Abstract
The metal-free polymeric semiconductor graphitic carbon nitride (g-C3N4) has emerged as a promising material for photocatalytic applications due to its responsiveness to visible light, adjustable electronic structure, and stability. This review systematically summarizes recent advances in preparation strategies, including thermal polycondensation, solvothermal synthesis, and template methods. Additionally, it discusses modification approaches such as heterojunction construction, elemental doping, defect engineering, morphology control, and cocatalyst loading. Furthermore, it explores the diverse applications of g-C3N4-based materials in photocatalysis, including hydrogen (H2) evolution, carbon dioxide (CO2) reduction, pollutant degradation, and the emerging field of piezoelectric photocatalysis. Particular attention is given to g-C3N4 composites that are rationally designed to enhance charge separation and light utilization. Additionally, the synergistic mechanism of photo–piezocatalysis is examined, wherein a mechanically induced piezoelectric field facilitates carrier separation and surface reactions. Despite significant advancements, challenges persist, including limited visible-light absorption, scalability issues, and uncertainties in the multi-field coupling mechanisms. The aim of this review is to provide guidelines for future research that may lead to the development of high-performance and energy-efficient catalytic systems in the context of environmental and energy applications.
1. Introduction
With the rapid development of human society and the acceleration of modernization, the impact of environmental pollution and energy shortage is deepening [1]. Since the Second Industrial Revolution, the rapid development of the global economy has been accompanied by excessive fossil fuel combustion, uncontrolled industrial wastewater discharge, and the misuse of antibiotics. These actions, akin to an invisible sword, have gradually eroded Earth, which is essential for human survival, triggering a series of severe energy crises and environmental pollution issues. The intensification of the greenhouse effect, the accumulation of heavy metals in soil, the eutrophication of aquatic systems, and other phenomena serve as silent warnings that we are confronting unprecedented ecological challenges. More critically, these pollutants continue to accumulate within the food chain and, even at very low concentrations, can inflict significant harm on the human body, thereby threatening human life and health. For nearly a century, the greenhouse effect has remained a major environmental issue afflicting humanity [2]. Nowadays, China’s “dual-carbon” goal has been elevated to the level of national strategy and has become an important part of building a strong modern socialist country [3,4]. Among all gases confirmed to contribute to the greenhouse effect, carbon dioxide (CO2) is the predominant emitter, responsible for approximately 60% of total greenhouse gas emissions [5]. The effective and efficient utilization of solar energy can significantly decrease the reliance on fossil fuels, thereby contributing to the achievement of the “dual carbon” goals [6]. Zou (2021) summarized international practices for achieving carbon neutrality, categorizing them into four main aspects: carbon substitution, carbon emission reduction, carbon sequestration, and carbon recycling [7]. The escalating energy crisis and environmental pollution underscore the urgency of exploring clean renewable energy carriers and reducing excessive dependence on traditional fossil fuels [8]. In light of this challenge, there is an urgent need to actively develop and utilize alternative clean energy sources. The primary objectives are to reduce carbon emissions, optimize the energy structure, and achieve resource recycling.
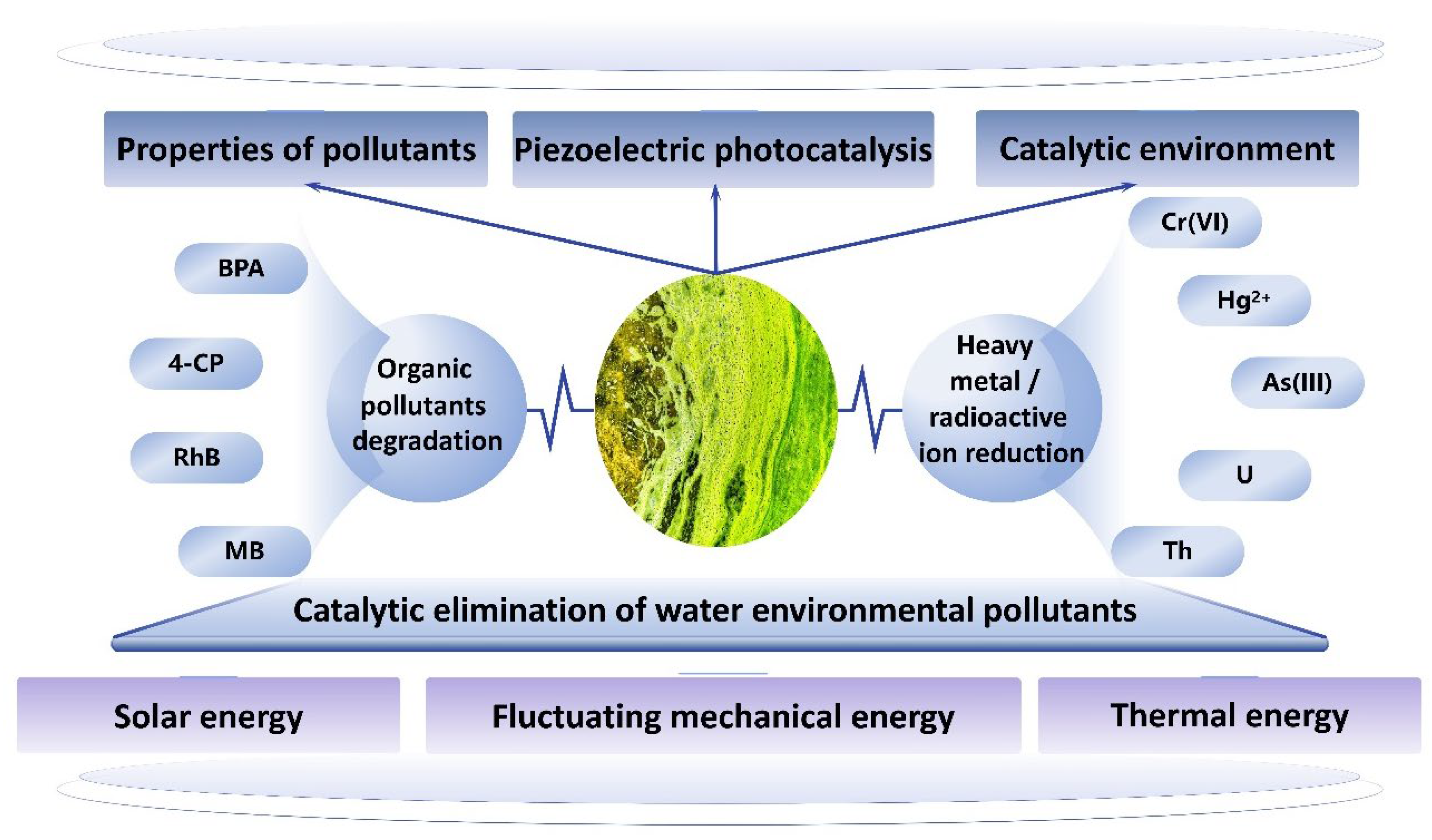
In the natural environment, solar energy, waste heat, and mechanical vibrations of water bodies represent significant potential energy sources. Through the implementation of efficient collection and conversion technologies, these forms of energy can be transformed into usable power. This approach not only reduces our dependence on fossil fuels but also addresses the pressing issue of environmental pollution [9,10,11]. Photocatalysis is a promising strategy for reducing dependence on fossil fuels and addressing environmental pollution issues [12]. The efficient conversion of clean solar energy to chemical energy through semiconductor photocatalytic technology is regarded as a viable technical approach to mitigate energy shortages and environmental pollution [13]. Solar-driven photocatalysis can effectively eliminate environmental pollutants by generating photogenerated electron–hole pairs and triggering a series of redox reactions. However, its practical application is significantly hindered by low photocatalytic activity, primarily due to the high recombination rate of photogenerated carriers [14,15]. The construction of internal electric fields in photocatalytic materials provides a driving force for charge separation, reduces the probability of recombination between photogenerated electron–hole pairs, and enhances the rates of reduction and oxidation reactions on their surfaces [16]. Although various methods have been employed to construct internal electric fields, including the design of heterojunctions, the effects of conventional internal electric fields are often short-lived due to the extreme saturation of external charges and internal photogenerated carriers [17,18]. Therefore, developing a strategy for the sustainable provision of carrier drives is essential. Notably, water bodies contain an abundance of fluctuating mechanical energies and converting these mechanical energies into a cyclic and continuous internal electric field through the piezoelectric effect can assist in the continuous separation of photogenerated charges. The polluted water environment mainly includes organic pollutants and heavy metal ions [19]. Organic pollutants primarily include bisphenol A (BPA), p-chlorophenol (4-CP), rhodamine B (RhB), and methylene blue (MB). Heavy metal ions predominantly consist of hexavalent chromium (Cr6+), mercury ions (Hg2+), trivalent arsenic (As3+), and certain radioactive elements such as uranium (U) and thorium (Th). The mechanical and catalytic environments provided by fluctuations in the aquatic ecosystem, along with natural light, facilitate the piezoelectric photocatalysis process, which enables the harmless degradation of organic pollutants and the reduction of heavy metal ions. Consequently, the integration of the piezoelectric effect and photocatalytic effect presents a pioneering solution for the sustainable degradation of pollutants in aquatic environments (Figure 1). This strategy harnesses renewable energy sources, including solar energy, fluctuating mechanical energy, and thermal energy, resulting in reaction products that do not generate secondary pollution. This approach aligns with the scientific objectives of achieving a green cycle and low carbon emissions, thereby possessing significant research and application value.
Figure 1.
Piezoelectric–photocatalytic solution to water pollution.
The sensitivity and response of photocatalytic outputs from single materials possessing photocatalytic properties are significantly below the actual demand. Therefore, optimizing the design of semiconductor photocatalytic materials, selecting appropriate piezoelectric materials, and integrating the properties of both semiconductors and piezoelectric materials are essential for enhancing carrier transfer efficiency. Such enhancements can improve the piezoelectric output capacity, bringing it closer to actual energy demands. Consequently, the design and development of a nano-piezoelectric photocatalyst that integrates a photosensitive semiconductor with a piezoelectric semiconductor to broaden its application scope is a topic of considerable contemporary interest.
Graphitic carbon nitride (g-C3N4) is a novel non-metallic polymer semiconductor material characterized by its unique two-dimensional layered structure and conjugated system. Its appropriate band gap of approximately 2.7 eV endows it with a significant ability to respond to visible light. Furthermore, g-C3N4 exhibits remarkable physical and chemical stability, along with distinctive electronic properties, which render it a promising candidate in the field of photocatalysis. Consequently, it has found widespread applications in energy conversion, environmental remediation, and catalysis [20]. In 2009, Wang et al. [21] were the first to apply graphitic carbon nitride (g-C3N4) in photocatalysis, demonstrating its capability to catalyze the decomposition of water under visible light irradiation, resulting in the production of H2 and O2. Ong et al. [22] synthesized g-C3N4 through thermal polymerization using various nitrogen-containing organic compounds. Numerous scholars have conducted continuous research on g-C3N4, revealing that this material possesses unique advantages in the field of photocatalysis. g-C3N4 can be synthesized from various nitrogen-rich precursors, such as melamine, urea, and dicyandiamide, through a straightforward thermal polymerization method. These raw materials are readily available and suitable for large-scale production [23]. As a metal-free catalyst, g-C3N4 is devoid of toxic heavy metal elements and does not generate secondary pollution during its application. It adheres to the principles of green chemistry, and its excellent biocompatibility further enhances its potential applications in biomedical and environmental fields [20]. Compared to traditional wide-band-gap semiconductors like TiO2 (3.2 eV), g-C3N4 features a narrower band gap of approximately 2.7 eV, enabling more effective utilization of visible light for photocatalytic reactions [24]. The optical absorption range, charge separation efficiency, and surface reactivity of g-C3N4 can be further optimized through strategies such as nanostructure design, element doping, and heterostructure construction [25]. These advantages position g-C3N4 materials as having significant potential for application in environmental remediation, particularly in water treatment and air purification. They can effectively degrade and remove a wide range of pollutants, including organic dyes, antibiotics, heavy metal ions, gaseous pollutants, pesticides, endocrine disruptors, and other organic compounds [20].
However, g-C3N4 also presents several limitations, including a small specific surface area, a narrow light response range, and the propensity for rapid recombination of photogenerated electrons and holes, which restrict its photocatalytic performance [26]. The primary reason for the deficiencies in the photocatalytic activity of g-C3N4 is its amorphous nature, which leads to a significant number of carrier complexation centers on its surface. Although numerous researchers have successfully synthesized high-crystallinity g-C3N4 photocatalysts that exhibit superior capabilities for photogenerated carrier separation, these high-crystalline g-C3N4 catalysts often lack reactive sites due to the elimination of many non-condensed amino groups. Consequently, this limitation restricts their efficiency in utilizing photogenerated carriers. To enhance the photocatalytic performance of g-C3N4, it is essential to optimize its structure by creating additional reactive sites. However, improvements in photocatalytic performance remain constrained by the modification of a single material, as the inherent issue of a high carrier complexation rate necessitates the development of more advanced structures. Techniques such as semiconductor modification and heterojunction construction can be employed to adjust the properties of individual materials, thereby enhancing the transfer and utilization efficiency of photogenerated carriers and facilitating charge separation between semiconductors. Hossain et al. successfully constructed heterojunctions using TiO2 and g-C3N4, which significantly inhibit the recombination of photogenerated carriers, leading to a marked improvement in photocatalytic activity under UV/visible light irradiation [27]. In polluted water, the flow is rich in mechanical energy, which can be harnessed through the piezoelectric effect to convert mechanical force into electric field force, thereby facilitating the migration of photogenerated carriers. This migration, during the photocatalytic reaction, is also influenced by the Coulomb force. The integration of the piezoelectric effect with photocatalysis offers a viable solution that aligns with the green “dual carbon” initiative. Semiconductor material complexes can establish a built-in electric field through the construction of heterojunctions, promoting the transfer of carriers near the interface. Furthermore, the direction of the polarized electric field generated by the piezoelectric effect periodically changes with the expansion and contraction of the device during the simple harmonic vibration of the piezoelectric material. Under the combined influence of the built-in electric field and the polarized electric field, the migration of carriers can be effectively driven, thereby minimizing the ineffective aggregation of carriers on the surface and enhancing the performance of the catalytic reaction.
This review systematically summarizes recent research progress on graphitic carbon nitride and its composites within the domains of photocatalysis and the emerging field of photo-pressure synergistic catalysis. It emphasizes the regulatory mechanisms associated with various material design strategies, including the construction of composite structures, control of morphology, surface modification, and element doping, and their effects on light absorption, charge carrier dynamics, and catalytic activity. Furthermore, it explores the construction principles, performance advantages, and fundamental synergistic mechanisms of g-C3N4-based photo-pressure synergistic catalysts. A comparative analysis of the efficiency of different systems in energy conversion and environmental purification applications is also provided. Additionally, this review critically addresses current research challenges, such as the necessity for a deeper understanding of complex coupling mechanisms, the large-scale synthesis of efficient and stable catalysts, and the adaptability to practical application scenarios. Finally, potential future development directions are discussed, including the creation of intelligent responsive materials, exploration of multi-field coupling mechanisms, and enhancement of energy utilization efficiency. It is anticipated that this review will serve as a valuable reference for future explorations in this field and for the design and development of high-performance catalysts, ultimately advancing green catalytic technology towards practical applications.
2. Introduction to Photocatalysis
2.1. Principles of Photocatalytic Technology
The pioneering technology of photocatalysis emerged in 1972 [28], marked by the discovery of hydrogen generation through water splitting by Fujishima and Honda. They proposed a theory suggesting that solar-powered photocatalysts, specifically oxide semiconductors with an appropriate band gap (Eg), can efficiently absorb light and generate photogenerated electron holes [29]. Since then, the application of photocatalytic technology to the degradation of organic pollutants has garnered significant attention. Over time, the applications of this technology have expanded extensively, encompassing not only the degradation of organic pollutants but also the photolysis of water to produce hydrogen, CO2 reduction, heavy metal remediation, and disinfection and sterilization [30,31]. The application of photocatalytic technology in water treatment enhances water quality safety and addresses the global issue of water scarcity [32].
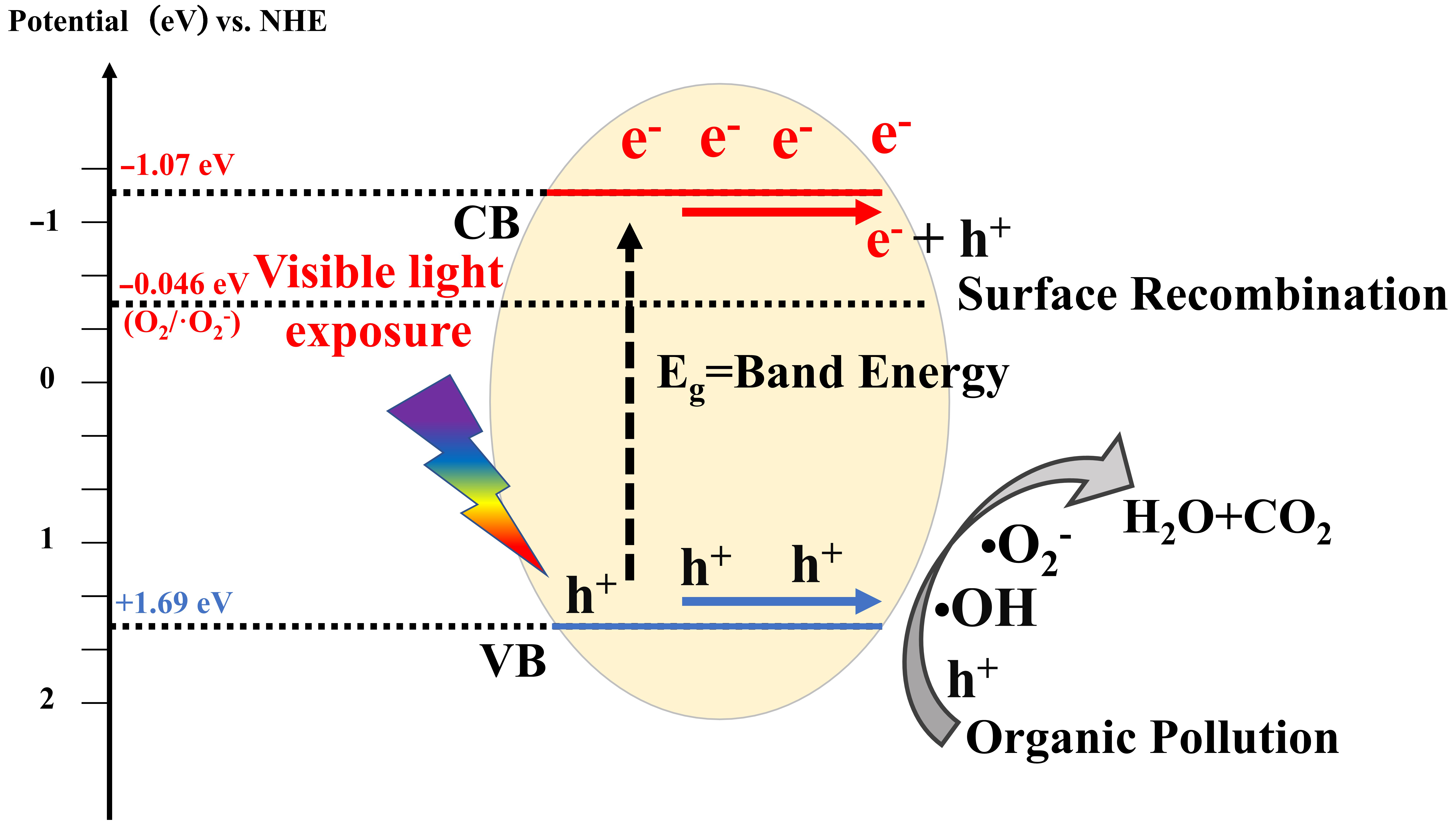
Photocatalysis refers to a series of photochemical reactions that occur under the influence of visible, ultraviolet, and even infrared light, facilitated by photocatalysts. This process involves the use of semiconductor materials to generate active species with oxidation and reduction capabilities under light exposure, resulting in the transformation of pollutants [33]. To date, research on the fundamental mechanisms of photocatalysis has reached a relatively advanced stage. Essentially, photocatalysis can be categorized into four primary stages: light collection, charge excitation, charge separation and transfer, and surface electrocatalytic reactions [34,35]. As illustrated in Figure 2, the core of photocatalytic degradation of organic matter lies in the interaction between active species and reactants. Thus, the effective utilization of visible light, efficient separation of photogenerated carriers, and the enhancement of the exposed specific surface area at various reaction sites are crucial for increasing the number of active species. These aspects have become focal points for further research by many experts and scholars, as well as challenges faced by photocatalytic technology.
