Temperature-Dependent Degradation of Volatile Organic Compounds Using Ga2O3 Photocatalyst
Abstract
1. Introduction
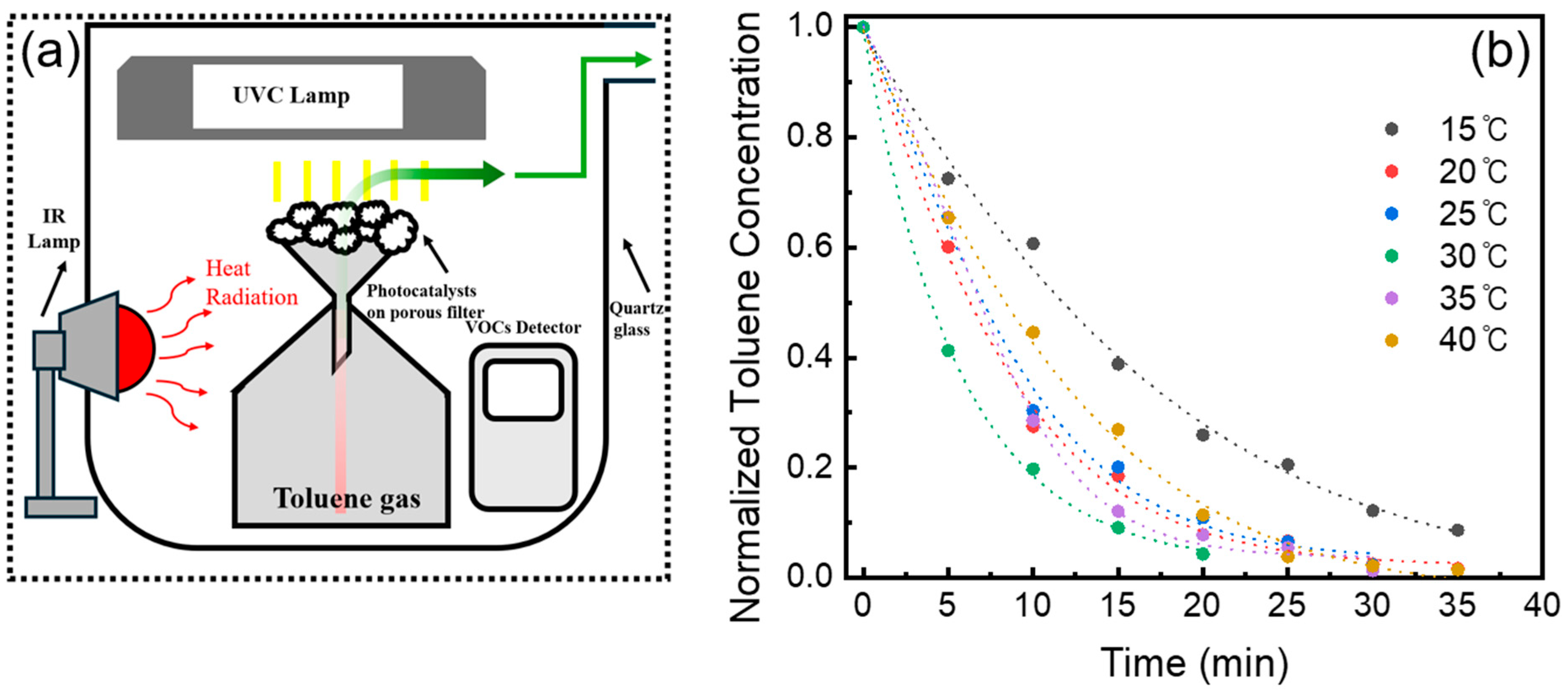
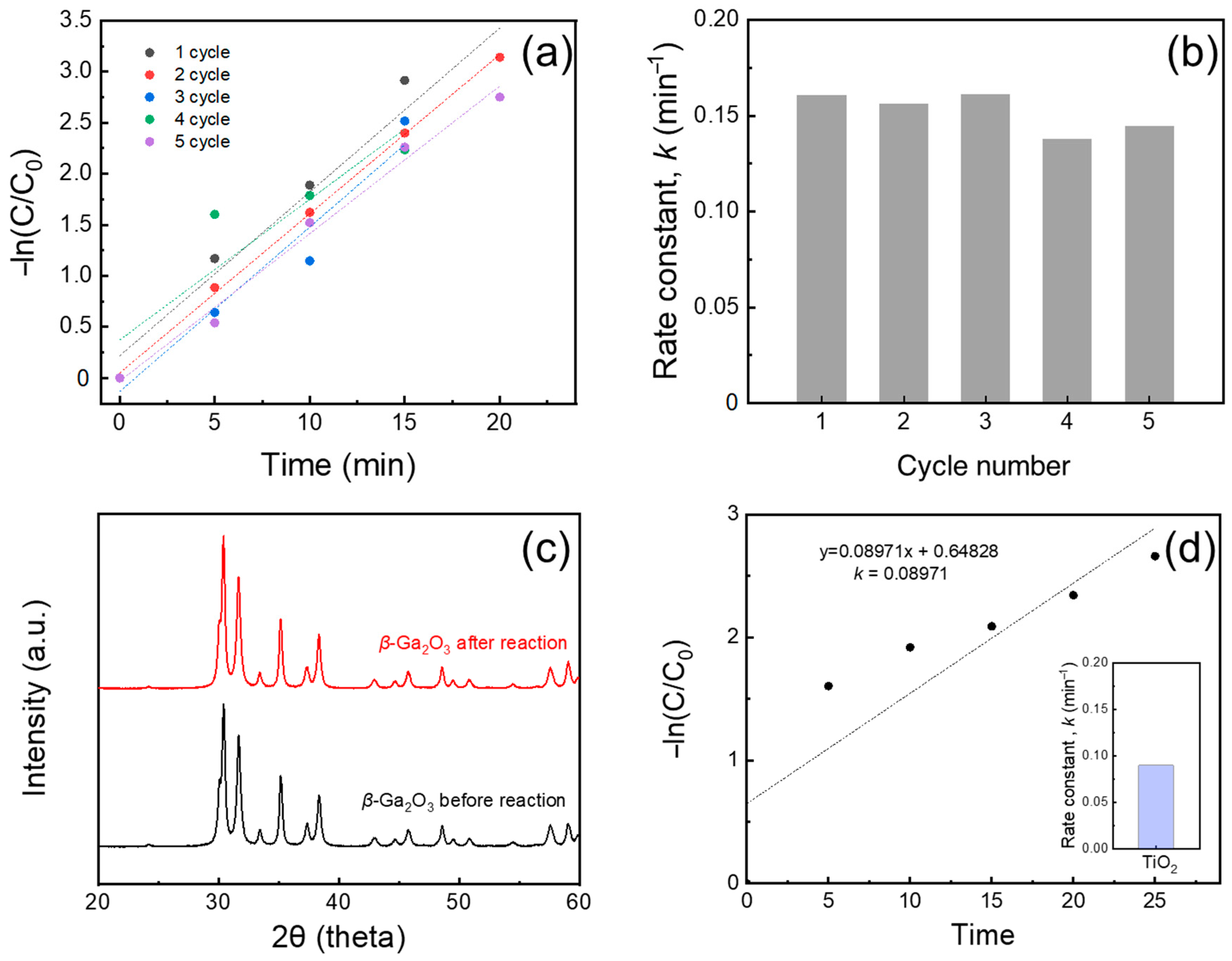
2. Results and Discussion
3. Materials and Methods
3.1. Materials Characterization
3.2. Photocatalytic Reactor Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef]
- Cao, L.; Men, Q.; Zhang, Z.; Yue, H.; Cui, S.; Huang, X.; Zhang, Y.; Wang, J.; Chen, M.; Li, H. Significance of Volatile Organic Compounds to Secondary Pollution Formation and Health Risks Observed during a Summer Campaign in an Industrial Urban Area. Toxics 2024, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-J.; Li, X.-Y.; Shen, Y.-C.; You, J.-X.; Wen, M.-Z.; Wang, J.-B.; Yang, X.-T. Assessing Volatile Organic Compounds Exposure and Chronic Obstructive Pulmonary Diseases in US Adults. Front. Public Health 2023, 11, 1210136. [Google Scholar] [CrossRef]
- Kim, S.; Park, E.; Song, S.-H.; Lee, C.-W.; Kwon, J.-T.; Park, E.Y.; Kim, B. Toluene concentrations in the blood and risk of thyroid cancer among residents living near national industrial complexes in South Korea: A population-based cohort study. Environ. Int. 2021, 146, 106304. [Google Scholar] [CrossRef]
- Saeedi, M.; Malekmohammadi, B.; Tajalli, S. Interaction of benzene, toluene, ethylbenzene, and xylene with human’s body: Insights into characteristics, sources and health risks. J. Hazard. Mater. Adv. 2024, 16, 100459. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef] [PubMed]
- Stranger, M.; Potgieter-Vermaak, S.S.; Van Grieken, R. Comparative overview of indoor air quality in Antwerp, Belgium. Environ. Int. 2007, 33, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of Indoor Volatile Organic Compounds via Photocatalytic Oxidation: A Short Review and Prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Wei, G.; Li, R.; Wang, N.; Yang, X.; Li, Z.; Zhang, Y.; Peng, Y. High visible light responsive ZnIn2S4/TiO2-x induced by oxygen defects to boost photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 622, 156839. [Google Scholar] [CrossRef]
- Cappelletti, G.; Pifferi, V.; Mostoni, S.; Falciola, L.; Di Bari, C.; Spadavecchia, F.; Meroni, D.; Davoli, E.; Ardizzone, S. Hazardous o-Toluidine Mineralization by Photocatalytic Bismuth-Doped ZnO Slurries. Chem. Commun. 2015, 51, 10459–10462. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–28. [Google Scholar] [CrossRef]
