Scalable High-Yield Exfoliation of Hydrophilic h-BN Nanosheets via Gallium Intercalation
Abstract
1. Introduction
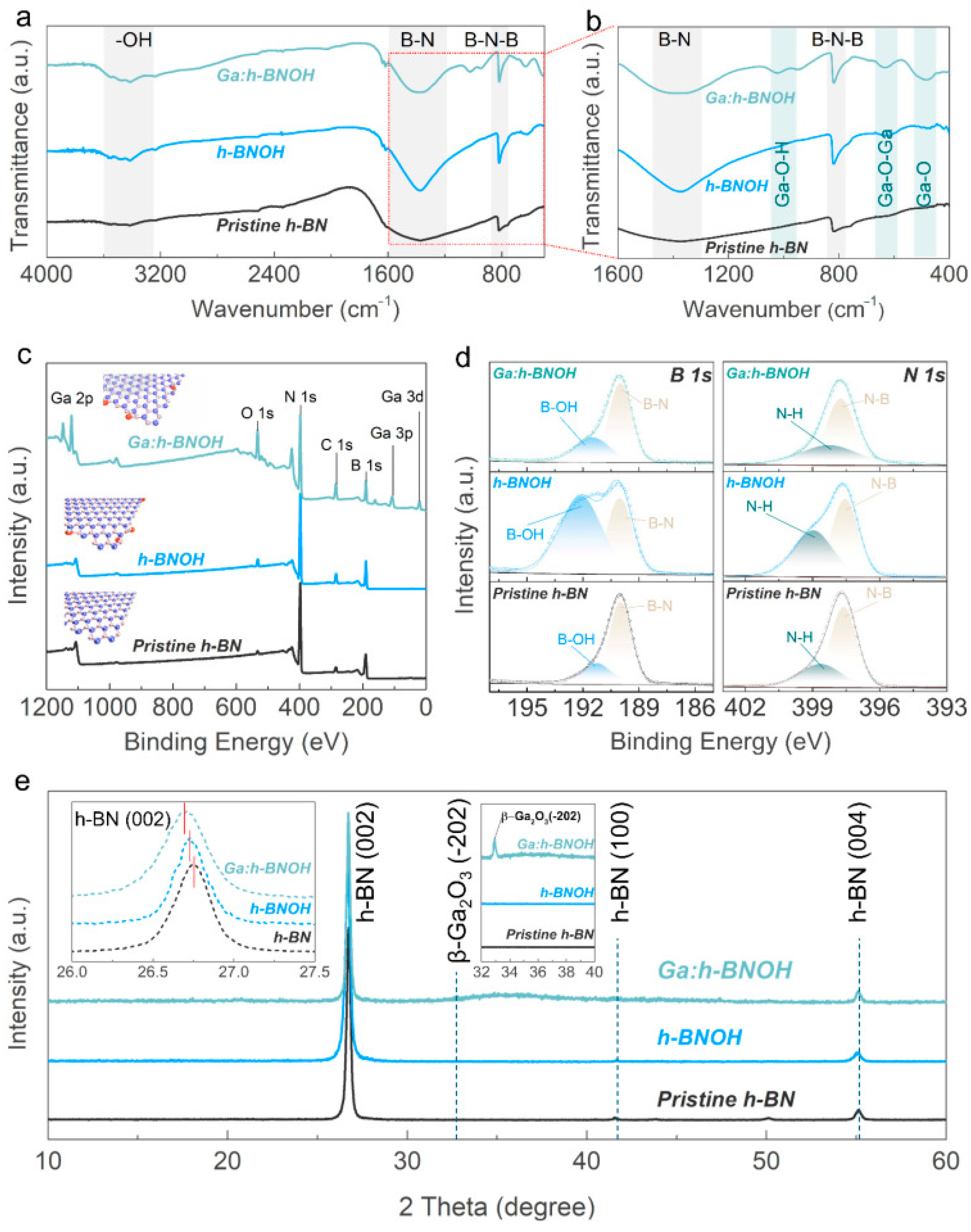
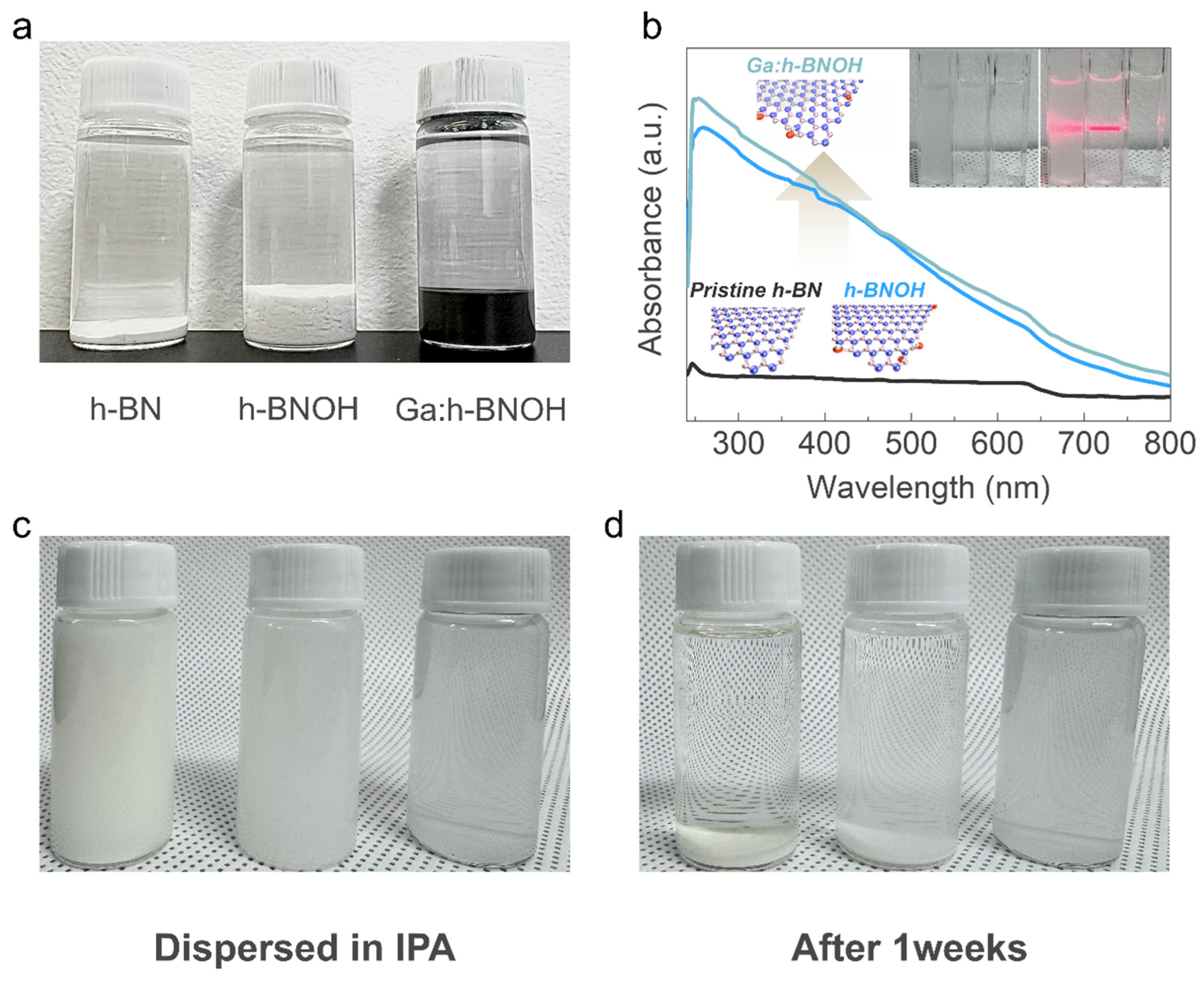
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Hydroxylation of h-BN
3.3. Gallium Intercalation and Liquid-Phase Exfoliation
3.4. Post-Treatment and Product Collection
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, Y.; Kim, T.; Eom, J.; Harm, O.; Byeon, J.; Lim, J.; Lee, J.; Pak, S.; Cha, S. Two-Terminal MoS2-Based Retinomorphic Devices with Enhanced Synaptic Plasticity. Adv. Electron. Mater. 2025, 11, 2400878. [Google Scholar] [CrossRef]
- Shin, W.; Byeon, J.; Koo, R.H.; Lim, J.; Kang, J.H.; Jang, A.R.; Lee, J.H.; Kim, J.J.; Cha, S.; Pak, S.; et al. Toward Ideal Low-Frequency Noise in Monolayer CVD MoS2 FETs: Influence of van der Waals Junctions and Sulfur Vacancy Management. Adv. Sci. 2024, 11, 2307196. [Google Scholar] [CrossRef]
- Lim, J.; Kim, T.; Park, H.; Eom, J.; Jung, M.; Byeon, J.; Lim, Y.; Pak, S.; Cha, S. Surface Wettability-Mediated Enhancement of Hydrogen Evolution Reaction Performance in Electron-Doped MoS2 Monolayers. ACS Appl. Energy Mater. 2024, 7, 2938–2945. [Google Scholar] [CrossRef]
- Byeon, J.; Eom, J.; Kim, T.; Lim, J.; Jung, M.; Lim, Y.; Park, H.; Hong, J.; Pak, S.; Cha, S. Achieving Adsorbate-Free Monolayered MoS2 Field Effect Transistors by Controlled Surface Gas Treatment. ACS Appl. Electron. Mater. 2024, 6, 1763–1769. [Google Scholar] [CrossRef]
- Pak, S. Controlled p-Type Doping of MoS2 Monolayer by Copper Chloride. Nanomaterials 2022, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, T.; Wang, W.; Zhang, Z. Miracle in “white”: Hexagonal boron nitride. Small 2024, 21, 2400489. [Google Scholar] [CrossRef]
- Jiang, P.; Qian, X.; Yang, R.; Lindsay, L. Anisotropic thermal transport in bulk hexagonal boron nitride. Phys. Rev. Mater. 2018, 2, 064005. [Google Scholar] [CrossRef]
- Lu, S.; Shen, P.; Zhang, H.; Liu, G.; Guo, B.; Cai, Y.; Chen, H.; Xu, F.; Zheng, T.; Xu, F. Towards n-type conductivity in hexagonal boron nitride. Nat. Commun. 2022, 13, 3109. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Liang, W.; Sun, M. Electrical properties and applications of graphene, hexagonal boron nitride (h-BN), and graphene/h-BN heterostructures. Mater. Today Phys. 2017, 2, 6–34. [Google Scholar] [CrossRef]
