Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors
Abstract
1. Introduction
2. Results
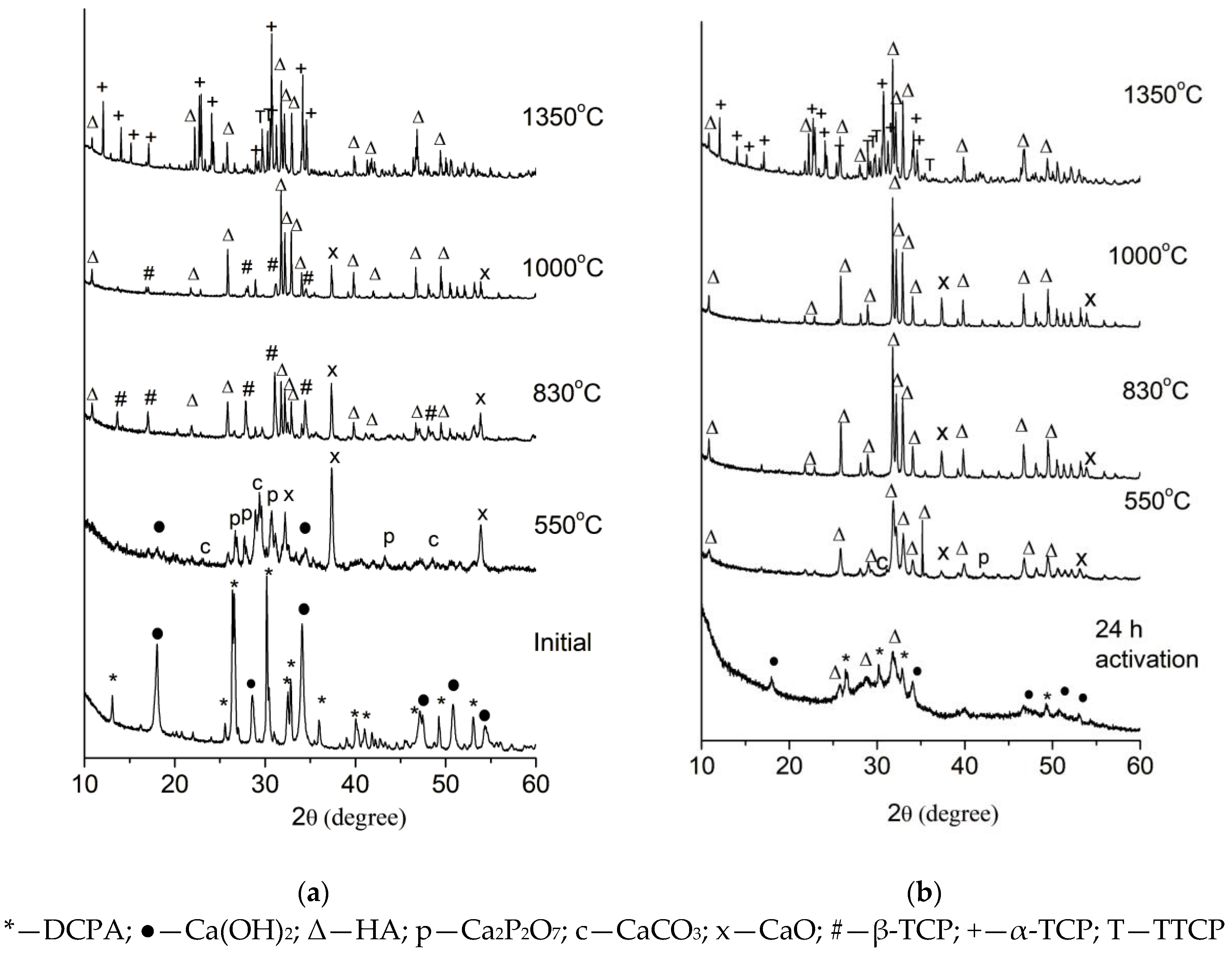
2.1. Effect of High-Energy Activation on the Phase Composition and Particle Morphology of the Activated Mixtures
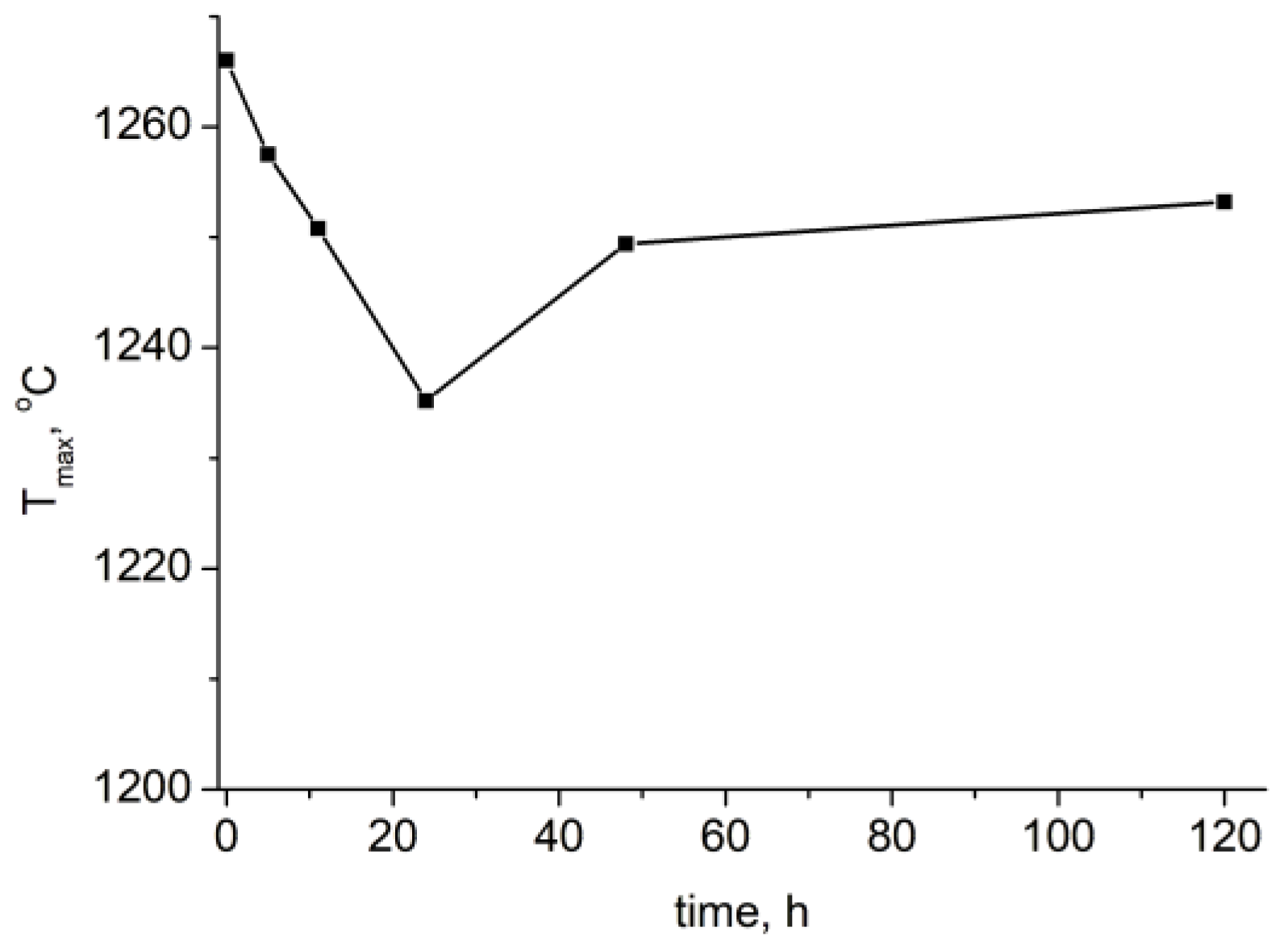
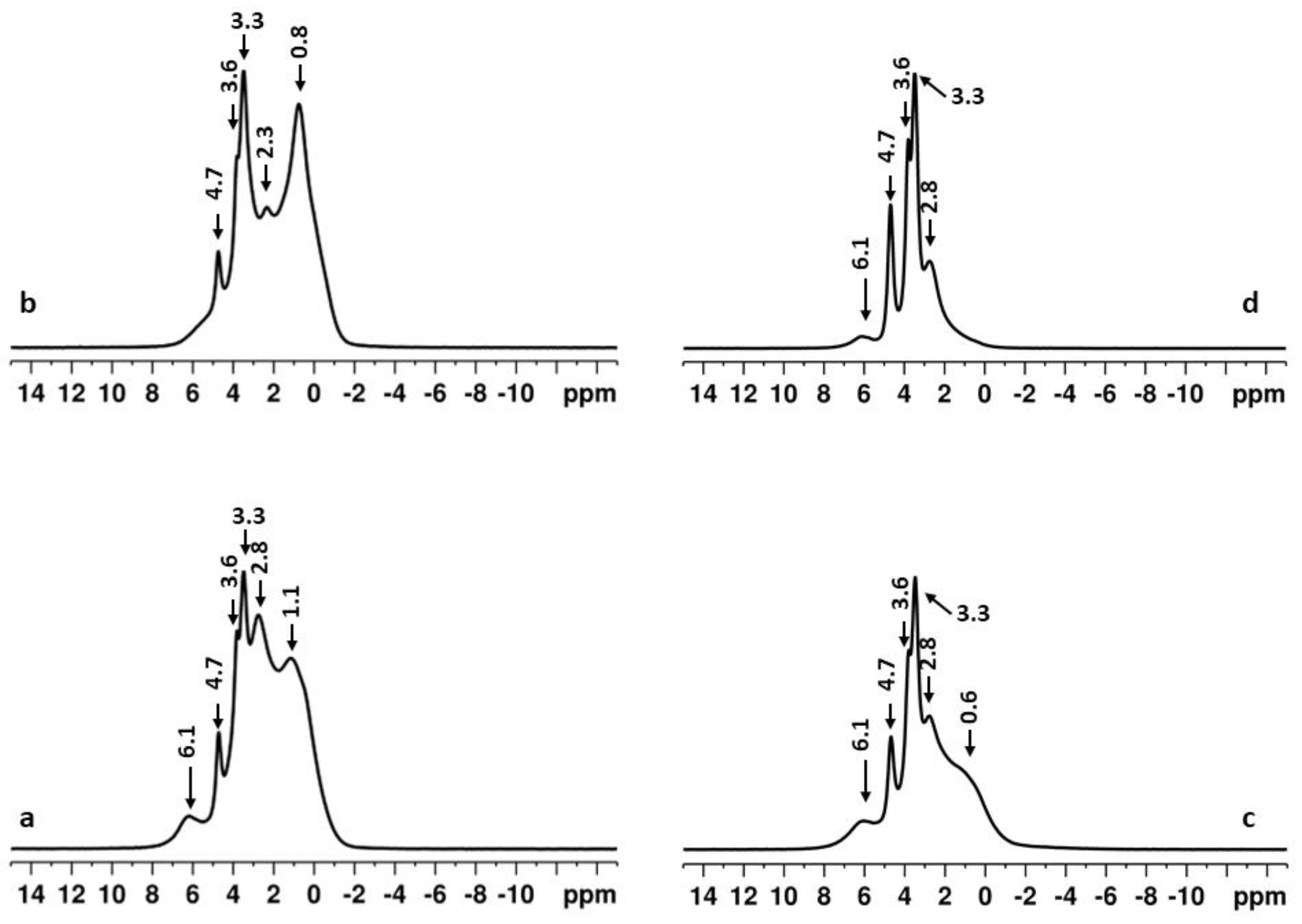