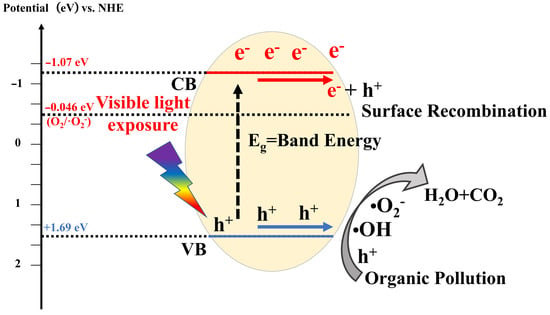
Figure 2.
Photocatalytic reaction mechanism.
2.2. Problems Faced by Photocatalytic Technology
Despite extensive research on photocatalytic technology, its practical application is still hindered by low quantum efficiency, complex catalyst design, and limited light response ranges [36]. The key challenges include low quantum efficiency resulting from the rapid recombination of photogenerated electron–hole pairs on picosecond to nanosecond timescales, which reduces carrier lifetimes below the requirements for effective reactions [37,38]; insufficient light utilization due to the wide band gaps of most materials, which restricts absorption primarily to ultraviolet light while underutilizing the visible and near-infrared spectra [39]; complex catalyst design characterized by ambiguous active sites and unclear synergistic mechanisms in composite materials; difficulties in energy band regulation, necessitating comprehensive control over composition, structure, and phase state; and practical application barriers such as high preparation costs, poor reproducibility, and limitations in scalability.
Addressing these issues necessitates a robust foundation in the research of structure–activity relationships and reaction mechanisms. It also requires the utilization of computational simulations and advanced analytics to facilitate precise catalyst design. Furthermore, fostering interdisciplinary collaboration is essential to integrate cross-domain knowledge for the advancement of material development.
2.3. Classification and Research Status of Common Photocatalysts
The advancement of photocatalytic technology requires a careful balance between enhancing light utilization efficiency and preserving the redox capabilities of materials. Photocatalytic materials have traditionally been classified into four categories: oxides, sulfides, nitrides, and emerging nanomaterials.
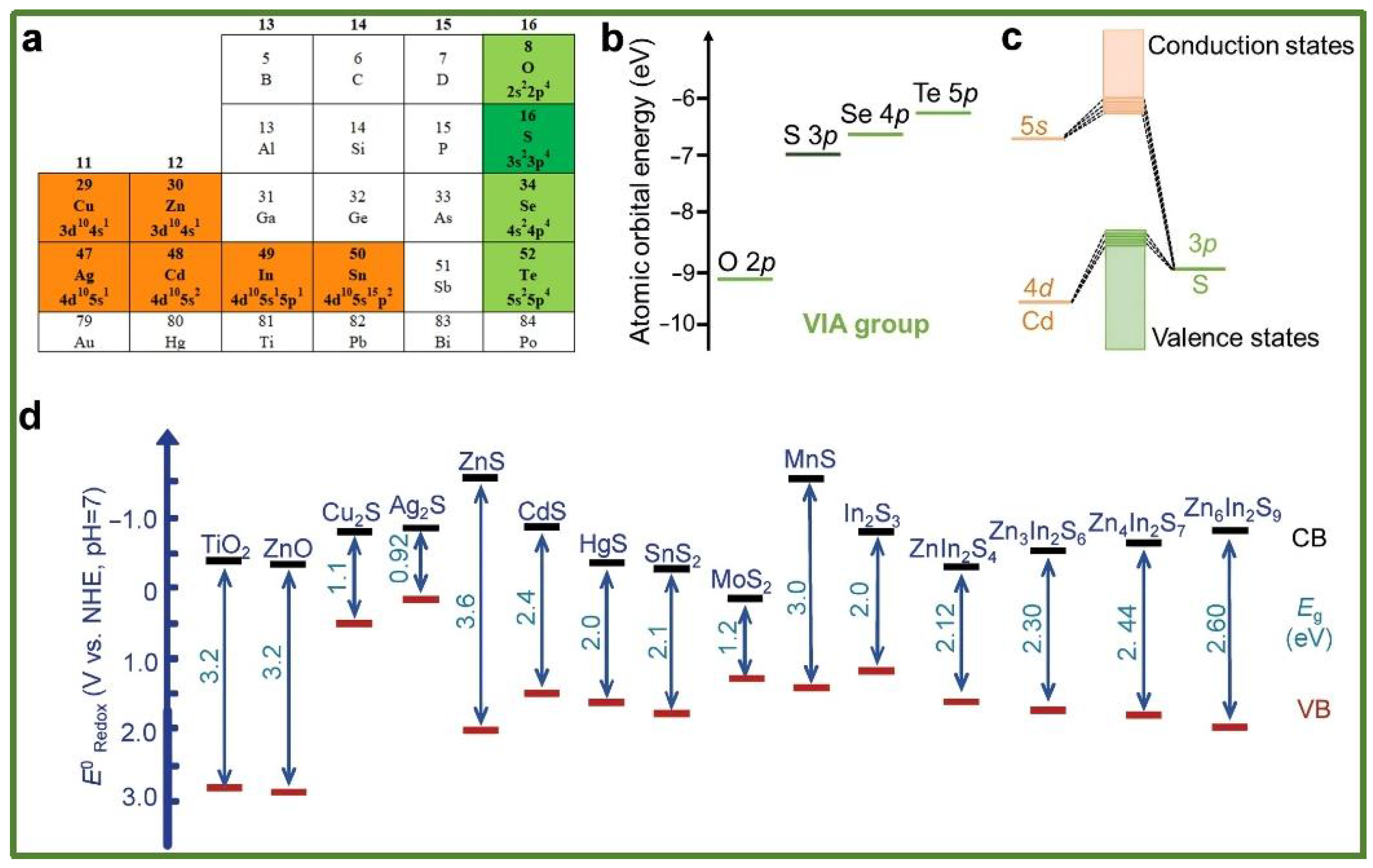
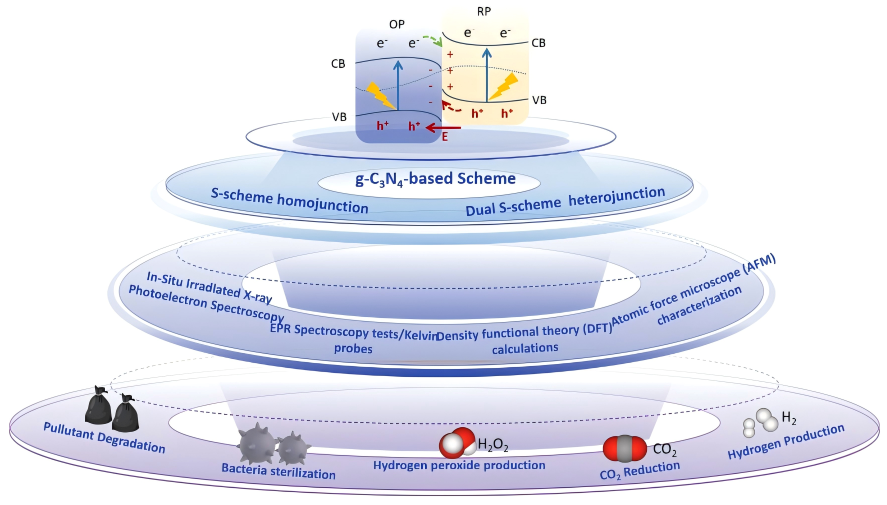
Although metal oxides (e.g., TiO2, ZnO) are widely utilized, their development is hindered by the toxicity of certain metallic elements and low reaction selectivity. Sulfides enhance visible-light absorption due to their narrow band gaps; however, they are plagued by severe photocorrosion phenomena, which necessitate improvements through strategies such as heterojunction construction. Metal sulfides are a class of highly dynamic semiconductor photocatalytic materials, characterized by cations primarily composed of metals with d10 electronic configurations, such as Zn, Cd, Cu, Ag, In, and Sn (Figure 3a). In metal sulfides, the cations hybridize with the atomic orbitals of S2−, resulting in numerous closely spaced molecular orbitals, which form conduction bands (CBs) and valence bands (VBs) [40]. The band edge, defined as the bottom of the CB and the top of the VB, is primarily characterized by the outermost orbitals of atoms. The atomic energy of the outermost orbital (np) for elements in the sulfur family typically increases as one moves down the group (as illustrated in Figure 3b, with energy values sourced from the NIST database). Figure 3c presents the electronic structure of cadmium sulfide (CdS). Notably, the conduction band energy of most metal sulfide semiconductors is negative, indicating their strong reducing capability, akin to that of metal oxides. Furthermore, the oxidation capacity of most metal sulfides is generally weaker than that of metal oxides, as the valence band position contributed by the sulfur 3p orbitals is higher than that of the oxygen 2p orbitals (refer to Figure 3d). Consequently, metal sulfides are characterized by a strong reducing ability, relatively weak oxidizing ability, and a narrow band gap. Nitrides (e.g., C3N4) can generate highly reactive electron–hole pairs owing to their wide band gap structures, demonstrating significant potential in catalytic efficiency. They have been extensively studied for applications including water treatment, air purification, and bacterial disinfection (Figure 4). Nevertheless, challenges persist, including a limited light-response range, a high charge carrier recombination rate, and elevated preparation costs. The applications of emerging nanomaterials (e.g., MOFs, MXenes) are also expanding.
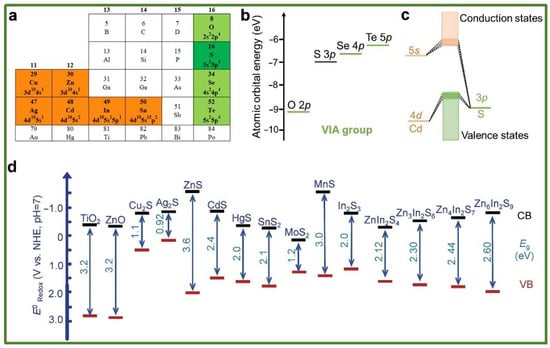
Figure 3.
(a) The main group of metal sulfides (highlighted); (b) atomic orbital energies of O, S, Se, and Te; (c) the electronic structure of CdS; and (d) the energy band positions of some typical oxides and sulfides [40] (reprint from Wu, X.; Xie, S.; Zhang, H.; et al. Metal Sulfide Photocatalysts for Lignocellulose Valorization. Adv. Mater. 2021, 33, 2007129).
Figure 4.
S-type heterostructured g-C3N4 photocatalysts for photocatalytic environmental pollutant abatement and sterilization.
Among numerous catalysts, graphitic carbon nitride (g-C3N4) has emerged as a focal point of research due to its cost-effective raw materials, namely, urea and melamine, as well as its straightforward single-step thermal polymerization preparation method. Additionally, g-C3N4 exhibits a remarkable combination of exceptional redox performance and structural stability. Future research will focus on optimizing its structural design to achieve a synergistic enhancement of light absorption and redox activity.
3. Study Progress of the g-C3N4 Photocatalysts
3.1. Introduction to g-C3N4 Photocatalysts
Carbon nitride is composed of carbon (C) and nitrogen (N), both of which are abundant on Earth [41]. Active carbon and nitrogen in soil serve as crucial components of the soil’s carbon and nitrogen reservoirs, playing a vital role in the carbon and nitrogen cycles within ecosystems [42]. Moreover, C and N are essential elements in living organisms, combining with hydrogen (H) and oxygen (O) to form organic molecular compounds such as proteins, nucleic acids, and amino acids. According to covalent bonding theory, C3N4 represents the optimal chemical formula for the formation of nitrides. Graphitic carbon nitride (g-C3N4) is a novel class of non-metallic polymer semiconductors that function as a visible light-responsive photocatalyst due to their unique optical properties [43]. In contrast to traditional semiconductor photocatalytic materials, the intentional synthesis of g-C3N4 photocatalysts primarily emphasizes the construction of catalytic sites and the modulation of charge transfer dynamics [44]. g-C3N4 offers several advantages, including a relatively simple preparation method, low-cost and readily available raw materials, corrosion and high-temperature resistance, stability, good chemical and thermal stability, and non-toxicity, indicating a promising application in energy and environmental fields. The band gap of g-C3N4 is approximately 2.7 eV, with the conduction band minimum around −1.1 eV and the valence band maximum at approximately +1.6 eV. g-C3N4 demonstrating excellent responsiveness to visible light, making it superior to other semiconductors and increasingly significant in photocatalytic technology.
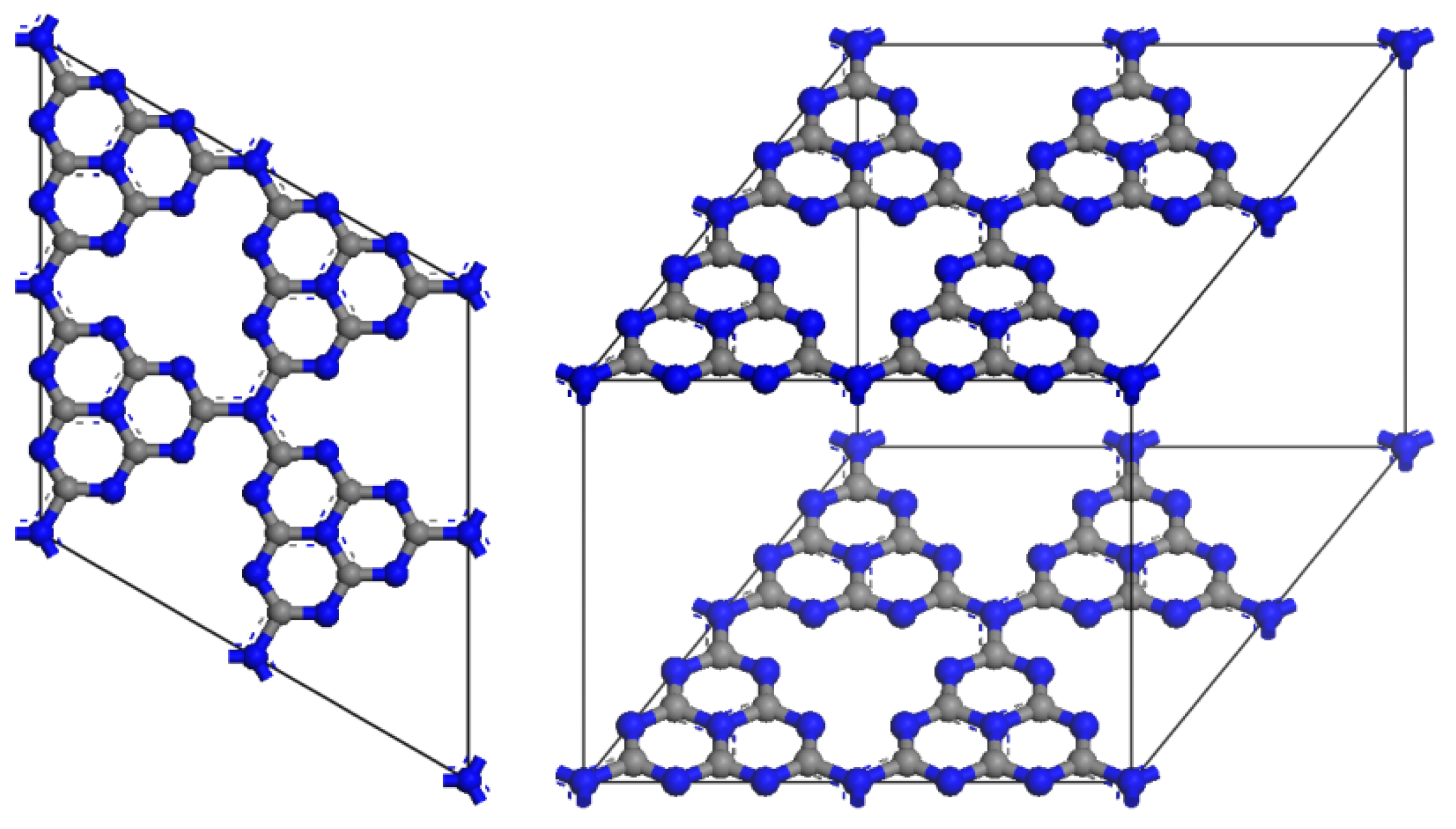
g-C3N4 exhibits a distinctive two-dimensional structure composed of carbon and nitrogen atoms, imparting unique structural characteristics essential for its photocatalytic efficiency. The core structure consists of stacked layers of carbon and nitrogen atoms arranged in a planar hexagonal lattice, akin to that of graphite, thus earning the designation “graphite-like” [45]. The structure consists of layers formed by tri-s-triazine (C3N3) units. Each layer is composed of these tri-s-triazine units, which are held together by weak van der Waals forces (Figure 5). This lamellar structure enhances the specific surface area, thereby providing a greater number of active and reactive sites for catalytic reactions [46]. In the g-C3N4 lattice, the carbon atoms are sp2 hybridized, which contributes to the planar triangular geometry of the crystal structure. g-C3N4 consists of two fundamental structural units: the triazine ring and the heptazine ring. Notably, calculations based on density functional theory indicate that the heptazine ring (triple-s-triazine) exhibits greater stability than the triazine ring. Furthermore, g-C3N4 can adsorb various functional groups, including amino, hydroxyl, and carboxyl groups, on its surface, allowing for modification through adsorption. These functional groups enhance the surface charge, hydrophilicity, and chemical reactivity of the material, rendering it suitable for specific photocatalytic applications [47,48,49]. The band gap of g-C3N4 is measured at 2.7 eV at pH 7.0, which significantly enhances its applicability. This band gap enables g-C3N4 to be excited by visible light with an excitation wavelength of approximately 475 nm, a crucial factor for photocatalytic reactions. Recent research has established effective control over the growth mechanism of g-C3N4 and the customized design of its structure. By adjusting parameters such as layer spacing, band gap, and surface chemistry, g-C3N4 emerges as a formidable candidate for addressing future environmental and energy challenges, thereby contributing significantly to a sustainable and cleaner future.
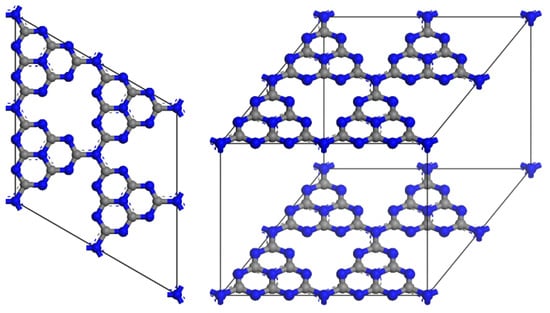
Figure 5.
Crystal structure of g-C3N4.
3.2. Preparation Method of g-C3N4 Photocatalysts
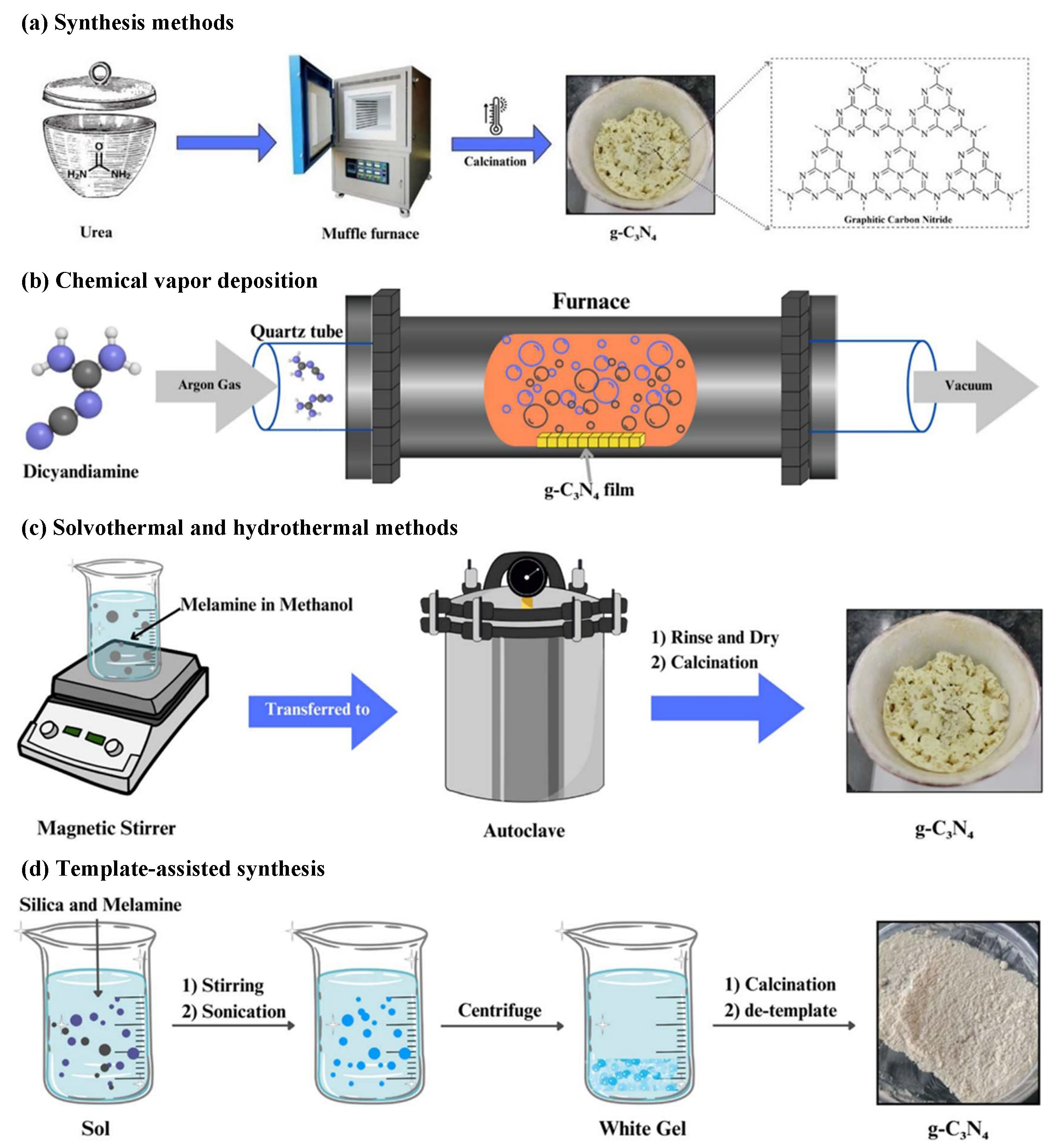
The synthesis of graphitic phase carbon nitride (g-C3N4) has experienced significant advancements since its initial proposal, enabling researchers to produce g-C3N4 materials with various morphologies and structures. These developments allow for the tailoring and design of desired properties for photocatalytic applications through diverse preparation methods. Notably, g-C3N4 has not been discovered in nature and must be synthesized using artificial methods. The mainstream synthetic preparation techniques, illustrated in Figure 6, include thermal polycondensation [50,51,52], chemical vapor deposition (CVD) [53,54], solvothermal versus hydrothermal methods [55,56], and the template-assisted synthesis method [57]. The raw materials utilized in these processes include melamine, urea, cyanamide, dicyandiamide, thiourea, and cyanuric acid.