- Cho, J.; Su, P.-C.; Kim, J. Highly thermally conductive and insulating composites fabricated through the hot-pressing of hollow structured h-BN/rGO hybrid filler. Appl. Mater. Today 2024, 37, 102149. [Google Scholar] [CrossRef]
- Liang, L.; Feng, Y.; Yang, K.; Wang, Z.; Zhang, Z.; Chen, X.; Chen, Q. High thermal conductivity electrical insulation composite EP/h-BN obtained by DC electric field induction. Polym. Compos. 2024, 45, 181–192. [Google Scholar] [CrossRef]
- Saji, V.S. 2D hexagonal boron nitride (h-BN) nanosheets in protective coatings: A literature review. Heliyon 2023, 9, e19362. [Google Scholar] [CrossRef]
- Kim, D.I.; Jeong, H.B.; Lim, J.; Jeong, H.S.; Kim, M.K.; Pak, S.; Lee, S.; An, G.H.; Chee, S.S.; Hong, J.P. A Practical Zinc Metal Anode Coating Strategy Utilizing Bulk h-BN and Improved Hydrogen Redox Kinetics. Energy Environ. Mater. 2025, 8, e12826. [Google Scholar] [CrossRef]
- Lim, J.; Heo, S.J.; Jung, M.; Kim, T.; Byeon, J.; Park, H.; Jang, J.E.; Hong, J.; Moon, J.; Pak, S. Highly Sustainable h-BN Encapsulated MoS2 Hydrogen Evolution Catalysts. Small 2024, 20, 2402272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Leng, J.; Jiang, Y.; Zhang, J. Experimental characterization of 3D printed PP/h-BN thermally conductive composites with highly oriented h-BN and the effects of filler size. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106586. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Pyo, J.; Lim, J.; Byeon, J.; Park, S.; Kang, S.; Park, S.; Lee, S.-T.; Kim, E.; Kim, M.K.; Sohn, J.I. Etchant-Free Wafer-Scale 2D Transfer and van der Waals 3D Integration via Peel-Off Force Engineering. ACS Nano 2025, 19, 25860–25869. [Google Scholar] [CrossRef] [PubMed]
- Deepika; Li, L.H.; Glushenkov, A.M.; Hait, S.K.; Hodgson, P.; Chen, Y. High-efficient production of boron nitride nanosheets via an optimized ball milling process for lubrication in oil. Sci. Rep. 2014, 4, 7288. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Kedawat, G.; Kanika; Gupta, S.; Kumar Gupta, B. An Innovative Method for Large-Scale Synthesis of Hexagonal Boron Nitride Nanosheets by Liquid Phase Exfoliation. ChemistrySelect 2020, 5, 12564–12569. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, F.; Wu, Y.; Hao, X.; Wang, Z.; Xu, X. One-step exfoliation and hydroxylation of boron nitride nanosheets with enhanced optical limiting performance. Adv. Opt. Mater. 2016, 4, 141–146. [Google Scholar] [CrossRef]
