Novel Strategy to Evaluate Platinum Photocatalysts for Hydrosilation-Curable Silicones
Abstract
1. Introduction
2. Results and Discussion
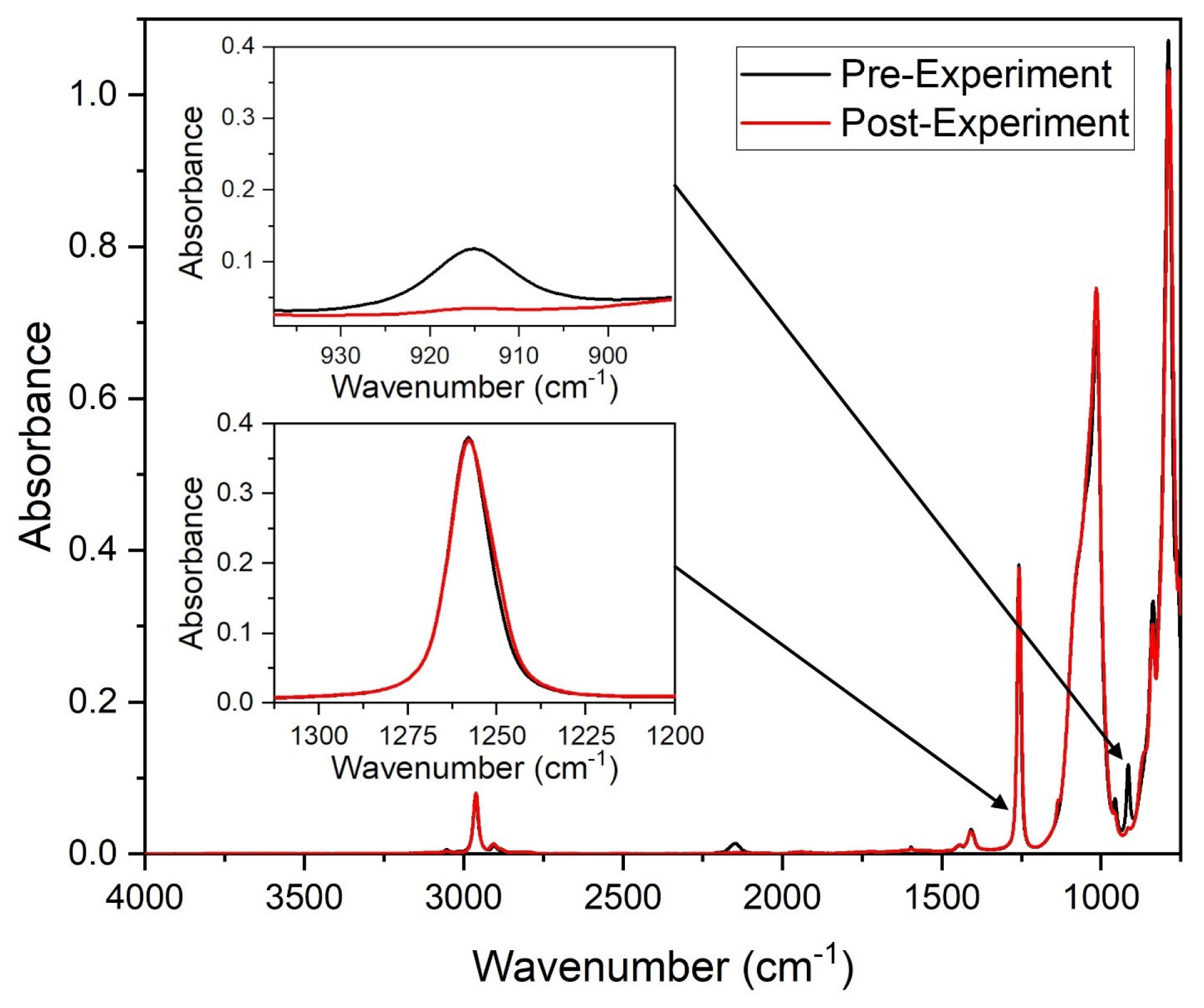
2.1. Kinetics of Polymerization
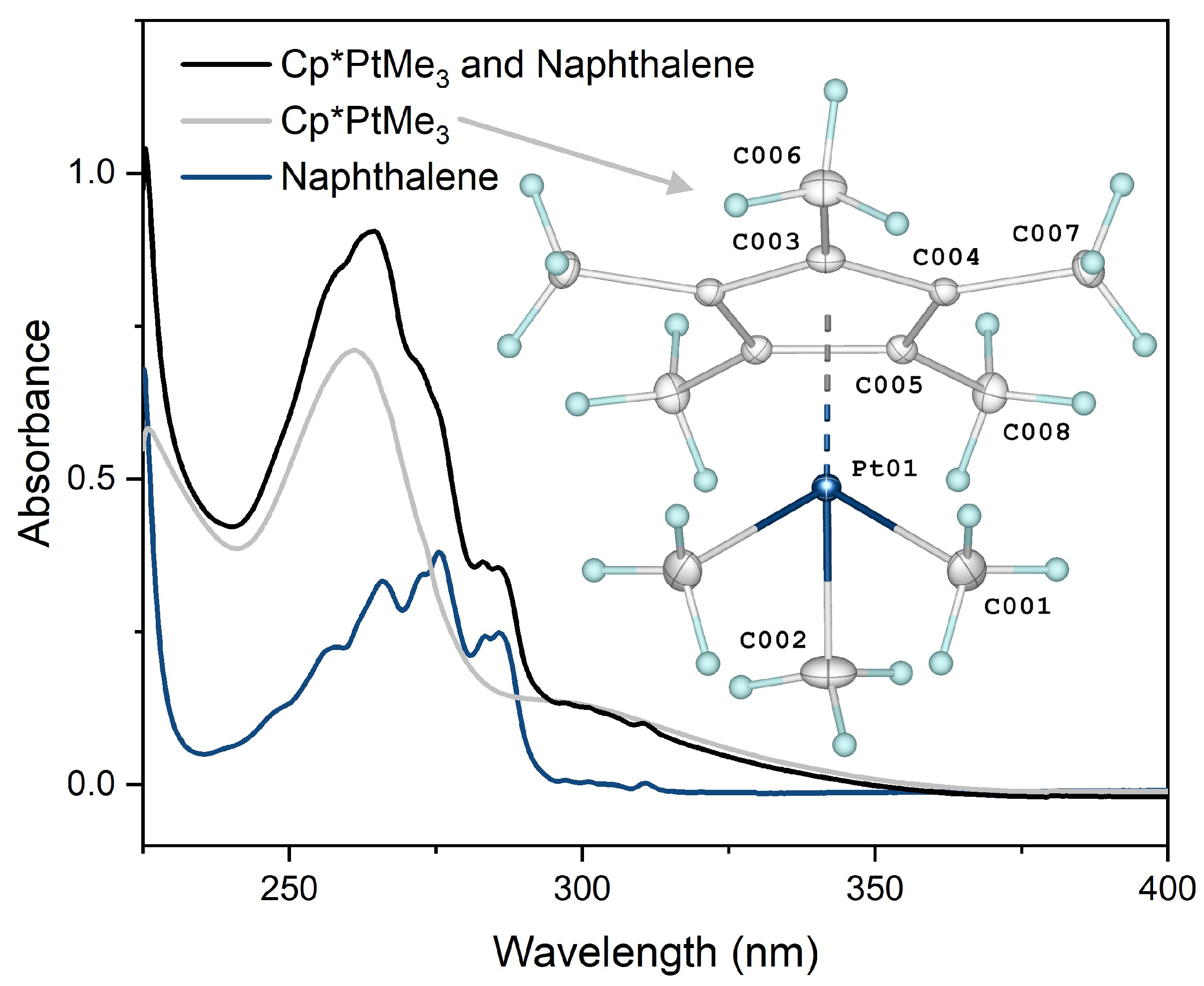
2.2. X-ray Crystallography
3. Materials and Methods
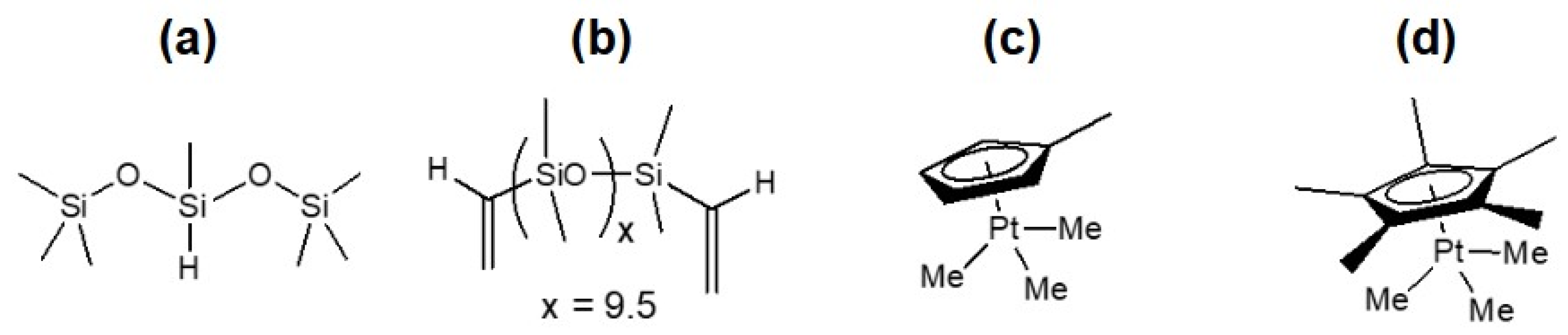
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- de Buyl, F. Silicone Sealants and Structural Adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Morari, C.; Balan, I.; Pintea, J.; Chitanu, E.; Iordache, I. Electrical Conductivity and Electromagnetic Shielding Effectiveness of Silicone Rubber Filled with Ferrite and Graphite Powders. Prog. Electromagn. Res. M 2011, 21, 93–104. [Google Scholar] [CrossRef]
- Owen, M.J. Interfacial Activity of Polydimethylsiloxane. In Surfactants in Solution; Springer: Boston, MA, USA, 1986; pp. 1557–1569. [Google Scholar] [CrossRef]
- Ekeland, R.A.; Tonge, J.S.; Gordon, G.V. Release Force Understanding-Recent Findings; Dow Corning Corporation: Midland, MI, USA, 2005; Available online: https://www.semanticscholar.org/paper/Release-Force-Understanding-%E2%80%93-Recent-Findings-Ekeland-Tonge/ae177a6d1e45cd12e36271a555a46d31999d2f71 (accessed on 18 July 2024).
- Eckberg, R.P. Coatings Technology Handbook, 3rd ed.; Tracton, A.A., Ed.; CRC Press Taylor Fr. Group: New York, NY, USA, 2005; Chapter 92 Silicone Release Coatings. [Google Scholar] [CrossRef]
- Marciniec, B.; Guliński, J. Recent Advances in Catalytic Hydrosilylation. J. Organomet. Chem. 1993, 446, 15–23. [Google Scholar] [CrossRef]