- Li, X.; Zhong, F.; Li, P.; Xiao, J.; Xi, J. Transition Metal Modified Al2O3 Mesoporous Nanospheres for Catalysis of Organic Reactions. Appl. Surf. Sci. 2024, 653, 159355. [Google Scholar] [CrossRef]
- Velinova, R.; Kaneva, N.; Ivanov, G.; Kovacheva, D.; Spassova, I.; Todorova, S.; Atanasova, G.; Naydenov, A. Synthesis and Characterization of Pd/La2O3/ZnO Catalyst for Complete Oxidation of Methane, Propane and Butane. Inorganics 2025, 13, 17. [Google Scholar] [CrossRef]
- Rajendran, S.; Palani, G.; Shanmugam, V.; Trilaksanna, H.; Kannan, K.; Nykiel, M.; Korniejenko, K.; Marimuthu, U. A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption. Molecules 2023, 28, 7922. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolobov, N.S.; Pershin, A.A.; Kozlov, D.V. TiO2-Mediated Photocatalytic Oxidation of Volatile Organic Compounds: Formation of CO as a Harmful By-Product. Appl. Catal. B Environ. 2017, 200, 503–513. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Zhang, C.; He, H. Effect of TiO2 Calcination Temperature on the Photocatalytic Oxidation of Gaseous NH3. J. Environ. Sci. 2014, 26, 673–682. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase—A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Warren, Z.; Wenk, J.; Mattia, D. Increased photocorrosion resistance of ZnO foams via transition metal doping. RSC Adv. 2023, 13, 2438–2450. [Google Scholar] [CrossRef]
- Bae, H.J.; Yoo, T.H.; Kim, S.; Choi, W.; Song, Y.S.; Kwon, D.-K.; Cho, B.J.; Hwang, W.S. Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide under Ultraviolet-C Irradiation. Catalysts 2019, 9, 449. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Ding, Z.; Fu, X. Efficient Decomposition of Benzene over a β-Ga2O3 Photocatalyst under Ambient Conditions. Environ. Sci. Technol. 2006, 40, 5799–5803. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Enhanced photocatalytic performance of novel self-assembled floral β-Ga2O3 nanorods. Curr. Appl. Phys. 2013, 13, 652–658. [Google Scholar] [CrossRef]