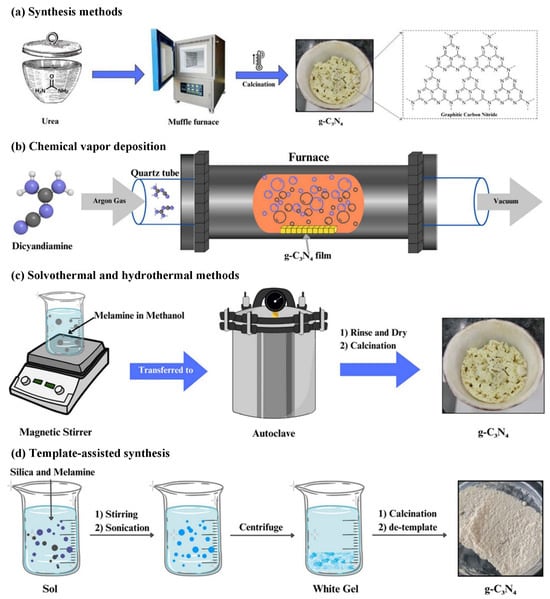
Figure 6.
Synthesis of graphite-phase carbon nitride (g-C3N4) by (a) thermal polycondensation [50,51,52]; (b) chemical vapor deposition [53,54]; (c) solvothermal versus hydrothermal [55,56] and (d) template-assisted synthesis [57].
Chemical vapor deposition (CVD) involves the vaporization of precursors containing carbon and nitrogen in a vacuum environment, allowing for chemical reactions to occur on the surface of a heated substrate, resulting in the deposition of a g-C3N4 film. CVD can produce g-C3N4 samples with exceptionally high crystallinity, and these samples exhibit carrier mobility that is several times greater than that of samples prepared using traditional thermal polymerization methods [58]. The high crystallinity of the material significantly reduces both internal and surface defects, effectively inhibiting the recombination of photogenerated electron–hole pairs, thereby greatly enhancing photocatalytic performance. Additionally, g-C3N4 synthesized via the CVD method exhibits excellent uniformity and potential for large-area fabrication. Researchers have demonstrated that a uniform film with a thickness fluctuation of less than 5% can be achieved through CVD deposition on a planar substrate. This uniformity is crucial for the development of high-performance optoelectronic devices [59]. However, traditional chemical vapor deposition (CVD) systems necessitate high vacuum conditions, sophisticated gas flow control systems, and high-temperature heating devices, leading to significantly higher equipment investment and maintenance costs compared to other methods. Furthermore, the CVD process typically demands high energy consumption to sustain the elevated temperature and vacuum environment, which further escalates production costs. Another limiting factor is the relatively low growth rate associated with CVD. Due to the reliance on surface reactions of gas-phase precursors, the deposition rate of g-C3N4 produced via the CVD method is generally only a few microns per hour. This results in the need for extended processing times to prepare thick film materials, thereby reducing production efficiency. In practical applications, g-C3N4 synthesized through CVD is primarily utilized in high value-added fields, such as high-performance optoelectronic devices, precision sensors, and efficient catalytic systems.
The thermal polycondensation method is the most traditional and widely utilized technique for the preparation of g-C3N4. This method involves the thermally induced polycondensation of organic precursors containing carbon and nitrogen—such as urea, dicyandiamide, and melamine—at elevated temperatures typically ranging from 500 to 600 °C, resulting in the formation of a g-C3N4 polymer network. A significant advantage of thermal polycondensation lies in its simplicity and minimal equipment requirements. In contrast to the conventional CVD method, which necessitates a complex vacuum system, the thermal polycondensation method can be executed using a standard muffle furnace or tube furnace. This considerably reduces both equipment investment and maintenance costs, thereby enabling the preparation of g-C3N4 materials even in laboratories with limited research facilities, which in turn fosters extensive research on this material. However, g-C3N4 synthesized via the traditional thermal polycondensation method exhibits issues such as low crystallinity and a restricted specific surface area. Various structural defects, including nitrogen vacancies, carbon vacancies, and incomplete heptazine units, are likely to arise during high-temperature polymerization. These defects serve as recombination centers for photogenerated electron–hole pairs, consequently diminishing photocatalytic efficiency [58]. The bulk g-C3N4 synthesized through traditional methods typically exhibits a limited specific surface area, generally less than 10 m2/g, and a reduced number of exposed active sites. These characteristics significantly constrain its catalytic performance [25]. The thermal condensation method is currently the sole approach for achieving large-scale industrial production of g-C3N4. This method is particularly significant in the domain of photocatalytic environmental remediation, where g-C3N4 synthesized through thermal polymerization has demonstrated substantial practical application value. Results indicate that the g-C3N4 photocatalyst, produced by an enhanced thermal condensation method, operates steadily in a custom-designed continuous flow device for a duration of 20 days under real outdoor sunlight, consistently maintaining a pollutant removal efficiency of nearly 100% [60]. Future research on thermal condensation will concentrate on optimizing the process further and minimizing energy consumption. This includes the development of low-temperature polymerization processes, the utilization of microwave-assisted heating to enhance thermal efficiency, and the investigation of more economical precursor sources.
The solvothermal and hydrothermal methods are categorized under liquid-phase synthesis technologies. By heating the precursor solution in a high-pressure reactor, these methods leverage the unique properties of the solvent under high-temperature and high-pressure conditions to facilitate the condensation reactions of carbon and nitrogen precursors, ultimately leading to the formation of g-C3N4. A significant advantage of the solvothermal and hydrothermal methods is their capability to synthesize g-C3N4 at relatively low temperatures. In contrast to the traditional thermal condensation method, which necessitates temperatures ranging from 500 to 600 °C, the hydrothermal and solvothermal methods typically achieve precursor polycondensation within the temperature range of 200 to 300 °C, thereby considerably reducing energy consumption. However, despite their numerous advantages, the low yield and crystallinity associated with the solvothermal and hydrothermal methods pose challenges for large-scale applications. The reaction occurring in a liquid-phase environment limits the processing capacity due to the volume of the solvent. Typically, laboratory-scale hydrothermal reactors can process only tens to hundreds of milliliters of precursor solution at a time, which restricts the yield of g-C3N4 [59]. In terms of application, g-C3N4 prepared via solvothermal or hydrothermal methods primarily demonstrates potential in high-performance fields. For instance, studies indicate that g-C3N4 nanosheets treated using the hydrothermal method exhibit significant activity in photocatalytic hydrogen production, achieving H2 yields of 20–60 μmol·h−1·g−1. Regarding CO2 photoreduction, g-C3N4-based catalysts synthesized through the solvothermal method have shown remarkable product selectivity, with CH4 yields increasing threefold [61]. However, these applications remain in the laboratory research phase, and there is still a significant gap to bridge before achieving large-scale industrial implementation.
Template-assisted synthesis is a preparation method that facilitates the formation of specific structures in g-C3N4 by utilizing a template. This method can be categorized into hard template and soft template approaches based on the type of template employed. Hard templates typically consist of inorganic materials with regular pore structures, such as mesoporous SiO2, while soft templates utilize organic molecules, including surfactants or block copolymers. The template method enables precise control over the pore structure and morphology of g-C3N4 at the nanoscale, making it the predominant technique for preparing porous g-C3N4. A significant advantage of template-assisted synthesis is its ability to accurately regulate the pore structure and specific surface area of g-C3N4. However, this method is also associated with certain limitations, including a complex processing workflow and high costs, as well as the potential for structural damage to g-C3N4 during the template removal phase. Consequently, this technique is primarily suited for laboratory research and high value-added applications.
The introduction of the four preparation methods reveals that the thermal polycondensation method has emerged as a more widely utilized approach in practical applications, owing to its straightforward process, favorable preparation conditions, and cost-effectiveness. Future large-scale production should prioritize process optimization, the selection of precursors (such as the development of economical precursor systems), the design of specialized reactors, waste management during production, and the establishment of recycling processes.
3.3. Defects of g-C3N4 Photocatalysts
g-C3N4 has garnered significant attention due to its remarkable thermal and chemical stability, unique electronic structure, responsiveness to visible light, and various other advantages [6]. This material has a wide range of applications in photocatalysis, bioimaging, optoelectronic sensors, and photovoltaic solar cells. It is undoubtedly a photocatalyst with exceptional capabilities and potential. However, as a standalone photocatalyst, it faces several challenges in transitioning from experimental settings to practical applications: (1) Limited utilization of the effective visible light spectrum: The band gap of 2.7 eV is broader than that of typical semiconductor photocatalysts, rendering it more sensitive to visible light. This wider band gap restricts its responsiveness to visible light. (2) Low quantum efficiency: While many semiconductors exhibit photocatalytic properties, their quantum efficiency is typically low. This limitation arises because the photogenerated conduction band electrons and valence band holes in semiconductors tend to recombine easily, making it difficult for them to react with organic substances on solid surfaces [62]. The photogenerated carriers in g-C3N4, following excitation by visible light, are prone to spontaneous complexation, which complicates the assurance of photostability. (3) Photocorrosion and aggregation present significant challenges: g-C3N4, typically a yellowish powder, is susceptible to hole oxidation or electron reduction when exposed to visible light. This photocorrosion and aggregation can lead to catalyst deactivation, as the oxidation of holes or reduction of electrons in g-C3N4 under visible light irradiation occurs readily, resulting in a decrease in specific surface area due to increased aggregation and stacking of the layered morphology. (4) Achieving high selectivity in complex reaction systems remains challenging; the fine-tuning of the material’s surface design and optimization limitations hinder g-C3N4 from attaining exceptional selectivity during catalytic reactions. In summary, considering these challenges, researchers continue to strive towards developing innovative approaches aimed at cost-effective fabrication methods and seamless integration with existing infrastructures. Currently, various modifications of g-C3N4 have been explored to increase its specific surface area, broaden its photoresponsive range, and enhance the efficiency of photogenerated carrier separation and migration.
4. g-C3N4 Photocatalyst Modification Measures
In recent years, semiconductor photocatalysis has emerged as a prominent research focus within the fields of functional ceramic materials, photochemistry, environmental protection, and biotechnology. However, the short lifetime of photogenerated carriers, low quantum efficiency, and limited visible light absorption of monolithic semiconductor photocatalysts continue to impede their further development [63]. Over the past decade, researchers have developed various strategies to enhance the photocatalytic performance of g-C3N4, including defect engineering, elemental doping, structural modulation, and the construction of heterojunctions.
4.1. Formal Modification
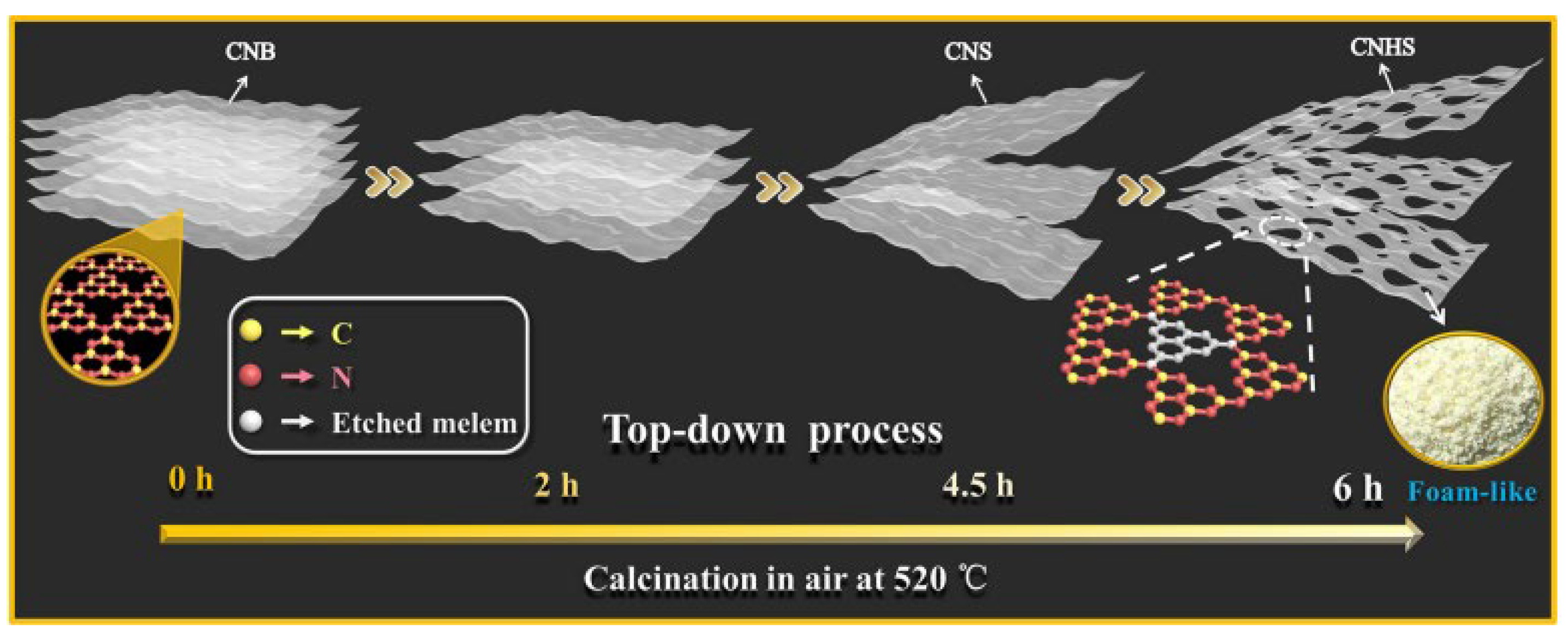
The morphology control method is one of the most commonly employed techniques; however, g-C3N4 synthesized via the traditional thermal polymerization method typically exists in bulk form, resulting in a limited specific surface area and a paucity of photocatalytic active sites. By fabricating g-C3N4 into spherical, nanosheet, or porous structures, one can significantly enhance both the specific surface area and the number of active sites, thereby improving its photocatalytic performance. Furthermore, varying the calcination temperatures allows for the regulation of the final exposed morphology and pore size of g-C3N4, with a positive correlation generally observed between pore size and specific surface area. As shown in Figure 7, Li et al. [64] fabricated porous g-C3N4 nanosheets (CNHS) with a thickness of 9.2 nm, featuring abundant micropores (1–2 nm), mesopores (2–50 nm), and macropores (50–100 nm) by thermally exfoliating native g-C3N4 (CNB) for a continuous duration of 6 h at 520 °C. The resulting specific surface area was 277.98 m2/g, nearly 26 times greater than that of CNB, which measured 10.89 m2/g.
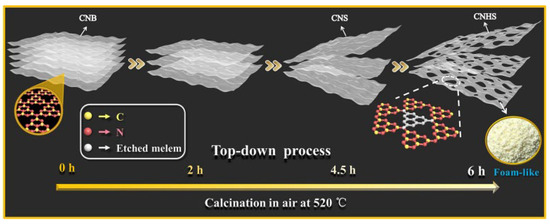
Figure 7.
Preparation of foamy porous ultrathin g-C3N4 nanosheets by top-down method [64] (reprint from Li, Y.; Jin, R.; Xing, Y.; et al. Macroscopic Foam-Like Holey Ultrathin g-C3N4 Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016, 6, 1601273).
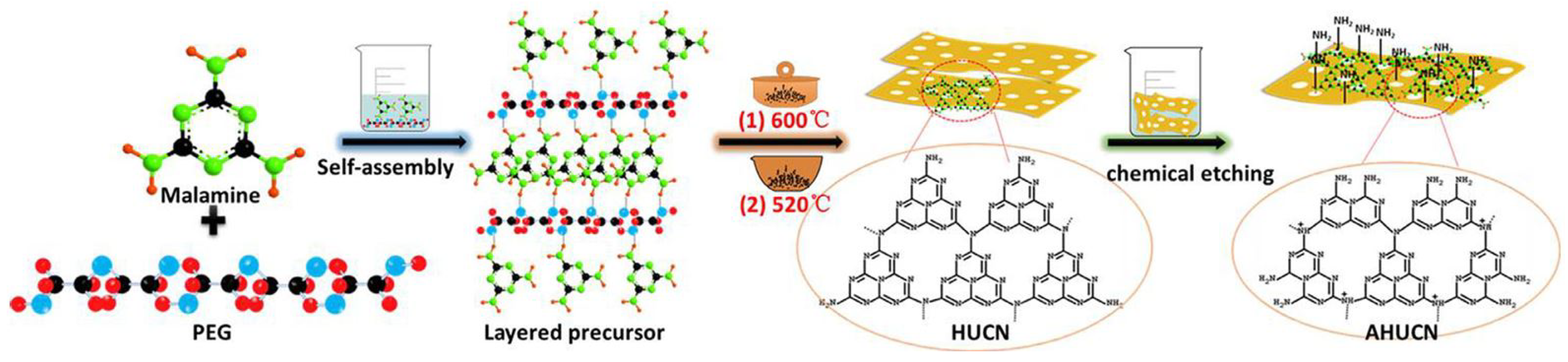
The significant presence of in-plane holes greatly enhances mass transfer and improves the mobility of photogenerated charges. Additionally, the larger pore size facilitates electron transfer orbitals, further improving the mobility of photogenerated charges. As shown in Figure 8, Xiao et al. [65] demonstrated that by controlling the photocatalytic pathway, efficient inactivation of Escherichia coli was achieved using amino acid-rich porous ultrathin g-C3N4 nanosheets (AHUCN). This method yielded the highest inactivation efficiency among monolithic g-C3N4 structures. The unique characteristics of the porous ultrathin nanosheets, combined with their amino-acid-rich surface, provided synergistic advantages by offering more active reaction sites, facilitating the separation of photogenerated electrons and holes, accelerating photoelectron transfer, and enhancing the yield of photocatalytic H2O2.
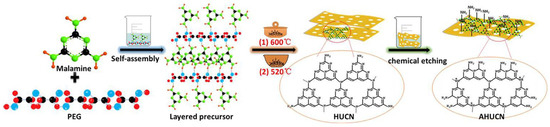
Figure 8.
Schematic diagram of the synthesis process of AHUCN [65] (reprint from Xiao, J.; Liu, Q.; Song, M.; et al. Directing Photocatalytic Pathway to Exceedingly High Antibacterial Activity in Water by Functionalizing Holey Ultrathin Nanosheets of Graphitic Carbon Nitride. Water Res. 2021, 198, 117125).
4.2. Defect Engineering
Defect engineering in g-C3N4 primarily involves the creation of nitrogen vacancies (N-vacancies) and carbon vacancies (C-vacancies). The introduction of nitrogen vacancies generates defect energy levels above the valence band of g-C3N4, thereby narrowing the band gap and enhancing its light capture capability. Conversely, the introduction of carbon vacancies can establish an intermediate energy level below the conduction band, facilitating long-wavelength photon absorption. Gao et al. [66] synthesized carbon defects (carbon vacancies) and amino groups in situ on the surface of ultrathin g-C3N4 nanosheets via thermal polymerization of urea aqueous solution and gas shock stripping. These carbon defects elevate the conduction band potential, promoting the reduction of protons by photogenerated electrons to generate hydrogen. The amino group enhances hydrophilicity and dispersibility, and its hydrogen production activity is 57 times greater than that of bulk g-C3N4. Xue et al. [67] developed g-C3N4 (g-C3N4-N3C) with three coordinated N vacancies. These N vacancies contribute to narrowing the band gap, improving light capture efficiency, accelerating the separation and transfer of photogenerated carriers, and providing additional active sites. Experimental results confirm that g-C3N4-N3C exhibits a commendable photocatalytic N2 fixation rate of 1915 μmol/h·g and a photocatalytic H2O2 production rate of 1098 μmol/h·g. Notably, at λ = 370 nm, the corresponding apparent quantum efficiencies (AQE) are 7.79% and 12.43%, respectively, surpassing those of most known photocatalysts.
