
| MNPs | Magnetic nanoparticles |
| AuNPs | Gold nanoparticles |
| CPME | Cyclopentyl methyl ether |
| THF | Tetrahydrofuran |
| 2-MeTHF | 2-Methyltetrahydrofuran |
| DBU | 1,5-Diazabicyclo(5.4.0)undec-7-ene |
| HMF | 5-Hydroxymethylfurfural |
| DMF | Dimethylformamide |
| DMA | Dimethylacetamide |
| DCM | Dichloromethane |
| PS | Polystyrene |
References
- Wanzlick, H.W.; Schönherr, H.J. Direct synthesis of a mercury salt-carbene complex. Angew. Chem. Int. Ed. 1968, 7, 141–142. [Google Scholar] [CrossRef]
- Öfele, K. 1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer Übergangsmetall-carben-komplex. J. Organomet. Chem. 1968, 12, 42–43. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The Development of L2X2Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; DeHope, A.; Donnadieu, B.; Bertrand, G. A Rigid Cyclic (Alkyl)(amino)carbene Ligand Leads to Isolation of Low-Coordinate Transition-Metal Complexes. Angew. Chem. Int. Ed. 2005, 44, 7236–7239. [Google Scholar] [CrossRef]
- Yamashita, M.; Goto, K.; Kawashima, T. Fixation of Both O2 and CO2 from Air by a Crystalline Palladium Complex Bearing N-Heterocyclic Carbene Ligands. J. Am. Chem. Soc. 2005, 127, 7294–7295. [Google Scholar] [CrossRef]
- Jazzar, R.F.R.; Macgregor, S.A.; Mahon, M.F.; Richards, S.P.; Whittlesey, M.K. C-C and C-H bond activation reactions in N-heterocyclic carbene complexes of ruthenium. J. Am. Chem. Soc. 2002, 124, 4944–4945. [Google Scholar] [CrossRef]
- Muehlhofer, M.; Strassner, T.; Herrmann, W.A. New Catalyst Systems for the Catalytic Conversion of Methane into Methanol. Angew. Chem. Int. Ed. 2002, 41, 1745–1747. [Google Scholar] [CrossRef]
- Boydston, A.J.; Rice, J.D.; Sanderson, M.D.; Dykhno, O.L.; Bielawski, C.W. Synthesis and Study of Bidentate Benzimidazolylidene−Group 10 Metal Complexes and Related Main-Chain Organometallic Polymers. Organometallics 2006, 25, 6087–6098. [Google Scholar] [CrossRef]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. Sterically demanding, bioxazoline-derived N-heterocyclic carbene ligands with restricted flexibility for catalysis. J. Am. Chem. Soc. 2004, 126, 15195–15201. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Diez-Gonzalez, S.; Nolan, S.P. N-heterocyclic carbenes as organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2988–3000. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S.; Nolan, S.P. Stereoelectronic parameters associated with N-heterocyclic carbene (NHC) ligands: A quest for understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar] [CrossRef]
- Biju, A.T.; Kuhl, N.; Glorius, F. Extending NHC-Catalysis: Coupling Aldehydes with Unconventional Reaction Partners. Acc. Chem. Res. 2011, 44, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Moratorio ’ de Moraes, R.S.; Martins, M.T.M.; Tavares de Almeida Pinto, G.; Rodrigues, S.C.; Antunes do Nascimento, P.; Cardoso Cruz, C.; D’Oliveira Goes, K.; Cunha, A.C. An overview on generation and general properties of N-heterocyclic carbenes: Applications of 1,2,4-triazolium carbenes as metal free organocatalysts. Arab. J. Chem. 2024, 17, 105527. [Google Scholar] [CrossRef]
