Recent Advances in Carbon Dots-Based Photocatalysts for Water Treatment Applications
Abstract
1. Introduction
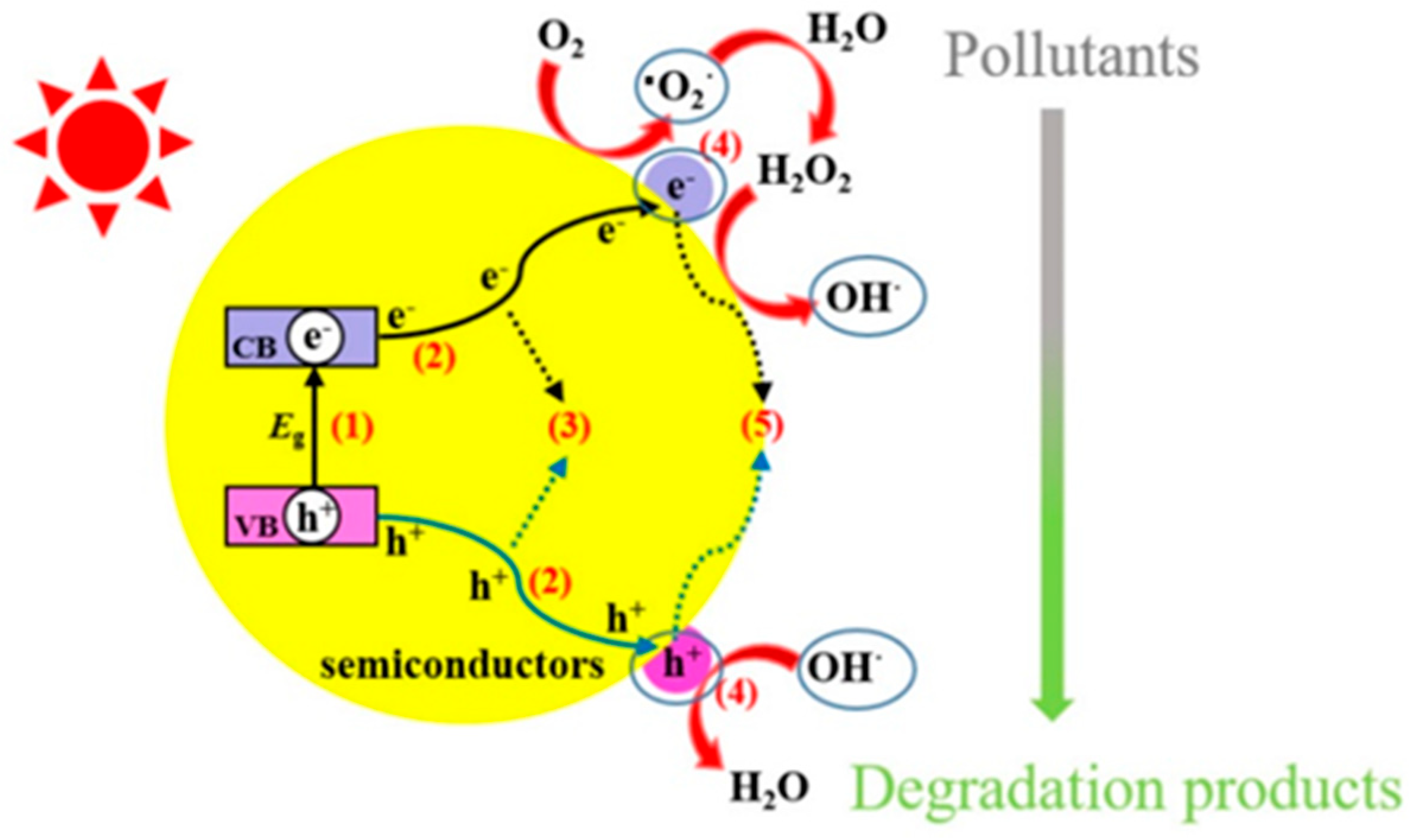
1.1. The Role of Photocatalysis in Water Treatment
1.2. Limitations of Inorganic Photocatalysts
2. The Combination of Inorganic Photocatalysts with Carbon Dots (CDs)
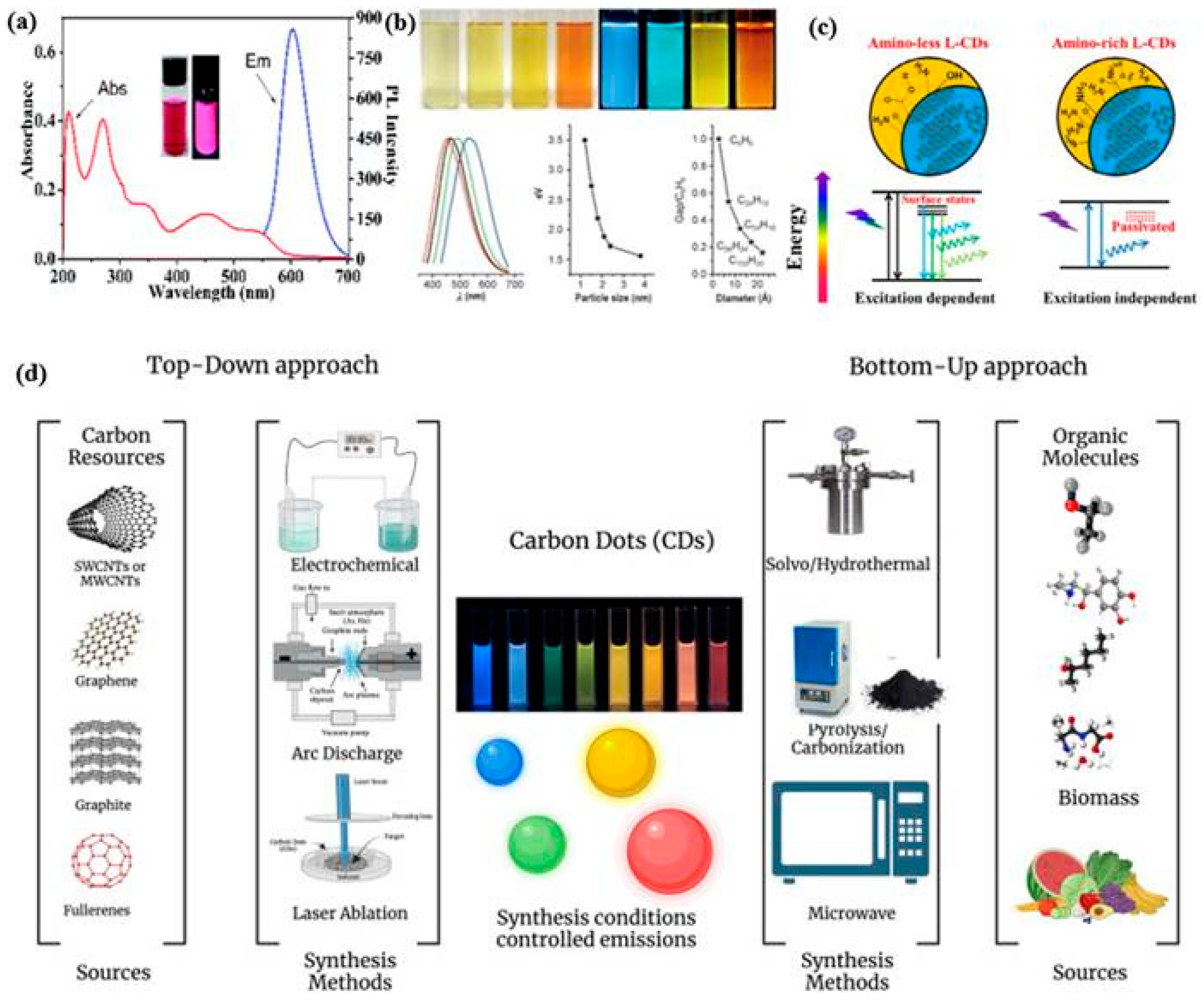
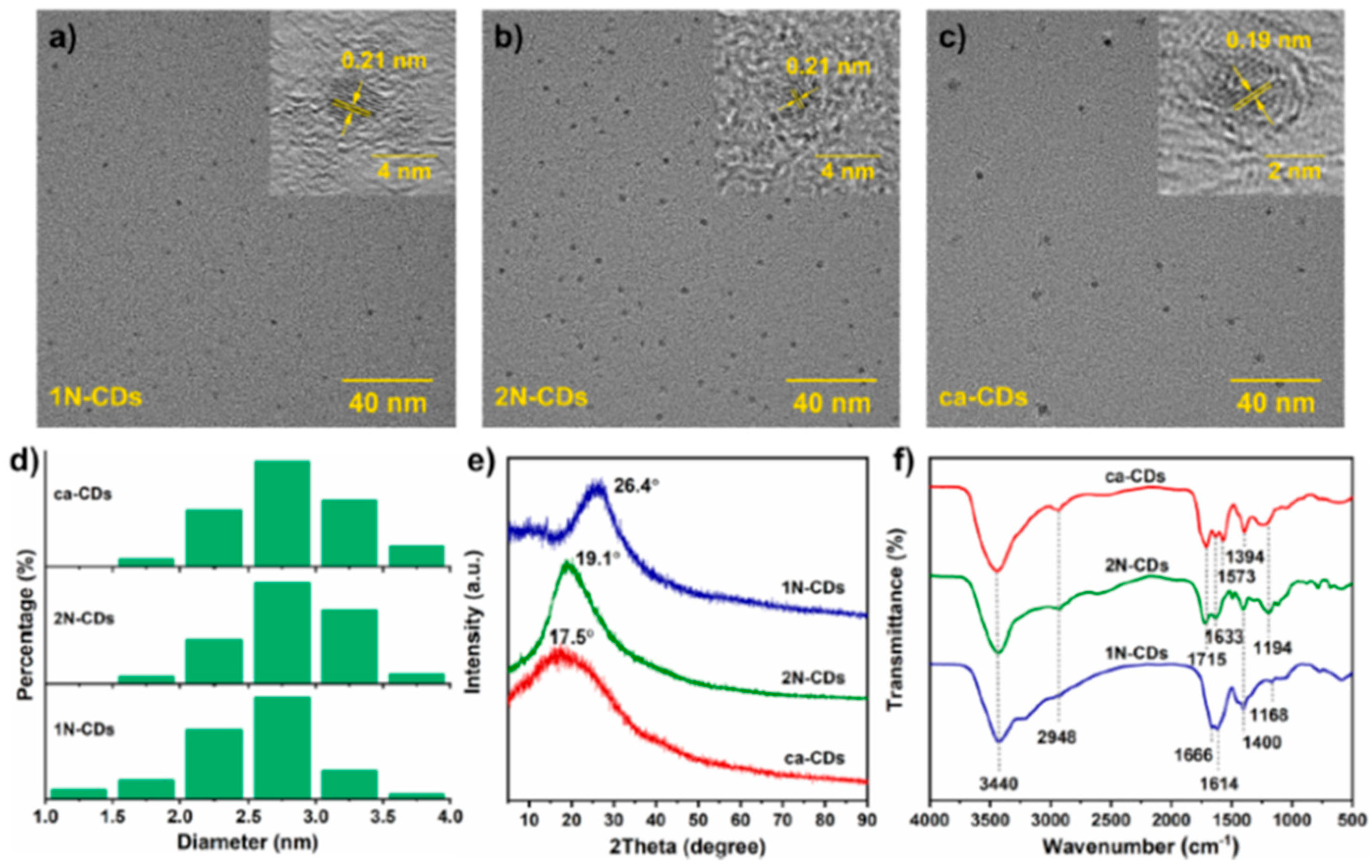
2.1. CDs (Structure, Properties, Synthesis)
2.2. In Situ and Ex Situ Synthesis of CDs-Based Heterostructures
2.3. CDs–Metal Oxide Heterostructures
2.4. CDs–Metal Chalcogenide Heterostructures
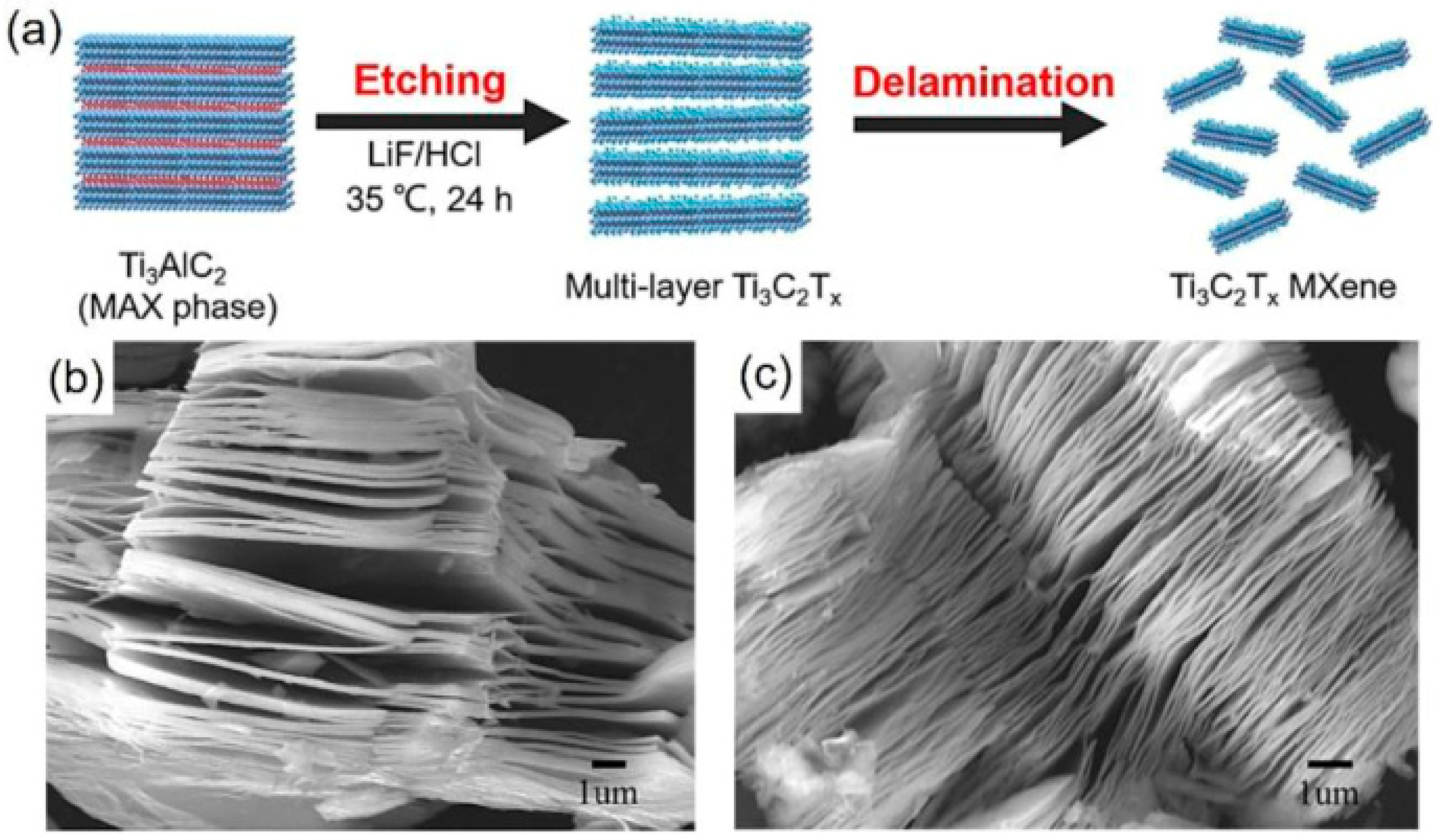
2.5. CDs–Mxene Heterostructures
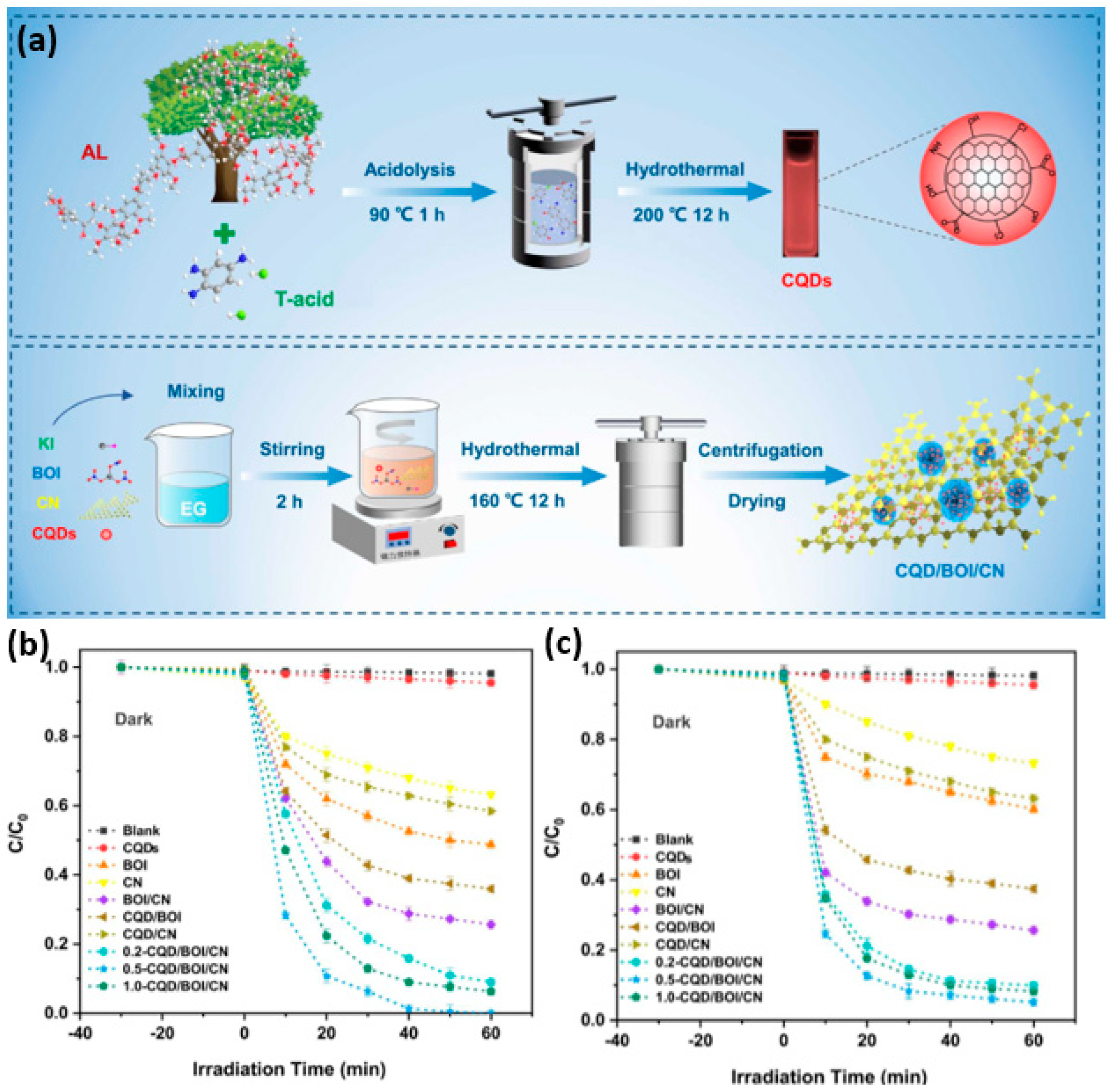
2.6. CDs–Metal Oxyhalide Heterostructures
2.7. CDs–Metal-Free Heterostructures
2.8. CDs-Based Ternary Heterostructures
| CD-Based Ternary Heterostructures | Water Pollutant | Light Source | Photocatalytic Degradation | References |
|---|---|---|---|---|
| N-doped GQDs–TiO2-Graphene Oxide | Methylene Blue (10 ppm) Crystal Violet (10 ppm) Basic Red 46 (10 ppm) | 300 W Xenon lamp >420 nm | 96.60% after 150 min 82.40% after 150 min >99.99% after 150 min (50 mg/100 mL photocatalyst) | [170] |
| GQDs–ZnO–NiO | Methylene Blue (30 ppm) Methyl Orange (30 ppm) | 1000 W Halogen lamp UV and Vis | 93.42% after 90 min 75.68% after 90 min (100 mg photocatalyst) | [171] |
| CQDs–CdS–Ta3N5 | Levofloxacin (15 ppm) | 300 W Xenon lamp >420 nm | 91.90% after 60 min (25 mg/100 mL photocatalyst) | [172] |
| CDs–CdS–g–C3N4 | Tetracycline (20 ppm) | 98.00% after 45 min (40 mg/50 mL photocatalyst) | [173] | |
| CDs–WO3–g–C3N4 | Malachite Green (20 ppm) | Vis | 96.30% after 80 min (50 mg/100 mL photocatalyst) | [174] |
| N-doped CDs–Co3O4–MoS2 | RhB (30 ppm) | * | 97.75% within 5 min (200 mg/L photocatalyst) | [175] |
| CQDs–Ag–MoS2 | Tartrazine (20 ppm) | Vis | >99.9% after 30 min (300 mg/L photocatalyst) | [176] |
| CQDs–BiFeO3–BiOBr | Imidacloprid (10 ppm) | 500 W Xenon lamp >400 nm | 95.7% after 180 min | [177] |
| CQDs–BiOBr–g–C3N4 | Tetracycline (10 ppm) | 500 W Xenon lamp Vis | >99.9% after 60 min (40 mg/40 mL photocatalyst) | [178] |
| CQDs–BiOBr-Ti3C2 | Moxifloxacin (10 ppm) | 500 W Xenon lamp >420 nm | 96.10% after 120 min (25 mg/50 mL photocatalyst) | [179] |
| CQDs–BiOBr–W18O49 | Tetracycline (20 ppm) | 300 W Xenon lamp | 97.30% after 45 min (20 mg/50 mL photocatalyst) | [180] |
| CQDs–Bi2MoO6–CuS | 300 W Xenon lamp >420 nm | 96.98% after 60 min (20 mg/100 mL photocatalyst) | [181] | |
| N-doped GQDs–TiO2–g–C3N4 | Ciprofloxacin (10 ppm) | >420 nm | 89.60% after 150 min (50 mg/100 mL photocatalyst) | [182] |
| F-doped CDs–TiO2–g–C3N4 | RhB (3 ppm) | 500 W Xenon lamp Vis | 74.00% after 50 min (10 mg/100 mL photocatalyst) | [183] |
| P-doped GQD–TiO2–AgI | Methyl Orange (10 ppm) | 300 W Xenon lamp >420 nm | 78.20% after 60 min (100 mg/100 mL photocatalyst) | [184] |
| N-doped CD–CuFe2O4–g–C3N4 | Tetracycline Hydrochloride (10 ppm) | 300 W Xenon lamp | 85.69% after 60 min (certain mass of photocatalyst in 100 mL of water pollutant) | [185] |
2.9. From Conventional Photocatalysts to Novel Heterostructures: The Added Value of CDs
3. Outlook
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMX | amoxicillin |
| BPA | bisphenol A |
| CB | conduction band |
| CDs | carbon dots |
| CHC | chrysin hydrochloride |
| CNDs | carbon nanodots |
| CPDs | carbon polymer dots |
| CPS | cationic polystyrene |
| CPX | ciprofloxacin |
| CQDs | carbon quantum dots |
| GQDs | graphene quantum dots |
| g–C3N4 | graphitic nitride |
| IUPAC | International Union of Pure and Applied Chemistry |
| EIS | electrochemical impedance spectroscopy |
| ESR | electron spin resonance |
| EST | estradiol |
| FT-IR | Fourier transform infrared spectroscopy |
| LEV | levofloxacin |
| MB | methylene blue |
| MG | malachite green |
| MO | methyl orange |
| MRSA | methicillin-resistant staphylococcus aureus |
| NFT | nitrofurantoin |
| NPs | nanoparticles |
| NTs | nanotubes |
| OFX | ofloxacin |
| OVs | oxygen vacancies |
| PL | photoluminescence |
| PANI | polyaniline |
| PMS | peroxymonosulfate |
| PNP | p-nitrophenol |
| RR141 | reactive red azo dye |
| Redox | reduction/oxidation |
| RhB | rhodamine B |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| TC | tetracycline |
| TCH | tetracycline hydrochloride |
| UV | ultraviolet |
| VB | valence band |
| Vis | visible |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic Heavy Metal Ions Contamination in Water and Their Sustainable Reduction by Eco-Friendly Methods: Isotherms, Thermodynamics and Kinetics Study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mathew, B.T.; Aljasmi, A.N.; Alhammadi, H.S.; Salem, H.M.; Abd El-Mageed, T.A.; et al. Ecological Impacts and Management Strategies of Pesticide Pollution on Aquatic Life and Human Beings. Mar. Pollut. Bull. 2024, 206, 116613. [Google Scholar] [CrossRef]
- Wada, O.Z.; Olawade, D.B. Recent Occurrence of Pharmaceuticals in Freshwater, Emerging Treatment Technologies, and Future Considerations: A Review. Chemosphere 2025, 374, 144153. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.-H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in Water Systems: A Review of Their Impacts on the Environment and Their Potential Hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sundaram, B. A Review of the Photocatalysis Process Used for Wastewater Treatment. Mater. Today Proc. 2024, 102, 393–409. [Google Scholar] [CrossRef]
- Li, B.; Qi, B.; Guo, Z.; Wang, D.; Jiao, T. Recent Developments in the Application of Membrane Separation Technology and Its Challenges in Oil-Water Separation: A Review. Chemosphere 2023, 327, 138528. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.-E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and Challenges in Adsorption-Based Wastewater Remediation: A Comprehensive Review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, Y.; Fang, H.; Jin, C.; Mo, Y.; Xiao, Z.; Zhang, N.; Sun, L.; Lu, H. A New Understanding on the Prerequisite of Antibiotic Biodegradation in Wastewater Treatment: Adhesive Behavior between Antibiotic-Degrading Bacteria and Ciprofloxacin. Water Res. 2024, 252, 121226. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, F.; Mohammad Amani, A. Photocatalytic Systems: Reactions, Mechanism, and Applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaki, R.S.R.; Jusoh, R.; Chanakaewsomboon, I.; Setiabudi, H.D. Recent Advances in Metal Oxide Photocatalysts for Photocatalytic Degradation of Organic Pollutants: A Review on Photocatalysts Modification Strategies. Mater. Today Proc. 2024, 107, 59–67. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, S.A.C.; Fan, J.; Lv, K. Research Progress in Metal Sulfides for Photocatalysis: From Activity to Stability. Chemosphere 2022, 303, 135085. [Google Scholar] [CrossRef]