- Tondreau, A.M.; Atienza, C.C.H.; Weller, K.J.; Nye, S.A.; Lewis, K.M.; Delis, J.G.P.; Chirik, P.J. Iron Catalysts for Selective Anti-Markovnikov Alkene Hydrosilylation Using Tertiary Silanes. Science 2012, 335, 567–570. [Google Scholar] [CrossRef]
- Xi, L.; Liu, Z.; Su, J.; Bei, Y.; Xiang, H.; Liu, X. UV-Activated Hydrosilylation of (Me-Cp)Pt(Me)3: Enhanced Photocatalytic Activity, Polymerization Kinetics, and Photolithography. J. Appl. Polym. Sci. 2019, 136, 48251. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Brokken-Zijp, J.; Laven, J.; Huinink, H.P.; Reuvers, N.J.W.; Van, M.P.; de With, G. Influence of Cross-Linker Concentration on the Cross-Linking of PDMS and the Network Structures Formed. Polymer 2009, 50, 3955–3966. [Google Scholar] [CrossRef]
- Yang, D.B. Kinetic Studies of Photopolymerization Using Real Time FT-IR Spectroscopy. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 199–208. [Google Scholar] [CrossRef]
- Mayer, T.; Burget, D.; Mignani, G.; Fouassier, J.P. Photohydrosilylation Reaction of Silicone Polymers. Platinum-Based Photocatalysts: Trimethyl(β-Dicarbonyl) Platinum IV Complexes. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3141–3146. [Google Scholar] [CrossRef]
- Burget, D.; Mayer, T.; Mignani, G.; Fouassier, J.P. Kinetic Study of the Photoactivated Hydrosilylation of Some β-Dicarbonyl Complexes of Trialkylplatinum(IV). J. Photochem. Photobiol. A Chem. 1996, 97, 163–170. [Google Scholar] [CrossRef]