2.2. Effect of High-Energy Activation on the Thermal Characteristics of the Mixtures
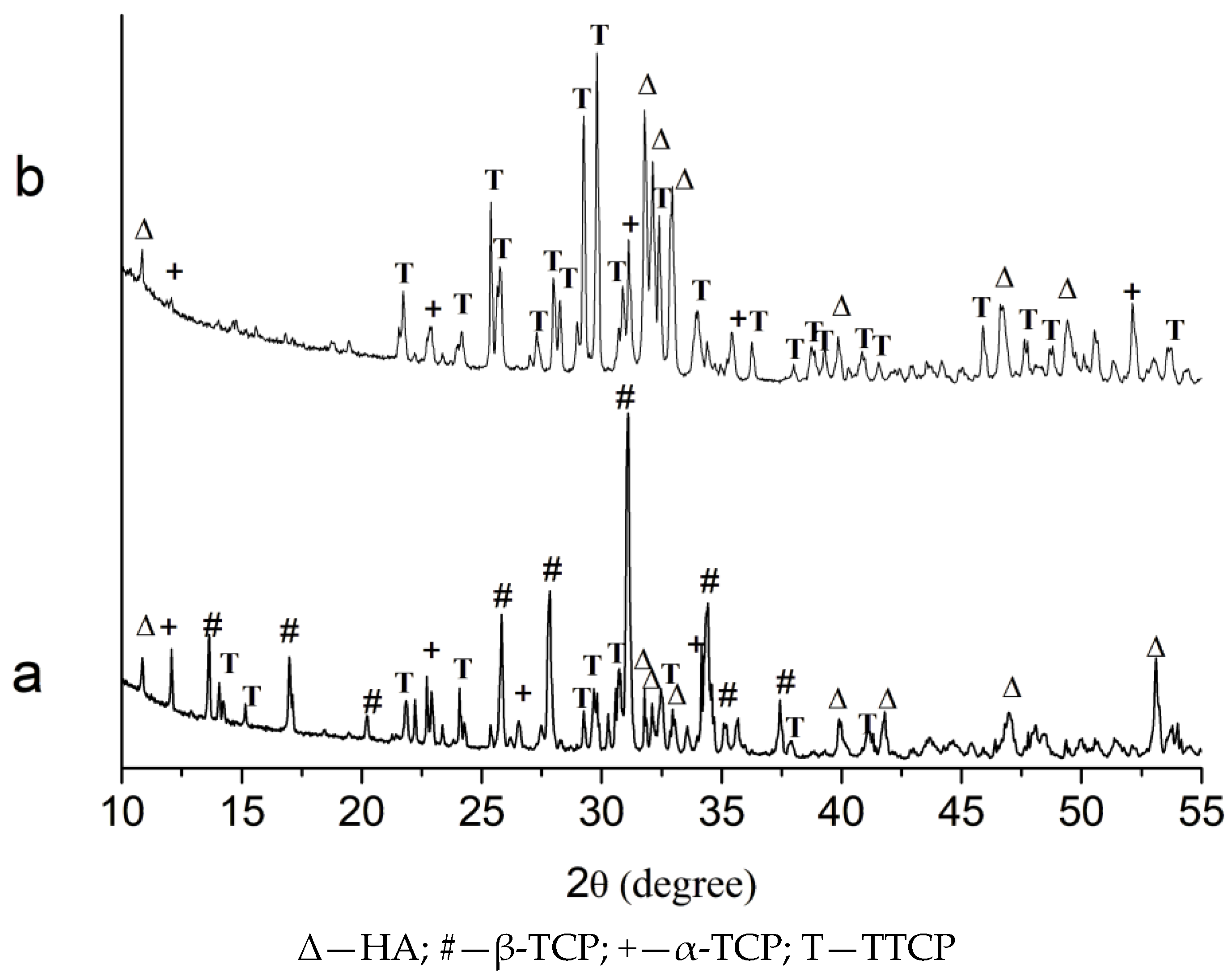
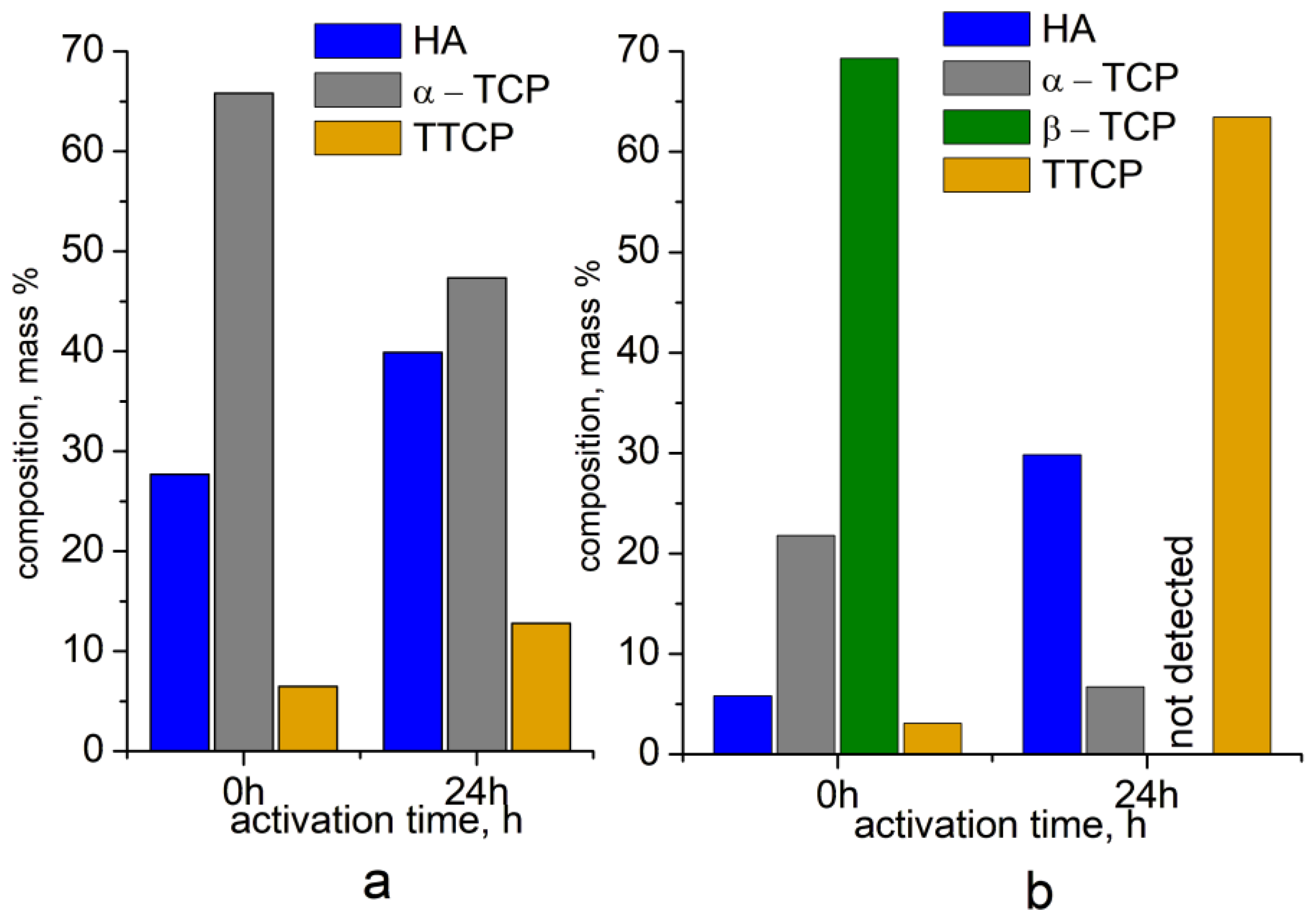
2.3. Effect of High-Energy Activation on the Phase Composition After Calcination
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. High Energy Activation
4.3. Calcination of Activated Precursors
- Step heating at a rate of 10 °C/min to a given temperature (determined by DTA-TG-MASS analysis) and hold for 3 h, followed by slow cooling (The samples were left to cool overnight in the furnace itself after it was turned off).