- Adabala, S.; Dutta, D.P. A Review on Recent Advances in Metal Chalcogenide-Based Photocatalysts for CO2 Reduction. J. Environ. Chem. Eng. 2022, 10, 107763. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Hoang, T.K.A.; Soto-Moscoso, M. Halides and Oxyhalides-Based Photocatalysts for Abatement of Organic Water Contaminants—An Overview. Environ. Res. 2022, 212, 113149. [Google Scholar] [CrossRef]
- Li, N.; Huo, J.; Zhang, Y.; Ye, B.; Chen, X.; Li, X.; Xu, S.; He, J.; Chen, X.; Tang, Y.; et al. Transition Metal Carbides/Nitrides (MXenes): Properties, Synthesis, Functional Modification and Photocatalytic Application. Sep. Purif. Technol. 2024, 330, 125325. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic Carbon Nitride (g-C3N4) as an Emerging Photocatalyst for Sustainable Environmental Applications: A Comprehensive Review. RSC Sustain. 2024, 2, 265–287. [Google Scholar] [CrossRef]
- Noureen, L.; Wang, Q.; Humayun, M.; Shah, W.A.; Xu, Q.; Wang, X. Recent Advances in Structural Engineering of Photocatalysts for Environmental Remediation. Environ. Res. 2023, 219, 115084. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Żebrowska, J.; Klimczuk, T.; Lisowski, W.; Żak, A.M.; Skowron, P.M.; Zaleska-Medynska, A. Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts. Nanomaterials 2024, 14, 409. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent Advances in Photocatalytic Self-Cleaning Performances of TiO2-Based Building Materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [CrossRef]
- Abhishek, B.; Jayarama, A.; Rao, A.S.; Nagarkar, S.S.; Dutta, A.; Duttagupta, S.P.; Prabhu, S.S.; Pinto, R. Challenges in Photocatalytic Hydrogen Evolution: Importance of Photocatalysts and Photocatalytic Reactors. Int. J. Hydrogen Energy 2024, 81, 1442–1466. [Google Scholar] [CrossRef]
- Kumagai, H.; Tamaki, Y.; Ishitani, O. Photocatalytic Systems for CO2 Reduction: Metal-Complex Photocatalysts and Their Hybrids with Photofunctional Solid Materials. Acc. Chem. Res. 2022, 55, 978–990. [Google Scholar] [CrossRef]
- Ansari, A.S.; Azzahra, G.; Nugroho, F.G.; Mujtaba, M.M.; Ahmed, A.T.A. Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal. Catalysts 2025, 15, 134. [Google Scholar] [CrossRef]
- Akbari, M.; Rasouli, J.; Rasouli, K.; Ghaedi, S.; Mohammadi, M.; Rajabi, H.; Sabbaghi, S. MXene-Based Composite Photocatalysts for Efficient Degradation of Antibiotics in Wastewater. Sci. Rep. 2024, 14, 31498. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, I.; El-Shamy, A.M. A Comparative Study for Optimizing Photocatalytic Activity of TiO2-Based Composites with ZrO2, ZnO, Ta2O5, SnO, Fe2O3, and CuO Additives. Sci. Rep. 2024, 14, 27175. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.; Siddique, M.; Panchal, S. A Review of Visible-Light-Active Zinc Oxide Photocatalysts for Environmental Application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- He, X.; Wang, A.; Wu, P.; Tang, S.; Zhang, Y.; Li, L.; Ding, P. Photocatalytic Degradation of Microcystin-LR by Modified TiO2 Photocatalysis: A Review. Sci. Total Environ. 2020, 743, 140694. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Gao, X.; Cao, X.; Wu, S.; Long, X.; Ma, Q.; Su, J. A Review of CdS Photocatalytic Nanomaterials: Morphology, Synthesis Methods, and Applications. Mater. Sci. Semicond. Process. 2024, 176, 108288. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, X.; Yu, H.-Q. Bismuth Oxyhalide Photocatalysts for Water Purification: Progress and Challenges. Coord. Chem. Rev. 2023, 493, 215339. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving Oxidation Stability of 2D MXenes: Synthesis, Storage Media, and Conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]
- Keneshbekova, A.; Smagulova, G.; Kaidar, B.; Imash, A.; Ilyanov, A.; Kazhdanbekov, R.; Yensep, E.; Lesbayev, A. MXene/Carbon Nanocomposites for Water Treatment. Membranes 2024, 14, 184. [Google Scholar] [CrossRef]
- Asrami, M.R.; Jourshabani, M.; Park, M.H.; Shin, D.; Lee, B. A Unique and Well-Designed 2D Graphitic Carbon Nitride with Sponge-like Architecture for Enhanced Visible-Light Photocatalytic Activity. J. Mater. Sci. Technol. 2023, 159, 99–111. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of Doping and Additive Applications on Photocatalyst Textural Properties in Removing Organic Pollutants: A Review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Ji, T.; Guan, P.; Weng, Y. Carbon Quantum Dots Modified MoS2 for High-Efficiency and Long-Endurance Persulfate Activation: Enhanced Electron Transfer and Piezoelectricity. Sep. Purif. Technol. 2025, 353, 128148. [Google Scholar] [CrossRef]
- Tran, V.V.; Nu, T.T.V.; Jung, H.-R.; Chang, M. Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers 2021, 13, 3031. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, R.; Jaiswal, R.K.; Singh, A.K.; Kumar, P.; Singh, K. Surface Modification of CdS Quantum Dots: An Effective Approach for Improving Biocompatibility. Mater. Res. Express 2019, 6, 055002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Xiao, Q.; Shi, L.; Zhao, Z.; Wang, H. Enhancement of CdS Resistance to Photocorrosion and Photocatalytic Removal of Uranyl by Complexation with N-Deficient g-C3N4 under Aerobic Conditions. Chemosphere 2023, 335, 139022. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, X.; Shen, L.; Zhao, D.L.; Kong, N.; Li, Y.; Qiu, X.; Chen, C.; Teng, J.; Xu, Y.; et al. Exceptional Self-Cleaning MXene-Based Membrane for Highly Efficient Oil/Water Separation. J. Membr. Sci. 2024, 700, 122691. [Google Scholar] [CrossRef]
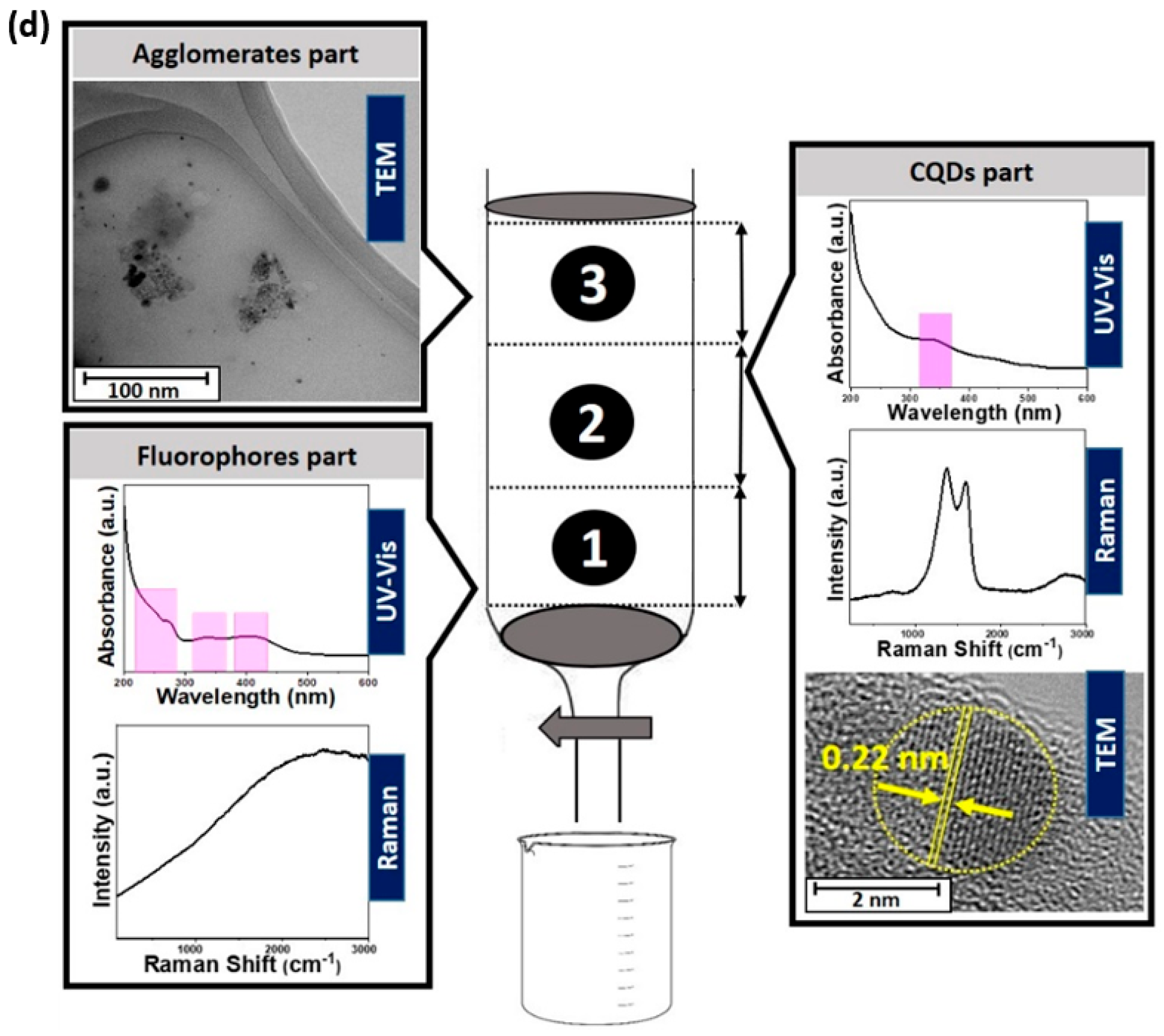
- Falara, P.P.; Zourou, A.; Kordatos, K.V. Recent Advances in Carbon Dots/2-D Hybrid Materials. Carbon 2022, 195, 219–245. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, B.; Shi, R.; Zhang, S.; Liu, Y.; Wang, B.; Zhang, K.; Waterhouse, G.I.N.; Zhang, T.; Lu, S. Carbon Dots as New Building Blocks for Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2022, 12, 2103426. [Google Scholar] [CrossRef]
- Gengan, S.; Ananda Murthy, H.C.; Sillanpää, M.; Nhat, T. Carbon Dots and Their Application as Photocatalyst in Dye Degradation Studies—Mini Review. Results Chem. 2022, 4, 100674. [Google Scholar] [CrossRef]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into Photoluminescence Mechanisms of Carbon Dots: Advances and Perspectives. Sci. Bull. 2021, 66, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Panniello, A.; Madonia, A.; Carbonaro, C.M.; Mocci, F.; Sibillano, T.; Giannini, C.; Comparelli, R.; Ingrosso, C.; Depalo, N.; et al. Photostable Carbon Dots with Intense Green Emission in an Open Reactor Synthesis. Carbon 2022, 198, 230–243. [Google Scholar] [CrossRef]
- Soledad-Flores, O.; Bailón-Ruiz, S.J.; Román-Velázquez, F. Rapid Synthesis of Non-Toxic, Water-Stable Carbon Dots Using Microwave Irradiation. Micro 2024, 4, 659–669. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; da Silva Abreu, F.O.M. Carbon Quantum Dots Synthesis from Waste and By-Products: Perspectives and Challenges. Mater. Lett. 2021, 282, 128764. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef] [PubMed]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef] [PubMed]