- Velazquez, H.D.; Verpoort, F. N-heterocyclic carbene transition metal complexes for catalysis in aqueous media. Chem. Soc. Rev. 2012, 41, 7032–7060. [Google Scholar] [CrossRef]
- Albright, E.L.; Malola, S.; Jacob, S.I.; Yi, H.; Takano, S.; Mimura, K.; Tsukuda, T.; Häkkinen, H.; Nambo, M.; Crudden, C.M. Enantiopure Chiral Au13 Nanoclusters Stabilized by Ditopic N-Heterocyclic Carbenes: Synthesis, Characterization, and Electrocatalytic Reduction of CO2. Chem. Mater. 2024, 36, 1279–1289. [Google Scholar] [CrossRef]
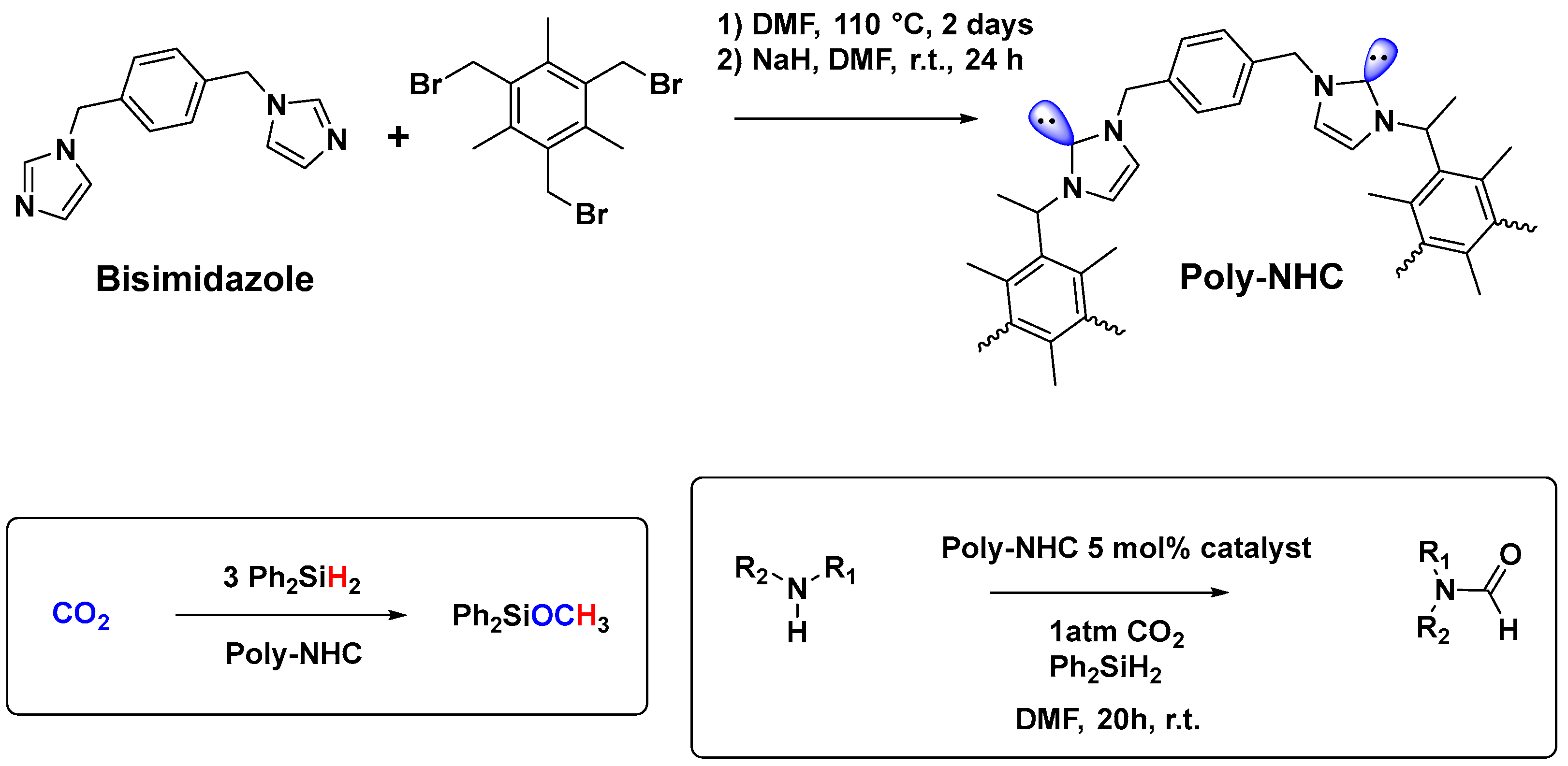
- Riduan, S.N.; Ying, J.Y.; Zhang, Y. Solid poly-N-heterocyclic carbene catalyzed CO2 reduction with hydrosilanes. J. Catal. 2016, 343, 46–51. [Google Scholar] [CrossRef]
- Feroci, M.; Chiarotto, I.; Inesi, A. Electrolysis of Ionic Liquids. A Possible Keystone for the Achievement of Green Solvent-Catalyst Systems. Curr. Org. Chem. 2013, 17, 204–219. [Google Scholar] [CrossRef]
- Hollóczki, O.; Gerhard, D.; Massone, K.; Szarvas, L.; Németh, B.; Veszprémi, T.; László Nyulászi, L. Carbene in ionic liquids. New J. Chem. 2010, 34, 3004–3009. [Google Scholar] [CrossRef]
- Stoppelman, J.P.; McDaniel, J.G. N-Heterocyclic Carbene Formation in the Ionic Liquid [EMIM+][OAc−]: Elucidating Solvation Effects with Reactive Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127, 5317–5333. [Google Scholar] [CrossRef]
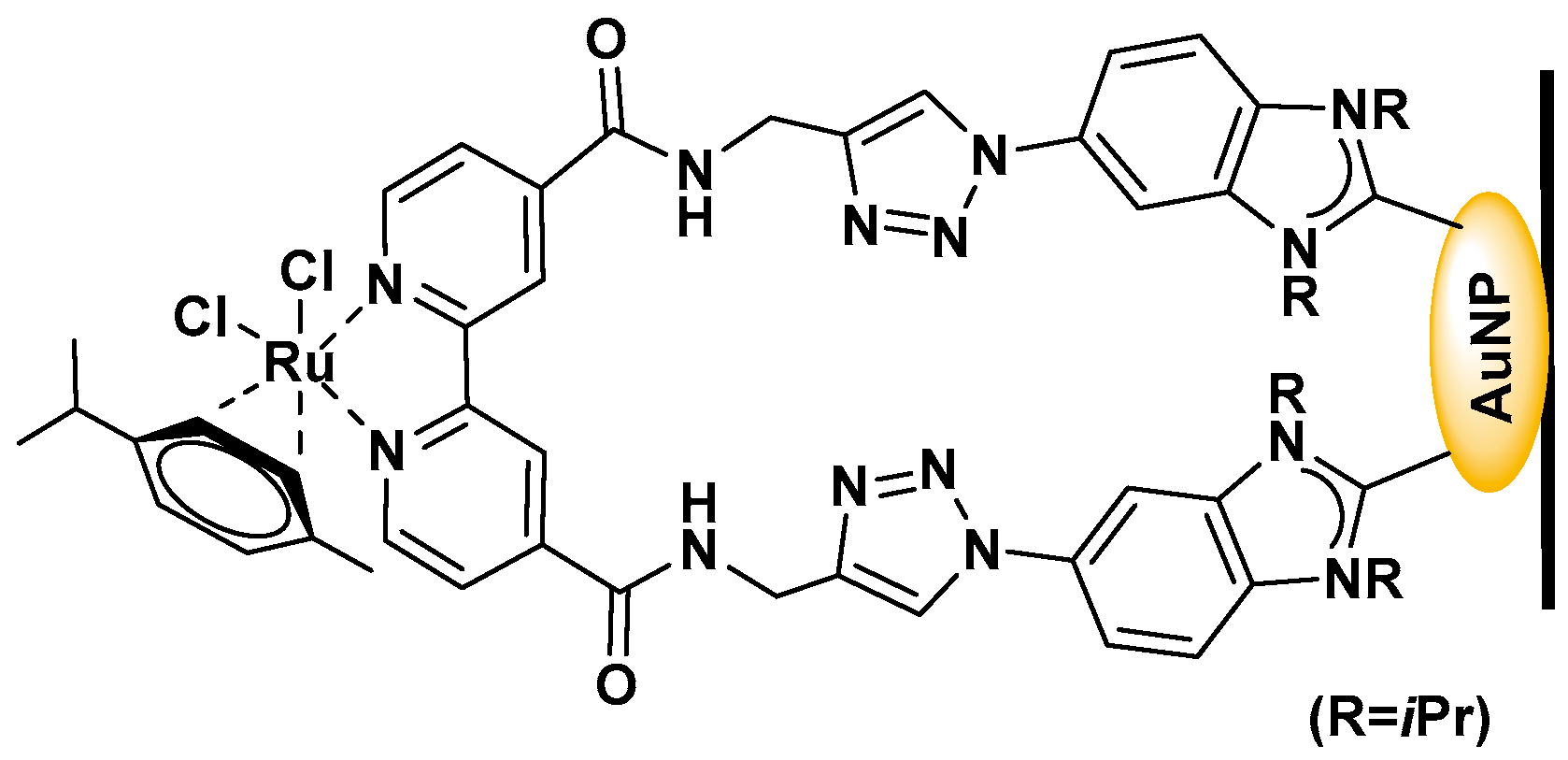
- Jun, H.; Choi, S.; Lee, J.B.; Nam, Y.S. Plasmonic Heterostructure Functionalized with a Carbene-Linked Molecular Catalyst for Sustainable and Selective Carbon Dioxide Reduction. ACS. Appl. Mater. Interfaces 2020, 12, 33817–33826. [Google Scholar] [CrossRef]
- Rapakousiou, A. N-Heterocyclic Carbenes in Advanced Electrocatalysis: From Molecular Complexes to Hybrid Metal Nanostructures. Organomet. Sci. 2025, 1, 2. [Google Scholar]
- Lei, P.; Ling, Y.; An, J.; Nolan, S.P.; Szostak, M. 2-Methyltetrahydrofuran (2-MeTHF): A Green Solvent for Pd-NHC-Catalyzed Amide and Ester Suzuki-Miyaura Cross-Coupling by N-C/O-C Cleavage. Adv. Synth. Catal. 2019, 361, 5654–5660. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes-Palladium (II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.; Khargharia, S.; Kar, R.; Borah, B.J.; Das, P. A new air-stable Pd-PEPPSI N-heterocyclic carbene complex: Synthesis, structure, computational and catalytical studies. J. Mol. Struct. 2024, 1295, 136678. [Google Scholar] [CrossRef]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst for Palladium Catalyzed Cross-Coupling: (η3-1-tBu-indenyl)2(μ-Cl)2Pd2’. ACS Catal. 2015, 5, 3680–3688. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.; Hartwig, J.F. Palladium-Catalyzed Synthesis of Arylamines from Aryl Halides. Mechanistic Studies Lead to Coupling in the Absence of Tin Reagents. Tetrahedron Lett. 1995, 36, 3609–3612. [Google Scholar] [CrossRef]
- Guram, A.S.; Rennels, R.A.; Buchwald, S.L. A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines. Angew. Chem. Int. Engl. Ed. 1995, 34, 1348–1350. [Google Scholar] [CrossRef]