- Orozco, S.; Rivero, M.; Montiel, E.; Espino Valencia, J. Gallium Oxides Photocatalysts Doped With Fe Ions for Discoloration of Rhodamine Under UV and Visible Light. Front. Environ. Sci. 2022, 10, 884758. [Google Scholar] [CrossRef]
- Kim, J.; Ryou, H.; Lee, J.; Kim, S.; Hwang, W.S. Optical and Structural Characterization of Cu-Doped Ga2O3 Nanostructures Synthesized via Hydrothermal Method. Inorganics 2025, 13, 231. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Zbudniewek, K.; Rynkowski, J.; Keller, V.; Grams, J.; Ruppert, A.M.; Keller, N. Wide Band Gap Ga2O3 as Efficient UV-C Photocatalyst for Gas-Phase Degradation Applications. Environ. Sci. Pollut. Res. 2017, 24, 26792–26805. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of β-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 364. [Google Scholar] [CrossRef]
- Kang, B.K.; Lim, G.-H.; Lim, B.; Yoon, D.H. Morphology Controllable Synthesis and Characterization of Gallium Compound Hierarchical Structures via Forced-Hydrolysis Method. J. Alloys Compd. 2016, 675, 57–63. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Akatsuka, M.; Yamamoto, M.; Yoshioka, K.; Ozawa, A.; Kato, Y.; Yoshida, T. Preparation of Gallium Oxide Photocatalysts and Their Silver Loading Effects on the Carbon Dioxide Reduction with Water. J. Photochem. Photobiol. A Chem. 2018, 358, 459–464. [Google Scholar] [CrossRef]
- Park, H.-A.; Choi, J.H.; Choi, K.M.; Lee, D.K.; Kang, J.K. Highly Porous Gallium Oxide with a High CO2 Affinity for the Photocatalytic Conversion of Carbon Dioxide into Methane. J. Mater. Chem. 2012, 22, 5304–5307. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, P.; Sun, X.; Chu, B.; He, Q. Properties and Photocatalytic Activity of β-Ga2O3 Nanorods under Simulated Solar Irradiation. J. Nanomater. 2015, 2015, 191793. [Google Scholar] [CrossRef]
- Mohamed, S.H.; El-Hagary, M.; Althoyaib, S. Growth of β-Ga2O3 Nanowires and Their Photocatalytic and Optical Properties Using Pt as a Catalyst. J. Alloys Compd. 2012, 537, 291–296. [Google Scholar] [CrossRef]
- Li, X.; Zhen, X.; Meng, S.; Xian, J.; Shao, Y.; Fu, X.; Li, D. Structuring β-Ga2O3 Photonic Crystal Photocatalyst for Efficient Degradation of Organic Pollutants. Environ. Sci. Technol. 2013, 47, 9911–9917. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, P. Photocatalytic Decomposition of Perfluorooctanoic Acid with β-Ga2O3 Wide Bandgap Photocatalyst. Catal. Commun. 2009, 10, 1184–1187. [Google Scholar] [CrossRef]