- Zourou, A.; Ntziouni, A.; Roman, T.; Tampaxis, C.; Steriotis, T.; Gkouzia, G.; Alff, L.; Sanchez, D.E.; Terrones, M.; Kordatos, K.V. Synthesis and Characterization of Magnetic Carbon Dots (CDs)-Based Hybrid Material as an Adsorbent for the Removal of Organic Dye from Water. Carbon 2024, 230, 119612. [Google Scholar] [CrossRef]
- Wang, C.; Yang, M.; Shi, H.; Yao, Z.; Liu, E.; Hu, X.; Guo, P.; Xue, W.; Fan, J. Carbon Quantum Dots Prepared by Pyrolysis: Investigation of the Luminescence Mechanism and Application as Fluorescent Probes. Dyes Pigments 2022, 204, 110431. [Google Scholar] [CrossRef]
- Nazar, M.; Hasan, M.; Wirjosentono, B.; Gani, B.A.; Nada, C.E. Microwave Synthesis of Carbon Quantum Dots from Arabica Coffee Ground for Fluorescence Detection of Fe3+, Pb2+, and Cr3+. ACS Omega 2024, 9, 20571–20581. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Wei, C.; Qu, Y.; Xiao, X.; Cheng, H. One-Step Synthesis of Red-Emitting Carbon Dots via a Solvothermal Method and Its Application in the Detection of Methylene Blue. RSC Adv. 2019, 9, 29533–29540. [Google Scholar] [CrossRef]
- Kaur, I.; Batra, V.; Bogireddy, N.K.R.; Baveja, J.; Kumar, Y.; Agarwal, V. Chemical- and Green-Precursor-Derived Carbon Dots for Photocatalytic Degradation of Dyes. iScience 2024, 27, 108920. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Pournemati, K.; Ahmasdi, Z.; Khatase, A. Decoration of Carbon Dots on Oxygen-Vacancy-Enriched S-Scheme TiO2 Quantum Dots/TiO2Oxygen Vacancies Photocatalysts: Impressive Quantum-Dot Sized Photocatalysts for Remediation of Antibiotics, Bacteria, and Dyes. Langmuir 2024, 40, 8503–8519. [Google Scholar] [CrossRef]
- Camilli, E.; Foglia, M.L.; Bravo, J.P.; Copello, G.J.; Villanueva, M.E. Comparison among OH, N and P functionalized carbon quantum dots/TiO2 nanocomposites for food industry wastewater remediation. Diam. Relat. Mater. 2024, 145, 111103. [Google Scholar] [CrossRef]
- Sendao, R.M.S.; Algarra, M.; Lazaro-Martinez, J.; Brandao, A.T.S.C.; Gil, A.; Pereira, C.; Esteves de Silva, J.C.G.; Pinto da Silva, L. Visible-light-driven photocatalytic degradation of organic dyes using a TiO2 and waste-based carbon dots nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136475. [Google Scholar] [CrossRef]
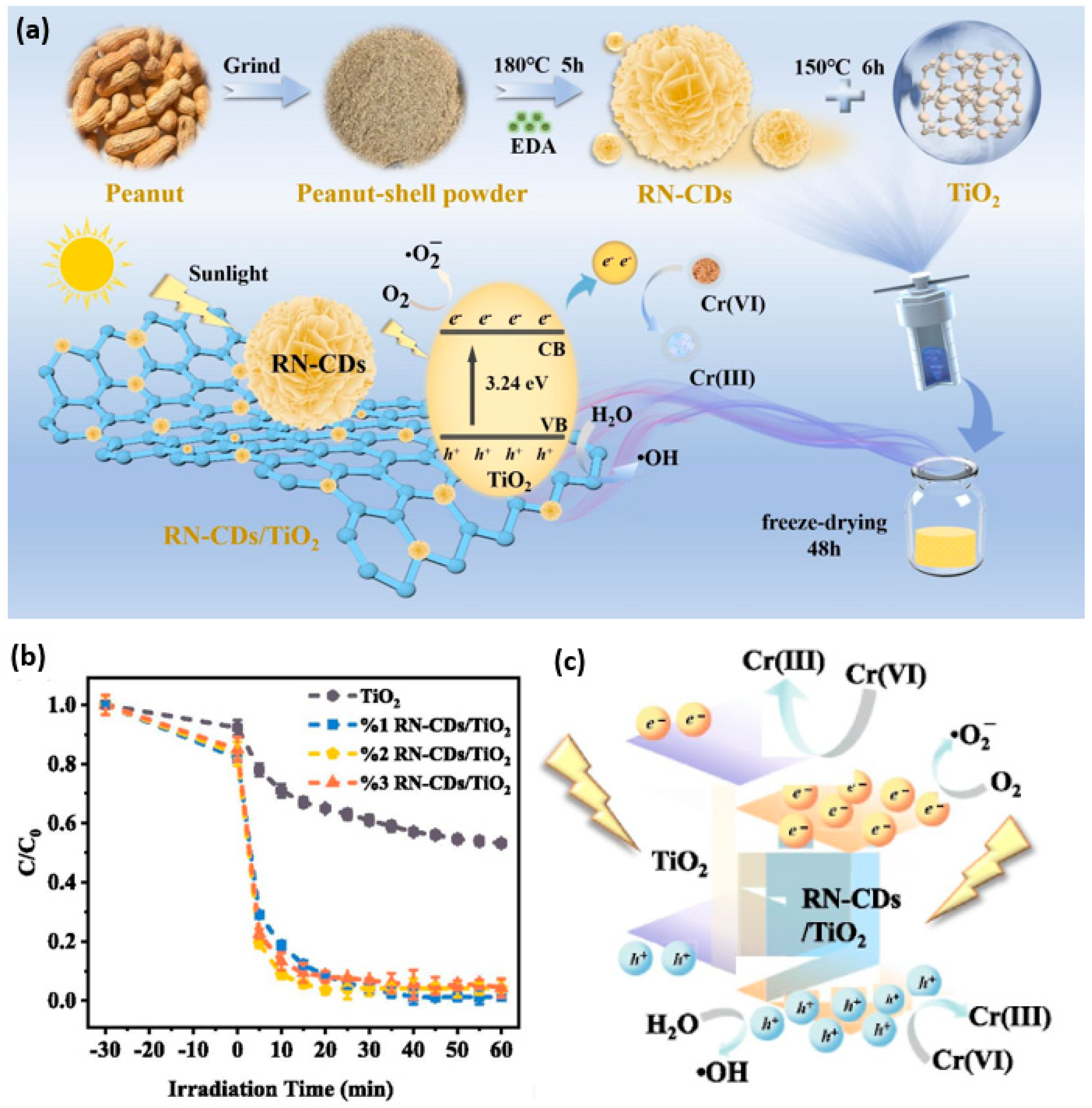
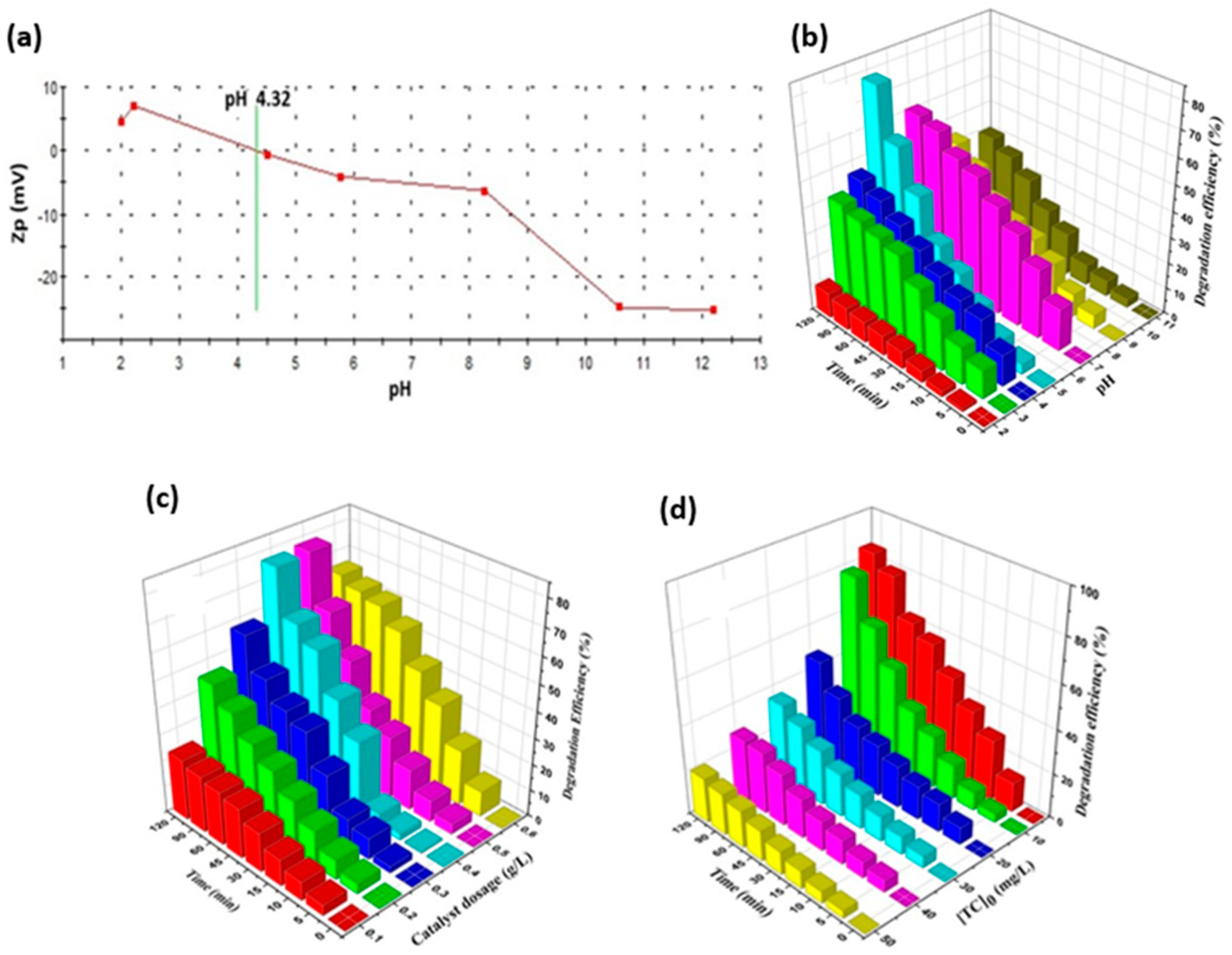
- Ma, R.; Xu, Z.; Zhang, C.; Li, H.; Chen, J.; Fan, J.; Shi, Q. Highly efficient reduction of Cr(VI) from industries sewage using novel biomass-driven carbon dots modified TiO2 under sunlight. Chem. Eng. J. 2024, 500, 157480. [Google Scholar] [CrossRef]
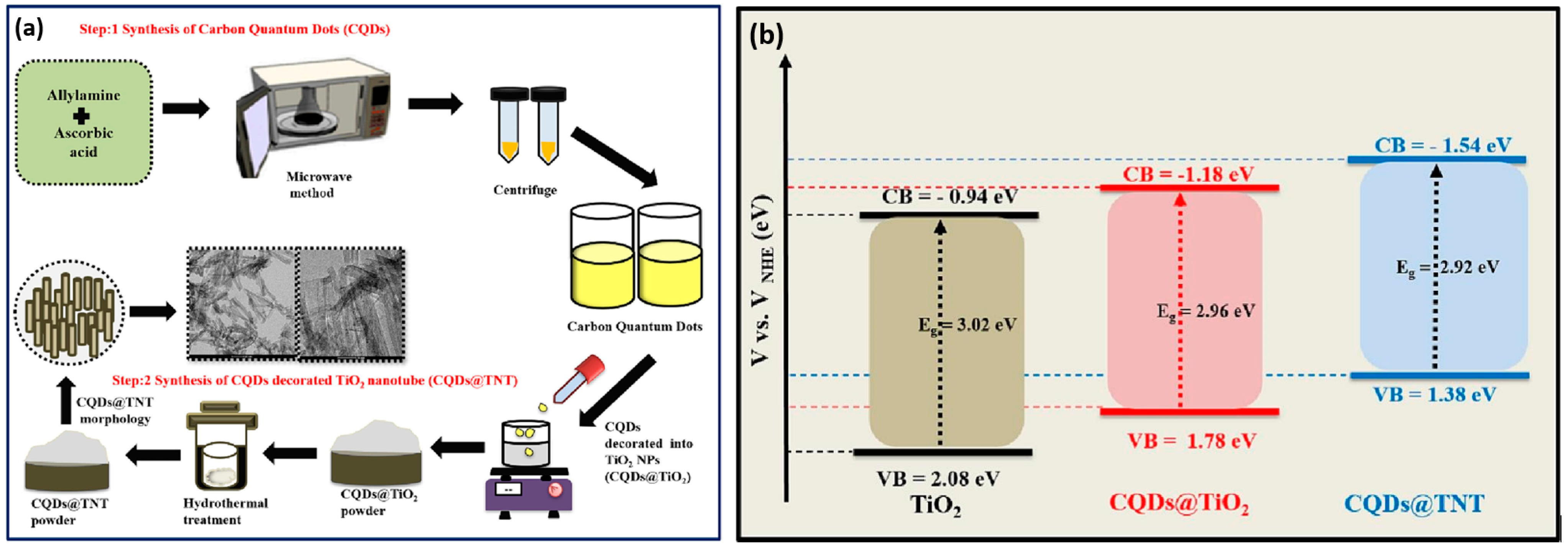
- Rawat, J.; Sharma, H.; Dwivedi, C. Microwave-assisted synthesis of carbon quantum dots and their integration with TiO2 nanotubes for enhanced photocatalytic degradation. Diam. Relat. Mater. 2024, 144, 111050. [Google Scholar] [CrossRef]
- Bui, B.-C.; Vu, N.-N.; Nemamchs, H.-E.; Nguyen, H.T.; Nguyen, V.-A.; Nguyen-Tri, P. Single nickel atoms doped into TiO2 decorating carbon quantum dots for boosting photodegradation of ciprofloxacin. J. Water Process Eng. 2025, 70, 106904. [Google Scholar] [CrossRef]
- Taghiloo, B.; Shahnazi, A.; Nabid, M.R. Construction of nanocomposite hydrogel by TiO2-carbon quantum dots encapsulated in alginate with a highly efficient adsorption and photodegradation of dye pollutants. J. Alloys Compd. 2024, 1005, 175859. [Google Scholar] [CrossRef]
- Ayu, D.G.; Gea, S.; Andriayani; Telaumbanua, D.J.; Piliang, A.F.R.; Harahap, M.; Yen, Z.; Goei, R.; Tok, A.I.Y. Photocatalytic Degradation of Methylene Blue Using NDoped ZnO/Carbon Dot (N-ZnO/CD) Nanocomposites Derived from Organic Soybean. ACS Omega 2023, 8, 14965–14984. [Google Scholar] [CrossRef]
- Hidayat, N.; Widiyandari, H.; Parasdila, H.; Prilita, O.; Astuti, Y.; Mufti, N.; Ogi, T. Green synthesis of ZnO photocatalyst composited carbon quantum dots (CQDs) from lime (Citrus aurantifolia). Catal. Commun. 2024, 187, 106888. [Google Scholar] [CrossRef]
- Parveen, S.; Latif, N.; Chotana, G.A.; Kanwal, A.; Hussain, S.; Habila, M.A.; Iqbal, A.; Manavalan, R.K.; Farooq, N. ZnO/carbon quantum dots nanocomposites derived from Moringa oleifera gum: An improved catalytic vitiation of methylene blue dye. Mater. Res. Bull. 2025, 181, 113106. [Google Scholar] [CrossRef]
- Kalifathullah, S.K.; Sundaramurthy, D. Exploration of biological activities of green NCarbon Quantum Dots and photocatalytic studies of ZnO@NCQDs. Emergent Mater. 2024, 7, 2755–2766. [Google Scholar] [CrossRef]
- Xu, J.-J.; Lu, Y.-N.; Tao, F.-F.; Liang, P.-F.; Zhang, P.-A. ZnO Nanoparticles Modified by Carbon Quantum Dots for the Photocatalytic Removal of Synthetic Pigment Pollutants. ACS Omega 2023, 8, 7845–7857. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, F.S.; Mapari, M.G.; Tivalekar, S.R.; Naz, E.G.; Babar, D.G. A Nanocomposite of ZnO and N,P-Co-Doped Carbon Dotsfor the Photocatalytic Degradation of Various Dyes and their Kinetic Study. ChemistrySelect 2023, 8, e202301821. [Google Scholar] [CrossRef]
- Karaca, C.; Eroğlu, Z.; Karaca, S. Anthraquinone-Rich Rheum ribes L. as a Source of Nitrogen-Doped Carbon Quantum Dots for ZnO-Based S-Scheme Heterojunction Photocatalysts in Tetracycline Degradation. J. Environ. Chem. Eng. 2025, 13, 115999. [Google Scholar] [CrossRef]
- Nugroho, D.; Wannakan, K.; Nanan, S.; Benchawattananon, R. The Synthesis of carbon dots//zincoxide (CDs/ZnOH400) by using hydrothermal methods for degradation of ofloxacin antibiotics and reactive red azo dye (RR141). Sci. Rep. 2024, 14, 2455. [Google Scholar] [CrossRef]
- Nugroho, D.; Khoris, I.M.; Yoskamtorn, T.; Nanan, S.; Lee, J.; Benchawattananon, R. Hybrid nanostructure carbon dots/zinc oxide from Rosa indica for photodegradation of various pharmaceuticals pollutants. J. Water Process Eng. 2025, 70, 106828. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, W.; Wang, J.; Ren, F.; Chen, Z.; Sun, Z. Construction of biomass derived carbon quantum dots modified TiO2 photocatalysts with superior photocatalytic activity for methylene blue degradation. J. Alloys Compd. 2023, 932, 167627. [Google Scholar] [CrossRef]
- Prabhakaran, P.K.; Balu, S.; Sridharan, G.; Ganapathy, D.; Sundramoorthy, A.K. Development of Eco-friendly CQDs/TiO2 nanocomposite for enhanced photocatalytic degradation of methyl orange dye. Eng. Res. Express 2025, 7, 015002. [Google Scholar] [CrossRef]
- Hsieh, M.-L.; Juang, R.-S.; Gansomi, Y.A.; Fu, C.-c.; Hsieh, C.-t.; Liu, W.-R. Synthesis and characterization of high performance ZnO/graphene quantum dot composites for photocatalytic degradation of metronidazole. J. Taiwan Inst. Chem. Eng. 2022, 131, 104180. [Google Scholar] [CrossRef]