- Jakubek, V.; Lees, A.J. Quantitative Photochemistry of Cp‘Pt(CH3)3 (Cp‘ = η5-C5H4CH3) in Solution: A Highly Efficient Organometallic Photoinitiator for Hydrosilylation. Inorg. Chem. 2004, 43, 6869–6871. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Marchi, S.; Meier, P.; Kornmann, X. UV-Activated Hydrosilation Reaction for Silicone Polymer Crosslinking. J. Appl. Polym. Sci. 2012, 128, 1521–1526. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Meier, P.; Kornmann, X. A Comparison of the Reactivity of Two Platinum Catalysts for Silicone Polymer Cross-Linking by UV-Activated Hydrosilation Reaction. Macromol. React. Eng. 2015, 9, 360–365. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Meier, P.; Kornmann, X. Visible Light-Activated Hydrosilation Reaction. J. Photochem. Photobiol. A Chem. 2015, 303–304, 86–90. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Ligorio, D.; Meier, P.; Kornmann, X. Impressive Rate Raise of the Hydrosilation Reaction through UV-Activation: Energy and Time Saving. Macromol. Mater. Eng. 2016, 301, 610–613. [Google Scholar] [CrossRef]
- Hofmann, J. IR Spectroscopic Method for Determination of Silicone Cross-Linking; Pressure Sensitive Tape Council: Chicago, IL, USA, 2016. [Google Scholar]
- Xiang, H.; Wang, X.; Lin, G.; Xi, L.; Yang, Y.; Lei, D.; Dong, H.; Su, J.; Cui, Y.; Liu, X. Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate. Polymers 2017, 9, 552. [Google Scholar] [CrossRef]