- Farina, V. High-Turnover Palladium Catalysts in Cross-Coupling and Heck Chemistry: A Critical Overview. Adv. Synth. Catal. 2004, 346, 1553–1582. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts 2019, 9, 840. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-Purpose Product. Catalysts 2022, 12, 48. [Google Scholar] [CrossRef]
- Pozsoni, N.B.; Nahra, F.; van Hecke, K.; Cazin, C.S.J.; Nolan, S.P. Impact of the N-Heterocyclic Carbene (NHC) Ligand on the trans-[Pd(NHC)(NH2nBu)Cl2] Precatalyst Architecture in C−N Bond-Forming Reactions. Organometallics 2024, 43, 2963–2971. [Google Scholar] [CrossRef]
- Bera, S.S.; Utecht-Jarzyńska, G.; Yang, S.; Nolan, S.P.; Szostak, M. Metal−N-Heterocyclic Carbene Complexes in Buchwald−Hartwig Amination Reactions. Chem. Rev. 2025, 125, 5349–5435. [Google Scholar] [CrossRef] [PubMed]
- Slimani, I.; Özdemir, I.; Gürbüz, N.; Alıcı, B.; Arslan, N.B.; Özdemir, N. An Eco-friendly Approach to C−H Bond Activation through Microwave Irradiation Employing Synthesized Palladium-PEPPSI-NHC Complexes. ACS Omega 2025, 10, 39994–40008. [Google Scholar] [CrossRef] [PubMed]
- Vasu, G.R.P.; Venkata, K.R.M.; Kakarla, R.R.; Ranganath, K.V.S.; Aminabhavi, T.M. Recent advances in sustainable N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) catalysts: A review. Environ. Res. J. 2023, 225, 115515. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.D.; Lammert, R.M.; Yirak, J.R.; Lyer, K.S.; Wong, M.J.; Lipshutz, B.H. Recent Green and Sustainable Pd-Catalyzed Aminations John Michael Saunders, Kylee. ChemSusChem 2025, 18, e202500184. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Diao, X.; Zeng, X.; Xiao, Y.; Yuan, J.; Mao, P. New chelating N-heterocyclic carbene palladium complexes immobilized on magnetic nanoparticles: Synthesis, characterization, and catalytic properties. Appl. Organomet. Chem. 2024, 38, e7636. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N. Ultrasound-assisted synthesis of 3D flower-like zinc oxide decorated T fMWCNTs for sensitive detection of toxic environmental pollutant 4-nitrophenol. Ultrason. Sonochem. 2020, 60, 104798. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, L.T.; Yang, R.; Wang, L.; Liu, J.H.; Huang, X.J. Single-Walled Carbon Nanotube/Pyrenecyclodextrin Nanohybrids for Ultrahighly Sensitive and Selective Detection of p-Nitrophenol. Langmuir 2011, 27, 10295–10301. [Google Scholar] [CrossRef]
- Liao, G.F.; Gong, Y.; Zhong, L.; Fang, J.S.; Zhang, L.; Xu, Z.S.; Gao, H.Y.; Fang, B.Z. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, M.; Ren, Y.; Xu, J.; Zheng, J.; Alsulami, H.; Kutbi, M.A.; Zhang, F.-Y. Carbon-supported nickel nanoparticles on SiO2 cores for protein adsorption and nitroaromatics reduction. ACS Appl. Nano Mater. 2020, 3, 4623–4634. [Google Scholar] [CrossRef]
- Salameh, N.; Ferlin, F.; Valentini, F.; Anastasiou, I.; Vaccaro, L. Waste-Minimized Continuous-Flow Synthesis of Oxindoles Exploiting a Polymer-Supported N Heterocyclic Palladium Carbene Complex in a CPME/Water Azeotrope. ACS Sustain. Chem. Eng. 2022, 10, 3766–3776. [Google Scholar] [CrossRef]