- Preethi, G.; Pillai, R.; Ramdas, B.; Ramamoorthy, S.; Patil, B.; Lekshmi, I.C.; Mohan Kumar, P.; Rangaraj, L. Role of Carbon Quantum Dots in a Strategic Approach to Prepare Pristine Zn2SnO4 and Enhance Photocatalytic Activity under Direct Sunlight. Diam. Relat. Mater. 2023, 131, 109554. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Q.; Zhao, L.; Fang, J.; Qi, J.; Fan, H.; Yue, X.; Li, G.; Qian, Y.; Li, H. Wood-Supported Nitrogen-Doped Carbon Quantum Dot @Cu2O Composites for Efficient Photocatalytic Degradation of Dye Wastewater. Cellulose 2024, 31, 7587–7600. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Ma, P.; Guo, Z.; Ma, Q.; Zhao, Q.; Guo, Y.; Zhao, J.; Guan, G. Carbon Quantum Dots/ Cu2O S-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline. Colloids Surf. Physicochem. Eng. Asp. 2024, 690, 133779. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Zang, J. Bi2MoO6 Nanoflower-like Microsphere Photocatalyst Modified by Boron Doped Carbon Quantum Dots: Improving the Photocatalytic Degradation Performance of BPA in All Directions. J. Alloys Compd. 2023, 962, 171167. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Wang, Q.; Dai, K. Graphene Quantum Dot Modified Bi2MoO6 Nanoflower for Efficient Degradation of BPA under Visible Light. Chin. J. Struct. Chem. 2024, 43, 100473. [Google Scholar] [CrossRef]
- Liu, J.; Ji, L.; He, Q.; Zang, S.; Sun, J.; Yang, H.; Dong, T.; Liu, T.; Wu, H.; Chen, X.; et al. Algal Carbon Quantum Dots/Bi2MoO6 S-Scheme Heterojunction with Enhanced Visible-Light Photocatalytic Degradation for Ciprofloxacin. Sep. Purif. Technol. 2025, 363, 132196. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Tian, M.; Wang, W.; Yu, J.; Chen, L. Efficient Combination of Carbon Quantum Dots and BiVO4 for Significantly Enhanced Photocatalytic Activities. Catalysts 2023, 13, 463. [Google Scholar] [CrossRef]
- Jiteshwaran, T.; Steffy, J.P.; Janani, B.; Syed, A.; Elgorban, A.M.; Abid, I.; Wong, L.S.; Khan, S.S. In Situ Growth of Carbon Quantum Dots on Acid/Base 3D Co2VO4 Nanoplates to Regulate Photocatalysis and Peroxymonosulfate Activation towards Highly Efficient Degradation of Ciprofloxacin. J. Water Process Eng. 2025, 71, 107336. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, G.; Yang, Z.; Guo, Q.; Zhang, B.; Nie, Y.; Wang, D. Preparation and Study of CDs-WO3 Composites with Enhanced Photocatalytic Antimicrobial Properties and Degradation of Dyes. Biochem. Eng. J. 2025, 217, 109670. [Google Scholar] [CrossRef]
- Aloni, P.; Venkatesan, P.; Sundaresan, A.P.; Roy, D.; Ranjan, R.K.; Sharma, A.; Doong, R.-A.; Clament Sagaya Selvam, N. Unveiling the Impact of Nitrogen-Doped Graphene Quantum Dots on Improving the Photocatalytic Performance of CuWO4 Nanocomposite. Appl. Surf. Sci. 2025, 686, 162130. [Google Scholar] [CrossRef]
- Sarwar, A.; Razzaq, A.; Zafar, M.; Idrees, I.; Rehman, F.; Kim, W.Y. Copper Tungstate (CuWO4)/Graphene Quantum Dots (GQDs) Composite Photocatalyst for Enhanced Degradation of Phenol under Visible Light Irradiation. Results Phys. 2023, 45, 106253. [Google Scholar] [CrossRef]
- Khan, A.; Valicsek, Z.; Horváth, O.; Khan, M.M.; Wafi, A. Ferrite-Based Photocatalysts: Synthesis, Modifications, and Key Parameters in Photocatalytic-Related Applications. Mater. Today Commun. 2024, 40, 109556. [Google Scholar] [CrossRef]
- Esmail, L.A.; Jabbar, H.S.; Salih, S.K. Synthesis of a New Carbon Dot Magnetic Nanocomposite (CDs@Fe3O4) from Crocus Cancellatus: Characterization and Its Photocatalytic Degradation of Fluorescein Dye. Inorg. Chem. Commun. 2024, 159, 111823. [Google Scholar] [CrossRef]
- Joga, S.B.; Korabandi, D.; Lakkaboyana, S.K.; Kumar, V. Synthesis of Iron Nanoparticles on Lemon Peel Carbon Dots (LP-CDs@Fe3O4) Applied in Photo-Catalysis, Antioxidant, Antidiabetic, and Hemolytic Activity. Inorg. Chem. Commun. 2025, 174, 113960. [Google Scholar] [CrossRef]
- Sari, E.K.; Tumbelaka, R.M.; Ardiyanti, H.; Istiqomah, N.I.; Chotimah; Suharyadi, E. Green Synthesis of Magnetically Separable and Reusable Fe3O4/Cdots Nanocomposites Photocatalyst Utilizing Moringa oleifera Extract and Watermelon Peel for Rapid Dye Degradation. Carbon Resour. Convers. 2023, 6, 274–286. [Google Scholar] [CrossRef]
- Monje, D.S.; Mercado, D.F.; Mesa, G.A.P.; Valencia, G.C. Carbon Dots Decorated Magnetite Nanocomposite Obtained Using Yerba Mate Useful for Remediation of Textile Wastewater through a Photo-Fenton Treatment: Ilex Paraguariensis as a Platform of Environmental Interest-Part 2. Environ. Sci. Pollut. Res. Int. 2023, 30, 3070–3087. [Google Scholar] [CrossRef]
- Akhter, T.; Aslam, M. Carbon Dot-Modified Ferrite (CDs@ZF): Efficient Visible-Light Photocatalyst for Dye Degradation and Antibacterial Activity. Inorg. Chem. Commun. 2025, 173, 113777. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Ammar-Merah, S.; Nkambule, T.T.I.; Kefeni, K.K.; Kuvarega, A.T. Synergistic Role of N-Doped Carbon Quantum Dots on Zn-Doped Cobalt Ferrite (N-CQDs/ZnCF) for the Enhanced Photodegradation of Oxytetracycline under Visible Light. Mater. Sci. Eng. B 2023, 294, 116538. [Google Scholar] [CrossRef]
- Anh, V.C.N.; Nhi, L.T.T.; Dung, L.T.K.; Hoa, D.T.N.; Son, N.T.; Uyen, N.T.T.; Thu, N.N.U.; Son, L.V.T.; Hieu, L.T.; Tuyen, T.N.; et al. Photocatalytic Degradation of Methylene Blue under Visible Light by Cobalt Ferrite Nanoparticles/Graphene Quantum Dots. Beilstein J. Nanotechnol. 2024, 15, 475–489. [Google Scholar] [CrossRef]
- Renu; Nidhi; Kaur, P.; Komal; Minakshi; Paulik, C.; Kaushik, A.; Singhal, S. Rational Design of Boerhavia diffusa Derived CoFe2O4-Carbon dots@Boehmite Platform for Photocatalysis and Ultra Trace Monitoring of Hazardous Pesticide and UO22+ Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 325, 125111. [Google Scholar] [CrossRef] [PubMed]
- Mmelesi, O.K.; Ammar-Merah, S.; Nkambule, T.T.I.; Nkosi, B.; Liu, X.; Kefeni, K.K.; Kuvarega, A.T. The Photodegradation of Naproxen in an Aqueous Solution Employing a Cobalt Ferrite-Carbon Quantum Dots (CF/N-CQDs) Nanocomposite, Synthesized via Microwave Approach. J. Water Process Eng. 2024, 59, 104968. [Google Scholar] [CrossRef]
- Malitha, M.D.; Molla, M.T.H.; Bashar, M.A.; Chandra, D.; Ahsan, M.S. Fabrication of a Reusable Carbon Quantum Dots (CQDs) Modified Nanocomposite with Enhanced Visible Light Photocatalytic Activity. Sci. Rep. 2024, 14, 17976. [Google Scholar] [CrossRef]
- Naghash-Hamed, S.; Arsalani, N.; Mousavi, S.B. Facile Fabrication of CuFe2O4 Coated with Carbon Quantum Dots Nanocomposite as an Efficient Heterogeneous Catalyst toward the Reduction of Nitroaniline Compounds for Management of Aquatic Resources. J. Photochem. Photobiol. Chem. 2023, 443, 114822. [Google Scholar] [CrossRef]
- Jamal, F.; Rafique, A.; Moeen, S.; Haider, J.; Nabgan, W.; Haider, A.; Imran, M.; Nazir, G.; Alhassan, M.; Ikram, M.; et al. Review of Metal Sulfide Nanostructures and Their Applications. ACS Appl. Nano Mater. 2023, 6, 7077–7106. [Google Scholar] [CrossRef]