- Rapid heating at a rate of 10 °C/min to 1350 °C and holding for 3 h, followed by rapid annealing (The samples were removed from the furnace at a temperature of 1350°C and placed in a desiccator at room temperature).
4.4. Characterisation
4.4.1. X-Ray Diffraction Analysis (XRD)
4.4.2. Differential Thermal Analysis Combined with Detection of Released Gases (DTA-TG-MASS)
4.4.3. Nuclear Magnetic Resonance (NMR) Studies
4.4.4. Transmission Electron Microscopy Analysis (TEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hydroxyapatite |
| DCPA | Dicalcium phosphate anhydrous |
| TCP | Thricalcium phosphate |
| TTCP | Tetracalcium phosphate |
| NMR | Nuclear Magnetic Resonance |
| XRD | X-ray diffraction analysis |
| TEM | Transmission electron microscopy |
| DTA-TG-MASS | Differential thermal analysis with thermogravimetry and mass spectrometry |
References
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4)-Based Bioceramics: Preparation, Properties, and Applications. Coatings 2022, 12, 1380. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Fürderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef]
- Wu, V.M.; Uskoković, V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro? Biochim. Biophys. Acta 2016, 1860, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C.; Eanes, E.D. (Eds.) Octacalcium Phosphate; Karger Medical and Scientific Publishers: Basel, Switzerland, 2001. [Google Scholar]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomat. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Ehara, A.; Ogata, K.; Imazato, S.; Ebisu, S.; Nakano, T.; Umakoshi, Y. Effects of α-TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization. Biomaterials 2003, 24, 831–836. [Google Scholar] [CrossRef]
- Medvecky, L.; Stulajterova, R.; Giretova, M.; Mincik, J.; Vojtko, M.; Balko, J.; Briancin, J. Effect of tetracalcium phosphate/monetite toothpaste on dentin remineralisation and tubule occlusion in vitro. Dent. Mater. 2018, 34, 442–451. [Google Scholar] [CrossRef]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Xu, H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Lukina, Y.; Safronova, T.; Smolentsev, D.; Toshev, O. Calcium Phosphate Cements as Carriers of Functional Substances for the Treatment of Bone Tissue. Materials 2023, 16, 4017. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.M.; Sayed, M.; Mansour, T.S.; Naga, S.M. Biodegradable ceramic materials for orthopedic and dentistry applications. Discov. Appl. Sci. 2025, 7, 990. [Google Scholar] [CrossRef]