- Eckberg, R.P. Photo-Initiated Addition Cure Silicone Release Coatings. In Proceedings of the Radtech Conference Proceeding, Disney Coronado Springs, Radtech UV+EB 2020, Orlando, FL, USA, 8–11 March 2020; Available online: https://radtech2020.com/wp-content/uploads/Papers/Materials%20II/Eckberg%20-%20Photo-Activated%20Hydrosilation%20-%20Prospects%20for%20UV%20Curable%20Silicone%20Coatings%20Applications.pdf (accessed on 8 July 2024).
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7, 100. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Martir, D.R.; Chen, S.; Xu, W.Z.; Oyeneye, O.O.; Chakrabarti, S.; Zysman-Colman, E.; Charpentier, P.A. Tuning the Optical Properties of Silicon Quantum Dots via Surface Functionalization with Conjugated Aromatic Fluorophores. Sci. Rep. 2018, 8, 3050. [Google Scholar] [CrossRef]
- Szabó, Á.; Szöllősi, J.; Nagy, P. Principles of Resonance Energy Transfer. Curr. Protoc. 2022, 2, e625. [Google Scholar] [CrossRef]
- Clegg, R.M. Fluorescence Resonance Energy Transfer. Curr. Opin. Biotechnol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. CCDC Mogul 2023.3.1 Retrieval of Crystallographically-Derived Molecular Geometry Information. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Ramamoorthy, V.; Sharp, P.R. Synthesis of Monomeric (η5-pentamethylcylopentadienyl)platinum(IV) Methyl and Bromo Complexes and of [Hydrotris(3,5-dimethyl-7-pyrazolyl)borato]trimethylplatinum. Inorg. Chem. 1990, 29, 3345–3349. [Google Scholar] [CrossRef]
- Rockensuss, W.; Roesky, H.W.; Gilje, J.W.; Noltemeyer, M. Chloro-dimethyl-(η5-pentamethyl-cyclopentadienyl)-platinum(IV), CSD Commun. 1992. Refcode: SOWTED. Available online: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=SOWTED&DatabaseToSearch=Published (accessed on 8 July 2024).