- Choudhary, M.; Saini, P.; Chakinala, N.; Surolia, P.K.; Gupta Chakinala, A. Carbon Dots Decorated Cadmium Sulfide Nanomaterials for Boosting Photocatalytic Activity for Ciprofloxacin Degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 319, 124572. [Google Scholar] [CrossRef]
- Orak, C.; Oğuz, T.; Horoz, S. Facile Synthesis of Mn-Doped CdS Nanoparticles on Carbon Quantum Dots: Towards Efficient Photocatalysis. J. Aust. Ceram. Soc. 2024, 60, 1657–1667. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, L.; Wang, D.; Wang, X.; Ji, C.; Zhou, Y.-H. CuS@Cu-CD Composites as Efficient Heterogeneous Fenton-like Catalysts for the Photodegradation of Tetracycline. Environ. Sci. Adv. 2023, 2, 495–507. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Cui, M.; Huang, R.; Ma, W.; Wang, Y.; Su, R.; Qi, W. Synergetic Assembly of a Molybdenum Disulfide/Carbon Quantum Dot Heterojunction with Enhanced Light Absorption and Electron Transfer Di-Functional Properties for Photocatalysis. Mater. Res. Bull. 2024, 171, 112627. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Chen, L.; Li, T.; Gao, F.; Chen, X.; Zhao, T.; Wang, F.; Jiang, Y. A Gradient Photothermal Hydrogel with Carbon-Dots Deposited Molybdenum Disulfide Nanoflowers as Photothermal Centers for Efficient Solar-Driven Water Evaporation and Treatment. Surf. Interfaces 2024, 55, 105433. [Google Scholar] [CrossRef]
- Fatima, T.; Husain, S.; Khanuja, M. Novel Ternary Z Scheme Carbon Quantum Dots (CQDs) Decorated WS2/PANI ((CQDs@WS2/PANI):0D:2D:1D) Nanocomposite for the Photocatalytic Degradation and Electrochemical Detection of Pharmaceutical Drugs. Nano Mater. Sci. 2025, 7, 259–275. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. An Overview of In2S3, In2S3-Based Photocatalyst: Characteristics Synthesis Modifications Design Strategies Catalytic Environmental Application. J. Environ. Chem. Eng. 2024, 12, 113449. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Z.; Cui, H.; Dong, W.; Li, Z.; Liao, G. Graphene Quantum Dots Sensitized In2S3 Nanohybrids for Improved Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2023, 34, 1923. [Google Scholar] [CrossRef]
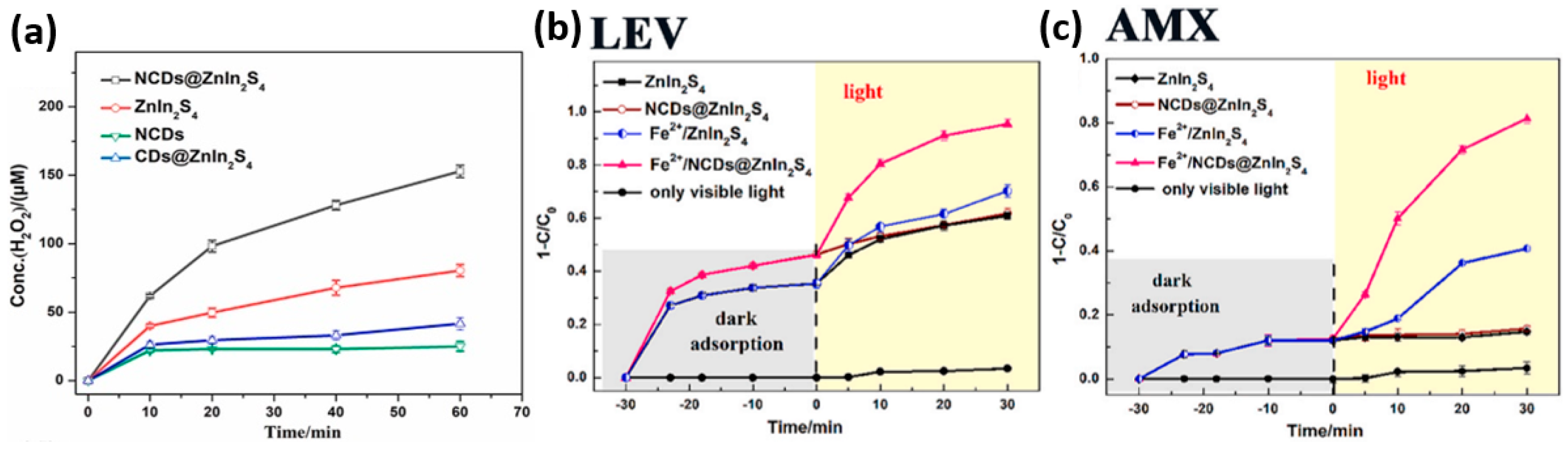
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Mini-Review on ZnIn2S4-Based Photocatalysts for Energy and Environmental Application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Huang, H.; Cheng, J. A Photocatalysis-Self-Fenton System Based on NCDs@ZnIn2S4 Composites at Neutral pH and Low Amount of Fe2+ for the Effective Degradation of Antibiotics. J. Environ. Manag. 2024, 370, 122580. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Bai, W.; Shi, L.; Li, Z.; Liu, D.; Liang, Y.; Han, B.; Qi, J.; Li, Y. Recent progress on the preparation and application in photocatalysis of 2D MXene-based materials. Mater. Today Energy 2024, 41, 101547. [Google Scholar] [CrossRef]
- Seling, T.R.; Songsart-Power, M.; Shringi, A.K.; Paudyal, J.; Yan, F.; Limbu, T.B. Ti3C2Tx MXene-Based Hybrid Photocatalysts in Organic Dye Degradation: A Review. Molecules 2025, 30, 1463. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, K.; Xu, M.; Fang, N.; Yu, W.; Chu, Y.; Shu, S. Photocatalysis for synergistic water remediation H2 production: A review. Chem. Eng. J. 2023, 61, 145066. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zheng, W. Qantum Dots Compete at the Acme of MXene Family for the Optimal Catalysis. Nano-Micro Lett. 2022, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Tawalbeh, M.; Mohammed, S.; Al-Othman, A.; Yusuf, M.; Mofijur, M.; Kamyab, H. MXenes and MXene-based materials for removal of pharmaceutical compounds from wastewater: Critical review. Environ. Res. 2023, 228, 115919. [Google Scholar] [CrossRef]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and Heterointerface Engineering of 2D MXenes and Their Nanocomposites: Insights into Electro- and Photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, J.; Zhao, K.; Cui, D.; Liu, P.; Yin, H.; Al-Mamun, M.; Lowe, S.E.; Zhang, W.; Zhong, Y.L. Ru(bpy)32+-sensitized {001} facets LiCoO2 nanosheets catalyzed CO2 reduction reaction with 100% carbonaceous products. Nano Res. 2022, 15, 1061–1068. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as Co-Catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Guo, T.; Wang, J.J.; Nicolosi, V. Multifunctional Ti3C2Tx MXene composite hydrogels with strain sensitivity toward absorption-dominated electromagnetic-interference shielding. ACS Nano. 2021, 15, 1465–1474. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAXphases MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Miri-Jahromi, A.; Didandeh, M.; Shekarsokhan, S. Capability of MXene 2Dmaterial as an amoxicillin ampicillin cloxacillin adsorbent in wastewater. J. Mol. Liq. 2022, 351, 118545. [Google Scholar] [CrossRef]
- Alyasi, H.; Wahib, S.; Alcantara Gomez, T.; Rasool, K.; Mahmoud, K.A. The power of MXene-based materials for emerging contaminant removal from water—A review. Desalination 2024, 586, 117913. [Google Scholar] [CrossRef]
- Elemike, E.E.; Adeyemi, J.; Onwudiwe, D.C.; Wei, L.; Oyedeji, A.O. The future of energy materials: A case of MXenes-carbon dots nanocomposites. J. Energy Storage 2022, 50, 104711. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Lu, Z.; Chao, Y.; Liu, W. High-performance MnO2@MXene/carbon nanotube fiber electrodes with internal external construction for supercapacitors. J. Mater. Sci 2022, 57, 3613–3628. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Gund, G.S.; Jung, M.G.; Roh, S.H.; Park, J.; Kim, J.K.; Park, H.S. Core-shell structured MXene@carbon nanodots as bifunctional catalysts for solar-assisted water splitting. ACS Nano 2020, 14, 17615–17625. [Google Scholar] [CrossRef]
- Cao, J.-M.; Zatovsky, I.V.; Gu, Z.-Y.; Yang, J.-L.; Zhao, X.-X.; Guo, J.-Z.; Xu, H.; Wu, X.-L. Two-dimensional MXene with multidimensional carbonaceous matrix: A platform for general-purpose functional materials. Prog. Mater. Sci. 2023, 135, 101105. [Google Scholar] [CrossRef]
- Amor, A.B.; Hemmami, H.; Amor, I.B.; Zeghoud, S.; Alhamad, A.A.; Belkacem, M.; Nair, N.S.; Sruthimol, A.B. Advances in carbon quantum dot applications:Catalysis, sensing, and biomedical innovations. Mater. Sci. Semicond. Process. 2025, 185, 108945. [Google Scholar] [CrossRef]
- Chen, H.-R.; Meng, W.-M.; Wang, R.-Y.; Chen, F.-L.; Li, T.; Wang, D.-D.; Wang, F.; Zhu, S.-E.; Wei, C.-X.; Lu, H.-D.; et al. Engineering highly graphitic carbon quantum dots by catalytic dehydrogenation and carbonization of Ti3C2Tx-MXene wrapped polystyrene spheres. Carbon 2022, 190, 319–328. [Google Scholar] [CrossRef]
- Dey, A.; Varagnolo, S.; Power, N.P.; Vangapally, N.; Elias, Y.; Damptey, L.; Jaato, B.N.; Gopalan, S.; Golrokhi, Z.; Sonar, P.; et al. Doped MXenes—A new paradigm in 2D systems: Synthesis, properties and applications. Prog. Mater. Sci. 2023, 139, 101166. [Google Scholar] [CrossRef]