- Tien, L.-C.; Chen, W.-T.; Ho, C.-H. Enhanced Photocatalytic Activity in β-Ga2O3 Nanobelts. J. Am. Ceram. Soc. 2011, 94, 3117–3122. [Google Scholar] [CrossRef]
- Ghodsi, V.; Jin, S.; Byers, J.C.; Pan, Y.; Radovanovic, P.V. Anomalous Photocatalytic Activity of Nanocrystalline γ-Phase Ga2O3 Enabled by Long-Lived Defect Trap States. J. Phys. Chem. C 2017, 121, 9433–9441. [Google Scholar] [CrossRef]
- Roberts, G.W.; Satterfield, C.N. Effectiveness Factor for Porous Catalysts. Langmuir–Hinshelwood Kinetic Expressions. Ind. Eng. Chem. Fundam. 1965, 4, 288–293. [Google Scholar] [CrossRef]
- Doucet, N.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Kinetics of Photocatalytic VOCs Abatement in a Standardized Reactor. Chemosphere 2006, 65, 1188–1196. [Google Scholar] [CrossRef]
- Blake, N.R.; Griffin, G.L. Selectivity Control during the Photoassisted Oxidation of 1-Butanol on Titanium Dioxide. J. Phys. Chem. 1988, 92, 5697–5701. [Google Scholar] [CrossRef]
- Wu, J.-F.; Hung, C.-H.; Yuan, C.-S. Kinetic Modeling of Promotion and Inhibition of Temperature on Photocatalytic Degradation of Benzene Vapor. J. Photochem. Photobiol. A Chem. 2005, 170, 299–306. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.; Kwak, J.; Cha, H.; Ryou, H.; Kim, S.; Hwang, W.S.; Kim, H. Temperature-Dependent Degradation of Volatile Organic Compounds Using Ga2O3 Photocatalyst. Inorganics 2025, 13, 326. https://doi.org/10.3390/inorganics13100326
Hong D, Kwak J, Cha H, Ryou H, Kim S, Hwang WS, Kim H. Temperature-Dependent Degradation of Volatile Organic Compounds Using Ga2O3 Photocatalyst. Inorganics. 2025; 13(10):326. https://doi.org/10.3390/inorganics13100326
Chicago/Turabian StyleHong, Dayoun, Jiwon Kwak, Hyeongju Cha, Heejoong Ryou, Sunjae Kim, Wan Sik Hwang, and Hyunah Kim. 2025. "Temperature-Dependent Degradation of Volatile Organic Compounds Using Ga2O3 Photocatalyst" Inorganics 13, no. 10: 326. https://doi.org/10.3390/inorganics13100326
APA StyleHong, D., Kwak, J., Cha, H., Ryou, H., Kim, S., Hwang, W. S., & Kim, H. (2025). Temperature-Dependent Degradation of Volatile Organic Compounds Using Ga2O3 Photocatalyst. Inorganics, 13(10), 326. https://doi.org/10.3390/inorganics13100326