- Howard, W.A.; Bergman, R.G. The Synthesis of Olefin, Carbonyl and Thiolate Complexes of Platinum(IV) in the [η5-C5Me5)Pt(IV)] System. Crystal and Molecular Structure of (η2-C5Me5)PtMe2[S(p-CH3(C6H4]. Polyhedron 1998, 17, 803–810. [Google Scholar] [CrossRef]
- Xue, Z.; Strouse, J.; Shuh, D.K.; Knobler, C.B.; Kaesz, H.D.; Hicks, R.F.; Williams, R.S. Characterization of (Methylcyclopentadienyl)trimethylplatinum and Low-temperature Organometallic Chemical Vapor Deposition of Platinum Metal. J. Am. Chem. Soc. 1989, 111, 8779–8784. [Google Scholar] [CrossRef]
- Kohler, K.; Steiner, A.; Roesky, H.W. Die Kristallstrukturen von (η5-C5Me5)MoMe4 und (η5-C5Me5)WMe4/The Crystal Structures of (η5-C5Me5)MoMe4 and (η5-C5Me5)WMe4. Z. für Naturforschung B 1995, 50, 1207–1209. [Google Scholar] [CrossRef]
- Maus, D.C.; Copie, V.; Sun, B.; Griffiths, J.M.; Griffin, R.G.; Luo, S.; Schrock, R.R.; Liu, A.H.; Seidel, S.W.; Davis, W.M.; et al. A Solid-State NMR Study of Tungsten Methyl Group Dynamics in [W(η5-C5Me5)Me4][PF6]. J. Am. Chem. Soc. 1996, 118, 5665–5671. [Google Scholar] [CrossRef]
- Decker, J.M.; Geib, S.J.; Meyer, T.Y. The Synthesis and Olefin Reactivity of Neutral and Cationic Tantalum Amidinate−pentamethylcyclopentadienyl Complexes. Organometallics 1999, 18, 4417–4420. [Google Scholar] [CrossRef]
- Schrock, R.R.; Liu, A.H.; O’Regan, M.B.; Finch, W.C.; Payack, J.F. Preparation and Characterization of Two Unsubstituted Hydrazido(1-) Complexes, W(η5-C5Me5)Me4(η2-NHNH2) and [W(η5-C5Me5)Me3(η2-NHNH2)]+[SO3CF3]−. Inorg. Chem. 1988, 27, 3574–3583. [Google Scholar] [CrossRef]
- Alaimo, P.J.; Bergman, R.G. Modeling the Proposed Intermediate in Alkane Carbon−hydrogen Bond Activation by Cp*(PMe3)Ir(Me)OTf: Synthesis and Stability of Novel Organometallic Iridium(V) Complexes. Organometallics 1999, 18, 2707–2717. [Google Scholar] [CrossRef]
- Glassman, T.E.; Vale, M.G.; Schrock, R.R. Synthesis and Structure of a Tungsten(IV) η2-dimethyldiazene Complex in which the Diazene Ligand Behaves as a Four-electron Donor. Inorg. Chem. 1992, 31, 1985–1986. [Google Scholar] [CrossRef]