- Yue, C.; Xing, Q.; Sun, P.; Zhao, Z.; Lv, H.; Li, F. Enhancing stability by trapping palladium inside N-heterocyclic carbene-functionalized hypercrosslinked polymers for heterogeneous C-C bond formations. Nat. Commun. 2021, 12, 1875. [Google Scholar] [CrossRef]
- You, L.; Tan, R.; Wang, X.; Hao, J.; Xie, S.; Xiong, G.; Ding, F.; Potapov, A.S.; Sun, Y. N-Heterocyclic Carbene–Palladium Functionalized Coordination Polymer (Pd-NHC@Eu-BCI) as an Efficient Heterogeneous Catalyst in the Suzuki–Miyaura Coupling Reaction. Crystals 2023, 13, 341. [Google Scholar] [CrossRef]
- Khandaka, H.; Sharma, K.N.; Joshi, R.K. Aerobic Cu and amine free Sonogashira and Stille couplings of aryl bromides/chlorides with a magnetically recoverable Fe3O4@SiO2 immobilized Pd (II)-thioether containing NHC. Tetrahedron Lett. 2021, 67, 152844. [Google Scholar] [CrossRef]
- Kempasiddhaiah, M.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Magnetite tethered mesoionic carbene-palladium (II): An efficient and reusable nanomagnetic catalyst for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions in aqueous medium. Appl. Organomet. Chem. 2019, 33, e4846. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.M.; Saeedi, S.; Panahi, F. New bis (N-heterocyclic carbene) palladium complex immobilized on magnetic nanoparticles: As a magnetic reusable catalyst in Suzuki-Miyaura cross coupling reaction. Appl. Organomet. Chem. 2017, 31, e3870. [Google Scholar] [CrossRef]
- Min, Q.; Miao, P.; Chu, D.; Liu, J.; Qi, M.; Kazemnejadi, M. Introduction of a Recyclable Basic Ionic Solvent with Bis-(NHC) Ligand Property and the Possibility of Immobilization on Magnetite for Ligand-And Base-Free Pd-Catalyzed Heck, Suzuki and Sonogashira Cross-Coupling Reactions in Water. Catal. Lett. 2021, 151, 3030–3047. [Google Scholar] [CrossRef]
- Nandeshwar, M.; Mandal, S.; Kuppuswamy, S.; Prabusankar, G. A Sustainable Approach for Graphene Oxide-supported Metal N-Heterocyclic Carbenes Catalysts. Chem. Asian J. 2023, 18, e202201138. [Google Scholar] [CrossRef]
- Axet, M.R.; Dechy-Cabaret, O.; Durand, J.J.; Gouygou, M.; Serp, P. Coordination chemistry on carbon surfaces. Coord. Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Sachdeva, H. Recent advances in the catalytic applications of GO/rGO for green organic synthesis. Green Process. Synth. 2020, 9, 515–537. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Shang, N.; Gao, S.; Feng, C.; Zhang, H.; Wang, C.; Wang, Z. Graphene oxide supported N-heterocyclic carbene-palladium as a novel catalyst for the Suzuki–Miyaura reaction. RSC Adv. 2013, 3, 21863–21868. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Catalyst Enhancement and Recyclability by Immobilization of Metal Complexes onto Graphene Surface by Noncovalent Interactions. ACS Catal. 2014, 4, 2038–2047. [Google Scholar] [CrossRef]
- Salameh, N.; Minio, F.; Rossini, G.; Marrocchi, A.; Vaccaro, L. Waste-minimized C(sp3)-H activation for the preparation of fused N-heterocycles. Green Synth. Catal. 2023, 4, 240–245. [Google Scholar] [CrossRef]