- Balbuena, O.B.F.; Paiva, L.F.S.; Ribeiro, A.A.; Monteiro, M.M.; de Oliveira, M.V.; Pereira, L.C. Sintering parameters study of a biphasic calcium phosphate bioceramic synthesized by alcoholic sol-gel technique. Ceram. Int. 2021, 47, 32979–32987. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I. Powder systems for calcium phosphate ceramics. Inorg. Mater. 2017, 53, 17–26. [Google Scholar] [CrossRef]
- Irfa’i, M.A.; Schmahl, W.W.; Pusparizkita, Y.M.; Muryanto, S.; Ismail, A.P.R.; Jamari, J.; Bayuseno, A.P. Hydrothermally synthesized-nanoscale carbonated hydroxyapatite with calcium carbonates derived from green mussel shell wastes. J. Mol. Struct. 2024, 1306, 137837. [Google Scholar] [CrossRef]
- Nikolenko, M.V.; Vasylenko, K.V.; Myrhorodska, V.D.; Kostyniuk, A.; Likozar, B. Synthesis of Calcium Orthophosphates by Chemical Precipitation in Aqueous Solutions: The Effect of the Acidity, Ca/P Molar Ratio, and Temperature on the Phase Composition and Solubility of Precipitates. Processes 2020, 8, 1009. [Google Scholar] [CrossRef]
- Ali, W.A.; Richards, S.E.; Alzard, R.H. Unlocking the potential of ball milling for nanomaterial Synthesis: An overview. J. Ind. Eng. Chem. 2025, 149, 63–93. [Google Scholar] [CrossRef]
- Chen, W.C.; Ju, C.P.; Tien, Y.C.; Lin, J.H. In vivo testing of nanoparticle-treated TTCP/DCPA-based ceramic surfaces. Acta Biomater. 2009, 5, 1767–1774. [Google Scholar] [CrossRef]
- Sargin, Y.; Kizilyalli, M.; Telli, C.; Güler, H. A new method for the solid-state synthesis of tetracalcium phosphate, a dental cement: X-ray powder diffraction and IR studies. J. Eur. Ceram. Soc. 1997, 17, 963–970. [Google Scholar] [CrossRef]
- Brosseau, C.; Liao, J.; Duan, X.; Li, Y.; Zheng, C.; Yang, Z.; Zhou, A.; Zou, D. Synthesis and Mechanism of Tetracalcium Phosphate from Nanocrystalline Precursor. J. Nanomater. 2014, 2014, 840102. [Google Scholar] [CrossRef]
- Jalota, S.; Tas, A.C.; Bhaduri, S.B. Synthesis of HA-seeded TTCP (CA4(PO4)2O) powders at 1230 °C from Ca(CH3COO)2.H2O and NH4H2PO4. J. Am. Ceram. Soc. 2005, 88, 3353–3360. [Google Scholar] [CrossRef]
- Romeo, H.E.; Fanovich, M.A. Synthesis of tetracalcium phosphate from mechanochemically activated reactants and assessment as a component of bone cements. J. Mater. Sci. Mater. Med. 2008, 19, 2751–2760. [Google Scholar] [CrossRef]
- Nizar, M.S.; Sofiyaningsih, N.; Manullang, R.J.; Wijayanti, R.B.; Rosmayanti, I.; Sumardan, D. Novel fast firing method to synthesize tetracalcium phosphate. AIP Conf. Proc. 2021, 2349, 020001. [Google Scholar] [CrossRef]