- Hui, J.; Wu, R.; Zhu, Y.; Zhang, Z.; Wei, S.; Ouyang, F. Citric acid-assisted in situ preparation of MoIn2S4/CQDs with few-layer promotes charge transfer and enhances photocatalytic activity. Appl. Surf. Sci. 2024, 667, 160420. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Al-Gheethi, A.A.; Morsin, M.; Tee, K.S. Recent progress and new perspective of MXene-based membranes for water purification: A review. Ceram. Int. 2022, 48, 16477–16491. [Google Scholar] [CrossRef]
- Wei, X.; Akbar, M.U.; Raza, A.; Li, G. A Review on Bismuth Oxyhalide Based Materials for Photocatalysis. Nanoscale Adv. 2021, 3, 3353–3372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Li, X. Carbon Quantum Dots Modified 3D Flower-like BiOCl Nanostructures with Enhanced Visible Light Photocatalytic Degradation of Rhodamine B. Discov. Mater. 2025, 5, 30. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, W.; Hu, Y.; Zhang, F.; Wang, L.; Zhou, D.; Chen, X.; Meng, S. Boosting Visible-Light Photocatalytic Activity of BiOCl Nanosheets via Synergetic Effect of Oxygen Vacancy Engineering and Graphene Quantum Dots-Sensitization. Molecules 2024, 29, 1362. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zhu, H.; Xiang, G. Robust Bi-Anchoring Carbon Dot/BiOCl Sheet Heterojunction Photocatalysts toward Superior Photocatalytic Activity. Nanoscale 2024, 16, 12670–12679. [Google Scholar] [CrossRef]
- Lu, T.; Huang, H.; Lv, G.; Meng, Z.; Zhu, L. Aerogel-Derived Carbon Quantum Dots Modified BiOCl Nanocomposites with Augmented Oxygen Vacancies for Enhanced Photodegradation of Antibiotics. J. Ind. Eng. Chem. 2024, 138, 586–600. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Lei, Q.; Zhong, D.; Ke, Y.; Liu, W.; Yang, L. Significantly Activated Persulfate by Novel Carbon Quantum Dots-Modified N-BiOCl for Complete Degradation of Bisphenol-A under Visible Light Irradiation. Sci. Total Environ. 2023, 870, 161804. [Google Scholar] [CrossRef]
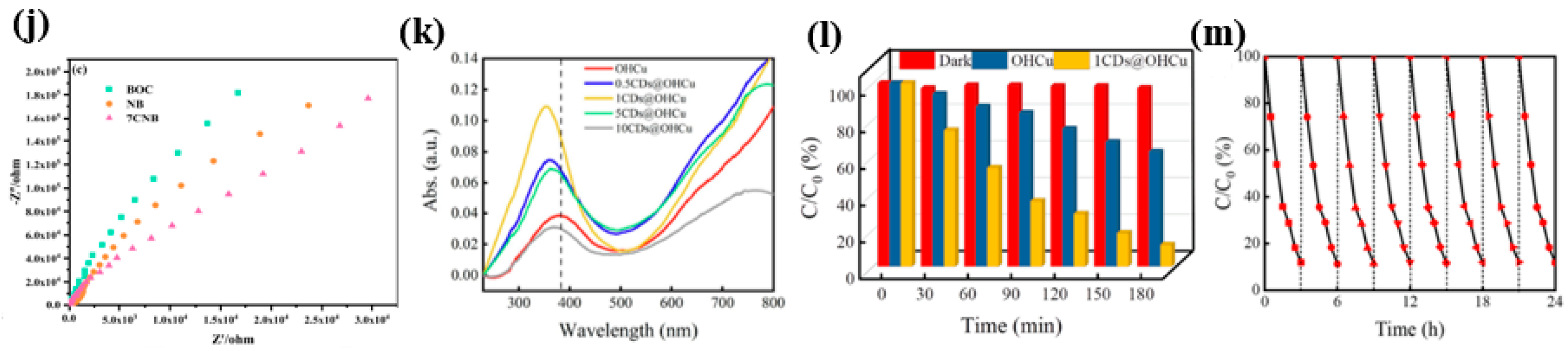
- Cui, M.-J.; Jiang, J.-Z.; Song, Z.-H.; Ren, T.-Z. Enhanced Photocatalytic Degradation of Methylene Blue Using Carbon Dots-Modified Copper Chloride Hydroxide Nanocomposite. Inorg. Chem. Commun. 2023, 158, 111559. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-Free Photocatalysts for Various Applications in Energy Conversion and Environmental Purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Si, Y.; Zhou, B.-X.; Huang, T.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Interfacial charge modulation: Carbon quantum dot implanted carbon nitride double-deck nanoframes for robust visible-light photocatalytic tetracycline degradation. Nanoscale 2020, 12, 3135. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Ya, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B 2017, 207, 103. [Google Scholar] [CrossRef]
- Li, B.; Fang, Q.; Si, Y.; Huang, T.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Ultra-thin tubular graphitic carbon Nitride-Carbon Dot lateral heterostructures: One-Step synthesis highly efficient catalytic hydrogen generation. Chem. Eng. J. 2020, 397, 125470. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Liu, X.; Zeng, H.; Zhang, H.; Crittenden, J. Efficient sulfadiazine degradation via in-situ epitaxial grow of Graphitic Carbon Nitride (g-C3N4) on carbon dots heterostructures under visible light irradiation: Synthesis mechanisms toxicity evaluation. J. Colloid Interface Sci. 2020, 561, 696. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.F.; Wang, D.N.; Dong, L.L.; Shen, H.B.; Lu, W.Y.; Chen, W.X. Graphitic carbon nitride co-modified by zinc phthalocyanine and graphene quantum dots for the efficient photocatalytic degradation of refractory contaminants. Appl. Catal. B Environ. 2019, 244, 96–106. [Google Scholar] [CrossRef]
- Yuan, A.L.; Lei, H.; Xi, F.N.; Liu, J.Y.; Qin, L.S.; Chen, Z.; Dong, X.P. Graphene quantum dots decorated graphitic carbon nitride nanorods for photocatalytic removal of antibiotics. J. Colloid Interface Sci. 2019, 548, 56–65. [Google Scholar] [CrossRef]
- Jourshabani, M.; Long, N.V.D.; Asrami, M.R.; Pho, Q.H.; Lee, B.-K.; Hessel, V. Nitrogen-Doped Carbon Quantum Dot as Electron Acceptor Anchored on Graphitic Carbon Nitride Nanosheet for Improving Rhodamine B Degradation. Mater. Sci. Eng. B 2024, 305, 117417. [Google Scholar] [CrossRef]
- Han, H.; Wang, B.; Tang, Q.; Jia, S.; Liu, J.; Li, H.; Wang, C.; Xu, H.; Hua, Y. Non-Metallic Nitrogen-Doped Graphene Quantum Dots Coupled with g-C3N4 Achieve Efficient Photocatalytic Performance. Appl. Surf. Sci. 2024, 649, 159171. [Google Scholar] [CrossRef]
- Ishak, N.; Galář, P.; Mekkat, R.; Grandcolas, M.; Šoóš, M. Fine-Tuning Photoluminescence and Photocatalysis: Exploring the Effects of Carbon Quantum Dots Synthesis and Purification on g-C3N4. Colloids Surf. Physicochem. Eng. Asp. 2025, 706, 135789. [Google Scholar] [CrossRef]
- Awang, H.; Peppel, T.; Strunk, J. Photocatalytic Degradation of Diclofenac by Nitrogen-Doped Carbon Quantum Dot-Graphitic Carbon Nitride (CNQD). Catalysts 2023, 13, 735. [Google Scholar] [CrossRef]
- Yang, P.; Shen, A.; Zhu, Z.; Wang, L.; Tang, R.; Yang, K.; Chen, M.; Dai, H.; Zhou, X. Construction of carbon nitride-based heterojunction as photocatalyst for peroxymonosulfate activation: Important role of carbon dots in enhancing photocatalytic activity. Chem. Eng. J. 2023, 464, 142724. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Sheng, L.; Wang, Z.; Ye, Y.; Zheng, J.; Fan, M.; Zhang, Y.; Sun, X. Carbon dots cooperatively modulating photocatalytic performance and surface charge of O-doped g-C3N4 for efficient water disinfection. J. Colloid Interface Sci. 2023, 631, 25–34. [Google Scholar] [CrossRef]
- Xu, Z.; Su, X.; Yang, P.; Zhong, J.; Li, M.; Burda, C.; Dou, L. Efficient Photocatalytic CO2 and Cr(VI) Reduction on Carbon Quantum Dots/Carbon Nitride Heterojunctions. Fuel 2025, 381, 133285. [Google Scholar] [CrossRef]
- Cheng, K.; Shao, W.; Li, H.; Guo, W.; Bian, H.; Han, J.; Wu, G.; Xing, W. Biomass Derived Carbon Dots Mediated Exciton Dissociation in Rose Flower-like Carbon Nitride for Boosting Photocatalytic Performance. Ind. Crops Prod. 2023, 192, 116086. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Ding, Y.; Zhang, B.; Zhang, S.; Liu, B. Metal-Free N-GQDs/P-g-C3N4 Photocatalyst with Broad-Spectrum Response: Enhanced Exciton Dissociation and Charge Migration for Promoting H2 Evolution and Tetracycline Degradation. Sep. Purif. Technol. 2023, 304, 122297. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Tan, C.; Fan, H.; Lu, J.; Li, Y.; Hu, H. Novel In-Situ Composites of Chlorine-Doped CQDs and g-C3N4 with Improved Interfacial Connectivity and Accelerated Charge Transfer to Enhance Photocatalytic Degradation of Tetracycline and Hydrogen Evolution. Appl. Surf. Sci. 2023, 638, 158060. [Google Scholar] [CrossRef]
- Aygun, A.; Tiri, R.N.E.; Bayat, R.; Sen, F. Hydrothermal Synthesis of BCQD@g-C3N4 Nanocomposites Supporting Environmental Sustainability: Organic Dye Removal and Bacterial Inactivation. J. Hazard. Mater. Adv. 2024, 16, 100464. [Google Scholar] [CrossRef]