- Ferlin, F.; Anastasiou, I.; Salameh, N.; Miyakoshi, T.; Baudoin, O.; Vaccaro, L. C(sp3)−H Arylation Promoted by a Heterogeneous Palladium-N-Heterocyclic Carbene Complex in Batch and Continuous Flow. ChemSusChem 2022, 15, e202102736. [Google Scholar] [CrossRef]
- Salameh, N.; Anastasiou, I.; Ferlin, F.; Minio, F.; Chen, S.; Santoro, S.; Liu, P.; Gu, Y.; Vaccaro, L. Heterogeneous palladium-catalysed intramolecular C(sp3) single bond-H α-arylation for the green synthesis of oxindoles. Mol. Catal. 2022, 522, 112211. [Google Scholar] [CrossRef]
- Hwang, S.J.; Cho, S.; Chang, S. Synthesis of Condensed Pyrroloindoles via Pd-Catalyzed Intramolecular C−H Bond Functionalization of Pyrroles. J. Am. Chem. Soc. 2008, 130, 16158–16159. [Google Scholar] [CrossRef]
- Let, S.; Dam, G.K.; Samanta, P.; Fajal, S.; Dutta, S.; Ghosh, S.K. Palladium-Anchored N-Heterocyclic Carbenes in a Porous Organic Polymer: A Heterogeneous Composite Catalyst for Eco-Friendly C−C Coupling. J. Org. Chem. 2022, 87, 16655–16664. [Google Scholar] [CrossRef]
- Inonu, Z.; Keskin, S.; Erkey, C. An Emerging Family of Hybrid Nanomaterials: Metal-Organic Framework/Aerogel Composites. ACS Appl. Nano Mater. 2018, 1, 5959–5980. [Google Scholar] [CrossRef]
- Wu, M.-B.; Zhang, C.; Xie, Y.; Huang, S.; Liu, C.; Wu, J.; Xu, Z. −.K. Janus Metal-Organic Frameworks/Wood AerogelComposites for Boosting Catalytic Performance by Le Châtelier’s Principle. ACS Appl. Mater. Interfaces 2021, 13, 51039–51047. [Google Scholar] [CrossRef]
- Li, F.; Ding, L.-G.; Yao, B.-J.; Huang, N.; Li, J.-T.; Fu, Q.-J.; Dong, Y.-B. Pd loaded and covalent-organic framework involved chitosan aerogels and their application for continuous flow-through aqueous CB decontamination. J. Mater. Chem. A 2018, 6, 11140–11146. [Google Scholar] [CrossRef]
- Ding, L.-G.; Yao, B.-J.; Li, F.; Shi, S.-C.; Huang, N.; Yin, H.-B.; Guan, Q.; Dong, Y.-B. Ionic liquid-decorated COF and its covalent composite aerogel for selective CO2 adsorption and catalytic conversion. J. Mater. Chem. A 2019, 7, 4689–4698. [Google Scholar] [CrossRef]
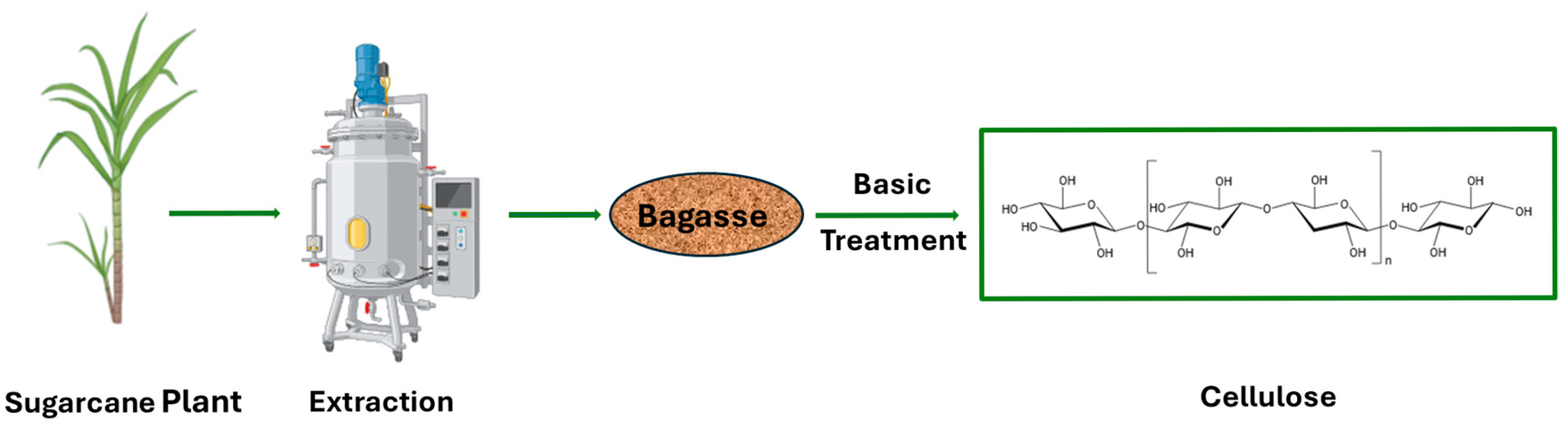
- Antony, A.M.; Chamanmalik, M.I.; Kandathil, V.; Sampatkumar, H.G.; Sasidhar, B.S.; Yelamaggad, B.S.; Patil, S.A. Biomacromolecule supported N-heterocyclic carbene-palladium (II) as a novel catalyst for Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions. Cellulose 2023, 30, 7551–7573. [Google Scholar] [CrossRef]
- Kandathil, V.; Kempasiddaiah, M.; Sasidhar, B.; Patil, S.A. From agriculture residue to catalyst support; a green and sustainable cellulose-based dip catalyst for C-C coupling and direct arylation. Carbohydr. Polym. 2019, 223, 115060. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Rafieian, F.; Mousavi, M.; Yu, Q.; Jonoobi, M. Amine functionalization of microcrystalline cellulose assisted by (3-chloropropyl) triethoxysilane. Int. J. Biol. Macromol. 2019, 130, 280–287. [Google Scholar] [CrossRef]
- Kandathil, V.; Fahlman, B.D.; Sasidhar, B.; Patil, S.A.; Patil, S.A. A convenient, efficient and reusable N-heterocyclic carbene-palladium (II) based catalyst supported on magnetite for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions. New J. Chem. 2017, 41, 9531–9545. [Google Scholar] [CrossRef]