- Yuan, F.; Guan, Q.; Dou, X.; Yang, H.; Hong, Y.; Xue, Y.; Cao, Z.; Li, H.; Xu, Z.; Qin, Y. High-yield synthesis of hydroxylated boron nitride nanosheets and their utilization in thermally conductive polymeric nanocomposites. RSC Adv. 2024, 14, 21230–21240. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Xu, H.; Chen, L.; An, Y.; Gao, M.; Zhou, H.; Chen, J. Hydroxylated hexagonal boron nitride nanoplatelets enhance the mechanical and tribological properties of epoxy-based composite coatings. Prog. Org. Coat. 2022, 165, 106731. [Google Scholar] [CrossRef]
- Li, Z.; Li, K.; Li, Y.; Yu, Y.; Lv, J.; Liu, X.; Guan, K.; Lei, W.; Zhang, S.; Zhang, H. Modified molten salt assisted exfoliation of large-size 2D materials. Adv. Funct. Mater. 2024, 34, 2310371. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Q.; Jiang, P.; Huang, X. Rapid, high-efficient and scalable exfoliation of high-quality boron nitride nanosheets and their application in lithium-sulfur batteries. Nano Res. 2021, 14, 2424–2431. [Google Scholar] [CrossRef]
- Zuo, K.; Zhang, X.; Huang, X.; Oliveira, E.F.; Guo, H.; Zhai, T.; Wang, W.; Alvarez, P.J.; Elimelech, M.; Ajayan, P.M. Ultrahigh resistance of hexagonal boron nitride to mineral scale formation. Nat. Commun. 2022, 13, 4523. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Smith, L.; Wei, Z.; Williams, C.D.; Chiricotto, M.; Pereira da Fonte, C.; Carbone, P. Relationship between Capillary Wettability, Mass, and Momentum Transfer in Nanoconfined Water: The Case of Water in Nanoslits of Graphite and Hexagonal Boron Nitride. ACS Appl. Mater. Interfaces 2024, 16, 56316–56324. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lei, Z.; Yao, Y.; Liu, J.; Wu, B.; Ouyang, W. Anisotropic interfacial force field for interfaces of water with hexagonal boron nitride. Langmuir 2023, 39, 18198–18207. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Cho, D.-H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.-G. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Song, C.; Zhou, S.; Yang, F.; Kong, Y. Engineering carbon-defects on ultrathin g-C3N4 allows one-pot output and dramatically boosts photoredox catalytic activity. Appl. Catal. B Environ. 2021, 295, 120272. [Google Scholar] [CrossRef]
- Yang, F.; Tang, J.; Ou, R.; Guo, Z.; Gao, S.; Wang, Y.; Wang, X.; Chen, L.; Yuan, A. Fully catalytic upgrading synthesis of 5-Ethoxymethylfurfural from biomass-derived 5-Hydroxymethylfurfural over recyclable layered-niobium-molybdate solid acid. Appl. Catal. B Environ. 2019, 256, 117786. [Google Scholar] [CrossRef]