- Sunil, S.; Mandal, B.K. Facile Synthesis of CQD/g-C3N4 as a Highly Effective Metal-Free Photocatalyst for the Degradation of Carmoisine and Indigo Carmine Dye. Inorg. Chem. Commun. 2025, 171, 113545. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xu, Q.; Yuan, P.; Zhang, P.; Zhuo, S.; Zhu, C.; Du, J. Facile Synthesis of Porous Graphitic Carbon Nitride Modulated by Up-Conversion Carbon Quantum Dots for Visible Light-Triggered Photocatalysis towards Bacteria Inactivation. Appl. Catal. Gen. 2024, 673, 119586. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Han, C.; Li, Z.; Lun, X.; Zhang, C. Photocatalysis-PMS Oxidation System Based on CQDs-Doped Carbon Nitride Nanosheets for Degradation of Residual Drugs in Water. Environ. Sci. Pollut. Res. 2023, 30, 108538–108552. [Google Scholar] [CrossRef]
- Chen, R.; Hu, R.; Gao, L.; Wei, H.; Zhao, S.; Liu, X.; Li, X.; Xu, A. Synergistic Photocatalytic Activation of Permanganate by Carbon Quantum Dots-Doped g-C3N4 under Visible Light Irradiation. J. Environ. Chem. Eng. 2025, 13, 117162. [Google Scholar] [CrossRef]
- Guan, J.; Liu, X.; Bai, N.; Wang, F.; Yang, Z.; Zhang, J.; Gao, F.; Zhang, P.; Wei, Z. Luminescence Properties of CQDs and Photocatalytic Properties of TiO2/ZnO/CQDs Ternary Composites. J. Mater. Sci. Mater. Electron. 2023, 34, 2169. [Google Scholar] [CrossRef]
- Hao, L.; Liang, Z.; Yu, Y.; Houy, H.; Min, D. Nano-island-like heterojunction of nitrogen-doped carbon quantum dots anchoring on titanium oxide nano sheets@Cu2O for enhancing visible light-driving photocatalytic degradation of methyl orange. J. Water Process Eng. 2024, 57, 104675. [Google Scholar] [CrossRef]
- Dou, S.; Wang, D.; Shang, Q.; Kong, X.; Fang, Y. Carbon Dots Modified Dendritic TiO2-CdS Heterojunction for Enhanced Photodegradation of Rhodamine and Hydrogen Evolution. Diam. Relat. Mater. 2023, 137, 110115. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Shi, Y.; Guo, F.; Tang, Y. Effect of different carbon dots positions on the transfer of photo-induced charges in type I heterojunction for significantly enhanced photocatalytic activity. Sep. Purif. Technol. 2023, 304, 122337. [Google Scholar] [CrossRef]
- Wang, G.; Li, X. Hao P, Liu W, Zhan H, Bi S, g-C3N4/Nitrogen-Doped Carbon Dot/Silver Nanoparticle-Based Ternary Photocatalyst for Water Pollutant Treatment. ACS Appl. Nano Mater. 2023, 6, 5747–5758. [Google Scholar] [CrossRef]
- Abdurahman, M.-H.; Zuhairi Abdullah, A.; Da Oh, W.; Fazliani Shopware, N.; Faisal Gasim, M.; Okoye, P.; Ul-Hamid, A.; Rahman Mohamed, A. Tunable band structure of synthesized carbon dots modified graphitic carbon nitride/bismuth oxychlorobromide heterojunction for photocatalytic degradation of tetracycline in water. J. Colloid Interface Sci. 2023, 629, 189–205. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Zhang, H.; Hong Luo, K.; Li, C. Fabrication of Z-scheme Bi7O9I3/g-C3N4 heterojunction modified by carbon quantum dots for synchronous photocatalytic removal of Cr (Ⅵ) and organic pollutants. J. Hazard. Mater. 2023, 446, 130663. [Google Scholar] [CrossRef]
- Tao, Y.; Mou, Z.; Lei, W.; Li, Y.; Shangguan, L.; Zhu, W.; Gong, J. Improving adsorption and visible light photocatalytic performance of TiO2 via synergistic effect of nitrogen-doped graphene quantum dots and reduced mildly oxidized graphene oxide for cationic dye pollutants removal. Surf. Interfaces 2024, 46, 104150. [Google Scholar] [CrossRef]
- Joulaee, S.; Mirzaei, M.; Hassanpour, A.; Safardoust-Hojaghan, H.; Khani, A. Efficient removal of anionic and cationic dyes from waste water using green ZnO/NiO/graphene quantum dots nano photocatalyst. Opt.-Int. J. Light Electron Opt. 2023, 290, 171324. [Google Scholar] [CrossRef]
- Li, S.; Rong, K.; Wang, X.; Shen, C.; Yang, F.; Zhang, Q. Design of Carbon Quantum Dots/CdS/Ta3N5 S-Scheme Heterojunction Nanofibers for Efficient Photocatalytic Antibiotic Removal. Acta Phys.-Chim. Sin. 2024, 40, 2403005. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.; Liu, X.; Wen, M.; Wang, G.; Zhan, H.; Chen, X.; Li, H.; Ma, J.; Liu, W. Bifunctional Nitrogen-Doped Carbon Quantum Dot-Modified Graphitic Carbon Nitride/Cadmium Sulfide Nanomaterials for Antibiotic Selective Detection and Photocatalytic Degradation. Mater. Today Chem. 2024, 38, 102123. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, Q.; Zhang, L.; Qi, Z. Fabrication of Z-Scheme Heterojunctions of CDs@WO3/g-C3N4 Nanocomposite Photocatalyst with Enhanced Visible-Light Photocatalytic Degradation of Malachite Green. J. Mater. Sci. Mater. Electron. 2024, 35, 598. [Google Scholar] [CrossRef]
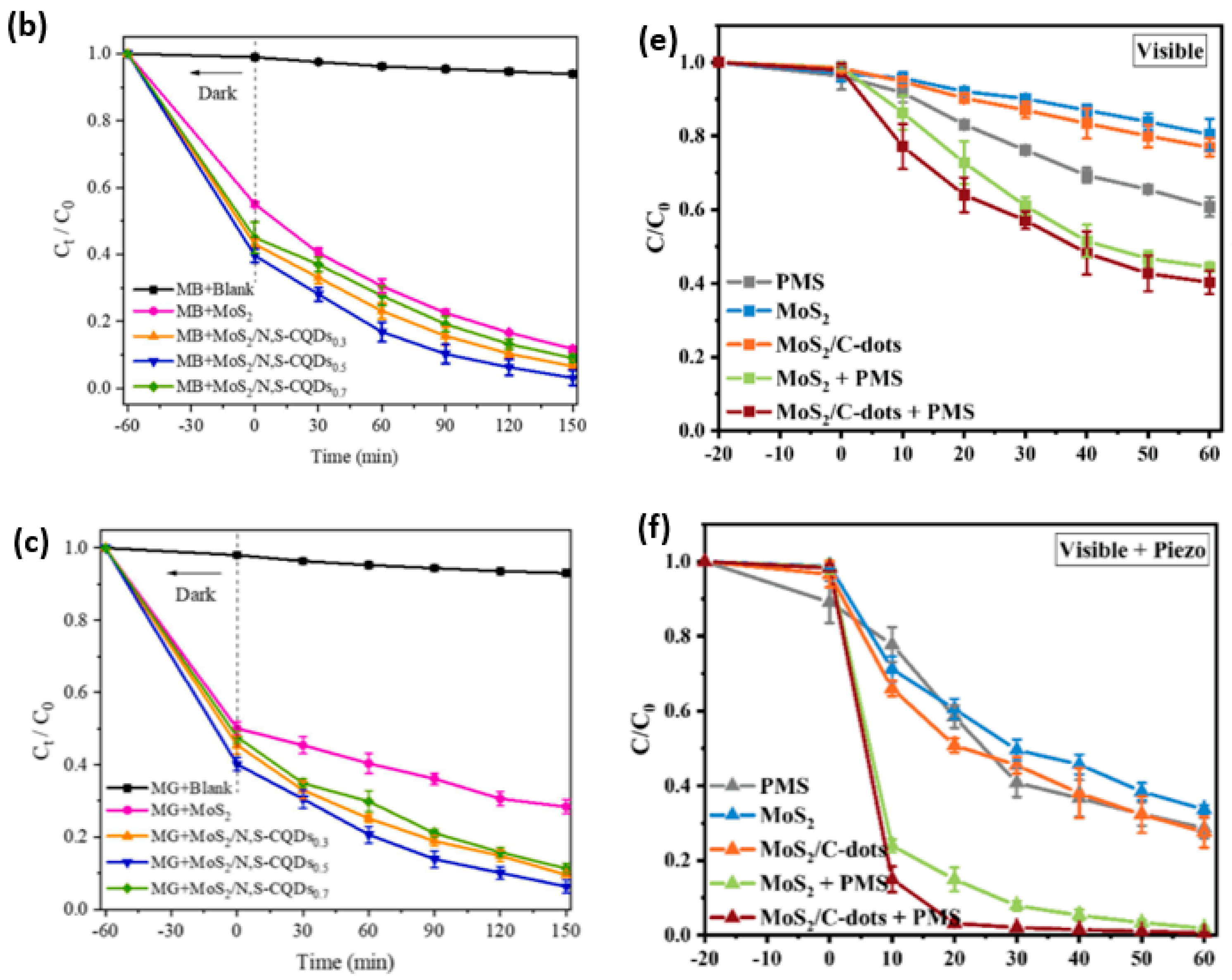
- Jiang, R.; Zhong, D.; Xu, Y.; Chang, H.; He, Y.; Zhang, J.; Liao, P. Chitosan Derived N-Doped Carbon Anchored Co3O4-Doped MoS2 Nanosheets as an Efficient Peroxymonosulfate Activator for Degradation of Dyes. Int. J. Biol. Macromol. 2024, 265, 130519. [Google Scholar] [CrossRef]
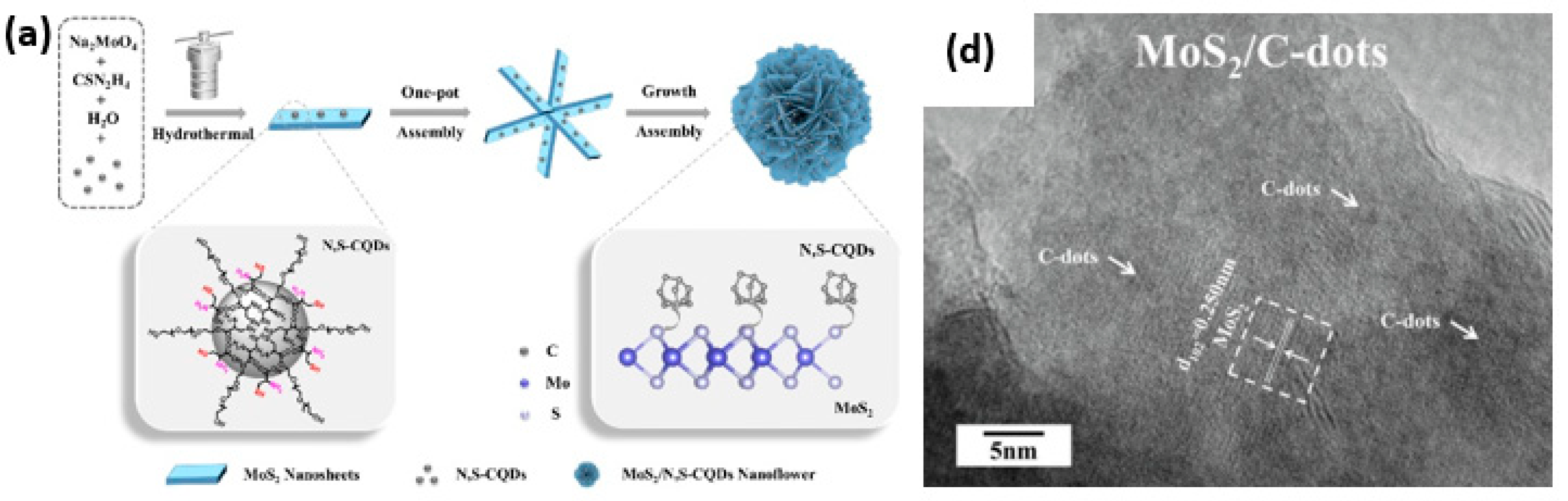
- Teymourinia, H.; Rtimi, S.; Ghalkhani, M.; Ramazani, A.; Aminabhavi, T.M. Flower-like Nanocomposite of Carbon Quantum Dots, MoS2, and Dendritic Ag-Based Z-Scheme Type Photocatalysts for Effective Tartrazine Degradation. Chem. Eng. J. 2023, 473, 145239. [Google Scholar] [CrossRef]
- Bai, S.; Lv, T.; Chen, M.; Li, C.; Wang, Z.; Yang, X.; Xia, T. Carbon Quantum Dots Assisted BiFeO3@BiOBr S-Scheme Heterojunction Enhanced Peroxymonosulfate Activation for the Photocatalytic Degradation of Imidacloprid under Visible Light: Performance, Mechanism and Biotoxicity. Sci. Total Environ. 2024, 915, 170029. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Yang, X.; Wang, Y.; Yang, T.; Que, M.; Ma, Y.; Li, Y. Synthesis of Highly Conductive Carbon Quantum Dot-Enhanced Z-Scheme BiOBr/g-C3N4 Heterojunction for Effective Photocatalytic Degradation of Tetracycline Hydrochloride. J. Phys. Chem. Solids 2024, 189, 111957. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Shi, J.; Huang, T.; Liu, K.; Tong, Z.; Zhang, H. Reinforced Built-in Electric Field and Mediated Schottky Barrier Height via Carbon Quantum Dots Electronic Bridges on BiOBr/Ti3C2 for Efficient Photocatalytic Quinolone Antibiotics Degradation. Chem. Eng. J. 2024, 500, 157168. [Google Scholar] [CrossRef]
- Tie, W.; Bhattacharyya, S.S.; Ma, T.; Yuan, S.; Chen, M.; He, W.; Lee, S.H. Improving Photoexcited Carrier Separation through Z-Scheme W18O49/BiOBr Heterostructure Coupling Carbon Quantum Dots for Efficient Photoelectric Response and Tetracycline Photodegradation. Carbon 2025, 231, 119707. [Google Scholar] [CrossRef]
- Xu, D.; Yu, C.; Peng, X.; Yan, H.; Zhang, Y. CQDs Modified Bi2MoO6/CuS p–n Heterojunction Photocatalytic Efficient Degradation of Tetracycline. Res. Chem. Intermed. 2024, 50, 2477–2499. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhang, X.; Zhang, J.; Zhang, S. N-Doped Graphene Quantum Dot-Decorated N-TiO2/P-Doped Porous Hollow g-C3N4 Nanotube Composite Photocatalysts for Antibiotic Photodegradation and H2 Production. Int. J. Miner. Metall. Mater. 2024, 31, 165–178. [Google Scholar] [CrossRef]