- Zhang, Y.; Ngeow, K.C.; Ying, J.Y. The First N-Heterocyclic Carbene-Based Nickel Catalyst for C−S Coupling. Org. Lett. 2007, 9, 3495–3498. [Google Scholar] [CrossRef]
- Neshat, A.; Khezri, R.; Yousefshahi, M.R.; Gholinejad, M.; Varmaghani, F. Carbon Sulfur Coupling Reactions Catalyzed by Nickel (II) N-Heterocyclic Carbene Complexes. Eur. J. Inorg. Chem. 2023, 26, e202300437. [Google Scholar] [CrossRef]
- Dzik, W.I.; Gooßen, J.L. Selective Crossed-Tishchenko Reaction—A Waste-Free Synthesis of Benzyl Esters. Angew. Chem. Int. Ed. 2011, 50, 11047–11049. [Google Scholar] [CrossRef]
- Ogata, Y.; Kawasaki, A. Alkoxide transfer from aluminium alkoxide to aldehyde in the Tishchenko reaction. Tetrahedron 1969, 25, 929–935. [Google Scholar] [CrossRef]
- Hoshimoto, Y.; Ohashi, M.; Ogoshi, S. Nickel-Catalyzed Selective Conversion of Two Different Aldehydes to Cross-Coupled Esters. J. Am. Chem. Soc. 2011, 133, 4668–4671. [Google Scholar] [CrossRef]
- Ogoshi, S.; Hoshimoto, Y.; Ohashi, M. Nickel-catalyzed Tishchenko reactionvia hetero-nickelacycles by oxidative cyclization of aldehydes with nickel (0) complex. Chem. Commun. 2010, 46, 3354–3356. [Google Scholar] [CrossRef]
- Zhang, J.; Rahman, M.; Zhao, Q.; Feliciano, J.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Szostak, M. N-Heterocyclic Carbene Complexes of Nickel (II) from Caffeine and Theophylline: Sustainable Alternative to Imidazol-2-ylidenes. Organometallics 2022, 41, 1806–1815. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Lake, B.R.M.; Laing, T.; Phillips, R.M.; Willans, C.W. Synthesis and anticancer activity of silver(I)−N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 2015, 44, 7563–7569. [Google Scholar] [CrossRef]
- Bertrand, B.; Stefan, L.; Pirrotta, M.; Monchaud, D.; Bodio, E.; Richard, P.; Le Gendre, P.; Warmerdam, E.; de Jager, M.H.; Groothuis, G.M.M.; et al. Caffeine-Based Gold(I) N-Heterocyclic Carbenes as Possible Anticancer Agents: Synthesis and Biological Properties. Inorg. Chem. 2014, 53, 2296–2303. [Google Scholar] [CrossRef]
- Scattolin, T.; Caligiuri, I.; Canovese, L.; Demitri, N.; Gambari, R.; Lampronti, I.; Rizzolio, F.; Santo, C.; Visentin, F. Synthesis of new allyl palladium complexes bearing purine-based NHC ligands with antiproliferative and proapoptotic activities on human ovarian cancer cell lines. Dalton Trans. 2018, 47, 13616–13630. [Google Scholar] [CrossRef] [PubMed]
- Skander, M.; Retailleau, P.; Bourrié, B.; Schio, L.; Mailliet, P.; Marinetti, A. N-Heterocyclic Carbene-Amine Pt (II) Complexes, a New Chemical Space for the Development of Platinum-Based Anticancer Drugs. J. Med. Chem. 2010, 53, 2146–2154. [Google Scholar] [CrossRef]
- Kale, D.; Rashinkar, G.; Patil, A.; Kumbhar, A.; Salunkhe, R. Facile Access to 2-Substituted Benzoxazoles Using Sawdust Supported N-Heterocyclic Carbene-Ni Complex via C-H Activation. Lett. Org. Chem. 2020, 17, 479–489. [Google Scholar] [CrossRef]