- Yang, F.; Shao, B.; Liu, X.; Gao, S.; Hu, X.; Xu, M.; Wang, Y.; Zhou, S.; Kong, Y. Nanosheet-like Ni-based metasilicate towards the regulated catalytic activity in styrene oxidation via introducing heteroatom metal. Appl. Surf. Sci. 2019, 471, 822–834. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Y.; Shen, Y.; Yuan, A.; Guo, Z.; Song, H.; Yang, F. Enabling room-temperature reductive C–N coupling of nitroarenes: Combining homogeneous and heterogeneous synergetic catalyses mediated by light. Green. Chem. 2022, 24, 4012–4025. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Sun, L.; Ward, Z.; Wang, H.; Ratnayake, G.; Wang, C.; Zhao, M.; He, H.; Gao, J. Two-dimensional Nanosheets by Liquid Metal Exfoliation. Adv. Mater. 2025, 37, 2416375. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Xue, J.; Ge, J.; He, J.; Hou, J.; Xie, Y.; Li, Y.; Zhang, H.; Sofer, Z. A library of 2D electronic material inks synthesized by liquid-metal-assisted intercalation of crystal powders. Nat. Commun. 2024, 15, 6388. [Google Scholar] [CrossRef]
- Nayak, A.P.; Dolocan, A.; Lee, J.; Chang, H.-Y.; Pandhi, T.; Holt, M.; Tao, L.; Akinwande, D. Inversion of the electrical and optical properties of partially oxidized hexagonal boron nitride. Nano 2014, 9, 1450002. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, B.; Li, F.; Yan, Y.; Wang, Y.; Li, R. Preparation of boron nitride nanosheets by glucose-assisted ultrasonic cavitation exfoliation. Nanoscale Adv. 2023, 5, 6582–6593. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized boron nitride nanosheets/poly (L-lactide) nanocomposites and their crystallization behavior. Polymers 2019, 11, 440. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Yuan, W.; Meng, Y.; Chu, Z.; Gan, W. A facile fabrication of nanocomposites with dual conductive networks based on 3D nickel foam, 1D silver nanowires and 2D boron nitride nanosheets. J. Mater. Sci. Mater. Electron. 2023, 34, 239. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3 (Sn) oxides for high-temperature gas sensors. Nanomaterials 2021, 11, 2938. [Google Scholar] [CrossRef]
- Matsumae, T.; Kurashima, Y.; Umezawa, H.; Tanaka, K.; Ito, T.; Watanabe, H.; Takagi, H. Low-temperature direct bonding of β-Ga2O3 and diamond substrates under atmospheric conditions. Appl. Phys. Lett. 2020, 116, 141602. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Yu, H.; Bulin, C.; Sun, H.; Li, R.; Ge, X.; Xing, R. High-efficient liquid exfoliation of boron nitride nanosheets using aqueous solution of alkanolamine. Nanoscale Res. Lett. 2017, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Garro Mena, L.; Hohn, K.L. Modification of hexagonal boron nitride by thermal treatment. J. Mater. Sci. 2021, 56, 7298–7307. [Google Scholar] [CrossRef]