- Vale, M.G.; Schrock, R.R. Synthesis and Reactions of Monomeric Hydrazine and Hydrazido Complexes that Contain the Cp*MoMe3 core. Inorg. Chem. 1993, 32, 2767–2772. [Google Scholar] [CrossRef]
- Schrock, R.R.; Glassman, T.E.; Vale, M.G.; Kol, M. High-Oxidation-State Pentamethylcyclopentadienyl Tungsten Hydrazine and Hydrazido Complexes and Cleavage of the Nitrogen-Nitrogen Bond. J. Am. Chem. Soc. 1993, 115, 1760–1772. [Google Scholar] [CrossRef]
- Moro, M.; Zardi, P.; Rossi, M.; Biffis, A. Evaluation of Heteroleptic Pt (II) β-Diketonate Complexes as Precatalysts for the Photoactivated Curing of Silicone Resins. Catalysts 2022, 12, 307. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
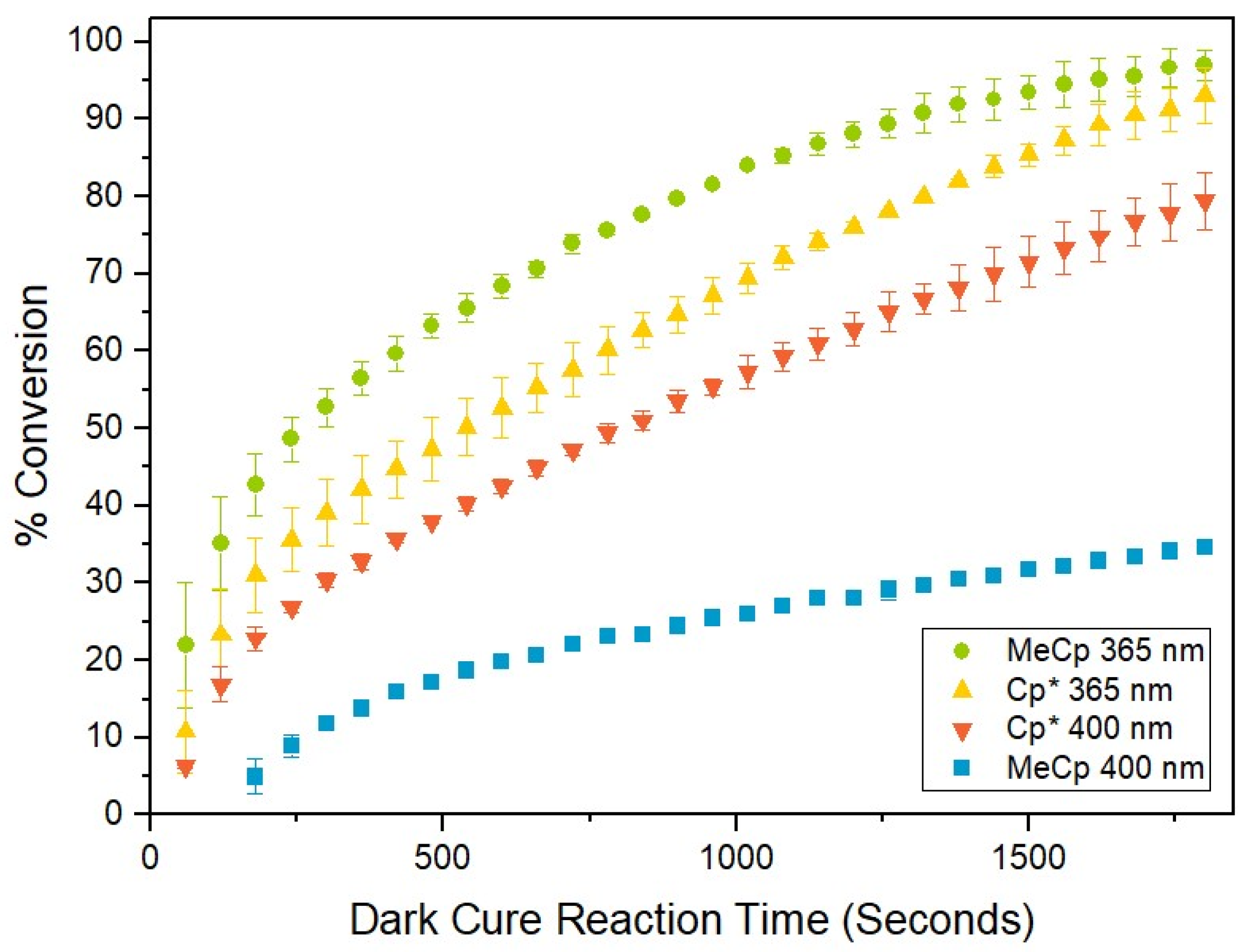

| Experiment | Ratei (×10−3 Ms−1) | t1/2 (s) | k (×10−3 s−1) | % Conversion |
|---|---|---|---|---|
| 365 nm, PS, 300 s | 3.01 | 351 | 1.98 | 96.8 |
| 365 nm, PS, 150 s | 2.09 | 501 | 1.38 | 95.3 |
| 365 nm, 300 s | 1.88 | 583 | 1.19 | 97.0 |
| 400 nm, PS, 300 s | 1.14 | 876 | 0.791 | 88.9 |
| 400 nm, 300 s | 0.417 | 6410 | 0.108 | 34.7 |
| Experiment | Ratei (×10−3 Ms−1) | t1/2 (s) | k (×10−3 s−1) | % Conversion |
|---|---|---|---|---|
| 365 nm, PS, 300 s | 1.39 | 692 | 1.00 | 96.0 |
| 365 nm, 300 s | 1.10 | 844 | 0.821 | 93.1 |
| 400 nm, 300 s | 0.864 | 1100 | 0.627 | 79.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidis, M.; Leman, J.; Bonitatibus, P.J., Jr. Novel Strategy to Evaluate Platinum Photocatalysts for Hydrosilation-Curable Silicones. Inorganics 2024, 12, 197. https://doi.org/10.3390/inorganics12070197
Michailidis M, Leman J, Bonitatibus PJ Jr. Novel Strategy to Evaluate Platinum Photocatalysts for Hydrosilation-Curable Silicones. Inorganics. 2024; 12(7):197. https://doi.org/10.3390/inorganics12070197
Chicago/Turabian StyleMichailidis, Melina, John Leman, and Peter J. Bonitatibus, Jr. 2024. "Novel Strategy to Evaluate Platinum Photocatalysts for Hydrosilation-Curable Silicones" Inorganics 12, no. 7: 197. https://doi.org/10.3390/inorganics12070197
APA StyleMichailidis, M., Leman, J., & Bonitatibus, P. J., Jr. (2024). Novel Strategy to Evaluate Platinum Photocatalysts for Hydrosilation-Curable Silicones. Inorganics, 12(7), 197. https://doi.org/10.3390/inorganics12070197