- Wilson, J.; Chen, E.Y.-X. Organocatalytic Cross-Coupling of Biofuranics to Multifunctional Difuranic C11 Building Blocks. ACS Sustain. Chem. Eng. 2016, 4, 4927–4936. [Google Scholar] [CrossRef]
- Bohre, A.; Dutta, S.; Saha, B.; Abu-Omar, M.M. Upgrading furfurals to drop-in biofuels: An overview. ACS Sustain. Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- King, A.E.; Brooks, T.J.; Tian, Y.-H.; Batista, E.R.; Sutton, A.D. Understanding ketone hydrodeoxygenation for the production of fuels and feedstocks from biomass. ACS Catal. 2015, 5, 1223–1226. [Google Scholar] [CrossRef]
- Sutton, A.D.; Waldie, F.D.; Wu, R.; Schlaf, M.; Silks, L.A.P., III; Gordon, J.C. The hydrodeoxygenation of bioderived furans into alkanes. Nat. Chem. 2013, 5, 428–432. [Google Scholar] [CrossRef]
- Liu, D.; Chen, E.Y.X. Integrated catalytic process for biomass conversion and upgrading to C12 furoin and alkane fuel. ACS Catal. 2014, 4, 1302–1310. [Google Scholar] [CrossRef]
- Cywar, R.M.; Wang, L.; Chen, E.Y.-X. Thermally Regulated Recyclable Carbene Catalysts for Upgrading of Biomass Furaldehydes. ACS Sustain. Chem. Eng. 2019, 7, 1980–1988. [Google Scholar] [CrossRef]
- Zang, H.; Chen, E.Y.X. Organocatalytic Upgrading of Furfural and 5-Hydroxymethyl Furfural to C10 and C12 Furoins with Quantitative Yield and Atom-Efficiency. Int. J. Mol. Sci. 2015, 16, 7143–7158. [Google Scholar] [CrossRef]
- Wang, L.; Chen, E.Y.X. Recyclable Supported Carbene Catalysts for High-Yielding Self-Condensation of Furaldehydes into C10 and C12 Furoins. ACS Catal. 2015, 5, 6907–6917. [Google Scholar] [CrossRef]
- Di Carmine, G.; Ragno, D.; Massi, A.; D’Agostino, C. Oxidative Coupling of Aldehydes with Alcohol for the Synthesis of Esters Promoted by Polystyrene-Supported N-Heterocyclic Carbene: Unraveling the Solvent Effect on the Catalyst Behavior Using NMR Relaxation. Org. Lett. 2020, 22, 4927–4931. [Google Scholar] [CrossRef]
- Ragno, D.; Brandolese, A.; Urbani, D.; Di Carmine, G.; De Risi, C.; Bortolini, O.; Giovannini, P.P.; Massi, A. Esterification of glycerol and solketal by oxidative NHC-catalysis under heterogeneous batch and flow conditions. React. Chem. Eng. 2018, 3, 816–825. [Google Scholar] [CrossRef]
- Belleflamme, M.; Hommes, J.; Dervisoglu, R.; Bartalucci, E.; Wiegand, T.; Beine, A.K.; Leitner, W.; Vorholt, A.J. Catalytic Upgrading of Acetaldehyde to Acetoin Using a Supported N-Heterocyclic Carbene Catalyst. ChemSusChem 2024, 17, e202400647. [Google Scholar] [CrossRef]
- Gu, L.; Lu, T.; Li, X.; Zhang, Y. A highly efficient thiazolylidene catalyzed acetoin formation: Reaction, tolerance and catalyst recycling. Chem. Commun. 2014, 50, 12308–12310. [Google Scholar] [CrossRef]
- Lu, T.; Li, X.; Gu, L.; Zhang, Y. Vitamin B1-Catalyzed Acetoin Formation from Acetaldehyde: A Key Step for Upgrading Bioethanol to Bulk C4 Chemicals. ChemSusChem 2014, 7, 2423–2426. [Google Scholar] [CrossRef]
- Awad, W.H.; Gilman, J.W.; Nyden, M.; Harris, R.H.; Sutto, T.E.; Callahan, J.; Trulove, P.C.; Delong, H.C.; Fox, D. Thermal Degradation Studies of Alkyl-Imidazolium Salts and Their Application in Nanocomposites. Thermochim. Acta 2004, 409, 3–11. [Google Scholar] [CrossRef]