4.3. Element Doping
The element doping strategies for g-C3N4 primarily encompass non-metal doping, metal doping, and co-doping (multi-element synergy). These strategies aim to optimize the electronic structure, light absorption capacity, and catalytic activity of g-C3N4. Metal doping enhances photocatalytic reactions by refining the active sites and energy band structure. For instance, Li et al. [68] employed a transition metal double anchoring configuration to induce asymmetric distortion in the g-C3N4 skeleton via sulfur atoms, thereby promoting electron delocalization and forming multiple active sites. Non-metal doping, on the other hand, broadens light absorption and facilitates charge propagation by adjusting the band gap. Additionally, the introduction of non-metal elements can create defects within the semiconductor structure, alter its crystallinity and light absorption characteristics, and provide traps or recombination centers for capturing electrons or holes. According to Mottammal et al. [69], sulfur–potassium co-doping, as well as sulfur doping alone, effectively reduced the band gap to 2.1 eV, resulting in a 50 nm red shift in the visible light absorption edge. Furthermore, the quantum efficiency of the modified g-C3N4 under 420 nm illumination reached 15.7%, which is 12 times greater than that of pristine g-C3N4. Fahim A. Qaraah et al. innovatively synthesized hexagonal porous g-C3N4 supported by Ag-Ni bimetallic sites through hydrothermal and calcination processes. The high porosity of g-C3N4, the synergistic effect of Ag-Ni bimetallic sites, and the increased surface area demonstrate excellent photocatalytic performance. The optimal results indicate that the yield of CO is 77.65 μmol/g, while the yield of CH4 is 17.89 μmol/g [70].
4.4. Heterojunctions Structure Construction
Heterojunctions can be classified into conventional types: type I, type II, type Z, and type S. The construction of semiconductor heterojunctions has garnered significant attention due to its remarkable effectiveness in improving photocatalytic activity. By forming semiconductor heterostructures, the separation of photogenerated electron–hole pairs can be significantly enhanced, and the visible light absorption range of carbon nitride can be broadened, thereby improving its catalytic activity. For g-C3N4, the development of heterojunction materials represents an effective approach to enhance its photocatalytic performance. The formation of g-C3N4-based heterojunction photocatalysts using g-C3N4 and other semiconductor materials can effectively improve the separation efficiency of photogenerated carriers, thereby enhancing the photocatalytic activity of the catalyst [71]. Shreerang Mishra et al. [72] constructed a double S-type heterojunction ternary system of g-C3N4-loaded NiTiO3 and NiSnO3 by ultrasonic calcination method, forming a two-way charge transfer path. The composite material achieved a hydrogen production rate of 137 μmol·g−1·h−1 under visible light, which was 5.49 times higher than that of a single component.
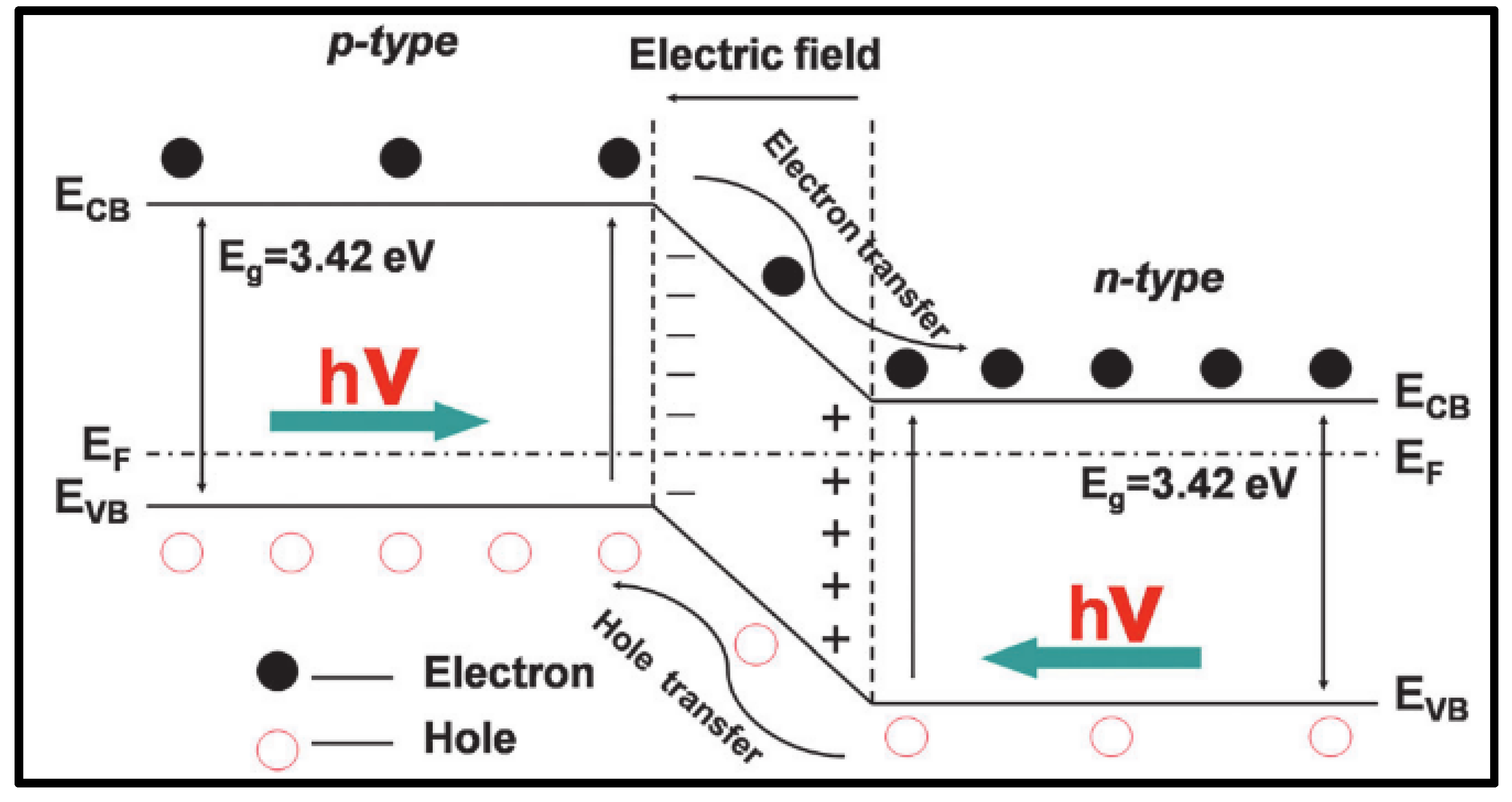
Due to the differences in band gap among various materials, semiconductors and semiconductor heterojunctions can convert light energy into electricity more efficiently, thereby enhancing photoelectric conversion efficiency. Traditionally, semiconductor heterostructures have garnered significant attention. As illustrated in Figure 9, semiconductor heterojunction systems can generally be categorized based on semiconductor type into two categories: p-n type and non-p-n type. Among these, the p-n junction in semiconductors serves as an effective structure for efficient charge collection and separation. Typically, when p-type and n-type semiconductors come into contact, the diffusion of electrons and holes leads to the formation of a p-n junction with a space charge region at the interface. This generates a built-in electric field that facilitates the movement of electrons and holes in opposite directions.
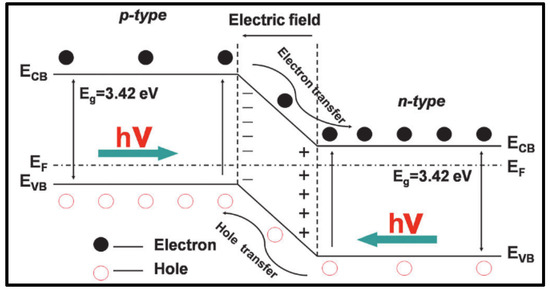
Figure 9.
Schematic representation of energy band structure and electron–hole pair separation in p-n heterojunction [73].
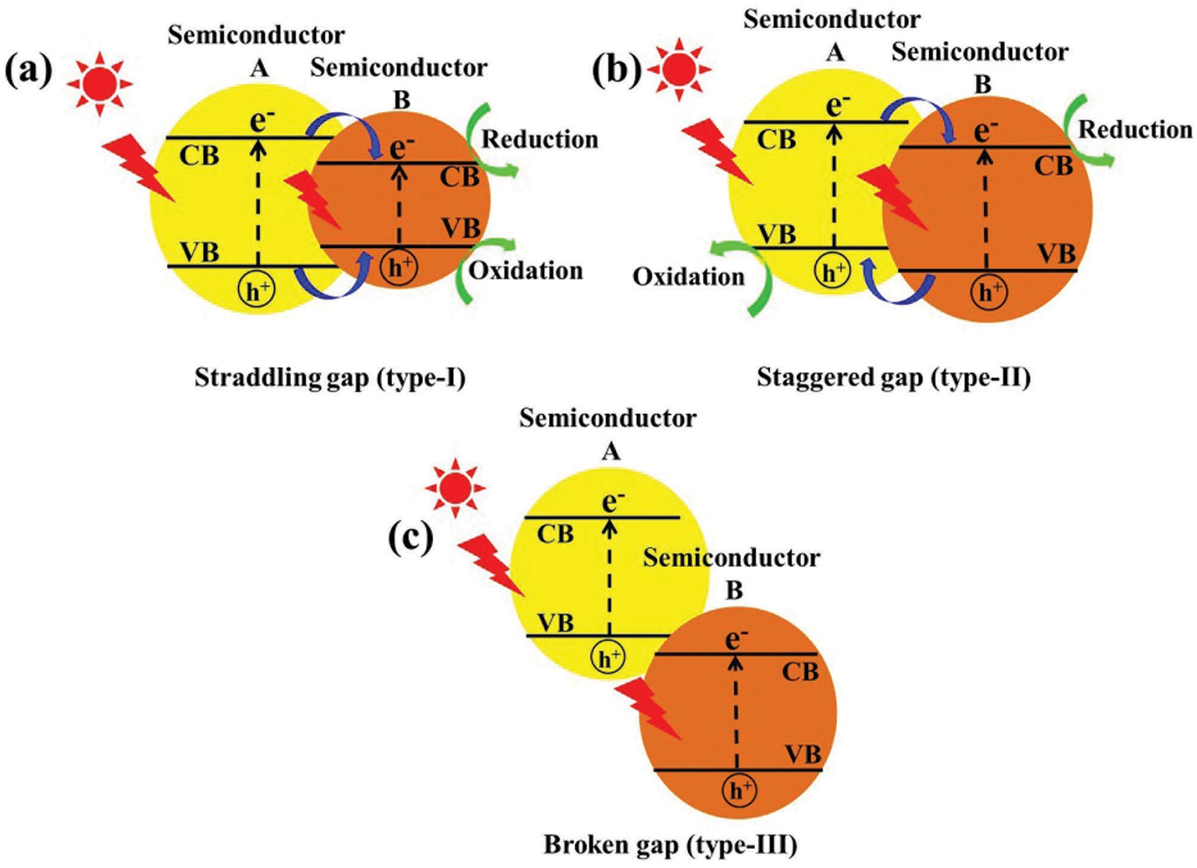
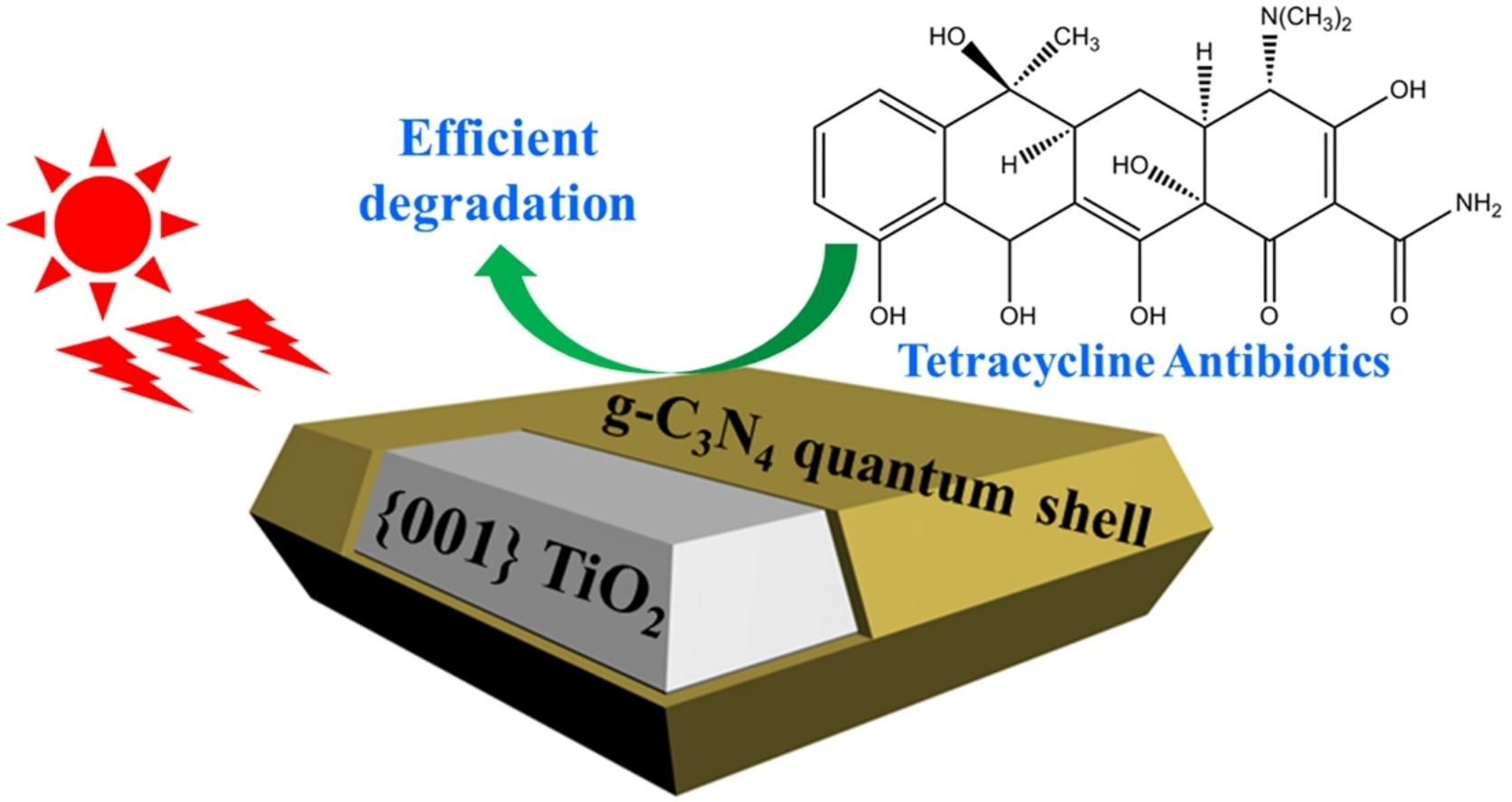
Semiconductor heterojunctions can be classified into three structural types: straddling gap (Type I), staggered gap (Type II), and broken gap (Type III) [73]. As illustrated in Figure 10, the Type I semiconductor heterojunction structure, under visible light irradiation, allows for the easy aggregation of excited electron–hole pairs within the valence band and conduction band of the same semiconductor. This results in photogenerated carriers being unable to achieve effective separation. Conversely, the energy band position of the Type III semiconductor heterojunction structure is staggered, preventing the overlap of band gaps between the two semiconductors. Consequently, carriers excited under visible light cannot migrate effectively between the semiconductors, failing to enhance the separation of electron–hole pairs. In contrast, the Type II heterojunction facilitates spatial separation of photogenerated electron–hole pairs due to the distinct positions of the valence band and conduction band. This characteristic makes Type II heterojunctions the most effective conventional heterojunctions for improving photocatalytic activity, as they are well-suited for the spatial separation of electron–hole pairs. Recent studies have increasingly focused on Type II heterostructures based on g-C3N4. Wang et al. [74] reported that g-C3N4 with quantum thickness was polymerized onto the surface of anatase titanium dioxide (TiO2) nanosheets to form TiO2@g-C3N4 (TCN) core–shell quantum II heterojunctions. The structural composition of these heterojunctions facilitated the effective transfer of electrons between the two materials, thereby enhancing the photocatalytic degradation activity of tetracycline. This performance was found to be 2-fold higher than that of TiO2 and 2.3-fold higher than that of pure g-C3N4 (see Figure 10 and Figure 11).
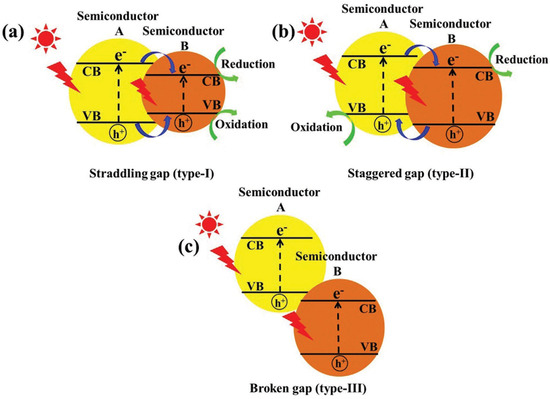
Figure 10.
Schematic representation of three different types of separation of electron–hole pairs in the case of heterojunction photocatalysts: (a) Type I; (b) Type II; and (c) Type III heterojunction [73] (Reprint from Low, J.; Yu, J.; Jaroniec, M.; et al. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694).
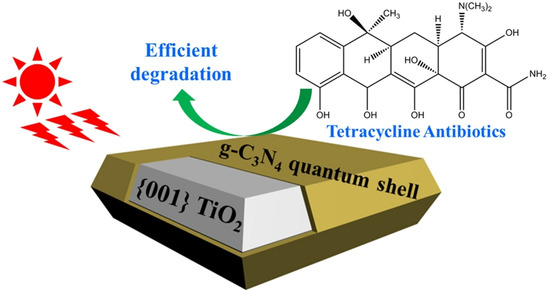
Figure 11.
Structures of prepared TiO2@g-C3N4 (TCN) core–shell quantum type II heterojunctions [74] (reprint from Wang, W.; Fang, J.; Shao, S.; et al. Compact and Uniform TiO2@g-C3N4 Core–Shell Quantum Heterojunction for Photocatalytic Degradation of Tetracycline Antibiotics. Appl. Catal. B 2017, 217, 57–64).