- Ayers, R.; Hannigan, N.; Vollmer, N.; Unuvar, C. Combustion synthesis of heterogeneous calcium phosphate bioceramics from calcium oxide and phosphate precursors. Int. J. Self-Propag. High-Temp. Synth. 2011, 20, 6–14. [Google Scholar] [CrossRef]
- Jeon, C.; Chun, S.; Lim, S.; Kim, S. Synthesis and Characterization of TTCP for Calcium Phosphate Bone Cement. Biomater. Res. 2011, 15, 1–6. [Google Scholar]
- Louati, B.; Hlel, F.; Guidara, K.; Gargouri, M. Analysis of the effects of thermal treatments on CaHPO4 by 31P NMR spectroscopy. J. Alloys Compd. 2005, 394, 13–18. [Google Scholar] [CrossRef]
- Edén, M. Structure and formation of amorphous calcium phosphate and its role as surface layer of nanocrystalline apatite: Implications for bone mineralization. Materialia 2021, 17, 101107. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, H.; Pujari-Palmer, M.; Stevensson, B.; Grins, J.; Engqvist, H.; Eden, M. Advanced solid-state 1H/31P NMR characterization of pyrophosphate-doped calcium phosphate cements for biomedical applications: The structural role of pyrophosphate. Ceram. Int. 2019, 45, 20642–20655. [Google Scholar] [CrossRef]
- Ben Osman, M.; Diallo-Garcia, S.; Herledan, V.; Brouri, D.; Yoshioka, T.; Kubo, J.; Millot, Y.; Costentin, G. Discrimination of surface and bulk structure of crystalline hydroxyapatite nanoparticles by NMR. J. Phys. Chem. C 2015, 119, 23008–23020. [Google Scholar] [CrossRef]
- Lee, J.; Bae, J.-S.; Kim, Y.-I.; Yoo, K.-H.; Yoon, S.-Y. Synthesis, Characterization, and Biological Performances of Magnesium-Substituted Dicalcium Phosphate Anhydrous. Materials 2024, 17, 4605. [Google Scholar] [CrossRef] [PubMed]
- Pesce, C.; Luca, G.P.; Molinari, M.; Richardson, A. Effects of organic additives on calcium hydroxide crystallisation during lime slaking. Cem. Concr. Res. 2021, 139, 106254. [Google Scholar] [CrossRef]
- Duff, E.J. Orthophosphates. XIII,* Thermal Decomposition of Secondary Calcium Orthophosphate (CaHPO4) and Secondary Calcium Orthophosphate Dihydrate (CaHPO4•2H2O). J. Appl. Chem. Biotechnol. 1971, 21, 233–235. [Google Scholar] [CrossRef]
- Khachani, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- El Hazzat, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Complex evolution of phase during the thermal investigation of brushite-type calcium phosphate CAHPO4•2H2O. Materialia 2021, 16, 101055. [Google Scholar] [CrossRef]
- Mulongo-Masamba, R.; El Kassri, T.; Khachani, M.; Arsalane, S.; Halim, M.; El Hamidi, A. Synthesis and thermal dehydroxylation kinetic of anhydrous calcium phosphate monetite CaHPO4. J. Therm. Anal. Calorim. 2016, 124, 171–180. [Google Scholar] [CrossRef]