- Yuan, S.; Yin, G.; Zhao, T.; Zhang, J.; Wei, S.; Zhang, H.; Liu, Z.; Zhang, J.; Lu, Q.; Sun, M. Preparation and Photocatalytic Degradation Properties of Z-Scheme Si-TiO2/g-C3N4 Heterojunction Modified with F-CDs. Solid State Sci. 2025, 160, 107832. [Google Scholar] [CrossRef]
- Shan, C.; Liu, Z.; Li, F.; Jia, W.; Cai, G.; Li, M.; Tang, T.; Li, S.; Wen, J.; Hu, G.; et al. Ternary TiO2/P-GQDs/AgI Nanocomposites with n-p-n Heterojunctions for Enhanced Visible Photocatalysis. J. Nanoparticle Res. 2023, 25, 128. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, C.; Zhang, A.; Zhang, Y.; Zhang, L.; Bai, H. Nitrogen-Doped Carbon Quantum Dots Modified Dual-Vacancy Z-Scheme CuFe2O4/g-C3N4 Photocatalyst Synergistic with Fenton Technique for Photothermal Degradation of Antibiotics. Chem. Eng. J. 2024, 497, 154497. [Google Scholar] [CrossRef]












| CDs–Metal Oxides | Water Pollutant | Light Source | Photocatalytic Degradation | References |
|---|---|---|---|---|
| N-doped CQDs–TiO2 | Methylene Blue (10 ppm) | 300 W Xenon lamp | 93.10% after 60 min (10 mg/100 mL photocatalyst) | [74] |
| CQDs–TiO2 | Methyl Orange (25 ppm) | 300 W Xenon lamp >400 nm | 85.00% after 130 min (20 mg/50 mL photocatalyst) | [75] |
| GQDs–ZnO | Metronidazole (100 ppm) | UV | >99.99% after 30 min (100 mg/100 mL photocatalyst) | [76] |
| CQDs–Zn2SnO4 | Methylene Blue (3 ppm) | Natural Sunlight | 95.00% after 150 min (200 mg/200 mL photocatalyst) | [77] |
| N-doped CDs–Cu2O | Methylene Blue (10 ppm) | 500 W Xenon lamp | 96.40% after 120 min * | [78] |
| CQDs–Cu2O | Tetracycline (10 ppm) | Xenon lamp Vis | 92.49% after 100 min (100 mg/90 mL photocatalyst) | [79] |
| B-doped CQDs–Bi2MoO6 | Bisphenol A (20 ppm) | 300 W Xenon lamp | 100% after 120 min (20 mg/60 mL photocatalyst) | [80] |
| GQDs–Bi2MoO6 | Bisphenol A (20 ppm) | 300 W Xenon lamp Vis | 95.00% after 120 min (50 mg/60 mL photocatalyst) | [81] |
| Sargassum horneri-derived CDs–Bi2MoO6 | Ciprofloxacin (20 ppm) | 300 W Xenon lamp | 97.70% after 180 min (50 mg/100 mL photocatalyst) | [82] |
| CQDs–BiVO4 | Benzyl Paraben (10 ppm) | 300 W Xenon lamp >420 nm | 85.40% after 150 min (100 mg/100 mL photocatalyst) | [83] |
| CQDs–Co2VO4 | Ciprofloxacin (20 ppm) | 500 W Halogen lamp Vis | 80.75% after 13 min (50 mg/L photocatalyst and peroxymonosulphate) | [84] |
| CDs–WO3 | Methylene Blue (10 ppm) Malachite Green (10 ppm) | 10 W Xenon lamp 400–800 nm | 87.00% after 120 min 88.04% after 120 min (10 mg photocatalyst) | [85] |
| N-doped GQDs–CuWO4 | Tetracycline (15 ppm) | 300 W Xenon lamp 300–800 nm | 99.00% after 90 min (10 mg photocatalyst) | [86] |
| GQDs–CuWO4 | Phenol (100 ppm) | 5 W LED light | 53.41% after 90 min (200 mg/100 mL photocatalyst) | [87] |
| CDs–Ferrites | Water Pollutant | Light Source | Photocatalytic Degradation | References |
|---|---|---|---|---|
| crocus cancellatus-derived CDs–Fe3O4 | Fluorescein (45 μΜ) | Natural Sunlight | 94.4% after 60 min (15 mg of photocatalyst and 0.05 M H2O2) | [89] |
| lemon peel-derived CDs–Fe3O4 | Methylene Blue (50 ppm) | 99.24% after 30 s (100 mg of photocatalyst and 0.045 M H2O2) | [90] | |
| watermelon peel derived CDs–Fe3O4 | Methylene Blue (7 ppm) | UV | 98.00% after 30 min (50 mg of photocatalyst) | [91] |
| yerba mate derived CDs–Fe3O4 | Methyl Orange (8.5 ppm) | >400 nm | 97.70% after 7 h (100 ppm photocatalyst and 150 mM H2O2) | [92] |
| mushrooms derived CDs–ZnFe2O4 | Methylene Blue (10 ppm) RhB (10 ppm) | 200 W Xenon lamp Vis | 94.25% after 30 min 96.10% after 60 min (10 mg/L photocatalyst) | [93] |
| N-doped CDs–Zn-doped CoFe2O4 | Oxytetracycline (10 ppm) | 250 W HPMVL lamp Vis | 98.00% after 100 min (200 mg/L photocatalyst) | [94] |
| GQDs–CoFe2O4 | Methylene Blue (10 ppm) | 160 W bulb | ~90% after 120 min (50 mg/100 mL photocatalyst) | [95] |
| Boerhavia diffusa derived CDs–CoFe2O4 | Tetracycline (50 ppm) | 150 W Xenon lamp | 92% after 120 min (50 mg/100 mL photocatalyst and H2O2) | [96] |
| N-doped CDs–CoFe2O4 | Naproxen (10 ppm) | Vis | 89.50% after 100 min (20 mg/100 mL photocatalyst) | [97] |
| mango peel derived CDs–Co0,5Zn0,5Fe2O4 | Reactive Blue 222 (50 ppm) Reactive Yellow 145 (50 ppm) | 500 W Halogen lamp Vis | ~95% after 25 min (1 g/L photocatalyst) | [98] |
| CDs–CuFe2O4 | 2-Nitroaniline (200 ppm) 4-Nitroaniline (200 ppm) | * | 96.70% after 45 s 96.50% after 15 s (7 mg/5 mL photocatalyst and NaBH4) | [99] |
| CD–g–C3N4 | Water Pollutant | Light Source | Photocatalytic Degradation | References |
|---|---|---|---|---|
| ginkgo leaves-derived CDs–g–C3N4 | Rhodamine B (10 ppm) | Vis | >99.99% after 60 min (* photocatalyst powder/50 mL) | [155] |
| N-doped GQDs– P-doped g–C3N4 | Tetracycline (20 ppm) | >420 nm | 89.90% after 60 min (50 mg/100 mL photocatalyst) | [156] |
| Cl-doped CQDs– g–C3N4 | 91.70% after 120 min (40 mg/40 mL photocatalyst) | [157] | ||
| B-doped CQDs– g–C3N4 | Rhodamine B (1 g/L) Methyl orange (1 g/L) | Vis | 65.58% after 120 min 73.56% after 120 min (10 mg/100 mL photocatalyst) | [158] |
| CQDs–g–C3N4 | Indigo Carmine (10 ppm) Carmoisine (10 ppm) | UV and Vis | 96.00% after 60 min 93.50% after 60 min (1.0 mg/mL photocatalyst) | [159] |
| E. coli (200 ppm) S. aureu (300 ppm) | 300 W Xenon lamp >420 nm | ** MIC of 12.5 ppm MIC of 40 ppm | [160] | |
| Meloxicam (10 ppm) Tetracycline (10 ppm) | Vis | 99.90% after 30 min 95.97% after 45 min (20 mg photocatalyst and 20 mg peroxymonosulfate/50 mL) | [161] | |
| Sulfadiazine (40 µM) | 300 W Xenon lamp >400 nm | 98.00% after 30 min (20 mg photocatalyst and KMnO4/50 mL) | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zourou, A.; Ntziouni, A.; Karagianni, A.; Alizadeh, N.; Argirusis, N.; Antoniadou, M.; Sourkouni, G.; Kordatos, K.V.; Argirusis, C. Recent Advances in Carbon Dots-Based Photocatalysts for Water Treatment Applications. Inorganics 2025, 13, 286. https://doi.org/10.3390/inorganics13090286
Zourou A, Ntziouni A, Karagianni A, Alizadeh N, Argirusis N, Antoniadou M, Sourkouni G, Kordatos KV, Argirusis C. Recent Advances in Carbon Dots-Based Photocatalysts for Water Treatment Applications. Inorganics. 2025; 13(9):286. https://doi.org/10.3390/inorganics13090286
Chicago/Turabian StyleZourou, Adamantia, Afrodite Ntziouni, Alexandra Karagianni, Niyaz Alizadeh, Nikolaos Argirusis, Maria Antoniadou, Georgia Sourkouni, Konstantinos V. Kordatos, and Christos Argirusis. 2025. "Recent Advances in Carbon Dots-Based Photocatalysts for Water Treatment Applications" Inorganics 13, no. 9: 286. https://doi.org/10.3390/inorganics13090286
APA StyleZourou, A., Ntziouni, A., Karagianni, A., Alizadeh, N., Argirusis, N., Antoniadou, M., Sourkouni, G., Kordatos, K. V., & Argirusis, C. (2025). Recent Advances in Carbon Dots-Based Photocatalysts for Water Treatment Applications. Inorganics, 13(9), 286. https://doi.org/10.3390/inorganics13090286