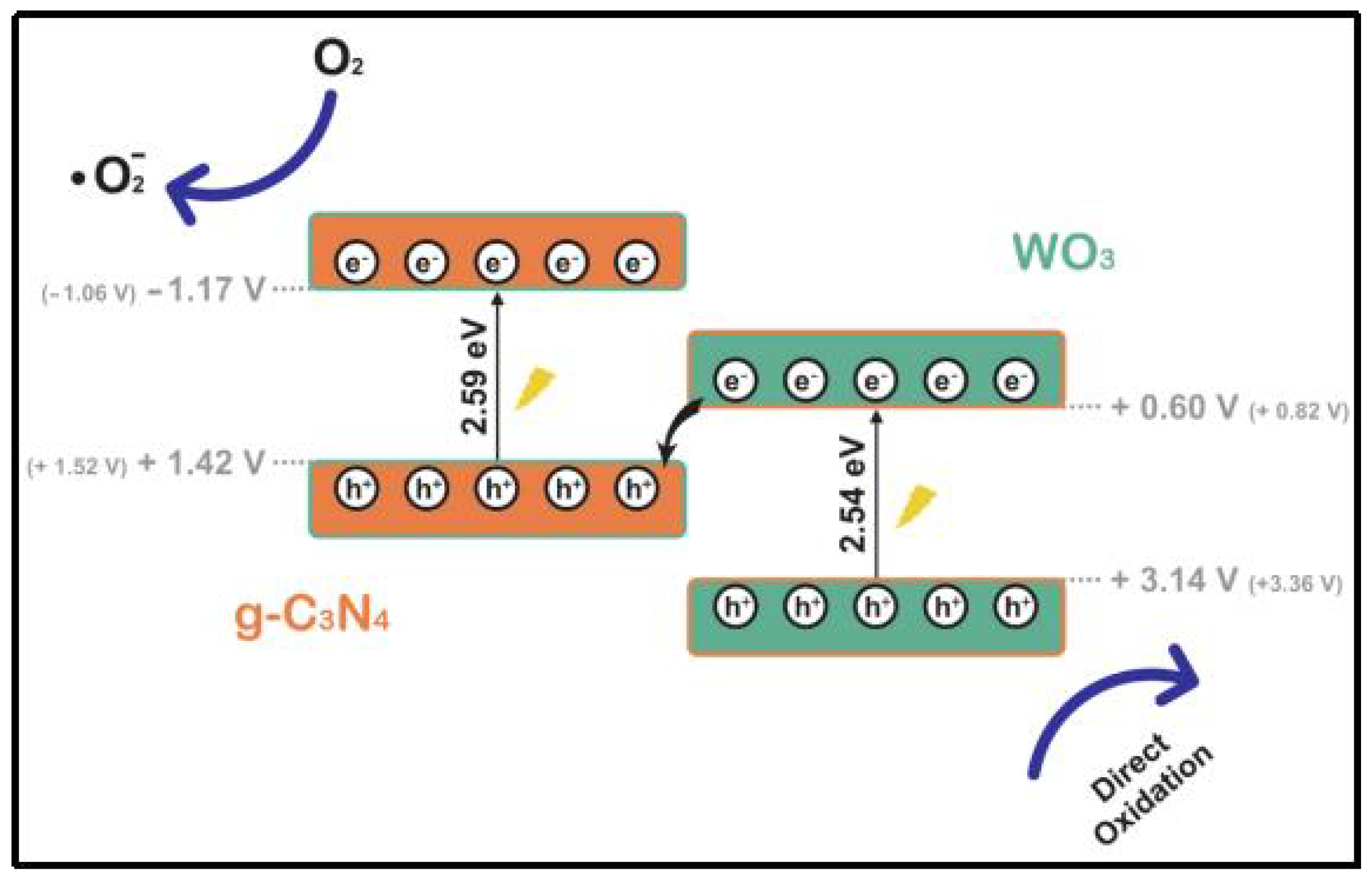
Although conventional type II and p-n heterojunctions effectively enhance carrier separation, their oxidation and reduction properties are compromised. Consequently, the photogenerated carriers ultimately reside in the valence band of the semiconductor, which has limited oxidation capability, and in the conduction band of the semiconductor, which has limited reduction capability after separation. To address these challenges, Bard et al. (1979) designed the Z-type heterojunction to enhance redox capacity by studying and simulating the photosynthesis process in plants [75]. As research progresses, various types of Z-heterojunctions have emerged, including conventional Z-heterojunctions, all-solid-state Z-heterojunctions, indirect Z-heterojunctions, and direct Z-heterojunctions. This work focuses on the conventional Z-heterojunction system, where the intermediate functions as both an electron acceptor and donor. This configuration effectively traps holes and electrons within the valence and conduction bands, respectively, thereby compensating for the weak redox capacity and facilitating the development of robust redox capabilities and spatially segregated redox reaction sites. Adan et al. [76] directly complexed WO3 with g-C3N4 using a straightforward sonication-assisted method (Figure 12). The resulting complexes exhibited excellent material homogeneity, effective interfaces, and appropriate energy levels. Furthermore, their Z-type heterojunction structure facilitated the formation of superoxide radicals, significantly enhancing the degradation of pollutants and achieving a degradation rate four times greater than that of pure g-C3N4.
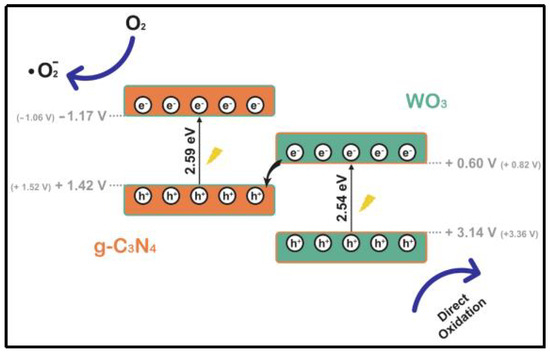
Figure 12.
Photocatalytic mechanism of g-C3N4/WO3 heterostructured photocatalysts (positions of theoretical CBs and VBs are shown inside the figure) [76].
Despite their potential advantages, the structural configuration and complex design of Z-type heterojunctions limit their large-scale preparation. Additionally, several shortcomings exist: (1) the presence of intermediate trapped electron–hole pairs makes the newly generated carriers susceptible to thermodynamic reversal; (2) the reaction environment is restricted to the liquid phase, which limits practical application scenarios; (3) during the migration process, photogenerated electron–hole pairs must traverse multiple energy levels, hindering carrier migration and resulting in poor stability of the photocatalytic reaction. Consequently, we shift our focus to a novel S-type heterojunction, characterized by two semiconductors with distinct valence and conduction band positions. When constructing the heterojunction in close contact, a potential energy difference in the energy band structure induces the energy band bending at the contact surface, thereby facilitating the efficient separation of electron–hole pairs. Chen et al. [77] synthesized sulfur-doped g-C3N4 and nitrogen-doped MoS2 using a one-step thermal polycondensation method, resulting in the formation of an S-type heterojunction structure. This structure effectively broadens the absorption of visible light and enhances the separation of photogenerated carriers, achieving a hydrogen generation rate of 658.5 μmol/g/h. This rate is significantly higher than that of pure g-C3N4 (28.8 μmol/g/h) and MoS2 (17.4 μmol/g/h), surpassing them by factors of 23 and 38, respectively (Figure 13). The convergence of Fermi energy levels within the S-type heterojunction generates built-in electric fields at the contact surfaces, leading to the bending of energy bands and Coulombic attraction. This phenomenon acts as a driving force for electrons in the conduction band of the composite and facilitates the recombination of holes in the valence band. Consequently, these mechanisms significantly reduce the ineffective recombination of photogenerated carriers, allowing for the retention of strong photogenerated carriers to participate in the photocatalytic reaction.
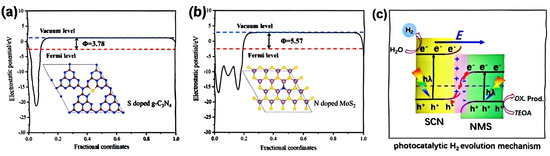
Figure 13.
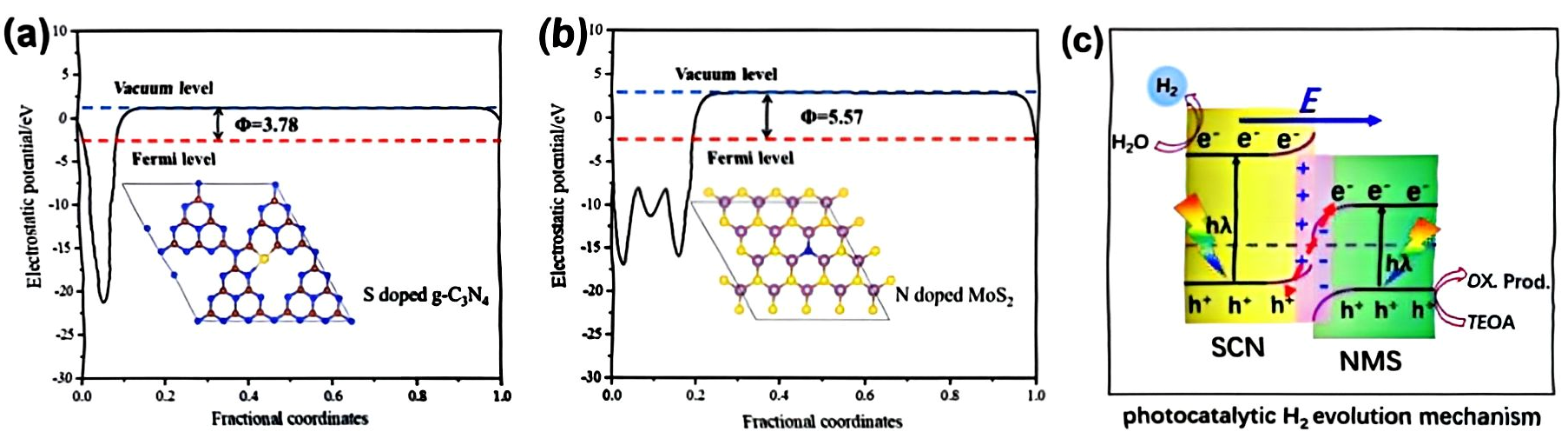
(a) Electrostatic potentials of CN (001) surface and (b) MoS2 (002) surface; (c) schematic of NMS/SCN photocatalytic mechanism S-type heterojunction [77] (reprint from Chen, Y.; Su, F.; Xie, H.; et al. One-Step Construction of S-Scheme Heterojunctions of N-Doped MoS2 and S-Doped g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2020, 404, 126498).
5. Integration of Multiple Catalytic Technologies: Piezoelectric–Photocatalytic Technology
Photocatalysis is recognized as a green technology that effectively mitigates excessive energy consumption while maintaining the long-term stability of aquatic ecosystems. Laboratory studies demonstrate that photocatalysis can completely oxidize a wide range of organic contaminants in both water and air streams at or near room temperature [78]. However, the low transport efficiency of charge carriers and the high recombination rate of photogenerated electron–hole pairs hinder the enhancement of degradation catalysis performance in conventional semiconductor materials. Currently, photocatalysis is being increasingly integrated with other catalytic technologies to achieve more efficient and environmentally friendly solutions. For instance, the combination of photocatalysis with electrocatalysis and thermal catalysis allows for more effective energy utilization. This synergistic approach, particularly in sewage treatment and organic waste degradation, can significantly enhance degradation rates and overall efficiency. Moreover, piezoelectric–photocatalytic technology utilizes the synergistic effect of piezoelectric and optical processes to effectively incorporate the piezoelectric effect into the photocatalytic process [79]. Piezoelectric–photocatalytic technology is a method for the catalytic degradation of organic pollutants that utilizes the synergistic effects of mechanical force and light in conjunction with a semiconductor catalyst. This technology offers significant advantages, including high efficiency and environmental sustainability [80].
In addition, various forms of energy are readily available in the surrounding environment, including mechanical vibrations, thermal energy, fluid flow, light, and electromagnetic radiation, such as radio waves. These energy sources can be harnessed to generate electrical energy for electronic devices through diverse energy conversion methods. Among these, mechanical vibrations are particularly prevalent in daily life, occurring in contexts such as human movements, vehicular transport, and the operation of industrial machinery [81,82]. Mechanical energy can be stored and converted into electrical energy, which can then be utilized to power electronic devices. This process provides a clean source of electrical energy. Currently, researchers are focusing on various fields, including electromagnetism [83], electrostatics [84], piezoelectrics [85], friction electricity [86], and thermoelectrics [87]. Various energy harvesting strategies at the meso-, micro-, and nanoscales have been proposed using piezoelectric materials, each exhibiting unique polarization properties. Notably, the self-polarization characteristic of piezoelectric materials enables them to convert external mechanical energy into electrical energy without requiring an external magnetic field or the addition of other substances. Furthermore, these materials demonstrate high sensitivity to external forces, making them particularly effective for energy harvesting applications [88]. Furthermore, the energy harvested by piezoelectric materials is not only stable but also exceptionally dense, yielding a higher energy output per unit area compared to other energy collection methods [89,90,91]. Therefore, the application of piezoelectric technology for harvesting external mechanical energy to enhance the degradation of pollutants has emerged as one of the environmentally friendly solutions for efficient energy utilization.
5.1. Materials Research for Piezoelectric–Photocatalytic Technology
Among the various catalytic technologies, the integration of piezoelectric technology is particularly significant. Piezoelectricity refers to a material property that enables the conversion of mechanical energy into electrical energy, finding extensive applications in sonar, sensors, and energy conversion. The synergy between photocatalysis and piezoelectric technology not only facilitates chemical reactions through photocatalysis but also harnesses piezoelectric technology to transform mechanical energy generated during these reactions into electrical energy. This approach enables the multi-level utilization of energy and contributes to the establishment of a theoretical framework for photoelectrochemical three-phase coupling catalysis. The choice of materials for piezoelectric energy harvesting plays a crucial role in determining performance. Consequently, a diverse range of materials, including inorganic, organic, and composite substances, has been explored in the research of piezoelectric energy harvesting. In the field of piezoelectric catalysis, commonly used piezoelectric materials include ceramic materials such as zirconate–titanate (PbTiO3-PbZrO3, PZT) [92], composite materials like zinc oxide (ZnO) [93], and polymers such as polyvinylidene fluoride (PVDF) [94]. Piezoelectric ceramic materials represent a novel category of intelligent materials that exploit their piezoelectric and inverse piezoelectric effects [95]. These ceramic materials can be classified into two main categories: lead-containing piezoelectric ceramics and lead-free piezoelectric ceramics. They have found extensive applications in various fields, including aerospace, ship sonar, high-speed trains, automobiles, precision instrument control, mobile communications, and both office and home electronic products [96]. Lead-containing ceramics exhibit toxicity due to the presence of lead, which contradicts the principles of green environmental protection. In contrast, lead-free ceramics demonstrate resistance to high pressure and high temperature; however, they are characterized by extreme brittleness, which poses challenges for safe daily use.
5.2. Research on Flexible Films of Polyvinylidene Fluoride
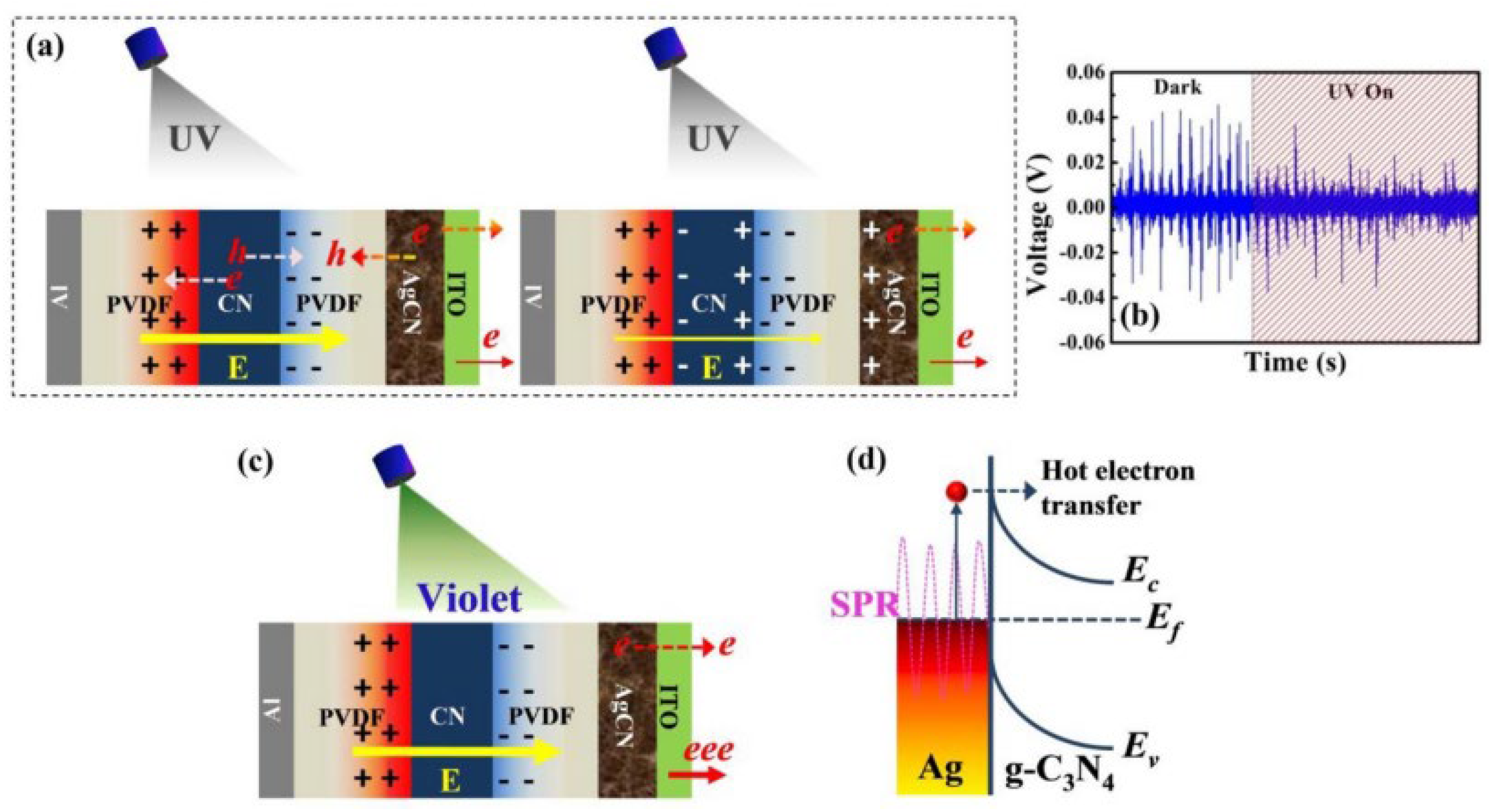
Flexible and non-polluting polymer polyvinylidene fluoride (PVDF) and its copolymers represent the most promising candidates for the preparation of piezoelectric materials. This is attributed to their unique electroactive properties, high flexibility, excellent processing characteristics, and long-term stability [97,98,99]. Bayan et al. [100] developed a self-polarized PVDF-based nanogenerator by incorporating pristine g-C3N4 nanosheets as filler materials within a PVDF matrix. The researchers demonstrated that this nanogenerator could efficiently generate a maximum voltage of approximately 2.3 V and a maximum power output of around 110 µW/cm2 when activated by a finger tap. Furthermore, the integration of an additional layer of plasmonic silver nanoparticle-loaded g-C3N4 nanosheets resulted in a significant enhancement in optical response. The hybrid plasma nanogenerator, exhibiting a strain of approximately 0.021%, achieved self-powered photodetection with a light-to-dark current ratio of approximately 60 (Figure 14), in contrast to the unstrained device, which exhibited a ratio of about 2.0. Dai et al. [101] prepared a sample of PVDF-Na0.5Bi0.5TiO3-BiOCl0.5Br0.5 (PV-NB) based on a flexible composite film of PVDF-Na0.5Bi0.5TiO3. The conversion of mechanical energy from flowing water into piezoelectric potential energy was utilized to enhance charge transfer in the photocatalytic composite film, resulting in a 2.33-fold increase in photocatalytic ability. The durability of the film material was verified through repeated degradation performance tests (Figure 15).
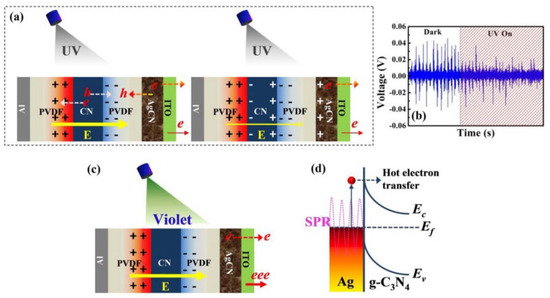
Figure 14.
Schematic diagram of the photoresponse mechanism by PENG: (a) charge redistribution under UV light under strain conditions; (b) output voltage generated by impacts under dark and UV irradiation conditions; (c) charge redistribution under UV light irradiation under strain conditions; (d) thermal electron transfer mechanism of silver nanoparticle [96].
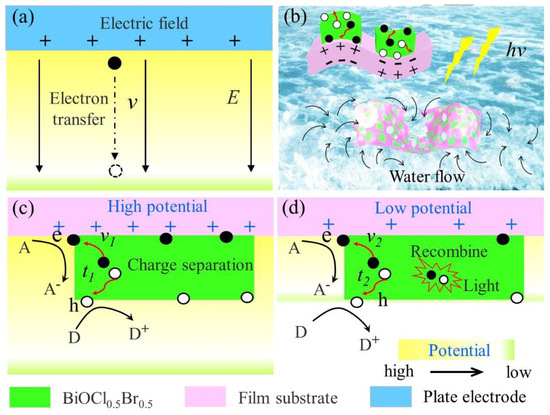
Figure 15.