- Ohtani, H.; Ishimura, S.; Kumai, M. Thermal Decomposition Behaviors of Imidazolium-type Ionic Liquids Studied by Pyrolysis-Gas Chromatography. Anal. Sci. 2008, 24, 1335–1340. [Google Scholar] [CrossRef]
- Deruer, E.; Duguet, N.; Lemaire, M. Thiazolylidene-Catalyzed Cleavage of Methyl Oleate-Derived α-Hydroxy Ketone to the Corresponding Free Aldehydes. ChemSusChem 2015, 8, 2481–2486. [Google Scholar] [CrossRef]
- Vu, N.D.; Bah, S.; Deruer, E.; Duguet, N.; Lemaire, M. Robust Organocatalysts for the Cleavage of Vegetable Oil Derivatives to Aldehydes through Retrobenzoin Condensation. Chem. Eur. J. 2018, 24, 8141–8150. [Google Scholar] [CrossRef]
- Chanda, T.; Zhao, J.C.-G. Recent Progress in Organocatalytic Asymmetric Domino Transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- Hubert, C.B.; Barry, S.M. New chemistry from natural product biosynthesis. Biochem. Soc. Trans. 2016, 44, 738–744. [Google Scholar] [CrossRef]
- Grossmann, A.; Enders, D. N-heterocyclic carbene catalyzed domino reactions. Angew. Chem. 2012, 124, 320–332, Erratum in: Angew. Chem. Int. Ed. 2012, 51, 314–325. [Google Scholar] [CrossRef]
- Wang, L.; Ni, Q.; Blümel, M.; Shu, T.; Raabe, G.; Enders, D. NHC-Catalyzed Asymmetric Synthesis of Functionalized Succinimides from Enals and α-Ketoamides. Chem. Eur. J. 2015, 21, 8033–8037. [Google Scholar] [CrossRef]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. The Catalytic, Enantioselective Michael Reaction. Synthesis 2017, 49, 451–471. [Google Scholar] [CrossRef]
- Chen, S.; Hao, L.; Zhang, Y.; Tiwari, B.; Chi, Y.R. Asymmetric Access to the Smallest Enolate Intermediate via Organocatalytic Activation of Acetic Ester. Org. Lett. 2013, 15, 5822–5825. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, H.; Grossmann, A.; Loh, C.C.J.; Merkens, C.; Enders, D. Organocatalytic Domino Oxa-Michael/1,6-Addition Reactions: Asymmetric Synthesis of Chromans Bearing Oxindole Scaffolds. Angew. Chem. 2016, 128, 12283–12287. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Li, F.; Wu, J.; Wang, J. Chemoselective N-Heterocyclic Carbene-Catalyzed Cascade of Enals with Nitroalkenes. Org. Lett. 2015, 17, 3588–3591. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Qami, A.; Kibongui-Fila, A.; Berteina-Raboin, S. Use of N-Heterocyclic Carbene Compounds (NHCs) Under Sustainable Conditions—An Update. Inorganics 2025, 13, 330. https://doi.org/10.3390/inorganics13100330
El Qami A, Kibongui-Fila A, Berteina-Raboin S. Use of N-Heterocyclic Carbene Compounds (NHCs) Under Sustainable Conditions—An Update. Inorganics. 2025; 13(10):330. https://doi.org/10.3390/inorganics13100330
Chicago/Turabian StyleEl Qami, Abdelkarim, Adrien Kibongui-Fila, and Sabine Berteina-Raboin. 2025. "Use of N-Heterocyclic Carbene Compounds (NHCs) Under Sustainable Conditions—An Update" Inorganics 13, no. 10: 330. https://doi.org/10.3390/inorganics13100330
APA StyleEl Qami, A., Kibongui-Fila, A., & Berteina-Raboin, S. (2025). Use of N-Heterocyclic Carbene Compounds (NHCs) Under Sustainable Conditions—An Update. Inorganics, 13(10), 330. https://doi.org/10.3390/inorganics13100330