- Abdullah, Y.; Husain, H.; Hak, C.R.C.; Alias, N.H.; Yusof, M.R.; Kasim, N.A.; Zali, N.M.; Mohamed, A.A. A short note on physical properties to irradiated nuclear fuel by means of X-ray diffraction and neutron scattering techniques. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; p. 040010. [Google Scholar]
- Cudziło, S.; Szermer-Olearnik, B.; Dyjak, S.; Gratzke, M.; Sobczak, K.; Wróblewska, A.; Szczygieł, A.; Mierzejewska, J.; Węgierek-Ciura, K.; Rapak, A. Combustion Synthesis of Functionalized Carbonated Boron Nitride Nanoparticles and Their Potential Application in Boron Neutron Capture Therapy. Materials 2024, 17, 2438. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Liu, T.; Jin, H.; Li, Z.; Liu, Y.; Liu, D.; Wang, D. Influence of O2 flow rate on the properties of Ga2O3 growth by RF magnetron sputtering. Micromachines 2023, 14, 260. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Exfoliation of hexagonal boron nitride (h-BN) in liquide phase by ion intercalation. Nanomaterials 2018, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tan, J.; Pan, Y.; Cai, X.; Zou, X.; Cheng, H.-M.; Liu, B. Mass production of 2D materials by intermediate-assisted grinding exfoliation. Natl. Sci. Rev. 2020, 7, 324–332. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Kim, D.; Park, S.; Lee, S.-T.; Hong, J.; Lee, S.; Pak, S. Scalable High-Yield Exfoliation of Hydrophilic h-BN Nanosheets via Gallium Intercalation. Inorganics 2025, 13, 314. https://doi.org/10.3390/inorganics13100314
Kang S, Kim D, Park S, Lee S-T, Hong J, Lee S, Pak S. Scalable High-Yield Exfoliation of Hydrophilic h-BN Nanosheets via Gallium Intercalation. Inorganics. 2025; 13(10):314. https://doi.org/10.3390/inorganics13100314
Chicago/Turabian StyleKang, Sungsan, Dahun Kim, Seonyou Park, Sung-Tae Lee, John Hong, Sanghyo Lee, and Sangyeon Pak. 2025. "Scalable High-Yield Exfoliation of Hydrophilic h-BN Nanosheets via Gallium Intercalation" Inorganics 13, no. 10: 314. https://doi.org/10.3390/inorganics13100314
APA StyleKang, S., Kim, D., Park, S., Lee, S.-T., Hong, J., Lee, S., & Pak, S. (2025). Scalable High-Yield Exfoliation of Hydrophilic h-BN Nanosheets via Gallium Intercalation. Inorganics, 13(10), 314. https://doi.org/10.3390/inorganics13100314