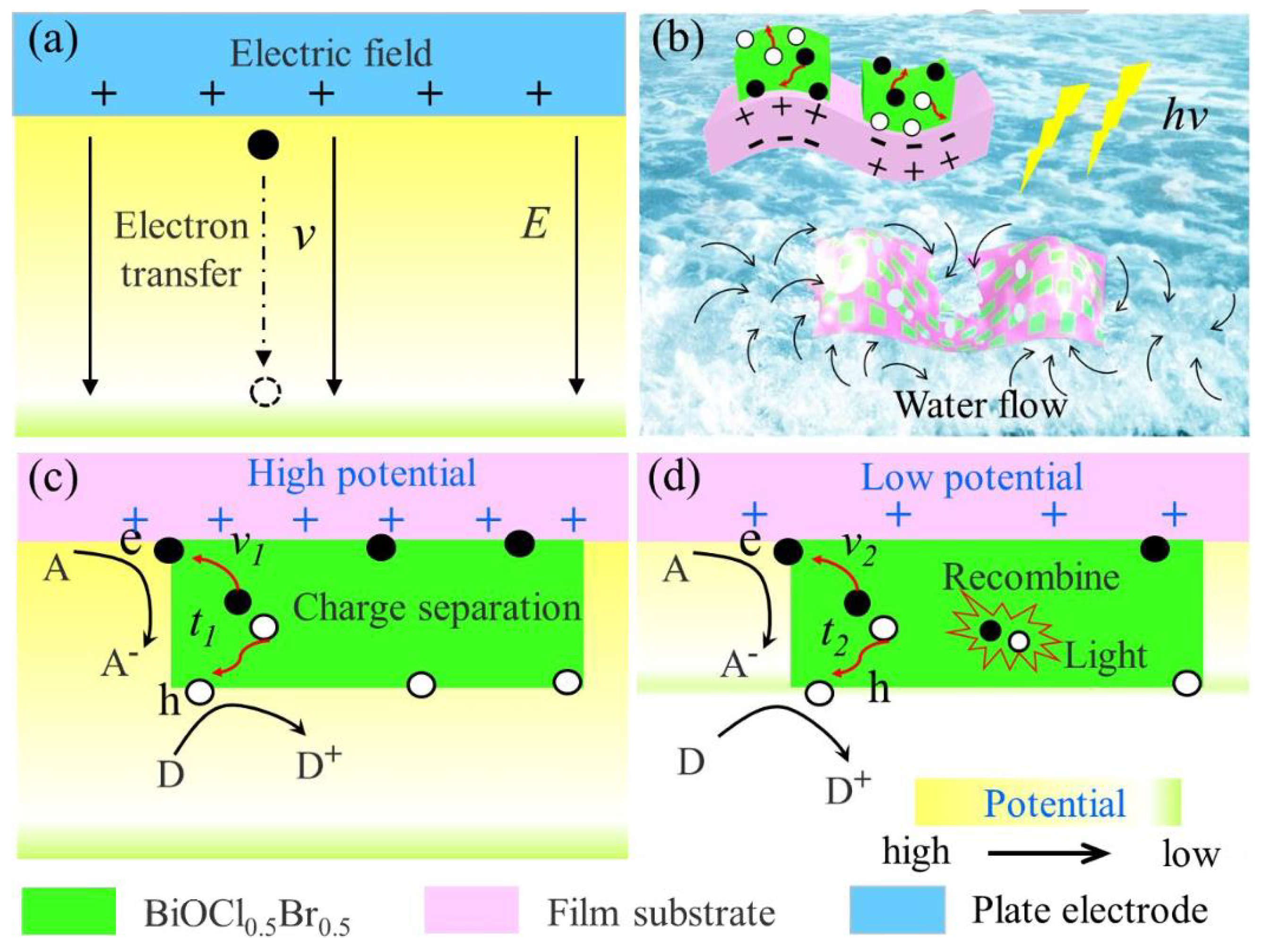
Mechanism of piezoelectric potential enhancing photocatalytic activity: (a) electron acceleration along electric field E with transfer rate ν; (b) light-induced deformation of flexible composite membrane and generation of photoexcited carriers; (c,d) carrier transfer behavior at high and low potentials (ν1, ν2: carrier migration rates; t1, t2: response times) [101] (reprint from Dai, B.; Huang, H.; Wang, F.; et al. Flowing Water Enabled Piezoelectric Potential of Flexible Composite Film for Enhanced Photocatalytic Performance. Chem. Eng. J. 2018, 347, 263–272).
5.3. Synergistic Effect of g-C3N4 in Piezoelectric Photocatalysis
Piezophotocatalysis, as an emerging coupled catalytic strategy, offers an innovative approach to address the critical challenge of rapid recombination of photogenerated carriers in traditional semiconductor photocatalysis. This method combines photoexcitation with the piezoelectric effect induced by mechanical stress. g-C3N4 and its composites have emerged as an ideal platform for constructing efficient piezophotocatalytic systems due to their unique structural and physicochemical properties, which demonstrate significant synergistic enhancement effects.
5.3.1. Theoretical Basis of Synergistic Mechanism
The photoexcitation process involves the transition of electrons from the valence band to the conduction band of g-C3N4 under visible light irradiation, resulting in the generation of electron (e−)–hole (h+) pairs. In the piezoelectric effect process, when the system experiences external mechanical stress—such as ultrasonic vibration, stirring, or fluid shear force—the piezoelectric component (g-C3N4 itself or modified/composite strong piezoelectric materials) with a non-centrosymmetric structure undergoes deformation. This deformation leads to the separation of internal positive and negative charge centers, creating a piezoelectric polarization field and potential. The coupling and synergy effects manifest primarily in three aspects: (1) Directional driving of charge carrier separation: The piezoelectric polarization field functions as a robust built-in electric field, with its direction dynamically changing according to the stress state. This field effectively drives photogenerated electrons and holes to migrate in opposite directions, significantly suppressing their spatial recombination. (2) Band bending and surface charge modulation: The piezoelectric potential can induce significant bending of the catalyst’s surface energy bands, thereby influencing the driving force of redox reactions. Concurrently, the polarized charges generated on the surface of the piezoelectric material, with positive and negative charges enriched on different crystal planes, facilitate the directional adsorption and activation of reactant molecules (such as H2O, O2, and pollutants) on the catalyst surface. (3) Broadening the light response range (in certain systems): Strong electric fields may indirectly enhance the material’s light-harvesting capabilities through the Franz–Keldysh effect or by promoting defect states to participate in light absorption. The synergistic conversion of light and mechanical energy into chemical energy significantly enhances overall catalytic efficiency, often surpassing the mere sum of individual photocatalysis and piezoelectric catalysis.
5.3.2. Construction Strategy of g-C3N4-Based Piezoelectric–Photocatalytic System
- (1)
- Intrinsic piezoelectric regulation of g-C3N4 can be achieved through structural optimization by adjusting high-temperature polycondensation conditions, selecting appropriate precursors such as melamine, urea, and thiourea, and introducing pore-forming agents. These modifications enhance the layered structure of g-C3N4, increase interlayer spacing, and introduce structural defects, such as nitrogen vacancies, which may marginally improve its inherent piezoelectric response. The primary sources of its piezoelectric contribution are interlayer shear slip and out-of-plane deformation. Additionally, elemental doping serves as a technique to modify the lattice of g-C3N4 [102]. The introduction of metal ions (e.g., K+, Na+) [103] or non-metal elements (e.g., P, B, O) into the lattice can disrupt the symmetry of g-C3N4 and alter its electronic structure, thereby enhancing both light absorption and piezoelectric properties. Liang et al. [104] synthesized g-C3N4 nanoplates by a new self-template process, and then ultrasonically exfoliated to produce porous g-C3N4 nanoplates with a thickness of 1.5–2.0 nm. Piezoelectric response force microscopy shows that the material exhibits excellent piezoelectric response. In addition, the material exhibits obvious piezoelectric–photocatalytic degradation of RhB, showing the best first-order rate constant. Under visible light and ultrasonic excitation, it is 2.3 times under visible light irradiation alone and 9.7 times under ultrasonic excitation alone. The piezoelectric field generated by ultrasonic excitation drives the opposite direction of photogenerated electrons and holes to migrate to the surface of the nanosheets, resulting in more reactive free radicals, thereby accelerating the degradation of RhB.
- (2)
- Composite materials incorporating strong piezoelectric substances, such as perovskite types (e.g., BaTiO3 [105], BiFeO3 [106], Bi4Ti3O12 [107]), exhibit notable piezoelectric properties, and their band structures can readily align with g-C3N4 to form effective heterojunctions. Wurtzite-type materials, including ZnO and ZnS, are straightforward to synthesize and possess commendable piezoelectric characteristics. Layered transition metal sulfides, such as MoS2 and WS2 [108], not only display piezoelectric properties but also exhibit outstanding photoelectric and electrochemical behaviors. In designing composite structures, a critical factor is the construction of tight heterointerfaces (e.g., 0D/2D, 2D/2D, 3D/0D) [109], which ensures efficient transfer of photogenerated carriers and effective penetration of the piezoelectric polarization field at the interface. Core–shell structures and Z-type heterojunction [110] designs have been demonstrated to utilize synergistic effects more efficiently. Cai Jing et al. [111] synthesized a Z-type heterojunction/g-C3N4/BiOCl material incorporating graphene quantum dots using a one-step hydrothermal technique. The material demonstrated high photocatalytic stability. The researchers assessed the piezoelectric–photocatalytic performance of the material by immobilizing N2 under simulated sunlight. The results indicated that the photocatalytic activity of the material was significantly superior to that of pure g-C3N4 and BiOCl, with a formation rate of 1773.8 μmol/(h·g), which is 7.3 times greater than that of pure g-C3N4 and 5.2 times greater than that of BiOCl, respectively.
- (3)
- Organic Piezoelectric Composite: Utilizing the excellent piezoelectric properties and flexibility of ferroelectric polymers, such as PVDF and PVDF-TrFE [112], these materials are combined with g-C3N4 to prepare flexible piezoelectric–photocatalytic films or fibers. This combination allows for the efficient collection of low-frequency mechanical energy from the environment, including wind energy, water flow energy, and human motion energy. Huang et al. [113] developed a modified g-C3N4/PVDF membrane, referred to as the MCU-g-C3N4/PVDF membrane, based on the original PVDF membrane. The experimental results demonstrated that the MCU-g-C3N4/PVDF membrane achieved 84.24% and 71.26% removal rates for rhodamine B and tetracycline hydrochloride, respectively, thereby confirming its excellent photocatalytic performance.
5.3.3. Application of g-C3N4 in Piezoelectric Photocatalysis
Furthermore, numerous studies have confirmed that the g-C3N4-based piezoelectric–photocatalytic system demonstrates exceptional synergistic properties across a variety of reactions.
Pollutant degradation under ultrasonic-assisted visible light irradiation has been shown to significantly enhance the degradation rates of organic dyes (e.g., rhodamine B, methyl orange) [114], antibiotics (e.g., tetracycline, ciprofloxacin), and persistent organic pollutants. Systems such as g-C3N4/BaTiO3, g-C3N4/BiFeO3 [115], and g-C3N4/MoS2 exhibit degradation rates that are typically 2–5 times greater, or even higher, than those achieved through single photocatalysis or piezoelectric catalysis. The piezoelectric effect not only accelerates the separation of charge carriers but also facilitates the continuous and efficient generation of reactive oxygen species, including ∙OH and ∙O2−. Kang et al. [116] constructed a Z-type heterojunction with a core–shell structure by growing Bi2WO6 nanosheets on g-C3N4 coated ZnO nanorods. This design has more active sites and carrier excitation concentration, showing excellent piezoelectric–photocatalytic efficiency, and the degradation efficiency of RhB is increased to 0.217 min−1. Huang et al. [117] designed Ag/NaNbO3/g-C3N4 photocatalytic material by doping Ag into NaNbO3 and g-C3N4 composites, which significantly reduced the carrier recombination efficiency. The photocatalytic performance was improved by using the plasma resonance polymerization effect of Ag nanoparticles and the piezoelectric effect of NaNbO3. The complete degradation of methyl orange was achieved within 1h after the addition of appropriate amount of Ag nanoparticles.
The combination of piezoelectricity and photocatalysis has demonstrated that graphite-phase carbon nitride is highly effective in air purification, particularly in the removal of nitrogen oxides and formaldehyde. The primary mechanism underlying this process is photocatalytic oxidation, wherein material modifications optimize the electronic structure, surface activity, and reaction pathways. Liu et al. synthesized a g-C3N4/BaTiO3 composite with nitrogen vacancies. The introduction of a built-in electric field enhanced the band gap, facilitated carrier separation, and improved the adsorption and activation of reactants, resulting in remarkable NO removal and NO2 piezoelectric–photocatalytic properties [118]. Di et al. [119] utilized wind energy to enhance the degradation of formaldehyde using piezoelectric materials. They prepared heterojunction composites of BiFeO3 nanocubes and oxygen vacancy g-C3N4 nanosheets. The concentration of formaldehyde was reduced from 1.5 ppm to 0.68 ppm within 90 min, demonstrating a degradation efficiency that is double that of pure carbon nitride. This efficient degradation behavior is attributed to the synergistic interaction between the piezoelectric field and the heterojunction containing oxygen vacancies, providing a novel approach for air purification.
In the realm of photocatalytic nitrogen fixation and energy conversion, carbon nitride—particularly graphite-phase carbon nitride—based photocatalysts exhibit significant potential when subjected to the synergistic effects of light and pressure [120]. Hydrogen production via water splitting is enhanced by the piezoelectric polarization field, which effectively mitigates the insufficient driving force for proton reduction (H+ to H2) in the conduction band of g-C3N4 while simultaneously inhibiting the reverse reaction. Systems such as g-C3N4/ZnO [121] and g-C3N4/BaTiO3 demonstrate markedly improved hydrogen production activity due to the synergistic effects of light and pressure, which extend carrier lifetimes by several orders of magnitude. Additionally, Zhang et al. [122] developed a novel TiO2/g-C3N4 composite catalyst. The piezoelectric properties of carbon nitride, when combined with ultrasonic and visible light, induce mechanical vibrations that alter molecular distribution and band bending, thereby significantly enhancing photocatalytic hydrogen production performance. This resulted in a hydrogen production rate of 43.57 mmol/h/g, with a quantum yield of 87.13% at 365 nm. Deng et al. [123] employed a combination of piezoelectric and photocatalytic strategies to construct an S-type heterostructure using Bi2MoO6 and C3N4, which effectively enhanced carrier migration and demonstrated an impressive nitric acid yield under ultrasound and light. Guo et al. [124] synthesized g-C3N4 with a surface pit structure through thermal polymerization, resulting in a 2.3-fold enhancement in piezoelectric response. Under piezoelectric photocatalysis conditions, a H2O2 yield of 189.8 μM/h was achieved in pure water, representing an increase of approximately 14.4 times compared to g-C3N4 under traditional photocatalysis.
5.3.4. Summary of g-C3N4 Photocatalytic Application Cases
Currently, numerous efforts have been directed towards the practical application of carbon nitride. To enhance the material’s properties, we present detailed parameters in Table 1. This material exhibits significant potential for development in the field of sewage treatment. The subsequent table illustrates parameters such as pollutant degradation, treatment efficiency, and the optimal reaction conditions for g-C3N4 in practical applications.

Table 1.
Summary of the practical application of g-C3N4 in the degradation of pollutants.
In addition to the modifications of the material itself, the application of piezoelectric photocatalysis can effectively degrade pollutants. Khadim et al. [130] prepared a Z-type heterojunction of AgBr and Br-doped g-C3N4 (AgBr/Br-g-C3N4). The catalyst, comprising 20 wt% AgBr/Br-g-C3N4, was designed in a simple structure utilizing a split-plate airlift contactor. Under visible light irradiation and ultrasonic conditions, its piezoelectric–photocatalytic degradation ability for methylene blue (MB) was evaluated. The results indicated that 99.7% of MB degradation could be achieved within 60 min. To intuitively illustrate the performance of g-C3N4 in the piezoelectric–photocatalytic degradation of organic matter, Table 2 summarizes the performance and mechanism of g-C3N4 in degrading pollutants.

Table 2.
Summary of the performance and mechanism of some g-C3N4 piezoelectric–photocatalytic degradation of pollutants.
According to Table 2, g-C3N4-based piezoelectric photocatalysts have both opportunities and challenges to move towards large-scale practical applications. For the optimization of the material itself, the activity and stability of the g-C3N4-based catalyst have been significantly improved by strategies such as element doping, heterojunction construction, and special morphology design. Many studies have also shown that it has good cycle stability (usually the efficiency remains above 90% after five cycles) [131]. This is the premise of large-scale application. At the same time, efficient catalysts require matching reactors to achieve large-scale efficiency. The split-plate airlift reactor used in the study is a positive direction [130]. It uses the principle of gas lifting to realize liquid circulation and mixing, which consumes less energy than traditional mechanical stirring, and is more conducive to the contact between catalyst and pollutants and the transfer of ultrasonic energy. How to efficiently input mechanical energy to induce piezoelectric effect is the key. In addition to ultrasound, low-frequency vibration can also effectively drive g-C3N4/PVDF composite membrane to degrade pollutants, which provides an idea for the utilization of low-grade mechanical energy such as flowing water energy and wave energy widely existing in the environment and is expected to reduce operating costs. For powder catalysts, the preparation of magnetic composite materials or thin film catalysts also avoids the difficulty of powder recovery. Despite the remarkable achievements in the laboratory, there are still many challenges to realize the large-scale application of g-C3N4-based piezoelectric–photocatalytic technology, which are as follows:
- At present, the understanding of basic scientific problems such as how to coordinate piezoelectric effect and photocatalytic effect, how to separate and transfer interface charge, and accurate identification of active species still needs to be deepened, especially in complex real water environment, the mechanism of catalytic process may be more complicated.
- Most of the research is still carried out in a single pollutant solution configured in the laboratory. The actual wastewater composition is complex, and there may be anions, natural organic matter, suspended solids, etc., which may compete with pollutants for active sites or quench active species, thereby significantly inhibiting catalytic efficiency. The anti-interference ability and long-term stability of the catalyst need to be more fully verified in the real environment.
- Although solar energy is free, the input of mechanical energy (ultrasound) still needs to consume electricity. Although airlift reactors and the use of environmental mechanical energy contribute to energy conservation, the overall energy consumption and economic costs of large-scale applications still need to be carefully evaluated. The preparation cost of high-performance catalysts also needs to be considered.
- Scale-up of laboratory reactors is a key step towards engineering applications. This involves the optimization of a series of engineering problems such as mass transfer, heat transfer, light distribution, and sound field distribution, which requires the cross-cooperation of materials, chemistry, environment, machinery, and other disciplines.
- At present, many studies focus on the removal rate of pollutants, but the identification of degradation intermediates, the degree of mineralization, and the environmental toxicity analysis of the final products are not sufficient. Ensuring that pollutants are completely mineralized or converted into non-toxic and harmless substances is the bottom-line requirement for environmental technology applications.
Future research should focus on the treatment effects of actual water bodies, the amplification effects of reactors, the energy efficiency of the system, and the environmental economic evaluation throughout the entire life cycle. Close collaboration across multiple disciplines and fields is essential for advancing this promising technology from the laboratory to practical water environment management.
The piezoelectric photocatalysis of g-C3N4 demonstrates notable efficacy in hydrogen production. To facilitate a comparison with conventional photocatalytic hydrogen production and to analyze the distinctions between the two methodologies, we summarize and contrast several representative studies on g-C3N4 piezoelectric photocatalysis and photocatalytic hydrogen production in Table 3.

Table 3.
Summary of some relevant data of g-C3N4 piezoelectric photocatalysis and photocatalytic hydrogen production.
The peak yield of piezoelectric photocatalysis, recorded at 5464 μmol·h−1·g−1, is slightly lower than that of conventional photocatalysis, which achieves 6095.1 μmol·h−1·g−1. However, piezoelectric photocatalysis significantly alleviates the limitations associated with conventional photocatalysis, which typically requires high light intensity conditions. By harnessing mechanical energy conversion, piezoelectric photocatalysis diminishes dependence on light and can sustain catalytic activity even in dark environments, facilitated by an ultrasonic independent drive. This capability positions piezoelectric photocatalysis as a superior alternative to conventional methods.
g-C3N4 exhibits significant potential for applications not only in piezoelectric–photocatalytic hydrogen production but also in hydrogen peroxide synthesis. To this end, we have compiled comprehensive data regarding the production of hydrogen peroxide by g-C3N4 under both pure photocatalytic and piezoelectric–photocatalytic conditions, followed by a comparative analysis. The detailed data is presented in Table 4.

Table 4.
Data summary of hydrogen peroxide production by g-C3N4 under partial photocatalytic/piezoelectric photocatalysis.
Through the comparison presented in the table above, it is evident that the highest yield of the piezoelectric–photocatalytic system reaches 4.86 mmol·g−1·h−1, significantly surpassing the maximum yield of the pure photocatalytic system, which is 1083 μmol·g−1·h−1. Furthermore, piezoelectric photocatalysis can reduce the requirements for light conditions and intensity, maintaining catalytic activity even in dark environments, thus demonstrating a highly efficient and versatile potential for H2O2 production.
5.3.5. Challenges and Prospects
In-depth mechanism analysis necessitates a more profound exploration of the microscopic dynamic processes involved in light-force-chemical energy conversion. Specifically, it is crucial to understand how the piezoelectric polarization field precisely regulates the behavior of charge carriers at heterointerfaces and surface reactions at the nano/atomic scale. This exploration still requires the application of in situ and operando characterization techniques, such as in situ X-ray photoelectron spectroscopy (XPS), in situ Kelvin probe force microscopy (KPFM), and time-resolved spectroscopy, alongside theoretical simulations.