- Mortier, A.; Lemaitre, J.; Rouxhet, P.G. Temmperature-Programmed Characterization of Synthetic Calcium-Deficient Phosphate Apatites. Thermochim. Acta 1989, 143, 265–282. [Google Scholar] [CrossRef]
- Miquel, J.L.; Facchini, L.; Legrand, A.P. Characterisation and Conversion Study into Natural Living Bone of Calcium Phosphate Bioceramics by Solid State NMR Spectroscopy. Clin. Mater. 1990, 5, 115. [Google Scholar] [CrossRef]
- Vogel, J.; Rüssel, C.; Günther, G.; Hartmann, P.; Vizethum, F.; Bergner, N. Characterization of plasma-sprayed hydroxyapatite by 31P-MAS-NMR and the effect of subsequent annealing. J. Mater. Sci. Mater. Med. 1996, 7, 495. [Google Scholar] [CrossRef]
- Guo, H.; Pujari-Palmer, M.; Yu, Y.; Stevensson, B.; Engqvist, H.; Edén, M. Quantitative phase analyses of biomedical pyrophosphate-bearing monetite and brushite cements by solid-state NMR and powder XRD. Ceram. Int. 2000, 46, 11000. [Google Scholar] [CrossRef]
- Francis, M.D.; Webb, N.C. Hydroxyapatite formation from a hydrated calcium monohydrogen phosphate precursor. Calc. Tis Res. 1970, 6, 335–342. [Google Scholar] [CrossRef]
- Dickens, B.; Bowen, J.S.; Brown, W.E. A refinement of the crystal structure of CaHPO4 (synthetic monetite). Acta Cryst. 1972, B28, 797–806. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Angeles, C.; De la Isla, A.; Castano, V.M. Effect of bio-calcium oxide on the morphology of hydroxyapatite. Int. J. Basic Appl. Sci. 2015, 4, 395. [Google Scholar] [CrossRef]
- Jinlong, N.; Zhenxi, Z.; Dazong, J. Investigation of Phase Evolution During the Thermochemical Synthesis of Tricalcium Phosphate. J. Mater. Synth. Process. 2001, 9, 235–240. [Google Scholar] [CrossRef]
- Hui, P.; Meena, S.L.; Singh, G.; Agarawal, R.D.; Prakash, S. Synthesis of Hydroxyapatite Bio-Ceramic Powder by Hydrothermal Method. J. Miner. Mater. Charact. Eng. 2010, 9, 683–692. [Google Scholar] [CrossRef]
- Kivrak, N.; Taş, A.C. Synthesis of Calcium Hydroxyapatite-Tricalcium Phosphate (HA-TCP) Composite Bioceramic Powders and Their Sintering Behavior. J. Am. Ceram. Soc. 1998, 81, 2245–2252. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Dohrn, M.; Neuroth, M.; Geisler, T. High-temperature phase transformations of hydroxylapatite and the formation of silicocarnotite in the hydroxylapatite–quartz–lime system studied in situ and in operando by Raman spectroscopy. J. Mater. Sci. 2022, 57, 15239–15266. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Celve, S.; Alonson, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
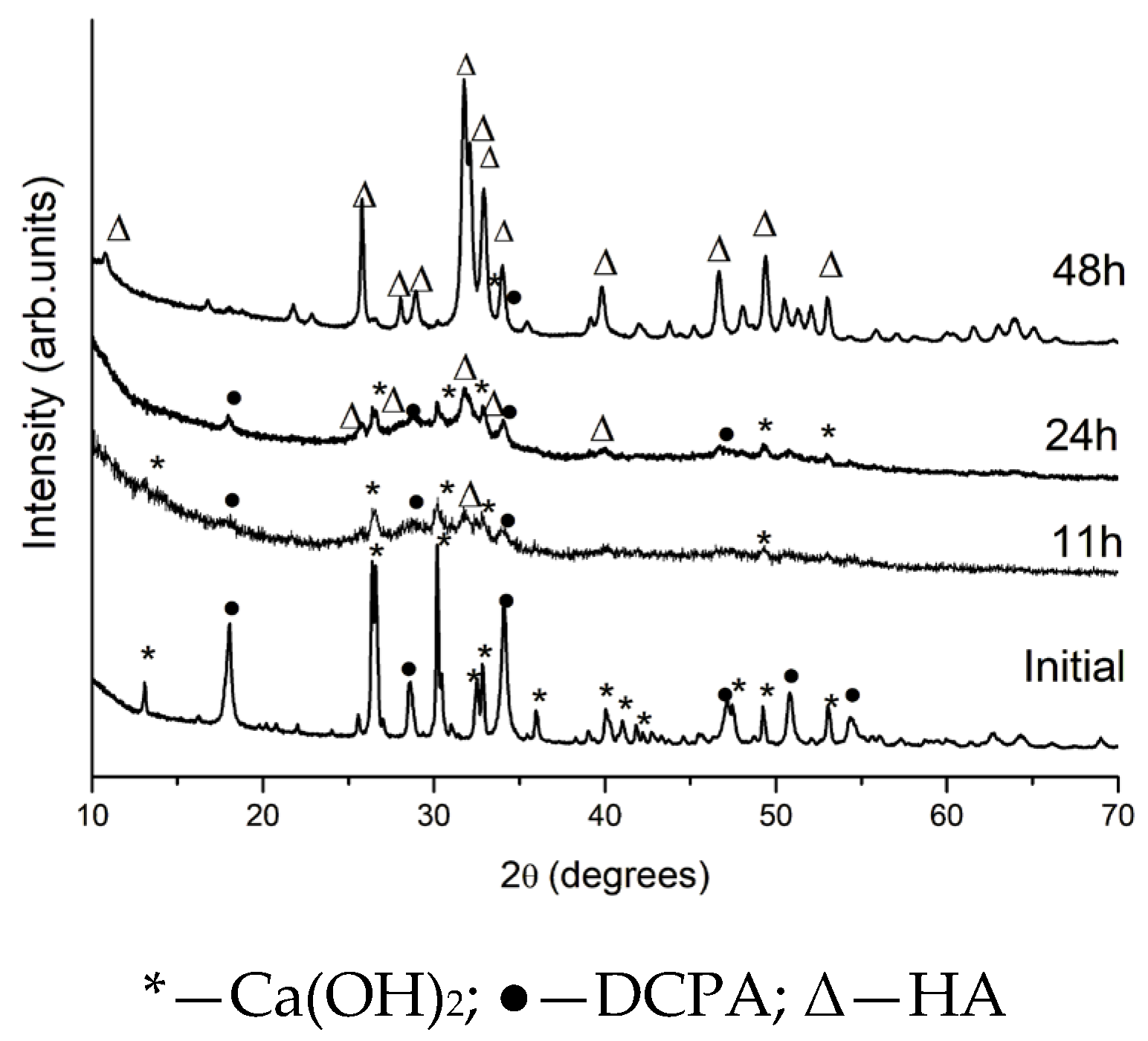
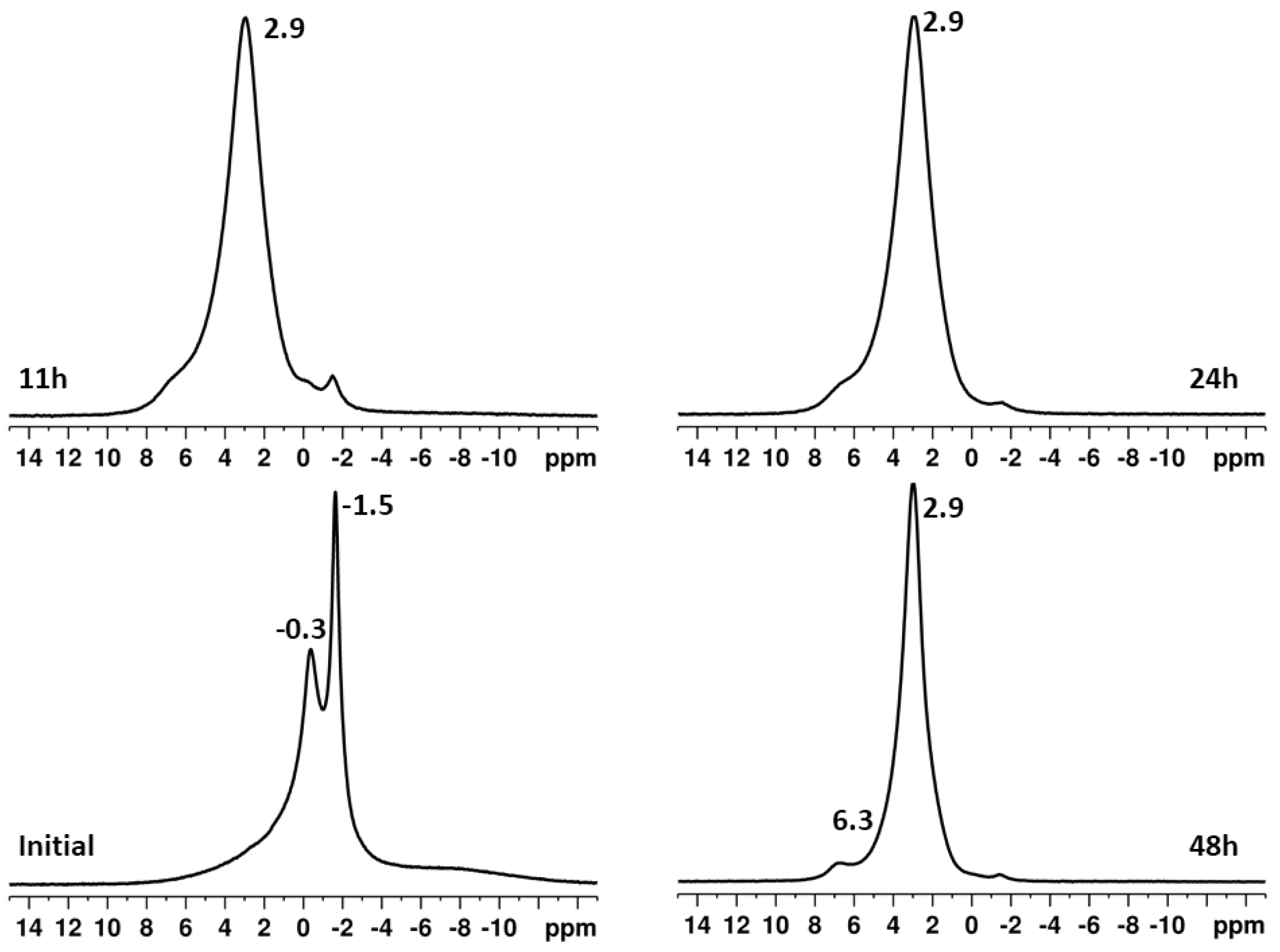