Designing efficient and stable materials presents a significant challenge, particularly in the development of novel g-C3N4-based composite materials that incorporate strong piezoelectric properties, a broad spectral response, high carrier mobility, and excellent chemical and mechanical stability. Achieving precise control over heterogeneous interface structures and optimizing stress transfer efficiency are essential for enhancing performance. Currently, efficient mechanical energy harvesting and utilization are impeded by the reliance on high-energy-density ultrasound in laboratory settings, which contrasts with the low-frequency, irregular mechanical energy found in real-world environments, such as natural water flow, wind, and ambient noise. Consequently, it is crucial to design specialized structures that can effectively capture and convert environmental mechanical energy, including flexible devices and integrated vibration energy harvesting systems, for practical applications.
Fabrication at scale and integration of systems are critical challenges that must be addressed to develop cost-effective and scalable processes for material synthesis and device manufacturing. Furthermore, focusing on the design of effective synergistic reactor systems that seamlessly integrate photonic and mechanical components is essential. Overcoming these challenges is vital for realizing practical applications that can advance the field and lead to innovative solutions. By addressing these issues, researchers can pave the way for more efficient production methods and improved technologies, ultimately contributing to advancements across various industries.
Exploration of multi-field-coupling future research has the potential to delve deeper into the interactions between light pressure synergy and other domains of study, such as thermocatalysis, electrocatalysis, and magnetic catalysis. By investigating these multi-field couplings, researchers aim to enhance energy conversion efficiency. This exploration focuses not only on improving the fundamental processes involved but also on expanding the practical applications of these technologies. By pushing the boundaries of these interdisciplinary connections, it is possible to develop solutions that operate effectively under a wider range of complex conditions. The ultimate goal is to create more efficient and versatile catalytic systems capable of addressing various energy challenges.
6. Conclusions
g-C3N4 has demonstrated significant potential as a low-cost, stable, and environmentally friendly photocatalyst. Through optimized preparation methods—including precursor selection, thermal modulation, and nanostructuring—along with strategic modifications such as forming heterojunctions with semiconductors (e.g., TiO2 or MoS2), doping with nonmetals or metals, and creating porous architectures, its photocatalytic efficiency has been markedly enhanced. These advancements have facilitated a wide range of applications, including solar fuel generation (hydrogen production and CO2 conversion), environmental remediation (degradation of organic pollutants and bacterial disinfection), and organic synthesis. Recent advancements in piezoelectric photocatalysis have also demonstrated significant potential. The combination of g-C3N4 with piezoelectric materials such as BaTiO3, ZnO, and MoS2, or the enhancement in intrinsic piezoelectricity through structural engineering, leads to the generation of built-in electric fields under mechanical stress (e.g., ultrasound or fluid flow). This phenomenon substantially suppresses the charge recombination rate and enhances redox kinetics. As a result, this innovative approach has achieved pollutant degradation activities that exceed those of traditional photocatalysis by more than threefold.
Despite significant advancements in the field, considerable challenges persist. The dynamics of carriers at various photo–piezo interfaces necessitate a clearer mechanistic understanding. Furthermore, the scalable synthesis of stable, high-surface-area composites continues to present formidable challenges. Mechanical energy harvesting from real-world sources, such as wind and water flow, demands innovative reactor designs. Additionally, the stability of coupled photo/mechanical stress requires thorough assessment. Future research should prioritize in situ characterization of coupling mechanisms, computationally guided material design, and flexible device engineering for ambient energy capture. This should be accompanied by the development of multi-field (photo/electro/piezo/thermal) catalytic systems. g-C3N4-based photocatalysts and photo–piezo catalysts are anticipated to demonstrate significant potential for scalable solar-to-chemical energy conversion and the advancement of sustainable environmental technologies through ongoing innovation.
Author Contributions
Conceptualization, M.L., L.Y. and D.L.; methodology, Y.L.; formal analysis, Y.S.; investigation, H.H. and L.X.; resources, D.L.; data curation, Y.F.; writing—original draft preparation, M.L.; writing—review and editing, D.L.; funding acquisition, D.L. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Basic Research Plan in the Shaanxi Province of China (No. 2023-JC-ZD-25) and the Science and Technology Guidance Project Plan of China National Textile and Apparel Council (No. 2022038, 2023018).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vasu, D.; Ravi, P.V.; Subramaniyam, V.; Moorthi, P.; You, Y.F.; Chiu, T.W. Green Fluorine-Confined 2D Layered Graphitic Carbon Nitride (g-C3N4) Nanosheets for Electrocatalysts and Photocatalysts toward the Detection and Degradation of Metol Drugs in Wastewater. ACS Appl. Eng. Mater. 2023, 1, 1866–1880. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, W.; Du, G. Effects of Greenhouse Effect on Freshwater Ecosystem. Beijing Water 2004, 1, 3. [Google Scholar]
- Zhao, Y.; Su, Q.; Li, B.; Zhang, Y.; Wang, X.; Zhao, H.; Guo, S. Have Those Countries Declaring “Zero Carbon” or “Carbon Neutral” Climate Goals Achieved Carbon Emissions-Economic Growth Decoupling? J. Clean. Prod. 2022, 363, 132450. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef]
- Heo, Y.J.; Seong, D.B.; Park, S.J. Synthesis of Polyethylenimine-Impregnated Titanate Nanotubes for CO2 Capture: Influence of Porosity and Nitrogen Content on Amine-Modified Adsorbents. J. CO2 Util. 2019, 34, 472–478. [Google Scholar] [CrossRef]
- Lin, B.; Xia, M.; Xu, B.; Chong, B.; Chen, Z.; Yang, G. Bio-inspired nanostructured g-C3N4-based photocatalysts: A comprehensive review. Chin. J. Catal. 2022, 43, 2141–2172. [Google Scholar] [CrossRef]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The Role of New Energy in Carbon Neutral. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Yu, W.; Gao, Y.; Chen, Z.; Zhao, Y.; Wu, Z.; Wang, L. Strategies for Optimizing Electrocatalytic Hydrogen Evolution Performance of Metal Phosphides. Chin. J. Catal. 2021, 42, 1876–1902. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Wang, J.; Zhou, Y.; Liu, X.; Hao, M.; Chen, Z.; Wang, S.; Yang, H.; Wang, X. Application of Single-Atom-Based Photocatalysts in Environmental Pollutant Removal and Renewable Energy Production. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1064–1109. [Google Scholar]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.; Wang, C. Polarization-Enhanced Photocatalytic Activity in Non-Centrosymmetric Materials Based Photocatalysis: A Review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for Removal of Environmental Pollutants and Fuel Production: A Review. Environ. Chem. Lett. 2020, 19, 441–463. [Google Scholar] [CrossRef]
- Yang, T.; Wang, B.; Zhu, J.; Xia, J.; Li, M. Enhanced Photocatalytic Performance of In2S3 Multi-Level Pore Structure Nanomaterials Derived from Sacrificial Metal–Organic Frameworks. Chin. J. Catal. 2024, 59, 204–213. [Google Scholar] [CrossRef]
- Guo, C.; Zhao, G.; Feng, Y.; Chen, D.; Loh, T.P.; Wang, D.; Jia, Z. Recyclable g-C3N4-Catalyzed Decarboxylative Alkenylation of N-Aryl Glycines with Vinyl Sulfones under Visible-Light Irradiation. Org. Chem. Front. 2025, 12, 2409–2414. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2018, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic Materials and Technologies for Air Purification. J. Hazard. Mater. 2016, 325, 340–366. [Google Scholar] [CrossRef]
- Xu, J.; Antonietti, M.; Shalom, M. Moving Graphitic Carbon Nitride from Electrocatalysis and Photocatalysis to a Potential Electrode Material for Photoelectric Devices. Chem. Asian J. 2016, 11, 2499–2512. [Google Scholar] [CrossRef]
- Wei, H.; Liang, L.; Ma, T.; Han, H.; Zhu, J.; Liu, Y.; Fang, Z.; Yang, Z.; Guo, K. Controllable-Morphology CoFe2O4/g-C3N4 p–n Heterojunction Photocatalysts with Built-In Electric Field Enhance Photocatalytic Performance. Appl. Catal. B 2022, 306, 121107. [Google Scholar]
- Li, Y.; Wang, L.; Gao, X.; Xue, Y.; Li, B.; Zhu, X. Construction of Graphitic Carbon Nitride-Based Photocatalyst with Strong Built-In Electric Field via π–π Stacking Interaction Boosting Photocatalytic CO2 Reduction. J. Mater. Chem. A 2024, 12, 7807–7816. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, Y.; Wang, H.; Zhang, B.; Hou, R.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Recent advances on energy and environmental application of graphitic carbon nitride (g-C3N4)-based photocatalysts: A review. J. Environ. Chem. Eng. 2023, 11, 110164. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- He, B.; Chen, S.; Cui, Y.; Chen, X.; Lei, Y.; Sun, J. Hollow polymeric ionic liquid spheres with hierarchical electron distribution: A novel composite of g-C3N4 for visible light photocatalytic water splitting enhancement. Chem. Eng. J. 2022, 440, 135625. [Google Scholar] [CrossRef]
- Rajput, Y.; Kumar, P.; Zhang, T.C.; Kumar, D.; Nemiwal, M. Recent advances in g-C3N4-based photocatalysts for hydrogen evolution reactions. Int. J. Hydrogen Energy 2022, 47, 38533–38555. [Google Scholar] [CrossRef]
- Dong, W.; Chang, Q.; Wang, W.; Yang, S. Research Progress in Preparing Methods of g-C3N4 Nanosheets by Exfoliation. J. Chin. Ceram. Soc. 2023, 51, 1868–1882. [Google Scholar]
- Sun, X.; Huang, H.; Shi, W.; Sun, L.; Song, Z. Research Progress on Preparation and Application of Modified g-C3N4 Photocatalytic Materials. Prog. Fine Petrochem. 2025, 26, 35–41. [Google Scholar]
- Hossain, S.M.; Park, H.; Kang, H.-J.; Mun, J.S.; Tijing, L.; Rhee, I.; Kim, J.-H.; Jun, Y.-S.; Shon, H.K. Synthesis and NOx Removal Performance of Anatase S–TiO2/g-C3N4 Heterojunction Formed from Dye Wastewater Sludge. Chemosphere 2021, 275, 130020. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.W.H.; Lotsch, B.V. A Tour-Guide through Carbon Nitride-Land: Structure- and Dimensionality-Dependent Properties for Photo(Electro)Chemical Energy Conversion and Storage. Adv. Energy Mater. 2022, 12, 2101078. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kamat, P.V. A Conversation with Akira Fujishima. ACS Energy Lett. 2017, 2, 1586–1587. [Google Scholar] [CrossRef]
- Suresh, R.; Gnanasekaran, L.; Rajendran, S.; Soto-Moscoso, M.; Chen, W.-H.; Show, P.L.; Khoo, K.S. Application of Nanocomposites in Integrated Photocatalytic Techniques for Water Pollution Remediation. Environ. Technol. Innov. 2023, 31, 103149. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, R.; Zhou, S.; Duan, Y.; Jiang, P.; Wang, K. Research Progress on the Application of Photocatalytic Technology in Water Treatment. Fine Chem. Ind. 2024, 41, 707–718. [Google Scholar]
- Xavier, M.M.; Mohanapriya, S.; Divya, K.S.; Adarsh, N.N.; Nair, P.R.; Mathew, S. Exploring the Effect of Precursors of Polymeric Carbon Nitride Nanosheets on Their Photo- and Electrocatalytic Applications. ChemistrySelect 2020, 5, 12679–12689. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Dong, W.; Zeng, G. Recent Advances in Round-the-Clock Photocatalytic System: Mechanisms, Characterization Techniques and Applications. J. Photochem. Photobiol. C 2019, 39, 58–75. [Google Scholar] [CrossRef]
- Xin, X.; Shunheng, T.; Mingli, L.; Zhong, H.; Zheng, C.; Zuo, X.; Nan, J. Discussion on the Reaction Mechanism of the Photocatalytic Degradation of Organic Contaminants from a Viewpoint of Semiconductor Photo-Induced Electrocatalysis. Appl. Catal. B 2016, 198, 124–132. [Google Scholar]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2021, 34, 2107668. [Google Scholar] [CrossRef]
- Agustina, M.; Lucila, M.S.; Orlando, M.A. Photocatalytic Reactors with Suspended and Immobilized TiO2: Comparative Efficiency Evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar]
- Jafari Foruzin, L.; Rezvani, Z.; Nejati, K.; Hou, Z.; Dai, H.; Asadpour-Zeynali, K. High Quantum Efficiency of Photocatalytic Water Oxidation over the TiO2/MMO Nanocomposite under Visible-Light Irradiation. J. Mol. Liq. 2019, 288, 111035. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/Graphitic Carbon Nitride Heterojunctions for High-Efficiency Photocatalytic and Electrocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 44317–44329. [Google Scholar] [CrossRef]
- Wu, X.; Xie, S.; Zhang, H.; Zhang, Q.; F.Sels, B.; Wang, Y. Metal Sulfide Photocatalysts for Lignocellulose Valorization. Adv. Mater. 2021, 33, 2007129. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. Catalytic Conversion of CO2 to Chemicals and Fuels: The Collective Thermocatalytic/Photocatalytic/Electrocatalytic Approach with Graphitic Carbon Nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Cui, Q.; Li, Z.; Zhang, B.; Zhao, Y.; Nan, F. A Meta-Analysis on the Effects of Freeze–Thaw Cycles on Soil Soluble Carbon and Nitrogen and Microbial Biomass Carbon and Nitrogen Content. J. Ecol. Environ. 2022, 31, e008031. [Google Scholar]
- Tian, H.F.; Song, L.M. Research Progress of g-C3N4 Photocatalyst. J. Tianjin Univ. Technol. 2012, 31, 5. [Google Scholar]
- Yang, N.; Chen, Z.; Zhao, Z.; Cui, Y. Electrochemical Fabrication of Ultrafine g-C3N4 Quantum Dots as a Catalyst for the Hydrogen Evolution Reaction. New Carbon Mater. 2022, 37, 392–399. [Google Scholar] [CrossRef]
- Miao, W.; Liu, Y.; Chen, X.; Zhao, Y.; Mao, S. Tuning Layered Fe-Doped g-C3N4 Structure through Pyrolysis for Enhanced Fenton and Photo-Fenton Activities. Carbon 2020, 159, 461–470. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.; Jiang, S.P. WOx/g-C3N4 Layered Heterostructures with Controlled Crystallinity towards Superior Photocatalytic Degradation and H2 Generation. Carbon 2020, 156, 488–498. [Google Scholar] [CrossRef]
- Wang, X.; Sang, L.; Zhang, L.; Yang, G.; Guo, Y.; Yang, Y. Controllable Synthesis for Carbon Self-Doping and Structural Defect Co-Modified g-C3N4: Enhanced Photocatalytic Oxidation Performance and Mechanism Insight. J. Alloys Compd. 2023, 941, 168921. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Tian, X.; Wang, Y.; Li, R.; Zhang, Y.; Song, Z.; Xu, B.; Chu, W.; Qi, F.; et al. Coupling Metal–Organic Frameworks and g-C3N4 to Derive Graphene-Like Carbon for Peroxymonosulfate Activation: Upgrading Framework Stability and Performance. Appl. Catal. B 2019, 255, 117763. [Google Scholar] [CrossRef]
- Oh, Y.; Hwang, J.; Lee, E.-S.; Yoon, M.; Le, V.-D.; Kim, Y.-H.; Kim, D.; Kim, S. Divalent Fe Atom Coordination in Two-Dimensional Microporous Graphitic Carbon Nitride. ACS Appl. Mater. Interfaces 2016, 8, 38. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.-T.; Shin, E.W. Effect of g-C3N4 Precursors on the Morphological Structures of g-C3N4/ZnO Composite Photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Zhang, G.; Hu, L.; Wang, P. Visible-Light Responsive g-C3N4 Coupled with ZnS Nanoparticles via a Rapid Microwave Route: Characterization and Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2019, 488, 360–369. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Liu, C.; Li, X.; Song, K.; Wang, R.; Chen, W.; Zhao, G.; Liu, R.; Wang, H.; et al. Oxygen-Doped Porous g-C3N4 via Oxalic Acid-Assisted Thermal Polycondensation as a Visible-Light-Driven Photocatalyst for Bisphenol A Degradation. ACS Appl. Nano Mater. 2023, 6, 16567–16579. [Google Scholar] [CrossRef]
- Cui, L.; Song, J.; McGuire, A.F.; Kang, S.; Fang, X.; Wang, J.; Yin, C.; Li, X.; Wang, Y.; Cui, B. Constructing Highly Uniform Onion-Ring-Like Graphitic Carbon Nitride for Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution. ACS Nano 2018, 12, 5551–5558. [Google Scholar] [CrossRef]
- Ye, L.; Chen, S. Fabrication and High Visible-Light-Driven Photocurrent Response of g-C3N4 Film: The Role of Thiourea. Appl. Surf. Sci. 2016, 389, 1076–1083. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.K.A.; Dao, D.Q.; Shin, E.W. Ethanol Solvothermal Treatment on Graphitic Carbon Nitride Materials for Enhancing Photocatalytic Hydrogen Evolution Performance. Nanomaterials 2022, 12, 179. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Iqbal, W.; Shi, M.; Yang, L.; Chang, N.; Qin, C. Morphology-Effects of Four Different Dimensional Graphitic Carbon Nitrides on Photocatalytic Performance of Dye Degradation, Water Oxidation and Splitting. J. Phys. Chem. Solids 2022, 173, 111109. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, K.; Lakshmanan, A.; Sarkar, D. One-Step Synthesis of Highly Reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2021, 33, 9116–9125. [Google Scholar] [CrossRef]
- Zhao, B.; Zhong, W.; Chen, F.; Wang, P.; Bie, C.; Yu, H. High-crystalline g-C3N4 photocatalysts: Synthesis, structure modulation, and H2-evolution application. Chin. J. Catal. 2023, 52, 127–143. [Google Scholar] [CrossRef]
- Tang, S.; Xing, Y.; Wang, Y.; Wei, G. Recent advances in graphitic carbon nitride-based nanocomposites for energy storage and conversion applications. Nanotechnology 2025, 36, 122002. [Google Scholar] [CrossRef]
- Liu, H.; Yang, B.; Liao, G.; Huang, B.; Li, J.; Rodriguez, R.D.; Jia, X. Facilitating carrier kinetics in ultrathin porous carbon nitride through shear-repair strategy for peroxymonosulfate-assisted water purification. Nat. Commun. 2025, 16, 5909. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Graphitic carbon nitride based Z scheme photocatalysts: Design considerations, synthesis, characterization and applications. J. Ind. Eng. Chem. 2019, 79, 383–408. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar]
- Gao, L.; Huang, X. Research Progress on Catalytic Activity of Nano-TiO2 Semiconductor. Electron. Compon. Mater. 2002, 21, 4. [Google Scholar]