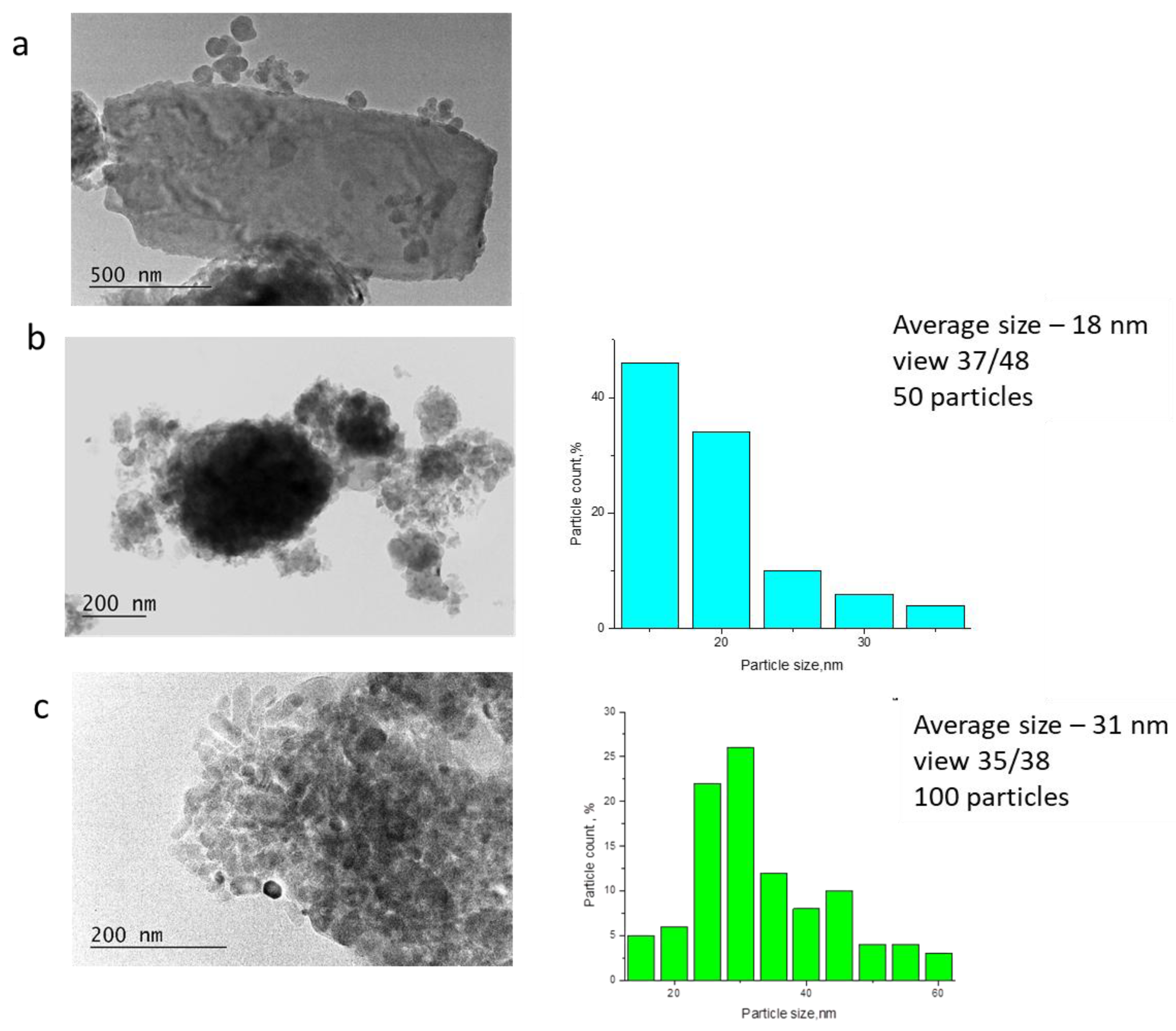
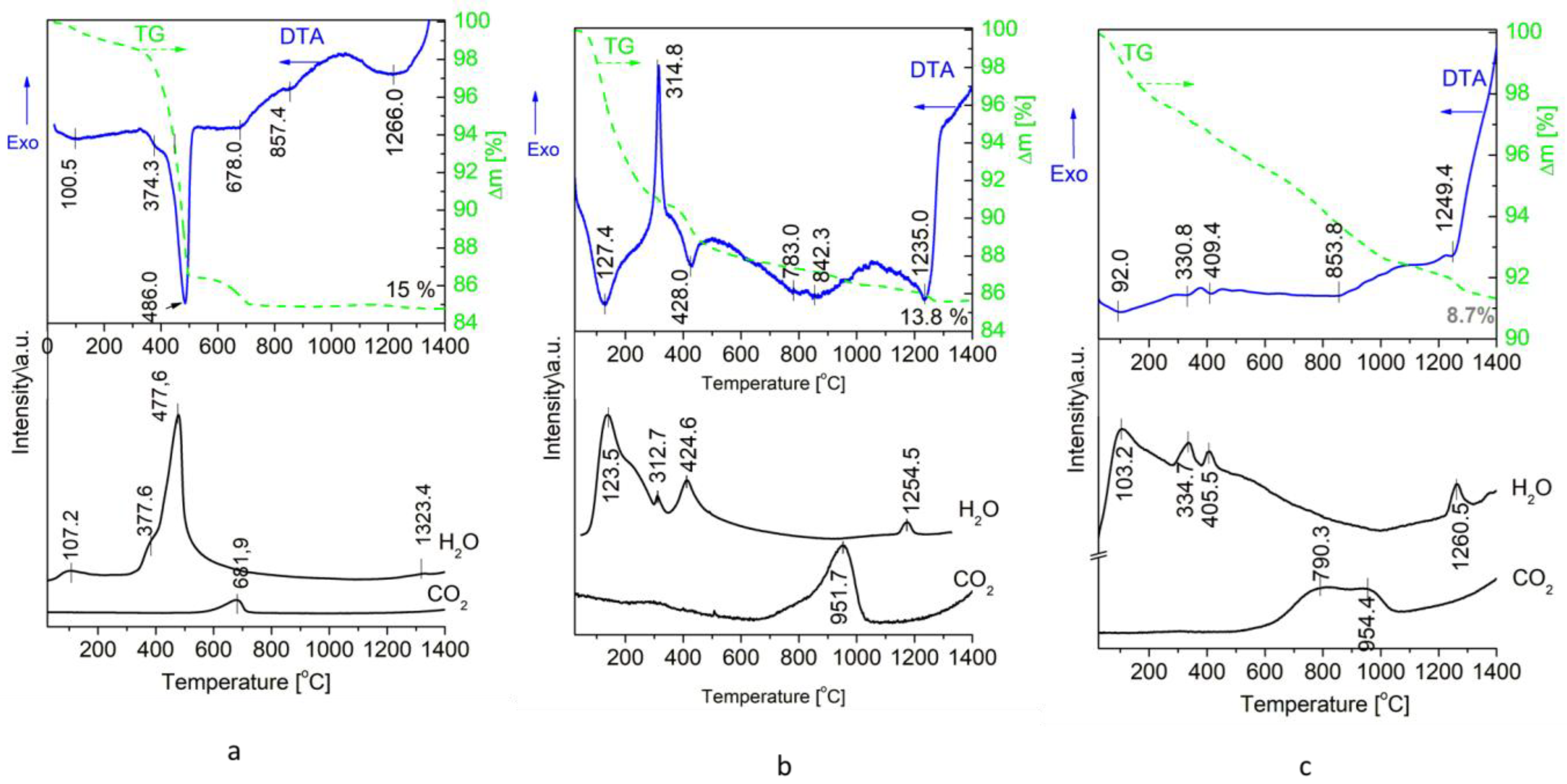

| Sample | Ca(OH)2 * (%) | DCPA * (%) | HA * (%) | HA Mean Size (nm) | Degree of Crystallinity |
|---|---|---|---|---|---|
| Initial | 34 | 66 | - | 100% | |
| 11 h activation | 18 | 45 | 37 | 19 | 8% |
| 24 h activation | 11 | 23 | 66 | 20 | 18% |
| 48 h activation | 4 | 2 | 94 | 33 | 100% |
| Temperature Region | Unactivated Mixture | 24 h Activated Sample | ||
|---|---|---|---|---|
| Up to 550 °C | 2CaHPO4 → β-Ca2P2O7 + H2O↑ | [33] | Ca5(PO4)3OH(ncr)—stabilization of the structure, increase of crystallinity | |
| Ca(OH)2 → CaO + H2O↑ | [34] | 2CaHPO4 → β-Ca2P2O7 + H2O↑ | [33] | |
| 2Ca(OH)2 + 3CaHPO4 → Ca5(PO4)3OH + 2H2O↑ | [43] | Ca(OH)2 → CaO + H2O↑ | [34] | |
| CaO + CO2(atm) → CaCO3 | 2Ca(OH)2 + 3CaHPO4 → Ca5(PO4)3OH + 2H2O↑ | [43] | ||
| CaO + CO2(atm) → CaCO3 | ||||
| 550–830 °C | β-Ca2P2O7 +CaO → 2β-Ca3(PO4)2 | [44] | β-Ca2P2O7 +CaO → 2β-Ca3(PO4)2 | [44] |
| Ca5(PO4)3OH—structure stabilization | Ca5(PO4)3OH—stabilization of the structure, increase of crystallinity | |||
| 830–1000 °C | 3β-Ca3(PO4)2 + CaO + H2O = 2Ca5(PO4)3OH (during cooling) | [45] | Ca5(PO4)3OH—starting the transphormation in which non-stoichiometric HA is formed (NMR, this study) | |
| Ca5(PO4)3OH—stabilization of the structure, increase of crystallinity | ||||
| 1000–1350 °C | 2Ca5(PO4)3OH → 3β-Ca3(PO4)2 + CaO + H2O | [46] | 2Ca5(PO4)3OH → 3β-Ca3(PO4)2 + CaO + H2O | [46] |
| β-Ca3(PO4)2 → α-Ca3(PO4)2 | [47] | β-Ca3(PO4)2 → α-Ca3(PO4)2 | [47] | |
| 2Ca5(PO4)3OH + 2CaO → 3Ca4(PO4)2O +H2O | [9] | 2Ca5(PO4)3OH + 2CaO → 3Ca4(PO4)2O +H2O | [9] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sezanova, K.; Tuparova, Y.; Shestakova, P.; Markov, P.; Kovacheva, D.; Rabadjieva, D. Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors. Inorganics 2025, 13, 313. https://doi.org/10.3390/inorganics13100313
Sezanova K, Tuparova Y, Shestakova P, Markov P, Kovacheva D, Rabadjieva D. Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors. Inorganics. 2025; 13(10):313. https://doi.org/10.3390/inorganics13100313
Chicago/Turabian StyleSezanova, Kostadinka, Yordanka Tuparova, Pavletta Shestakova, Pavel Markov, Daniela Kovacheva, and Diana Rabadjieva. 2025. "Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors" Inorganics 13, no. 10: 313. https://doi.org/10.3390/inorganics13100313
APA StyleSezanova, K., Tuparova, Y., Shestakova, P., Markov, P., Kovacheva, D., & Rabadjieva, D. (2025). Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors. Inorganics, 13(10), 313. https://doi.org/10.3390/inorganics13100313