- Li, Y.; Jin, R.; Xing, Y.; Li, J.; Song, S.; Liu, X.; Li, M.; Jin, R. Macroscopic Foam-Like Holey Ultrathin g-C3N4 Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016, 6, 1601273. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.; Song, M.; Li, X.; Li, Q.; Shang, J. Directing Photocatalytic Pathway to Exceedingly High Antibacterial Activity in Water by Functionalizing Holey Ultrathin Nanosheets of Graphitic Carbon Nitride. Water Res. 2021, 198, 117125. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, X.; Song, C.; Zhou, S.; Yang, F.; Kong, Y. Engineering Carbon-Defects on Ultrathin g-C3N4 Allows One-Pot Output and Dramatically Boosts Photoredox Catalytic Activity. Appl. Catal. B 2021, 295, 120272. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, C.; Yang, Q.; Wang, X.; An, S.; Zhang, X.; Tian, J. Construction of g-C3N4 with Three-Coordinated Nitrogen (N3C) Vacancies for Excellent Photocatalytic Activities of N2 Fixation and H2O2 Production. Chem. Eng. J. 2023, 457, 141146. [Google Scholar] [CrossRef]
- Li, W.; Zeng, H.; Zhou, Z.; Li, L.; Tang, R.; Ding, C.; Gong, D.; Deng, Y. Sulfur-Synergized Dual-Cobalt Anchoring Configuration in Carbon Nitride: Deciphering Cooperative Mechanisms for Boosted Peroxymonosulfate Activation. Chem. Eng. J. 2025, 520, 166214. [Google Scholar] [CrossRef]
- Mottammal, D.; Cherusseri, J.; Thomas, S.A.; R.S., R.L.; N.Rajendran, D.; Choi, M.Y. Toward Doping in Graphitic Carbon Nitride: Progress and Perspectives on Catalytic Hydrogen Production. Adv. Mater. Technol. 2025, e00667. [Google Scholar] [CrossRef]
- Qaraah, F.A.; Mahyoub, S.A.; Shen, H.; Yin, X.; Salah, A.; A.Onaizi, S.; A.Drmosh, Q.; Xin, F. Synergistic Role of Dual-Metal Sites (Ag–Ni) in Hexagonal Porous g-C3N4 Nanostructures for Enhanced Photocatalytic CO2 Reduction. Carbon 2025, 232, 119735. [Google Scholar] [CrossRef]
- Niu, W.; Yang, Y. Graphitic Carbon Nitride for Electrochemical Energy Conversion and Storage. ACS Energy Lett. 2018, 3, 2796–2815. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, A.; Verma, N. g-C3N4-Supported NiBO3 (B = Ti, Sn) Perovskite-Based Dual-S-Scheme Heterostructure for Efficient Hydrogen Production via Water Splitting. Fuel 2025, 399, 135673. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and Uniform TiO2@g-C3N4 Core–Shell Quantum Heterojunction for Photocatalytic Degradation of Tetracycline Antibiotics. Appl. Catal. B 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and Heterogeneous Photocatalysis at Semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Cadan, F.M.; Ribeiro, C.; Azevedo, E.B. Improving g-C3N4:WO3 Z-Scheme Photocatalytic Performance under Visible Light by Multivariate Optimization of g-C3N4 Synthesis. Appl. Surf. Sci. 2021, 537, 147904. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Xie, H.; Wang, R.; Ding, C.; Huang, J.; Xu, Y.; Ye, L. One-Step Construction of S-Scheme Heterojunctions of N-Doped MoS2 and S-Doped g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2020, 404, 126498. [Google Scholar] [CrossRef]
- Zeltner, W.A.; Tompkins, D.T. Shedding Light on Photocatalysis. ASHRAE Trans. 2005, 111 Pt 2, 523–534. [Google Scholar]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic Carbon Nitride Materials: Controllable Synthesis and Applications in Fuel Cells and Photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Song, S.; Zhuo, Z.; Huang, M. Research Progress on Piezoelectric-Photocatalytic Technology and Its Application in Degradation of Organic Dye Wastewater. Green Technol. 2022, 24, 86–94. [Google Scholar]
- Wang, Z.; Hu, X.; Liu, Z.; Zou, G.; Wang, G.; Zhang, K. Recent Developments in Polymeric Carbon Nitride-Derived Photocatalysts and Electrocatalysts for Nitrogen Fixation. ACS Catal. 2019, 9, 10260–10278. [Google Scholar] [CrossRef]
- Besharat, F.; Ahmadpoor, F.; Nezafat, Z.; Nasrollahzadeh, M.; Manwar, N.; Fornasiero, P.; Gawande, M. Advances in Carbon Nitride-Based Materials and Their Electrocatalytic Applications. ACS Catal. 2022, 12, 5605–5660. [Google Scholar] [CrossRef]
- Xie, W.; Gao, L.; Wu, L.; Chen, X.; Wang, F.; Tong, D.; Zhang, J.; Lan, J.; He, X.; Mu, X.; et al. A Nonresonant Hybridized Electromagnetic-Triboelectric Nanogenerator for Irregular and Ultralow Frequency Blue Energy Harvesting. Research 2021, 2021, 5963293. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T. Electrostatics of Optical Rectification in Metallic Particles. J. Opt. Soc. Am. B 2022, 39, 990–1002. [Google Scholar] [CrossRef]
- Toprak, A.; Tigli, O. Piezoelectric Energy Harvesting: State-of-the-Art and Challenges. Appl. Phys. Rev. 2014, 1, 031104. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, Q.; Wang, Z.L. The Current Development and Future Outlook of Triboelectric Nanogenerators: A Survey of Literature. Adv. Mater. Technol. 2019, 4, 1800588. [Google Scholar] [CrossRef]
- Bowen, C.R.; Taylor, J.; LeBoulbar, E.; Zabek, D.; Chauhan, D. Pyroelectric Materials and Devices for Energy Harvesting Applications. Energy Environ. Sci. 2014, 7, 3836–3856. [Google Scholar] [CrossRef]
- Ng, T.; Liao, W. Sensitivity Analysis and Energy Harvesting for a Self-Powered Piezoelectric Sensor. J. Intell. Mater. Syst. Struct. 2005, 16, 785–795. [Google Scholar] [CrossRef]
- Caliò, R.; Rongala, U.B.; Camboni, D.; Milazzo, M.; Stefanini, C.; Petris, G.; Oddo, C. Piezoelectric Energy Harvesting Solutions. Sensors 2014, 14, 4755–4796. [Google Scholar] [CrossRef]
- Ali, F.; Raza, W.; Li, X.; Gul, H.; Kim, K. Piezoelectric Energy Harvesters for Biomedical Applications. Nano Energy 2019, 57, 879–902. [Google Scholar] [CrossRef]
- Wang, J.; Geng, L.; Zhou, S.; Zhang, Z.; Lai, Z.; Yurchenko, D. Design, Modeling and Experiments of Broadband Tristable Galloping Piezoelectric Energy Harvester. Acta Mech. Sin. 2020, 36, 592–605. [Google Scholar] [CrossRef]
- Panda, P.; Sahoo, B. PZT to Lead-Free Piezo Ceramics: A Review. Ferroelectrics 2015, 474, 128–143. [Google Scholar] [CrossRef]
- Le, A.T.; Ahmadipour, M.; Pung, S.-Y. A Review on ZnO-Based Piezoelectric Nanogenerators: Synthesis, Characterization Techniques, Performance Enhancement and Applications. J. Alloys Compd. 2020, 844, 156172. [Google Scholar] [CrossRef]
- Lu, L.J.; Ding, W.Q.; Liu, J.Q.; Yang, B. Flexible PVDF-Based Piezoelectric Nanogenerators. Nano Energy 2020, 78, 105251. [Google Scholar] [CrossRef]
- Piao, H.; Choi, G.; Jin, X.; Hwang, S.; Song, Y.; Cho, S.; Choy, J. Monolayer Graphitic Carbon Nitride as Metal-Free Catalyst with Enhanced Performance in Photo- and Electro-Catalysis. Nano-Micro Lett. 2022, 14, 55. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, C.; Gao, J.; Xue, D.; Zhang, L.; Li, S.; Ren, X. Research Progress of Lead-Free Piezoelectric ceramics. Prog. Chin. Mater. 2016, 35, 423–428. [Google Scholar]
- Kumar, A.; Choudhary, P.; Chhabra, T.; Kaur, H.; Kumar, A.; Qamar, M.; Krishnan, V. Frontier Nanoarchitectonics of Graphitic Carbon Nitride Based Plasmonic Photocatalysts and Photoelectrocatalysts for Energy, Environment and Organic Reactions. Mater. Chem. Front. 2023, 7, 1197–1247. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Lv, Z.; Lu, K. Enhanced Piezo-Phototronic Effect of ZnO Nanorod Arrays for Harvesting Low Mechanical Energy. Ceram. Int. 2019, 45, 15065–15072. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Zhao, H.; Zhang, Z.; An, B.; Bai, C.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; et al. Synthesis of Ternary ZnO/ZnS/MoS2 Piezoelectric Nanoarrays for Enhanced Photocatalytic Performance by Conversion of Dual Heterojunctions. Appl. Surf. Sci. 2021, 556, 149695. [Google Scholar] [CrossRef]
- Bayan, S.; Bhattacharya, D.; Mitra, R.K.; Ray, S. Self-Powered Flexible Photodetectors Based on Ag Nanoparticle Loaded g-C3N4 Nanosheets and PVDF Hybrids: Role of Plasmonic and Piezoelectric Effects. Nanotechnology 2020, 31, 365401. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Huang, H.; Wang, F.; Lu, C.; Kou, J.; Wang, L.; Xu, Z. Flowing Water Enabled Piezoelectric Potential of Flexible Composite Film for Enhanced Photocatalytic Performance. Chem. Eng. J. 2018, 347, 263–272. [Google Scholar] [CrossRef]
- Nagar, O.P.; Chouhan, N. Non-Metal Doped Graphitic Carbon Nitride (g-C3N4): Prospects and Review on Hydrogen Generation via Water Splitting. Int. J. Hydrogen Energy 2024, 96, 533–565. [Google Scholar] [CrossRef]
- Thennarasu, G.; Rajendran, S.; Kalairaj, A.; Rathore, H.; Panda, R.; Senthivelan, T. A Comprehensive Review on the Application of Semiconductor Nanometal Oxides Photocatalyst for the Treatment of Wastewater. Clean Technol. Environ. Policy 2025, 27, 495–516. [Google Scholar] [CrossRef]
- Liang, F.; Chen, Z.; Lu, Z.; Wang, X. Piezoelectric-Enhanced Photocatalytic Performance of Porous Carbon Nitride Nanosheets. J. Colloid Interface Sci. 2023, 630, 191–203. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Z.; Ma, J.; Zhou, M.; Wu, Z.; You, H.; Zhang, H.; Li, N.; Wang, F. High piezo-photocatalysis of BaTiO3 nanofibers for organic dye decomposition. Surf. Interfaces 2024, 48, 104308. [Google Scholar] [CrossRef]
- Lan, S.; Yu, C.; Sun, F.; Chen, Y.; Chen, D.; Mai, W.; Zhu, M. Tuning piezoelectric driven photocatalysis by La-doped magnetic BiFeO3-based multiferroics for water purification. Nano Energy 2022, 93, 106792. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, X.; Shi, J.; Wang, Y.; Yuan, G.; Liu, J.-M. Excellent piezo-photocatalytic performance of Bi4Ti3O12 nanoplates synthesized by molten-salt method. Nano Energy 2022, 98, 107247. [Google Scholar] [CrossRef]
- Abdollahpour, R.; Bazyari, A. Synergistic Photocatalysis: Enhanced Degradation of Organic Dyes Using a Heterojunction Nanocomposite of High-Surface-Area g-C3N4 and TiO2–WO3–Bi2O3/SiO2. Environ. Sci. Pollut. Res. 2025, 32, 17466–17486. [Google Scholar] [CrossRef]
- Tong, H.; Li, F.-F.; Du, M.; Song, H.; Han, B.; Jia, G.; Xu, X.; Zou, X.; Ji, L.; Kai, J.; et al. Interface Engineering, Charge Carrier Dynamics, and Solar-Driven Applications of Halide Perovskite/2D Material Heterostructured Photocatalysts. ACS Appl. Mater. Interfaces 2025, 17, 23431–23465. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, J.; Zhang, L.; Sun, Z.; Ge, C. Dual Sulfur Defect Engineering of Z-Scheme Heterojunction on Ag-CdS1−x@ZnIn2S4−x Hollow Core–Shell for Ultra-Efficient Selective Photocatalytic H2O2 Production. J. Colloid Interface Sci. 2023, 647, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Maimaitizi, H.; Okitsu, K.; Tursun, Y.; Abulizi, A. Z-Type Heterojunction of Graphene Quantum Dots/g-C3N4/BiOCl Has Excellent Nitrogen Fixation Photocatalytic Performance. Int. J. Energy Res. 2022, 46, 12147–12159. [Google Scholar] [CrossRef]
- Abdolmaleki, H.; Haugen, A.B.; Buhl, K.B.; Daasbjerg, K.; Agarwala, S. Interface Engineering of PVDF-TrFE to Achieve Higher Piezoelectric, Ferroelectric and Dielectric Properties for Sensing and Energy Harvesting Applications. Adv. Sci. 2023, 10, 2205942. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, J.; Shi, Y.; Zeng, G.; Cheng, W.; Yu, H.; Gu, Y.; Shi, L.; Yi, K. Evaluation of Self-Cleaning and Photocatalytic Properties of Modified g-C3N4-Based PVDF Membranes Driven by Visible Light. J. Colloid Interface Sci. 2019, 541, 356–366. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light. Molecules 2023, 28, 123456. [Google Scholar] [CrossRef]
- Ye, H.; Guo, T.; Zuo, G. Preparation and Photocatalytic Properties of g-C3N4/BiFeO3 Composite Visible-Light Catalyst. China Ceram. 2016, 52, 5. [Google Scholar]
- Kang, Z.; Ke, K.; Lin, E.; Qin, N.; Wu, J.; Huang, R.; Bao, D. Piezoelectric Polarization Modulated Novel Bi2WO6/g-C3N4/ZnO Z-Scheme Heterojunctions with g-C3N4 Intermediate Layer for Efficient Piezo-Photocatalytic Decomposition of Harmful Organic Pollutants. J. Colloid Interface Sci. 2022, 607, 1589–1602. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, F.; Yu, M.; Zhou, Y.; Xu, J.; Liu, J. Synthesis of Ag-Loaded NaNbO3/g-C3N4 Heterojunction for Enhanced Photocatalytic Degradation of Methyl Orange. Mater. Sci. Semicond. Process. 2025, 192, 109401. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, T.; Wu, J.; Chang, M.; Fei, H.; Li, F.; Yang, S.; Li, Q. Enhanced Removal and Selective Conversion for NO with N-Vacancies g-C3N4/BaTiO3 by Piezo-Photocatalysis. Sep. Purif. Technol. 2025, 360, 130914. [Google Scholar] [CrossRef]
- Di, B.; Wang, Z.; Wang, H.; Gong, S.; Zheng, L.; Min, Y.; Li, H. Piezoelectric Photocatalytic Degradation of Formaldehyde Based on BFO@OCN Heterojunctions. Nano Energy 2024, 132, 110384. [Google Scholar] [CrossRef]
- Kishore, A.; Sunil, S.M.; Viswanathan, R.; Kailaperumal, A.; Ali, B. Different Synthetic Routes and Band-Gap Engineering of Photocatalysts. In Photocatalysts and Electrocatalysts in Water Remediation: From Fundamentals to Full-Scale Applications; Wiley: New York, NY, USA, 2022; pp. 39–80. [Google Scholar]
- Li, H.; Luo, K.Y.; Hu, W.Y.; Li, J.; Wang, Z.; Ma, C.; Pan, Z.; Zhang, Q.; Yuan, H.; Yu, F.; et al. Facile Synthesis of Core–Shell Structure ZnO/g-C3N4 Nanocomposites with Enhanced Photocatalytic Activity. Acta Photonica Sin. 2021, 50, 1116001. [Google Scholar]
- Zhang, B.; Jin, X.; Li, X.; Dong, L.; Liu, D.; Zhang, Y.; Zhao, Z.; Ge, Q.; Zhang, F. Piezoelectric Assisted Boosting Photocatalytic Hydrogen Evolution over a Finely Prepared Pt/TiO2/g-C3N4 Composite System Using Lactic Acid as Sacrificial Agent. Int. J. Hydrogen Energy 2024, 84, 480–490. [Google Scholar] [CrossRef]
- Deng, X.; Chen, P.; Cui, R.; Gong, X.; Li, X.; Wang, X.; Deng, C. Synergistic Polarity Interaction and Structural Reconstruction in Bi2MoO6/C3N4 Heterojunction for Enhancing Piezo-Photocatalytic Nitrogen Oxidation to Nitric Acid. Appl. Catal. B 2024, 351, 123977. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, K.; Zou, S.; Xiong, B.; Wang, L.; Shi, W.; Sun, Y.; Guo, F. Construction of Surface Pit-Structured g-C3N4 by Induced SiO2 Hard Template for Boosted Piezoelectric-Assisted Photocatalytic H2O2 Production. J. Colloid Interface Sci. 2025, 699, 138118. [Google Scholar] [CrossRef]
- Sun, P.; Xiong, J.; Sun, P.; Fang, Y.; Liu, H.; Liu, H.; Xiong, B.; Wang, H.; Li, X.A. Additional O doping significantly improved the catalytic performance of Mn/O co-doped g-C3N4 for activating periodate and degrading organic pollutants. Sep. Purif. Technol. 2024, 331, 125593. [Google Scholar] [CrossRef]
- Wu, H.-H.; Chang, C.-W.; Lu, D.; Maeda, K.; Hu, C. Synergistic Effect of Hydrochloric Acid and Phytic Acid Doping on Polyaniline-Coupled g-C3N4 Nanosheets for Photocatalytic Cr (VI) Reduction and Dye Degradation. ACS Appl. Mater. Interfaces 2019, 11, 35702–35712. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Fan, J.; Zheng, H. Preparation and performance of In(OH)3/g-C3N4 catalyst containing oxygen vacancies for photodegradation of pollutants in water. Journal of civil and environmental engineering. 2020, 42, 207–208. [Google Scholar]
- Zhang, X.; Wu, H.; Lin, L.; Wu, Y.; Wang, M. Degradation mechanism of azo dyes -contaminated wastewater by g-C3N4-P25/photosynthetic bacteria composite. Acta Sci. Circumstantiae 2018, 38, 2632–2640. [Google Scholar]
- Khadim, H.J.; Ammar, S.H.; Al-Farraji, A.; Mohammed, M.S.; Jabbar, Z.H.; Alabdly, H.A. Fabrication of AgBr/Br-doped g-C3N4 hybrids for efficient piezo-photocatalytic degradation of organic pollutants in split-plate airlift reactor. J. Water Process Eng. 2025, 76, 108118. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Ammar, S.H.; Kareem, Y.S.; Al-Farraji, A. Assembly of Fe-doped g-C3N4 as a robust piezophotocatalyst system for degradation of organic dyes. J. Mol. Struct. 2025, 1344, 142962. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Z.; Shao, B.; He, Q.; Pan, Y.; Zhang, X.; Sun, J.; He, M.; Ge, L.; Cheng, C.; et al. Enhanced piezo-photocatalytic degradation of organic pollutants by cambered wall lamellar structure of porous tubular g-C3N4. Nano Energy 2024, 120, 109137. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, W.; Liang, Z.; Zhang, Y.; Gan, T.; Hu, H.; Huang, Z. Construction of a BaTiO3/tubular g-C3N4 dual piezoelectric photocatalyst with enhanced carrier separation for efficient degradation of tetracycline. Chem. Eng. J. 2023, 461, 141947. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Zhou, Y.; Deng, Y.; Feng, C.; Xiong, S.; Huang, Y.; Peng, G.; Li, L.; Zhou, Z. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B. Environ. 2022, 303, 120929. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Li, B.; Yan, Y.; Xu, R.; Meng, M.; Yan, Y. Fluid-induced piezoelectric field enhancing photocatalytic hydrogen evolution reaction on g-C3N4/LiNbO3/PVDF membrane. Nano Energy 2022, 99, 107429. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Tong, R.; Sun, Z.; Wang, X.; Yang, L.; Zhai, J.; Wang, S.; Pan, H. Mo incorporated Ni nanosheet as high-efficiency co-catalyst for enhancing the photocatalytic hydrogen production of g-C3N4. Int. J. Hydrogen Energy 2020, 45, 18912–18921. [Google Scholar] [CrossRef]
- Xu, H.; She, X.; Fei, T.; Song, Y.; Liu, D.; Li, H.; Yang, X.; Yang, J.; Li, H.; Song, L.; et al. Metal-Oxide-Mediated Subtractive Manufacturing of Two-Dimensional Carbon Nitride for High-Efficiency and High-Yield Photocatalytic H2 Evolution. ACS Nano 2019, 13, 11294–11302. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Li, H.; Zhang, J.; Zhang, R. Preparation and Photocatalytic Hydrogen Production Properties of Cyano-modified g-C3N4. J. Synth. Cryst. 2020, 49, 631. [Google Scholar]
- Meng, L.; Zhao, C.; Chu, H.; Li, Y.-H.; Fu, H.; Wang, P.; Wang, C.-C.; Huang, H. Synergetic piezo-photocatalysis of g-C3N4/PCN-224 core-shell heterojunctions for ultrahigh H2O2 generation. Chin. J. Catal. 2024, 59, 346–359. [Google Scholar] [CrossRef]
- Fu, M.; Luo, J.; Shi, B.; Tu, S.; Wang, Z.; Yu, C.; Ma, Z.; Chen, X.; Li, X. Promoting Piezocatalytic H2O2 Production in Pure Water by Loading Metal-Organic Cage-Modified Gold Nanoparticles on Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2024, 63, e202316346. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fan, S.; Li, X.; Duan, J.; Zhang, D. Modulating the Molecular Structure of Graphitic Carbon Nitride for Identifying the Impact of the Piezoelectric Effect on Photocatalytic H2O2 Production. ACS Catal. 2023, 13, 9515–9523. [Google Scholar] [CrossRef]
- Liu, P.; Liang, T.; Bian, J.; Li, Z.; Zhang, Z.; Jing, L. Improved photocatalytic H2O2 production of boron doped g-C3N4 by controllably co-modifying nanosized PDI and Ag. Mater. Res. Bull. 2023, 167, 112370. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, J.; Qiu, B.; Zhou, Y.; Lei, J.; Xing, M.; Wang, L.; Zhou, Y.; Liu, Y.; Zhang, J. Ultrathin g-C3N4 nanosheet with hierarchical pores and desirable energy band for highly efficient H2O2 production. Appl. Catal. B Environ. 2020, 267, 118396. [Google Scholar] [CrossRef]
- Chu, C.; Miao, W.; Li, Q.; Wang, D.; Liu, Y.; Mao, S. Highly efficient photocatalytic H2O2 production with cyano and SnO2 co-modified g-C3N4. Chem. Eng. J. 2022, 428, 132531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).